Abstract
We have recently documented that treatment with the alternative biofuel, acetyl-l-carnitine (ALC, 300 mg/kg), as late as 1 hr after T10 contusion spinal cord injury (SCI), significantly maintained mitochondrial function 24 hrs after injury. Here we report that following more severe contusion SCI centered on the L1/L2 segments that are postulated to contain lamina X neurons critical for locomotion (the “central pattern generator”), ALC treatment resulted in significant improvements in acute mitochondrial bioenergetics and long-term hindlimb function. While control-injured rats were only able to achieve slight movements of hindlimb joints, ALC-treated animals produced consistent weight-supported plantar steps one month after injury. Such landmark behavioral improvements were significantly correlated with increased tissue sparing of both gray and white matter proximal to the injury, as well as preservation of choline acetyltransferase (ChAT)-positive neurons in lamina X rostral to the injury site. These findings signify that functional improvements with ALC treatment are mediated, in part, by preserved locomotor circuitry rostral to upper lumbar contusion SCI. Based on beneficial effects of ALC on mitochondrial bioenergetics after injury, our collective evidence demonstrate that preventing mitochondrial dysfunction acutely “promotes” neuroprotection that may be associated with the milestone recovery of plantar, weight-supported stepping.
Keywords: mitochondrial bioenergetics, L1/L2 SCI, locomotor function, locomotor neuronal circuitry, central pattern generator, neuroprotection, hindlimb function, ChAT-positive neurons
1.1 Introduction
Traumatic spinal cord injury (SCI) results in highly dynamic and complex patterns of secondary destructive biochemical and pathophysiological processes that result in extensive tissue damage and often permanent loss of function (Rabchevsky et al., 2011). Post-traumatic disruption of Ca2+ homeostasis, generation of reactive oxygen species (ROS), and oxidative damage are arguably among the best possible secondary targets for pharmacotherapeutics (Hall and Springer, 2004, Sullivan et al., 2007). Increased excitotoxicity following traumatic SCI is also linked directly to oxidative stress elicited by mitochondrial dysfunction (Luo et al., 2004, McEwen et al., 2007, Sullivan et al., 2007). In addition to their vital energy production (e.g. ATP synthesis) essential for cell survival, a conundrum lies in the fact that mitochondria are also a principle source of free radical production and, thus, primary targets for free radical attack.
Mitochondria play a pivotal role in the secondary injury cascade in SCI models (Azbill et al., 1997, Sullivan et al., 2007, Yu et al., 2009, Vaishnav et al., 2010) and pharmacological targeting of mitochondrial dysfunction with therapeutic agents provides critical neuroprotection (McEwen et al., 2007, Patel et al., 2009, Xiong et al., 2009, Patel et al., 2010, Springer et al., 2010). We recently reported that administration of acetyl-l-carnitine (ALC) as late as 1 hr after thoracic (T10) SCI prolonged near-normal mitochondrial function at the injury site, and daily ALC treatment for one week significantly increased spinal cord tissue sparing compared to vehicle treatment (Patel et al., 2010). ALC is synthesized in human brain, liver, heart and kidney (Farrell et al., 1986, Rebouche, 2004, Jones et al., 2010). It has been used clinically to improve various neurological disorders (Bonavita, 1986, Tempesta et al., 1987, Rai et al., 1990, Spagnoli et al., 1991, Sano et al., 1992, Lowitt et al., 1995, Onofrj et al., 1995, Swamy-Mruthinti and Carter, 1999). Experimentally, it is documented that ALC can cross the blood-brain barrier with the help of a second isoform of organic cation transporters (OCTN2) in a sodium-dependant manner (Ohashi et al., 1999, Kido et al., 2001). Recently it has been reported that ALC can be metabolized for brain energy metabolism in 21 – 22 days old rats, however extent of its contribution to energy metabolism following brain injury is not clear (Scafidi et al., 2010a). Moreover, following traumatic brain injury in immature rats, treatment with ALC during first 24 hrs improved behavioral outcomes and reduced brain lesion volume (Scafidi et al., 2010b). ALC increases acetyl-CoA levels by donating an acetyl group to Coenzyme A (CoA) to form acetyl CoA and free carnitine, a reaction catalyzed by carnitine acetyltransferase in mitochondria (Jones et al., 2010, Scafidi et al., 2010a). Acetyl-CoA then enters into the citric acid cycle and forms NADH that is used by NADH dehydrogenase (complex I) in the mitochondrial electron transport chain (ETC) for ATP production, thus acting as an alternative bio-fuel.
ALC is reported to increase the endogenous pool of antioxidant glutathione (GSH) (Aureli et al., 1999) and the activities of antioxidant enzymes (El-Awady el et al., 2011, Hao et al., 2011). This helps to maintain cellular redox system components (NAD+/NADH and NADP+/NADPH) (Elanchezhian et al., 2009), while stabilizing membrane lipid/phospholipids to optimize mitochondrial enzyme complex activities (Paradies et al., 1992, Aureli et al., 2000, Hao et al., 2011). Accordingly, ALC is reported to alter metabolic processes in diseases correlated with mitochondrial-related disorders (Dhitavat et al., 2002, Virmani and Binienda, 2004, Di Cesare Mannelli et al., 2007, Di Cesare Mannelli et al., 2008). Based on this evidence and our previous findings that targeting mitochondrial dysfunction after SCI is neuroprotective, in the current study we evaluated the effects of daily ALC treatment on long-term hindlimb locomotor function and tissue sparing following contusion SCI.
1.2 Experimental procedures
1.2.1 Spinal Cord Injury and Treatments
Female Sprague-Dawley rats (n=76) (Harlan Labs, IN) weighing 200–250g were housed in the animal facility, Biomedical and Biological Sciences Research Building, University of Kentucky and allowed ad libitum access to water and food. All animal housing conditions, surgical procedures, and postoperative care techniques were conducted according to the University of Kentucky Institutional Animal Care and Use Committee and the National Institutes of Health animal care guidelines. Prior to surgeries, rats were anesthetized with ketamine (80 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and hair on dorsal surface was shaven and skin disinfected. A dorsal laminectomy was performed at the tenth (T10, n=12) or twelfth (T12, n=64) thoracic level to expose the T9/T10 or L1/L2 spinal cord, respectively. Contusions (200 kdyn for T10 or 250 kdyn for L1/L2) were performed with the Infinite Horizon impactor (PSI, LLC, Lexington, KY), as previously described (Sullivan et al., 2007, Patel et al., 2009, Patel et al., 2010). i. For biochemical assessments of mitochondrial integrity, sham rats (n=8) received a dorsal laminectomy and showed normal hindlimb locomotion post-surgery. Injured rats were treated (i.p.) with either vehicle (saline, n=8) or 300 mg/kg acetyl-L-carnitine (ALC, n=8; Sigma, St. Louis, MO) at 15 min after contusion SCI. A booster injection of ALC or control saline was also given at 6 hr post-injury before euthanizing at 24hr post-SCI. ii. For the long-term behavioral and histological experiments, injured rats (n=14 per group) received i.p. injection of vehicle (saline) or 300 mg/kg ALC at 15 min post-SCI and additional boosters after 6 hrs. This was then followed by i.p. injections of either saline or 300 mg/kg ALC once daily for 21 days post-SCI. One week after the last injections, the rats were euthanized and perfused to isolate spinal cord tissue for histology. ALC was dissolve in saline to get final concentration of 300 mg/ml; pH was adjusted to 5.0 with NaOH. Injured rats were administered ALC or saline 1 μl/g body weight.
1.2.2 Mitochondrial Isolation
At 24 hrs following sham operation or injury, rats were euthanized with CO2 and decapitated for isolation and characterization of total (both neuronal and glial somata) mitochondrial populations (n=8/group), as described previously (Jin et al., 2004, Sullivan et al., 2007). Briefly, the spinal cords were rapidly dissected and placed in ice cold isolation buffer with 1mM EGTA (215mM mannitol, 75mM sucrose, 0.1% BSA, 20mM HEPES, 1mM EGTA and pH is adjusted to 7.2 with KOH). Each spinal cord was dissected into 1.5 cm segments centered on the injury site and homogenized in 2 ml of ice cold isolation buffer with EGTA. The homogenate was then centrifuged at 1,300 × G for 3 min @ 4°C. Supernatant was removed and the pellet was resuspended in isolation buffer and centrifuged at 1,300 × G for 3 min @ 4°C. The resulting two supernatants were then combined and centrifuged at 13,000 × G for 10 min @ 4°C. The mitochondria pellets from two spinal cords in each group were pooled and resuspended in 350 μl of isolation buffer containing EGTA and synaptosomes rupturing using a nitrogen disruption chamber (1200 psi, 10 min) cooled to 4°C. The resulting fractions were then placed atop a discontinuous Ficoll gradient (7.5%/10%) and centrifuged at 100,000 × G for 30 min @ 4°C. The resulting mitochondrial pellet was resuspended in isolation buffer without EGTA and centrifuged for 10 min at 10,000 × G. The final mitochondrial pellet was resuspended in EGTA-free isolation buffer at a concentration of ~10 mg/ml and stored on ice during assessments of mitochondrial function. The protein concentration was determined using the BCA protein assay kit (Thermo Scientific, Rockford, IL) by measuring absorbance at 560 nm with a Biotek Synergy HT plate reader (Winooski, Vermont).
