Abstract
Mice with genetic deletion of the cholesterol transporter ATP Binding Cassette (ABC)G1 have pulmonary lipidosis and enhanced innate immune responses in the airway. Whether ABCG1 regulates adaptive immune responses to the environment is unknown. To this end, Abcg1+/+ and Abcg1−/− mice were sensitized to ovalbumin via the airway using low-dose lipopolysaccharide as an adjuvant, and then challenged with ovalbumin aerosol. Naive Abcg1−/− mice displayed increased B cells, CD4+ T cells, CD8+ T cells, and dendritic cells (DCs) in lung and lung-draining mediastinal lymph nodes, with lung CD11b+ DCs displaying increased CD80 and CD86. Upon allergen sensitization and challenge, the Abcg1−/− airway, compared to Abcg1+/+, displayed reduced Th2 responses (IL-4, IL-5, eosinophils), increased neutrophils and IL-17, but equivalent airway hyperresponsiveness. Reduced Th2 responses were also found using standard intraperitoneal ovalbumin sensitization with aluminum hydroxide adjuvant. Mediastinal lymph nodes from airway-sensitized Abcg1−/− mice produced reduced IL-5 upon ex vivo ovalbumin challenge. Abcg1−/− CD4+ T cells displayed normal ex vivo differentiation, whereas Abcg1−/− DCs were found paradoxically to promote Th2 polarization. Th17 cells, IL-17+ γδT cells, and IL-17+ neutrophils were all increased in Abcg1−/− lungs, suggesting Th17 and non-Th17 sources of IL-17 excess. Neutralization of IL-17 prior to challenge normalized eosinophils and reduced neutrophilia in the Abcg1−/− airway. We conclude that Abcg1−/− mice display IL-17-mediated suppression of eosinophilia and enhancement of neutrophilia in the airway following allergen sensitization and challenge. These findings identify ABCG1 as a novel integrator of cholesterol homeostasis and adaptive immune programs.
Introduction
Whereas regulation of intracellular cholesterol has long drawn its most attention in the fields of macrophage biology and atherosclerosis, emerging reports have begun to reveal an unexpectedly central role for cholesterol in T cell biology and adaptive immunity. Cell proliferation and cholesterol homeostasis were recently shown to be coupled in T cells insofar as intracellular cholesterol levels change during T cell activation and serve as a metabolic checkpoint on cell division (1). Liver X Receptor (LXR)2, a nuclear receptor that serves as the primary cell sensor for oxidized cholesterol, suppresses T cell proliferation via inducing its target gene, the cholesterol efflux transporter ATP Binding Cassette (ABC)G1, to reduce intracellular cholesterol levels; conversely, Lxr−/− and Abcg1−/− mice have lymphoid hyperplasia and enhanced vaccine-driven T lymphocyte proliferation (1, 2). Synthetic LXR agonists have moreover recently been shown to exert complex effects on immune polarization, suppressing Th17 differentiation (3, 4), modifying dendritic cell (DC) maturation in favor of a Th2 program (5), and reducing IgE production by B cells (6).
Although the role of LXR in T cell proliferation has begun to be elucidated, the physiologic relevance of the LXR-ABCG1 axis as an endogenous regulator of allergic disease has yet to be demonstrated. ABCG1 is in particular highly expressed in the lung, wherein it has recently been shown by us and others not only to guard against cholesterol overload in macrophages, but also to regulate constitutive cytokine expression and leukocyte trafficking (7-9), as well as the innate immune response (9). Abcg1−/− mice have low-level induction in the airspace of a complex array of cytokines, including those that are Th1 (interleukin [IL]-12)-, Th2 (granulocyte macrophage-colony stimulating factor [GM-CSF])-, and Th17 (IL-6)-promoting (7, 9). Conversely, both GM-CSF and transforming growth factor [TGF]-β, a T regulatory- and Th17-promoting cytokine, upregulate ABCG1 (10, 11). ABCG1 thus participates in complex feedback with T cell-polarizing cytokines. As the lung is continually exposed to aeroallergens and innate immune stimuli, and atopic asthma results from unchecked Th2 and Th17 responses that are primed by the innate immune system (12), we speculated that ABCG1 might be centrally positioned as a regulator of asthma pathogenesis.
Herein, we report that ABCG1 regulates the response to allergen in the airway. Following ovalbumin (OVA) sensitization and challenge, a widely used model of atopic asthma, the Abcg1−/− airway, compared to wild type, displays reduced Th2 responses, but increased neutrophils (PMNs) and IL-17. We rule out changes in CD4+ T cell-intrinsic differentiation, and find, paradoxically, that Abcg1−/− DCs promote Th2 polarization. Neutralization of IL-17 prior to OVA challenge ‘rescues’ some aspects of the Abcg1−/− phenotype, normalizing intra-airway and peribronchiolar eosinophils. Taken together, we propose that ABCG1 coordinates airway adaptive immunity, its deletion suppressing adaptive lung eosinophilia through an IL-17-dependent mechanism.
Materials and Methods
Animals
Age- (8-12 weeks) and sex-matched mice were used for all experiments. Abcg1−/− mice backcrossed >6 generations onto C57BL/6 (98.2-99.1% C57BL/6 across 110 microsatellite markers [data not shown]) were generated as described (13). C57BL/6 controls were purchased from The Jackson Laboratory. All experiments were performed in accordance with the Animal Welfare Act and the U.S. PHS Policy on Humane Care and Use of Laboratory Animals after approval by the NIEHS Animal Care and Use Committee.
OVA Sensitization and Challenge
Similar to a previous report (12), mice were sensitized to OVA on days 0 and 7, and challenged on day 14. For airway sensitizations, anesthetized mice underwent oropharyngeal aspiration of 100 μg low-endotoxin (<1 EU/mg) OVA (Profos AG, Regensburg, Germany), supplemented with 0.1 μg E. coli 0111:B4 LPS (Sigma, St.Louis, MO) in PBS vehicle (50 μl volume). For intraperitoneal (i.p.) sensitizations, mice were injected with 100 μg OVA (Sigma) complexed in 50% aluminum hydroxide (Pierce, Rockford, IL) in 200 μl. In all experiments, mice were challenged on a single occasion (day 14) for 1 hour with an aerosol of 1% OVA (Sigma) in saline. Mice were then harvested at the indicated times post-challenge.
Bronchoalveolar lavage fluid (BALF) collection and analysis
BALF was collected as previously described, and airway leukocytes counted and typed by morphology (9). BALF cytokines/chemokines were measured by ELISA or Bio-Plex assay (Bio-Rad).
Flow cytometric analysis
Lungs were extracted, minced, and digested with collagenase XI, hyaluronidase, DNase, and Liberase (1 hr, 37°C), and the reaction was stopped with ethylenediaminetetraacetic acid. Single cell suspensions were enriched on a discontinuous density gradient using Histopaque (Sigma). Washed cells were diluted to 2×107/ml and incubated with a blocking cocktail of purified rat anti-mouse CD16/CD32 (BD Pharmingen, San Jose, CA), normal mouse serum, and normal rat serum (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 20 minutes. For staining of surface antigens, cells were labeled with antibodies against mouse CD4 (clone GK1.5, eBioscience, or clone GK1.5, BD Pharmingen), TCR β (clone H57–597, BD Pharmingen), TCR γδ (clone GL3, BD Pharmingen), or the appropriate isotype control antibodies. For intracellular staining, cells were stimulated with 50 ng/ml phorbol myristate acetate and 500 ng/ml ionomycin (Sigma) for 4 hours before staining and incubated with GolgiStop (BD Pharmingen) during the last 3 hours of stimulation. Cells were fixed and permeabilized using Cytofix/Cytoperm (BD Biosciences) and labeled with antibodies against IL-17A (clone eBio17B7, eBioscience), IL-5, or IFN-γ (Ebioscience). Additional antibodies used include anti-Siglec-F (PE, clone E50-2440, BD Pharmingen), anti-CD11b (PE, clone M1/70, BioLengend), anti-CD45 (APC/Cy7, clone 30-F11, BioLegend), anti-B220 (APC, clone RA3-6B2, Biolegend), anti-CD11c (FITC [Ebioscience] or PE [Biolegend], clone N418), anti-CD103 (PE, clone 2E7, Biolegend), anti-CD80 (APC, clone 16-1OA1, Biolegend), anti-CD86 (APC, clone GL-1, Biolegend), anti-Gr1 (FITC, clone RB6-8C5, Biolegend), and anti-F4/80 (PE, clone BM8, Biolegend). CD4+ lymphocytes were identified as non-autofluorescent cells within a lymphocyte gate based on forward and side scatter, DCs as CD11c+, non-autofluorescent cells, and γδT cells as γδTCR+TCRβ−. Cells were collected using a BD LSR II cytometer (BD Biosciences) and data were analyzed using FlowJo 7.2.2 software (Tree Star, Inc., Ashland, OR).
