Abstract
The importance of the epicardium for myocardial and valvuloseptal development has been well established; perturbation of epicardial development results in cardiac abnormalities, including thinning of the ventricular myocardial wall and malformations of the atrioventricular valvuloseptal complex. To determine the spatiotemporal contribution of epicardially derived cells to the developing fibroblast population in the heart we have used a mWt1/IRES/GFP-Cre mouse to trace the fate of EPDCs from embryonic day (ED)10 until birth. EPDCs begin to populate the compact ventricular myocardium around ED12. The migration of epicardially-derived fibroblasts toward the interface between compact and trabecular myocardium is completed around ED14. Remarkably, epicardially-derived fibroblasts do not migrate into the trabecular myocardium until after ED17. Migration of EPDCs into the atrioventricular cushion mesenchyme commences around ED12. As development progresses, the number of EPDCs increases significantly, specifically in the leaflets which derive from the lateral atrioventricular cushions. In these developing leaflets the epicardially-derived fibroblasts eventually largely replace the endocardially-derived cells. Importantly, the contribution of EPDCs to the leaflets derived from the major AV cushions is very limited. The differential contribution of EPDCs to the various leaflets of the atrioventricular valves provides a new paradigm in valve development and could lead to new insights into the pathogenesis of abnormalities that preferentially affect individual components of this region of the heart. The notion that there is a significant difference in the contribution of epicardially and endocardially derived cells to the individual leaflets of the atrioventricular valves has also important pragmatic consequences for the use of endocardial and epicardial cre-mouse models in studies of heart development.
Keywords: Wt1, epicardium, EPDC, valves, fibroblasts, heart, mouse
INTRODUCTION
Fibroblasts and fibroblast-like cells form the largest non-cardiomyocyte cell population in the developed heart (Banerjee et al., 2007) and play an important role in cardiac function, remodeling, and disease (Prunotto et al., ; Vasquez et al.). Not all fibroblast/fibroblast-like cells in the heart are the same (Krenning et al., 2010; Snider et al., 2009; Zeisberg and Kalluri, 2010). The fibroblasts found embedded in the interstitial space of the myocardium are generally referred to as cardiac fibroblasts (CFs) while the fibroblast-like cells in the interstitium of the respective cardiac valves are commonly known as valve interstitial cells (VICs) or valvular fibroblasts (VFs),
In the initial papers that reported on the contribution of the epicardium to the developing heart using a variety of cell fate tracing techniques, results were presented that convincingly showed that after an epicardial-to-mesenchymal transformation a subset of epicardially-derived cells (EPDCs) migrate into the ventricular walls (Dettman et al., 1998; Gittenberger-de Groot et al., 1998; Manner, 1999). Here they give rise to a number of more differentiated cell types including the CFs and the coronary smooth muscle cells (Lie-Venema et al., 2007; Wessels and Perez-Pomares, 2004). During this process, differentiating EPDCs gradually lose expression of genes typically expressed in the epicardium, including cytokeratin, Tbx18, Wt1 and RALDH2 (Perez-Pomares et al., 2002) while at the same time upregulating genes associated with the more differentiated cell types. A comprehensive spatiotemporal analysis of the contribution of the EPDCs to the CF population has not, however, been published. Similarly, although several studies have demonstrated that EPDCs also populate the mesenchyme of the developing atrioventricular (AV) cushions (Gittenberger-de Groot et al., 1998; Manner, 1999; Perez-Pomares et al., 2002), the question of whether EPDCs also materially contribute to the VICs in the developed AV valve leaflets has not been resolved.
Most of our current understanding on the importance of the epicardium in the formation of the different fibroblast subpopulations is based on experimental studies using avian model systems (Manner, 1999) (Gittenberger-de Groot et al., 2000; Lie-Venema et al., 2007; Perez-Pomares et al., 2002). More recently, however, our ability to study the specific contribution of EPDCs to the developing heart has been furthered by transgenic mouse technology, including the cGATA5-cre (Merki et al., 2005), the Tbx18-cre (Cai et al., 2008), the inducible Wt1-cre (Zhou et al., 2008), the inducible Tcf21-cre mouse (Acharya et al., 2011), and the Sema3DeGFPcre knock-in and ScxGFPCre BAC transgenic mice (Katz et al., 2012). Studies utilizing these mouse models have, however, primarily focused on the possible contribution of EPDCs to the cardiac muscle, the role of epicardial EMT in the development of the AV sulcus, and/or the role of the epicardium in coronary development (Cai et al., 2008; Smart et al., 2011; Zhou et al., 2008), (Zhou et al., 2010), (Merki et al., 2005) (Katz et al., 2012; Mellgren et al., 2008). As a result, still little is known regarding the contribution of EPDCs to the respective fibroblast populations in the developing and mature mammalian heart.
In this study we focused on the contribution of EPDCs to emerging populations of fibroblasts and fibroblast-like cells with a specific emphasis on the developing AV valves. The AV valves ensure unidirectional flow of blood from atria to ventricles and are largely derived from two sets of mesenchymal AV cushions. The two major AV cushions are the first to develop while the two lateral AV cushions appear later (de Lange et al., 2004; Wessels et al., 1996; Wessels and Sedmera, 2003). The mesenchyme found in the early AV cushions derives from an endocardial epithelial-to-mesenchymal transition (EMT) (de Lange et al., 2004; Markwald et al., 1977; Runyan and Markwald, 1983; Snarr et al., 2008). During valvuloseptal morphogenesis, the major AV cushions fuse, take part in the formation of the AV mesenchymal complex and contribute to the formation of the aortic leaflet of the left AV valve and the septal leaflet of the right AV valve (Snarr et al., 2007a; Snarr et al., 2007b). The lateral AV cushions do not fuse with each other or other mesenchymal tissues, but contribute to the parietal leaflet of the right AV valve and the mural leaflet of the left AV valve (de Lange et al., 2004; Wessels and Sedmera, 2003). Eventually, the cushion mesenchyme differentiates and gives rise to the valve interstitial cells (VICs). For many years, it was generally accepted that all VICs were endocardially derived. More recent studies, however, have demonstrated that circulating bone marrow-derived cells can engraft into the valves and also contribute to the VIC population (Hajdu et al., 2011). Although several studies in avian model systems have demonstrated that EPDCs contribute to the mesenchyme of the developing AV cushions (Gittenberger-de Groot et al., 1998; Manner, 1999; Perez-Pomares et al., 2002), a contribution of the EPDCs to the VIC population in the formed AV valves has never been reported. In fact, based on immunohistochemical studies on quail-to-chick epicardial chimeras it has been suggested that the epicardial contribution to the developing valves is only be temporary and that the formed leaflets of the AV valves do not contain cells with an epicardial origin (de Lange et al., 2004). The development of the AV valves is intrinsically related to the formation of the annulus fibrosus, a sheet of fibrous tissue physically separating the atrial and ventricular working myocardium at the AV junction. This fibrous tissue is important to the prevention of ventricular pre-excitation, allowing sequential contraction of atrial and ventricular chambers and aiding in the unidirectional flow of blood through the heart (Wessels et al., 1991) (Wessels et al., 1996). While recent publications have provided new insights into the role of the epicardium in the formation of the annulus fibrosus (Kolditz et al., 2008; Zhou et al., 2010) it is surprising how little information exists regarding the role of the epicardium in the formation of the AV valve leaflets which are in fibrous continuity with the annulus fibrosus. In this paper we focus on this important aspect of valvuloseptal development.
