Abstract
The ventral striatum (VS) is a critical brain region for reinforcement learning and motivation. Intrinsically motivated subjects performing challenging cognitive tasks engage reinforcement circuitry including VS even in the absence of external feedback or incentives. However, little is known about how such VS responses develop with age, relate to task performance, and are influenced by task difficulty. Here we used fMRI to examine VS activation to correct and incorrect responses during a standard n-back working memory task in a large sample (n= 304) of healthy children, adolescents and young adults aged 8–22. We found that bilateral VS activates more strongly to correct than incorrect responses, and that the VS response scales with the difficulty of the working memory task. Furthermore, VS response was correlated with discrimination performance during the task, and the magnitude of VS response peaked in mid-adolescence. These findings provide evidence for scalable intrinsic reinforcement signals during standard cognitive tasks, and suggest a novel link between motivation and cognition during adolescent development.
Keywords: Ventral striatum, working memory, adolescence, reward, motivation
INTRODUCTION
Intrinsic motivation is critical for cognitive performance and achievement across domains (Nicholls, 1984). Intrinsic motivation is also impaired in neuropsychiatric disorders including addiction, mood disorders, and the negative symptoms of schizophrenia (Barch, 2005). As disorders of motivation often begin in adolescence, understanding how the neurobiological substrates of motivation develop during this period is of particular importance (Insel, 2009). Adolescence is a critical period for reward system development (Ernst et al., 2005; Ernst and Fudge, 2009; Bjork et al., 2004; Bjork et al., 2007), where increased risk taking results in substantial morbidity and mortality (Steinberg, 2008). One notable recent study has demonstrated that prediction errors in an explicit reward task peaked during mid-adolescence (Cohen et al., 2010). However, intrinsic (as opposed to explicit) reinforcement responses have not previously been evaluated in children and adolescents.
A growing literature has delineated a neural circuit centered on the ventral striatum (VS) that responds to a wide range of explicit rewards and is tightly tied to motivated behavior (Knutson and Cooper, 2005). Extending this literature, other reports have detailed VS responses to correct>incorrect performance feedback (Ullsperger and von Cramon 2003; Tricomi and Fiez 2008). More recently, Han et al. (2010) described VS activation to correct responses in cognitive tasks in the absence of explicit rewards or even performance feedback; we have subsequently reported similar effects (Wolf et al., 2011). However, most studies of cognition do not typically report VS activation, perhaps due to relatively small sample sizes and modulation of VS activity by specific parameters such as task difficulty. In non-human primates, the responses of midbrain dopaminergic neurons projecting to VS are governed in part by reward prediction errors (Hollerman and Schultz, 1998). Prediction errors have provided a critical theoretical framework for understanding reward system responses, and effectively index the difference between expected and received rewards (Schultz, 1998). Thus, studies in both primates (Fiorillo et al., 2003) and humans (Dreher et al., 2005) have shown that unexpected rewards generate a greater reward response. Analogously, during difficult trials in a cognitive task, correct responses are less expected, suggesting that differential VS responses may scale with task demand. This may relate in part to variation in response confidence, as responses in a difficult task are likely to be less certain; Daniel and Pollmann (2012) recently demonstrated that response confidence modulates VS responses in a manner consistent with prediction errors. Additionally, difficulty impacts intrinsic motivation: correct responses are reported as more satisfying during a challenging compared to an easy task (Shalley and Oldham, 1985). However, no prior research has directly investigated the link between task difficulty and VS reinforcement responses, nor related such VS responses to task performance accuracy.
We investigated VS responses during the n-back working memory task in a large sample of children, adolescents and young adults. We selected the n-back task as it parametrically varies working memory load and therefore task difficulty. In addition, the n-back paradigm provides neither performance feedback nor explicit rewards, allowing investigation of intrinsic reinforcement responses outside of the context of a classic reward paradigm. We hypothesized that even without any feedback, VS would respond more to correct trials than incorrect trials, and that these responses would scale with task difficulty. Additionally, we examined whether VS response magnitude is positively associated with task performance, consistent with a measure of intrinsic motivation and task engagement. Finally, we investigated how such VS responses evolve with age during adolescence.
