Abstract
Cocaine addiction is characterized by compulsive drug seeking, including relapse after a period of withdrawal. The relapse response requires increased glutamate transmission in the nucleus accumbens (NAc). Consistent with this view, GLT1, the transporter responsible for >90% of glutamate uptake, is down-regulated in NAc after several days of withdrawal in rats previously trained to self-administer cocaine under limited access conditions (1–2 hr/day). Human addiction, however, appears to be better modeled by extending daily drug access (6–8 hr/day) and introducing long periods of withdrawal. Here, we determined the combined effects of manipulating cocaine access and withdrawal on GLT1 expression in NAc core and shell. Rats were trained to self-administer cocaine (0.25 mg per intravenous infusion) in daily limited or extended access sessions for 11 days followed by a period of short (1 day) or long (40–45 days) withdrawal. We found that although cocaine withdrawal decreases GLT1 expression in both core and shell, only in core is GLT1 down-regulation sensitive to both access and withdrawal. In fact, after long withdrawal, GLT1 in core is down-regulated more than in shell in either the limited or extended access condition. Thus, glutamate regulation in core appears to be a critical factor in the drug-seeking behavior that follows relatively long periods of cocaine withdrawal.
Keywords: cocaine, GLT1, nucleus accumbens, self-administration, withdrawal, glutamate
1
Cocaine addiction is a chronic relapsing disorder characterized by compulsive drug seeking. Key components of the underlying neural circuitry include prefrontal cortex, which processes the signals that trigger relapse (Goldstein and Volkow, 2002, Sun and Rebec, 2006), and nucleus accumbens (NAc), which is implicated in goal-directed behavior (Childress et al., 1999). The NAc can be divided into core and shell regions distinguishable in morphology and connectivity (Zahm and Heimer, 1993, Meredith, 1999). Core and shell also play different roles in behavior with evidence favoring a role for the core in relapse to drug seeking (Everitt and Robbins, 2005). In fact, drug-induced changes in glutamate transmission in core appear to underlie different aspects of cocaine addiction (Kalivas et al., 2009). In rats, cocaine self-administration (SA) decreases basal glutamate levels in core (McFarland et al., 2003) likely due to decreased expression of the cystine-glutamate exchanger (Knackstedt et al., 2010), the protein responsible for the majority of extracellular glutamate in NAc (Baker et al., 2002). Core glutamate, however, increases during cocaine relapse (Hotsenpiller et al., 2001, McFarland et al., 2003), and can be explained by down-regulation of GLT1 (Sari et al., 2009, Knackstedt et al., 2010), the transporter responsible for the removal of at least 90% of extracellular glutamate (Rothstein et al., 1995, Danbolt, 2001, Rothstein et al., 2005).
In commonly used models of cocaine relapse, rats self-administer the drug for 1–2 hrs/day for one or two weeks and then are tested for reinstatement of cocaine seeking after several days of extinction training (Shaham et al., 2003). Although this model has shed light on the leading causes of relapse, it elicits a pattern of controlled drug use that may not parallel key features of human addiction. In contrast, extending drug access to 6+ hrs/day elicits behaviors that closely resemble compulsive drug seeking (Ahmed and Koob, 1998, Ferrario et al., 2005). Moreover, introducing withdrawal periods in the extended access model results in a time-dependent increase in drug relapse, also known as incubation of cocaine craving (Conrad et al., 2008). Collectively, these findings suggest that increasing drug access and withdrawal promotes the transition to addiction. Here, we assessed the effects of limited and extended cocaine access in conjunction with periods of short (SW) and long withdrawal (LW) on GLT1 expression in NAc core and shell.
2 - Experimental Procedures
2.1 - Animals
Data were obtained from 40, male, Sprague-Dawley rats (350–400g at the start of experimentation) bred from animals supplied by Harlan Industries. Rats were single-housed in a temperature- and humidity-controlled vivarium. Food and water were available ad libitum, and lights operated on a 12 h cycle (on at 7:00 A.M.). All housing and experimental procedures were approved by the Institutional Animal Care and Use Committee.
2.2 - Behavioral chambers
Rats were tested in eight, standard operant chambers [27 cm (length) × 22.5 cm (width) × 23.5 cm (height)] supplied by MED Associates. One wall of each chamber was equipped with two levers [active and inactive (spaced 13 cm apart and 10 cm above the grid floor)] and 1 W cue light (located 3.5 cm above each lever). The number of inactive lever presses was < 5% of that of active lever presses throughout the entire study. A food hopper, located between the two levers, was connected to a food dispenser installed outside each chamber. A programmable speaker, used to deliver a tone (54 dB), was installed on the opposite wall along with a 5 W house light. Each chamber was housed in a light- and sound-attenuating cubicle. A fluid pump, positioned outside each cubicle, was used to deliver cocaine.
2.3- Animal surgery
Each rat was anesthetized with xylazine (10 mg/kg, i.p.) and ketamine (80 mg/kg, i.p.) for surgical implantation of a jugular vein catheter as previously described (Sun and Rebec, 2003). After the incision site was sutured, the animals were closely monitored for one week, during which the catheters were flushed twice daily with heparinized physiological saline (30 U/ml heparin). To assess catheter patency during the period of cocaine SA, 0.1 ml of brevital (1%) was injected as necessary. Loss of muscle tone within 5 s after injection indicated a patent catheter.
