Abstract
In this study, we examined the relationship between the level of daily cigarette consumption and the startle response to affective and cigarette-related cues among treatment-seeking smokers. Before receiving any behavioral or pharmacological treatment, 136 smokers attended a baseline laboratory session, during which we recorded their reflexive eyeblink responses to acoustic startle probes while they were viewing pleasant, unpleasant, neutral, and cigarette-related pictures. We found that 1) cigarette-related and pleasant pictures similarly reduced the startle magnitude compared to neutral pictures; 2) the magnitude of startle modulation rendered by pleasant or unpleasant pictures did not differ among light, moderate, and heavy smokers; and 3) startle attenuation by cigarette-related pictures was greater in heavy smokers than in light smokers. These results suggest that similar to pleasant stimuli, cigarette-related cues are motivationally salient for smokers, and that this salience increases with nicotine dependence.
Keywords: Cue reactivity, Affect, Nicotine addiction, Startle response
1. Introduction
Unfortunately, relapse is the most frequent outcome of a smoking cessation attempt. Many smokers have reported that their smoking relapses were associated with situations in which cigarette-related cues were present (Shiffman, et al., 2007). Given the prevalence of environmental smoking-related cues, it is important to better understand smokers’ reactivity to these cues in order to prevent relapse more effectively. Consistent with this reported association between relapse and the presence of smoking cues, many laboratory studies have demonstrated that smokers have greater reactivity to cigarette-related cues than non-cigarette-related cues by measuring self-reported craving (Payne, Smith, Sturges, & Holleran, 1996; Sayette, Martin, Wertz, Shiffman, & Perrott, 2001; Tidey, Rohsenow, Kaplan, & Swift, 2005), attentional bias (Ehrman, et al., 2002; Hogarth, Mogg, Bradley, Duka, & Dickinson, 2003; Waters, Shiffman, Bradley, & Mogg, 2003), event-related potentials (Parker & Gilbert, 2008; Versace, Minnix, et al., 2011; Versace, et al., 2010) and hemodynamic response (David, et al., 2005; Due, Huettel, Hall, & Rubin, 2002; Smolka, et al., 2006).
In addition to these various measures, a psychophysiological methodology called the affectively modulated startle response has been frequently used to evaluate the motivational salience of cigarette-related cues in smokers. The startle eyeblink response, the magnitude of which is usually measured by voltages changes in the orbicularis oculi electromyography (EMG) in response to an abrupt and loud acoustic probe, is sensitive to foreground motivationally relevant cues. The startle response is enhanced to unpleasant pictures and attenuated to pleasant pictures (Vrana, Spence, & Lang, 1988). By including cigarette-related pictures in this paradigm, researchers can compare the salience of these smoking cues in relation to other motivationally significant stimuli. Several studies have demonstrated that in smokers, cigarette-related pictures and pleasant pictures led to comparable reductions in startle magnitude, suggesting the appetitive salience of the smoking cues (Cinciripini, et al., 2006; Dempsey, Cohen, Hobson, & Randall, 2007; Geier, Mucha, & Pauli, 2000; Rehme, et al., 2009), although one study reported the opposite finding using this method (Munoz, et al., 2010).
Although cigarette-related cues have generally been found to be appetitive for smokers, findings concerning the relationship between cigarette-related cue reactivity and the severity of nicotine dependence have been equivocal. Several studies have found that smokers who were more dependent on nicotine were more reactive (e.g., greater self-reported craving) to cigarette-related cues than less dependent smokers (Payne, et al., 1996; Sayette, et al., 2001; Smolka, et al., 2006). Other studies have found the opposite: less-dependent smokers were more reactive (e.g., larger attentional bias) to cigarette-related cues than more-dependent smokers (Hogarth, et al., 2003; Mogg, Field, & Bradley, 2005; Rehme, et al., 2009; Vollstädt-Klein, et al., 2011). It is unclear what factors may account for these discrepant findings. For example, there are considerable methodological differences across these studies, ranging from self-report (Sayette, et al., 2001) to various psychophysiological assessments (e.g., blood flow level in Vollstädt-Klein, et al., 2011 and attentional bias in Hogarth, et al., 2003). Although they were used to assess cue reactivity in general terms, these methods may have measured distinct aspects of cue reactivity, such as the subjective evaluation of craving and attentional allocation. In addition, sample characteristics, such as smoking deprivation and its duration as well as basic demographics (e.g., sex and race), may have interacted with the above-mentioned methodological differences to make the relationship between nicotine dependence and cue reactivity more complicated.