The mouse model used in this study, the mWt1/IRES/GFP-Cre (Wt1Cre) mouse, was designed based on the preferential expression of the transcription factor Wilm’s Tumor 1 (Wt1) in the epicardium (Moore et al., 1999; Perez-Pomares et al., 2002). To trace the fate of epicardially-derived cells we crossed this mouse with the R26-mT/mG reporter mouse (Muzumdar et al., 2007) and used immunofluorescence to follow the fate of epicardially-derived cells in detail.
METHODS
Generation and characterization of the mWt1/IRES/GFP-Cre mouse
To determine the fate of EPDCs in the valvuloseptal complex of the developing murine heart, bacterial artificial chromosome (BAC) transgenesis was used to generate a mWt1/IRES/GFP-Cre (Wt1Cre) mouse (see also (del Monte et al., 2011)). To achieve this, an IRES/EGFP-Cre cassette was inserted 17bp downstream of the translation stop site of the Wt1 gene in the BAC clone RP23-266M16 of the mouse RPCI-23 (C57BL/6J) BAC library (http://bacpac.chori.org). This BAC clone ranged from −127kbp to +11.5kbp relative to the transcription start site of Wt1.
Epicardial and endocardial cell-fate tracing
To trace the fate of epicardially and endocardially derived cells the mWt1/IRES/GFP-Cre mouse and the Tie2-cre mouse (Kisanuki et al., 2001) were used in combination with the B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J reporter mouse, or R26-mT/mG mouse. Expression of EGFP after cre-recombination in the Wt1cre::R26-mT/mG mouse (Wt1cre-mG) and Tie2::R26- mT/mG mouse (Tie2cre-mG) specimens was detected by immunofluorescence.
Immunofluorescent detection of EGFP and other antigens on Wt1cre-mG and Tie2cre-mG specimens
After sacrificing time-pregnant dams, embryos were isolated in phosphate-buffered saline (PBS), fixed overnight in freshly dissolved paraformaldehyde (4% w/v in PBS), processed through a graded series of ethanol, cleared in toluene, embedded in Paraplast Plus (Fisher Brand, catnr: 23-021-400), serially sectioned, mounted on Superfrost/Plus microscope slides (Fisherbrand catnr: 12-550-15) and stored at room temperature. In addition we collected Wt1cremG hearts of neonates right after birth (ND0). Staging and hematoxylin/eosin staining were performed as previously described (Waller and Wessels, 2000)(Snarr 2007a,b). To determine the developmental fate of the EPDCs, as well as to establish their relationship to other cell populations in the developing and postnatal heart, a panel of antibodies against a series of antigens was applied, including EGFP (Abcam ab 13970), Wt1 (Santa Cruz; catnr sc-192), Myosin Heavy Chain (MF20; DSHB), Troponin I (TnI; MilliPore; catnr MB1691), Sox9 (Santa Cruz, catnr sc-20095), FilaminA_(Epitomics, catnr 2242-1), Vimentin (Epitomics; catnr 27071), Collagen I (MD Bioscience; catnr 203002), Collagen I α1 Telopeptide (PhospoSolutions; catnr 322-COLT), and pSMAD1,5,8 (rabbit polyclonal, Millipore; catnr AB3848). Prior to incubation with primary antibodies, slides were typically pretreated with 15 ml of Vector Biolabs antigen unmasking solution (cat # H-3300) in 1600 ml of distilled water for five minutes in a pressure cooker (Fagor Splendid, item# 918060616), followed by preincubation with 1% bovine serum albumin (BSA) in PBS. Secondary antibodies (Jackson Immunoresearch) used included donkey anti-rabbit TRITC (711-025-152), donkey anti-chicken 649 (703-495-155), and donkey anti-mouse FITC (715-095-151). Nuclei were visualized using either Topro3 (Molecular Probes; 1:500) or DAPI (Invitrogen; Slowfade Gold Antifade Reagent with DAPI). Fluorescence was visualized using either a Leica SPE confocal laser scanning microscope or a Zeiss AxioImager II microscope.
In situ hybridization
After sacrifice of time-pregnant dams, embryos were isolated in ice-cold phosphate-buffered saline (PBS) and dissected, fixed overnight in freshly dissolved 4%(w/v) paraformaldehyde (PFA) in PBS, and embedded in paraplast. In situ hybrization was performed essentially as previously described (Kruithof et al., 2006). The embryos were sectioned at 10µm, deparaffinized, rehydrated in a graded series of alcohol and incubated with 10 mg/ml proteinase K dissolved in PBS for 15min at 37°C. The proteinase K activity was blocked by rinsing the sections in 0.2% glycine in PBST (PBS + 0.05%Tween-20) for 5 min. After rinsing in PBS, the sections were post-fixed for 10min in 4% PFA and 0.2% glutaraldehyde in PBS, followed by rinsing in PBS. After pre-hybridization for at least 1 hr at 70°C in hybridization mix (50%formamide, 5×SSC (20×SSC; 3M NaCl, 0.3M tri-sodium citrate, pH 4.5), 1% blocking solution (Roche), 5mM EDTA, 0.1% 3-[(3-Cholamidopropyl) dimethylammonio]-1-propanesulfonate (Sigma; Steinheim, Germany), 0.5 mg/ml heparin (BD Biosciences; Erembodegem, Belgium), and 1mg/ml yeast totalRNA (Roche), a digoxigenin (DIG)-labeled probe was added to the hybridization mix in a final concentration of 1 ng/ml. For the detection of Wt1, a 3.1 kb full length cDNA probe (Genebank, NM_144783) was used recognizing all Wt1 isoforms. Additionally, probes were used specific to cardiac Troponin I (cTnI), and SOX9. After overnight hybridization, the sections were rinsed with 2×SSC, followed by two washes with 50% formamide, 2×SSC, pH 4.5, at 65°C, and rinsing in TNT (0.1M Tris-HCl, pH=7.5, 0.15M NaCl, 0.05% Tween-20) at room temperature. Subsequently, the sections were incubated for 1 hr in MABT-block (100mM Maleic Acid, 150mM NaCl, pH7.4, 0.05% Tween-20, 2% blocking solution), followed by 2 hours incubation in MABT-block containing 100 mU/ml alkaline phosphatase-conjugated anti-DIG Fab fragments (Roche catnr: 1093274). After rinsing in TNT and subsequently in NTM (100mM Tris pH9.0 100mM NaCl, 50mM MgCl2), probe binding was visualized using nitro blue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl-phosphate (Roche catnr: 1681451). Colour development was stopped by rinsing in double-distilled water. The sections were dehydrated in a graded ethanol series, rinsed in xylene, and embedded in Entellan. Images were recorded using a Leica DFC320 camera mounted on an AxioPhot microscope (Zeiss).
