Abstract
We have previously reported that experimental mild traumatic brain injury results in increased sensitivity to stressful events during the first post injury weeks, as determined by analyzing the hypothalamic-pituitary-adrenal (HPA) axis regulation following restraint-induced stress. This is the same time period when rehabilitative exercise has proven to be ineffective after a mild fluid-percussion injury (FPI). Here we evaluated effects of stress on neuroplasticity.
Adult male rats underwent either a FPI or sham injury. Additional rats were only exposed to anesthesia. Rats were exposed to 30-min of restraint stress, followed by tail vein blood collection at post-injury days (PID) 1, 7 and 14. The response to dexamethasone (DEX) was also evaluated. Hippocampal tissue was collected 120 min after stress onset. Brain derived neutrophic factor (BDNF) along with glucocorticoid (GR) and mineralocorticoid (MR) receptors were determined by western blot analysis. Results indicated injury dependent changes in glucocorticoid and mineralocorticoid receptors that were influenced by the presence of dexamethasone. Control and FPI rats responded differentially to DEX in that GR increases after receiving the lower dose of DEX were longer lasting in the FPI group. A suppression of MR was found at PID 1 in vehicle treated FPI and Sham groups. Decreases in the precursor form of BDNF were observed in different FPI groups at PIDs 7 and 14, These findings suggest that the increased sensitivity to stressful events during the first post injury weeks, after a mild FPI, has an impact on hippocampal neuroplasticity.
Traumatic brain injury (TBI) is a significant health concern in our society. Clinical findings indicate that even those injuries that are categorized as mild result in neurophysiological disruptions that will have on impact on the affective response to environmental stimuli (Ashman et al., 2006, Bay and Xie, 2009, Englander et al., 2010). Likewise, similar findings have been observed following experimental brain injury in rats. In an earlier paper, we described an increased sensitivity to restraint-induced stress during the first two post injury weeks as indicated by increases in corticosterone (CORT) and adrenocorticotropic hormone (ACTH) compared to uninjured rats. This hyper-responsiveness to stress was evident even when baseline CORT and ACTH values were similar to uninjured controls (Griesbach et al., 2011). The stress response involves the activation of the hypothalamic-pituitary-adrenal (HPA) axis resulting in the release of ACTH from pituitary cells. ACTH stimulates the adrenal gland to release glucocorticoids, such as CORT, which in turn results in the inhibition of ACTH secretion (Dallman et al., 1987).
It has been well characterized that stress decreases neuronal plasticity and favors neurodegeneration (Goodman et al., 1996, McEwen and Magarinos, 2001). The effects of stress on the central nervous system are most notable within the hippocampus where it substantially influences neuronal excitability and long-term potentiation (LTP) (Diamond et al., 1992, Joels and de Kloet, 1992, Diamond et al., 1994). The hippocampus has a high density of glucocorticoid receptors (McEwen, 1999). These receptors exert a variety of effects besides the autoregulation of the stress response; such as influencing mood, learning and memory (de Kloet, 2000). Moreover, stress related increases in glucocorticoids have been associated with cell death and cognitive impairments (McEwen, 1999, Sapolsky, 1999). Among the effects of stress is the inhibition of hippocampal brain derived neurotrophic factor (BDNF) (Schaaf et al., 1998, Gronli et al., 2006). It should be noted that corticosterone administration does not have an influence on neurotrophn-3, which is the other member of the neurotrophin familiy that is highly expressed in the hippocampus (Schaaf et al., 1997). Indeed, corticosteroids regulate BDNF expression via activation of the mineralocorticoid (MR) and glucocorticoid receptors (GR), which can act directly as transcription factors (Hansson et al., 2000, Hansson et al., 2006). GR and MR are members of the nuclear receptor superfamily ligand-activated transcription factors that provide a link between extracellular steroid levels and cell transcriptional activity. CORT is the endogenous ligand for these receptors, as is cortisol for humans.
The anatomical and vascular characteristics of the hypothalamic-pituitary complex increase its vulnerability during TBI. Particularly, diffuse TBI, where metabolism and neural connectivity are compromised (Bazarian et al., 2006, Barkhoudarian et al., 2011). Both human and animal studies have shown alterations in HPA function following TBI, as indicated by severity dependent changes in plasma cortisol/corticosterone (Steinbok and Thompson, 1979, Cernak et al., 1999, Taylor et al., 2008). Given the above-mentioned effects of glucocorticoids on hippocampal synaptic plasticity and BDNF expression, it is feasible that impaired neuroendocrine function interferes with BDNF mediated restorative processes after TBI. For example, a hyper-response to stress following TBI may play a role in the inability to increase BDNF during the subacute period, as seen in rats with a mild injury (Griesbach et al., 2004, Griesbach et al., 2007).
Here we report hippocampal GR and MR changes following acute restraint-induced stress at different post TBI periods. Changes in levels of the mature (m) and precursor (pre) forms of BDNF were also explored. BDNF is first synthesized as a precursor and undergoes posttranslational modifications to reach its mature form (Meyer et al., 1996, Seidah et al., 1996). It is the mature form of BDNF that increases synaptic efficacy and plays a crucial role in LTP (Lu, 2003, Barco et al., 2005). Tissue samples for these studies were obtained from the animals of a previous study analyzing ACTH and CORT (Griesbach et al., 2011). Thus, this study will help determine if the hyper-response to stress that was previously reported in these animals is associated to changes in GR and MR proteins.
EXPERIMENTAL PROCEDURES
Subjects
A total of 220 male Sprague-Dawley rats (mean weight: 302g + 2.46 SEM) from Charles River Breeding Labs (Hollister, CA) were utilized in these experiments. Rats underwent surgery to induce either sham injury (n=81) or FPI (n=81). Additional control rats were only exposed to anesthesia in order to control for surgical stress effects associated with the craniotomy procedure (DeKeyser et al., 2000) (n=54). Animals were randomly assigned to the different experimental groups described below. Rats were handled daily and habituated to a reversed lighting schedule (lights off: 09:00 to 21:00 hrs) commencing the week prior to experimental procedures. During the experiments, rats were single-housed in opaque plastic bins (50.8 × 25.4 × 25.4 cm), which were lined with bedding material. Rats had ad libitum access to water and rat chow. All procedures were performed in accordance with the United States National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the UCLA Chancellor’s Animal Research Committee.