1.2.3 Measurements of Mitochondrial Function
Mitochondrial respiration was assessed using a miniature Clark-type electrode (Hansatech Instruments, Norfolk, England) in a sealed, thermostatically controlled chamber at 37°C. Mitochondria were added to the chamber to yield a final protein concentration of ~200–300 μg/mL respiration buffer (125 mM KCl, 2 mM MgCl2, 2.5 mM KH2PO4, 20 mM HEPES and 0.1% BSA, pH 7.2). The oxidative substrates and inhibitors for different enzyme complexes of mitochondrial electron transport system (ETS) were added to assess mitochondrial respiration, as detailed previously (Sullivan et al., 2007, Patel et al., 2009, Patel et al., 2010). Two independent oxymetric traces for each sample were taken and the respiration rates averaged.
1.2.4 Behavioral Assessments
The Basso, Beattie, Bresnahan Locomotor Rating Scale (BBB LRS) (Basso et al., 1996) was used to test the hindlimb function of injured rats following SCI at both T10 and L1/2. BBB LRS testing began at 2 days after SCI and continued weekly for up to 4 (L1/L2) or 5 (T10) weeks post-SCI. Assessments were made by individuals blinded to treatment groups.
1.2.5 Spinal Cord Tissue Processing
Rats were overdosed with 0.2 ml Fatal-Plus solution containing sodium pentobarbital (390mg/ml) (Vortech Pharma Ltd, Dearborn, MI) and transcardially perfused with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde (PFA) in PBS. To maintain consistent sampling, each spinal cord from the L1/L2 injury cohorts was transected at the rostral T11 spinal root and a 30 mm segment of spinal cord was immediately dissected and post fixed for 2–4 hr in 4% PFA/PBS at 4°C. This was followed by overnight washing 0.1 M PB at 4°C to remove excess 4% PFA before cryoprotecting in 20% sucrose/PBS at 4°C and embedding tissues as described (Rabchevsky et al., 2002, Rabchevsky et al., 2007). Frozen spinal cords were then serially cryosectioned at 20 μm in the coronal plane and processed for stereological assessment of tissue sparing and neuronal cell counts.
1.2.6 Histology and Immunohistochemistry
A modified eriochrome cyanine-cresyl violet (EC-CV) staining protocol for myelin that differentiates both white matter and cell bodies was used to calculate the amount of spared spared white matter in transaxial sections of injured spinal cord from all the experimental groups (Rabchevsky et al., 2002, Rabchevsky et al., 2007). Each series of EC-CV stained sections was assessed for spared tissue based on positive staining for myelin or normal cytoarchitecture of gray matter using Scion Imaging analysis software (Scion Corporation, Frederick, MD). All histological analyses were assessed blindly with respect to treatment. Using the Cavalieri method (Michel and Cruz-Orive, 1988), the volumes of injured/spared tissues was first calculated from a series of evenly spaced sections (1 mm apart) centered on the injury site. To accomplish this, cross sectional area measurements of spared gray and white matter were each quantified separately to calculate their respective subvolumes in the injured spinal cords (Rabchevsky et al., 2002). The % tissue sparing at the injury epicenter was calculated (Rabchevsky et al., 1999, Rabchevsky et al., 2000) by dividing the total circumferential area of the spinal cord section minus the area of injured tissue by the total circumferential area of the spinal cord, multiplied by 100.
Randomly chosen slide series mounted with evenly spaced tissue sections were processed for immunohistochemical staining of choline acetyltransferase (ChAT)-positive neurons in spinal cord gray matter. Sections were pre-incubated in 0.1M PBS containing 5% normal donkey serum (Vector Laboratories, Burlingame, CA), and 0.2% triton-X (Ttx) for one hour at room temperature. They were then incubated overnight at 4°C with polyclonal Goat Anti-ChAT (Millipore, Billerica, MA) diluted in blocking buffer. The next day, the slides were thoroughly rinsed and incubated with anti-goat Texas Red (Jackson Immuno, Philadelphia, PA) in 0.1M PBS and 0.2%Ttx overnight at 4°C. The slides were rinsed again and coverslipped with Vectashield (Vector Laboratories, Burlingame, CA) containing 5 μM Hoechst 33342 nuclear dye (Sigma, St. Louis, MO) for visualizing cellular distribution under an Olympus BX51 microscope (Olympus Corp. Melville, NY) equipped with epifluorescent illumination and connected to an Olympus `Magnafire' digital camera (Olympus Corp., Melville, NY, USA) to capture images.
1.2.7 Stereological Quantification of ChAT-Positive Neurons
We initially counted ChAT-positive cells in the bilateral ventral gray matter and found no differences between the Saline- versus ALC-treated groups. We subsequently analyzed Lamina X regions using the same rostral and caudal sections by employing the optical fractionator technique (West et al., 1991) to the same serial sections.
The area of Lamina X in each cross section, containing presumptive central pattern generator (CPG) neurons, was carefully circumscribed and measured using Bioquant® image analysis computer program (Nova Prime, V6.70.10; Bioquant Image Analysis Corp., Nashville, TN) using an Olympus BX51 microscope (Olympus Corp., Melville, NY), and images were captured using an Optronics digital video camera (Optronics Corp., Goleta, CA). For each section, the rectangular region of interest (lamina X) was standardized by using the central canal as a reference point (see schematic representation in Figure 7). For each analyzed section, a uniform counting grid was placed over the region of interest with a counting frame setting of 150 × 150 μm (22,500 μm2). The disector area was set at 50 × 50 μm (2,500 μm2) and the height of the disector was set at 10 μm, with a 2 μm thick guard zone on the top and bottom. The criteria for ChAT-positive cell body inclusion were positive immunostaining of the soma and a prominent nucleus, visualized with Hoechst nuclear dye under separate UV emission. To count labeled cells, each disector was viewed under 40× objective magnification (10× eye piece). As detailed previously (Hou et al., 2008), all cell nuclei within the counting frame or touching the upper or right inclusive sides of the frame were counted through the depth of the disector. Nuclei touching the left or lower forbidden sides of the frame were not counted. All cells with nuclei in focus within the 10 μm disector were counted. The total number of cells in each cord was calculated using the specific parameters detailed above and our published equation (McCullers et al., 2002).
1.2.8 Statistical Analysis
For mitochondrial bioenergetics and histology experiments, differences among sham, injured vehicle-treated, and injured ALC-treated groups were investigated using analysis of variance (ANOVA) and the Newman-Keuls post-hoc when warranted. Two-way repeated measures ANOVA analyses were used to determine statistical differences among the data collected over time (behavioral data) or across spinal cord levels (cell counts). Significance was set at p < 0.05 for all analyses.
1.3 RESULTS
1.3.1 Effects of ALC on Mitochondrial Bioenergetics
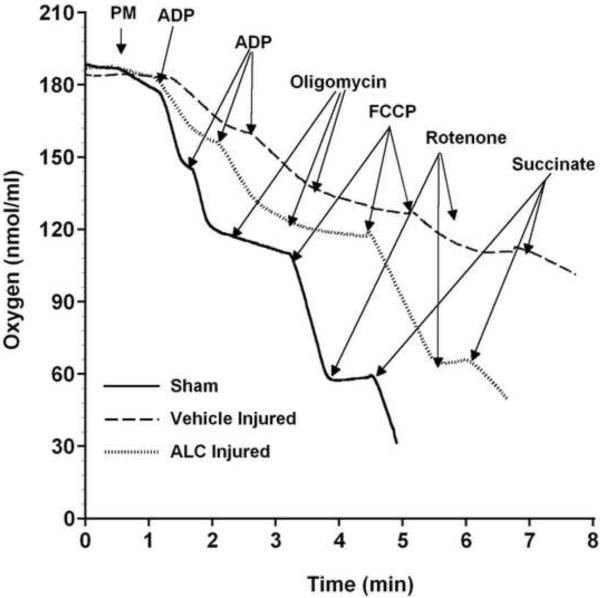
ALC or saline was injected (i.p.) 15 min after L1/L2 SCI, alongside sham operations. Mitochondria were then isolated 24 hr later and subjected to assessments of bioenergetics in terms of respiration rates (oxygen consumption) under various experimental conditions. Typical respiration traces taken at 24 hrs post-laminectomy from sham versus ALC- or saline (vehicle)-treated injured rats are shown in Figure 1. Dramatically compromised mitochondrial respiratory activity was observed in vehicle-treated injured rats compared to shams, whereas ALC treatment conspicuously maintained mitochondrial respiration rates.
Figure 1.
Effects of ALC on mitochondrial respiration following L1/L2 contusion SCI. Representative oxymetric traces show the profound loss of respiration in mitochondria isolated from vehicle-treated injured spinal cords ( ) compared to shams (
) compared to shams ( ). Treatment with ALC 15 min post-injury increased mitochondrial respiration (
). Treatment with ALC 15 min post-injury increased mitochondrial respiration ( ) compared to vehicle treatment. Mitochondrial respiration was assessed by addition of substrates and inhibitors for enzyme complexes of mitochondrial electron transport system (ETS). State II respiration is initiated using complex I (NADH-linked) substrates pyruvate and malate (PM). This is followed by the addition of ADP (state III) to the chamber, which is rapidly converted to ATP in spinal cord mitochondria, resulting in state IV respiration. The addition of oligomycin locks the mitochondria in state IV respiration. Maximum NADH-linked ETS activity/capacity is then assessed by the addition of uncoupler FCCP [state V (complex-I)]. Complex I respiration is then inhibited by the addition of rotenone (Rot) followed by complex II-driven maximum ETS activity [state V (complex II)] assessed by addition of succinate.