Ex vivo CD4+ T cell differentiation and DC/OT-II co-cultures
Naïve CD4+ T cells were purified from mouse spleens using the autoMACS system (Miltenyi Biotec, Auburn, CA) and appropriate antibodies per manufacturer’s instructions. For ex vivo CD4+ T cell differentiation studies, naïve CD4+ T cells from Abcg1+/+ and Abcg1−/− mice were cultured in plates pre-coated with anti-CD3 (1 μg/ml) and –CD28 (5 μg/ml) antibodies for 5 days under Th1 (2 ng/ml IL-2, 5 μg/ml anti-IL-4, 10 ng/ml IL-12) or Th2 (2 ng/ml IL-2, 5 ng/ml IL-4, 5 μg/ml anti-IFN-γ) polarizing conditions. Flow cytometry was then performed to quantitate intracellular expression of IL-4 and IFN-γ. For DC/OT-II T cell cultures, CD11b+ and CD103+ DCs were sorted from the CD11c+/autofluorescence– gate of disaggregated lung from naïve mice and pulsed for 16 hours with OVA (50 μg/ml) in the presence of LPS (60 ng/ml). The following morning, OVA-pulsed DCs were co-cultured with naïve splenic CD4+ T cells (5×104 DCs : 1×105 T cells, 200 μl volume) that had been purified from OT-II mice, a transgenic strain in which the TCR of CD4+ T cells is restricted to OVA. Following 5 days of co-culture, IL-4, IFN-γ, and IL-17A were quantified in culture supernatants by ELISA.
Measurement of nuclear factor (NF)-κB activation in lung parenchymal nuclear isolates
Lungs were harvested from naïve mice, and then p65 NF-κB DNA binding activity quantified (p65 TransAM™, Active Motif) in nuclear isolates (Nuclear Extract Kit, Active Motif) according to the manufacturer’s instructions, as previously reported (9).
In vivo IL-17 neutralization
Fifty μg of anti-IL17A antibody (clone 50104, R&D systems) or isotype control (clone 54447, R&D systems, Minneapolis, MN) was injected i.p. 30 minutes prior to challenge.
Invasive airway measurements
Airway responses to aerosolized methacholine were invasively measured on anesthetized mice as previously described (12), using the Flexivent system (Scireq, Montreal, PQ, Canada). A single compartment model was used to assess total respiratory system resistance (R) after delivery of increasing doses of methacholine (0–50 mg/ml), and individual peak responses were determined at each dose for each mouse.
DC migration studies
Ovalbumin, Alexa Fluor (AF) 647-conjugate (Invitrogen, Carlsbad, CA) was administered directly to the airways via oropharyngeal aspiration. Lung-draining thoracic lymph nodes were harvested 24 hours later, enzymatically digested, and AF-OVA+ DCs quantified by flow cytometry. OVA+ DC counts in the MLN were also normalized to the number of lung-resident DCs in naïve Abcg1+/+ and Abcg1−/− mice in order to standardize DC migration propensity to number of lung-resident DCs.
Ex vivo OVA restimulation of mediastinal lymph nodes (MLNs) and splenocytes
For MLN studies, mice were airway sensitized as described above. On day 14, MLNs were collected and mechanically disrupted. Lymph node cells were cultured in the presence of OVA (100 μg/ml, 5 days), after which cytokines were quantified in culture supernatants (ELISA). For splenocyte studies, mice were i.p. sensitized as described above. On day 14, spleens were harvested and disaggregated splenocytes were similarly assayed.
Serum IgG1, IgG2a, and IgE measurement
Mice were sensitized via the airways or the peritoneum as described above and serum was collected on day 14. For measurement of OVA-specific IgG1 and IgG2a, serum was serially diluted and applied for 2 hours to ELISA plates that were pre-coated with OVA (50 ug/ml). The plate was then washed and a biotinylated anti-IgG1 (or anti-IgG2a) antibody was added for 1 hour. After another wash, the plate was loaded with streptavidin-HRP (30 min), and the reaction then developed with HRP substrate. OVA-specific IgE in serum was measured as previously described (14).
Immunoblotting
Equal protein whole cell lysates were resolved by 10% SDS-PAGE, transferred to nitrocellulose (Bio-Rad, Hercules, CA), and probed (1:1000) with rabbit anti-ABCG1 antibody (Novus Biologicals, Littleton, CO) or rabbit anti-β-actin antibody (Cell Signaling, Danvers, MA). Membranes were then washed in Tween 20 and Tris-buffered saline and exposed (60 min) to 1:5000 HRP-conjugated secondary antibody (GE Healthcare, Piscataway, NJ) in 5% milk/Tween 20 and Tris-buffered saline. After further washes, signal was detected with ECL Western Blot Detection Reagents (GE Healthcare), followed by film exposure (GE Healthcare).
RNA analysis
Tissues were preserved in RNAlater (Ambion/Applied Biosystems, Austin, TX). RNA was extracted (Ambion/Applied Biosystems) and quantified spectrophotometrically. cDNA was transcribed using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). 50 ng of cDNA was used for qPCR (in duplicate) using Taqman probe-and-primer combinations for IL-13 (Mm00434204_m1) and GAPDH (Mm99999915_g1) (Applied Biosystems). Ct values were determined using ABI 7500 Real Time PCR System with SDS software 1.3.1. Change in expression was calculated using the 2−ΔΔCt method normalized to GAPDH expression and expressed as fold-change compared to the control group.
Quantitation of airway goblet cells
Formalin fixed paraffin embedded lung tissues were sectioned at 5 μm thickness and stained with Alcian blue/Periodic acid-Schiff (AB/PAS) stain for quantitating the number of mucus-positive cells. High-resolution digital scans of glass slides were made using the Aperio Scanscope XT Scanner (Aperio Technologies, Inc., Vista, CA) at 40X. Only those bronchi with a length-to-width ratio of <2.5 were selected for counting PAS-positive cells so as to minimize error that might arise from tangential sectioning (15). The circumferences of each of the bronchi were annotated and the length of the bronchial epithelial perimeter was measured using Aperio’s Imagescope. The number of goblet cells was manually enumerated and normalized to the length of the annotated bronchial circumference as the number of AB/PAS-positive goblet cells per mm of the bronchial basement membrane.
Quantitation of peribronchiolar eosinophils
Representative 5 μm formalin fixed paraffin embedded lung sections were stained with the eosinophil-specific histochemical stain Sirius Red (16), and digitally scanned (Aperio) as described above. The same airways that were annotated for AB/PAS-positive goblet cell estimation, as described above, were evaluated for eosinophil counts within Sirius Red-stained serial sections. The number of peribronchiolar eosinophils was manually enumerated within the annotated peribronchiolar regions up to 100 μm from the basement membranes and normalized to the length of the annotated bronchial circumference.