2.4 - Cocaine Self-Administration
Rats, food restricted for one week to reduce their weight to ~85%, were trained to press the active lever for food (rodent food pellet, formula A/I) on a fixed-ratio 1 (FR1) schedule of reinforcement. After food responding stabilized, rats began cocaine SA on the following day. Before the first SA session, rats were divided into one of two access groups: a limited group (2 hrs/session) or an extended group (6 hrs/session). All rats participated in one daily SA session for exactly 11 days. Pressing the active lever was reinforced by an infusion of cocaine (0.25 mg of cocaine in a volume of 0.1 ml over 2.8 s) on a FR1 schedule. Each session lasted 2 or 6 hrs or until animals received 60 (limited) or 180 (extended) infusions of cocaine. Thus, maximum amounts of cocaine per limited and extended SA session were 15.0 and 45.0 mg, respectively. Food groups with identical handling and food training were run on either limited or extended schedules, but were reinforced with food pellets. Limited rats were run on a cocaine SA schedule for 11 days while concurrently displaying stable responding, defined as less than 10% variation in the number of active lever presses for four consecutive days (Sari et al., 2009). Because responding does not stabilize in extended rats (Ahmed and Koob, 1998, Ferrario et al., 2005), they were run on this schedule for exactly 11 days. Similar to others using the extended access model (Ahmed and Koob, 1998, 2004, Ferrario et al., 2005, Ahmed and Cador, 2006, Ben-Shahar et al., 2008), we defined escalation in cocaine consumption as a significantly greater intake on subsequent cocaine administration trials compared to the first trial. In both cocaine and both food access groups, withdrawal began on the following day.
2.5 - Withdrawal
Rats were assigned to one of two withdrawal groups: SW (1 day of withdrawal) or LW (40–45 days of withdrawal). The range for LW was chosen based on the incubation of cocaine craving model (McCutcheon et al., 2010, Ferrario et al., 2011a, Ferrario et al., 2011b). All withdrawal took place in the home environment.
2.6 - Tissue Extraction
Immediately after the appropriate withdrawal period, rats were euthanized by rapid decapitation, brains were removed and snap frozen in an isopentane bath cooled on dry ice. The NAc from both hemispheres was dissected for subsequent immunoblotting. Brains were sliced into 1 mm coronal sections via a brain-slicing matrix (Plastics One), and core (+2.5 to +0.7 mm from bregma) and shell (+3.0 to +2.5 mm from bregma) were extracted free-hand according to standard coordinates (Paxinos, 1998).
2.7 - Western Blots
Extracted tissue was immersed in a lysis buffer (100µL) and frozen on dry ice. Once frozen, tissue was sonicated and re-frozen twice to homogenize. The samples were then centrifuged for 20 min at 16,100 × g at 4°C. Supernatant was extracted and frozen over dry ice. Protein quantification was carried out by the Bio-Rad DC Protein Assay Kit. A bovine serum albumin standard curve was constructed and the unknown sample concentrations (core and shell tissue) were determined via the absorbance at a wavelength of 750 nm. Extracted proteins were separated in 4 – 20% glycine gel (Invitrogen) electrophoretically at 200 V for 1 hr. Proteins were then transferred onto a nitrocellulose membrane electrophoretically at 20 V for 30 min. Unbound sites on the membrane were blocked by incubating with 3% milk in TBST (0.5 M Tris-HCl; 1.5 M NaCl, pH 7.4; 10 ml of 10% Tween 20) for 30 min at room temperature. The guinea pig anti-GLT1 antibody (Millipore Bioscience Research Reagents) diluted 1:5000 in 3% milk in TBST was incubated overnight at 4°C. Horseradish peroxidase (HRP) secondary antibody was used at 1:10,000 dilution with 3% milk in TBST. Equivalent protein loading was assessed by glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Millipore Bioscience Research Reagents) immunoblotting as a loading control. After incubation with HRP kit (SuperSignal West Pico; Pierce) for 1 min, membranes were exposed to Kodak BioMax MR film (Thermo Fisher Scientific), and the films were developed on a SRX-101A machine. Digitized images of immunoreactive proteins were quantified using an image analysis system. Film exposure times were carefully monitored to rule out saturation effects. A ratio of the optical densities of GLT1/GAPDH was determined for each sample and compared to the ratio of the optical densities of GLT1/GAPDH of food controls (food control ratio of the optical densities of GLT1/GAPDH was set to 100%).
Protein extractions and Western blots were performed in parallel for each group, including one food control per gel for comparison. Each gel included both core and shell samples from individual experimental animals.
2.8 - Statistical Analyses
One and two-way ANOVAs were used to analyze behavioral data. Bonferroni-pairwise comparisons were made within individual groups. Western blot data were analyzed by ANOVA followed by Games-Howell Multiple Comparisons Tests. All statistical tests were based on p < 0.05 level of significance.