Nomenclature
The anatomy of the AV valves in the mouse resembles to a certain extent that of the AV valves in the human heart. However, while the right AV valve in the human has a pronounced three leaflet configuration, and hence is referred to as the tricuspid valve, in the mouse the anatomy of the right AV valve is better described as a two-leaflet valve comprised of a septal leaflet, deriving from the fused major cushions and a parietal leaflet, deriving from the right lateral AV cushion respectively (Icardo et al., 1993). The left AV valve, is, like in humans, also a two-leaflet valve and consists of the aortic leaflet, which, like the septal leaflet of the right AV valve, derives from the major AV cushions, and the mural leaflet, deriving from the left lateral AV cushion. In this paper we will, to facilitate description and discussion, on occasion refer to the parietal and mural leaflets as the lateral AV leaflets, while the septal leaflet of the right AV valve and the aortic leaflet of the left AV valve may be referred to as the septal AV leaflets.
RESULTS
Wilm’s Tumor 1 (Wt1) transcription factor is expressed in epicardial and epicardially-derived cells
One of the genes critically important for epicardial development is the transcription factor Wilm’s Tumor 1 (Wt1) (Moore et al., 1999). Wt1 expression has been demonstrated in the epicardium and a subset of epicardially-derived cells in the avian and mouse heart (Moore et al., 1999; Perez-Pomares et al., 2002) (Zeng et al., 2011; Zhou et al., 2008). Based on the “epicardial-specific” expression of Wt1 mRNA and protein, an mWt1/IRES/GFP-Cre (Wt1cre for short) mouse was constructed to enable cell fate tracing of epicardially-derived cells.
In order to put the results of our cell fate studies with the Wt1cre mouse into context, we performed in situ hybridization and immunofluorescence studies to establish endogenous Wt1 mRNA and protein expression (Fig.1). The tissue section in situ experiments demonstrated cardiac expression of Wt1 mRNA in the epicardium (Fig.1B,D), in a subset of cells within the ventricular myocardial wall, and in cells located within the interventricular septum (Fig.1D). Wt1 mRNA expression was also seen in non-cardiac tissues, including the pericardial membranes. The expression pattern of Wt1 protein, detected by immunofluorescence, was in accordance with that of Wt1 mRNA. Thus, Wt1 protein expression was observed in the epicardium and subepicardium (Fig.1E,F), in isolated cells migrating into the ventricular walls (Fig.1F), and in a number of cells throughout the interventricular septum (Fig.1E). Importantly, Wt1-positive cells were not observed in derivatives of the major and lateral AV cushions (Fig.1E, see also Fig 5).
Figure 1. Wilm’s Tumor 1 (Wt1) mRNA and protein expression in the developing mouse heart.
Non-radioactive in situ hybridization on serial sections of mouse embryos at 11.5ED (A,B) and 13.5ED (C,D) for cardiac Troponin I (cTnI; A,C) and Wt1 (B,D) shows expression of Wt1 mRNA in the epicardium covering the surface of the heart (white arrow heads in B and D), in isolated cells in the interventricular septum (black arrows in D), and cells in the ventricular free wall (white arrows in D). Immunolabeling for Wt1 at 13.5ED (green nuclei in E,F) shows the expression of Wt1 protein in the epicardial epithelium (white arrowheads in F) as well as cells in the ventricular wall (white arrows in E and F) mimicking the expression profile observed for Wt1 mRNA in the in situ hybridization. Double immunofluorescent labeling for Wt1 (green nuclei in G) and Wt1cre-mG cells (red in G) at 10ED shows the Wt1 driven expression of EGFP in the epicardium covering the myocardium of the early embryonic heart. IVS=interventricular septum, LA=left atrium, LV=left ventricle, RA=right atrium; RV=right ventricle
Figure 5. Mesenchyme of the developing AV valve leaflets does not express Wt1.
Non-radioactive in situ hybridization on serial sections of ED14.5 (A,B) and neonatal hearts collected right after birth (C,D) with probes for cardiac Troponin I (A,C) and Wt1 (B,D) shows the absence of Wt1 mRNA expression in the developing parietal and septal leaflets of the right AV valve. IVS=interventricular septum, LV=left ventricle, RA=right atrium, RV=right ventricle
For cell fate experiments we used the R26-mT/mG mouse line, in which EGFP is expressed in cells after cre-recombination (Muzumdar et al., 2007). EGFP was detected immunofluorescently and cells that express EGFP in the reporter line after recombination with the Wt1cre mouse are in this paper referred to as Wt1cre-mG cells (Fig.1G). It is important to note that using the EGFP antibody, we did not detect the mWt1/IRES/GFP-Cre fusion protein itself. Thus, no antibody-binding was detected in Wt1cre mice that were not crossed with the R26-mT/mG reporter mouse.