Statistical Analysis
Data are represented as mean ± SEM. A two-tailed student’s t test was applied for comparisons of two groups (GraphPad Prism software, San Diego, CA). ANOVA was used for comparisons of >2 groups. For all tests, p<0.05 was considered significant.
Results
Abcg1−/− mice display altered steady state lung and MLN leukocyte populations
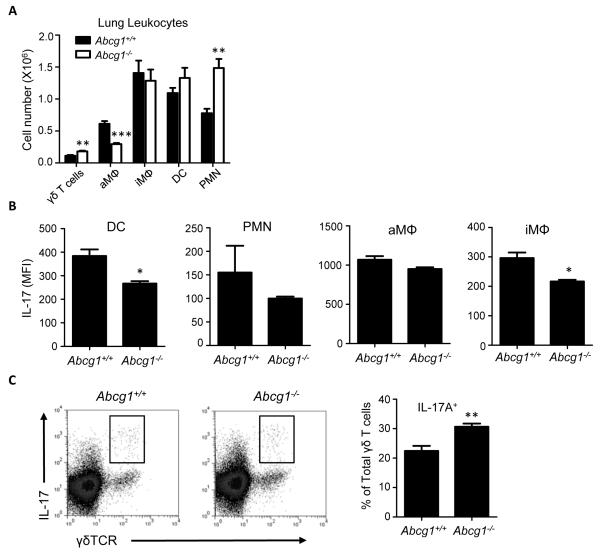
We and others recently reported that unexposed Abcg1−/− mice have elevated recruitment of several leukocyte subtypes to the airspace in the steady state including PMNs and lymphocytes, as well as low-level induction in the airway of a wide array of cytokines (7-9). This phenotype is thought to stem from overproduction of cyto-/chemokines by ABCG1-deficient hematopoietic cells in the lung, perhaps due to cholesterol overload (7-9). In order to more comprehensively explore the effect of ABCG1 deletion on thoracic immune cell populations as a backdrop to investigating ABCG1 in the airway response to allergen, we profiled the cellular composition of lung parenchyma from unexposed Abcg1+/+ and Abcg1−/− mice using flow cytometric markers. Initial studies revealed that 45.1 +/− 1.4% (mean +/− SEM) of total lung parenchymal cells were CD45+ in naïve Abcg1−/− mice, as compared to 35.8 +/− 2.3% in naïve Abcg1+/+ mice (p<0.05), consistent with a global increase in leukocyte composition of the resting Abcg1−/− lung. As shown in Figure 1A, this reflects significant increases in CD4+ T cells, CD8+ T cells, B cells, DCs, and PMNs, whereas other leukocyte subpopulations are unchanged in number (γδT cells, alveolar macrophages, interstitial macrophages, eosinophils).
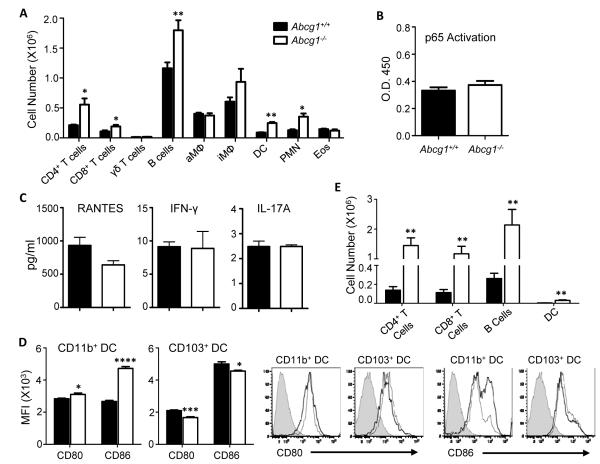
Figure 1. ATP-binding cassette (Abc)g1−/− mice have expanded steady state leukocyte populations in the lung and lung-draining lymph nodes.
(A) Total WBC were isolated from lungs of naïve Abcg1+/+ and Abcg1−/− mice. The indicated leukocyte populations were enumerated by flow cytometry (CD4+ T cells [CD3+CD4+]; B cells [CD19+B220+]; dendritic cells (DC)[CD11c+autofluorneg], neutrophils (PMNs)[Gr-1hi]; CD8+ T cells [CD3+CD8+]; γδT cells [γδTCR+TCRβ−]; alveolar macrophages (aMΦ)[CD11c+autofluorhi]; interstitial macrophages (iMΦ)[CD11c–CD11b+F4/80+]; eosinophils [Siglec-F+]). Data represents mean +/− SEM (n=5 mice/group), and is representative of 3 independent experiments. (B) The nuclear fraction was isolated from lung parenchyma of naïve Abcg1+/+ and Abcg1−/− mice, and assayed for p65 NF-κB DNA binding activity (n=5/genotype). (C) Cytokines were quantified in lung parenchyma (RANTES, IFN-γ) or BALF (IL-17) of naïve mice (n=4-6/genotype). (D) CD11b+ and CD103+ dendritic cells from lungs of naïve mice were assayed by flow cytometry for cell surface display (mean fluorescence intensity [MFI]) of CD86 and CD80 (n=5/genotype). Representative histograms are shown (unstained controls [shaded trace], Abcg1+/+ [light line], Abcg1−/− [dark line]). (E) Leukocytes were enumerated as in A from mediastinal lymph nodes of naïve mice. *, p<0.05; **, p<0.01; ***, p<0.001.
Despite the marked increase in select immune cell populations, NF-κB activation was not different in the nuclear fraction of Abcg1−/− lung parenchyma than in that of wild type (wt) counterparts (Figure 1B), arguing against significant induction of type-1 inflammation. Consistent with this, we found wt levels of IL-17 in BALF, and of IFN-γ and RANTES in lung parenchyma (Figure 1C). Nonetheless, upon examining the DC populations in the Abcg1−/− lung more closely, we found significantly increased display of the co-stimulatory molecules CD86 and CD80 that was restricted to CD11bhiCD103lo (hereafter, CD11b+) DCs, consistent with increased maturation of this DC subset (Figure 1D). By contrast, there was a modest reciprocal downregulation of CD80 and CD86 in CD103hiCD11blo (hereafter, CD103+) DCs. The lung-draining mediastinal lymph nodes (MLNs) of naïve Abcg1−/− mice also contained significant increases above wt in CD4+ T cells, CD8+ T cells, B cells, and DCs (Figure 1E). Taken together, these data suggest that ABCG1 plays a broadly spanning role in steady state immune homeostasis in the lung and MLNs, regulating the size of several leukocyte populations as well as the maturity status of DCs.
Abcg1−/− mice have reduced airway Th2 and elevated IL-17 responses to allergen
We next used an OVA sensitization model to determine whether the adaptive airway response to allergens is modified by ABCG1 deletion. For our primary studies, rather than sensitize via standard i.p. injection of OVA complexed to aluminum hydroxide (alum) adjuvant, we used an alternate model in which mice are OVA-sensitized through the airway using admixed low-dose (0.1 μg) LPS as adjuvant, followed by a single OVA airway challenge (12, 17). In addition to its environmental and physiologic (sensitization via airway) relevance, we favored this model as it allows for physiologic interactions of the innate and adaptive immune systems of the lung (17).
