Abstract
Tea flavonoids such as epigallocatechin gallate (EGCG) protect against vascular diseases such as atherosclerosis via their antioxidant and anti-inflammatory functions. Persistent and widespread environmental pollutants, including polychlorinated biphenyls (PCB), can induce oxidative stress and inflammation in vascular endothelial cells. Even though PCBs are no longer produced, they are still detected in human blood and tissues and thus considered a risk for vascular dysfunction. We hypothesized that EGCG can protect endothelial cells against PCB-induced cell damage via its antioxidant and anti-inflammatory properties. To test this hypothesis, primary vascular endothelial cells were pretreated with EGCG, followed by exposure to the coplanar PCB 126. Exposure to PCB 126 significantly increased cytochrome P450 1A1 (Cyp1A1) mRNA and protein expression and superoxide production, events which were significantly attenuated following pretreatment with EGCG. Similarly, EGCG also reduced DNA binding of NF-κB and downstream expression of inflammatory markers such as monocyte chemotactic protein-1 (MCP-1) and vascular cell adhesion protein-1 (VCAM-1) after PCB exposure. Furthermore, EGCG decreased endogenous or base-line levels of Cyp1A1, MCP-1 and VCAM-1 in endothelial cells. Most of all, treatment of EGCG upregulated expression of NF-E2-related factor 2 (Nrf2)-controlled antioxidant genes, including glutathione S transferase (GST) and NAD(P)H:quinone oxidoreductase 1 (NQO1), in a dose-dependent manner. In contrast, silencing of Nrf2 increased Cyp1A1, MCP-1 and VCAM-1 and decreased of GST and NQO1 expression, respectively. These data suggest that EGCG can inhibit AhR regulated genes and induce Nrf2-regulated antioxidant enzymes, thus providing protection against PCB-induced inflammatory responses in endothelial cells.
Keywords: PCB, EGCG, polyphenol, endothelial cell, inflammation, atherosclerosis
Introduction
Epidemiological studies provide substantial evidence that vascular diseases are linked to exposure to environmental pollutants. For example, a recent study reported increased hospitalization rates for acute myocardial infarction and diabetes mellitus in populations residing near areas contaminated with persistent organic pollutants (Sergeev and Carpenter, 2010). The lining of blood vessels is protected by the endothelium, and endothelial cells play an active role in physiological processes such as regulation of vessel tone, blood coagulation, and vascular permeability. Dysfunction of endothelial cells is a critical underlying cause of the initiation of cardiovascular diseases such as atherosclerosis. One functional change in atherosclerosis is the activation of the endothelium, which is manifested as an increase in the expression of specific cytokines and adhesion molecules.
PCBs are persistent organic pollutant found in soil, air, water and food, and a major source of human exposure to PCBs is from dietary intake of contaminated foods. Because PCBs are lipid soluble, humans can accumulate them in tissues and such bioaccumulation is a human health concern (Hopf et al., 2009). We have demonstrated previously that polychlorinated biphenyls (PCBs), and in particular coplanar PCBs can cause endothelial cell dysfunction as determined by inflammatory markers such as expression of cytokines and adhesion molecules (Han et al., 2010a; Hennig et al., 2005b; Majkova et al., 2009). Coplanar PCBs (e.g., PCB 126) exert their toxicity through binding to the aryl hydrocarbon receptor (AhR). AhR target genes, including cytochrome P450 1A1 (Cyp1A1), are considered a source for oxidative stress in endothelial cells and subsequent vascular dysfunction (Kopf et al., 2010; Kopf and Walker, 2010).
Because the endothelium is in immediate contact with the blood, endothelial cells are particularly susceptible to the effect of environmental contaminants and their downstream mediators present in the bloodstream. Endothelial cells are also exposed to circulating nutrients and diet-derived bioactive metabolites, thus providing an opportunity for nutritional modulation of chemical insults to the endothelium. Our laboratory has provided strong evidence that nutrition can modulate the toxicity of environmental pollutants. For example, we have found that specific dietary fats can further compromise endothelial dysfunction induced by selected PCBs and that antioxidant nutrients such as dietary flavonoids can protect against endothelial cell damage mediated by these persistent organic pollutants (Choi et al., 2010; Hennig et al., 2005a; Majkova et al., 2011; Wang et al., 2008). Diets high in polyphenols (e.g., flavonoids) are associated with a reduced risk of chronic diseases, such as cardiovascular diseases, by affecting molecular mechanisms involved in the initiation and progression of these diseases (Kris-Etherton et al., 2004). Particularly, green tea consumption is associated with mortality due to cardiovascular disease (Kuriyama et al., 2006). Flavonoids constitute a subclass of bioactive compounds rich in fruits and vegetables, soy food, legumes, tea and cocoa. Green tea consumption has been shown to be significantly greater in healthy subjects compared to those with coronary artery disease, suggesting that green tea might be protective against coronary atherosclerosis (Sasazuki et al., 2000). Catechins are the major constituents of the polyphenols in green tea, and the most abundant catechin in green tea is epigallocatechin gallate (EGCG). EGCG has antioxidant and anti-inflammatory effects, at least in part, due to its ability to upregulate genes encoding for phase II enzymes, including glutathione S transferase (GST) and NAD(P)H:quinone oxidoreductase 1 (NQO1). These enzymes contain antioxidant response elements (ARE) in the promoter region of the genes (Jones et al., 2007). NF-E2-related factor 2 (Nrf2) is a transcription factor that binds to ARE and thus plays a regulatory role in gene transcription.
Mechanisms responsible for EGCG-induced endothelial cell protection against proinflammatory environmental pollutants are not fully understood. In the current study we provide evidence that EGCG may reduce inflammatory markers induced by PCB exposure via elevated expression of antioxidant genes in primary endothelial cells. In particular, we investigated how EGCG modulates transcription factors such as AhR, NF-κB and Nrf2 to protect endothelial cells against PCB 126-induced cellular inflammation.
Materials and Methods
Materials and chemicals
EGCG was purchased from Cayman Chemical (Ann Arbor, MI). 3,3’,4,4’,5-pentachlorobiphenyl (PCB 126) was obtained from AccuStandard Inc. (New Haven, CT). Primary antibodies for VCAM-1 and Cyp1A1, and horse radish peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). β-actin antibody and dihydroethidium (DHE) were purchased from Sigma (St. Louis, MO).
Cell culture and experimental media
Primary vascular endothelial cells were isolated from porcine pulmonary arteries. Cells were cultures in M199 (Gibco, Grant Island, NY), supplemented with fetal bovine serum (FBS; Gibco, Carlsbad, CA). Endothelial cells were grown to confluence, followed by incubation overnight in medium containing 5% FBS before initiation of cell treatments. Stock solutions of PCB 126 were prepared in DMSO. The same amounts of DMSO as in PCB-treated cells were added to control cultures. The levels of DMSO in experimental media were less than 0.05%. Cells were pre-treated with EGCG at 25–50 µM for 3 h and then treated with PCB 126 at 0.25 µM for 4–16 h.