Nevertheless, no reports so far have studied the relationship between cigarette-related cue reactivity and nicotine dependence exclusively in an adequately sized clinical sample of smokers. In general, these smokers wish to quit, but are subject to potential smoking cue-related relapse once they start to quit. Therefore, a better understanding of their reactivity to cigarette-related cues may improve intervention outcomes among these treatment-seeking smokers by preventing cue-related relapse. In the current study, we enrolled smokers who were participating in a clinical cessation program and evaluated the impact of daily cigarette consumption levels on cigarette-related and affective cue reactivity among these smokers. Given the role of smoking cues in relapse (Shiffman, et al., 2007) and higher relapse probability among heavier smokers (Dale, et al., 2001), we hypothesized that heavier smokers would have higher reactivity (i.e., greater startle attenuation) to cigarette-related cues than lighter smokers and that responses to pleasant and unpleasant cues would not differ between the groups.
2. Material and Methods
2.1 Participants
Participants from the Houston metropolitan area were recruited into a smoking cessation clinical study using newspaper, radio, and Internet advertisements. Only smokers, who wished to quit smoking, were aged 18–65 years, were fluent in English, smoked ≥5 cigarettes per day (CPD), had an expired carbon monoxide (CO) level ≥6 ppm, and reported no uncontrolled medical illnesses were included in the treatment study. Individuals were excluded from the program if they were taking psychotropic medications, had psychiatric disorders or were abusing substances other than nicotine, were involved in any other smoking cessation treatment, or had contraindications for bupropion or varenicline (these two drugs were administered randomly after the laboratory session assessments reported herein). A total of 182 treatment-seeking smokers met these inclusion and exclusion criteria and attended the initial laboratory session, before which they were asked to smoke ad libitum. Because of unsatisfactory data quality (see details in Section 2.3 Data acquisition and reduction), 46 participants were excluded, resulting in 136 participants being included in the current report. Using criteria established by the Centers for Disease Control and Prevention (Maurice, et al., 2006), we divided these participants into three groups based on their CPD: heavy smokers (N=30, CPD: ≥25); moderate smokers (N=61, CPD: 15–24); and light smokers (N=45, CPD: 5–14). The smoking group distribution of the participants who were excluded (N=46) and included (N=136) in the data analysis was similar to that in the Centers for Disease Control and Prevention report (p=0.17 for both) (Maurice, et al., 2006). This study was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board. All participants provided written informed consent.
2.2 Experimental design and stimuli
E-Prime software (v1.2; Psychology Software Tools, Sharpsburg, PA, USA) running on a Pentium 4 computer was used to deliver the experimental stimuli (i.e., pictures and acoustic startle probes). The picture set was created from the International Affective Picture System (Lang, Bradley, & Cuthbert, 2005) and cigarette-related picture collections previously used by our laboratory (Carter, et al., 2006; Cinciripini, et al., 2006; Versace, et al., 2010) and others (Gilbert, et al., 2005). Each set included equal numbers (N=24) of pictures in the following four categories: pleasant, unpleasant, neutral, and cigarette-related. Pleasant and unpleasant pictures were matched according to arousal level on the normative data (e.g., erotica and mutilations as high arousing pictures for pleasant and unpleasant categories, respectively) from the International Affective Picture System (Lang, et al., 2005). The content of the neutral and cigarette-related pictures was perceptually and conceptually matched (e.g., similar scenarios). The acoustic stimulus consisted of a 50-ms 100 dB(A) burst of white noise with instantaneous rise time binaurally delivered through insert earphones (model 3A, 10Ω; E-A-R Auditory Systems, Indianapolis, IN, USA).
We presented the entire picture set twice in two blocks, and each block contained two segments. Each picture was presented for 4 seconds followed by a random inter-trial interval of 3–5 seconds. For each category of 48 pictures, 12 of them were randomly probed between 2.5 and 3.5 seconds after picture onset by acoustic stimuli, yielding 48 startle probes for each participant. No consecutive pictures were probed by the acoustic stimulus, and the minimum and average time between startle probes was about 9 and 21 seconds, respectively. The orders of both pictures and startle probes were counter-balanced, and no two pictures of the same category were presented in two consecutive trials.
Besides the startle reflex assessment, high-density electroencephalography and corrugator EMG activity were recorded, and some of these results have been the subject of other reports (Versace, Lam, et al., 2011; Versace, Minnix, et al., 2011).