Contribution of epicardially-derived cardiac fibroblasts to compact and trabeculated ventricular myocardium
Studies in the developing avian and murine heart have demonstrated that EPDCs contribute to the developing cardiac fibroblast (CFs) population (Dettman et al., 1998; Gittenberger-de Groot et al., 1998; Manner, 1999; Perez-Pomares et al., 2002) (Cai et al., 2008; Wu et al., 2010; Zhou et al., 2010). However, partly because of research emphases, as well as the nature of cell-fate techniques used in the respective papers, these studies have not provided a comprehensive insight into the extent of the epicardial contribution to the CF population in the developing heart. Furthermore, this aspect of epicardial development has not received wide attention in studies in the mouse. To achieve a better understanding of the involvement of EPDCs in this process we performed a cell fate analysis in hearts of Wt1cre-EGFP specimens from ED10 until the neonatal stage.
The formation of the proepicardium in the mouse begins around ED9 (Viragh and Challice, 1981). At this stage, the ventricular wall is but a layer of thin myocardium lined by endocardium on the luminal side. Shortly thereafter, at ED 10–10.5, epicardial cells have largely covered the entire myocardial surface of the developing atria and ventricles (Figs 1G and 2A,A’,A”). The myocardial ventricular wall of the right and left ventricle at this stage is just a few cell layers thick and sparsely trabeculated. At ED12.5, two separate components of the ventricular wall can be clearly distinguish; the compact and the trabeculated myocardium (Fig 2B,B’,B”). Analysis of Wt1cre-mG hearts at this stage demonstrate the appearance of EGFP expressing cells within the compact ventricular myocardium (Fig 2B’,B”). Slightly later in development, at ED13–14, EPDCs have migrated toward the boundary between the compact and trabecular myocardium and, as such, can be found throughout the compact layer of the ventricular free walls (Fig 2C,C’,C”). This restricted distribution of Wt1cre-mG cells to the compact myocardium is maintained through ED17 (Figs 2E and 3) and it is not until the neonatal stage that Wt1cre-mG positive cells are found in the ventricular trabecular myocardium (Fig 2 D,D’,D”,F).
Figure 2. Epicardial cell fate tracing with the Wt1cre mouse demonstrates timeline of how epicardially derived interstitial fibroblasts populate the compact and trabecular ventricular myocardium.
Tissue sections of 10.5ED (A-A”), 12.5ED (B-B”), 14ED (C-C”) and neonatal (D-D”) hearts of Wt1cre-mG specimens histologically stained for HE (A,B,C,D), and immunofluorescently for EGFP (red) and MF20 (green) demonstrate how EGFP-labeled EPDCs start to populate the compact ventricular myocardium between 12.5ED (B’,B”) and 14ED (C’,C”). Panels E and F show the distribution of EGFP expressing cells (white) at 17ED and in the neonatal heart at low magnification. Comparison between the distribution of EGFP labeled cells at 14ED ((C’,C”) and 17ED (E) show that the EPDCs remain confined to the compact myocardium within that developmental window. The sections of the neonatal heart show that by this time the migration of EPDCs into the trabecular myocardium is complete (D’-D”, F). Arrows in panels A–D” demonstrate the thickness of compact myocardium at the respective stages.
CM=compact myocardium, IVS=interventricular septum, TM=trabecular myocardium
Figure 3. Wt1cre-mG expressing EPDCs within the ventricular myocardium of the late fetal heart are restricted to the compact myocardium and form the population of interstitial cardiac fibroblasts.
Panels A–D show immunofluorescent labeling of Wt1cre-mG hearts at 17ED for EGFP expression (red, A–C), MF20 (green,B–D), and nuclear DAPI staining (blue, A–B), and demonstrates that at this late fetal stage, EPDCs have not yet populated the trabecular myocardium (A,B). The Wt1cre-mG cells have a characteristic mesenchymal/fibroblastic phenotype, and do not express myocardial specific genes (arrows in B,C,D). Co-labeling of sections of 17ED Wt1cre-mG hearts for EGFP expression (red in E,H,K,N), for FilaminA (green in F,G,I,J) and Vimentin (green in L,M,O,P) antibodies as well as the staining for Collagen I α1 Telopeptide Z (green in Q,R) in combination with the staining for EGFP expression (red in Q,R) confirms the fibroblastic nature of the majority of EGFP labeled cells (H–J are enlargements of boxed areas in E–G; N–P are enlargements of boxed areas in K–M; R is an enlargement of boxed area in Q). CM=compact myocardium, TM=trabecular myocardium
The Wt1cre-mG positive EPDCs in the ventricular walls have a mesenchymal/fibroblastic phenotype, are highly polarized, with their major axis lying parallel to neighboring cardiomyocytes (Fig 3A,B) and do not express myocardial specific genes (e.g. myosin heavy chain, Fig 3C,D). To determine whether, in addition to their morphological appearance, the EPDCs located within the compact myocardial wall also express fibroblast-associated genes and to confirm that the EGFP-positive cells are indeed cardiac fibroblasts, immuonohistochemical studies were performed. While there are no unique markers for CFs, a wide variety of genes have been described that are typically expressed by these cells including Collagen I, Vimentin and FilaminA (Kim et al., 2010; Krenning et al., 2010; Norris et al., 2010; Snider et al., 2009; Zeisberg and Kalluri, 2010). These experiments showed co-expression of fibroblast-associated genes with the EGFP-positive EPDCs (Fig 3 E–R).
Epicardially-derived cells preferentially contribute to the AV valve leaflets derived from the lateral AV cushions
Valve interstitial cells (VICs) form an important group of fibroblasts in the heart and are found in the cardiac valves. Although several studies have demonstrated that epicardially-derived cells (EPDCs) contribute to the mesenchyme of the developing AV cushions (Gittenberger-de Groot et al., 1998; Manner, 1999; Perez-Pomares et al., 2002), their contribution to the formed AV valves of the four-chambered heart is a matter of debate (de Lange et al., 2004). Using our Wt1cre mouse we have revisited this important question.
In Wt1cre-mG hearts at ED12–13, at which point the two major as well as the two lateral AV cushions have formed, the Wt1cre-mG positive cells have densely populated the AV sulcus (Fig 4A,B). The distribution of Wt1cre-mG positive cells within the lower boundary of the lateral AV junctional myocardium suggests that this population of cells is actively migrating from the AV sulcus through the AV myocardium toward the cushion/myocardium interface of the right (Fig 4B) and left (not shown) lateral AV cushions which, at this stage, are relatively small compared to the major AV cushions. At this stage, only a few Wt1cre-mG positive cells can be found within the right lateral AV cushion (Fig.4B). The material contribution of Wt1cre-mG positive cells to the AV cushions changes dramatically between ED13 and ED15. Hearts at these stages show increasingly larger numbers of Wt1cre-mG cells in the developing AV valve leaflets (Fig.4C–D). This influx of Wt1cre-mG cells is essentially restricted to the leaflets that derive from the lateral cushions, only very few Wt1cre-EGF positive cells are seen in the leaflets that derived from the major AV cushions at this and subsequent stages (Fig.4D,F,H). The dramatic difference in the contribution of Wt1cre-mG positive cells to the AV leaflets derived from the lateral cushions versus those derived from the major cushions persists and becomes even more pronounced as the heart matures throughout fetal development and into the neonatal stage (Fig.4E–H and Fig.8 A,B).