Real-time PCR
The levels of mRNAs expression were assessed by real-time PCR using 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA) and SYBR Green master mix (Applied Biosystems) as described earlier (Han, Eum et al. 2010). Sequences for mRNAs were designed using the Primer Express Software 3.0 for real-time PCR (Applied Biosystems) and synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). Sequences for porcine CYP1A1, VCAM-1, MCP-1 and β-actin were described in earlier articles published from our laboratory (Majkova et al., 2009; Ramadass et al., 2003). Porcine NQO1 sequences were: sense, 5’-CCCGGGAACTTTCAGTATCCT-3’; and antisense, 5’-CTGCGGCTTCCACCTTCTT-3’; porcine GST-omega 1 sequences were: sense, 5’-GCTGAGCCAAGTGGGAGACA-3’; and antisense, 5’-CCTCGGCCATTGAAATAGTGA-3’; porcine Nrf2 sequences were: sense, 5’-CTGCATATCCCATTCCCTGATGA-3’; and antisense, 5’-ATGCAAGCTGAGCCTCATTGA-3’.
Western blot analysis and ELISA
Western blot analyses for Cyp1A1, VCAM-1 and β-actin were performed as described previously (Han et al., 2010a). MCP-1 protein levels were measured in cell culture media using Quantikine ELISA kit (R&D Systems, Minneapolis, MN) as described previously (Majkova et al., 2009).
Preparation of nuclear extracts and electrophoretic mobility shift assay (EMSA)
DNA binding activities of NF-κB were analyzed using DNA-protein binding detection kit (Gibco-BRL). Preparation of nuclear extracts and EMSA were carried out as described earlier (Han et al., 2010b). Binding activities of AhR to xenobiotic response element (XRE) were assessed using a consensus XRE sequence (5’-CGGCTCTTCTCACGCAACTCCG-3’ and 5’-CGGAGTTGCGTGAGAAGAGCCG-3’) from the Cyp1A1 gene as described previously (Gouedard et al., 2004; Takahashi et al., 1997).
Assessment of oxidative stress using dihydroethidium (DHE)
Cells were grown to confluence in 8-chamber slides (BD Biosciences, Bedford, MA). After treatments, cells were incubated with 5 µM DHE in CO2 incubator at 37°C for 30 min, followed by washing with ice-cold PBS buffer. Cells were then fixed with 10% buffered formalin and washed with PBS. Slides were mounted with ProLong Gold Antifade reagent containing DAPI (Invitrogen, Carlsbad, CA) to visualize the nuclei. The slides were then evaluated under an Olympus BX61W1 fluorescence microscope, and the images were captured using the QCapture Pro software (QImaging, Surrey, BC, Canada). DHE red fluorescence was quantified by GelQuant software (BiochemLabSolutions, San Francisco, CA).
Monocyte adhesion assay
The monocyte adhesion assay was performed as described earlier (Han et al., 2010a). Briefly, human THP-1 monocytes were activated with TNF-α and loaded with the fluorescent probe calcein (Molecular Probes, Carlsbad, CA). Monocytes were added to endothelial cell monolayers, and incubated for 30 m at 37 °C. Unbound monocytes were washed away with PBS and the monolyer was fixed with 4% formaldehyde for 30 m. Attached fluorescent monocytes were counted in randomly selected fields (a total of 20 fields from 4 different wells per each treatment group) using a fluorescent microscope (Olympius IX70, Center Valley, PA).
Nrf2 siRNA and transfection studies
Double stranded small interfering RNA targeting Nrf2 was synthesized by (Applied Biosystems (sense, 5’-GAAUUACAGUGUCUUAAUAtt-3’; antisense, 5’-UAUUAAGACACUGUAAUUCtg-3’). Primary endothelial cells were transfected with control or Nrf2 siRNA at a final concentration of 80 nM using GeneSilencer transfection reagent (Genlantis, San Diego, CA) in OptiMEM serum free media. Cells were incubated with the transfection mixture for 4 h followed by adding FBS to achieve a final FBS concentration of 10%. Forty-eight hours after transfection, cells were used for treatments.
Statistical analysis
Data were analyzed using SigmaStat software (Systat Software, Point Richmond, CA). Comparisons between treatments were made by one-way or two-way ANOVA with post-hoc comparisons of the means. A probability value of p < 0.05 was considered statistically significant.
Results
EGCG attenuates PCB 126-mediated induction of Cyp1A1 and cellular oxidative stress
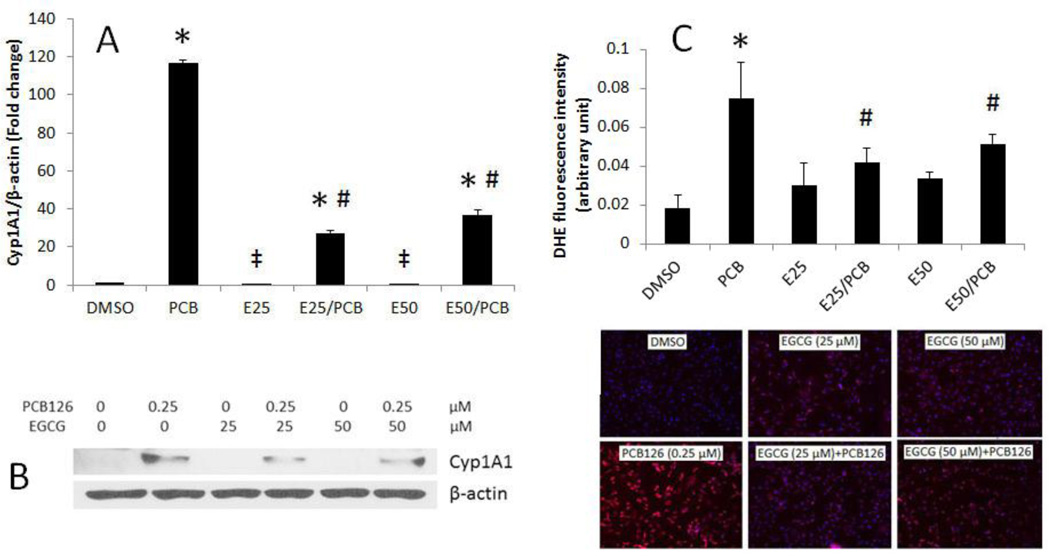
To determine whether EGCG can modulate PCB-induced induction of Cyp1A1, endothelial cells were pretreated with EGCG at concentrations of 25 or 50 µM, followed by treatment with PCB 126. Concentrations of EGCG were chosen based on preliminary data showing maximum endothelial cell protection against PCB exposure without cell death (Ramadass et al., 2003). These EGCG concentrations also were used by others in endothelial cells (Lee et al., 2009; Ludwig et al., 2004). Exposure of cells to PCB 126 significantly increased expression of Cyp1A1 at the transcriptional and translational levels (Figures 1A and B). PCB 126-induced Cyp1A1 expression was markedly reduced when cells were pretreated with EGCG at either 25 or 50 µM. Oxidative stress is a critical event of endothelial inflammation, and induction of Cyp1A1 leads to oxidative stress as a result of increased generation of reactive oxygen species. The fluorescent dye DHE is sensitive to reactive oxygen species and in particular to superoxide anions. Once this dye is oxidized by superoxide, it stains the cell a bright fluorescent red. Figure 1C shows that PCB 126, at a concentration of 0.25 µM, significantly upregulated superoxide production. PCB 126-induced overproduction of reactive oxygen species was significantly reduced by pretreatment of EGCG.
Figure 1.