2.3 Data acquisition and reduction
Two recording electrodes (Ag-AgCl) were placed on the orbicularis oculi muscle under the right eye, and the EMG activity was acquired and amplified with an EMG100C module connected to an MP150WSW bioamplifier (BIOPAC Systems, Goleta, CA, USA) sampled at 1,000 Hz. AcqKnowledge III data acquisition software (v3.8.2; BIOPAC Systems) was used to record data that had been filtered (28–500 Hz), rectified and smoothed by a five-sample boxcar filter (Blumenthal, et al., 2005). Before starting the experiment, we checked sensor impedance (<30 KΩ) and visually examined data quality to ensure proper data collection.
Startle responses were first examined offline on a trial-by-trial basis using AcqKnowledge software. Trials with excessive noise or no scoreable startle response were considered missing, and 46 of 182 participants who had over 33% missing trials were considered startle nonresponders and excluded from subsequent analysis. The relatively high loss of data may have been due to the age of our sample, as older subjects tend to respond less to startle probes (Ellwanger, Geyer, & Braff, 2003; Ford, et al., 1995; Ludewig, et al., 2003), and relatively high sensor impedance, which may have attenuated the EMG signal and permitted electromagnetic interference intrusion that reduced the data quality (Blumenthal, et al., 2005). The former hypothesis is supported by the age difference (t(180)=2.76, p<0.01) between nonresponders (mean [M]=48.39 years, standard deviation [SD]=10.15 years) and responders (M=43.44 years, SD=10.50 years). There were no other differences in sex, race, or smoking-related variables (e.g., daily cigarette consumption and years of smoking) between the responders and nonresponders.
The startle magnitude was defined as the difference between the peak (the maximal EMG activity within 20–120 ms after the probe onset) and the baseline (the average EMG activity within a 25-ms time window before the probe onset) of the startle trial. A total of 9.7% of all the trials from all included participants were excluded due to unstable baseline activity (>2 SDs above the mean baseline within the subject) or outlier response (>3 SDs above the mean startle magnitude within the picture category for each subject), and we confirmed that there were at least five valid trials in each picture category to ensure proper estimation of the associated startle response.
2.4 Data analysis
To reduce between-subject variations of the raw eyeblink response magnitudes, we standardized the startle responses using the formula for computing Cohen’s d (Cohen, 1988): d score = (M1–M2)/SDp, where M1 is the raw startle data in the presence of cigarette-related, pleasant or unpleasant pictures; M2 is the raw startle data in the presence of neutral pictures; and SDp is the pooled SD of raw startle data in the presence of neutral and corresponding category pictures: cigarette-related, pleasant or unpleasant. Before standardizing the startle responses to cigarette-related, pleasant, and unpleasant pictures with those to neutral pictures, we ruled out the possibility that the startle responses to neutral pictures differed among the smoking dependence groups (p>0.99).
Statistical analyses were conducted using mixed models in SAS software (PROC MIXED, v9.2; SAS Institute, Carey, NC, USA). The two independent variables in the models were picture category (pleasant, unpleasant, and cigarette-related pictures) and group (light, moderate, and heavy smokers). All models included subject as a random effect. The main and interaction terms of these two variables were examined in the mixed models. Significant main and interaction effects were examined using pairwise contrasts of the estimated least square means. All the statistical analyses were run twice, once with, and once without basic demographic information (e.g., age, sex, and race) being covariates. Unless specified, all the p values for multiple pairwise contrast comparisons were adjusted using the Bonferroni procedure. The significance level was 0.05.
3. Results
3.1 Participant characteristics
Table 1 summarizes the basic demographic information and smoking variables of our participants. Light, moderate, and heavy smokers did not differ in their age at the time of the study or the age at which they started smoking. There were fewer women and African Americans in the heavy smokers group, consistent with previous findings (Centers for Disease Control and Prevention, 2002; Maurice, et al., 2006; Novotny, Warner, Kendrick, & Remington, 1988). Heavier smokers smoked more CPD, reported higher Fagerström Test for Nicotine Dependence scores, had a longer smoking history, and had higher levels of expired CO than lighter smokers.
Table 1.
Demographics and smoking variables of light, moderate, and heavy smokers.