Lateral Fluid Percussion Injury
As previously done (Griesbach et al., 2011), rats were anesthetized with isofluorane (4% for induction, 2.0% for maintenance, in 100% O2) via a nose mask. The level of anesthesia was monitored by level of respiration, muscular relaxation and the corneal and pedal reflexes. After loss of corneal and pedal reflexes the scalp and scapular regions were shaved, the animal was secured in a stereotaxic head frame, and the scalp was cleansed with ethanol and betadine. Rectal temperature was monitored and maintained between 36.5-38.0°C with a thermostatically controlled heating pad (Braintree Scientific Inc., Braintree, MA, USA). The scalp and temporal muscle were reflected and a 3-mm diameter circular craniotomy was made over the left parietal cortex, centered at 3 mm posterior to bregma and 6 mm lateral to the midline. The bone flap was removed and the dura left intact in all animals to receive FPI. The dura was inspected with the aid of a microscope (Wild, Heerburg, Switzerland). A plastic injury cap was placed over the craniotomy with silicone adhesive, cyanoacrylate, and dental cement. When the dental cement hardened, the cap was filled with 0.9% NaCl solution. Anesthesia was discontinued and the animal was removed from the stereotaxic device. The injury cap was attached to the fluid percussion device. At the first sign of hind-limb withdrawal to a paw pinch, a mild fluid percussion pulse (1.5 atm) was administered. Apnea times were determined as the time from injury to the return of spontaneous breathing. Time of unconsciousness was determined from the time of injury until the return of the hind-limb withdrawal reflex. Immediately upon responding to a paw pinch, anesthesia was restored, the injury cap removed, and the scalp was sutured. Sham animals underwent an identical preparation with the exception of the FPI. Additional animals were used to control for the effects of the craniotomy. These control (Ctrl) rats were placed under anesthesia for a period of time similar to that of the rats that underwent FPI or sham surgery. A skin incision was also made, as described above, to assure that there would be experimenter blindness for the following procedures. After suturing, Bupivacaine (0.25 mg) was injected into the margins of the scalp incision and triple antibiotic ointment was applied over the incision. The rat was placed in a recovery chamber for approximately one hour before being returned to its cage.
Dexamethasone Sensitivity Test, Restraint Stress
At post-injury days (PID) 1 (20-24 hrs after surgery), 7 or 14 rats were subjected to restraint stress and DEX experiments were performed. DEX is a synthetic glucocorticoid with a high affinity to the glucocorticoid receptor. Prior to restraint, basal blood collections were obtained by tail venepuncture. The basal blood collections are obtained immediately after placing the rat in a flat-bottom tube (13×6×8 cm, Harvard Apparatus, Co). The duration of the blood collection process, beginning from the removal of the rat from the home-cage to its completion does not exceed 2 minutes. Immediately thereafter, rats were tightly restrained in the tubes for 30 min. Rats remained in the plexiglass tubes, in a relaxed position, for an additional 90 min. Blood was collected by tail venepuncture at 30, 60, 90 and 120 min after onset of restraint. Rats remained in their individual homecages between the blood collections. All blood samples were obtained between 14:00 and 16:00 hours during the active dark phase. Blood samples were processed and analyzed for ACTH and CORT as previously described (Griesbach et al., 2011).
In the DEX experiments, immediately following basal blood collection, DEX was administered by s.c. injection of DEX (Gensia Pharmaceuticals; Irvine CA) [0.01 (low) or 0.1 (high) mg/kg diluted in physiological saline] or saline vehicle (Sal). Animals were randomly assigned to each drug group (FPI and Sham: n = 9 per group, Ctrl: n=6 per group). The 0.1 mg/kg dose has been found to significantly suppress stress-induced increases in CORT and ACTH in control rats (Houshyar et al., 2001, Taylor et al., 2010). Two hours later rats were restrained for 30 min as described above. Rats were sacrificed by decapitation after the last blood collection at 120 min after restraint onset and brains were saved for further analysis.
Western Blots
Hippocampal tissue within the injured hemisphere was dissected and immediately placed on dry ice. Tissue samples from the entire hippocampus were weighed and homogenized in lysis buffer (100 mM Tris/HCL, pH 7, containing 2% bovine serum albumin (BSA), 1M NaCl, 4mM EDTA. Na2, 2% Triton X-100, 0.1% sodium azide and Protease Inhibitor Cocktail Tablets (Roche Applied Science, Indianapolis, IN, USA). Homogenates were centrifuged for 30 minutes at 4°C. The resulting supernatants were collected and immediately processed for total protein concentration according to the Micro BCA procedure (Pierce, Rockford, IL, USA) using bovine serum albumin as the standard. All chemicals were obtained from Sigma (St. Louis, MO, USA) unless otherwise noted.
Homogenates were separated by SDS-Page on 15% and 10% Tris-HCl Criterion Precast Gels (Bio-Rad Laboratories Inc., Hercules, CA, USA). Twenty-five μg protein was loaded to each well. Proteins were transferred to PVDF membranes (Millipore, Bedford, MA, USA) for Western blot analysis and stained for total protein using Ruby Red Sypro protein blot stain (Invitrogen Molecular Probes, Eugene, OR, USA) according to manufacturer’s instructions. Non-specific binding sites were blocked in TBS with 0.1% Tween-20 and 5% milk for 1h at room temperature. Membranes were incubated at 4°C overnight, with rabbit polyclonal anti-BDNF N-20 (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), or 2h at RT with rabbit polyclonal anti-GR M-20 (1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and rabbit anti-MR H-300 (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) followed by a 1h incubation at room temperature with anti-Rabbit IgG horseradish peroxidase conjugate secondary antibody (1:10,000, Pierce Biotechnology, Rockford, IL, USA). All washing steps were carried out with TBS with 0.1% Tween-20. Blots were developed using a chemiluminescent detection method with Super Signal West Femto Maximum Sensitivity Substrate kit (Thermo-Pierce Biotechnology, Rockford, IL, USA) according to manufacturer’s instructions. Blots were processed on the Chemi Doc XRS Imaging System (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Total protein per lane was determined after transfer by using Ruby Red Sypro Stain. Raw values for the proteins of interest were thus controlled to total protein from the same lane on the same blot. Each immunoblot contained the same Ctrl-Sal samples and an experimental group. In order to be able to compare across different experimental groups, the average immunoblot signal for each control sample (Ctrl-Sal) was obtained across all immunoblots. This also allowed for the normalization of Ctrl--Sal samples across the different gels. The experimental group was normalized to the average of Ctrl-Sal values. The final data was expressed as the percent change from the mean Ctrl-Sal values.
Statistical Analysis
Hippocampal BDNF, GR and MR group individual percent values were analyzed by conventional two-factor [Injury (FPI, Sham and Ctrl) and Drug (Sal, low and high)] analysis of variance. Differences in individual group means were then detected with Bonferroni corrected comparisons. Please note that for multiple comparisons the SPSS Bonferroni adjusted p-values are quoted (SPSS Inc, v.19., NY, USA).