EGCG attenuates PCB 126-mediated induction of Cyp1A1 and cellular oxidative stress. (A) Expression of mRNA was analyzed in endothelial cells pretreated with 25–50 µM of EGCG for 3 h, followed by treatment with PCB 126 at 0.25 µM for 4 h. Real-time PCR technique was used to measure expressed mRNA levels. (B) Expression of CYP1A1 protein in endothelial cells pretreated with 0–50 µM of EGCG for 3 h, followed by treatment with PCB 126 for 4 h using Western blot technique. Densitometry results were normalized to β-actin. The Western blot picture shown is a representative of three independent blots. Real-time PCR and Western blot results represent the mean ± SEM, with n=3. (C) Superoxide production in endothelial cells using DHE staining. DHE red fluorescence was assessed using fluorescence microscopy and the strength of signal was quantified. Experiments were repeated a minimum of three times. *Significantly increased compared to DMSO control. ‡Significantly decreased compared to DMSO control. # Significantly different compared to the PCB treatment group.
EGCG attenuates PCB 126-induced activation of NF-κB and AhR
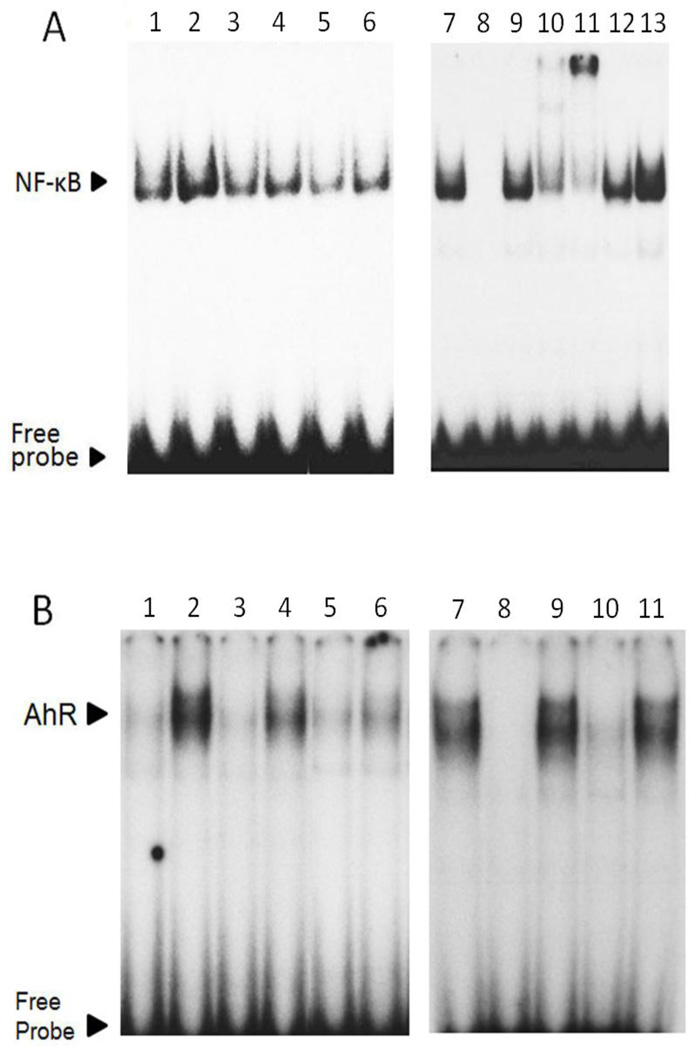
Because EGCG decreased PCB 126-induced oxidative stress, we determined the transcriptional activation of NF-κB which is a redox-sensitive transcription factor that upregulates endothelial inflammatory genes including VCAM-1 and MCP-1. Our EMSA results showed that PCB 126 markedly increased NF-κB DNA binding activity, which was completely blocked when cells were pretreated with EGCG (Figure 2A). To ensure the specificity and subunit composition of NF-κB, competition and supershift assays also were conducted (Figure 2A). In addition, we examined the transcriptional activation of AhR which is responsible for upregulation of PCB 126-induced Cyp1A1 expression. EMSA results demonstrated that EGCG reduced PCB 126-induced AhR-XRE binding in a dose-dependent manner (Figure 2B). The specificity of AhR-XRE binding was also confirmed (Figure 2B).
Figure 2.
EGCG attenuates PCB 126-induced activation of NF-κB and AhR. (A) Endothelial cells were pretreated with EGCG (25–50 µM) for 3 h, followed by PCB 126 exposure at 0.25 µM for 3 h. EMSA for NF-κB was performed with nuclear proteins extracted from endothelial cells. EMSA competition assay demonstrated the specificity of NF-κB probe using a competitor (unlabeled oligonucleotide probe), and a mutator (unlabelled probe with a mutation) and antibodies. Figure 2A: Lane 1, DMSO; lane 2, PCB 126; lane 3, EGCG 25 µM; lane 4, EGCG 25 µM/PCB 126; lane 5, EGCG 50 µM; lane 6, EGCG 50 µM/PCB 126; lane 7, PCB 126; lane 8, PCB and competitor; lane 9, PCB and mutator; lane 10, PCB and p50 antibody; lane 11, PCB and p65 antibody; lane 12, PCB and c-Rel antibody; lane 12, PCB and p300 antibody. (B) Endothelial cells were pretreated with EGCG (25–50 µM) for 3 h, followed by PCB 126 exposure at 0.25 µM for 6 h. EMSA for AhR was performed with nuclear proteins extracted from endothelial cells. EMSA competition assay showed the specificity of AhR probe using a competitor and a mutator and antibodies. Figure 2B: Lane 1, DMSO; lane 2, PCB 126; lane 3, EGCG 25 µM; lane 4, EGCG 25 µM/PCB 126; lane 5, EGCG 50 µM; lane 6, EGCG 50 µM/PCB 126; lane 7, PCB 126; lane 8, PCB and competitor; lane 9, PCB and mutator; lane 10, PCB and AhR antibody; lane 11, PCB and Myc antibody. Images are representative of three independent experiments, and image splicing was carried out only for the same experiment, same gel and the same exposure times.