) compared to vehicle treatment. Mitochondrial respiration was assessed by addition of substrates and inhibitors for enzyme complexes of mitochondrial electron transport system (ETS). State II respiration is initiated using complex I (NADH-linked) substrates pyruvate and malate (PM). This is followed by the addition of ADP (state III) to the chamber, which is rapidly converted to ATP in spinal cord mitochondria, resulting in state IV respiration. The addition of oligomycin locks the mitochondria in state IV respiration. Maximum NADH-linked ETS activity/capacity is then assessed by the addition of uncoupler FCCP [state V (complex-I)]. Complex I respiration is then inhibited by the addition of rotenone (Rot) followed by complex II-driven maximum ETS activity [state V (complex II)] assessed by addition of succinate.
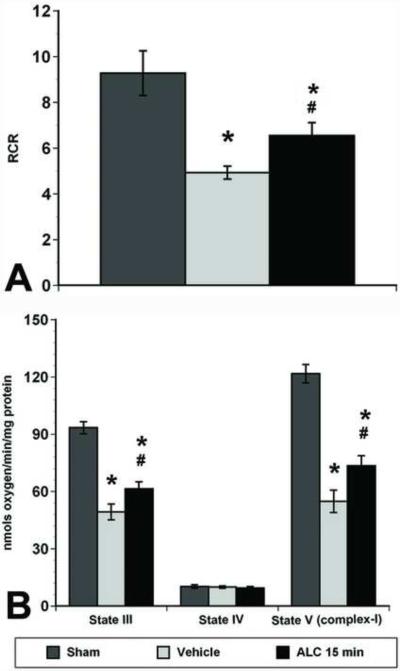
The respiratory control ratios (RCR), one of the most sensitive measurements of mitochondrial integrity, were calculated as the ratio of state III (presence of ADP) to state IV (absence of ADP and/or presence of oligomycin) respiration (Figure 2A). Significant differences in mitochondrial RCR [F(2,9)=29.43, p<0.001] were noted after SCI. Post hoc analysis confirmed that ALC treatment significantly (p<0.05) maintained mitochondrial integrity compared to vehicle treatment 24 hrs after SCI, though the values remained significantly (p<0.05) lower than shams.
Figure 2.
Effects of ALC on mitochondrial bioenergetics following L1/L2 contusion spinal cord injury. A. The respiratory control ratio (RCR; calculated as the ratio of State III / State IV slopes) is a measure of mitochondrial integrity and coupling of the ETS to oxidative phosphorylation. The RCR was significantly decreased in vehicle-treated injured rats at 24 hrs post-injury, whereas ALC treatment 15 min and 6 hrs post-injury significantly (p<0.05) maintained the RCR after 24 hrs. B. Respiration rates from mitochondria were calculated as nmols oxygen/min/mg protein 24 hrs post-injury following treatment with either vehicle or ALC. Compared to the significantly impaired mitochondrial respiration rates (State III & State V (complex I)) observed with vehicle treatment, ALC treatment significantly (p<0.05) preserved mitochondrial respiration rates. However, the RCR and respiration rates (State III and State V) for all the ALC-treated groups remained significantly (p<0.05) lower than shams. Bars represent group means ± SEM, n=4/group. *p<0.05 compared to sham group; <# p<0.05 compared to vehicle-treated injured group.
Quantification of mitochondrial respiration rates showed a significant decrease in ADP phosphorylation (state III) [F(2, 9)=78.72, p<0.0001] and NADH-linked (complex I-driven) ETS capacity (State V-complex I) [F(2,9)=90.119, p<0.0001] following SCI (Figure 2B). Post-hoc analysis revealed a significant decrease in both State III and State V-complex I respiration in vehicle-treated injured spinal cord mitochondria compared to Shams. Treatment with ALC significantly (p<0.05) maintained state III and state V-complex I respiration compared to vehicle-treated injured rats, although the values remained significantly lower than Shams. State V-complex II respiration rates were not statistically different across the groups (data not shown). These results are comparable to our previous report after T10 SCI followed by ALC treatment (Patel et al., 2010).
1.3.2 Effects of ALC Treatment on Behavioral Recovery
Based on the neuroprotective properties of ALC that we previously documented (Patel et al., 2010), we assessed the efficacy of ALC treatment on functional recovery after T10 SCI. The results showed significant acute bilateral hindlimb impairment that increased over time post-injury in both groups, but terminal BBB scores reflected weight-supported hindlimb stepping that were not significantly different [F(1,10)=0.816, p>0.5] in ALC- versus vehicle-treated animals. Since the hindlimb deficits observed following T10 SCI are likely mediated more by damage to surrounding white matter rather than central gray matter, we next chose to carryout experiments using an injury model with functional locomotor consequences due to gray matter loss/sparing. Rats were contused at upper lumbar (L1/L2) spinal cord levels because this region contains propriospinal neurons suggested to be involved in locomotion in rats (Kremer and Lev-Tov, 1997, Magnuson et al., 1998, Bertrand and Cazalets, 2002, Magnuson et al., 2005).
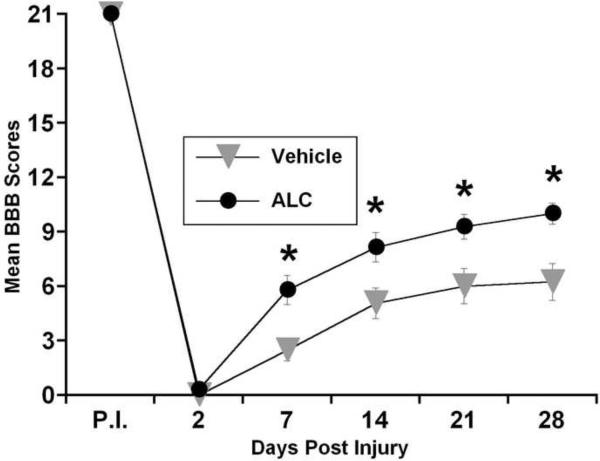
At day 2 post-injury, all injured animals demonstrated complete hindlimb paralysis (Figure 3). Compared to vehicle-treated injured animals, ALC treatment promoted enhanced recovery in hindlimb movements as early as 7 days post-injury. Critically, after 4 weeks, ALC-treated injured rats were able to consistently step with weight support compared to vehicle-treated rats that demonstrated only slight movement of hindlimb joints. There was a significant treatment x days effect on BBB scores [F (1, 26) = 7.151; p<0.05], and post-hoc analysis showed that ALC treatment significantly (p<0.05) improved hindlimb locomotor function (BBB scores) compared to vehicle controls, beginning 7 days-post SCI. Importantly, ALC-treated rats were able to functionally walk with hindlimb weight bearing, whereas vehicle-treated injured rats demonstrated only slight hindlimb movements.
Figure 3.
Graphic representation of the mean behavioral (BBB) scores over time post injury for vehicle- and ALC-treated rats before and after L1/L2 spinal cord injury. ALC treatment significantly improved hindlimb locomotor function beginning 7 days-post injury and after 3 weeks of daily ALC treatment, injured rats were able to consistently step with weight support on their hindlimbs compared to vehicle-treated rats that demonstrated only slight movement of hindlimb joints. These results show that with ALC treatment the early preservation of spinal cord tissue from secondary damage after injury was the important factor for the functional recovery over time. Symbols represent group means ± SEM, n=14 per group. *p<0.05 compared to vehicle-treated injured group.
1.3.3 Effects of ALC Treatment on Tissue Sparing
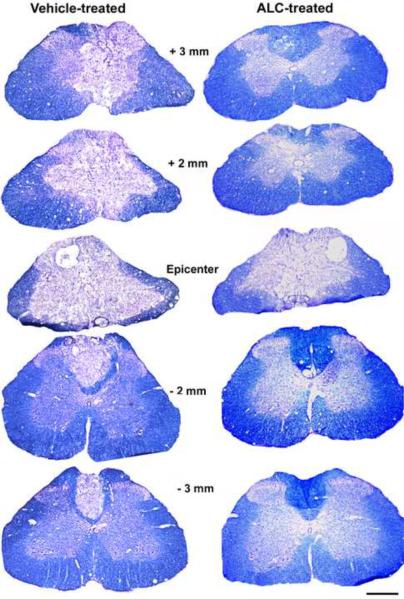
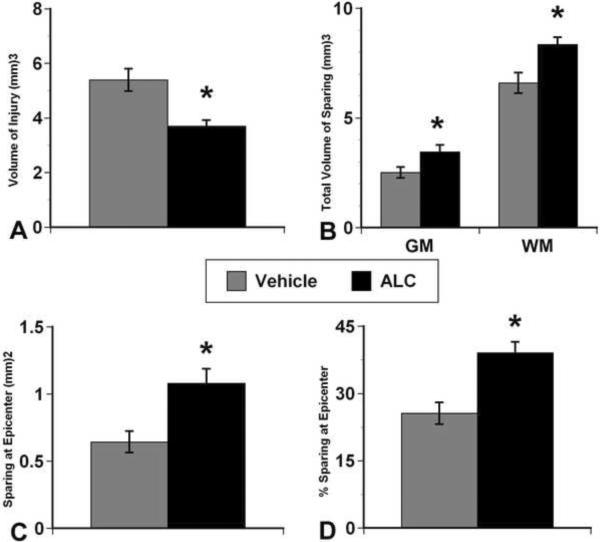
Histological assessment of spinal cord sections revealed remarkable tissue sparing with ALC compared to vehicle (Figure 4). Stereological analyses showed that the ALC-treated injured group had significantly (p<0.05) less injured tissue volume compared to vehicle treatment (Figure 5A), reflected in significant (p<0.05) sparing of both gray matter and white matter volumes (Figure 5B). ALC significantly (p<0.05) increased tissue sparing at the injury epicenter (Figures 5C & D), and gray and white matter sparing were more prominent rostral to the epicenter in the ALC-treated group (not shown).