3 - Results
3.1 - Extended rats display an escalation in cocaine consumption
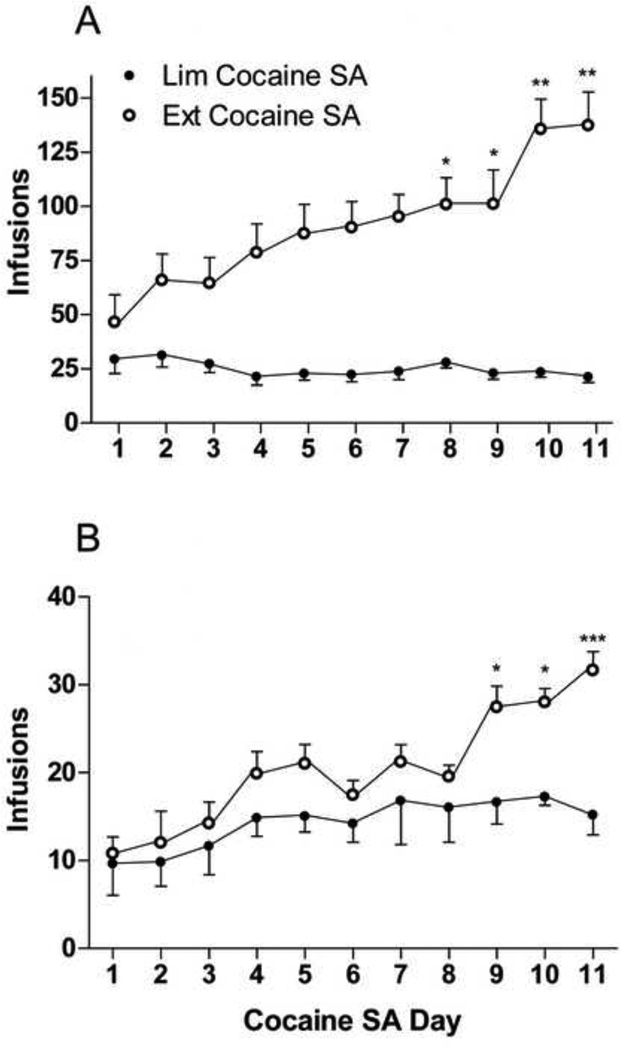
As revealed by one-way ANOVA, all limited rats exhibited stable responding across cocaine SA days (F(10, 131) = 0.4911; p = 0.8899), whereas all extended rats exhibited significant differences in cocaine consumption across SA days (F(10, 131) = 4.580; ***p < 0.0001) [Fig. 1A]. Two-way, repeated-measures ANOVA revealed a significant difference in cocaine consumption during the first hour between access condition and SA day (F(1, 132) = 29.12; ***p < 0.0001) [Fig. 1B]. Bonferroni’s Multiple Comparison revealed an escalation in cocaine consumption in extended rats on days 8–11 compared with day 1 (Fig. 1A, days 8–9, p < 0.05; days 10–11, p < 0.001) as well as during the first hr of SA on days 9–11 compared with limited SA rats (Fig. 1B, days 9–10, p < 0.05; day 11, p < 0.0001). None of the groups differed in body weight; all rats were within 15 g of each other.
Figure 1. Effect of drug access on cocaine intake.
(A) Cocaine infusions received during each day of limited and extended SA. Error bars indicate SEM. N = 16 rats/group. One-way ANOVA revealed no significant effects between or within limited access groups (F(10, 131) = 0.4911; p = 0.8899). One-way ANOVA revealed significant differences between and within extended access groups (F(10, 131) = 4.580; ***p < 0.0001). Bonferroni’s Multiple Comparison revealed that the number of cocaine infusions during extended SA sessions 8–11 is significantly higher than session 1 (*p < 0.05, **p < 0.001). (B) Cocaine infusions received each day during the first hour of limited and extended SA. Error bars indicate SEM. N = 16 rats/group. A two-way, repeated-measures ANOVA revealed a significant difference between limited and extended access as well as SA day (F(1, 132) = 29.12; ***p < 0.0001). Bonferroni’s Multiple Comparison revealed that the number of cocaine infusions during the first hour in extended SA rats is significantly higher on days 9–11 compared with limited SA rats (*p < 0.05, ***p < 0.0001).