MATERIALS AND METHODS
Participants
The present study is a collaboration between the Center for Applied Genomics (CAG) at Children’s Hospital of Philadelphia (CHOP) and the Brain Behavior Laboratory at the University of Pennsylvania (Penn). Study procedures were reviewed and approved by the Institutional Review Board of both CHOP and Penn. The target population-based sample is of 10,000 youths who presented to the CHOP network for a pediatric visit and volunteered to participate in genomic studies of complex pediatric disorders (Gur et al., 2011). A subsample of 1,000 subjects, stratified by age and gender, were randomly selected for neuroimaging. This report represents an interim analysis of the first 346 consecutively imaged participants who completed the n-back task and did not have either a) a history of a medical disorder that might affect brain function or b) a psychiatric disorder that required current psychotropic medication or previous inpatient psychiatric hospitalization. All subjects or their parent or guardian provided informed consent and minors provided assent. Of these 346 subjects, 42 subjects were excluded from the present analysis: nine subjects due to a failure to perform the task at a minimum level of performance (>15 non-responses, equivalent to >3 S.D.), one for perfect performance (precluding analysis of error trials), 29 for excessive in-scanner motion (either relative mean displacement >0.5mm or relative maximum displacement >6mm), two for poor registration quality, and one for incomplete image coverage. This resulted in a final sample of 304 subjects aged 8–22 (mean age 15.7 years, S.D. 3.3 years; 131 males).
Task paradigm
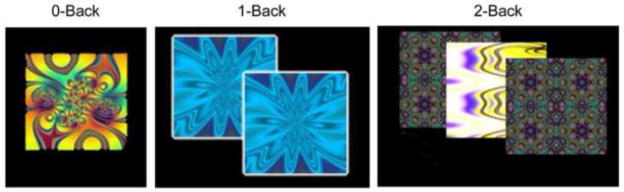
The n-back task (Figure 1) was adapted from prior studies where it has been described in detail (Ragland et al., 2002). Briefly, it involved presentation of complex geometric figures (fractals) for 500ms, followed by an interstimulus interval of 2500ms. This occurred under three conditions: 0-back, 1-back, and 2-back. In the 0-back condition, participants responded with a button press to a specified target fractal. For the 1-back condition, participants responded if the current fractal was identical to the previous one; in the 2-back condition, participants responded if the current fractal was identical to the item presented two trials previously. Each condition consisted of a 20-trial block (60 s); each level was repeated over three blocks. A target-foil ratio of 1:3 was maintained in all blocks; overall there were 45 targets and 135 foils. Visual instructions (9 s) preceded each block, informing the participant of the upcoming condition. The task included a total of 72 s of rest while a fixation crosshair was displayed. Total task duration was 693 s. Subjects did not receive any feedback about performance at any point.
Figure 1.
Task paradigm. Fractals were displayed under three conditions: 0-back, 1-back, and 2-back. Each condition consisted of a 20-trial block (60 s); each level was repeated over three blocks. Subjects did not receive any feedback about performance at any point.
Behavioral data analysis
Subject performance during trials was evaluated using a measure of discrimination index, Pr, and a measure of response bias, Br (Snodgrass and Corwin, 1988). These variables were computed with the following formulas, which were modified from the original as previously described (Sergerie et al., 2010):
Pr provides a measure of discrimination accuracy that is unbiased by a subject’s response bias; higher values reflect a greater degree of accuracy in discriminating between target and foil items. Response bias Br measures the overall tendency of a subject to respond that any given stimulus is a target or a foil. Positive Br values indicate a tendency to identify stimuli as targets (a liberal bias), while negative Br values denote a tendency to identify stimuli as foils (a conservative bias).
Pr, Br, and response times were calculated across all trials and also for each level of working memory load (0-back, 1-back, 2-back). The effect of working memory load on performance was evaluated with a mixed-effects analysis implemented in SAS (Cary, North Carolina) using the MIXED procedure, with load as a fixed factor, subject as a random factor, and gender and age as covariates. Post-hoc t-tests were used for comparisons of means for significant effects.
Post-error slowing
In this study design there was an inherent tension between the goals of studying intrinsic reinforcement and the explicit assessment of performance monitoring. In order to obtain an indirect measure of subject performance monitoring, we evaluated post-error slowing. Post-error slowing is a well-described phenomenon whereby subjects respond more slowly following the known commission of an error (Danielmeier and Ullsperger, 2011). While there was no explicit feedback presented in this study, we expected that post-error slowing would occur, reflecting the fact that subjects would have an internal representation of response confidence generated through ongoing performance monitoring. Specifically, we compared median response times on correct target trials following errors to correct target trials following correct responses on a within-subject basis using a paired t-test. This analysis was completed only for the 2-back condition, as there were an insufficient number of relevant trial pairs to complete this analysis for 0- and 1-back blocks.