RESULTS
Subjects
Injured rats had a mean (± SEM) unconsciousness time of 108 ± 5.1 s and a mean apnea time of 15 ± 2.5 s. Four animals were deleted from the study after FPI because the severity of injury was determined to be too high (over 300 s unconsciousness) and it was the purpose of these experiments to study mild TBI (Griesbach et al., 2011). Additionally, severely injured animals have an increased risk of cardiac arrest. Although we did not measure cardiac output in our animals, by eliminating severely injured subjects we eliminated cardiac arrest-induced increases in CORT (Neigh et al., 2009). No significant differences in weight gain were observed between the groups. No gross motor impairments of ambulatory ability were observed in any of the injured rats. Findings obtained for CORT and ACTH were previously published (Griesbach et al., 2011).
Glucocorticoid Receptor
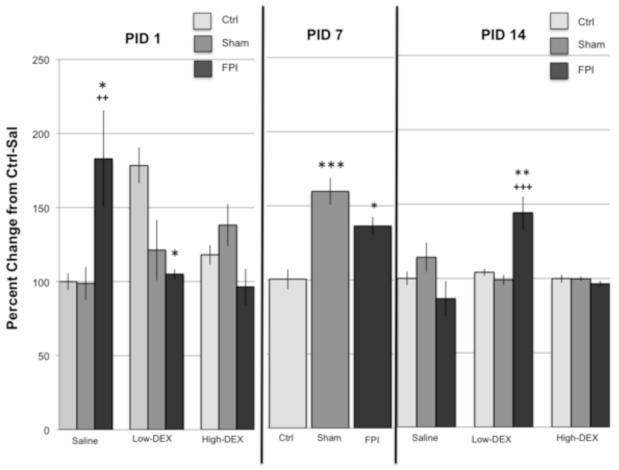
DEX reversed an FPI-related increase in GR at PID1. Analysis of GR at PID1 indicated a significant interaction for Injury × Drug (F4,70 = 6.22, p<0.0005). Further analysis indicated significant group effects under the Sal and low-DEX conditions. GR was significantly elevated in the FPI-Sal group compared to Ctrl-Sal (p < 0.05) and Sham-Sal (p < 0.005). As for the animals receiving the lower dose of DEX, GR was significantly lower in the FPI-low group compared to Ctrl-low (p < 0.05). Analysis of drug effects indicated that GR levels were higher in the Ctrl-low group compared to Ctrl-Sal (p < 0.05). In contrast, GR was significantly higher in the FPI-Sal group compared to FPI-low (p < 0.05) and FPI-high (p < 0.005).
At PID 7, increases in GR were observed in both in FPI and Sham injured groups. Analysis of GR at PID7 indicated a significant main Injury effect (F2,67 = 13.35, p < 0.0005). GR values for the Ctrl group were significantly lower compared to FPI (p < 0.05) and Sham (p < 0.0005) groups. No significant interactions were observed (Figure 1).
Fig. 1.
Levels of glucocorticoid receptor (GR) following restraint stress in Control (Ctrl), Sham, and fluid percussion injured (FPI) rats at post-injury days (PID) 1, 7 and 14. Effects on GR of dexamethasone (DEX) at a high (0.1 mg/kg) and low (0.01 mg/kg) dose are indicated. DEX or saline (Sal) were administered 2 hr prior to 30-min restraint. Each value represents the mean ± SEM. Injury related changes in GR were dependent on drug and PID. * P<0.05, **P<0.005, ***P<0.0005 compared to Control under the corresponding drug condition and PID. ++P<0.005, +++P<0.0005, compared to Sham under the corresponding drug condition and PID.
At PID 14, the FPI group responded differentially to the lower dose of DEX compared to other groups. Analysis of GR at PID14 indicated a significant interaction for Injury × Drug (F4,70 = 7.52, p < 0.0005). GR levels were higher in the FPI-low group compared to Ctrl-low (p < 0.005) and Sham-low (p < 0.0005). A strong drug effect was observed for the FPI-low group compared to FPI-Sal (p < 0.0005) and FPI-high (p < 0.0005). This result was supported by a significant main effect for Drug (F2,70 = 4.58, P<0.05).
Mineralocorticoid Receptor
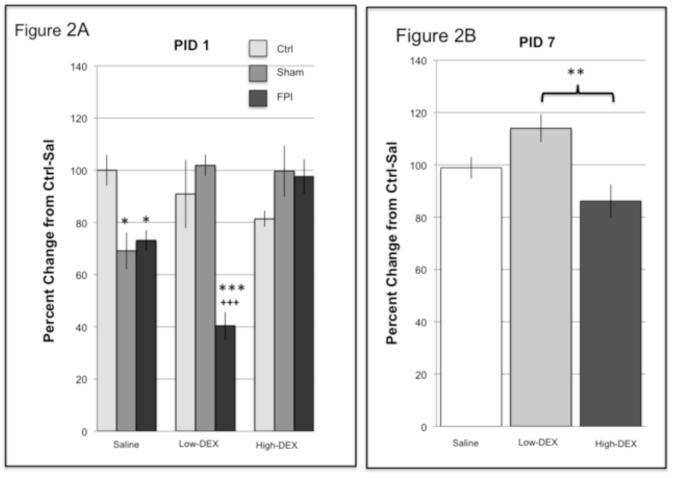
Acute increases in MR, observed in the FPI animals, were prevented with a higher dose of DEX. Analysis of MR at PID1 indicated a significant interaction for Injury × Drug (F4,71 = 10.08, p < 0.0005). Further analysis indicated significant group effects under the Sal and low-DEX conditions. MR was significantly lower in the Sham-Sal (p < 0.05) and FPI-Sal (p < 0.05) compared to the Ctrl-Sal group. As for the animals receiving the lower dose of DEX, MR was significantly lower in the FPI-low group compared to Ctrl-low (p < 0.0005) and Sham-low (p < 0.0005). Analysis of drug effects indicated that MR levels were lower in the FPI-low group compared to FPI-Sal (p < 0.005) and FPI-high (p < 0.0005). In addition, MR levels were elevated with DEX in the Sham animals; in that MR was higher in Sham-low (p < 0.005) and Sham-high (p < 0.05) compared to Sham-Sal. These findings were reflected as main effects for Injury (F2,71 = 8.51, p < 0.005) and Drug (F2,71 = 3.56, p < 0.05) (Figure 2a).
Fig. 2.
Levels of mineralocorticoid receptor (MR) following restraint stress in Control (Ctrl), Sham, and fluid percussion injured (FPI) rats at post-injury day (PID) (A) 1 and (B) 7. Effects on MR of dexamethasone (DEX) at a high (0.1 mg/kg) and low (0.01 mg/kg) dose are indicated. DEX or saline (Sal) were administered 2 hr prior to 30-min restraint. Each value represents the mean ± SEM of 6-9 rats per group. * P<0.05, **P<0.005, ***P<0.0005 compared to Control under the corresponding drug condition and PID.+++P<0.0005, compared to Sham under the corresponding drug condition and PID.