3.2 - Increasing access and withdrawal periods enhance GLT1 down-regulation in core
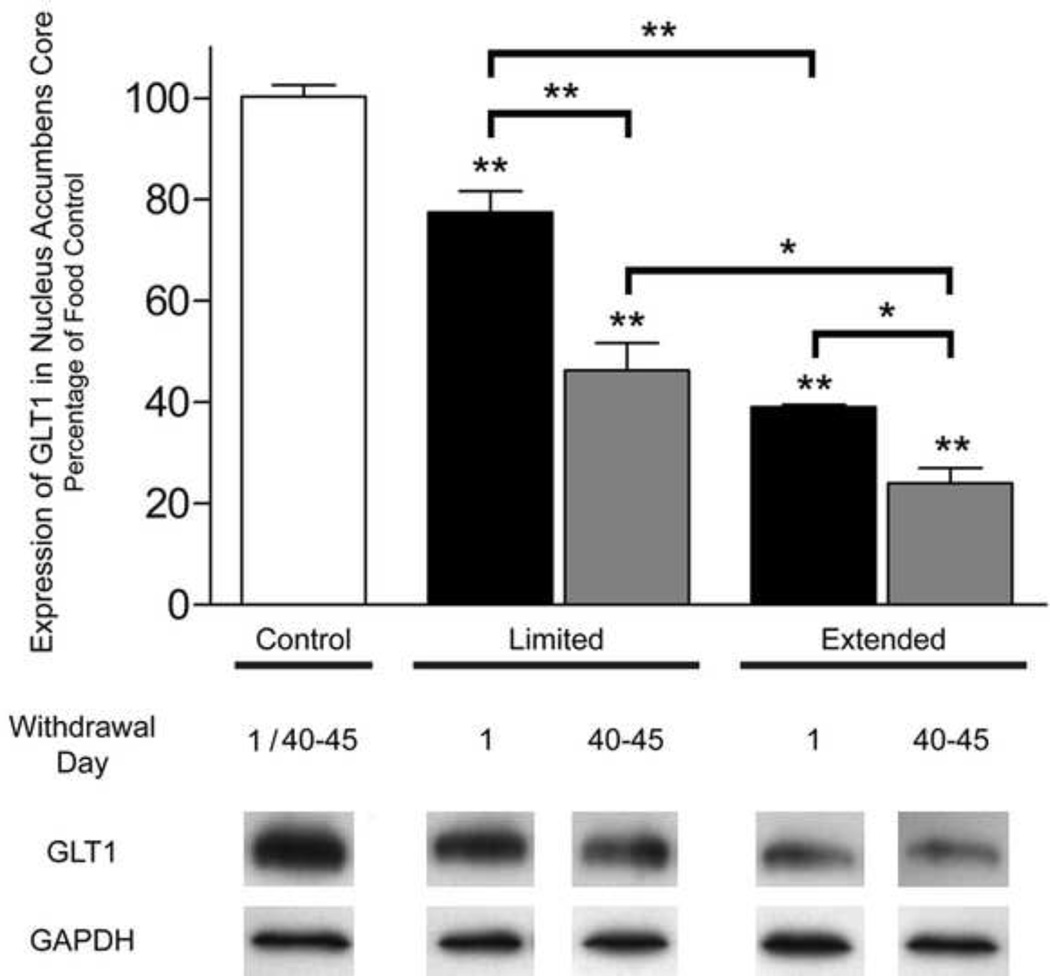
One-way ANOVA revealed significant differences in core GLT1 expression between both access and withdrawal groups (F(4, 35) = 69.794; p < 0.0001) [Fig. 2]. No significant differences were found between food controls in both access and withdrawal conditions and are represented as a single control group. Games-Howell Multiple Comparisons showed that both access and both withdrawal groups were significantly reduced compared with food controls (p < 0.001). Additionally, Games-Howell Multiple Comparisons indicated NAc core GLT1 expression to be significantly lower in the extended vs. limited groups (LW p < 0.05, SW p < 0.001) as well as in the LW vs. SW groups (extended, p < 0.05; limited, p < 0.001).
Figure 2. Effects of SW and LW in limited and extended cocaine access rats on core GLT1 expression.
Immunoblots for GAPDH, which was used as a loading control, and GLT1 are displayed. Error bars indicate SEM. N = 8 rats/group. One-way ANOVA revealed significant differences between and within groups (F(4, 35) = 69.794; ***p < 0.0001). Games-Howell Multiple Comparisons Test revealed a significant reduction in NAc core GLT1/GAPDH ratio in LW and SW limited and extended cocaine access rats compared to food controls (**p < 0.001). No changes in GLT1 expression occurred between the food groups (p > 0.05). Games-Howell Multiple Comparisons Test revealed a significant difference between extended vs. limited access groups (*p<0.05, **p < 0.001) and between LW vs. SW groups (*p < 0.05, **p < 0.001).
3.3 - Increasing access, but not withdrawal periods, enhances GLT1 down-regulation in shell
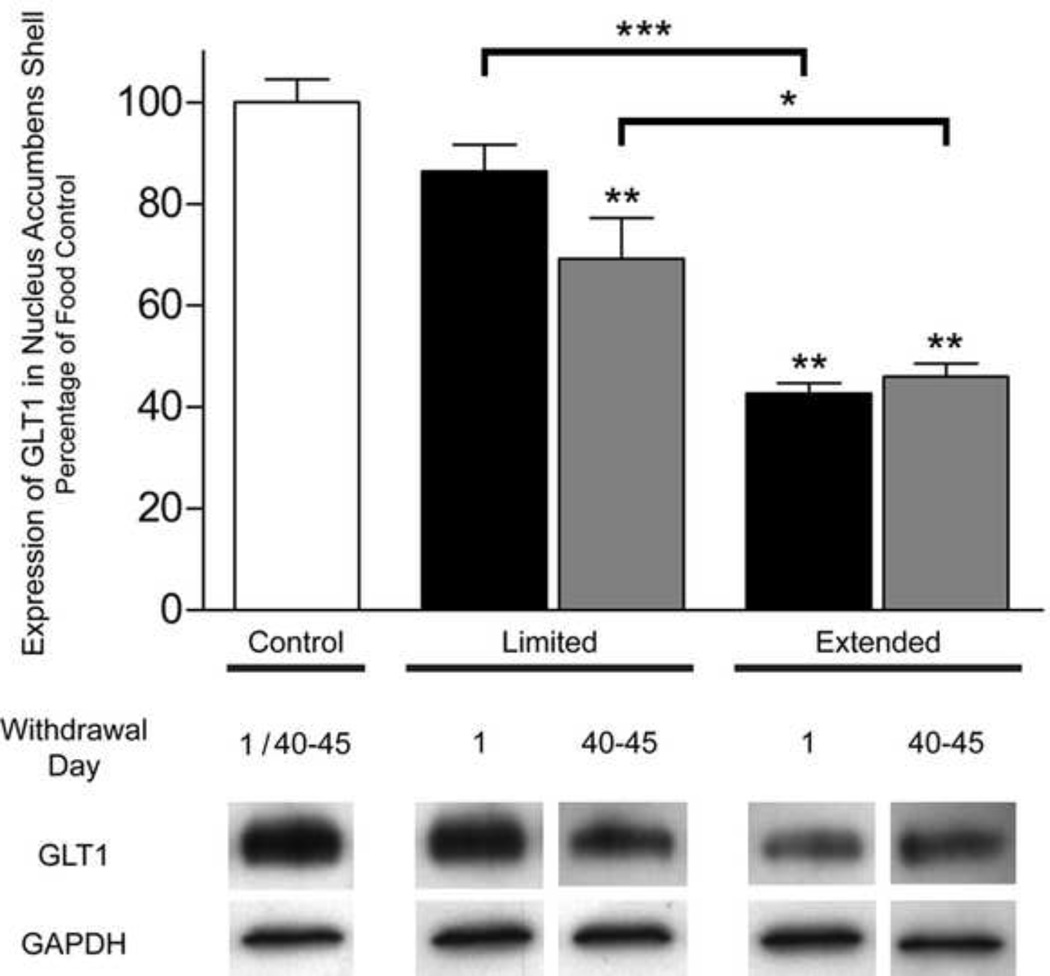