Figure 4.
Effects of ALC on tissue sparing 4 weeks after L1/L2 contusion spinal cord injury. Photomicrographs of EC-CV stained injured spinal cord sections illustrating that the ALC-treated injured group demonstrated increased sparing of spinal cord tissue at the injury epicenter, as well as rostral (+) and caudal (−) compared to vehicle treatment.
Figure 5.
Morphometric quantification of histopathology 4 weeks post-injury. A. The ALC treatment group showed less volume of injured tissue compared to the vehicle-treated group. B. Accordingly, ALC treatment significantly spared both gray matter (GM) and white matter (WM) tissues. C and D demonstrate that ALC significantly increased tissue sparing at the injury epicenter. Bars represents mean ± SEM, n=14 per group. * p<0.05 compared to vehicle-treated injured group.
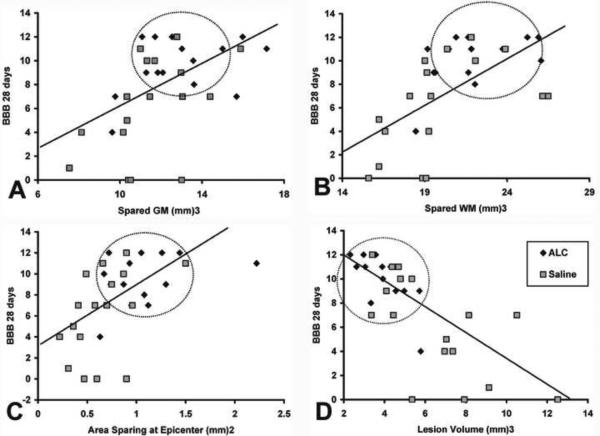
The correlation analyses of final BBB scores versus spared gray matter, white matter, tissue at epicenter and/or lesion volume are all shown in Figure 6. Significant (p<0.005) positive correlations were found between the higher BBB scores and increased sparing of gray matter, white matter and at epicenter (r=0.520, 0.652 and 0.546, respectively). Conversely, a significant (p<0.0001) negative correlation (r=0.761) was found between BBB scores and lesion volumes. Notably, encircled symbols in Figure 6A–D illustrate that ALC-treated injured animals showed significant neuroprotection that was positively correlated with higher BBB scores compared to vehicle treatment.
Figure 6.
Correlation analysis: spared tissues and lesion volume versus BBB scores. Regression plots for final mean BBB scores versus (A) spared gray matter (GM), (B) spared white matter (WM), tissue sparing at injury epicenter (C) and lesion volume (D). Significant positive correlations were found between final BBB scores and spared gray matter (p<0.005), white matter (p<0.0001) and spared tissue at epicenter (p<0.005), while total injured tissue volume showed a significant negative correlation with final BBB scores (p<0.0001). Notably, encircled symbols illustrate that increased tissue sparing with ALC treatment was significantly correlated with higher BBB scores, compared to vehicle treatment.
1.3.4 Effects of ALC Treatment on ChAT-Positive Cell Counts
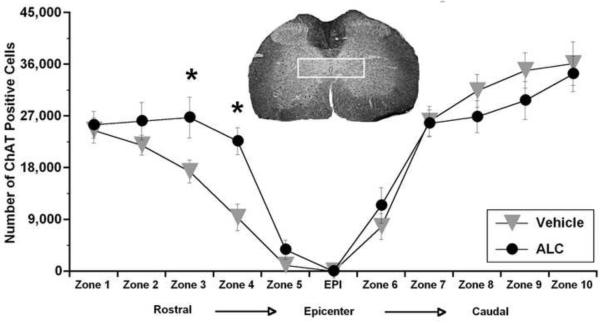
Preliminary stereological quantification of ChAT-positive cells in bilateral ventral horns rostral and caudal to the L1/L2 injury epicenter after 4 weeks showed no significant difference between treatment groups (565,238 ± 60,574 for ALC; 582,400 ± 59,089 vehicle; n=8/group). This indicated that sparing of lower motor neurons was not correlated with differential BBB scores. We next focused on presumptive CPG neurons in lamina X and found that the number of ChAT-positive cells was conspicuously higher in the ALC-treated group rostral to the injury epicenter compared to vehicle treatment, whereas no differences were apparent in caudal regions (Figure 7). A repeated measures ANOVA for ChAT-positive cell numbers in lamina X across all zones (each representing 1 mm3 segments rostral and caudal to the injury epicenter) confirmed a significant treatment effect rostral to the injury [F (1, 26) = 6.806; p<0.05], but no differences were found caudal [F (1, 26) = 0.249; p<0.5]. Post-hoc analysis confirmed significantly (p<0.05) higher ChAT-positive cell counts in rostral zones 3 and 4 in the ALC treatment group (Figure 7).
Figure 7.
Effects of ALC on ChAT-positive neurons in Lamina X after L1/L2 contusion spinal cord injury. When assessed stereologically as total cell numbers across zones in lamina X (each zone on the X-axis represents 1mm in length between analyzed sections), ChAT-positive cell numbers were significantly (p<0.05) higher in the zones rostral to the injury epicenter (EPI) after ALC treatment compared to vehicle; however, no differences were noted caudal to the epicenter. White box in spinal cord cross section indicates region of interest for ChAT-positive cell counting (lamina X). Symbols represent group means ± SEM. n=14 per group. *p<0.05 compared to vehicle-treated injured group.
Collectively, these results demonstrate that after severe contusion SCI, improved mitochondrial function following ALC treatment may be associated with greater tissue sparing at the injury site, as well as direct neuroprotection of lamina X neurons and white matter, notably rostral to the injury site. Such pronounced histological preservation ultimately resulted in the extraordinary recovery of weight-bearing hindlimb function.
1.4 DISCUSSION
The present study is an extension of our previous reports which all confirm that mitochondrial dysfunction plays a pivotal role in the development of secondary pathophysiological events following experimental contusion SCI (Sullivan et al., 2007, Patel et al., 2009, Patel et al., 2010). In the last couple of decades, many of the neurochemical and pathophysiological components of secondary injury cascades following SCI have been elucidated. This has resulted in the testing of several potential “neuroprotective” pharmacological strategies that target different aspects of the acute neurodegenerative processes in animal models and, more importantly, human clinical trials for SCI (Hall and Springer, 2004, McEwen et al., 2011). In spite of many promising therapeutics showing beneficial effects in animal models of SCI, most have not translated into clinically effective therapies. The exception is methylprednisolone sodium succinate (MPSS), the only clinically approved compound reported to show modest efficacy on certain functional outcome measures when administered within the first eight hours after SCI (Bracken et al., 1984, Bracken et al., 1985, Bracken, 1990, Bracken et al., 1990, Bracken et al., 1992, Bracken et al., 1997, Bracken, 2001). Experimentally, it has been reported that MPSS acts by reducing lipid peroxidation (Hall and Braughler, 1982, Braughler and Hall, 1992, Hall, 1992, 1993, Diaz-Ruiz et al., 2000) and inflammatory cytokine production (Xu et al., 1998). However, such effects have demonstrated inconsistent efficacy regarding both neuroprotection and/or functional recovery in rodent models of SCI (Akdemir et al., 1993, Liu and McAdoo, 1993, Ross and Tator, 1993, Ross et al., 1993, Behrmann et al., 1994, Koc et al., 1999, Diaz-Ruiz et al., 2000, Chikawa et al., 2001, Rabchevsky et al., 2002). Such lack of consistency in experimental and clinical evidence for neurological improvement, in addition to evidence of increased mortality and morbidity by high dose of MPSS, has resulted in the reevaluation of its clinical use for acute SCI (Hurlbert, 2000, Short et al., 2000).
It is apparent that multiple independent, yet interrelated biochemical and pathophysiological pathways are involved in the secondary injury cascade following contusion SCI. Thus, to foster neuroprotection against such complex events would appear to require systematic and multi-targeted strategies. However, most treatment approaches to date have focused on the inhibition or promotion of particular steps in one of many molecular signaling cascades, with little to no attention focused on the critical role that compromised organelle systems have in neurodegenerative processes (McEwen et al., 2011). Accordingly, in the current study we targeted one of the principle sources of free radical production, the mitochondria. This approach was based entirely on our recent reports that mild uncoupling of mitochondria after contusion SCI significantly reduces their oxidative damage (Patel et al., 2009) and, more critically, that post traumatic supplementation of ALC resulted in improved bioenergetics and significant spinal cord tissue sparing (Patel et al., 2010).
Increased excitotoxicity after SCI promotes increased oxidative stress and depletion of the endogenous anti-oxidant, GSH (Kamencic et al., 2001, Lucas et al., 2002). Since mitochondria are a primary source of free radical production, as well as themselves being targets for free radical attack, they play an important role in governing neuronal cell death/survival after SCI (Finkel, 2001, Hunot and Flavell, 2001, Sullivan et al., 2005). Specifically, we have reported that oxidative stress after SCI results in diminished activities of key mitochondrial enzyme complexes; i.e. pyruvate dehydrogenase complex (PDHC), NADH dehydrogenase (complex I) and cytochrome oxidase (complex IV) (Patel et al., 2009, Patel et al., 2010). In particular, inactivation and dysfunction of PDHC leads to metabolic failure and increased oxidative stress in neurodegenerative disorders and CNS injury models (Blass and Gibson, 1978, Sorbi et al., 1983, Ksiezak-Reding et al., 1984, Sheu et al., 1985, De Meirleir et al., 1993, Zaidan et al., 1998, Bogaert et al., 2000, Pocernich and Butterfield, 2003, Rosenthal and Henderson, 2003, Martin et al., 2005, Richards et al., 2006, Opii et al., 2007, Robertson et al., 2007). Compromised PDHC activity, consequently, could result in decreased conversion of pyruvate into acetyl-CoA required for the TCA cycle to synthesize the reducing equivalent NADH, essential for mitochondrial energy production in the form of ATP.