Given the increased DC co-stimulatory molecule display in the resting ABCG1-deficient lung, we initially speculated that Abcg1−/− mice might not require adjuvant to generate an adaptive response. However, like wt, Abcg1−/− mice that had either been airway-sensitized with LPS-free OVA or not sensitized did not recruit leukocytes (WBCs) to the airway upon OVA challenge (Figure 2A). OVA-sensitized and -challenged Abcg1−/− mice recruited greater numbers of WBCs to the airway than wt counterparts. Interestingly, this reflects a >2-fold greater expansion of airway PMNs in the face of significantly fewer eosinophils (Figure 2B-C). Consistent with reduced Th2 and Th1 but increased Th17 responses in the ABCG1-deficient lung, Abcg1−/− mice had reduced BALF IL-4, IL-5, and IFN-γ, but higher BALF IL-17 (Figure 2D). Serum OVA-specific IgG1 and IgG2a were similar between genotypes following airway sensitization (Figure 2E), whereas OVA-specific IgE was not consistently detected in either strain after airway sensitization (data not shown). Airway Th2 and Th17 responses cooperate in promoting allergen-induced airway hyperresponsiveness (AHR) (12). Perhaps reflecting counterbalancing effects of the decreased Th2/increased IL-17 phenotype of the ABCG1-deficient lung, sensitized- and-challenged Abcg1+/+ and Abcg1−/− mice had equivalent AHR (Supplemental Figure 1).
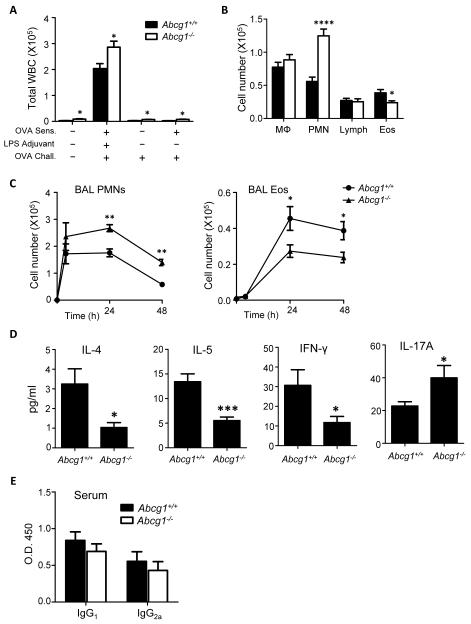
Figure 2. Pulmonary responses are dysregulated in Abcg1−/− mice following allergen sensitization and challenge.
(A) Mice were either mock-sensitized or sensitized via airway administration of PBS with or without ovalbumin (OVA) and/or LPS. All mice were then challenged with aerosolized OVA. BALF total leukocytes (white blood cells [WBCs]) were counted 48 hours after challenge. (B-D) Mice were sensitized via intratracheal administration of OVA with LPS and then challenged with aerosolized OVA followed by quantitation of pulmonary responses. (B) BALF macrophages (MΦ), neutrophils (PMN) lymphocytes (Lymph), and eosinophils (Eos) were counted 48 hours after challenge. (C) BALF neutrophils and eosinophils were enumerated at indicated times after challenge. (D) BALF was collected at 4 hours after challenge to measure IL-4, IL-5, and IL-17 by ELISA. IFN-γ was measured in BALF harvested 48 hours after challenge. Data represents mean +/− SEM (n=5-15 mice in each group). (E) Serum OVA-specific IgG1 and Ig2a were quantified (day 14) after airway-OVA/LPS sensitization on days 0 and 7. Data are representative of 2 independent experiments (n=9-10/genotype). *, p<0.05; **, p<0.01; ***, p<.001; ****, p<.0001.
Different results were observed using a more traditional model of i.p. OVA/alum sensitization followed by one-time airway OVA challenge. Using this Th2-permissive, Th17-nonpermissive model (12), increased PMNs in the airway; decreased production of IL-4, IL-5, and IFN-γ in the BALF; and normal serum levels of OVA-specific IgG1, IgG2a, and IgE were observed in Abcg1−/− mice after OVA challenge (Supplemental Figure 2A-D). However, unlike the airway OVA sensitization model, airway eosinophils and IL-17A were equivalent between genotypes in the i.p. OVA/alum model.
Abcg1−/− mice have reduced Th2 cells in lung and reduced Th2 responses in MLN
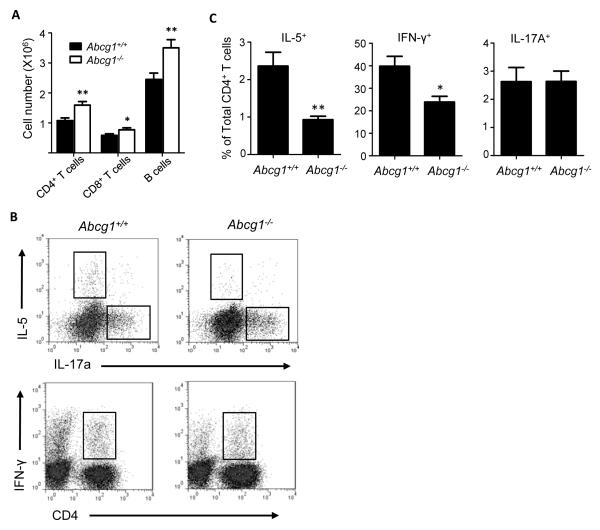
Having found lower IL-4, IL-5, and IFN-γ, and higher IL-17 in the BALF of allergen-sensitized and -challenged Abcg1−/− mice compared to wt, we next sought to confirm whether these differences reflect underlying changes in recruitment of Th1, Th2, and Th17 cells. As shown in Figure 3A, after airway sensitization and challenge, Abcg1−/− lungs contained more CD4+ T cells than wt. Compared to wt, however, they contained reduced percentages of IL-5+ and IFN-γ+ CD4+ T cells, consistent with lesser recruitment of Th2 and Th1 cells (Figure 3B-C). By contrast, Abcg1+/+ and Abcg1−/− lungs had equivalent percentages of IL17+ CD4+ T cells, suggesting intact recruitment of Th17 cells in Abcg1−/− lungs (Figure 3B-C).
Figure 3. In vivo Th1 and Th2 differentiation is impaired in airway-sensitized and challenged Abcg1−/− mice.
Mice were sensitized via airway administration of OVA/LPS and then challenged with aerosolized OVA. Leukocytes were prepared from whole lungs harvested at 24 hours after challenge. (A) Total CD4+ T cells, CD8+ T cells, and B cells were enumerated by flow cytometry. (B) Representative dot plots depict IL-5, IL-17A, IFN-γ expression in CD4+ T cells. (C) Bar graphs represent cytokine-positive CD4+ T cells measured as a percentage of total CD4+ T cells in the lung. Data represent mean +/− SEM (n=5-15 mice in each group). *, p<0.05; **, p<0.01.
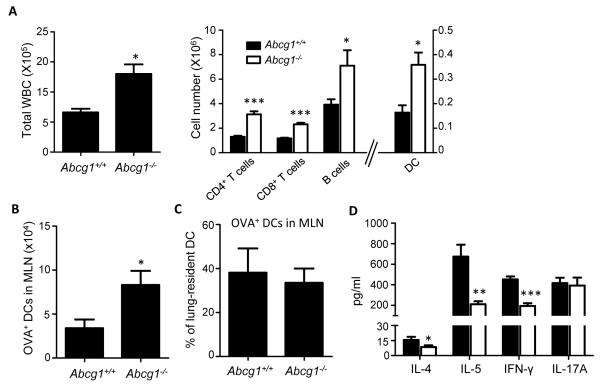
Several leukocyte subtypes, including DCs, were increased in sensitized Abcg1−/− MLNs compared to wt (Figure 4A). In order to examine whether DC trafficking from lung to MLN during sensitization is altered in Abcg1−/− mice, we monitored the appearance of Alexa Fluor (AF) 647-OVA+ DCs in MLNs 24 hrs after airway exposure to AF-OVA (18). Higher numbers of AF-OVA+ DCs were found in MLNs of Abcg1−/− mice than in those of wt counterparts (Figure 4B); however, upon normalization to lung DC number, we determined that an equal percentage of lung-resident DCs are migratory in Abcg1+/+ and Abcg1−/− mice (Figure 4C). This suggests that DC trafficking is not intrinsically altered in Abcg1−/− mice, but that increased numbers of sentinel DCs in the resting Abcg1−/− lung nevertheless account for enhanced absolute flux of antigen-positive DCs to the MLN upon sensitization.