One-way ANOVA revealed significant differences in shell GLT1 expression between both access and withdrawal groups (F(4, 35) = 36.3; ***p < 0.0001) [Fig. 3]. No significant differences were found between food controls in both access and withdrawal conditions and are represented as a single control group. Games-Howell Multiple Comparisons showed significant differences between both withdrawal groups as well as the limited LW group compared to food controls (p < 0.001). Additionally, Games-Howell Multiple Comparisons between access and withdrawal groups indicated significant differences only between extended and limited LW (p < 0.05) and between extended and limited SW groups (p < 0.0001).
Figure 3. Effects of SW and LW in limited and extended cocaine access rats on shell GLT1 expression.
Immunoblots for GAPDH, which was used as a loading control, and GLT1 are displayed. Error bars indicate SEM. N = 8 rats/group. One-way ANOVA revealed significant differences between and within groups (F(4, 35) = 36.3; ***p < 0.0001). Games-Howell Multiple Comparisons Test revealed a significant reduction in NAc shell GLT1/GAPDH ratio in LW limited and LW and SW extended cocaine access rats compared to food controls (**p < 0.001). No changes in GLT1 expression occurred between the food groups (p > 0.05). Games-Howell Multiple Comparisons Test revealed a significant difference between extended vs. limited access groups (*p < 0.05, ***p < 0.0001).
3.4 - Increasing withdrawal enhances GLT1 down-regulation in core vs. shell
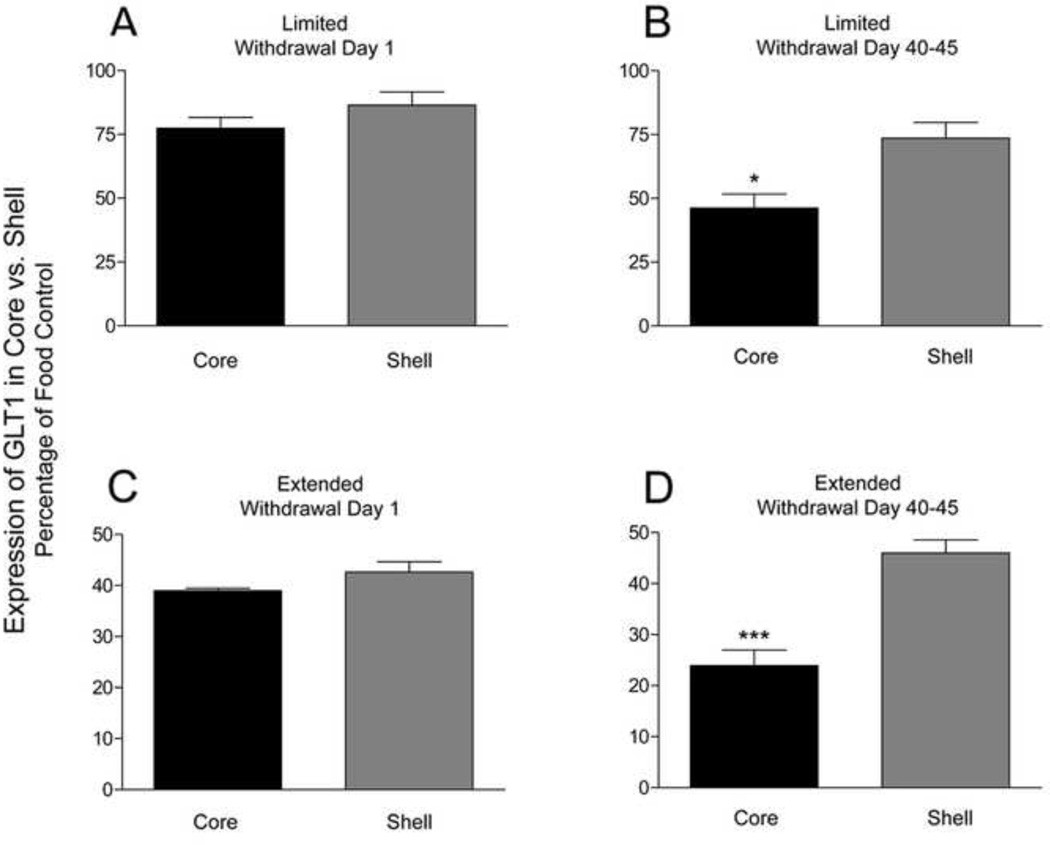
One-way ANOVA revealed significant differences between core and shell GLT1 expressions across access and withdrawal conditions (F(7, 56) = 31.738; ***p < 0.0001) [Fig. 4]. Games-Howell Multiple Comparisons between core and shell showed that core GLT1 down-regulation in both LW groups is significantly greater than in shell (limited, p < 0.05; extended, p < 0.0001).