ALC is involved in mitochondrial energy metabolism by increasing mitochondrial acetyl CoA by directly contributing acetyl group to coenzyme A to form acetyl CoA and free carnitine (Jones et al., 2010, Scafidi et al., 2010a). Free carnitine helps in carnitine/acylcarnitine exchange across the mitochondrial membrane catalyze by carnitine-acylcarnitine translocase as well as by intramitochondrial exchange of carnitine/acylcarnitine by carnitine palmitoyl transferase II to form acylCoA which ultimately forms acetyl CoA via β-oxidation (Pettegrew et al., 2000) which, in turn, can increases the pool of NADH and FADH2, thus acting as an alternative bio-fuel (Patel et al., 2010). Moreover, ALC is also reported to stabilize mitochondrial membrane composition by the synthesis of lipids/phospholipids (Paradies et al., 1992, Aureli et al., 2000, Hao et al., 2011). ALC supplementation also reverses age-related alterations in the expression of ten mitochondrial proteins that participate in oxidative phosphorylation and antioxidant systems in rat liver (Musicco et al., 2011). Consequently, we reported that ALC treatment following acute SCI significantly preserved the activities of key mitochondrial enzymes, notably PDHC, complex I and complex IV (Patel et al., 2010), likely due to reduced protein oxidation (Patel et al., 2009). Alternatively, ALC may possess anti-inflammatory and anti-excitatory properties which could also reduce the secondary injury cascade (Arafa et al., 2009, Tufekci et al., 2009, Hota et al., 2011).
Although acute ALC administration improved mitochondrial bioenergetics and increased tissue sparing following T10 contusion SCI (Patel et al., 2010), results from the present study show that daily ALC treatment did not improve long-term behavioral recovery. This may be due to the fact that neurons at mid-thoracic spinal levels do not directly participate in generating hindlimb movements during locomotion that are assessed by the BBB LRS (Magnuson et al., 1998). Thus, the hindlimb deficits observed following T10 SCI are primarily mediated by damage to surrounding white matter rather than central gray matter, notably innervating trunk musculature.
On the contrary, in addition to improved mitochondrial bioenergetics with acute ALC treatment, we found that that similar ALC treatment significantly restored hindlimb weight bearing and functional locomotion following L1/L2 contusion SCI. Importantly, the upper lumbar injury paradigm induced hindlimb locomotor deficits due to damage of critical locomotor circuitry (central pattern generating circuitry) that extends from the low thoracic (T12/T13) to mid-lumbar (L3/L4) segments in the rat spinal cord (Kremer and Lev-Tov, 1997, Magnuson et al., 1998, Bertrand and Cazalets, 2002, Magnuson et al., 2005). We submit that, by sparing white matter at the injury epicenter and gray matter over the injury length, notably rostral to the injury, the treatment likely spared intersegmental short distance propriospinal neurons that, in turn, lead to improved hindlimb function (Bareyre et al., 2004, Conta Steencken and Stelzner, 2010). Importantly, ALC significantly improved functional recovery (BBB scores) within first week of administration, supporting reports that maximal propriospinal axonal loss occurs by two weeks post-contusion SCI (Conta Steencken and Stelzner, 2010). Accordingly, our findings indicate that even a small, yet significant amount of gray matter sparing over the injury length following ALC treatment had a major impact on long-term functional recovery.
Correlation analyses between terminal BBB scores and spared spinal cord tissues provided strong evidence that improved hindlimb locomotion with ALC treatment following injury was a direct result of greater tissue sparing. Stereological quantification showed no differences in the number of ChAT-positive cells located in ventral horn gray matter following ALC treatment, indicating no effects on lower motor neurons innervating hindlimb musculature. However, significantly higher numbers of ChAT-positive cells were found in lamina X neurons within the presumptive locomotor circuitry, notably rostral to the injury site. The sparing of such critical upper lumbar interneurons with ALC treatment appeared to have a direct impact on the significant hindlimb functional recovery. These data are supported by reports that the extreme lower thoracic and upper lumbar spinal cord segments are the key areas for the genesis of locomotor activity in neonatal rats (Cazalets et al., 1995, 1996, Kjaerulff and Kiehn, 1996, Cowley and Schmidt, 1997). It has also been reported that specific glutamate receptor blockers alter locomotor patterns generated by lumbar regions when applied to the rostral segments of isolated neonatal spinal cord (Cazalets et al., 1996). Likewise, in adult rats, it has been shown that kainic acid-induced interneuron loss centered on the L2 segment induces lasting paraplegia independent of motor neuron loss or white matter damage (Hadi et al., 2000). Because more prominent oxidative damage (4-hydroxynonenal expression) is found caudal versus rostral to a contusion SCI (Baldwin et al., 1998) the higher oxidative stress below the injury may render a greater threshold for any neuroprotective agent, in this report ALC. Accordingly, ongoing studies include analyses of mitochondrial oxidative markers in this SCI model with or without ALC treatment.
Collectively, our data demonstrate that therapeutic interventions such as ALC that can target mitochondrial dysfunction hold great potential as pharmacological therapies for acute SCI. In particular, since SCI triggers a series of complex destructive biochemical and pathophysiological events, we believe that traditional pharmacological strategies targeting specific downstream events may not provide the best approach to foster neuroprotection following SCI (Rabchevsky et al., 2011). Moreover, results from the present study assessing two different injury sites (T10 & L1/L2) illustrates that selection of appropriate injury paradigms is vital for evaluating experimental neuroprotective strategies. Thus, in order to further investigate the efficacy of potential therapeutics for acute SCI, it will be necessary to employ combinatorial approaches using known pharmacological agents that target mitochondrial dysfunction, such as the mitochondrial uncoupler, 2,4-dinitro phenol (Patel et al., 2009), or N-methyl-isoleucine-cyclosporin (NIM811) (McEwen et al., 2007, Ravikumar et al., 2007), which blocks the mitochondrial permeability transition pore, in combination with alternative bio-fuel substrates such as ALC or β-hydroxybutyrate (ketones). In addition, based on our previous results showing improved mitochondrial function with starting dose of ALC 1 hr post-injury (Patel et al., 2010), ongoing study evaluate the effects of ALC on long-term behavioral studies with the starting dose at 1 hr post-SCI to make it more clinically relevant. Looking ahead, it will be equally important to examine the differential effects of such neuroprotective agents on synaptic (neuronal) versus non-synaptic (neuronal soma and glial) mitochondria to pinpoint key mitochondrial events as potential targets for interventions to more effectively treat SCI and, perhaps, other CNS injuries and/or disease states.
Highlights
Acetyl-l-carnitine maintained mitochondrial function in spinal cord injured rats.
Daily ALC treatment increased tissue sparing and hindlimb locomotion after SCI.
ALC fostered specific neuroprotection of cholinergic neurons in spinal cord lamina X.
Sparing of “CPG” propriospinal neurons was associated with behavioral recovery.
Targeting mitochondrial dysfunction with ALC after SCI renders neuroprotection.