Image acquisition
All subject data were acquired on the same scanner (Siemens Tim Trio 3 Tesla, Erlangen, Germany; 32 channel head coil) using the same imaging sequences. Blood oxygen level dependent (BOLD) fMRI was acquired using a whole-brain, single-shot, multi-slice, gradient-echo (GE) echoplanar (EPI) sequence of 231 volumes with the following parameters: TR/TE=3000/32 ms, flip=90°, FOV=192×192 mm, matrix=64X64, slice thickness/gap =3mm/0mm. The resulting nominal voxel size was 3.0×3.0×3.0mm. Prior to timeseries acquisition, a 5-minute magnetization-prepared, rapid acquisition gradient-echo T1-weighted (MPRAGE) image (TR 1810ms, TE 3.51 ms, FOV 180×240 mm, matrix 256×192, effective voxel resolution of 1 × 1 × 1mm) was acquired to aid spatial normalization to standard atlas space. Prior to scanning, in order to acclimate subjects to the MRI environment, a mock scanning session where subjects practiced the task was conducted using a decommissioned MRI scanner and head coil. Mock-scanning was accompanied by acoustic recordings of the noise produced by gradient coils for each scanning pulse sequence. During these sessions, feedback regarding head movement was provided using the MoTrack (Psychology Software Tools, Inc, Sharpsburg, PA) motion tracking system.
Image preprocessing
All fMRI data processing was conducted using tools that are part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl; Smith et al., 2004). BET was used to remove non-brain areas (Smith, 2002). Functional timeseries were motion corrected to the median image using a tri-linear interpolation with six degrees of freedom (Jenkinson et al., 2002), spatially smoothed (6mm FWHM), and grand-mean scaled using mean-based intensity normalization. The median functional and anatomical volumes were co-registered, and the anatomical image was transformed into standard space (T1 2mm MNI template) using a nonlinear registration algorithm (FNIRT). Transformation parameters were later applied to statistical images for group-level analyses.
Subject-level timeseries analysis
Our analysis consisted of three separate general linear models (GLMs). The first GLM modeled the effect of correct (COR) versus incorrect (INCOR) trials across levels of working memory load (two regressors), whereas the second GLM modeled correct and incorrect trials separately for each level (six regressors), allowing between-level comparisons. Finally, in order to evaluate the potential influence of subject motor responses, we conducted a third GLM where correct and incorrect responses to target and foil trials were modeled separately (four regressors). This allowed us to evaluate responses to correct targets (HIT), correct foils (correct rejection; CR), incorrect targets (MISS), and incorrect foils (false positive; FP). In this third model, motor responses were associated with HIT and FP trials. All models included temporal derivatives of task regressors, a regressor modeling task instructions, as well as six motion parameters. Task regressors were convolved with a canonical (double-gamma) hemodynamic response function. Subject-level statistical analyses were carried out using FILM with local autocorrelation correction (Woolrich et al., 2001) as implemented in FEAT.
Voxelwise group level analysis
Voxelwise group-level analysis of the across-level model was conducted using FLAME. In this model, the contrast of interest was COR>INCOR. To investigate the effects of age and performance on brain activation, a voxelwise linear mixed-effects analysis using linear and quadratic components of age (age and age2), sex, Pr, and Br was performed. Given our focus on activation related to correct responding, the group-level analysis was limited to voxels that had a non-negative response to correct trials (e.g., brain voxels with COR>baseline at z>0). Type I error was controlled using a whole-brain family-wise error (FWE) correction of p<0.05 (equivalent to z>4.7). All images are displayed without masking and rendered using MANGO (San Antonio, TX).
ROI analysis
As described below, the group level analysis of the across-level model identified the ventral striatum as the region that responded most robustly to the COR>INCOR contrast across all trials. In order to evaluate the effect of task difficulty on VS responses, as well as further evaluate the effects of age and performance, we conducted a region of interest (ROI) analysis using the between-level GLM that separated the COR>INCOR response by level of working memory load. We chose to use this ROI-based approach for the second model for two reasons. First, our a priori hypotheses focused on ventral striatum, and the first across-level GLM confirmed the hypothesized COR>INCOR response in this region. Second, the use of ROI summary data allowed us to use statistical software that could tolerate empty cells, which were present in some subjects due to ceiling performance at some levels of working memory load. In contrast, FSL and other voxelwise analysis approaches require that each subject have data present for every trial type.