| Measure | Light (N=45) | Moderate (N=61) | Heavy (N=30) | χ2 or F-Value | p value |
|---|---|---|---|---|---|
| Age in years, mean (SD) | 41.6 (10.9) | 42.8 (10.7) | 47.0 (9.4) | 2.49 | 0.09 |
| Gender, percent female | 60.0 | 39.3 | 16.7 | 14.11 | <0.001 |
| Race, percent | 12.62 | 0.03 | |||
| Black, non-Hispanic | 35.6 | 18.0 | 3.3 | ||
| White, non-Hispanic | 48.9 | 68.9 | 80.0 | ||
| Hispanic | 6.7 | 9.8 | 10.0 | ||
| Other | 8.8 | 3.3 | 6.7 | ||
| CPD, mean (SD) | 9.9 (2.2) | 19.3 (2.0) | 31.5 (6.1) | 363.59 | <0.0001 |
| CO in ppm, mean (SD) | 18.5 (9.6) | 27.5 (15.0) | 35.7 (18.0) | 13.52 | <0.0001 |
| FTND scores, mean (SD) | 3.0 (1.7) | 4.9 (1.6) | 6.3 (2.2) | 33.33 | <0.0001 |
| Years of smoking, mean (SD) | 19.9 (12.2) | 24.3 (11.6) | 27.1 (9.5) | 3.93 | 0.02 |
| Age at smoking initiation, mean (SD) | 18.3 (6.1) | 17.4 (4.6) | 18.0 (5.1) | 0.35 | 0.70 |
CPD: cigarettes per day; CO in ppm: carbon monoxide in parts per million; FTND: Fagerström Test for Nicotine Dependence; SD: standard deviation.
3.2 Startle modulation by picture category
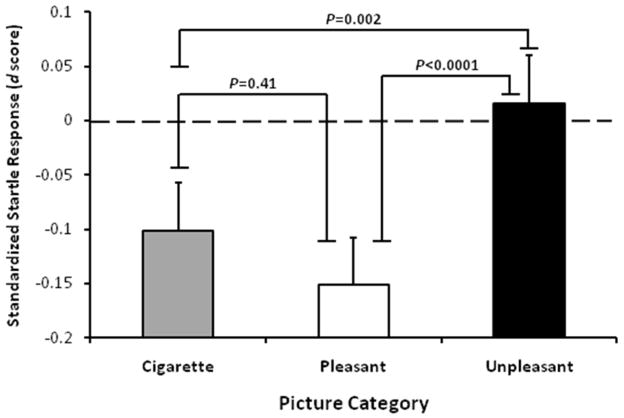
There was a main effect of picture category on the standardized startle responses (F(2,270)=13.28, p<0.0001; Fig. 1). Pairwise post hoc contrasts indicated that the startle magnitudes in the presence of pleasant and cigarette-related pictures were significantly lower than those in the presence of unpleasant pictures (pleasant vs. unpleasant, t(270)=−5.02, p<0.0001; cigarette-related vs. unpleasant, t(270)=−3.52, p=0.002). Cigarette-related and pleasant pictures did not differ in startle magnitude (cigarette-related vs. pleasant, t(270)=0.14, p=0.41). Both cigarette-related and pleasant pictures attenuated startle response in comparison to neutral pictures (cigarette-related, t(270)=−2.32, p=0.02; pleasant, t(270)=−3.47, p<0.001).
Figure 1.
Startle magnitude attenuation in the presence of cigarette-related and pleasant pictures.
Startle magnitude (estimated means with standard errors) as a function of picture category. The dashed line (d=0) indicates the startle magnitude in the presence of neutral pictures.
3.3 Startle cue reactivity
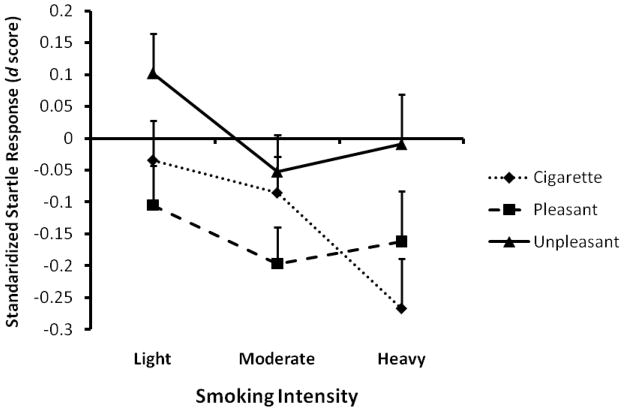
To examine whether the smoking groups differed in their standardized startle response as a function of picture category, we tested the two-way interaction between picture category (pleasant, unpleasant, and cigarette-related) and smoking group (light, moderate, and heavy) on the standardized startle responses. This interaction was significant: F(4,266)=2.43, p<0.05, suggesting that light, moderate, and heavy smokers had differential startle reactivity patterns to the pleasant, unpleasant, and cigarette-related pictures (Fig. 2). To determine how affective and smoking cue reactivity differed among these groups of smokers, we used post hoc pairwise contrasts, which showed that the interaction was primarily driven by the differences among the groups in response to cigarette-related pictures (F(2,266)=3.21, p=0.04) but not to pleasant (F(2,266)=0.74, p=0.48) or unpleasant pictures (F(2,266)=2.10, p=0.12). No significant differences were noted between the startle responses of light and moderate smokers (t(266)=0.68, p>0.05) to cigarette-related pictures, although startle attenuation appeared to be greater in heavy smokers than in moderate smokers (t(266)=−2.12, p<0.04 and p<0.11, before and after Bonferroni adjustment). Importantly, compared to light smokers, heavy smokers had greater startle attenuation in the presence of cigarette-related pictures (t(266)=−2.44, p<0.05, Cohen’s d=−0.36). Results were not altered by including age, sex, and race as covariates in the models.