Analysis of MR at PID7 indicated a significant main Drug effect (F2,69 = 6.07, p < 0.005). MR values for those animals receiving low-DEX were significantly higher compared to high-DEX (p < 0.005)(Figure 2b). No significant interactions were observed at PID 14 for MR.
BDNF
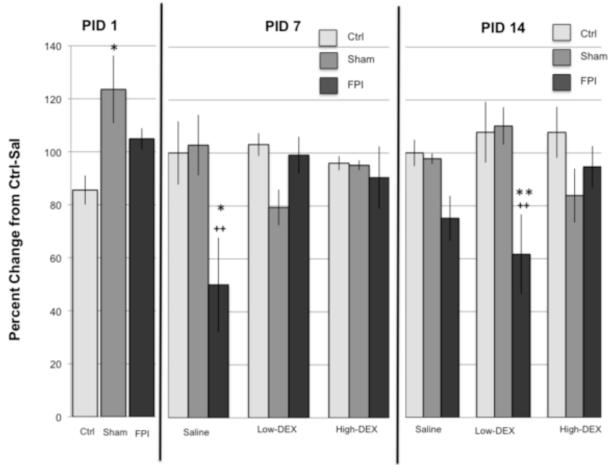
Sham injured rats had an acute increase in proBDNF. Analysis of proBDNF at PID1 indicated a main Injury effect (F2,72 = 4.0, p < 0.05). This effect was due to higher levels of proBDNF in the Sham group compared to Ctrl (p < 0.05).
A decrease in proBDNF was observed 1 week after FPI. Analysis of proBDNF at PID7 indicated a significant Injury × Drug interaction (F4,69 = 3.9, p < 0.05). Differences were observed under the Sal condition; in that proBDNF levels in the FPI-Sal group were lower compared to Ctrl-Sal (p < 0.05) and Sham-Sal (p < 0.005). Drug effects were only observed in FPI animals. Levels of proBDNF were lower in the FPI-Sal group compared to FPI-low (p < 0.005) and FPI-high (p < 0.05).
FPI effects on proBDNF were observed 2 weeks after injury. Analysis of proBDNF at PID14 indicated a significant Injury × Drug interaction (F4,71 = 2.96, p < 0.05). Differences were observed under the low-DEX condition; in that proBDNF levels in the FPI-low group was lower compared to Ctrllow (p < 0.005) and Sham-low (p < 0.005). Levels of proBDNF were lower in the FPI-low group compared to FPI-high (p < 0.05). These findings were reflected as main effects for Injury (F2,71 = 7.16, p < 0.005) (Figure 3).
Fig. 3.
Levels of the precursor form of brain derived neurotrophic factor (proBDNF) following restraint stress in Control (Ctrl), Sham, and fluid percussion injured (FPI) rats at post-injury days (PID) 1, 7 and 14. Effects on proBDNF of dexamethasone (DEX) at a high (0.1 mg/kg) and low (0.01 mg/kg) dose are indicated. DEX or saline (Sal) were administered 2 hr prior to 30-min restraint. Each value represents the mean ± SEM of 6-9 rats per group. Injury related decreases of the proBDNF in the FPI group were dependent on drug and PID. * P<0.05, **P<0.005 compared to Control under the corresponding drug condition and PID. ++P<0.005 compared to Sham under the corresponding drug condition and PID.
The early increase in proBDNF, observed in the Sham group, was accompanied by an increase in mBDNF. Analysis of mBDNF at PID1 indicated a main Injury effect (F2,75 = 6.36, p < 0.005). This effect was due to higher levels of mBDNF in the Sham group compared to Ctrl (p < 0.005). Comparison of FPI and Ctrl indicated a trend (p = 0.08) (Figure 4). No significant effects for mBDNF were observed at PIDs 7 and 14.
Fig. 4.
Levels of the mature form of brain derived neurotrophic factor (mBDNF) following restraint stress in Control (Ctrl), Sham, and fluid percussion injured (FPI) rats at post-injury day (PID) 1. Each value represents the mean ± SEM. **P<0.005 compared to Control.
DISCUSSION
These experiments evaluated the changes in BDNF, GR and MR within the hippocampus of brain-injured rats. These FPI rats presented a dysregulation of ACTH and CORT and a heightened response to restraint as indicated by CORT and ACTH plasma measurements (Tables 1 and 2) (Griesbach et al., 2011). Here we report marked injury effects in GR, MR and BDNF during the first postinjury week.
Table 1.
Basal levels of plasma CORT and ACTH on PID 1T 7 and 14
| Group | CORT, ng/ml | ACTHt pg/m1 | ||||
|---|---|---|---|---|---|---|
| PID1 | PID7 | PID 14 | PID1 | PID7 | PID 14 | |
| Ctrl | 251±19 | 251±19 | 251±19 | 130±16 | 75±3 | 75±3 |
| Sham | 216±18 | 207±23 | 238±24 | 132 ±8 # | 92±5 | 76±3 |
| FPI | 259±15 | 176±18* | 188±17 | 188±16* | 101±7** | 75±4 |
Blood samples were collected prior to restraint and DEX injection. Results are means + SEM. Ctrl values were pooled across days with no significant differences.
p<0.05 FPI vs Ctrl
p<0.005 FPI vs Ctrl
p<0.05 Sham vs FPI. These findings have been previously reported (Griesbach et al 2011).
Table 2.
CORTandACTH Responses to Restraint and DEX on PID 1P 7 and 14
| Group | CORT AUG | ACTH AUC | ||||
|---|---|---|---|---|---|---|
| PID1 | PID 7 | PID 14 | PID1 | PID 7 | PID 14 | |
| Ctrl-Sal | 552 ± 63 | 552 ± 63 | 552 ± 63 | 344 ±139† | 193 ±31 | 193 ±31 |
| Sham-Sal | 338 ±175 | 507 ± 79 | 612±82 | 316±140 | 528 ± 82* | 293 ± 32 |
| FPl-Sal | 620 ± 61 | 739 ± 76* | 964 ±110* | 319±118 | 546± 100* | 413 ± 113* |
| Ctrl-low | 495 ± 80 | 495 ± 80 | 495 ± 80 | 158 ±52 | 170 ±36 | 170 ±36 |
| Sham-low | 506 ± 92 | 323±112 | 261 ±200 | 76 ±72 | 166 ± 81§ | 69 ±37 |
| FPI-low | 624 ±153 | 510 ± 124 | 569±123 | −209± 126 | 148 ± 64§ | 218±64 |
| Ctrl-high | −374 ± 99§ | −374 ± 99§ | −374 i 99§ | −115 ±35§ | −105 +26§ | −105 ±26§ |
| Sham-high | −164 ± 171§ | −341 ± 129§ | −277 ±104§ | −250 ± 69§ | −140 ±24§ | −54 ± 11§ |
| FPI-high | 33 ± 101§ | −428 ± 66§ | −383 ± 101§ | −205 ± 82§ | −111 ±31§ | −80 ± 28§ |
Area under the curve (AUC) for CORT and ACTH is normalized to pre-stress basal levels. Each value represents the mean ± SEM of 6-9 rats per group.