A structural VS ROI was used in order to avoid the potential for bias imposed by a functional ROI from the across-level model, where correct and incorrect events were composed of variable proportions of trials from each level of working memory load. The VS ROI was defined using the antero-ventral (anterior and ventral to the anterior commissure at y=0, and z=0) aspect of the of the caudate, putamen, and nucleus accumbens. All regions were defined using the Harvard-Oxford subcortical atlas thresholded at p<0.25. Mean percent signal change was calculated for the contrast of COR>INCOR at each level of working memory load. One extreme outlier was excluded from this analysis. These values were then entered as a dependent variable in a mixed model analysis of variance, with working memory load (0, 1, 2-back) and laterality (L, R) as fixed factors, and subject as a random factor. The Kenward-Roger correction for degrees of freedom was applied (Kenward and Roger, 1997). Three models investigating specific hypotheses were conducted. In the first model we examined the effect of working memory load, hypothesizing that activation to correct versus incorrect responses would be greater under higher levels of working memory load. Second, we evaluated the influence of overall discrimination performance (Pr), to test the prediction that higher levels of activation would be related to better behavioral performance. Finally, following upon research suggesting that reward prediction errors peak in mid-adolescence (Cohen et al., 2010), we investigated linear and quadratic effects of subject age to test the prediction that age might be related to ventral striatum activation. Subject sex, response bias, and interaction terms were evaluated as well. As large samples can reveal significant results of small effect sizes, we additionally report effect sizes for between-subject variables such as performance and age. Standard measures of effect sizes (such as eta2) cannot be derived from linear mixed models; therefore, as in Raudenbush and Byrk (1992) we report effect sizes in terms of percentage variance explained.
Inclusion of motion as a confound variable
Even after excluding subjects with gross in-scanner motion, age and motion (as summarized by mean relative displacement) remained significantly related (r=−0.35). As has been previously noted, developmental differences in-scanner motion may systematically bias results (Church et al., 2010; Satterthwaite et al., 2012). Accordingly, as in Satterthwaite et al. (2012), in order to investigate the potential influence of motion, we re-conducted both the across-level voxelwise analysis and the between-level VS ROI analysis including mean relative displacement as a confound variable in the models.
Target/Foil analysis
Finally, using the third GLM (four-parameter model), we investigated whether the VS response to correct > incorrect trials was present for both target and foil trials separately. Accordingly, we conducted one-sample t-tests within both left and right VS ROIs for the contrasts of HIT > MISS (e.g., COR>INCOR for targets only) and CR>FP (foils only). In order to evaluate the potential influence of subject motor responses on VS response, we similarly investigated the COR > INCOR response for trials where a motor response was present for both the correct and incorrect trials (HIT>FP) and where no motor response was present for either side of the contrast (CR>MISS).
RESULTS
Behavioral results
Measures of behavioral performance are reported in Table 1. As expected, subjects had a lower Pr (indicating less accurate discrimination) at higher levels of working memory load [F (2, 606)= 469.83; p<0.001)]. Discrimination also improved with age [F (2, 301)= 27.36; p<0.001)]. Response bias was most conservative at the 1-back level [F (2, 606)= 4.35 p=0.01], but did not change with age. Subjects were slower at the highest levels of working memory load [F (2, 606)= 117.13; p<0.001)], and responded more quickly with age [F (2, 301)= 8.97; p<0.003)]. Sex did not have an effect on any measure. Finally, in the two-back condition there was strong evidence for post-error slowing, with significantly greater response times to correct targets following errors trials compared to correct trials [t(172)=9.08; p<0.001].
Table 1.
Behavioral Performance
| Variable [Mean (S.D.)] | 0-Back | 1-Back | 2-Back | Total |