Figure 2.
Differential cigarette-related cue reactivity among light, moderate, and heavy smokers.
4. Discussion and Conclusions
The current study showed that treatment-seeking smokers react to cigarette-related and pleasant cues in a similar fashion, suggesting that cigarette-related cues carry a level of incentive salience similar to that of pleasant stimuli. This finding confirms the results of our previous study (Cinciripini, et al., 2006) and those of others (Dempsey, et al., 2007; Geier, et al., 2000; Rehme, et al., 2009). A new contribution from this study is the finding that compared to light smokers, heavy smokers had greater startle attenuation while viewing cigarette-related pictures. This finding suggests that cigarette-related cues are more salient for heavy smokers than for light smokers.
Affectively modulated startle methodology has frequently been used to study whether cigarette-related cues have increased salience among smokers. Although a potentiation of startle response in an affectively modulated startle paradigm and a lack of startle modulation in a prepulse inhibition paradigm by cigarette-related pictures have been reported (Elash, Tiffany, & Vrana, 1995; Munoz, et al., 2010; Orain-Pelissolo, Grillon, Perez-Diaz, & Jouvent, 2004), most studies have shown that cigarette-related cues attenuate the startle magnitude to a similar degree that pleasant stimuli do (Cinciripini, et al., 2006; Dempsey, et al., 2007; Geier, et al., 2000; Rehme, et al., 2009). The attenuation of startle response in the presence of cigarette-related cues among smokers is consistent with a rodent study in which the authors found that the presence of appetitive cues (i.e. food cues) induced startle attenuation in rats, which is dependent on the nucleus accumbens (Koch, Schmid, & Schnitzler, 1996). This brain region has been suggested to play a critical role in the development of addiction of various drugs of abuse, including nicotine (Dani & Harris, 2005). Together with several functional neuroimaging studies in humans showing the activation of reward circuits such as the mesolimbic areas by cigarette-related cues among smokers (David, et al., 2005; Due, et al., 2002; Vollstädt-Klein, et al., 2011), this convergent body of evidence suggests that cigarette-related cues act as salient stimuli among smokers.
Our finding that heavy smokers had greater startle attenuation in response to cigarette-related cues than light smokers suggests that these cues are more salient for heavy smokers. This could be due to the sensitized midbrain dopaminergic circuits (David, et al., 2005; Due, et al., 2002; Smolka, et al., 2006; cf., Vollstädt-Klein, et al., 2011), as suggested by the incentive-sensitization theory of drug addiction with experimental evidence from several other studies (Payne, et al., 1996; Sayette, et al., 2001; Smolka, et al., 2006). In contrast to our results, one study using the affectively modulated startle methodology found that light smokers had greater cigarette-related cue reactivity than heavy smokers (Rehme, et al., 2009). Potential causes for the discrepancy between our findings and results reported by Rehme and colleagues could include distinct picture sets and different abstinence durations. First, distinct picture sets, particularly cigarette-related pictures, may lead to differential findings. For example, Stippekohl and colleagues reported different brain activation profiles by cigarette-related stimuli that depicted the beginning and end of smoking rituals (Stippekohl, et al., 2010). Second, the participants in our study typically smoked their last cigarettes 1–2 hours before the laboratory session, compared to 4–6 hours reported in the study by Rehme and colleagues. It appears that abstinence duration may modulate cigarette-related cue reactivity. While 24 hours of nicotine abstinence led to increased cigarette-related cue reactivity (McClernon, Kozink, Lutz, & Rose, 2009), results of cue reactivity modulation by 12 hours of abstinence or overnight abstinence have been mixed (Cinciripini, et al., 2006; McBride, Barrett, Kelly, Aw, & Dagher, 2006; McClernon, Hiott, Huettel, & Rose, 2005). It is unclear whether abstinence duration, particularly short-term abstinence, influences cigarette-related cue reactivity as a function of nicotine dependence severity.