P<0.05 compared to Ctrl-Sal at PID 7 and 14.
P<0.05 compared to Ctrl-Sal under the corresponding PID.
P<0.005 compared to equivalent Sal-treated injury condition under the corresponding PID.
These findings have been previously reported (Griesbach et al 2011).
Restraint Induced Stress increases hippocampal GR
The GR and MR have differential responses to CORT. MRs have a high affinity for CORT and are principally occupied during basal or resting conditions. In contrast, the lower affinity GRs are occupied when CORT levels are high in a peak-dependant manner i.e. stress (Reul and de Kloet, 1985, Conway-Campbell et al., 2010). The characteristics of the GR receptor, within the hippocampus, allow for its function in glucocorticoid negative feedback, but primarily to facilitate plasticity (McEwen, 1999, Revest et al., 2010, Spyrka and Hess, 2010). The reported findings appear to be in agreement with receptor characteristics; in that the GR was more affected by postinjury stress. Here we observed that injury related changes in GR were longer lasting compared to the MR. This suggests, that the sensitivity of the GR receptor to stress may be accentuated by TBI.
An increase in levels of GR was observed in the FPI-Sal rats at PID 1. This early elevation in GRs could be in response to the acute increases in transcription regulating processes such as inflammation, gliosis and neuroplasticty that are characteristic of brain injury; and that have been observed as increases in gene expression following a FPI (Lowenstein et al., 1994, Harris et al., 2009). It is also plausible that the acute increase of GR, in the FPI-Sal animals, was in response to the CORT decreases that were observed, prior to the induction of stress, at PID 1 (Table 1). Because the tissue for these studies was collected 120 min after restraint, which elevated CORT and ACTH, it is possible that GR protein levels were somewhat reduced to changes in protein translation during this period. Future studies analyzing GR mRNA would provide some clarification.
The FPI and sham groups showed increases in GR at the later time points. A craniotomy effect was observed at PID 7 as levels of GR were increased in the Sham groups compared to the Ctrl groups. These Sham animals also had significant increases in ACTH (Table 2). Activation of the HPA axis due to a craniotomy has been observed both clinically and in animal models (Shenkin et al., 1971, DeKeyser et al., 2000, Hirasawa et al., 2000). Moreover, long-lasting craniotomy effects have been reported (Cole et al., 2011). These reported sham effects are illustrative of why it is necessary to have appropriate controls when utilizing experimental brain injury models to enhance our understanding of human TBI. The GR returned to Ctrl levels in all the Sham groups by PID 14. However, GR elevations persisted in the FPI-low group.
The response to DEX changed across time
Contrasting with the Sal condition, GR was not elevated in the FPI groups that received DEX. This suggests the involvement of hippocampal glucocorticoid negative feedback at PID1. These data along with previous findings, indicating that the high dose of DEX suppressed CORT (Table 2), imply that the hyper-response to restraint was not associated with reduced sensitivity of CORT feedback regulation. Alternatively, an increase in CORT feedback sensitivity remains a possibility as suggested by the robust decrease in MR in the FPI-low group at PID1, along with the decreases in ACTH that have been previously reported in this group (Table 2).
Control and FPI animals responded differentially to the lower dose of DEX
An increase in GR was observed in the Ctrl groups that received the lower dose of DEX at PID1. This effect was not observed at later time points and can only be attributable to a prolonged isofluorane response. DEX associated increases were still observed at PID 14 in the FPI-low group. Increases in GR receptors may provide some insight on the hyper-response to stress that has been previously reported. Hippocampal GR receptors are involved in regulating the magnitude and duration of the stress response by regulating the release of ACTH (Jacobson and Sapolsky, 1991, Joels and de Kloet, 1994). The presence of a higher dose of DEX may have lessened the GR response because elevations of hippocampal GR were not observed in the FPI-high group as in the FPI-low group at PID14.
BDNF was acutely elevated in Sham animals
Glucocorticoid receptors underlie the control of synaptic plasticity by stress. For example, electrophysiological changes are observed within 30 min, in the hippocampus, as a response to stress (Xu et al., 1998). Once glucocorticoid hormones bind to the receptor they are transported to the nucleus and activate target genes. Among these genes are those associated to BDNF. Increases in the precursor and mature form of BDNF were observed at PID 1. However, it was only in Sham groups, and not the FPI, where these increases obtained significance. The increase in BDNF at this time point may be indicative of the activation of restorative processes in response to the craniotomy. However this is purely speculative and remains to be further explored. The differential response in BDNF between the FPI and Sham animals also remains to be determined. It is likely that ongoing disruptions in neuronal activation and metabolism play a role (Golding et al., 1999, Avramescu and Timofeev, 2008). Interestingly, an acute increase in GR was observed in the FPI-Sal rats that may have also participated in BDNF suppression. In spite of this, a strong relationship between GR and BDNF was not found; in that the groups showing significant increases in GR did not have a significant decrease in BDNF. This may be because the increases in GR were not strong enough and/or sustained for a sufficient period in order to decrease BDNF.
Restraint- induced stress decreased BDNF in FPI animals
The precursor form of BDNF was decreased in the FPI-Sal group at PID 7, when compared to Sal treated Ctrl and Sham animals. Decreases in BDNF mRNA have been observed after 6 hours of restraint stress (Murakami et al., 2005); contrasting with the 30 minutes of restraint that the animals underwent in these experiments. It is likely that the duration of the restraint in these experiments was insufficient to have an effect on the Ctrl and Sham groups. However, it might have been sufficient to affect FPI animals. Particularly, given that these were hyper-responsive to restraint as indicated by increases in CORT (Table 2). A decrease in the precursor for BDNF was also observed in the FPI-low group at PID14. This group also showed an increase in GR. It should be mentioned that this laboratory has not found decreases in BDNF, either in its precursor or mature form, in non-stressed FPI rats. (Griesbach et al., 2009, Griesbach et al., In Press). Thus, supporting the hypothesis that the stress response suppressed BDNF in the FPI animals. Future study of molecules downstream to BDNF as well as its receptor tyrosine kinase B (TrkB) should provide more insight on the cognitive repercussions of glucorocorticoid-induced decreases in BDNF. In turn, BDNF signaling is also likely to have an influence on HPA regulation. A recent study suggests that hypothalamic BDNF/TrkB signaling influences corticotropin releasing hormone expression (Jeanneteau et al., 2011).