Figure 4. In vivo Th1 and Th2 priming in lung draining lymph nodes is impaired following airway sensitization.
(A) Mice were sensitized on days 0 and 7 via intratracheal administration of OVA/LPS and cells were analyzed in mediastinal lymph nodes harvested on day 14. Total WBCs and subpopulations were enumerated by flow cytometry. (B-C) Ovalbumin, Alexa Fluor (AF) 647-conjugate was administered to the airways of naïve Abcg1+/+ and Abcg1−/− mice via oropharyngeal aspiration. Lung-draining mediastinal lymph nodes (MLNs) were harvested 24 hours later, enzymatically digested, and AF-OVA+ DCs quantified by flow cytometry. In B, the absolute number of OVA+ DCs in the MLN is shown. In C, these cell numbers are shown as a percentage of the number of DCs present in the lung of the respective genotypes at baseline. The data are representative of 2 independent experiments, n=5/genotype; *, p<0.05. (D) Equal numbers of mediastinal lymph node cells from OVA-sensitized mice were cultured in the presence of OVA (100 ug/ml) for 5 days followed by quantitation of indicated cytokines in culture supernatant by ELISA. ELISAs were performed in triplicate wells and data is presented as mean +/− SEM. The data shown are representative of 3 independent experiments. *, p<0.05; **, p<0.01; ***, p<0.001.
In order to model antigen presentation events in the MLN, equal numbers of MLN cells from airway-sensitized Abcg1+/+ and Abcg1−/− mice were next plated and restimulated with OVA. Consistent with the results for lung CD4+ T cells (Figure 3), OVA-restimulated MLNs from Abcg1−/− mice produced lower IL-4, IL-5, and IFN-γ, but equal IL-17 compared to wt (Figure 4D). By contrast, splenocytes from i.p. OVA/alum-sensitized Abcg1+/+ and Abcg1−/− mice produced equivalent IL-4, IL-5, IFN-γ, and IL-17 upon OVA restimulation (Supplemental Figure 2E), suggesting that ABCG1 deletion modifies the response to allergen in the lung and MLN differently from that in the spleen.
Taken together, these findings suggest that Th1 and Th2 polarization are reduced in the lungs and MLNs of Abcg1−/− mice, and that Th17 polarization is intact. Although the percentage of IL-17+ CD4+ T cells was normal in Abcg1−/− lungs after sensitization and challenge, absolute numbers of CD4+ T cells were increased in the Abcg1−/− lung (Figure 3A), suggesting that the excess airspace IL-17 in these mice may, at least in part, stem from increased Th17 cells. Nonetheless, the nearly 2-fold increase in BALF IL-17 above wt in Abcg1−/− mice (Figure 1) suggested that the increased airspace IL-17 might also derive from a cellular source other than Th17 cells.
IL-17 expression is selectively increased in Abcg1−/− γδT cells
Multiple lung cell types have the capacity to express IL-17. In addition to the well-studied Th17 effector type of (αβ)CD4+ T cells, macrophages, γδT cells, PMNs, and invariant NKT cells also express IL-17 in different contexts (19-22), although the latter are not a significant source of IL-17 after airway OVA sensitization/challenge (12). After sensitization and challenge, Abcg1+/+ and Abcg1−/− lungs had equal numbers of both interstitial macrophages and DCs; however, both cell types expressed lesser IL-17 in Abcg1−/− lungs (Figure 5A-B). Abcg1−/− alveolar macrophages expressed IL-17 at normal levels, but were reduced in number compared to wt. PMNs were increased ~2-fold in Abcg1−/− lungs and expressed comparable IL-17 to wt. Finally, γδT cells were increased both in number and in the percentage that were IL-17+ (Figure 5A, 5C). Taken together, these findings suggest that γδT cells along with PMNs likely account at least in part for the increased BALF IL-17 in Abcg1−/− mice after OVA sensitization and challenge.
Figure 5. IL-17 production from non-Th17 cells in the Abcg1−/− lung.
Mice were sensitized via intratracheal administration of OVA/LPS and then challenged with aerosolized OVA. (A) Leukocytes were prepared from whole lungs harvested at 24 hours after challenge and indicated subsets were quantified by flow cytometry (n=10/genotype). (B) IL-17 expression in non-T cell leukocyte subsets was measured by flow cytometry. (C) Representative dot plots depicting IL-17 expression in lung-resident γδT cells (γδTCR+TCRβ−), and bar graph depicting quantitation of % IL-17+ γδT cells. Data are mean +/− SEM (n=5-10 mice/group), and is representative of 2-3 separate experiments. *, p<0.01; **, p<0.001; ***; p<0.0001.
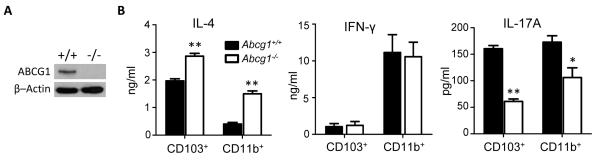
Abcg1−/− CD4+ T cells have normal differentiation whereas Abcg1−/− DCs are Th2-polarizing
Having detected fewer Th2 cells in lung and lesser Th2 cytokines in OVA-restimulated MLNs from Abcg1−/− mice, we next sought to better define the mechanism underlying reduced Th2 differentiation. As cytokine-driven Th2 polarization of cultured naïve splenic CD4+ T cells from Abcg1+/+ and Abcg1−/− mice was equivalent (Supplemental Figure 3), we examined whether ABCG1 expression modifies the capacity of DCs to instruct CD4+ T cells along different Th programs. This appeared plausible given the increased numbers of CD86hiCD11b+ DCs in the lungs of naïve Abcg1−/− mice, and prior reports that CD86 is Th2-promoting (23). Immunoblotting of whole cell lysates of sorted (>95% purity) naïve splenic DCs confirmed DC expression of ABCG1 (Figure 6A). We purified CD103+ and CD11b+ DCs from the lungs of Abcg1+/+ and Abcg1−/− mice, pulsed them with OVA in the presence of LPS, and then co-cultured them with naïve splenic CD4+ T cells from OT-II mice, a transgenic strain in which the TCR of CD4+ T cells is restricted to OVA. Abcg1−/− DC co-cultures of both the CD103+ and CD11b+ variety produced heightened IL-4 and reduced IL-17, whereas IFN-γ release did not differ between genotypes in either DC subpopulation (Figure 6B). This finding was somewhat unexpected, as it indicates that ABCG1-deficient DCs display increased capacity to program Th2 differentiation, and thus that altered DC function cannot account for, and may mitigate against, the Th2-suppressed phenotype observed in Abcg1−/− mice in vivo.
Figure 6. DCs from Abcg1−/− mice selectively induce the differentiation of Th2 cells.
(A) Whole cell lysates prepared from sorted naïve splenic DCs were subjected to SDS-PAGE and immunoblotted for ABCG1 and β–Actin. (B) CD103+ and CD11b+ DCs sorted from the lungs of naïve Abcg1+/+ or Abcg1−/− mice were pulsed ex vivo with OVA in the presence of LPS, and then co-cultured with naïve CD4+ T cells isolated from spleens of OT-II mice for 5 days. IL-4, IFN-γ, and IL-17A were quantified in culture supernatant by ELISA. Experiments were performed in triplicate wells and data is presented as mean +/− SEM. Representative of 3 independent experiments. *, p<0.01; **, p<0.001.