Figure 4. Wt1cre-mG cells significantly contribute to the formation of the atrioventricular valve leaflets that derive from the lateral atrioventricular cushions.
Sections through the atrioventricular region of hearts at 12.5ED (A–B), 14.5ED (C–D), 17ED (E–F) and the neonatal stage (G,H) were histologically stained for HE (A,C,E,G) and immunofluorescently for EGFP expression (red, B, D,F,H) and MF20 (green, B,D,F,H). The distribution of Wt1cre-mG cells clearly demonstrates the increased and preferential contribution of EPDCs to the developing parietal leaflet of the right AV valve (B, D, F, H). The small arrows in B point to the EPDCs that migrate into the interface between the AV and ventricular myocardium. Note in B, D, F and H the virtual absence of Wt1cre-mG cells in the septal leaflet of the right AV valve. LA=left atrium, LV=left ventricle, RA=right atrium; RV=right ventricle
Figure 8. Epicardially derived cells replace the endocardially derived mesenchyme of the developing parietal leaflet of the right AV valve.
EGFP immunofluorescent labeling (red) of sections of a neonatal Wt1cre-mG heart (A,B) shows the contribution of EPDCs to the parietal leaflet of the right AV valve (A) and the mural leaflet of the left AV valve. The panels also show the absence in these sections of EPDCs to the septal leaflet of the right AV valve (A) and the aortic leaflet of the left AV valve (B). Panels C through F show EGFP immunofluorescent labeling (red) of sections of Tie2cre-mG hearts at 12ED, 15ED and neonatal stage co-labeled with MF20 (green). The plate illustrates that at the right AV junction while initially the mesenchyme of all the AV cushions is largely endocardially derived (C,D), the endocardially derived mesenchyme of the parietal leaflet, but not that of the septal leaflet, becomes replaced by epicardially derived cells (A, E,F). Note in E and F that the endocardial lining of the parietal leaflet continues to be marked by Tie2cre-mG.
iAVC=inferior AV cushion, sAVC=superior AV cushion, llAVC=left lateral AV cushion, rl=right lateral AV cushion
It is theoretically possible that EGFP expression in the AV valve leaflets is not related to the influx of EPDCs but rather results from a transcriptional event that would activate the Wt1cre driver in the resident population of endocardially derived cells. In that case, one would expect to see an upregulation of endogenous Wt1 in these cells as well. To exclude this possibility, we performed in situ hybridization to detect Wt1 mRNA on sections of wildtype ED14.5 and neonatal hearts and determined that Wt1 mRNA is not expressed in any of the leaflets of the AV valves (Fig.5).
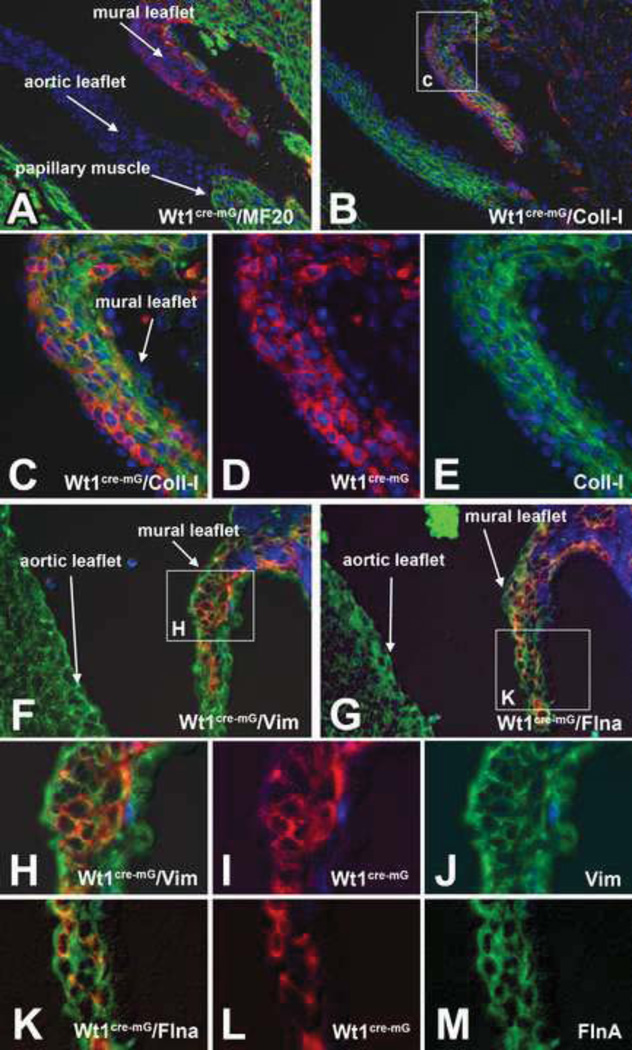
To verify that the Wt1cre-EGF positive EPDC population in the lateral leaflets is indeed, like the endocardially derived cells in the cushion derived tissues, giving rise to valve fibroblasts, we immunofluorescently stained serial sections at ED17 for the presence of genes characteristically expressed in the AV valves, including Collagen I (Fig.6 B–E), Vimentin (Fig.6F, H–J), FilaminA (Fig.6G, K–M), Periostin (not shown) and Versican (not shown) and established that all these genes are indeed expressed by the Wt1cre-EGF positive EPDCs. The expression patterns of these genes showed a similar staining pattern in the major and lateral cushions. Since we have shown that the mesenchyme of the major cushions is largely endocardially derived cells and the lateral cushion mesenchyme mainly consists of epicardially derived cells, this shows that the endocardially and epicardially derived cells have a very similar molecular profile as they differentiate into VICs.
Figure 6. Wt1cre-mG cells in the lateral leaflets of the AV valves express genes associated with differentiating valve fibroblasts.