There were several limitations to and concerns about the current study. First, as a clinical trial of smoking cessation, the study did not include nonsmokers and non-treatment-seeking smokers. Therefore, we were not able to evaluate the specificity of the cigarette-related cue reactivity, although nonsmokers have not been found to respond to smoking stimuli as smokers do (David, et al., 2005; Due, et al., 2002; Ehrman, et al., 2002; Hogarth, et al., 2003; Parker & Gilbert, 2008). We were also unable to evaluate whether intention to quit may modulate cigarette-related cue and affective reactivity. This question is interesting as Dempsey and colleagues found differential reactivity profiles across affective and cigarette-related conditions between smokers who did and did not intend to quit smoking (Dempsey, et al., 2007). Second, we did not observe significant startle potentiation in the presence of unpleasant pictures. The inclusion of ~30% mutilation pictures may be the reason for this null finding, as these pictures do not potentiate startle responses effectively (Bradley, Codispoti, Cuthbert, & Lang, 2001; Sarlo, Buodo, & Palomba, 2010). Third, although relatively higher cigarette-related cue salience may be one of the causes for higher relapse rates among heavy smokers compared to light smokers observed in several studies (Berg, et al., 2010; Dale, et al., 2001), a strong link between cue reactivity and relapse is yet to be established (Perkins, 2009). Fourth, although our data support a positive, but not negative, relationship between cigarette-related cue reactivity and nicotine dependence severity, future studies in larger clinical and nonclinical samples with more variation in dependence severity would be important to clarify the inconsistent relationship.
In summary, we found that cigarette-related stimuli acted as salient stimuli among treatment-seeking smokers, and heavy smokers had greater reactivity than light smokers to cigarette-related cues. The results suggest that increased salience may have been attributed to cigarette-related stimuli in smokers, particularly in heavy smokers. Future studies may focus on evaluating cigarette-related cue reactivity over long periods of abstinence and in former smokers to determine if such neural responses normalize long after the smokers quit. Future studies may also identify whether there might be genetic or biological factors that render smokers respond to cigarette-related cues differentially.
Cigarette-related stimuli are salient stimuli for smokers.
Cigarette-related stimuli are more salient in heavy smokers than in light smokers while they do not differ in their responses to pleasant and unpleasant stimuli.
Acknowledgments
Role of Funding Sources
This project was supported by grant R01DA017073 from the National Institute on Drug Abuse (NIDA) awarded to Paul M. Cinciripini and in part by the National Institutes of Health (NIH) through MD Anderson’s Cancer Center Support Grant CA016672. NIDA and NIH had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
We wish to thank the clinic team, including Tim Evans, Michelle Underwood, Jerell Jones, Christine Jeria, Jennifer Canul, Janeene Frerking, Kevin Winslow, Lauren Baker, Sandra Stautberg, Samuel Miller, Kevin Mulpur, Erin Glueckert, Silky Joshi, Katie McMahon, Beda Jean-Francois, Natalie Nathan, Blanca Navarro, Tiffany Rattler, John Avalos, for study coordination and data collection, Rudel Rymer for database programming and management, Markeda Wade for proof reading of the manuscript, and Sherry Allison for administrative support.
Footnotes
Contributors
Paul Cinciripini, Jason Robinson, Francesco Versace, Cho Lam, Jennifer Minnix and Victoria Brown designed the experiment and wrote the protocol. Yong Cui and the clinic team listed in the Acknowledgements section collected the data. Yong Cui, Jason Robinson, Francesco Versace, and Paul Cinciripini processed and analyzed the data. Yong Cui wrote the first draft of the manuscript and all authors contributed to and have approved the final manuscript.