Considerations
Interpretation of these findings is complex, given that GR and MR function is regulated by multiple posttranslational modifications that occur at the nuclear level. Future studies distinguishing protein translocation to the nucleus would aid in the interpretation of these findings. It should also be noted that these studies were performed in an acute stress model and not a model of repeated or chronic stress. Within the context of chronic stress, findings are controversial; with some studies indicating decreases of GR following chronic stress (Sapolsky et al., 1984, Kitraki et al., 1999) and others studies indicating no change (Herman and Spencer, 1998). As for acute stress, findings have also been controversial; and seem to be dependant on the experimental protocol and time of observation (Han et al., 2002, Murakami et al., 2005, Furay et al., 2008). It has been reported that the GR and MR response to acute restraint stress is biphasic, peaking initially at 30 min post restraint along with CORT and ACTH (Fujikawa et al., 2000). Others have shown decreases of GR mRNA at 120 after the onset of 30min of restraint, with levels returning to baseline by 240 min, with no changes in MR mRNA (Paskitti et al., 2000). In contrast to mRNA, observation of hippocampal GR protein after restraint indicated no change (Murakami et al., 2005). These diverse findings are illustrative of the sensitivity of hippocampal GR changes with experimental variables. It should be noted that the tissue for this study was not collected when CORT and ACTH levels were at their highest, which is during the first 30 min following restraint. Given that tissue was not obtained immediately after restraint and that the duration of restraint was relatively short, it is highly probable that GR and MR levels were similar to those without restraint in the Control animals. Regarding the injured rats, it remains unknown if GR and MR levels differed significantly from conditions prior to restraint. To our knowledge there are no studies observing hippocampal GR and MR after a FPI. There is however a study describing the effects of a controlled cortical impact injury on GR and MR mRNA within the hippocampus; where, a decrease in GR was observed 24 hrs after the injury on the hippocampus ipsilateral to the injury. No changes were observed for MR mRNA (McCullers et al., 2002). A caveat is that this decrease may have been associated with hippocampal cell death (Morales et al., 2005, Hall et al., 2008), which is characteristic of cortical impact injury model. In contrast the FPI is a more diffuse injury model that in our hands has resulted in minimal cell death both in adult and developing rats (Osteen et al., 2001, Griesbach et al., 2004, Gurkoff et al., 2006).
Implications
Affective disorders and cognitive impairments after TBI play a substantial part in decreasing quality of life. A dysregulation of the stress response has been linked to affective disorders in TBI patients (Jorge et al., 2007). In effect, alterations in the regulation of the HPA axis contribute to negative mood states associated with depression (Sapolsky, 2000, Ising et al., 2005, Vreeburg et al., 2009). In addition to showing a hyper-responsiveness to stress after TBI, we now provide evidence that post injury stress has an effect on BDNF regulation within the hippocampus. The effects of stress on cognitive abilities also needs to be considered, particularly given BDNF’s effects on plasticity, An increase in glucocorticoids, in human subjects, is associated with impaired memory (Newcomer et al., 1999, de Quervain et al., 2000) and hippocampal deterioration (Lupien et al., 1998). These data add some pieces to the puzzle in understanding the hyper-response and the delay of exercise-induced increases in BDNF during the subacute period. However, they also emphasize the need for more studies regarding HPA dysregulation after a mild TBI. Understanding some of the molecular mechanisms influencing the response to stress will allow us to better address posttraumatic affective and behavioral disorders as well as enhancing rehabilitative therapies.
Traumatic brain injury alters the dexamethasone response.
Traumatic brain injury alters the glucocorticoid receptor response to stress.
Stress-induced effects on the precursor for BDNF are time dependent.
Acknowledgements
This study was supported by NINDS award NS6190 (GSG) and the UCLA Brain Injury Research Center. We would wish to thank David Garfinkel for his excellent technical help.
Abbreviations
- ACTH
adrenocorticotropic hormone
- BDNF
brain derived neurotrophic factor
- CORT
corticosterone
- DEX
dexamethasone
- FPI
fluid-percussion injury
- GR
glucocorticoid receptor
- HPA
hypothalamic-pituitary-adrenal
- MR
mineralocorticoid receptor
- PID
postinjury day
- Sal
saline
- TBI
traumatic brain injury
- TrkB
tyrosine kinase B receptor
- UCLA
University of California at Los Angeles
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ashman TA, Gordon WA, Cantor JB, Hibbard MR. Neurobehavioral consequences of traumatic brain injury. Mt Sinai J Med. 2006;73:999–1005. [PubMed] [Google Scholar]
- Avramescu S, Timofeev I. Synaptic strength modulation after cortical trauma: a role in epileptogenesis. J Neurosci. 2008;28:6760–6772. doi: 10.1523/JNEUROSCI.0643-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco A, Patterson S, Alarcon JM, Gromova P, Mata-Roig M, Morozov A, Kandel ER. Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron. 2005;48:123–137. doi: 10.1016/j.neuron.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clin Sports Med. 2011;30:33–48. vii–iii. doi: 10.1016/j.csm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Bay E, Xie Y. Psychological and biological correlates of fatigue after mild-to-moderate traumatic brain injury. West J Nurs Res. 2009;31:731–747. doi: 10.1177/0193945909334856. [DOI] [PubMed] [Google Scholar]
- Bazarian JJ, Blyth B, Cimpello L. Bench to bedside: evidence for brain injury after concussion--looking beyond the computed tomography scan. Acad Emerg Med. 2006;13:199–214. doi: 10.1197/j.aem.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Cernak I, Savic VJ, Lazarov A, Joksimovic M, Markovic S. Neuroendocrine responses following graded traumatic brain injury in male adults. Brain Inj. 1999;13:1005–1015. doi: 10.1080/026990599121016. [DOI] [PubMed] [Google Scholar]