Sections of 17ED Wt1cre-mG hearts were immunofluorescently labeled for EGFP (red), MF20 (green in A and blue in F–M), Collagen I (green in B,C,E), Vimentin (green in F,H,J), and FilaminA (green in G,K,M). The nuclei in sections A–E were visualized by DAPI staining (blue), Panel C is an enlargement of the boxed area in B, panel H is an enlargement of the boxed area in F, and panel K is an enlargement of the boxed area in G. The co-labeling of the valve leaflets at the left AV junction of shows that the Wt1cre-mG positive cells are co-expressing characteristic fibroblast-associated proteins that are also found in the non-epicardially derived cushions, including Collagen 1 (B–E), Vimentin (F and H–J) and FilaminA (G and K–M).
Sox9, a member of the high-mobility-group of transcription factors, is widely expressed in the mesenchyme of the AV cushions (Lincoln et al., 2007; Smith et al., 2011) where it is reportedly involved in the regulation of endocardial EMT (Akiyama et al., 2004). More recent studies have also indicated a role for Sox9 in epicardial development (Smith et al., 2011). To obtain a better insight into the possible role of Sox9 in the epicardial involvement of AV valve formation we performed Sox9 immunolabeling and in situ hybridization (Fig.7). These experiments showed that while Sox9 is expressed at a very low level in the epicardial epithelium itself (Fig.7D,G), the expression of Sox9 is much higher in the Wt1cre-mG expressing EPDCs that are closer in proximity to the AV myocardium and those that are migrating into the myocardial junction (Fig.7B,C,E). Within the developing leaflets no difference was observed between the levels of Sox9 expression in the epicardially derived mesenchyme and the endocardially derived mesenchyme (Fig.7D).
Figure 7. Sox9 is expressed in the Wt1cre-mG cells that populate the lateral leaflets of the AV valves.
Tissue sections of 13ED (A–C) and 17ED (D–F) hearts of Wt1cre-mG specimens were immunofluorescently triple-labeled for EGFP expression (red), MF20 (blue) and Sox9 (green). The panels demonstrate that as EGFP labeled EPDCs migrate into the AV sulcus the level of Sox9 expression increases and that once the EPDCs have populated the AV valve leaflets their Sox9 expression profile is indistinguishable from that of the endocardially-derived valve mesenchymal cells. Tissue-section in situ hybridization for Sox9 in a 13.5ED heart (G,H) also shows that mesenchymal cells in the AV sulcus that are in close proximity to the AV junctional myocardium express a higher level of Sox9 mRNA when compared to the epicardial and epicardially-derived cells located further away from the junctional myocardium. Panel I shows a control in situ labeling with a cTnI probe. LA=left atrium, LV=left ventricle, RA=right atrium; RV=right ventricle
Epicardially-derived cells preferentially populate the AV valve leaflets that derive from the lateral AV cushions
It has been well-established that the early formation of the AV cushions involves an epithelial-to-mesenchymal transition (EMT) of the endocardium and that the mesenchyme found in these extracellular matrix-rich tissues is entirely endocardially-derived (Eisenberg and Markwald, 1995). Furthermore, we and others have previously suggested that as this endocardially-derived mesenchyme expands and differentiates, it also provides the tissue base for the VICs found in the leaflets of the developed AV valves (Wessels et al., 1996) (de Lange et al., 2004). These studies, have led to the paradigm that mature valve leaflets do not contain a substantial amount of cells from the myocardial, neural crest, or epicardial cell lineages (de Lange et al., 2004). The results from our Wt1cre-mG cell fate study described in this report, however, demonstrate that during late fetal development the developing VIC population in a subset of the AV valve leaflets, i.e. the parietal leaflet of the right AV valve and the mural leaflet of the left AV valve, largely consists of epicardially-derived cells (Fig.8A,B). This brought into question the conclusions regarding the endocardial origin of all valvular mesenchymal/fibroblasts in the developed AV valves. To revisit this matter we used the Tie2cre mouse (Kisanuki et al., 2001) in combination with the R26-mT/mG mouse for tracing of endocardial and endocardially-derived cells and compared the expression of Tie2cre-mG (Fig. 8C–F) to the Wt1cre-mG expression in hearts at two prenatal developmental stages (ED12–12.5 and ED14.5–15) and in the neonatal heart (see also Fig.4).
In ED12 Tie2cre-mG hearts, the entire endocardium, and virtually all mesenchymal cells of the AV cushions, express EGFP demonstrating the endocardial origin of nearly all cells that form the lateral and major AV cushions at this stage (Fig.8C). As described above, very few EGFP positive cells are found in Wt1cre-mG hearts at this stage (Fig. 4B), indicating a very limited contribution of the epicardium to the developing leaflets in this early developmental period.
As discussed earlier, and as demonstrated in ED14–14.5 Wt1cre-mG hearts, the lateral cushions become populated with EGFP labeled cells, whereas virtually no labeled cells are found in the derivatives of the major AV cushions at this stage (Fig.4D). In Tie2cre-mG hearts around the same developmental stage, all developing AV valve leaflets still contain large numbers of EGFP-expressing endocardially-derived cells (Fig.8D). Thus, at this mid-embryonic stage, epicardially and endocardially derived cells are intermingled in the lateral cushions, but the mesenchyme of the major cushions continues to be predominantly endocardially derived.
Analysis of Wt1cre-mG and Tie2cre-mG neonatal hearts demonstrates that epicardially- and endocardially-derived cells differentially contribute to the valvuloseptal complex of the developed heart. The parietal leaflets of neonatal Wt1cre-mG hearts show intense staining for EGFP indicating that the vast majority of the VICs are of epicardial origin. Very few Wt1cre-mG labeled cells are observed in the septal leaflet of the right AV valve and the aortic leaflet of the left AV valve, indicating that these leaflets do not receive a significant contribution from the epicardium (Fig.4 G,H and 8A,B). The labeling of the Tie2cre-mG labeled neonatal hearts demonstrated that at this stage only few Tie2cre-mG positive cells can be observed within the mesenchyme of the parietal leaflet and that Tie2cre-mG staining is virtually restricted to the endocardial lining (Fig 8E,F). Thus, whereas the mesenchyme of all leaflets is initially derived from an endocardial EMT, the population of VICs in the parietal leaflets of the post natal heart largely consists of EPDCs. In contrast, the vast majority of the VICs in the leaflets that derive from the major cushions, i.e. the septal leaflet of the right AV valve and the aortic leaflet of the left AV valve (Fig.8F), remain Tie2cre-mG positive, indicating that this cell population is still largely endocardially-derived cells in the postnatal heart (Fig 8C,D).