|---|---|---|---|---|
| Discrimination (Pr) | 0.79 (0.14) | 0.82 (0.15) | 0.60 (0.20) | 0.79 (0.14) |
| Response bias (Br) | −0.19(0.16) | −0.22 (0.20) | −0.19 (0.19) | −0.20 (0.19) |
| Number correct | 58.46 (2.8) | 56.77 (3.6) | 51.12 (4.7) | 166.3 (5.4) |
| Number incorrect* | 3.0 (3.4) | 3.8 (3.6) | 8.7 (4.6) | 13.4 (9.2) |
| Response time (ms) | 509.8 (80.1) | 538.1 (134.1) | 629.4 (210.1) | 536.0 (107.0) |
= excluding empty cells (subjects with no errors at a given level)
Across-level voxelwise results
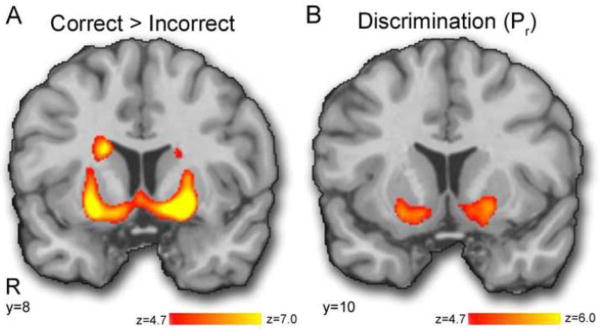
The first model examining the contrast of COR>INCOR was initially evaluated using an a priori FWE-corrected threshold; this resulted in a large, confluent cluster of activation, with peak activation in the ventral striatum (peak z=8.92, MNI coordinates: −22, 10, −8; Figure 2A). It should be noted that cortical brain regions typically associated with working memory function did not show a COR>INCOR response, but in fact displayed a robust INCOR>COR response. These prefrontal regions included a large right-lateralized cluster (encompassing the anterior cingulate, right dorsolateral prefrontal cortex, right frontal pole, and right anterior insula; peak z=13.32; 2,28, 38; 12,156 voxels), the left anterior insula (peak z= 9.95; −32, 20, −12; 1,541 voxels), the left dorsolateral prefrontal cortex (peak z=7.57; −42, 22, 30; 907 voxels), and the left frontal pole (peak z=4.37; −26, 50, 14; 257 voxels). Previous research has shown that these prefrontal regions undergo major changes during adolescence (Geier et al., 2009; Giedd, 2004); the developmental trajectory of the function of these brain regions will be examined in a separate manuscript upon completion of the study sample. Notably, there was a significant and anatomically specific relationship between discrimination performance (Pr) and COR>INCOR response that was strongest in bilateral ventral striatum: those who performed better demonstrated a greater COR>INCOR response (Figure 2B; left: peak z=5.64, 226 voxels at −15, 9, −10; right: peak z=5.40, 113 voxels at 18, 10, −11). There was a similar association between COR>INCOR and Pr in the right dorsal caudate (150 voxels). There were no other associations with sex, age, age2, Pr, or Br that were of a comparable magnitude (e.g., >100 voxels). When motion was included as a confound variable, the results were unchanged. In order to establish that the signal from the relatively more limited number of incorrect trials was meaningful, we also examined the contrast of INCOR > baseline. As seen in Supplementary Figure 1, this contrast revealed robust activation in task-active regions.
Figure 2.
Results from across-level model. All results displayed unmasked using a z-threshold equivalent to FWE p<0.05. A) Correct versus incorrect responses are associated with VS activation. B) VS responses to correct versus incorrect trials are associated with task discrimination performance: subjects with better discrimination performance exhibit greater VS activation to correct versus incorrect trials.
Between-level VS ROI results
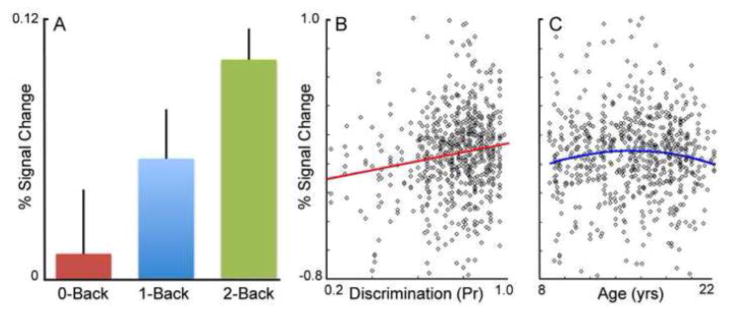
The analysis of the between-level model using a structurally defined VS ROI tested for an effect of working memory load, and provided a more sensitive estimate of age and performance effects. VS laterality, subject sex, and response bias (Br) did not contribute significantly and were thus removed from the model. In the bilateral ventral striatum, there was a significant effect of working memory load on the correct versus incorrect response, with greater VS activation seen in association with greater load [Figure 3A; F (2, 528)=4.80; p=0.0086]. Post-hoc t-tests indicated that this was driven by a significant difference between the 2-back condition and both the 0-back [T(556)=2.97, p=0.0031] and 1-back conditions [T(489)=1.97, p=0.049]. As in the across-level model, discrimination performance (Pr) had a significant relationship with VS activation [Figure 3B; F (1, 300)= 7.77; p=0.0057; 10% variance explained]. No linear effects with age were found (p=0.22), but a quadratic relationship between age and COR>INCOR response was present [Figure 3C; F (1, 306)= 4.28; p=0.039; 6% variance explained]. Motion did not significantly predict VS response, nor impact results when included in the model.
Figure 3.