- Cole JT, Yarnell A, Kean WS, Gold E, Lewis B, Ren M, McMullen DC, Jacobowitz DM, Pollard HB, O’Neill JT, Grunberg NE, Dalgard CL, Frank JA, Watson WD. Craniotomy: true sham for traumatic brain injury, or a sham of a sham? J Neurotrauma. 2011;28:359–369. doi: 10.1089/neu.2010.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway-Campbell BL, Sarabdjitsingh RA, McKenna MA, Pooley JR, Kershaw YM, Meijer OC, De Kloet ER, Lightman SL. Glucocorticoid ultradian rhythmicity directs cyclical gene pulsing of the clock gene period 1 in rat hippocampus. J Neuroendocrinol. 2010;22:1093–1100. doi: 10.1111/j.1365-2826.2010.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Jacobson L, Levin N, Cascio CS, Shinsako J. Characterization of corticosterone feedback regulation of ACTH secretion. Ann N Y Acad Sci. 1987;512:402–414. doi: 10.1111/j.1749-6632.1987.tb24976.x. [DOI] [PubMed] [Google Scholar]
- de Kloet ER. Stress in the brain. Eur J Pharmacol. 2000;405:187–198. doi: 10.1016/s0014-2999(00)00552-5. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat Neurosci. 2000;3:313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- DeKeyser FG, Leker RR, Weidenfeld J. Activation of the adrenocortical axis by surgical stress: involvement of central norepinephrine and interleukin-1. Neuroimmunomodulation. 2000;7:182–188. doi: 10.1159/000026437. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Fleshner M, Rose GM. Psychological stress repeatedly blocks hippocampal primed burst potentiation in behaving rats. Behav Brain Res. 1994;62:1–9. doi: 10.1016/0166-4328(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Englander J, Bushnik T, Oggins J, Katznelson L. Fatigue after traumatic brain injury: Association with neuroendocrine, sleep, depression and other factors. Brain Inj. 2010;24:1379–1388. doi: 10.3109/02699052.2010.523041. [DOI] [PubMed] [Google Scholar]
- Fujikawa T, Soya H, Fukuoka H, Alam KS, Yoshizato H, McEwen BS, Nakashima K. A biphasic regulation of receptor mRNA expressions for growth hormone, glucocorticoid and mineralocorticoid in the rat dentate gyrus during acute stress. Brain Res. 2000;874:186–193. doi: 10.1016/s0006-8993(00)02576-2. [DOI] [PubMed] [Google Scholar]
- Furay AR, Bruestle AE, Herman JP. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology. 2008;149:5482–5490. doi: 10.1210/en.2008-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding EM, Steenberg ML, Contant CF, Jr., Krishnappa I, Robertson CS, Bryan RM., Jr. Cerebrovascular reactivity to CO(2) and hypotension after mild cortical impact injury. Am J Physiol. 1999;277:H1457–1466. doi: 10.1152/ajpheart.1999.277.4.H1457. [DOI] [PubMed] [Google Scholar]
- Goodman Y, Bruce AJ, Cheng B, Mattson MP. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid beta-peptide toxicity in hippocampal neurons. J Neurochem. 1996;66:1836–1844. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Gomez-Pinilla F, Hovda DA. Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. J Neurotrauma. 2007;24:1161–1171. doi: 10.1089/neu.2006.0255. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009;1288:105–115. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125:129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Tio DL, Taylor AN. Heightening of the stress response during the first weeks after a mild traumatic brain injury. Neuroscience. 2011;178:147–158. doi: 10.1016/j.neuroscience.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbach GS, Tio DL, Vincelli BS, McArthur DL, Taylor AN. Differential Effects of Voluntary and Forced Exercise after Traumatic Brain injury on Stress Responses. Journal of Neurotrauma. doi: 10.1089/neu.2011.2229. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronli J, Bramham C, Murison R, Kanhema T, Fiske E, Bjorvatn B, Ursin R, Portas CM. Chronic mild stress inhibits BDNF protein expression and CREB activation in the dentate gyrus but not in the hippocampus proper. Pharmacol Biochem Behav. 2006;85:842–849. doi: 10.1016/j.pbb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Gurkoff GG, Giza CC, Hovda DA. Lateral fluid percussion injury in the developing rat causes an acute, mild behavioral dysfunction in the absence of significant cell death. Brain Res. 2006;1077:24–36. doi: 10.1016/j.brainres.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Hall ED, Bryant YD, Cho W, Sullivan PG. Evolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methods. J Neurotrauma. 2008;25:235–247. doi: 10.1089/neu.2007.0383. [DOI] [PubMed] [Google Scholar]
- Han JS, Bizon JL, Chun HJ, Maus CE, Gallagher M. Decreased glucocorticoid receptor mRNA and dysfunction of HPA axis in rats after removal of the cholinergic innervation to hippocampus. Eur J Neurosci. 2002;16:1399–1404. doi: 10.1046/j.1460-9568.2002.02191.x. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cintra A, Belluardo N, Sommer W, Bhatnagar M, Bader M, Ganten D, Fuxe K. Gluco- and mineralocorticoid receptor-mediated regulation of neurotrophic factor gene expression in the dorsal hippocampus and the neocortex of the rat. Eur J Neurosci. 2000;12:2918–2934. doi: 10.1046/j.1460-9568.2000.00185.x. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Sommer WH, Metsis M, Stromberg I, Agnati LF, Fuxe K. Corticosterone actions on the hippocampal brain-derived neurotrophic factor expression are mediated by exon IV promoter. J Neuroendocrinol. 2006;18:104–114. doi: 10.1111/j.1365-2826.2005.01390.x. [DOI] [PubMed] [Google Scholar]
- Harris JL, Reeves TM, Phillips LL. Injury modality, survival interval, and sample region are critical determinants of qRT-PCR reference gene selection during long-term recovery from brain trauma. J Neurotrauma. 2009;26:1669–1681. doi: 10.1089/neu.2009.0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Spencer R. Regulation of hippocampal glucocorticoid receptor gene transcription and protein expression in vivo. J Neurosci. 1998;18:7462–7473. doi: 10.1523/JNEUROSCI.18-18-07462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa K, Kasuya H, Hori T. Change in circulating blood volume following craniotomy. J Neurosurg. 2000;93:581–585. doi: 10.3171/jns.2000.93.4.0581. [DOI] [PubMed] [Google Scholar]
- Houshyar H, Galigniana MD, Pratt WB, Woods JH. Differential responsivity of the hypothalamic-pituitary-adrenal axis to glucocorticoid negative-feedback and corticotropin releasing hormone in rats undergoing morphine withdrawal: possible mechanisms involved in facilitated and attenuated stress responses. J Neuroendocrinol. 2001;13:875–886. doi: 10.1046/j.1365-2826.2001.00714.x. [DOI] [PubMed] [Google Scholar]