PCB 126 induces expression of MCP-1 and VCAM-1, and adhesion of monocytes to endothelial cells is modulated by EGCG
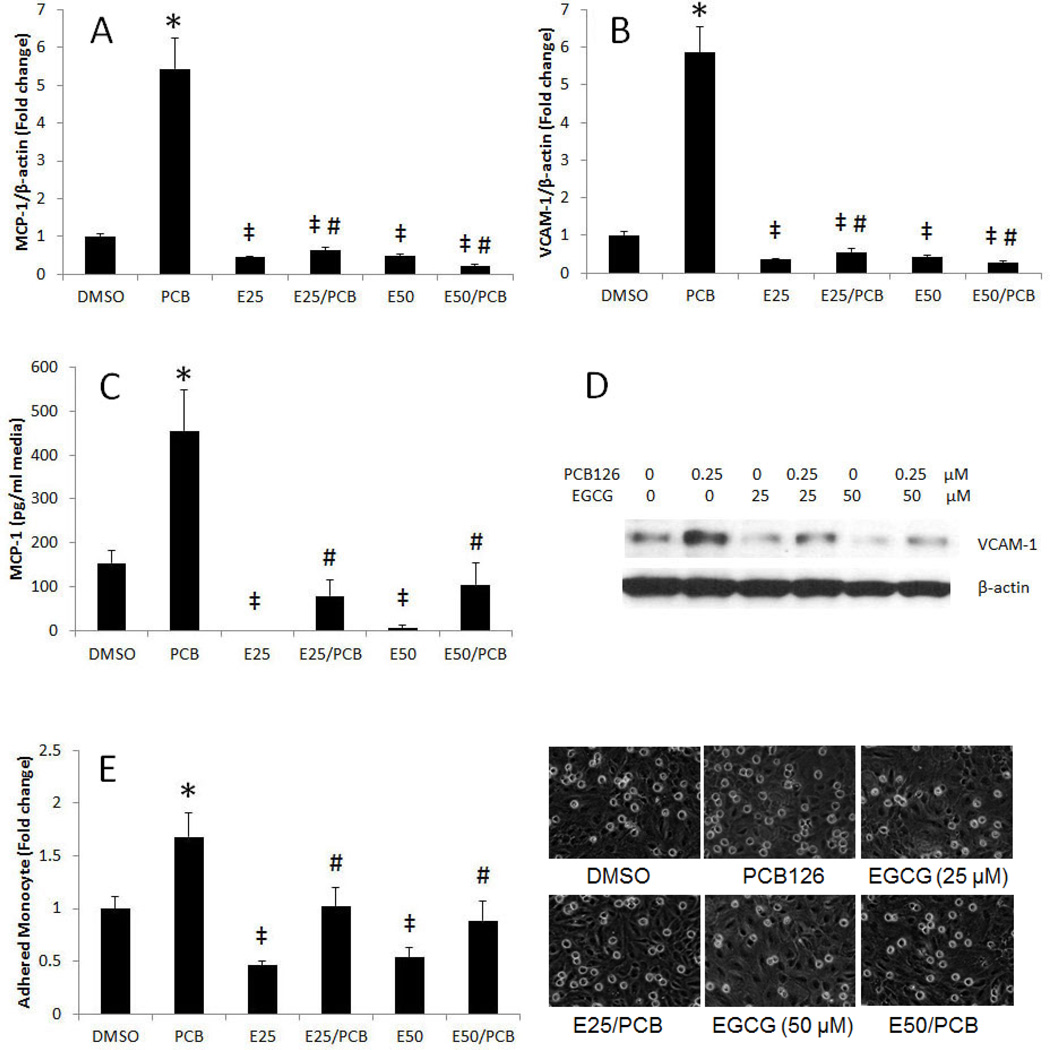
To determine whether EGCG can modulate PCB 126-induced inflammatory parameters, endothelial cells were exposed to PCB 126 with or without pretreatment with EGCG. MCP-1 is a chemokine that plays a critical role in the recruitment of monocytes to the site of endothelial inflammation which is one of the earliest events in the pathology of atherosclerosis (Majkova et al., 2009). Overexpression of VCAM-1 is also involved in the initial step of monocyte recruitment to the atherosclerotic lesions (Han et al., 2010a). A significant increase of MCP-1 and VCAM-1 mRNA was observed following exposure to PCB 126 (Figures 3A and B). Pretreatment with EGCG significantly reduced PCB-induced mRNA expression. Protein expression of MCP-1 and VCAM-1 was detected by ELISA and Western blot analysis, respectively. Similar to mRNA, protein expression of MCP-1 and VCAM-1 was markedly increased by PCB 126 but attenuated by pretreatment with EGCG (Figures 3C and D). Endothelial secretion of chemokines and the expression of adhesion molecules can trigger the recruitment of circulating monocytes to the endothelium that leads to adhesion and transmigration into the arterial wall (Badimon et al., 2011). Thus, monocyte adhesion onto the endothelium is a critical initial step in the pathology of atherosclerosis. In the present study, PCB 126 significantly increased the adhesion of monocytes (activated and fluorescence labeled THP-1 cells) to the endothelial monolayer, while pretreatment with EGCG abolished this atherogenic event (Figures 3E). In addition to the reduction of PCB-induced Cyp1A1, MCP-1 and VCAM-1 by EGCG, we also noted that EGCG markedly decreased the expression of these genes at base-line levels (Figures 1A, 3A and 3B). Overall, our data imply that a decrease of Cyp1A1 by EGCG (Figure 1A) leads to a decrease of NF-κB DNA binding activity (Figure 2A), followed by downregulation of MCP-1 and VCAM-1 gene expression (Figures 3A and B).
Figure 3.
PCB 126-induced expression of MCP-1 and VCAM-1, and adhesion of monocytes to endothelial cells is modulated by EGCG. mRNA expression of (A) MCP-1 and (C) VCAM-1 was analyzed in endothelial cells pretreated with 0–50 µM of EGCG for 3 h, followed by treatment with PCB 126 at 0.25 µM for 16 h. Real-time PCR technique was used to measure mRNA levels. Figure 3 shows protein expression of (B) MCP-1 and (D) VCAM-1 in endothelial cells pretreated with 0–50 µM of EGCG for 3 h, followed by treatment with PCB 126 at 0.25 µM for 16 h. MCP-1 protein levels in cell culture media were assessed using ELISA. Western blot was used to detect VCAM-1 protein in whole cell lysates. The Western blot picture shown is a representative of three independent blots. (E) Endothelial cells were pretreated with EGCG (25–50 µM) for 3 h, followed by treatment with PCB 126 at 0.25 µM for 16 h. Human THP-1 monocytes were activated with TNF-α and loaded with the fluorescent probe calcein. Activated and calcein loaded monocytes were added to endothelial monolayers for activation. After washing, adhered monocytes were counted using a fluorescent microscope. Results represent the mean ± SEM, with n=3. Experiments were repeated a minimum of three times. *Significantly increased compared to DMSO control. ‡Significantly decreased compared to DMSO control. #Significantly different compared to PCB 126 treatment group.