Results from between-level analysis of structurally-defined bilateral ventral striatum. A) VS activation to correct versus incorrect trials scale by task difficulty; greater VS activation is seen under higher levels of working memory load. Error bars represent SEM. B) Scatterplot illustrating the relationship between task discrimination performance (Pr) and VS COR>INCOR activation, collapsed across level of working memory load and hemisphere. Subjects who perform better also have greater VS COR>INCOR activation C) Scatterplot illustrating the quadratic relationship between subject age and VS activation. VS activation to COR>INCOR peaks in mid-adolescence.
Target/Foil analysis
The VS ROI analysis of the third GLM revealed that correct>incorrect activation was seen bilaterally in the VS for both targets [HIT>MISS contrast; left VS: T(303)=3.30, p<0.001; right VS: T(303)=1.97, p=0.025 ] and foils [CR>FP contrast; left VS T(303)=6.20, p<0.001; right VS: T(303)=6.87, p<0.001]. The correct > incorrect effect was seen in trials where there was a motor response present [HIT>FP contrast; left VS: T(303)=6.91, p<0.001; right VS: T(303)=6.93, p<0.001 ] and also in trials where there were no motor responses [CR>MISS contrast; left VS: T(303)=3.61, p<0.001; right VS: T(303)=2.67, p<0.004]. There was not a significant difference between correct trials where a motor response was made compared to correct trials when a motor response was not made (HIT vs. CR).
DISCUSSION
In this study we examined BOLD activation associated with correct compared to incorrect responses across and between levels of difficulty during a working memory task. Overall, the results demonstrate robust VS activation that scales with task difficulty and is correlated with task performance. Furthermore, the magnitude of VS activation peaked at mid-adolescence. These effects occurred in a task lacking extrinsic rewards or explicit feedback, suggesting that they may represent intrinsic reward signals.
Intrinsic reinforcement responses in the VS scale with task difficulty
Despite the widespread use of the n-back task in functional neuroimaging (Owen et al., 2005), to our knowledge this is the first demonstration of VS activation to correct versus incorrect trials in this task. Others have reported VS responses to correct>incorrect performance feedback (Ullsperger and von Cramon 2003; Tricomi and Fiez 2008). Notably, the effects reported here were present despite the lack of explicit feedback. This finding follows upon previous reports of VS activation to correct responses in recognition memory paradigms in the absence of feedback or explicit rewards (Han et al.,2010; Wolf et al., 2011). As in these studies, we suggest that VS responses in this context may reflect intrinsic reinforcement signals.
Here we extend this literature by demonstrating for the first time that the presence of such signals is modulated by the difficulty of the cognitive task. The n-back task allowed us to demonstrate that VS activation scales in proportion to working memory load and hence difficulty. These results accord with a substantial body of literature demonstrating reward prediction error responses (Fiorillo et al., 2003; Dreher et al., 2005; Satterthwaite et al., 2007), and suggest that the prediction-error framework may generalize to situations where explicit rewards are not present. A reward prediction error account would predict the present results (Schultz, 1998), where correct responses are less expected at higher levels of difficulty, and therefore provoke a larger reinforcement response. Consistent with this account, Daniel and Pollmann (2012) recently demonstrated VS prediction error responses in the absence of explicit feedback when response confidence was higher than expected. These findings provide a plausible neural correlate for the prior observation and common experience that accurate performance is reinforcing in proportion to the difficulty of the task (Shalley and Oldham, 1985). The effect of difficulty may also help explain why VS activation to correct responses without feedback is not consistently reported -- robust VS activation may only occur in tasks more difficult than those commonly used in many fMRI studies.
VS activation is correlated with task performance
While no prior work has demonstrated it directly, it is intuitively plausible that intrinsic reinforcement signals are related to task engagement and performance: subjects who are more rewarded by correct performance are likely to also be those who are more engaged in the task (French, 1958). Here, we found that subjects who performed better (using the discrimination metric Pr) also had higher VS activation to correct versus incorrect responses. The present results expand on the study by Han et al. (2010), which found that intrinsic VS activations were correlated with individual difference measures of reward-seeking. In a recent study in healthy adults and patients with schizophrenia (Wolf et al., 2011) using a very difficult recognition memory paradigm, we observed a correlation between VS activation on correct trials and response bias, but not discrimination accuracy. However, the sample size in that study was very small and underpowered to detect the Pr effect seen here. Furthermore, the task parameters in that study differed in multiple ways from the current study, including a discrimination accuracy only slightly above chance. In the present study, we benefited from a much larger sample size as well as a better distribution of discrimination responses. The current data suggest that VS activation may reflect performance-related variables such as motivation and task engagement. Notably, neuropsychiatric disorders that affect motivation (such as the negative symptoms of schizophrenia) are often associated with prominent cognitive deficits, suggesting a potential link between aberrations of reinforcement signaling and cognitive dysfunction (Wolf, 2006).