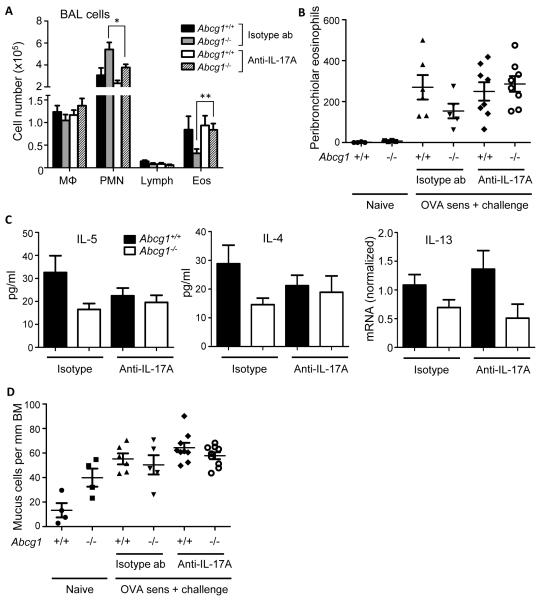
Neutralization of IL-17 prior to challenge normalizes eosinophils in the Abcg1−/− lung
Having found no indication that changes intrinsic to CD4+ T cells or DCs account for reduced Th2 cells in the Abcg1−/− lung, we next speculated that the high-IL-17 environment of the Abcg1−/− lung might suppress Th2 polarization and eosinophilia. Several groups including prior work by the authors in the airway OVA sensitization model have reported that deficient IL-17 signaling increases eosinophils and Th2 cytokines and reduces PMNs in the lung (12, 21, 24), whereas IL-17 supplementation has the opposite effect (12, 21). In particular, challenge-phase IL-17 has been convincingly shown to suppress Th2 responses (25, 26).
In order to address this, we treated OVA-sensitized Abcg1+/+ and Abcg1−/− mice i.p. with anti-IL-17A antibody or isotype control 30 minutes preceding OVA challenge (24, 25, 27). As shown in Figure 7A, IL-17 neutralization normalized the relative reduction of airway eosinophils in Abcg1−/− mice. A similar effect was seen for peribronchiolar eosinophils, as evaluated by Sirius Red stain of lung (Figure 7B, Supplemental Figure 4). Conversely, IL-17 neutralization significantly reduced airway PMNs in Abcg1−/− but not Abcg1+/+ mice. BALF IL-4 and IL-5 were also equalized in Abcg1+/+ and Abcg1−/− mice after IL-17A neutralization, but this appeared to reflect modest and statistically nonsignificant reciprocal decreases in the Abcg1+/+ airway and increases in Abcg1−/− airway (Figure 7C). IL-13 mRNA was lower in Abcg1−/− than Abcg1+/+ lungs under both isotype control antibody and anti-IL-17A antibody treatment conditions (Figure 7C). Finally, there were increased numbers of mucus-positive cells in naïve Abcg1−/− bronchioles compared to naive Abcg1+/+ bronchioles, but no significant differences across genotypes/treatment conditions after OVA (Figure 7D, Supplemental Figure 4). Taken together, these findings suggest that challenge-phase IL-17 overproduced likely by both Th17 and non-Th17 cells is responsible at least in part for the reduced eosinophils and increased PMNs observed after allergen sensitization and challenge in Abcg1−/− mice. However, at least within the parameters of our study, other features of the Th2 phenotype were either not reduced in the Abcg1−/− lung compared to wt after OVA-sensitization/challenge (airway mucus cells), or were not clearly reversed in the Abcg1−/− lung following IL-17A blockade (Th2 cytokines).
Figure 7. IL-17 neutralization during the challenge phase corrects allergen-induced leukocyte trafficking to Abcg1−/− airway.
(A) Airway OVA-sensitized Abcg1+/+ and Abcg1−/− mice were treated i.p. with 50 μg of anti-IL-17A antibody or isotype control antibody (ab) 30 minutes before OVA challenge. Leukocyte subpopulations were enumerated in the airway 24 hours post-challenge. Data represent mean +/− SEM from n=5-10 mice/condition over the course of 3 independent experiments. *, P=0.05; **, P<0.01. (B) Lungs from Abcg1+/+ and Abcg1−/− mice that had been treated as in A, or that were untreated (‘naïve’) were stained for eosinophils with Sirius Red. Tissue eosinophils within 100 μm of the major airways were then manually counted (n=4-8/condition; p=NS for all comparisons among OVA-exposed mice). (C) Mice treated as in A had BALF IL-5 and IL-4 quantified by Bioplex assay and lung parenchymal IL-13 mRNA (normalized to GAPDH) quantified by qPCR 24 hrs post-challenge. Data are mean +/− SEM (n=5/genotype), and are representative of three independent experiments. (D) Lungs from mice that were treated as in B were stained with Alcian Blue/PAS to identify mucus-positive goblet cells in the airway. Mucus-positive cells were quantified per 1 mm of bronchial basement membrane (BM) (n=4-8/condition; p<.05 [ANOVA] for naive Abcg1+/+ vs. naïve Abcg1−/−, but p=NS for all comparisons among OVA-exposed mice).
Discussion
We identify the cholesterol transporter ABCG1 as a novel regulator of adaptive immunity in the lung that exerts complex effects on several interacting cell types in the airway. Despite increased numbers of CD86hiCD11b+ DCs in the Abcg1−/− lung that display enhanced Th2-promoting capacity ex vivo, Abcg1−/− mice have a reduced Th2 response to allergen in the airway. We provide evidence that increased IL-17 in the Abcg1−/− lung is likely produced by both Th17 and non-Th17 cells and acts during the challenge phase of allergen exposure to suppress some features of the Th2 phenotype, in particular, airspace and peribronchiolar eosinophilia. This mechanism is consistent with several prior reports that challenge-phase IL-17 suppresses Th2 responses in the airway in other settings (25, 26).
Our use of two alternate routes/methods for sensitization, i.p. OVA/alum and intra-airway OVA/LPS, reveals further complexity in regulation of airway adaptive immunity by ABCG1. We previously reported that the i.p. OVA/alum method does not recruit Th17 cells to (or induce IL-17 in) the C57BL/6 lung following a single airway OVA challenge, whereas these responses occur robustly after challenge in airway OVA/LPS-sensitized mice (12). Thus, bypassing the airway during sensitization is not permissive to Th17 responses to a one-time allergen challenge. The wt-equivalent, low-level BALF IL-17A in Abcg1−/− mice after i.p. sensitization suggests that ABCG1 deletion does not bypass this requirement for airway sensitization in Th17 induction. Increased IL-17A also appeared to derive from non-Th17 cells in airway-sensitized and challenged Abcg1−/− mice, including PMNs, which were increased in the naïve Abcg1−/− lung. Thus, notwithstanding the immune dysregulation of the naïve Abcg1−/− lung, enhanced IL-17A production by non-Th17 cells in response to OVA challenge also appears to require sensitization via the airway.
As Abcg1−/− mice displayed reduced airway Th2 cytokines and eosinophils in the low IL-17 state following OVA sensitization by the traditional i.p. route, additional mechanisms likely contribute to reduced Th2 polarization in the Abcg1−/− lung. Indeed, although the restimulation studies of MLNs from sensitized mice (Figure 4) do involve ex vivo OVA challenge, they nevertheless suggest that ABCG1 may also regulate Th2 programming during sensitization. If so, such effects may interestingly be restricted to sensitization via the airway as Th2 responses were normal upon OVA restimulation of splenocytes from Abcg1−/− mice sensitized via the i.p. route.
ABCG1 is expressed in several of the lung cell types that contribute to asthma pathogenesis, including CD4+ T cells, DCs, macrophages, epithelial cells, and airway smooth muscle cells (1, 13, 28, 29) whereas we recently reported minimal ABCG1 expression in PMNs (9), and are unaware of prior reports of ABCG1 expression in eosinophils and γδT cells. Known consequences of ABCG1 deficiency that may impact cell function include expansion of lipid rafts with consequent enhancement of raft-dependent signaling (30, 31), and cell overload with oxysterols leading to secondary LXR activation (2). In the case of DCs, treatment with oxidized low density lipoprotein and synthetic LXR agonists are both reported to enhance Th2-polarizing function (5, 32), similar to what we observed in Abcg1−/− DCs. ABCG1 deficiency also enhances GM-CSF signaling in hematopoietic cells through expansion of lipid rafts (30), and thus would also be expected to promote maturation of DCs by this Th2 cytokine. Thus, LXR activation and expanded lipid rafts, both consequences of lipid overload, may possibly contribute to the Th2 bias we observed in Abcg1−/− DCs.