EGCG increases expression of the phase II enzymes, GST and NQO1
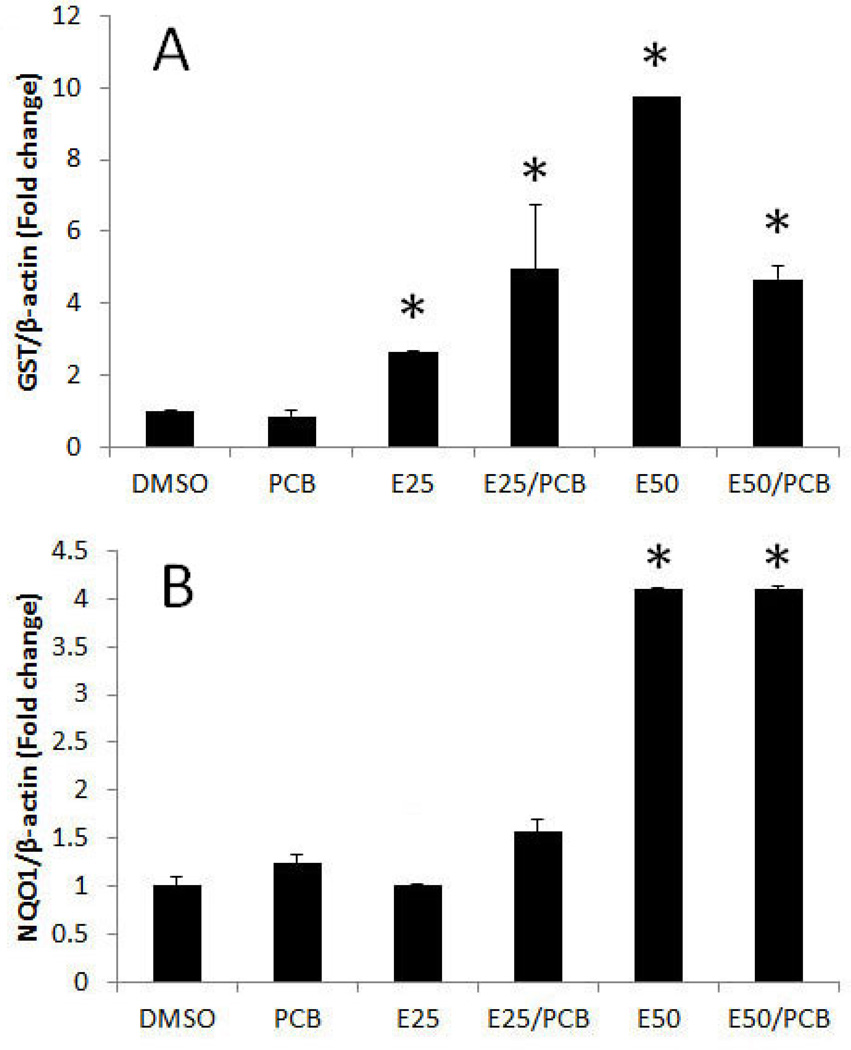
To identify mechanisms EGCG-mediated reduction of oxidative stress caused by PCB 126, expression of phase II antioxidant enzymes were determined. The expression of phase II detoxifying and antioxidant enzymes, such as glutathione-S-transferase (GST) and NAD(P)H:quinone oxidoreductase 1 (NQO1), is regulated by a cis-acting DNA regulatory element, named antioxidant response element (ARE). Nrf2 is the transcription factor that binds to ARE, resulting in induction of the expression of GST and NQO1. Thus, the gene expression of GST and NQO1 is an indicator of the activation of Nrf2-ARE gene battery. EGCG increased the expression of GST and NQO1 genes (Figure 4A and B). These data indicate that the Nrf2-ARE gene battery is activated by EGCG exposure, and this EGCG-induced activation remained elevated in the presence of PCB 126.
Figure 4.
EGCG increases expression of the phase II enzymes GST and NQO1. Expression of (A) GST and (B) NQO1 was analyzed in endothelial cells pretreated with 25–50 µM of EGCG for 3 h, followed by treatment with PCB 126 at 0.25 µM for 16 h. Real-time PCR technique was used to measure expressed mRNA levels. Results represent the mean ± SEM, with n=3. Experiments were repeated a minimum of three times. *Significantly different compared to DMSO control.
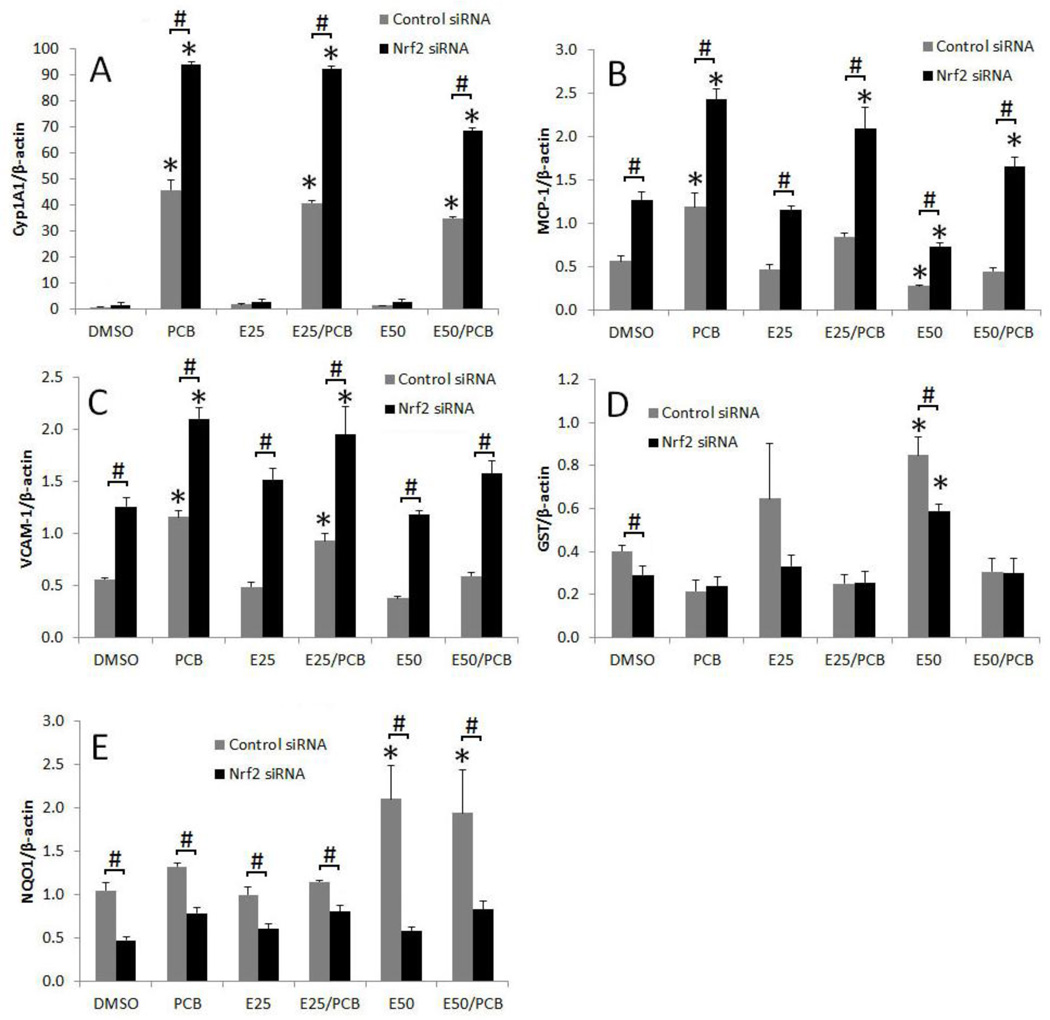
Nrf2 silencing increases expression of Cyp1A1, VCAM-1 and MCP-1
In order to identify the function of Nrf2 in EGCG-mediated protection against PCB exposure, we used siRNA gene silencing technique in primary porcine endothelial cells. In the control siRNA group, PCB 126 significantly upregulated Cyp1A1 gene expression, and pretreatment of EGCG significantly attenuated the expression of the Cyp1A1 gene (Figure 5A). Nrf2 silencing further increased PCB 126-induced Cyp1A1 gene expression (Figure 5A). A similar expression pattern was observed for MCP-1 and VCAM-1 (Figure 5B and C). As expected, Nrf2 silencing significantly decreased the gene expression of the Nrf2-regulated genes GST and NQO1 after pretreatment with EGCG (Figure 5D and E).
Figure 5.