ACKNOWLEDGMENTS
We are thankful for the expert technical assistance of Chris R. O'Dell and Khalid C. Eldhan. This study was supported by KSCHIRT #8-13 (AGR), NIH/NINDS R01NS069633 (AGR & PGS), NIH/NINDS P30 NS051220 and a generous donation from the Michael and Helen Schaffer Foundation, Boston, MA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akdemir H, Pasaoglu H, Arman F, Coksevim B, Pasaoglu A. Effects of TRH and high-dose corticosteroid therapy on evoked potentials, and tissue Na+,K+ and water content in experimental spinal injury. Res Exp Med, (Berl) 1993;193:297–304. doi: 10.1007/BF02576237. [DOI] [PubMed] [Google Scholar]
- Arafa HM, Hemeida RA, Hassan MI, Abdel-Wahab MH, Badary OA, Hamada FM. Acetyl-L-carnitine ameliorates caerulein-induced acute pancreatitis in rats. Basic Clin Pharmacol Toxicol. 2009;105:30–36. doi: 10.1111/j.1742-7843.2009.00399.x. [DOI] [PubMed] [Google Scholar]
- Aureli T, Di Cocco ME, Capuani G, Ricciolini R, Manetti C, Miccheli A, Conti F. Effect of long-term feeding with acetyl-L-carnitine on the age-related changes in rat brain lipid composition: a study by 31P NMR spectroscopy. Neurochem Res. 2000;25:395–399. doi: 10.1023/a:1007501306623. [DOI] [PubMed] [Google Scholar]
- Aureli T, Puccetti C, Di Cocco ME, Arduini A, Ricciolini R, Scalibastri M, Manetti C, Conti F. Entry of [(1,2-13C2)acetyl]-L-carnitine in liver tricarboxylic acid cycle and lipogenesis: a study by 13C NMR spectroscopy in conscious, freely moving rats. Eur J Biochem. 1999;263:287–293. doi: 10.1046/j.1432-1327.1999.00524.x. [DOI] [PubMed] [Google Scholar]
- Azbill RD, Mu X, Bruce-Keller AJ, Mattson MP, Springer JE. Impaired mitochondrial function, oxidative stress and altered antioxidant enzyme activities following traumatic spinal cord injury. Brain Res. 1997;765:283–290. doi: 10.1016/s0006-8993(97)00573-8. [DOI] [PubMed] [Google Scholar]
- Baldwin SA, Broderick R, Osbourne D, Waeg G, Blades DA, Scheff SW. The presence of 4-hydroxynonenal/protein complex as an indicator of oxidative stress after experimental spinal cord contusion in a rat model. J Neurosurg. 1998;88:874–883. doi: 10.3171/jns.1998.88.5.0874. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ, Nockels R, Perot PL, Salzman SK, Young W. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. Journal of neurotrauma. 1996;13:343–359. doi: 10.1089/neu.1996.13.343. [DOI] [PubMed] [Google Scholar]
- Behrmann DL, Bresnahan JC, Beattie MS. Modeling of acute spinal cord injury in the rat: neuroprotection and enhanced recovery with methylprednisolone, U-74006F and YM-14673. Experimental neurology. 1994;126:61–75. doi: 10.1006/exnr.1994.1042. [DOI] [PubMed] [Google Scholar]
- Bertrand S, Cazalets JR. The respective contribution of lumbar segments to the generation of locomotion in the isolated spinal cord of newborn rat. Eur J Neurosci. 2002;16:1741–1750. doi: 10.1046/j.1460-9568.2002.02233.x. [DOI] [PubMed] [Google Scholar]
- Blass JP, Gibson GE. Studies of the pathophysiology of pyruvate dehydrogenase deficiency. Adv Neurol. 1978;21:181–189. [PubMed] [Google Scholar]
- Bogaert YE, Sheu KF, Hof PR, Brown AM, Blass JP, Rosenthal RE, Fiskum G. Neuronal subclass-selective loss of pyruvate dehydrogenase immunoreactivity following canine cardiac arrest and resuscitation. Experimental neurology. 2000;161:115–126. doi: 10.1006/exnr.1999.7250. [DOI] [PubMed] [Google Scholar]
- Bonavita E. Study of the efficacy and tolerability of L-acetylcarnitine therapy in the senile brain. Int J Clin Pharmacol Ther Toxicol. 1986;24:511–516. [PubMed] [Google Scholar]
- Bracken MB. Methylprednisolone in the management of acute spinal cord injuries. Med J Aust. 1990;153:368. [PubMed] [Google Scholar]
- Bracken MB. Methylprednisolone and acute spinal cord injury: an update of the randomized evidence. Spine. 2001;26:S47–54. doi: 10.1097/00007632-200112151-00010. [DOI] [PubMed] [Google Scholar]
- Bracken MB, Collins WF, Freeman DF, Shepard MJ, Wagner FW, Silten RM, Hellenbrand KG, Ransohoff J, Hunt WE, Perot PL, Jr., et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA. 1984;251:45–52. [PubMed] [Google Scholar]
- Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, Eisenberg HM, Flamm E, Leo-Summers L, Maroon J, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- Bracken MB, Shepard MJ, Collins WF, Jr., Holford TR, Baskin DS, Eisenberg HM, Flamm E, Leo-Summers L, Maroon JC, Marshall LF, et al. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second National Acute Spinal Cord Injury Study. J Neurosurg. 1992;76:23–31. doi: 10.3171/jns.1992.76.1.0023. [DOI] [PubMed] [Google Scholar]
- Bracken MB, Shepard MJ, Hellenbrand KG, Collins WF, Leo LS, Freeman DF, Wagner FC, Flamm ES, Eisenberg HM, Goodman JH, et al. Methylprednisolone and neurological function 1 year after spinal cord injury. Results of the National Acute Spinal Cord Injury Study. J Neurosurg. 1985;63:704–713. doi: 10.3171/jns.1985.63.5.0704. [DOI] [PubMed] [Google Scholar]
- Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, Fehlings M, Herr DL, Hitchon PW, Marshall LF, Nockels RP, Pascale V, Perot PL, Jr., Piepmeier J, Sonntag VK, Wagner F, Wilberger JE, Winn HR, Young W. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. Jama. 1997;277:1597–1604. [PubMed] [Google Scholar]
- Braughler JM, Hall ED. Involvement of lipid peroxidation in CNS injury. Journal of neurotrauma. 1992;9(Suppl 1):S1–7. [PubMed] [Google Scholar]
- Cazalets JR, Borde M, Clarac F. Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. J Neurosci. 1995;15:4943–4951. doi: 10.1523/JNEUROSCI.15-07-04943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalets JR, Borde M, Clarac F. The synaptic drive from the spinal locomotor network to motoneurons in the newborn rat. J Neurosci. 1996;16:298–306. doi: 10.1523/JNEUROSCI.16-01-00298.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikawa T, Ikata T, Katoh S, Hamada Y, Kogure K, Fukuzawa K. Preventive effects of lecithinized superoxide dismutase and methylprednisolone on spinal cord injury in rats: transcriptional regulation of inflammatory and neurotrophic genes. Journal of neurotrauma. 2001;18:93–103. doi: 10.1089/089771501750055802. [DOI] [PubMed] [Google Scholar]
- Conta Steencken AC, Stelzner DJ. Loss of propriospinal neurons after spinal contusion injury as assessed by retrograde labeling. Neuroscience. 2010;170:971–980. doi: 10.1016/j.neuroscience.2010.06.064. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. Regional distribution of the locomotor pattern-generating network in the neonatal rat spinal cord. J Neurophysiol. 1997;77:247–259. doi: 10.1152/jn.1997.77.1.247. [DOI] [PubMed] [Google Scholar]
- De Meirleir L, Lissens W, Denis R, Wayenberg JL, Michotte A, Brucher JM, Vamos E, Gerlo E, Liebaers I. Pyruvate dehydrogenase deficiency: clinical and biochemical diagnosis. Pediatr Neurol. 1993;9:216–220. doi: 10.1016/0887-8994(93)90088-t. [DOI] [PubMed] [Google Scholar]
- Dhitavat S, Ortiz D, Shea TB, Rivera ER. Acetyl-L-carnitine protects against amyloid-beta neurotoxicity: roles of oxidative buffering and ATP levels. Neurochem Res. 2002;27:501–505. doi: 10.1023/a:1019800703683. [DOI] [PubMed] [Google Scholar]
- Di Cesare Mannelli L, Ghelardini C, Calvani M, Nicolai R, Mosconi L, Toscano A, Pacini A, Bartolini A. Neuroprotective effects of acetyl-L-carnitine on neuropathic pain and apoptosis: A role for the nicotinic receptor. J Neurosci Res. 2008 doi: 10.1002/jnr.21815. [DOI] [PubMed] [Google Scholar]
- Di Cesare Mannelli L, Ghelardini C, Calvani M, Nicolai R, Mosconi L, Vivoli E, Pacini A, Bartolini A. Protective effect of acetyl-L-carnitine on the apoptotic pathway of peripheral neuropathy. Eur J Neurosci. 2007;26:820–827. doi: 10.1111/j.1460-9568.2007.05722.x. [DOI] [PubMed] [Google Scholar]
- Diaz-Ruiz A, Rios C, Duarte I, Correa D, Guizar-Sahagun G, Grijalva I, Madrazo I, Ibarra A. Lipid peroxidation inhibition in spinal cord injury: cyclosporin-A vs methylprednisolone. Neuroreport. 2000;11:1765–1767. doi: 10.1097/00001756-200006050-00033. [DOI] [PubMed] [Google Scholar]
- El-Awady el SE, Moustafa YM, Abo-Elmatty DM, Radwan A. Cisplatin-induced cardiotoxicity: Mechanisms and cardioprotective strategies. Eur J Pharmacol. 2011;650:335–341. doi: 10.1016/j.ejphar.2010.09.085. [DOI] [PubMed] [Google Scholar]
- Elanchezhian R, Sakthivel M, Isai M, Geraldine P, Thomas PA. Evaluation of lenticular antioxidant and redox system components in the lenses of acetyl-L-carnitine treatment in BSO-induced glutathione deprivation. Mol Vis. 2009;15:1485–1491. [PMC free article] [PubMed] [Google Scholar]
- Farrell S, Vogel J, Bieber LL. Entry of acetyl-L-carnitine into biosynthetic pathways. Biochim Biophys Acta. 1986;876:175–177. doi: 10.1016/0005-2760(86)90332-2. [DOI] [PubMed] [Google Scholar]