Figure 4. Effects of SW and LW in limited and extended cocaine access rats on core vs. shell GLT1 expression.
Error bars indicate SEM. N = 8 rats/group. One-way ANOVA revealed significant differences between and within groups (F(7, 56) = 31.738; ***p < 0.0001). Games-Howell Multiple Comparisons Test revealed a significant reduction in LW extended and limited access groups (*p < 0.05, ***p < 0.0001).
4 – Discussion
Our results indicate that although cocaine withdrawal decreases GLT1 expression in both core and shell, only in core is GLT1 down-regulated after SW and LW periods in both limited and extended rats. The down-regulation in core, moreover, is significantly more pronounced in extended than limited rats after LW periods. Thus, core GLT1 is responsive to both the amount of cocaine received and the length of time away from the drug. In shell, only extended rats show GLT1 down-regulation in both withdrawal conditions, and unlike core, LW does not enhance the effect. Thus, core shows a progressive decrease in GLT1 expression as withdrawal increases, implicating this mechanism in the incubation of craving that develops over the course of drug withdrawal.
Ample evidence indicates a role for NAc glutamate in addiction-related behaviors including sensitization and drug seeking (Kalivas et al., 2009). Changes in glutamate transmission, including a decrease in NAc basal glutamate levels following withdrawal from repeated cocaine SA, have been detected (Baker et al., 2003a). This is consistent with clinical literature showing that enduring hypofrontality (deficient activation of prefrontal cortex) may be a feature of cocaine addiction (Goldstein et al., 2007). On the other hand, cue-induced reinstatement is associated with an increase in prefrontal cortex neuronal firing (Sun and Rebec, 2006). While this may seem incongruous with reports of hypofrontality, it is conceivable that hypofrontality provides a low level of background activity from which prefrontal cortex activation appears enhanced (Bowers et al., 2003). Furthermore, rats withdrawn from repeated exposure to cocaine respond to a cocaine challenge with an increase in NAc core extracellular glutamate (Pierce et al., 1996, Bowers and Kalivas, 2003), which likely results from decreased tone on mGlu 2/3 inhibitory autoreceptors (Baker et al., 2003a, Bowers and Kalivas, 2003). In fact, a decline in basal glutamate levels reduces activation of mGluR2/3 which in turn increases NAc core glutamate release probability during reinstatement (Moussawi et al., 2011). Additionally, presentation of cues previously associated with cocaine elevates NAc extracellular glutamate (Bell et al., 2000, Hotsenpiller et al., 2001). Consistent with these findings, intra-core infusions of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) promote cue-induced cocaine reinstatement (Cornish et al., 1999), whereas this behavior is diminished by AMPA receptor blockade in core (Cornish et al., 1999, Di Ciano and Everitt, 2001). Collectively, these findings indicate a role for NAc core glutamate in cocaine-seeking behaviors.
Although glutamate accumulation in extracellular fluid is controlled by a family of transporter proteins (Gegelashvili et al., 1997, Seal and Amara, 1999, Anderson and Swanson, 2000), GLT1, a sodium-dependent transporter found on astrocytes (Rothstein et al., 1994, Anderson and Swanson, 2000), is responsible for the removal of at least 90% of extracellular glutamate (Rothstein et al., 1994, Rothstein et al., 1995, Danbolt, 2001, Mitani and Tanaka, 2003). Because ample research indicates that the leading cause of cocaine relapse is an increase in NAc core glutamate levels (McFarland et al., 2003), removal of this excess extracellular glutamate would be expected to prevent relapse behaviors. Consistent with this hypothesis, upregulation of GLT1 attenuates cue- and cocaine-induced reinstatement (Sari et al., 2009, Knackstedt et al., 2010) following treatment with ceftriaxone, a beta-lactam antibiotic known to up-regulate GLT1 expression and function in mice and rats (Rothstein, 1995, Miller et al., 2008, Knackstedt et al., 2010). It is likely that up-regulation of GLT1 serves to diminish the overflow of extracellular glutamate that occurs during reinstatement. Furthermore, cue- and cocaine-induced reinstatement are also inhibited by treatment with N-acetylcysteine (Baker et al., 2003b, Kupchik et al., 2011), which activates the cystine-glutamate exchanger; activation of this exchanger increases basal glutamate levels and decreases the heightened core glutamate release that occurs during reinstatement (Xi et al., 2002, Kupchik et al., 2011). It has been suggested that restoration of NAc glutamate by increasing basal glutamate levels and dampening the heightened glutamate release during reinstatement is key to attenuation of the reinstatement response, with GLT1 playing a pertinent role in preventing the overflow of NAc glutamate (Knackstedt et al., 2010).