Nrf2 silencing increases expression of Cyp1A1, VCAM-1 and MCP-1. Primary endothelial cells were treated with Nrf2 siRNA or control siRNA, and then pretreated with 25–50 µM of EGCG for 3 h, followed by treatment with PCB 126 at 0.25 µM for 16 h. Expression of Cyp1A1 (A), MCP-1 (B), VCAM-1 (C), GST (D) and NQO1 (E) were analyzed in cells. Real-time PCR technique was used to measure expressed mRNA levels. Results represent the mean ± SEM, with n=3. Experiments were repeated a minimum of three times. *Significantly different compared to corresponding DMSO control. #Significantly different between control siRNA and Nrf2 siRNA within the same treatment.
Discussion
Cardiovascular disease is the leading cause of death in the United States. Some factors contributing to the etiology of this disease have been found including environmental contaminants, such as PCBs (Everett et al., 2008). Particularly coplanar PCBs exhibit their toxicity by binding to AhR and subsequent increase of Cyp1A1 gene expression. Coplanar PCBs stimulate production of ROS by uncoupling the catalytic cycle in their metabolism by Cyp1A1 (Schlezinger et al., 2006). We have shown previously that coplanar PCBs, including PCB 77 and PCB 126, are proinflammatory and atherogenic in vascular endothelial cells. In addition to PCBs being able to induce an inflammatory response, these persistent environmental pollutants also can promote obesity and atherosclerosis (Arsenescu et al., 2008).
Many mechanisms and signaling pathways associated with the pathology of inflammatory diseases are modulated by both dietary habits and environmental pollutants. In fact, numerous genes induced in diseases associated with vascular dysfunction such as atherosclerosis are oxidative stress-sensitive, suggesting that an imbalance in cellular oxidative stress and antioxidant status is a critical underlying factor. Evidence is emerging which suggests that antioxidant nutrients and related bioactive compounds common in fruits and vegetables protect against environmental toxic insult to the vascular endothelium by down-regulation of signaling pathways involved in inflammatory responses associated with vascular diseases such as atherosclerosis (Hennig et al., 2007). There are some epidemiological data that long-term consumption of flavonoids can decrease the incidence of cardiovascular diseases (Hertog et al., 1993; Mennen et al., 2004; Sesso et al., 2003). In addition to the human data, flavonoids can inhibit cytokine-induced oxidative stress and adhesion molecule expression in endothelial cells (Crespo et al., 2008; Lotito and Frei, 2006).
Data from the current study provide further evidence of the anti-inflammatory properties of tea catechins such as EGCG in endothelial cells. We demonstrated that EGCG can decrease PCB-induced expression of Cyp1A1 and endothelial inflammatory parameters, MCP-1 and VCAM-1. Exposure to PCB 126 significantly increased both message and protein expression of Cyp1A1 as well as production of reactive oxygen species, which were markedly attenuated when endothelial cells were first pre-enriched with EGCG (25–50 µM). It has been shown that EGCG can decrease the binding of AhR to XRE (Palermo et al., 2003a; Williams et al., 2000). Our EMSA studies revealed that the AhR-XRE binding complexes formed by PCB exposure were reduced by treatment with EGCG (Figure 2B), and as described in earlier studies from our laboratory (Ramadass et al., 2003), suggesting that EGCG can antagonize the AhR pathway. However, it is not clear how EGCG can alter the AhR-XRE binding. One plausible explanation is a direct binding of EGCG to the AhR by which the AhR-XRE binding is challenged. However, EGCG may not compete directly with TCDD for binding to the AhR (Palermo et al., 2003b; Palermo et al., 2005). Similarly, EGCG does not competitively inhibit 3-methylcholanthrene binding to the AhR (Fukuda et al., 2007). Our data suggest that EGCG antagonizes the AhR pathway indirectly through Nrf2-induced antioxidant genes. EGCG can also inhibit the activation of AhR-XRE gene battery through its ability to bind to Hsp90 (Palermo et al., 2005; Yin et al., 2009). Hsp90 is an essential component of AhR signaling pathway due to its ability to stabilize the AhR complex. As a result, EGCG can inhibit the recognition of AhR to the corresponding gene regulatory elements. EGCG also attenuated PCB 126-mediated induction of inflammatory markers such as MCP-1 and VCAM-1. The observed reduction in inflammatory parameters was due in part to the ability of EGCG to down-regulate the DNA-binding activity of NF-κB (Lee et al., 2009). In fact, our data confirmed that EGCG can markedly reduce the PCB 126-induced NF-κB DNA binding, which subsequently resulted in the down-regulation of the chemokine MCP-1 and the adhesion molecules VCAM-1. More importantly, EGCG also decreased the PCB 126-induced adhesion of monocytes to endothelial monolayers. Interestingly, EGCG also decreased baseline levels of Cyp1A1, MCP-1 and VCAM-1, suggesting that cellular protection by EGCG occurs even in the absence of a toxic stressor.
Mechanisms of protective properties of plant-derived flavonoids such as EGCG are not simple but may involve induction of phase II antioxidant enzymes (Tan et al., 2010). Indeed, in our endothelial cell system, EGCG treatment markedly induced both GST and NQO1 in a dose-dependent manner. The EGCG-mediated increase in these phase II antioxidant enzymes was in part maintained even in the presence of PCB 126. GST, NQO1 and other phase II antioxidant genes, such as heme oxigenase (HO-1), are regulated by the transcription factor Nrf2 and its binding to ARE (Giudice et al., 2010). Thus, the expression of these genes is an important indicator for the activation of Nrf2. In order to further identify the function of Nrf2 in EGCG-mediated protection against PCB exposure, we used the siRNA gene silencing technique. Indeed, we were able to demonstrate that endothelial cell treatment with Nrf2 siRNA could further increase the PCB 126-induced expression of Cyp1A1, MCP-1 and VCAM-1. These results may be at least in part due to down-regulation of NQO1 and GST after Nrf2 gene silencing (Figure 5), suggesting that ARE-driven antioxidant enzymes play an important protective role against PCB-induced inflammatory responses in endothelial cells.