Myocardial expression of Wt1cre-mG in cells of the interventricular septum
While it is generally accepted that EPDCs give rise to coronary smooth muscle cells and cardiac fibroblasts, their contribution to the coronary endothelium and cardiomyocyte population remains contentious, particularly because studies in the avian model systems and the mouse appear to lead to different conclusions. The apparent contradictory observation regarding the coronary endothelium was addressed in a recent paper (Katz et al., 2012) in which, using Sema3DeGFPcre knock-in and ScxGFPCre BAC transgenic mice, it was demonstrated that the Scx and Sema3D cell populations from the proepicardium give rise to the coronary endothelial cells, an observation which seems to largely resolve the hitherto unresolved divergent observations in the epicardial cell fate studies in the developing avian and murine heart.
The question of whether the epicardium materially contributes to the developing myocardium remains highly contentious. The initial cell fate studies in the avian heart never indicated a contribution of EPDCs to the developing myocardium (Dettman et al., 1998; Gittenberger-de Groot et al., 1998; Perez-Pomares et al., 2002). A few years ago, however, a number of epicardial cell-fate studies were published in which, using respectively a Tbx18:cre mouse and a Wt1GFPCre mouse, it was suggested that the epicardium significantly contributes to subpopulations of cardiomyocytes (Cai et al., 2008; Zhou et al., 2008) (Cai et al., 2008; Zhou et al., 2008). More recently, this observation seemed to be confirmed in a study by the Tabin lab (Katz et al., 2012). To determine whether, and if so how, cells that are labeled with our Wt1cre construct contribute to the developing myocardium we analyzed Wt1cre-mG hearts at different stages. At early stages, Wt1cre-mG positive cardiomyocytes are predominantly seen in the ventricular apex (Fig.9A–E), but as ventricular septation progresses, these Wt1cre-EGFP positive embryonic cardiomyoctes are also found dispersed throughout the interventricular septum, often in small clusters suggesting a clonal origin (Fig.9G,H,I). The morphology of these Wt1cre- EGFP/MF20 positive cells resembles that of the neighboring Wt1cre-EGFP-negative cardiomyocytes and is quite distinct from the Wt1cre-EGFP cells with a fibroblastic phenotype and which do not express myocardial markers (see e.g. Fig 3B,C).
Figure 9. A small subpopulation of cardiomyocytes is EGFP labeled in Wt1cre-mG hearts.
Immunofluorescent co-labeling for EGFP (red), MF20 (green) and DAPI nuclear stain (blue) of ED12.5 (A–E) and ED17 Wt1cre-mG hearts shows that a subpopulation of MF20 positive cardiomyocytes are also Wt1cre-mG positive. Panel B is an enlargement of boxed area in A, panels C,D, and E are enlargements of boxed area in B. Panel G is an enlargement of boxed area in F, panels H, I, and J are enlargements of boxed area in G. The respective panels demonstrate that Wt1cre-mG/MF20 positive cardiomyocytes are predominantly located in the interventricular septum. IVS=interventricular septum, LV=left ventricle, RV=right ventricle
DISCUSSION
Until a few years ago, our knowledge of the contribution of EPDCs to cardiac development was, by and large, limited to the avian heart. In a series of studies using dyelabeling and quail-to-chick proepicardial explant techniques, in which donor tissues containing quail proepicardium were transplanted into stage-matched chick host embryos, EPDCs were shown to migrate into the ventricular myocardium and into the AV cushions (Dettman et al., 1998; Gittenberger-de Groot et al., 1998; Manner, 1999; Perez-Pomares et al., 2002; Wessels and Perez-Pomares, 2004). These and other studies also demonstrated the potential of EPDCs to differentiate into a number of different cell types leading to the notion that the EPDC can be considered a multipotent cardiac stem cell (Wessels and Perez-Pomares, 2004; Winter and Gittenberger-de Groot, 2007; Winter et al., 2009). Notwithstanding the significant insight these studies yielded in the avian system, the technical nature of these approaches left many unanswered questions. While the development of Cre mice has broadened our understanding of the role of epicardium in the murine heart models (Cai et al., 2008; Katz et al., 2012; Zhou et al., 2008; Zhou et al., 2010), these studies have also led to new questions regarding specific aspects of the role of the epicardium in cardiac development.
In this paper we report the use of another cre-mouse which, like the one generated by Zhou and colleagues (Zhou et al., 2008; Zhou et al., 2010), is based on the characteristic epicardial expression of the Wilm’s Tumor 1 transcription factor (Wt1). Although this mWt1/IRES/GFP-Cre mouse has previously been used (del Monte et al., 2011; Norden et al., 2010), this is the first report in which its potential in the study of epicardial cell fate is documented in detail. The use of this mouse in combination with the R26-mT/mG reporter mouse (Muzumdar et al., 2007) enabled us to trace in great detail the fate of EPDCs and to address a number of important issues, specifically related to the contribution of the EPDCs to the developing fibroblast population in the murine heart.