Increasing cocaine access and withdrawal periods have been shown to induce neuroadaptations linked to cocaine craving and relapse. Animals exposed to extended vs. limited cocaine access not only display increased addiction-related symptoms, including escalation in drug consumption and relapse, but also show an increase in core and shell dendritic spines (Ahmed et al., 2000, Ferrario et al., 2005). Extended access to cocaine has also been shown induce a core and shell mGluR2/3 up-regulation (Hao et al., 2010), likely due to down-regulation of cystine-glutamate exchanger expression (Knackstedt et al., 2010), which plays a role in regulating mGluR2/3 tone (Kupchik et al., 2011). Furthermore, time-dependent changes in NAc core AMPA receptors occur following long withdrawal periods from cocaine-seeking behaviors and are directly related to cocaine craving (Conrad et al., 2008). Group 1 mGluR activation in NAc core down-regulates core Ca2+ -permeable AMPA receptors and up-regulates Ca2+ - impermeable AMPARs (McCutcheon et al., 2011), which likely normalizes the post-synaptic response to the excess glutamate that occurs during the relapse response. Collectively, these findings indicate a role for access in core and shell and for withdrawal in core. Our results show a similar pattern within the NAc, with access having a similar effect on core and shell GLT1 expression and withdrawal having a greater effect on core GLT1 expression. Given the importance of glutamate and its removal during addiction-related behaviors (Knackstedt et al., 2010), it is not surprising that we found changes in NAc GLT1 expression as a function of increased cocaine access and withdrawal. It is important to note that because GLT1 was found to be down-regulated in core regardless of cocaine access history, one could argue that a drop in GLT1 is less involved in the development of addiction. This interpretation is unlikely, however, given the significant decrease in core GLT1 expression that occurs from increasing access to cocaine. Furthermore, because NAc core is specifically linked to the incubation of cocaine craving following long withdrawal periods (Conrad et al., 2008), the effects withdrawal had on core GLT1 expression would be expected. The time-dependent changes in core GLT1 expression found here may also be directly related to cocaine-craving, although this was not directly assessed.
Although our findings and those of others (Conrad et al., 2008, McCutcheon et al., 2011) indicate a key role for glutamate-mediated mechanisms in NAc core in the incubation of cocaine craving, we cannot rule out a role for NAc shell in this effect. In fact, compared to food controls, limited or extended access to cocaine produced a down-regulation of GLT1 in shell after LW. As in core, moreover, the down-regulation in shell was significantly greater in the extended access group. Interestingly, however, the shell down-regulation after LW was not significantly different from the corresponding SW period in either access condition, suggesting that GLT1 down-regulation may not be the key feature underlying shell involvement in incubation of cocaine craving. Consistent with this view, recent evidence suggests that shell participation in cocaine craving may involve increased signaling through muscarinic acetylcholine receptors (Yee et al., 2011). Thus, multiple mechanisms are likely to mediate cocaine craving, but our results indicate a clear reduction in NAc GLT1 expression following increased access and withdrawal conditions.
Although a change in GLT1 expression does not necessarily indicate a change in function, such change is likely in light of an increase in extracellular glutamate following extinction training after cocaine SA (Knackstedt et al., 2010). However, glutamate uptake assays would more definitively determine the functional significance of the varied levels of expression discussed in this report. Although we did not measure prefrontal cortex GLT1 expression, no changes in prefrontal cortex GLT1 occur following limited cocaine SA (Knackstedt et al., 2010). However, a recent study indicates changes in glutamate-associate proteins including Homer1b/c and NR2a/b in medial prefrontal cortex following extended access to cocaine (Ben-Shahar et al., 2009). Thus, future work should assess whether GLT1 expression changes in prefrontal cortex as a function of increasing drug access. In conclusion, our data indicate a role for NAc GLT1 on cocaine-seeking behaviors, but with regional differences in GLT1 expression depending on the length of daily cocaine access and the duration of cocaine withdrawal. Thus, whereas length of access had a similar effect on core and shell GLT1 expression, core was more sensitive to duration of withdrawal.
Increasing cocaine access and withdrawal periods enhance core GLT1 down-regulation
Increasing cocaine access, but not withdrawal periods, enhances shell GLT1 down-regulation
Increasing withdrawal enhances GLT1 down-regulation in core vs. shell
Acknowledgements
This research was supported, in part, by NIDA (R01 DA 02451, P50 DA 05312, R01 DA 12964) and by the METACyt Initiative of Indiana University funded, in part, through a major grant from the Lilly Endowment, Inc. The authors would like to thank Faye Caylor for administrative assistance and Paul Langley for technical support.