VS activation peaks in mid-adolescence
In contrast to the robust association between discrimination performance and VS activation seen in both the voxelwise and ROI analysis, we only observed an effect of subject age in the more sensitive VS ROI analysis. This analysis revealed an “inverted-U” quadratic relationship between subject age and VS activity, with VS activation peaking in mid-adolescence at approximately age 16. These results are consistent with prior studies that have described increased VS activation among adolescents in explicit reward tasks (Cohen et al., 2010; Galvan et al., 2006). Our results extend this prior work by demonstrating age-related effects in intrinsic (rather than explicit) reinforcement responses. While statistically significant, this effect was relatively subtle (explaining 6% of model variance), and required both a large sample and a sensitive ROI analysis. This suggests the possibility that adolescence may differentially affect reward system responses to extrinsic compared to intrinsic rewards, and that VS reward-sensitivity in adolescence may thus be more prominent with explicit rewards.
Limitations, Future Directions, and Conclusion
This study has two main limitations. First, while the presence of VS activation in the absence of explicit feedback is very intriguing, it does not provide a direct link between the observed VS response and subject-level factors such as motivation. We predicted the observed pattern of VS responses using a reward framework and interpret it in the same context. Han et al. (2010) directly manipulated reward in their task design, clearly linking enhanced VS activation during correct trials to reward processing, and bolstering our interpretation of VS activation as reward-related. Furthermore, the presence of significant post-error slowing provides indirect evidence that subjects were monitoring performance on an ongoing basis, and were to some extent aware of when they were have likely to have made an error or a correct response (Danielmeier and Ullsperger, 2011). Nonetheless, our experimental design does not allow us to definitively exclude other potential interpretations. For example, VS responses might be related to increased attention present on correct trials. However, classic brain regions involved in attention including the dorsolateral prefrontal cortex and the anterior cingulate display the opposite response as the VS (i.e., incorrect > correct), which argues against such an interpretation. Similarly, a simple motor confound was excluded by the analysis of the third GLM, where the correct > incorrect effect was seen for both trials where motor responses were absent and for trials where there was a motor response. Nonetheless, future studies should vary reward contingencies as part of the paradigm design, and also obtain subjective measures of response confidence, reward sensitivity, and intrinsic motivation.
A second limitation is that the n-back task produced few errors at lower levels of working memory load, resulting in few trials and the presence of empty cells. This limitation stemmed from the wide range of ages being studied (Luna et al., 2010). However, three aspects of our approach minimize the impact of this limitation. First, our across-level GLM collapsed incorrect trials across levels, providing an adequate number of incorrect trials. Second, our between-level analysis of VS activation utilized an (unbiased, structurally-defined) ROI analysis that could accommodate empty cells. Finally and perhaps most important, while an analysis of a condition with a small number of trials might not be practical in an fMRI study of typical size, the large size of the present sample makes such analyses tractable.
In summary, the present data provide novel evidence for a link between reward system responses and cognition in adolescent development. It should be noted that the current results were obtained within the context of a large ongoing study; in the final sample we will be able to examine the relationship of VS activation to diverse, dimensionally defined psychiatric symptoms. Given the centrality of motivation and cognitive deficits to many neuropsychiatric disorders, the present results suggest that future studies in clinical populations should directly examine aberrations of intrinsic reinforcement signals. Furthermore, the impact of intrinsic reinforcement should be considered when interpreting performance and brain activation patterns in cognitive tasks.
Supplementary Material
Acknowledgments
FINANCIAL SUPPORT: Supported by RC2 grants from the National Institute of Mental Health MH089983 and MH089924, as well as T32 MH019112. Dr. Satterthwaite was supported by APIRE and the Marc Rapport Family Investigator grant through NARSAD. Dr. Wolf was also supported by MH085096, APIRE, and the Sidney R. Baer, Jr. Foundation through NARSAD.
Many thanks to the acquisition and recruitment team: Karthik Prabhakaran, Jeff Valdez, Marisa Riley, Ryan Hopson, Jack Keefe, and Nick DeLeo. Also, thanks to Jan Richard, James Dickson, Chad T. Jackson, Mark Griffin, Larry B. Macy, and Tianyi Du for data management and systems support. Rosetta Chiavacci provided study coordination. We additionally thank our anonymous reviewers for suggestions that strengthened this work.