- Finkel E. The mitochondrion: is it central to apoptosis? Science. 2001;292:624–626. doi: 10.1126/science.292.5517.624. [DOI] [PubMed] [Google Scholar]
- Hadi B, Zhang YP, Burke DA, Shields CB, Magnuson DS. Lasting paraplegia caused by loss of lumbar spinal cord interneurons in rats: no direct correlation with motor neuron loss. J Neurosurg. 2000;93:266–275. doi: 10.3171/spi.2000.93.2.0266. [DOI] [PubMed] [Google Scholar]
- Hall ED. The neuroprotective pharmacology of methylprednisolone. J Neurosurg. 1992;76:13–22. doi: 10.3171/jns.1992.76.1.0013. [DOI] [PubMed] [Google Scholar]
- Hall ED. Lipid antioxidants in acute central nervous system injury. Ann Emerg Med. 1993;22:1022–1027. doi: 10.1016/s0196-0644(05)82745-3. [DOI] [PubMed] [Google Scholar]
- Hall ED, Braughler JM. Effects of intravenous methylprednisolone on spinal cord lipid peroxidation and Na+ + K+)-ATPase activity. Dose-response analysis during 1st hour after contusion injury in the cat. J Neurosurg. 1982;57:247–253. doi: 10.3171/jns.1982.57.2.0247. [DOI] [PubMed] [Google Scholar]
- Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004;1:80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Shen W, Sun L, Long J, Sharman E, Shi X, Liu J. Mitochondrial dysfunction in the liver of type 2 diabetic Goto-Kakizaki rats: improvement by a combination of nutrients. Br J Nutr. 2011:1–8. doi: 10.1017/S0007114511000493. [DOI] [PubMed] [Google Scholar]
- Hota KB, Hota SK, Chaurasia OP, Singh SB. Acetyl-L-carnitine-mediated neuroprotection during hypoxia is attributed to ERK1/2-Nrf2-regulated mitochondrial biosynthesis. Hippocampus. 2011 doi: 10.1002/hipo.20934. [DOI] [PubMed] [Google Scholar]
- Hou S, Duale H, Cameron AA, Abshire SM, Lyttle TS, Rabchevsky AG. Plasticity of lumbosacral propriospinal neurons is associated with the development of autonomic dysreflexia after thoracic spinal cord transection. J Comp Neurol. 2008;509:382–399. doi: 10.1002/cne.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunot S, Flavell RA. Apoptosis. Death of a monopoly? Science. 2001;292:865–866. doi: 10.1126/science.1060885. [DOI] [PubMed] [Google Scholar]
- Hurlbert RJ. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. Journal of Neurosurgery. 2000;93:1–7. doi: 10.3171/spi.2000.93.1.0001. [DOI] [PubMed] [Google Scholar]
- Jin Y, McEwen ML, Nottingham SA, Maragos WF, Dragicevic NB, Sullivan PG, Springer JE. The mitochondrial uncoupling agent 2,4-dinitrophenol improves mitochondrial function, attenuates oxidative damage, and increases white matter sparing in the contused spinal cord. Journal of neurotrauma. 2004;21:1396–1404. doi: 10.1089/neu.2004.21.1396. [DOI] [PubMed] [Google Scholar]
- Jones LL, McDonald DA, Borum PR. Acylcarnitines: role in brain. Prog Lipid Res. 2010;49:61–75. doi: 10.1016/j.plipres.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Kamencic H, Griebel RW, Lyon AW, Paterson PG, Juurlink BH. Promoting glutathione synthesis after spinal cord trauma decreases secondary damage and promotes retention of function. FASEB J. 2001;15:243–250. doi: 10.1096/fj.00-0228com. [DOI] [PubMed] [Google Scholar]
- Kido Y, Tamai I, Ohnari A, Sai Y, Kagami T, Nezu J, Nikaido H, Hashimoto N, Asano M, Tsuji A. Functional relevance of carnitine transporter OCTN2 to brain distribution of L-carnitine and acetyl-L-carnitine across the blood-brain barrier. J Neurochem. 2001;79:959–969. doi: 10.1046/j.1471-4159.2001.00621.x. [DOI] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci. 1996;16:5777–5794. doi: 10.1523/JNEUROSCI.16-18-05777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koc RK, Akdemir H, Karakucuk EI, Oktem IS, Menku A. Effect of methylprednisolone, tirilazad mesylate and vitamin E on lipid peroxidation after experimental spinal cord injury. Spinal Cord. 1999;37:29–32. doi: 10.1038/sj.sc.3100732. [DOI] [PubMed] [Google Scholar]
- Kremer E, Lev-Tov A. Localization of the spinal network associated with generation of hindlimb locomotion in the neonatal rat and organization of its transverse coupling system. J Neurophysiol. 1997;77:1155–1170. doi: 10.1152/jn.1997.77.3.1155. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H, Peterson C, Gibson GE. The pyruvate dehydrogenase complex during aging. Mech Ageing Dev. 1984;26:67–73. doi: 10.1016/0047-6374(84)90165-9. [DOI] [PubMed] [Google Scholar]
- Liu D, McAdoo DJ. Methylprednisolone reduces excitatory amino acid release following experimental spinal cord injury. Brain Res. 1993;609:293–297. doi: 10.1016/0006-8993(93)90885-q. [DOI] [PubMed] [Google Scholar]
- Lowitt S, Malone JI, Salem AF, Korthals J, Benford S. Acetyl-L-carnitine corrects the altered peripheral nerve function of experimental diabetes. Metabolism. 1995;44:677–680. doi: 10.1016/0026-0495(95)90128-0. [DOI] [PubMed] [Google Scholar]
- Lucas JH, Wheeler DG, Guan Z, Suntres Z, Stokes BT. Effect of glutathione augmentation on lipid peroxidation after spinal cord injury. Journal of neurotrauma. 2002;19:763–775. doi: 10.1089/08977150260139138. [DOI] [PubMed] [Google Scholar]
- Luo J, Borgens R, Shi R. Polyethylene glycol improves function and reduces oxidative stress in synaptosomal preparations following spinal cord injury. Journal of neurotrauma. 2004;21:994–1007. doi: 10.1089/0897715041651097. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Green DM, Sengoku T. Lumbar spinoreticular neurons in the rat: part of the central pattern generator for locomotion? Ann N Y Acad Sci. 1998;860:436–440. doi: 10.1111/j.1749-6632.1998.tb09069.x. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Lovett R, Coffee C, Gray R, Han Y, Zhang YP, Burke DA. Functional consequences of lumbar spinal cord contusion injuries in the adult rat. Journal of neurotrauma. 2005;22:529–543. doi: 10.1089/neu.2005.22.529. [DOI] [PubMed] [Google Scholar]
- Martin E, Rosenthal RE, Fiskum G. Pyruvate dehydrogenase complex: metabolic link to ischemic brain injury and target of oxidative stress. J Neurosci Res. 2005;79:240–247. doi: 10.1002/jnr.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullers DL, Sullivan PG, Scheff SW, Herman JP. Mifepristone protects CA1 hippocampal neurons following traumatic brain injury in rat. Neuroscience. 2002;109:219–230. doi: 10.1016/s0306-4522(01)00477-8. [DOI] [PubMed] [Google Scholar]
- McEwen ML, Sullivan PG, Rabchevsky AG, Springer JE. Targeting mitochondrial function for the treatment of acute spinal cord injury. Neurotherapeutics. 2011;8:168–179. doi: 10.1007/s13311-011-0031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen ML, Sullivan PG, Springer JE. Pretreatment with the cyclosporin derivative, NIM811, improves the function of synaptic mitochondria following spinal cord contusion in rats. Journal of neurotrauma. 2007;24:613–624. doi: 10.1089/neu.2006.9969. [DOI] [PubMed] [Google Scholar]
- Michel RP, Cruz-Orive LM. Application of the Cavalieri principle and vertical sections method to lung: estimation of volume and pleural surface area. Journal of microscopy. 1988;150:117–136. doi: 10.1111/j.1365-2818.1988.tb04603.x. [DOI] [PubMed] [Google Scholar]
- Musicco C, Capelli V, Pesce V, Timperio AM, Calvani M, Mosconi L, Cantatore P, Gadaleta MN. Rat liver mitochondrial proteome: Changes associated with aging and acetyl-L-carnitine treatment. J Proteomics. 2011 doi: 10.1016/j.jprot.2011.05.041. [DOI] [PubMed] [Google Scholar]
- Ohashi R, Tamai I, Yabuuchi H, Nezu JI, Oku A, Sai Y, Shimane M, Tsuji A. Na(+)-dependent carnitine transport by organic cation transporter, (OCTN2): its pharmacological and toxicological relevance. J Pharmacol Exp Ther. 1999;291:778–784. [PubMed] [Google Scholar]
- Onofrj M, Fulgente T, Melchionda D, Marchionni A, Tomasello F, Salpietro FM, Alafaci C, De Sanctis E, Pennisi G, Bella R, et al. L-acetylcarnitine as a new therapeutic approach for peripheral neuropathies with pain. Int J Clin Pharmacol Res. 1995;15:9–15. [PubMed] [Google Scholar]
- Opii WO, Nukala VN, Sultana R, Pandya JD, Day KM, Merchant ML, Klein JB, Sullivan PG, Butterfield DA. Proteomic identification of oxidized mitochondrial proteins following experimental traumatic brain injury. Journal of neurotrauma. 2007;24:772–789. doi: 10.1089/neu.2006.0229. [DOI] [PubMed] [Google Scholar]
- Paradies G, Ruggiero FM, Gadaleta MN, Quagliariello E. The effect of aging and acetyl-L-carnitine on the activity of the phosphate carrier and on the phospholipid composition in rat heart mitochondria. Biochim Biophys Acta. 1992;1103:324–326. doi: 10.1016/0005-2736(92)90103-s. [DOI] [PubMed] [Google Scholar]