Regarding CD4+ T cells, LXR agonists reduce IFN-γ expression (33), also similar to our present findings for Abcg1−/− CD4+ T cells. However, unlike the case for Abcg1−/− CD4+ T cells, LXR activation also suppresses Th17 differentiation (3, 4). Thus, activation of LXR in Abcg1−/− CD4+ T cells, shown previously by another group using target gene profiling (2), is unlikely to be a central determinant of the overall in vivo phenotype of the Abcg1−/− lung, and it appears likely that other changes in the Abcg1−/− lung (e.g., increased IL-6 (9)) or perhaps intrinsic to the Abcg1−/− CD4+ T cell itself, may counterbalance LXR to preserve Th17 differentiation.
Finally, IL-17+ γδT cells differentiate in the thymus (34) and ABCG1 is reported to regulate thymocyte function (2). Thus, it is interesting to speculate that ABCG1 deficiency in γδT cells or in other thymic cells may account for the increased numbers of IL-17+ γδT cells we observed. In the end, given our finding that IL-17 acts as an exogenous suppressor of Th2 differentiation in the ABCG1-deficient lung, it seems likely that paracrine communications within the complex environment of the Abcg1−/− lung confer the in vivo phenotype. It is noteworthy in this regard that γδT cell-derived IL-17 was also recently reported to play a critical role in suppressing Th2 responses in the OVA-exposed lung of wt mice (21).
Of interest, human ABCG1 expression is altered during several conditions, and thus may possibly influence human disease through effects on adaptive immune programming. In pulmonary alveolar proteinosis, a rare disease in which patients display alveolar lipid overload reminiscent of the lung phenotype of the Abcg1−/− mouse, alveolar macrophages are ABCG1-deficient due to insufficient PPARγ-dependent gene induction by GM-CSF (11). While pulmonary alveolar proteinosis is rare, emerging reports of ABCG1 polymorphisms in humans (35) suggest that more widespread variation in ABCG1 expression/function likely exists in human populations. Indeed, macrophages from patients with diabetes mellitus are ABCG1-deficient (36), whereas the thiazolidinediones, PPARγ agonists that are widely prescribed in the treatment of diabetes, upregulate ABCG1 (11). As Th17 cells are increased in both type I and type II diabetes (37, 38), and IL-17 promotes islet cell injury (39), further investigation of the role of ABCG1 both in the inflammatory state of diabetes and in the therapeutic response to thiazolidinediones may be warranted. Notably, thiazolidinediones were recently reported to reduce IL-17 expression and inflammation in a murine model of asthma (27). Statins (HMG CoA reductase inhibitors), drugs widely prescribed for atherosclerotic cardiovascular disease and dyslipidemia but recently studied for their anti-inflammatory effects in the lung and other tissues (40), also downregulate ABCG1 in human macrophages (41, 42). Thus, ABCG1 may regulate the immunomodulatory response to statins, with possible consequences on the lung adaptive immune response.
In summary, we identify ABCG1 as an important common link among cholesterol homeostasis, innate immunity, and adaptive immunity, and demonstrate that ABCG1 is a critical regulator of both immune homeostasis and response to environment in the lung. It is noteworthy that the neutrophilic, high IL-17 asthma phenotype of Abcg1−/− mice is reminiscent of that described in severe asthma in human subjects (43). Future investigations will be necessary to better clarify the cell-specific roles of ABCG1 in the adaptive immune response of the lung; to test whether ABCG1 expression/function is altered in human asthma; and to determine whether pharmacologic manipulation of ABCG1 may be a feasible strategy in the treatment of human lung disease.
Supplementary Material
Acknowledgments
The authors thank Norris Flagler and Elizabeth Ney for assistance with histological image acquisition and editing.
Footnotes
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (Z01 ES102005).
ABCG1, ATP binding cassette G1; alum, aluminum hydroxide; BALF, bronchoalveolar lavage fluid; DC, dendritic cell; LXR, liver X receptor; MLN, mediastinal lymph node; PMN, neutrophil
References
- 1.Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD, Tontonoz P. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong AJ, Gebre AK, Parks JS, Hedrick CC. ATP-binding cassette transporter G1 negatively regulates thymocyte and peripheral lymphocyte proliferation. J Immunol. 2010;184:173–183. doi: 10.4049/jimmunol.0902372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui G, Qin X, Wu L, Zhang Y, Sheng X, Yu Q, Sheng H, Xi B, Zhang JZ, Zang YQ. Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation. J Clin Invest. 2011;121:658–670. doi: 10.1172/JCI42974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J, Wagoner G, Douglas JC, Drew PD. Liver X receptor agonist regulation of Th17 lymphocyte function in autoimmunity. J Leukoc Biol. 2009;86:401–409. doi: 10.1189/jlb.1008600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geyeregger R, Zeyda M, Bauer W, Kriehuber E, Saemann MD, Zlabinger GJ, Maurer D, Stulnig TM. Liver X receptors regulate dendritic cell phenotype and function through blocked induction of the actin-bundling protein fascin. Blood. 2007;109:4288–4295. doi: 10.1182/blood-2006-08-043422. [DOI] [PubMed] [Google Scholar]
- 6.Heine G, Dahten A, Hilt K, Ernst D, Milovanovic M, Hartmann B, Worm M. Liver X receptors control IgE expression in B cells. J Immunol. 2009;182:5276–5282. doi: 10.4049/jimmunol.0801804. [DOI] [PubMed] [Google Scholar]
- 7.Wojcik AJ, Skaflen MD, Srinivasan S, Hedrick CC. A critical role for ABCG1 in macrophage inflammation and lung homeostasis. J Immunol. 2008;180:4273–4282. doi: 10.4049/jimmunol.180.6.4273. [DOI] [PubMed] [Google Scholar]
- 8.Baldan A, Gomes AV, Ping P, Edwards PA. Loss of ABCG1 results in chronic pulmonary inflammation. J Immunol. 2008;180:3560–3568. doi: 10.4049/jimmunol.180.5.3560. [DOI] [PubMed] [Google Scholar]
- 9.Draper DW, Madenspacher JH, Dixon D, King DH, Remaley AT, Fessler MB. ATP-binding cassette transporter G1 deficiency dysregulates host defense in the lung. Am J Respir Crit Care Med. 2010;182:404–412. doi: 10.1164/rccm.200910-1580OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antonson P, Jakobsson T, Almlof T, Guldevall K, Steffensen KR, Gustafsson JA. RAP250 is a coactivator in the transforming growth factor beta signaling pathway that interacts with Smad2 and Smad3. J Biol Chem. 2008;283:8995–9001. doi: 10.1074/jbc.M707203200. [DOI] [PubMed] [Google Scholar]
- 11.Thomassen MJ, Barna BP, Malur AG, Bonfield TL, Farver CF, Malur A, Dalrymple H, Kavuru MS, Febbraio M. ABCG1 is deficient in alveolar macrophages of GM-CSF knockout mice and patients with pulmonary alveolar proteinosis. J Lipid Res. 2007;48:2762–2768. doi: 10.1194/jlr.P700022-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180:720–730. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy MA, Barrera GC, Nakamura K, Baldan A, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Hamada K, Suzaki Y, Goldman A, Ning YY, Goldsmith C, Palecanda A, Coull B, Hubeau C, Kobzik L. Allergen-independent maternal transmission of asthma susceptibility. J Immunol. 2003;170:1683–1689. doi: 10.4049/jimmunol.170.4.1683. [DOI] [PubMed] [Google Scholar]