Footnotes
DISCLOSURES: Drs. Gur report investigator-initiated grants from Pfizer and AstraZeneca. All other authors report no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barch DM. The relationships among cognition, motivation, and emotion in schizophrenia: how much and how little we know. Schizophr Bull. 2005;31:875–881. doi: 10.1093/schbul/sbi040. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Danube CL, Hommer DW. Developmental differences in posterior mesofrontal cortex recruitment by risky rewards. J Neurosci. 2007;27:4839–4849. doi: 10.1523/JNEUROSCI.5469-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JA, Petersen SE, Schlaggar BL. The “Task B problem” and other considerations in developmental functional neuroimaging. Hum Brain Mapp. 2010;31:852–862. doi: 10.1002/hbm.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, Asarnow RF, Sabb FW, Bilder RM, Bookheimer SY, Knowlton BJ, Poldrack RA. A unique adolescent response to reward prediction errors. Nat Neurosci. 2010;13:669–671. doi: 10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel R, Pollmann S. Striatal activations signal prediction errors on confidence in the absence of external feedback. Neuroimage. 2011;59:3457–3467. doi: 10.1016/j.neuroimage.2011.11.058. [DOI] [PubMed] [Google Scholar]
- Danielmeier C, Ullsperger M. Post-error adjustments. Front Psychol. 2011;2:233. doi: 10.3389/fpsyg.2011.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, Berman KF. Neural Coding of Distinct Statistical Properties of Reward Information in Humans. Cereb Cortex. 2005;16:561–573. doi: 10.1093/cercor/bhj004. [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neurosci Biobehav Rev. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Garver K, Terwilliger R, Luna B. Development of working memory maintenance. J Neurophysiol. 2009;101:84–99. doi: 10.1152/jn.90562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, Loughead J, Connolly JJ, Qiu H, Mentch FD, Abou-Sleiman PM, Hakonarson H, Gur RE. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. doi: 10.1037/a0026712. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Huettel SA, Raposo A, Adcock RA, Dobbins IG. Functional significance of striatal responses during episodic decisions: recovery or goal attainment? J Neurosci. 2010a;30:4767–4775. doi: 10.1523/JNEUROSCI.3077-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1:304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- Insel TR. Translating scientific opportunity into public health impact: a strategic plan for research on mental illness. Arch Gen Psychiatry. 2009;66:128–133. doi: 10.1001/archgenpsychiatry.2008.540. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997:983–997. [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Luna B, Velanova K, Geier CF. Methodological approaches in developmental neuroimaging studies. Hum Brain Mapp. 2010;31:863–871. doi: 10.1002/hbm.21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls JG. Achievement motivation: Conceptions of ability, subjective experience, task choice, and performance. Psychol Rev. 1984;91:328. [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Turetsky BI, Gur RC, Gunning-Dixon F, Turner T, Schroeder L, Chan R, Gur RE. Working memory for complex figures: An fMRI comparison of letter and fractal n-back tasks. Neuropsychology. 2002;16:370. [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: applications and data analysis methods. Thousand Oaks: SAGE; 2002. [Google Scholar]
- Satterthwaite TD, Green L, Myerson J, Parker J, Ramaratnam M, Buckner RL. Dissociable but inter-related systems of cognitive control and reward during decision making: evidence from pupillometry and event-related fMRI. Neuroimage. 2007;37:1017–1031. doi: 10.1016/j.neuroimage.2007.04.066. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE. Impact of in-scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2011.12.063. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Armony JL, Menear M, Sutton H, Lepage M. Influence of emotional expression on memory recognition bias in schizophrenia as revealed by fMRI. Schizophr Bull. 2010;36:800–810. doi: 10.1093/schbul/sbn172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalley CE, Oldham GR. Effects of goal difficulty and expected external evaluation on intrinsic motivation: A laboratory study. Academy of Management Journal. 1985:628–640. [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A Social Neuroscience Perspective on Adolescent Risk-Taking. Dev Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi E, Fiez JA. Feedback signals in the caudate reflect goal achievement on a declarative memory task. Neuroimage. 2008;41:1154–1167. doi: 10.1016/j.neuroimage.2008.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. J Neurosci. 2003;23:4308–4314. doi: 10.1523/JNEUROSCI.23-10-04308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DH. Anhedonia in schizophrenia. Curr Psychiatry Rep. 2006;8:322–328. doi: 10.1007/s11920-006-0069-0. [DOI] [PubMed] [Google Scholar]
- Wolf DH, Gerraty RT, Satterthwaite TD, Loughead J, Campellone T, Elliott MA, Turetsky BI, Gur RC, Gur RE. Striatal intrinsic reinforcement signals during recognition memory: relationship to response bias and dysregulation in schizophrenia. Front Behav Neurosci. 2011;5:81. doi: 10.3389/fnbeh.2011.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.