Abbreviations
- GAPDH
Glyceraldehyde-3-Phosphate Dehydrogenase
- GLT1
Glutamate Type 1 Transporter
- HRP
Horseradish Peroxidase
- LW
Long Withdrawal, 40–45 days of cocaine withdrawal
- NAc
Nucleus Accumbens
- SA
Self-Administration
- SW
Short Withdrawal, 1 day of cocaine withdrawal
- TBST
Tris-Buffered Saline with Tween 20
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Changes in response to a dopamine receptor antagonist in rats with escalating cocaine intake. Psychopharmacology (Berl) 2004;172:450–454. doi: 10.1007/s00213-003-1682-9. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003a;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Toda S, Kalivas PW. N-acetyl cysteine-induced blockade of cocaine-induced reinstatement. Ann N Y Acad Sci. 2003b;1003:349–351. doi: 10.1196/annals.1300.023. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell K, Duffy P, Kalivas PW. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharmacology. 2000;23:335–344. doi: 10.1016/S0893-133X(00)00100-7. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL, Szumlinski KK. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse. 2009;63:598–609. doi: 10.1002/syn.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Posthumus EJ, Waldroup SA, Ettenberg A. Heightened drug-seeking motivation following extended daily access to self-administered cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:863–869. doi: 10.1016/j.pnpbp.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, Kalivas PW. Forebrain astroglial plasticity is induced following withdrawal from repeated cocaine administration. Eur J Neurosci. 2003;17:1273–1278. doi: 10.1046/j.1460-9568.2003.02537.x. [DOI] [PubMed] [Google Scholar]
- Bowers MS, Lake RW, McFarland K, Peterson YK, Lanier SM, Lapish CC, Kalivas PW. AGS3: a G-Protein regulator of addiction-associated behaviors. Ann N Y Acad Sci. 2003;1003:356–357. doi: 10.1196/annals.1300.025. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, Kalivas PW. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93:1359–1367. doi: 10.1016/s0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25:341–360. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galinanes GL, Heng LJ, Tseng KY, Wolf ME. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca(2)-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 2011a;61:1141–1151. doi: 10.1016/j.neuropharm.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Loweth JA, Milovanovic M, Wang X, Wolf ME. Distribution of AMPA receptor subunits and TARPs in synaptic and extrasynaptic membranes of the adult rat nucleus accumbens. Neurosci Lett. 2011b;490:180–184. doi: 10.1016/j.neulet.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegelashvili G, Schousboe A, Seal RP, Amara SG, Anderson CM, Swanson RA. High affinity glutamate transporters: regulation of expression and activity. Mol Pharmacol. 1997;52:6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, Telang F, Alia-Klein N, Volkow ND. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144:1153–1159. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Martin-Fardon R, Weiss F. Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: factor in the transition to dependence. Biol Psychiatry. 2010;68:240–248. doi: 10.1016/j.biopsych.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci. 2001;14:1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Moussawi K, Tang XC, Wang X, Kalivas BC, Kolokithas R, Ogburn KB, Kalivas PW. The Effect of N-Acetylcysteine in the Nucleus Accumbens on Neurotransmission and Relapse to Cocaine. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Loweth JA, Ford KA, Marinelli M, Wolf ME, Tseng KY. Group I mGluR Activation Reverses Cocaine-Induced Accumulation of Calcium-Permeable AMPA Receptors in Nucleus Accumbens Synapses via a Protein Kinase C-Dependent Mechanism. J Neurosci. 2011;31:14536–14541. doi: 10.1523/JNEUROSCI.3625-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2010;31:5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE. The synaptic framework for chemical signaling in nucleus accumbens. Ann N Y Acad Sci. 1999;877:140–156. doi: 10.1111/j.1749-6632.1999.tb09266.x. [DOI] [PubMed] [Google Scholar]
- Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR, Kennedy RT, Rebec GV. Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington's disease phenotype in the R6/2 mouse. Neuroscience. 2008;153:329–337. doi: 10.1016/j.neuroscience.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani A, Tanaka K. Functional changes of glial glutamate transporter GLT-1 during ischemia: an in vivo study in the hippocampal CA1 of normal mice and mutant mice lacking GLT-1. J Neurosci. 2003;23:7176–7182. doi: 10.1523/JNEUROSCI.23-18-07176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci U S A. 2011;108:385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1998. [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD. Excitotoxicity and neurodegeneration in amyotrophic lateral sclerosis. Clin Neurosci. 1995;3:348–359. [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Amara SG. Excitatory amino acid transporters: a family in flux. Annu Rev Pharmacol Toxicol. 1999;39:431–456. doi: 10.1146/annurev.pharmtox.39.1.431. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. Neuroscience. 2003;23:10258–10264. doi: 10.1523/JNEUROSCI.23-32-10258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J Neurosci. 2006;26:8004–8008. doi: 10.1523/JNEUROSCI.1413-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther. 2002;300:162–171. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- Yee J, Famous KR, Hopkins TJ, McMullen MC, Pierce RC, Schmidt HD. Muscarinic acetylcholine receptors in the nucleus accumbens core and shell contribute to cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2011;650:596–604. doi: 10.1016/j.ejphar.2010.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. Specificity in the efferent projections of the nucleus accumbens in the rat: comparison of the rostral pole projection patterns with those of the core and shell. J Comp Neurol. 1993;327:220–232. doi: 10.1002/cne.903270205. [DOI] [PubMed] [Google Scholar]