Conflict of Interest
Dr. Paul M. Cinciripini has served on the Scientific Advisory Board of Pfizer Pharmaceuticals, has received grant support from Pfizer, and has conducted educational talks sponsored by Pfizer. All the other authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berg CJ, Thomas JL, Guo HF, An LC, Okuyemi KS, Collins TC, Ahluwalia JS. Predictors of smoking reduction among Blacks. Nicotine & Tobacco Research. 2010;12:423–431. doi: 10.1093/ntr/ntq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Carter BL, Robinson JD, Lam CY, Wetter DW, Tsan JY, Day SX, Cinciripini PM. A psychometric evaluation of cigarette stimuli used in a cue reactivity study. Nicotine & Tobacco Research. 2006;8:361–369. doi: 10.1080/14622200600670215. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Women and smoking: a report of the Surgeon General (Executive summary) MMWR. 2002;51:i–iv. 1–13. [PubMed] [Google Scholar]
- Cinciripini PM, Robinson JD, Carter BL, Lam C, Wu X, de Moor CA, Baile WF, Wetter DW. The effects of smoking deprivation and nicotine administration on emotional reactivity. Nicotine & Tobacco Research. 2006;8:379–392. doi: 10.1080/14622200600670272. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum; 1988. [Google Scholar]
- Dale LC, Glover ED, Sahs DPL, Schroeder DR, Offord KP, Croghan IT, Hurt RD. Bupropion for smoking cessation - Predictors of successful outcome. Chest. 2001;119:1357–1364. doi: 10.1378/chest.119.5.1357. [DOI] [PubMed] [Google Scholar]
- Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nature Neuroscience. 2005;8:1465–1470. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- David SP, Munafo MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, Walton RT. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;58:488–494. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JP, Cohen LM, Hobson VL, Randall PK. Appetitive nature of drug cues re-confirmed with physiological measures and the potential role of stage of change. Psychopharmacology. 2007;194:253–260. doi: 10.1007/s00213-007-0839-3. [DOI] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. American Journal of Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Bromwell MA, Lankford ME, Monterosso JR, O’Brien CP. Comparing attentional bias to smoking cues in current smokers, former smokers, and non-smokers using a dot-probe task. Drug and Alcohol Dependence. 2002;67:185–191. doi: 10.1016/s0376-8716(02)00065-0. [DOI] [PubMed] [Google Scholar]
- Elash CA, Tiffany ST, Vrana SR. Manipulation of smoking urges and affect through a brief-imagery procedure: Self-report, psychophysiological, and startle probe responses. Experimental and Clinical Psychopharmacology. 1995;3:156–162. [Google Scholar]
- Ellwanger J, Geyer MA, Braff DL. The relationship of age to prepulse inhibition and habituation of the acoustic startle response. Biological Psychology. 2003;62:175–195. doi: 10.1016/s0301-0511(02)00126-6. [DOI] [PubMed] [Google Scholar]
- Ford JM, Roth WT, Isaacks BG, White PM, Hood SH, Pfefferbaum A. Elderly men and women are less responsive to startling noises: N1, P3 and blink evidence. Biological Psychology. 1995;39:57–80. doi: 10.1016/0301-0511(94)00959-2. [DOI] [PubMed] [Google Scholar]
- Geier A, Mucha RF, Pauli P. Appetitive nature of drug cues confirmed with physiological measures in a model using pictures of smoking. Psychopharmacology. 2000;150:283–291. doi: 10.1007/s002130000404. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Izetelny A, Radtke R, Hammersley J, Rabinovich NE, Jameson TR, Huggenvik JI. Dopamine receptor (DRD2) genotype-dependent effects of nicotine on attention and distraction during rapid visual information processing. Nicotine & Tobacco Research. 2005;7:361–379. doi: 10.1080/14622200500125245. [DOI] [PubMed] [Google Scholar]
- Hogarth LC, Mogg K, Bradley BP, Duka T, Dickinson A. Attentional orienting towards smoking-related stimuli. Behavioural Pharmacology. 2003;14:153–160. doi: 10.1097/00008877-200303000-00007. [DOI] [PubMed] [Google Scholar]
- Koch M, Schmid A, Schnitzler HU. Pleasure-attenuation of startle is disrupted by lesions of the nucleus accumbens. Neuroreport. 1996;7:1442–1446. doi: 10.1097/00001756-199605310-00024. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-6. University of Florida; 2005. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Ludewig K, Ludewig S, Seitz A, Obrist M, Geyer MA, Vollenweider FX. The acoustic startle reflex and its modulation: effects of age and gender in humans. Biological Psychology. 2003;63:311–323. doi: 10.1016/s0301-0511(03)00074-7. [DOI] [PubMed] [Google Scholar]
- Maurice E, Trosclair A, Merritt R, Caraballo R, Malarcher A, Husten C, Pechacek T. Cigarette smoking among adults - United States, 2004 (Reprinted from MMWR, vol 54, pg 1121–1124, 2005) Jama-Journal of the American Medical Association. 2006;295:749–751. [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology (Berl) 2009;204:25–35. doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Field M, Bradley BP. Attentional and approach biases for smoking cues in smokers: an investigation of competing theoretical views of addiction. Psychopharmacology. 2005;180:333–341. doi: 10.1007/s00213-005-2158-x. [DOI] [PubMed] [Google Scholar]