- 15.Brass DM, Savov JD, Gavett SH, Haykal-Coates N, Schwartz DA. Subchronic endotoxin inhalation causes persistent airway disease. American journal of physiology. Lung cellular and molecular physiology. 2003;285:L755–761. doi: 10.1152/ajplung.00001.2003. [DOI] [PubMed] [Google Scholar]
- 16.Meyerholz DK, Griffin MA, Castilow EM, Varga SM. Comparison of histochemical methods for murine eosinophil detection in an RSV vaccine-enhanced inflammation model. Toxicol Pathol. 2009;37:249–255. doi: 10.1177/0192623308329342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wikstrom ME, Batanero E, Smith M, Thomas JA, von Garnier C, Holt PG, Stumbles PA. Influence of mucosal adjuvants on antigen passage and CD4+ T cell activation during the primary response to airborne allergen. J Immunol. 2006;177:913–924. doi: 10.4049/jimmunol.177.2.913. [DOI] [PubMed] [Google Scholar]
- 19.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003;170:2106–2112. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- 20.Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, Wong CH, Schneider E, Dy M, Leite-de-Moraes MC. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murdoch JR, Lloyd CM. Resolution of allergic airway inflammation and airway hyperreactivity is mediated by IL-17-producing {gamma}{delta}T cells. Am J Respir Crit Care Med. 2010;182:464–476. doi: 10.1164/rccm.200911-1775OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song C, Luo L, Lei Z, Li B, Liang Z, Liu G, Li D, Zhang G, Huang B, Feng ZH. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol. 2008;181:6117–6124. doi: 10.4049/jimmunol.181.9.6117. [DOI] [PubMed] [Google Scholar]
- 23.Broeren CP, Gray GS, Carreno BM, June CH. Costimulation light: activation of CD4+ T cells with CD80 or CD86 rather than anti-CD28 leads to a Th2 cytokine profile. J Immunol. 2000;165:6908–6914. doi: 10.4049/jimmunol.165.12.6908. [DOI] [PubMed] [Google Scholar]
- 24.Hellings PW, Kasran A, Liu Z, Vandekerckhove P, Wuyts A, Overbergh L, Mathieu C, Ceuppens JL. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003;28:42–50. doi: 10.1165/rcmb.4832. [DOI] [PubMed] [Google Scholar]
- 25.Besnard AG, Sabat R, Dumoutier L, Renauld JC, Willart M, Lambrecht B, Teixeira MM, Charron S, Fick L, Erard F, Warszawska K, Wolk K, Quesniaux V, Ryffel B, Togbe D. Dual Role of IL-22 in Allergic Airway Inflammation and its Cross-Talk with IL-17A. Am J Respir Crit Care Med. 2011;183:1153–1163. doi: 10.1164/rccm.201008-1383OC. [DOI] [PubMed] [Google Scholar]
- 26.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SJ, Lee KS, Kim SR, Min KH, Choe YH, Moon H, Chae HJ, Yoo WH, Lee YC. Peroxisome proliferator-activated receptor gamma agonist down-regulates IL-17 expression in a murine model of allergic airway inflammation. J Immunol. 2009;183:3259–3267. doi: 10.4049/jimmunol.0900231. [DOI] [PubMed] [Google Scholar]
- 28.Delvecchio CJ, Bilan P, Nair P, Capone JP. LXR-induced reverse cholesterol transport in human airway smooth muscle is mediated exclusively by ABCA1. Am J Physiol Lung Cell Mol Physiol. 2008;295:L949–957. doi: 10.1152/ajplung.90394.2008. [DOI] [PubMed] [Google Scholar]
- 29.Torocsik D, Barath M, Benko S, Szeles L, Dezso B, Poliska S, Hegyi Z, Homolya L, Szatmari I, Lanyi A, Nagy L. Activation of liver X receptor sensitizes human dendritic cells to inflammatory stimuli. J Immunol. 2010;184:5456–5465. doi: 10.4049/jimmunol.0902399. [DOI] [PubMed] [Google Scholar]
- 30.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shamshiev AT, Ampenberger F, Ernst B, Rohrer L, Marsland BJ, Kopf M. Dyslipidemia inhibits Toll-like receptor-induced activation of CD8alpha-negative dendritic cells and protective Th1 type immunity. J Exp Med. 2007;204:441–452. doi: 10.1084/jem.20061737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walcher D, Kummel A, Kehrle B, Bach H, Grub M, Durst R, Hombach V, Marx N. LXR activation reduces proinflammatory cytokine expression in human CD4-positive lymphocytes. Arterioscler Thromb Vasc Biol. 2006;26:1022–1028. doi: 10.1161/01.ATV.0000210278.67076.8f. [DOI] [PubMed] [Google Scholar]
- 34.Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, Baumgarth N, Chien YH. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furuyama S, Uehara Y, Zhang B, Baba Y, Abe S, Iwamoto T, Miura S, Saku K. Genotypic Effect of ABCG1 gene promoter -257T>G polymorphism on coronary artery disease severity in Japanese men. J Atheroscler Thromb. 2009;16:194–200. doi: 10.5551/jat.e380. [DOI] [PubMed] [Google Scholar]
- 36.Mauldin JP, Nagelin MH, Wojcik AJ, Srinivasan S, Skaflen MD, Ayers CR, McNamara CA, Hedrick CC. Reduced expression of ATP-binding cassette transporter G1 increases cholesterol accumulation in macrophages of patients with type 2 diabetes mellitus. Circulation. 2008;117:2785–2792. doi: 10.1161/CIRCULATIONAHA.107.741314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jagannathan-Bogdan M, McDonnell ME, Shin H, Rehman Q, Hasturk H, Apovian CM, Nikolajczyk BS. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol. 2011;186:1162–1172. doi: 10.4049/jimmunol.1002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marwaha AK, Crome SQ, Panagiotopoulos C, Berg KB, Qin H, Ouyang Q, Xu L, Priatel JJ, Levings MK, Tan R. Cutting edge: Increased IL-17-secreting T cells in children with new-onset type 1 diabetes. J Immunol. 2010;185:3814–3818. doi: 10.4049/jimmunol.1001860. [DOI] [PubMed] [Google Scholar]
- 39.Honkanen J, Nieminen JK, Gao R, Luopajarvi K, Salo HM, Ilonen J, Knip M, Otonkoski T, Vaarala O. IL-17 immunity in human type 1 diabetes. J Immunol. 2010;185:1959–1967. doi: 10.4049/jimmunol.1000788. [DOI] [PubMed] [Google Scholar]
- 40.Fessler MB, Young SK, Jeyaseelan S, Lieber JG, Arndt PG, Nick JA, Worthen GS. A role for hydroxy-methylglutaryl coenzyme a reductase in pulmonary inflammation and host defense. Am J Respir Crit Care Med. 2005;171:606–615. doi: 10.1164/rccm.200406-729OC. [DOI] [PubMed] [Google Scholar]
- 41.Wong J, Quinn CM, Brown AJ. Statins inhibit synthesis of an oxysterol ligand for the liver x receptor in human macrophages with consequences for cholesterol flux. Arterioscler Thromb Vasc Biol. 2004;24:2365–2371. doi: 10.1161/01.ATV.0000148707.93054.7d. [DOI] [PubMed] [Google Scholar]
- 42.Wong J, Quinn CM, Gelissen IC, Jessup W, Brown AJ. The effect of statins on ABCA1 and ABCG1 expression in human macrophages is influenced by cellular cholesterol levels and extent of differentiation. Atherosclerosis. 2008;196:180–189. doi: 10.1016/j.atherosclerosis.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 43.Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, Ceuppens JL. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.