- Patel SP, Sullivan PG, Lyttle TS, Rabchevsky AG. Acetyl-L-carnitine ameliorates mitochondrial dysfunction following contusion spinal cord injury. J Neurochem. 2010;114:291–301. doi: 10.1111/j.1471-4159.2010.06764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Sullivan PG, Pandya JD, Rabchevsky AG. Differential effects of the mitochondrial uncoupling agent, 2,4-dinitrophenol, or the nitroxide antioxidant, Tempol, on synaptic or nonsynaptic mitochondria after spinal cord injury. J Neurosci Res. 2009;87:130–140. doi: 10.1002/jnr.21814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettegrew JW, Levine J, McClure RJ. Acetyl-L-carnitine physical-chemical, metabolic, and therapeutic properties: relevance for its mode of action in Alzheimer's disease and geriatric depression. Mol Psychiatry. 2000;5:616–632. doi: 10.1038/sj.mp.4000805. [DOI] [PubMed] [Google Scholar]
- Pocernich CB, Butterfield DA. Acrolein inhibits NADH-linked mitochondrial enzyme activity: implications for Alzheimer's disease. Neurotox Res. 2003;5:515–520. doi: 10.1007/BF03033161. [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG, Fugaccia I, Fletcher-Turner A, Blades DA, Mattson MP, Scheff SW. Basic fibroblast growth factor, (bFGF) enhances tissue sparing and functional recovery following moderate spinal cord injury. Journal of neurotrauma. 1999;16:817–830. doi: 10.1089/neu.1999.16.817. [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG, Fugaccia I, Sullivan PG, Blades DA, Scheff SW. Efficacy of methylprednisolone therapy for the injured rat spinal cord. J Neurosci Res. 2002;68:7–18. doi: 10.1002/jnr.10187. [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG, Fugaccia I, Turner AF, Blades DA, Mattson MP, Scheff SW. Basic fibroblast growth factor, (bFGF) enhances functional recovery following severe spinal cord injury to the rat. Experimental neurology. 2000;164:280–291. doi: 10.1006/exnr.2000.7399. [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG, Patel SP, Springer JE. Pharmacological interventions for spinal cord injury: where do we stand? How might we step forward? Pharmacol Ther. 2011;132:15–29. doi: 10.1016/j.pharmthera.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG, Sullivan PG, Scheff SW. Temporal-spatial dynamics in oligodendrocyte and glial progenitor cell numbers throughout ventrolateral white matter following contusion spinal cord injury. Glia. 2007;55:831–843. doi: 10.1002/glia.20508. [DOI] [PubMed] [Google Scholar]
- Rai G, Wright G, Scott L, Beston B, Rest J, Exton-Smith AN. Double-blind, placebo controlled study of acetyl-l-carnitine in patients with Alzheimer's dementia. Curr Med Res Opin. 1990;11:638–647. doi: 10.1185/03007999009112690. [DOI] [PubMed] [Google Scholar]
- Ravikumar R, McEwen ML, Springer JE. Post-treatment with the cyclosporin derivative, NIM811, reduced indices of cell death and increased the volume of spared tissue in the acute period following spinal cord contusion. Journal of neurotrauma. 2007;24:1618–1630. doi: 10.1089/neu.2007.0329. [DOI] [PubMed] [Google Scholar]
- Rebouche CJ. Kinetics, pharmacokinetics, and regulation of L-carnitine and acetyl-L-carnitine metabolism. Ann N Y Acad Sci. 2004;1033:30–41. doi: 10.1196/annals.1320.003. [DOI] [PubMed] [Google Scholar]
- Richards EM, Rosenthal RE, Kristian T, Fiskum G. Postischemic hyperoxia reduces hippocampal pyruvate dehydrogenase activity. Free Radic Biol Med. 2006;40:1960–1970. doi: 10.1016/j.freeradbiomed.2006.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CL, Saraswati M, Fiskum G. Mitochondrial dysfunction early after traumatic brain injury in immature rats. J Neurochem. 2007;101:1248–1257. doi: 10.1111/j.1471-4159.2007.04489.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal PB, Henderson R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J Mol Biol. 2003;333:721–745. doi: 10.1016/j.jmb.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Ross IB, Tator CH. Spinal cord blood flow and evoked potential responses after treatment with nimodipine or methylprednisolone in spinal cord-injured rats. Neurosurgery. 1993;33:470–476. doi: 10.1227/00006123-199309000-00017. discussion 476–477. [DOI] [PubMed] [Google Scholar]
- Ross IB, Tator CH, Theriault E. Effect of nimodipine or methylprednisolone on recovery from acute experimental spinal cord injury in rats. Surg Neurol. 1993;40:461–470. doi: 10.1016/0090-3019(93)90048-6. [DOI] [PubMed] [Google Scholar]
- Sano M, Bell K, Cote L, Dooneief G, Lawton A, Legler L, Marder K, Naini A, Stern Y, Mayeux R. Double-blind parallel design pilot study of acetyl levocarnitine in patients with Alzheimer's disease. Arch Neurol. 1992;49:1137–1141. doi: 10.1001/archneur.1992.00530350051019. [DOI] [PubMed] [Google Scholar]
- Scafidi S, Fiskum G, Lindauer SL, Bamford P, Shi D, Hopkins I, McKenna MC. Metabolism of acetyl-L-carnitine for energy and neurotransmitter synthesis in the immature rat brain. J Neurochem. 2010a;114:820–831. doi: 10.1111/j.1471-4159.2010.06807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scafidi S, Racz J, Hazelton J, McKenna MC, Fiskum G. Neuroprotection by acetyl-L-carnitine after traumatic injury to the immature rat brain. Dev Neurosci. 2010b;32:480–487. doi: 10.1159/000323178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu KF, Kim YT, Blass JP, Weksler ME. An immunochemical study of the pyruvate dehydrogenase deficit in Alzheimer's disease brain. Ann Neurol. 1985;17:444–449. doi: 10.1002/ana.410170505. [DOI] [PubMed] [Google Scholar]
- Short DJ, El Masry WS, Jones PW. High dose methylprednisolone in the management of acute spinal cord injury - a systematic review from a clinical perspective. Spinal Cord. 2000;38:273–286. doi: 10.1038/sj.sc.3100986. [DOI] [PubMed] [Google Scholar]
- Sorbi S, Bird ED, Blass JP. Decreased pyruvate dehydrogenase complex activity in Huntington and Alzheimer brain. Ann Neurol. 1983;13:72–78. doi: 10.1002/ana.410130116. [DOI] [PubMed] [Google Scholar]
- Spagnoli A, Lucca U, Menasce G, Bandera L, Cizza G, Forloni G, Tettamanti M, Frattura L, Tiraboschi P, Comelli M, et al. Long-term acetyl-L-carnitine treatment in Alzheimer's disease. Neurology. 1991;41:1726–1732. doi: 10.1212/wnl.41.11.1726. [DOI] [PubMed] [Google Scholar]
- Springer JE, Rao RR, Lim HR, Cho SI, Moon GJ, Lee HY, Park EJ, Noh JS, Gwag BJ. The functional and neuroprotective actions of Neu2000, a dual-acting pharmacological agent, in the treatment of acute spinal cord injury. Journal of neurotrauma. 2010;27:139–149. doi: 10.1089/neu.2009.0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Krishnamurthy S, Patel SP, Pandya JD, Rabchevsky AG. Temporal characterization of mitochondrial bioenergetics after spinal cord injury. Journal of neurotrauma. 2007;24:991–999. doi: 10.1089/neu.2006.0242. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Rabchevsky AG, Waldmeier PC, Springer JE. Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death? J Neurosci Res. 2005;79:231–239. doi: 10.1002/jnr.20292. [DOI] [PubMed] [Google Scholar]
- Swamy-Mruthinti S, Carter AL. Acetyl- L -carnitine decreases glycation of lens proteins: in vitro studies. Exp Eye Res. 1999;69:109–115. doi: 10.1006/exer.1999.0680. [DOI] [PubMed] [Google Scholar]
- Tempesta E, Casella L, Pirrongelli C, Janiri L, Calvani M, Ancona L. L-acetylcarnitine in depressed elderly subjects. A cross-over study vs placebo. Drugs Exp Clin Res. 1987;13:417–423. [PubMed] [Google Scholar]
- Tufekci O, Gunes D, Ozogul C, Kolatan E, Altun Z, Yilmaz O, Aktas S, Erbayraktar Z, Kirkim G, Mutafoglu K, Soylu A, Serbetcioglu B, Guneri EA, Olgun N. Evaluation of the effect of acetyl L-carnitine on experimental cisplatin nephrotoxicity. Chemotherapy. 2009;55:451–459. doi: 10.1159/000240020. [DOI] [PubMed] [Google Scholar]
- Vaishnav RA, Singh IN, Miller DM, Hall ED. Lipid peroxidation-derived reactive aldehydes directly and differentially impair spinal cord and brain mitochondrial function. Journal of neurotrauma. 2010;27:1311–1320. doi: 10.1089/neu.2009.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virmani A, Binienda Z. Role of carnitine esters in brain neuropathology. Mol Aspects Med. 2004;25:533–549. doi: 10.1016/j.mam.2004.06.003. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Singh IN, Hall ED. Tempol protection of spinal cord mitochondria from peroxynitrite-induced oxidative damage. Free Radic Res. 2009;43:604–612. doi: 10.1080/10715760902977432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Fan G, Chen S, Wu Y, Xu XM, Hsu CY. Methylprednisolone inhibition of TNF-alpha expression and NF-kB activation after spinal cord injury in rats. Brain Res Mol Brain Res. 1998;59:135–142. doi: 10.1016/s0169-328x(98)00142-9. [DOI] [PubMed] [Google Scholar]
- Yu WR, Liu T, Fehlings TK, Fehlings MG. Involvement of mitochondrial signaling pathways in the mechanism of Fas-mediated apoptosis after spinal cord injury. Eur J Neurosci. 2009;29:114–131. doi: 10.1111/j.1460-9568.2008.06555.x. [DOI] [PubMed] [Google Scholar]
- Zaidan E, Sheu KF, Sims NR. The pyruvate dehydrogenase complex is partially inactivated during early recirculation following short-term forebrain ischemia in rats. J Neurochem. 1998;70:233–241. doi: 10.1046/j.1471-4159.1998.70010233.x. [DOI] [PubMed] [Google Scholar]