- Ising M, Kunzel HE, Binder EB, Nickel T, Modell S, Holsboer F. The combined dexamethasone/CRH test as a potential surrogate marker in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1085–1093. doi: 10.1016/j.pnpbp.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Jeanneteau FD, Lambert WM, Ismaili N, Bath KG, Lee FS, Garabedian MJ, Chao MV. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proc Natl Acad Sci U S A. 2011;109:1305–1310. doi: 10.1073/pnas.1114122109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M, de Kloet ER. Control of neuronal excitability by corticosteroid hormones. Trends Neurosci. 1992;15:25–30. doi: 10.1016/0166-2236(92)90345-9. [DOI] [PubMed] [Google Scholar]
- Joels M, de Kloet ER. Mineralocorticoid and glucocorticoid receptors in the brain. Implications for ion permeability and transmitter systems. Prog Neurobiol. 1994;43:1–36. doi: 10.1016/0301-0082(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Jorge RE, Acion L, Starkstein SE, Magnotta V. Hippocampal volume and mood disorders after traumatic brain injury. Biol Psychiatry. 2007;62:332–338. doi: 10.1016/j.biopsych.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Kitraki E, Karandrea D, Kittas C. Long-lasting effects of stress on glucocorticoid receptor gene expression in the rat brain. Neuroendocrinology. 1999;69:331–338. doi: 10.1159/000054435. [DOI] [PubMed] [Google Scholar]
- Lowenstein DH, Gwinn RP, Seren MS, Simon RP, McIntosh TK. Increased expression of mRNA encoding calbindin-D28K, the glucose-regulated proteins, or the 72 kDa heat-shock protein in three models of acute CNS injury. Brain Res Mol Brain Res. 1994;22:299–308. doi: 10.1016/0169-328x(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Lu B. Pro-region of neurotrophins: role in synaptic modulation. Neuron. 2003;39:735–738. doi: 10.1016/s0896-6273(03)00538-5. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- McCullers DL, Sullivan PG, Scheff SW, Herman JP. Traumatic brain injury regulates adrenocorticosteroid receptor mRNA levels in rat hippocampus. Brain Res. 2002;947:41–49. doi: 10.1016/s0006-8993(02)02904-9. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001;16:S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- Meyer A, Chretien P, Massicotte G, Sargent C, Chretien M, Marcinkiewicz M. Kainic acid increases the expression of the prohormone convertases furin and PC1 in the mouse hippocampus. Brain Res. 1996;732:121–132. doi: 10.1016/0006-8993(96)00502-1. [DOI] [PubMed] [Google Scholar]
- Morales DM, Marklund N, Lebold D, Thompson HJ, Pitkanen A, Maxwell WL, Longhi L, Laurer H, Maegele M, Neugebauer E, Graham DI, Stocchetti N, McIntosh TK. Experimental models of traumatic brain injury: do we really need to build a better mousetrap? Neuroscience. 2005;136:971–989. doi: 10.1016/j.neuroscience.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res. 2005;53:129–139. doi: 10.1016/j.neures.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Neigh GN, Karelina K, Zhang N, Glasper ER, Owens MJ, Plotsky PM, Nemeroff CB, Devries AC. Cardiac arrest and cardiopulmonary resuscitation dysregulates the hypothalamic-pituitary-adrenal axis. J Cereb Blood Flow Metab. 2009;29:1673–1682. doi: 10.1038/jcbfm.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Richards K, Alderson AL. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch Gen Psychiatry. 1999;56:527–533. doi: 10.1001/archpsyc.56.6.527. [DOI] [PubMed] [Google Scholar]
- Osteen CL, Moore AH, Prins ML, Hovda DA. Age-dependency of 45calcium accumulation following lateral fluid percussion: acute and delayed patterns. J Neurotrauma. 2001;18:141–162. doi: 10.1089/08977150150502587. [DOI] [PubMed] [Google Scholar]
- Paskitti ME, McCreary BJ, Herman JP. Stress regulation of adrenocorticosteroid receptor gene transcription and mRNA expression in rat hippocampus: time-course analysis. Brain Res Mol Brain Res. 2000;80:142–152. doi: 10.1016/s0169-328x(00)00121-2. [DOI] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Revest JM, Kaouane N, Mondin M, Le Roux A, Rouge-Pont F, Vallee M, Barik J, Tronche F, Desmedt A, Piazza PV. The enhancement of stress-related memory by glucocorticoids depends on synapsin-Ia/Ib. Mol Psychiatry. 2010;15:1125, 1140–1151. doi: 10.1038/mp.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp Gerontol. 1999;34:721–732. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Stress down-regulates corticosterone receptors in a site-specific manner in the brain. Endocrinology. 1984;114:287–292. doi: 10.1210/endo-114-1-287. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, de Jong J, de Kloet ER, Vreugdenhil E. Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res. 1998;813:112–120. doi: 10.1016/s0006-8993(98)01010-5. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, Hoetelmans RW, de Kloet ER, Vreugdenhil E. Corticosterone regulates expression of BDNF and trkB but not NT-3 and trkC mRNA in the rat hippocampus. J Neurosci Res. 1997;48:334–341. [PubMed] [Google Scholar]
- Seidah NG, Benjannet S, Pareek S, Chretien M, Murphy RA. Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertases. FEBS Lett. 1996;379:247–250. doi: 10.1016/0014-5793(95)01520-5. [DOI] [PubMed] [Google Scholar]
- Shenkin HA, Gutterman P, Bouzarth WF. The adrenocortical response to craniotomy for brain tumor. J Neurosurg. 1971;34:657–664. doi: 10.3171/jns.1971.34.5.0657. [DOI] [PubMed] [Google Scholar]
- Spyrka J, Hess G. Repeated restraint-induced modulation of long-term potentiation in the dentate gyrus of the mouse. Brain Res. 2010;1320:28–33. doi: 10.1016/j.brainres.2010.01.031. [DOI] [PubMed] [Google Scholar]
- Steinbok P, Thompson G. Serum cortisol abnormalities after craniocerebral trauma. Neurosurgery. 1979;5:559–565. doi: 10.1227/00006123-197911000-00003. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Rahman SU, Sanders NC, Tio DL, Prolo P, Sutton RL. Injury severity differentially affects short- and long-term neuroendocrine outcomes of traumatic brain injury. J Neurotrauma. 2008;25:311–323. doi: 10.1089/neu.2007.0486. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Rahman SU, Tio DL, Gardner SM, Kim CJ, Sutton RL. Injury severity differentially alters sensitivity to dexamethasone after traumatic brain injury. J Neurotrauma. 2010;27:1081–1089. doi: 10.1089/neu.2009.1252. [DOI] [PubMed] [Google Scholar]
- Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, Smit JH, Zitman FG, Penninx BW. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry. 2009;66:617–626. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- Xu L, Holscher C, Anwyl R, Rowan MJ. Glucocorticoid receptor and protein/RNA synthesis-dependent mechanisms underlie the control of synaptic plasticity by stress. Proc Natl Acad Sci U S A. 1998;95:3204–3208. doi: 10.1073/pnas.95.6.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]