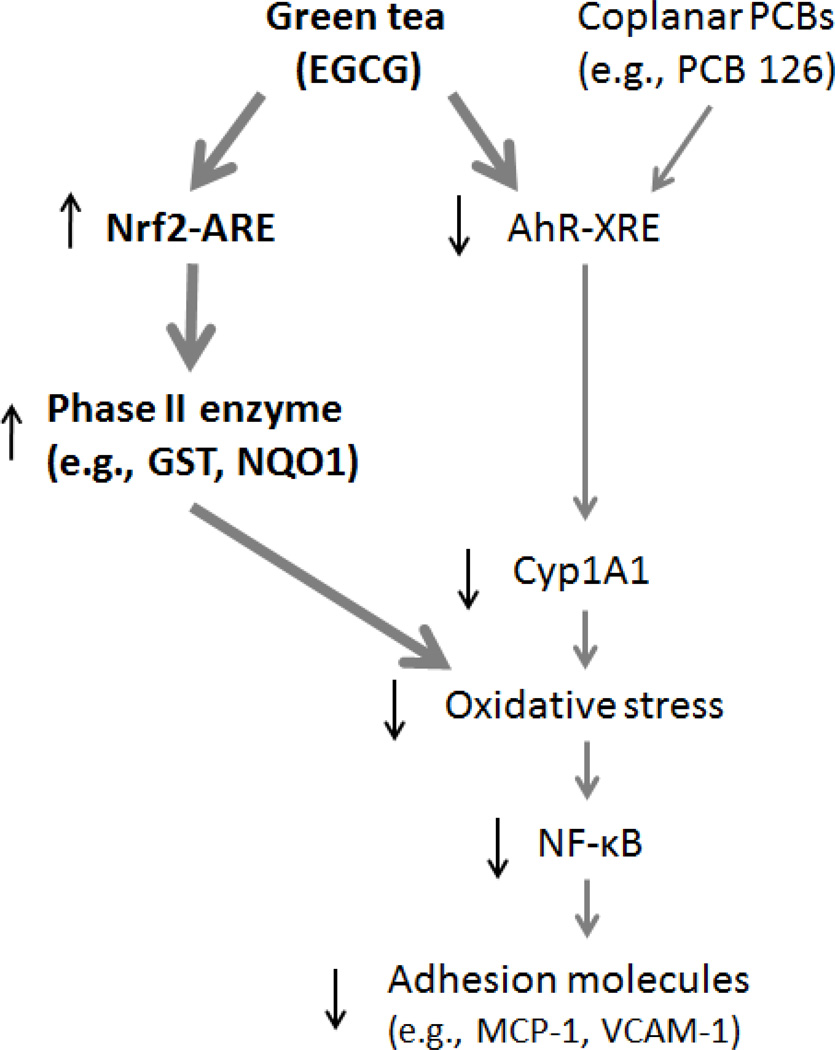
Our data suggest that EGCG can decrease PCB-induced inflammatory events in vascular endothelial cells by inhibiting the activation of AhR which resulted in downregulation of Cyp1A1 and decreased oxidative stress. We also provide evidence that the protective properties of EGCG against PCB 126-induced inflammation are regulated in part via Nrf2-ARE signaling and the induction of phase II antioxidant enzymes, events which can down-regulate oxidative stress-sensitive transcription factors and inflammatory genes (Figure 6).
Figure 6.
Schematic illustration of the inhibition of PCB 126-induced endothelial inflammatory responses by EGCG. EGCG reduces oxidative stress through up-regulation of Nrf2-regulated genes, such as phase II enzymes, and down-regulation of AhR-regulated genes, such as Cyp1A1. These cellular events lead to decreased NF-κB signaling, including reduced expression of adhesion molecules.
Highlights.
PCBs cause endothelial inflammation, an important event in the pathology of atherosclerosis.
Nutrition can modulate toxicity by environmental pollutants.
We demonstrated that the tea catechin EGCG can down-regulate PCB-induced inflammation.
EGCG protection was via inhibition of AhR and induction of Nrf2 regulatory genes.
Acknowledgements
This study was supported in part by grants from NIEHS (P42ES007380), and the Kentucky Agricultural Experimental Station.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare no conflict of interest.
References
- Arsenescu V, Arsenescu RI, King V, Swanson H, Cassis LA. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ Health Perspect. 2008;116:761–768. doi: 10.1289/ehp.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badimon L, Storey RF, Vilahur G. Update on lipids, inflammation and atherothrombosis. Thromb Haemost. 2011 doi: 10.1160/THS10-11-0717. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Arzuaga X, Kluemper CT, Caraballo A, Toborek M, Hennig B. Quercetin blocks caveolae-dependent pro-inflammatory responses induced by co-planar PCBs. Environ Int. 2010;36:931–934. doi: 10.1016/j.envint.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo I, Garcia-Mediavilla MV, Gutierrez B, Sanchez-Campos S, Tunon MJ, Gonzalez-Gallego J. A comparison of the effects of kaempferol and quercetin on cytokine-induced pro-inflammatory status of cultured human endothelial cells. Br J Nutr. 2008;100:968–976. doi: 10.1017/S0007114508966083. [DOI] [PubMed] [Google Scholar]
- Everett CJ, Mainous AG, 3rd, Frithsen IL, Player MS, Matheson EM. Association of polychlorinated biphenyls with hypertension in the 1999–2002 National Health and Nutrition Examination Survey. Environ Res. 2008;108:94–97. doi: 10.1016/j.envres.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Fukuda I, Mukai R, Kawase M, Yoshida K, Ashida H. Interaction between the aryl hydrocarbon receptor and its antagonists, flavonoids. Biochem Biophys Res Commun. 2007;359:822–827. doi: 10.1016/j.bbrc.2007.05.199. [DOI] [PubMed] [Google Scholar]
- Giudice A, Arra C, Turco MC. Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Methods Mol Biol. 2010;647:37–74. doi: 10.1007/978-1-60761-738-9_3. [DOI] [PubMed] [Google Scholar]
- Gouedard C, Barouki R, Morel Y. Dietary polyphenols increase paraoxonase 1 gene expression by an aryl hydrocarbon receptor-dependent mechanism. Mol Cell Biol. 2004;24:5209–5222. doi: 10.1128/MCB.24.12.5209-5222.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SG, Eum SY, Toborek M, Smart E, Hennig B. Polychlorinated biphenyl-induced VCAM-1 expression is attenuated in aortic endothelial cells isolated from caveolin-1 deficient mice. Toxicol Appl Pharmacol. 2010a;246:74–82. doi: 10.1016/j.taap.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SS, Yun H, Son DJ, Tompkins VS, Peng L, Chung ST, Kim JS, Park ES, Janz S. NF-kappaB/STAT3/PI3K signaling crosstalk in iMyc E mu B lymphoma. Mol Cancer. 2010b;9:97. doi: 10.1186/1476-4598-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B, Oesterling E, Toborek M. Environmental toxicity, nutrition, and gene interactions in the development of atherosclerosis. Nutr Metab Cardiovasc Dis. 2007;17:162–169. doi: 10.1016/j.numecd.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Hennig B, Reiterer G, Majkova Z, Oesterling E, Meerarani P, Toborek M. Modification of environmental toxicity by nutrients: implications in atherosclerosis. Cardiovasc Toxicol. 2005a;5:153–160. doi: 10.1385/ct:5:2:153. [DOI] [PubMed] [Google Scholar]
- Hennig B, Reiterer G, Toborek M, Matveev SV, Daugherty A, Smart E, Robertson LW. Dietary fat interacts with PCBs to induce changes in lipid metabolism in mice deficient in low-density lipoprotein receptor. Environ Health Perspect. 2005b;113:83–87. doi: 10.1289/ehp.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- Hopf NB, Ruder AM, Succop P. Background levels of polychlorinated biphenyls in the U.S. population. Sci Total Environ. 2009;407:6109–6119. doi: 10.1016/j.scitotenv.2009.08.035. [DOI] [PubMed] [Google Scholar]
- Jones CI, 3rd, Zhu H, Martin SF, Han Z, Li Y, Alevriadou BR. Regulation of antioxidants and phase 2 enzymes by shear-induced reactive oxygen species in endothelial cells. Ann Biomed Eng. 2007;35:683–693. doi: 10.1007/s10439-007-9279-9. [DOI] [PubMed] [Google Scholar]
- Kopf PG, Scott JA, Agbor LN, Boberg JR, Elased KM, Huwe JK, Walker MK. Cytochrome P4501A1 is required for vascular dysfunction and hypertension induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2010;117:537–546. doi: 10.1093/toxsci/kfq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf PG, Walker MK. 2,3,7,8-tetrachlorodibenzo-p-dioxin increases reactive oxygen species production in human endothelial cells via induction of cytochrome P4501A1. Toxicol Appl Pharmacol. 2010;245:91–99. doi: 10.1016/j.taap.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kris-Etherton PM, Lefevre M, Beecher GR, Gross MD, Keen CL, Etherton TD. Bioactive compounds in nutrition and health-research methodologies for establishing biological function: the antioxidant and anti-inflammatory effects of flavonoids on atherosclerosis. Annu Rev Nutr. 2004;24:511–538. doi: 10.1146/annurev.nutr.23.011702.073237. [DOI] [PubMed] [Google Scholar]
- Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, Tsuji I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. Jama. 2006;296:1255–1265. doi: 10.1001/jama.296.10.1255. [DOI] [PubMed] [Google Scholar]
- Lee AS, Jung YJ, Kim DH, Lee TH, Kang KP, Lee S, Lee NH, Sung MJ, Kwon DY, Park SK, Kim W. Epigallocatechin-3-O-gallate decreases tumor necrosis factor-alpha-induced fractalkine expression in endothelial cells by suppressing NF-kappaB. Cell Physiol Biochem. 2009;24:503–510. doi: 10.1159/000257494. [DOI] [PubMed] [Google Scholar]
- Lotito SB, Frei B. Dietary flavonoids attenuate tumor necrosis factor alpha-induced adhesion molecule expression in human aortic endothelial cells. Structure-function relationships and activity after first pass metabolism. J Biol Chem. 2006;281:37102–37110. doi: 10.1074/jbc.M606804200. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Lorenz M, Grimbo N, Steinle F, Meiners S, Bartsch C, Stangl K, Baumann G, Stangl V. The tea flavonoid epigallocatechin-3-gallate reduces cytokine-induced VCAM-1 expression and monocyte adhesion to endothelial cells. Biochem Biophys Res Commun. 2004;316:659–665. doi: 10.1016/j.bbrc.2004.02.099. [DOI] [PubMed] [Google Scholar]
- Majkova Z, Layne J, Sunkara M, Morris AJ, Toborek M, Hennig B. Omega-3 fatty acid oxidation products prevent vascular endothelial cell activation by coplanar polychlorinated biphenyls. Toxicol Appl Pharmacol. 2011;251:41–49. doi: 10.1016/j.taap.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majkova Z, Smart E, Toborek M, Hennig B. Up-regulation of endothelial monocyte chemoattractant protein-1 by coplanar PCB77 is caveolin-1-dependent. Toxicol Appl Pharmacol. 2009;237:1–7. doi: 10.1016/j.taap.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennen LI, Sapinho D, de Bree A, Arnault N, Bertrais S, Galan P, Hercberg S. Consumption of foods rich in flavonoids is related to a decreased cardiovascular risk in apparently healthy French women. J Nutr. 2004;134:923–926. doi: 10.1093/jn/134.4.923. [DOI] [PubMed] [Google Scholar]
- Palermo CM, Hernando JI, Dertinger SD, Kende AS, Gasiewicz TA. Identification of potential aryl hydrocarbon receptor antagonists in green tea. Chem Res Toxicol. 2003a;16:865–872. doi: 10.1021/tx025672c. [DOI] [PubMed] [Google Scholar]
- Palermo CM, Hernando JI, Dertinger SD, Kende AS, Gasiewicz TA. Identification of potential aryl hydrocarbon receptor antagonists in green tea. Chem Res Toxicol. 2003b;16:865–872. doi: 10.1021/tx025672c. [DOI] [PubMed] [Google Scholar]
- Palermo CM, Westlake CA, Gasiewicz TA. Epigallocatechin gallate inhibits aryl hydrocarbon receptor gene transcription through an indirect mechanism involving binding to a 90 kDa heat shock protein. Biochemistry. 2005;44:5041–5052. doi: 10.1021/bi047433p. [DOI] [PubMed] [Google Scholar]
- Ramadass P, Meerarani P, Toborek M, Robertson LW, Hennig B. Dietary flavonoids modulate PCB-induced oxidative stress, CYP1A1 induction, and AhR-DNA binding activity in vascular endothelial cells. Toxicol Sci. 2003;76:212–219. doi: 10.1093/toxsci/kfg227. [DOI] [PubMed] [Google Scholar]
- Sasazuki S, Kodama H, Yoshimasu K, Liu Y, Washio M, Tanaka K, Tokunaga S, Kono S, Arai H, Doi Y, Kawano T, Nakagaki O, Takada K, Koyanagi S, Hiyamuta K, Nii T, Shirai K, Ideishi M, Arakawa K, Mohri M, Takeshita A. Relation between green tea consumption and the severity of coronary atherosclerosis among Japanese men and women. Ann Epidemiol. 2000;10:401–408. doi: 10.1016/s1047-2797(00)00066-1. [DOI] [PubMed] [Google Scholar]
- Schlezinger JJ, Struntz WD, Goldstone JV, Stegeman JJ. Uncoupling of cytochrome P450 1A and stimulation of reactive oxygen species production by co-planar polychlorinated biphenyl congeners. Aquat Toxicol. 2006;77:422–432. doi: 10.1016/j.aquatox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Sergeev AV, Carpenter DO. Residential proximity to environmental sources of persistent organic pollutants and first-time hospitalizations for myocardial infarction with comorbid diabetes mellitus: a 12-year population-based study. Int J Occup Med Environ Health. 2010;23:5–13. doi: 10.2478/v10001-010-0010-y. [DOI] [PubMed] [Google Scholar]
- Sesso HD, Gaziano JM, Liu S, Buring JE. Flavonoid intake and the risk of cardiovascular disease in women. Am J Clin Nutr. 2003;77:1400–1408. doi: 10.1093/ajcn/77.6.1400. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Nakayama K, Itoh S, Fujii-Kuriyama Y, Kamataki T. Inhibition of the transcription of CYP1A1 gene by the upstream stimulatory factor 1 in rabbits. Competitive binding of USF1 with AhRArnt complex. J Biol Chem. 1997;272:30025–30031. doi: 10.1074/jbc.272.48.30025. [DOI] [PubMed] [Google Scholar]
- Tan XL, Shi M, Tang H, Han W, Spivack SD. Candidate dietary phytochemicals modulate expression of phase II enzymes GSTP1 and NQO1 in human lung cells. J Nutr. 2010;140:1404–1410. doi: 10.3945/jn.110.121905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Reiterer G, Toborek M, Hennig B. Changing ratios of omega-6 to omega-3 fatty acids can differentially modulate polychlorinated biphenyl toxicity in endothelial cells. Chem Biol Interact. 2008;172:27–38. doi: 10.1016/j.cbi.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SN, Shih H, Guenette DK, Brackney W, Denison MS, Pickwell GV, Quattrochi LC. Comparative studies on the effects of green tea extracts and individual tea catechins on human CYP1A gene expression. Chem Biol Interact. 2000;128:211–229. doi: 10.1016/s0009-2797(00)00204-0. [DOI] [PubMed] [Google Scholar]
- Yin Z, Henry EC, Gasiewicz TA. (-)-Epigallocatechin-3-gallate is a novel Hsp90 inhibitor. Biochemistry. 2009;48:336–345. doi: 10.1021/bi801637q. [DOI] [PMC free article] [PubMed] [Google Scholar]