- Munoz MA, Viedma-del-Jesus MI, Fernandez-Santaella MC, Peralta-Ramirez MI, Cepeda-Benito A, Vila J. Assessment of tobacco craving by means of the affective image visualization paradigm. Motivation and Emotion. 2010;34:93–103. [Google Scholar]
- Novotny TE, Warner KE, Kendrick JS, Remington PL. Smoking by blacks and whites-socioeconomic and demographic differences. American Journal of Public Health. 1988;78:1187–1189. doi: 10.2105/ajph.78.9.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orain-Pelissolo S, Grillon C, Perez-Diaz F, Jouvent R. Lack of startle modulation by smoking cues in smokers. Psychopharmacology. 2004;173:160–166. doi: 10.1007/s00213-003-1715-4. [DOI] [PubMed] [Google Scholar]
- Parker A, Gilbert D. Brain activity during anticipation of smoking-related and emotionally positive pictures in smokers and nonsmokers: A new measure of cue reactivity. Nicotine & Tobacco Research. 2008;10:1627–1631. doi: 10.1080/14622200802412911. [DOI] [PubMed] [Google Scholar]
- Payne TJ, Smith PO, Sturges LV, Holleran SA. Reactivity to smoking cues: Mediating roles of nicotine dependence and duration of deprivation. Addictive Behaviors. 1996;21:139–154. doi: 10.1016/0306-4603(95)00043-7. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Does smoking cue-induced craving tell us anything important about nicotine dependence? Addiction. 2009;104:1610–1616. doi: 10.1111/j.1360-0443.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- Rehme AK, Frommann I, Peters S, Block V, Bludau J, Quednow BB, Maier W, Schutz C, Wagner M. Startle cue-reactivity differentiates between light and heavy smokers. Addiction. 2009 doi: 10.1111/j.1360-0443.2009.02668.x. [DOI] [PubMed] [Google Scholar]
- Sarlo M, Buodo G, Palomba D. Lack of startle blink potentiation to mutilation pictures irrespective of fearfulness. Biological Psychology. 2010;85:338–343. doi: 10.1016/j.biopsycho.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Shiffman S, Perrott MA. A multidimensional analysis of cue-elicited craving in heavy smokers and tobacco chippers. Addiction. 2001;96:1419–1432. doi: 10.1080/09652140120075152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Balabanis MH, Gwaltney CJ, Paty JA, Gnys M, Kassel JD, Hickcox M, Paton SM. Prediction of lapse from associations between smoking and situational antecedents assessed by ecological momentary assessment. Drug and Alcohol Dependence. 2007;91:159–168. doi: 10.1016/j.drugalcdep.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka MN, Buhler M, Klein S, Zimmermann U, Mann K, Heinz A, Braus DF. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology. 2006;184:577–588. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- Stippekohl B, Winkler M, Mucha RF, Pauli P, Walter B, Vaitl D, Stark R. Neural responses to BEGIN- and END-stimuli of the smoking ritual in nonsmokers, nondeprived smokers, and deprived smokers. Neuropsychopharmacology. 2010;35:1209–1225. doi: 10.1038/npp.2009.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM. Subjective and physiological responses to smoking cues in smokers with schizophrenia. Nicotine & Tobacco Research. 2005;7:421–429. doi: 10.1080/14622200500125724. [DOI] [PubMed] [Google Scholar]
- Versace F, Lam CY, Engelmann JM, Robinson JD, Minnix JA, Brown VL, Cinciripini PM. Beyond cue reactivity: blunted brain responses to pleasant stimuli predict long-term smoking abstinence. Addiction Biology. 2011:no–no. doi: 10.1111/j.1369-1600.2011.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Minnix JA, Robinson JD, Lam CY, Brown VL, Cinciripini PM. Brain reactivity to emotional, neutral and cigarette-related stimuli in smokers. Addiction Biology. 2011;16:296–307. doi: 10.1111/j.1369-1600.2010.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Robinson JD, Lam CY, Minnix JA, Brown VL, Carter BL, Wetter DW, Cinciripini PM. Cigarette cues capture smokers’ attention: Evidence from event-related potentials. Psychophysiology. 2010;47:435–441. doi: 10.1111/j.1469-8986.2009.00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Kobiella A, Bühler M, Graf C, Fehr C, Mann K, Smolka MN. Severity of dependence modulates smokers’ neuronal cue reactivity and cigarette craving elicited by tobacco advertisement. Addiction Biology. 2011;16:166–175. doi: 10.1111/j.1369-1600.2010.00207.x. [DOI] [PubMed] [Google Scholar]
- Vrana SR, Spence EL, Lang PJ. The startle probe response: a new measure of emotion? Journal of Abnormal Psychology. 1988;97:487–491. doi: 10.1037//0021-843x.97.4.487. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Bradley BP, Mogg K. Attentional shifts to smoking cues in smokers. Addiction. 2003;98:1409–1417. doi: 10.1046/j.1360-0443.2003.00465.x. [DOI] [PubMed] [Google Scholar]