The epicardium and the development of the interstitial cardiac fibroblasts
Although there is still some debate regarding the origin of the respective fibroblast populations in the developing and adult heart in health and disease (Krenning et al., 2010; Snider et al., 2009; Zeisberg and Kalluri, 2010) a series of studies in recent years has demonstrated that the epicardium should be considered as a significant source of these cells (Dettman et al., 1998; Gittenberger-de Groot et al., 1998; Manner, 1999; Perez-Pomares et al., 2002) (Cai et al., 2008; Katz et al., 2012; Wu et al., 2010; Zhou et al., 2010). The data presented here, provide new information on how EPDCs populate the ventricular myocardial wall and septum to contribute to the ventricular interstitium. We show that EPDCs begin to invade the ventricular myocardial walls around ED12.5. Using a quail-to-chick chimera model at Hamburger/Hamilton stage 26 (H/H 26), which is comparable to murine ED12.5 (Wessels and Markwald, 2000), we found that in the avian heart EPDCs start to invade the ventricular walls around this stage as well (Wessels and Perez-Pomares, 2004). By ED 14, epicardially-derived fibroblasts have populated the compact layer of the myocardium. The formation of this densely packed layer of ventricular cardiomyocytes is frequently linked to the development of the epicardium as mice with disrupted epicardial development typically present with a thin compact ventricular wall (for a review see (Wessels and Perez-Pomares, 2004)). Likewise, when epicardial development in the developing avian heart is perturbed by microsurgical procedures, the experimental hearts also are found to have a thin ventricular wall (Gittenberger-de Groot et al., 2000),(Perez-Pomares et al., 2002). Here we show that the early development of the compact layer precedes the ingrowth of EPDCs into the ventricular wall. This indicates that the initial formation of the compact layer does not rely on the migration of EPDCs into the myocardial wall. The subsequent increase in wall thickness does coincide with the influx of EPDCs into the compact layer. This might suggest that this second phase of ventricular wall development, which involves continued proliferation of the myocardium (Ieda et al., 2009) and the establishment of a coronary network, does depend on concomitant development of the EPDC-derived interstitium. In a recent study on the role of PDGF receptor signaling in epicardial development, however, the paradigm that epicardially derived cardiac fibroblasts are important for normal myocardial development was challenged (Smith et al., 2011). It is obvious that more research is needed to unravel the importance of EPDCs in this process. The migration of EPDCs into the trabecular myocardium was found to be a very late process, taking place right before birth as at ED17 the EPDCs are still largely confined to the compact myocardium and it is not until the neonatal stage that significant numbers of EPDCs in the trabecular myocardium are observed. Based on these observations we infer that there are two important developmental windows related to the migration of EPDCs into the compact and trabecular ventricular myocardium. The first, ED12 throughED14, is a critical phase in the development of the EPDCs that populate the compact ventricular myocardium and give rise to the cardiac interstitial fibroblasts (CFs) and other epicardially-derived cell types, specifically the vascular smooth muscle cells of the coronary network. The second phase, the migration of these cells into the trabecular myocardium, is a very late gestational event, occurring around birth. How this phased development of the cardiac interstitial fibroblasts is regulated is unknown. The initial step for the first phase in the entire process is epicardial EMT. Factors regulating this process and subsequent migration of EPDCs into the subepicardial space and into the underlying myocardium have been identified and include PDGF-B and PDGFRβ, Tbx5, thymosin β4, Ets factors, and Sox9 (Lie-Venema et al., 2003; Lie-Venema et al., 2007; Smith et al., 2011). A mechanism of particular interest is the FGF10/FGFR2b signaling pathway. In a recent publication by the Ornitz lab (Vega-Hernandez et al., 2011) it was demonstrated that myocardial FGF10 signaling to epicardial and epicardially-derived cells through FGFR2b induces cardiac fibroblast development. Whether, and if so how, these and other candidates are involved in the spatial and temporal regulation of the migration of the epicardially derived cells into the compact myocardium remains to be established. Another challenge is the elucidation of the mechanism responsible for the delayed migration of EPDCs into the trabecular myocardium. Genes that during development show elevated levels of expression in the trabecular myocardium include ANF, BMP10, and p57Kip2 (Gaussin et al., 2002; Moorman and Christoffels, 2003; Snider et al., 2007). Whether these or other genes play a role in regulating EPDC immigration into the trabeculae, and whether EPDCs play a specific role in postnatal cardiac growth and remodeling of the trabecular component of the heart, will be subject for further research. Based on the observation that EPDCs appear in the trabecular myocardium very late in developmental terms, we infer that they likely do not play a significant role in trabecular development.
The epicardium and AV insulation
As the primary heart tube develops and loops, there is initially myocardial continuity between the developing atrial and ventricular myocardium (Bakker et al., 2010). The cardiomyocytes of the AV junctional myocardium are characterized by a series of unique molecular and functional features which serve to delay the cardiac impulse, generated in the sinoatrial region, enabling coordinated contraction of the atrial and ventricular chambers (Wessels et al., 1996; Wessels et al., 1992; Wessels et al., 1991; Wessels et al., 1990) (Bakker et al., 2010; de Jong et al., 1992) (Aanhaanen et al., 2011). Later in development, a ring of insulating fibrous tissue, the annulus fibrosus serves this purpose. Incomplete separation of atrial and ventricular myocardium, or de-novo formation of myocardial atrioventricular connections, can result in accessory atrioventricular pathways which can serve as anatomical substrates for ventricular pre-excitation as observed in patients with Wollf-Parkinson-White (WPW) syndrome. In earlier papers on the developing human heart, we showed that the separation of atrial and ventricular tissues occurs at the lower boundary of the atrioventricular junction and results in the incorporation of the embryonic AV myocardium into the lower rim of the atrial chambers (Wessels et al., 1996; Wessels et al., 1992). The data presented here confirms earlier studies that this separation of ventricular and atrioventricular/atrial myocardium is achieved by active migration of EPDCs into the lower boundary of the junctional myocardium (Zhou et al., 2010). It also demonstrates that after doing so, the immigrating EPDCs create a portal of entry that allows them to populate the lateral AV cushions. As the EPDCs migrate away from the epicardial epithelium into the AV sulcus and toward the AV myocardium, they gradually assume a mesenchymal phenotype similar to that of the endocardially-derived cushion mesenchymal cells. This includes the expression of Sox9, FilaminA, collagen I, and vimentin. It is important to note that the separation of atrial and ventricular myocardium does not take place in the postero-inferior aspect of the heart. Here, AV myocardial continuity is maintained through the bundle of His (or AV bundle), an integral part of the AV conduction system.
The epicardium and the development of the AV valves
Despite our increasing knowledge on the role of the epicardium in heart development, many contentious topics still remain, including the involvement of EPDCs in the formation of the AV valves. Although initial quail-to-chick chimera studies, strongly suggested that during valvulogenesis, EPDCs migrate into the developing AV valve leaflets, little attention was given to the specific contribution of these cells to the individual leaflets (Gittenberger-de Groot et al., 1998; Manner, 1999; Perez-Pomares et al., 2002). The importance of EPDCs for proper valvuloseptal morphogenesis was demonstrated in studies in which the development of the epicardium was perturbed by either ablation of the proepicardium or through insertion of a piece of eggshell in between the proepicardium and the heart proper to delay the attachment of proepicardial cells to the myocardial surface of the heart. These studies showed that inhibition of normal epicardial growth results in cardiac abnormalities including malformations of the valvuloseptal apparatus (Perez-Pomares et al., 2002). A few years ago, however, a paper was published in which it was suggested that the mesenchyme found in the derivatives of the major and lateral AV cushions is entirely endocardially derived (de Lange et al., 2004). In this study, which was mainly based on cre-lineage tracing experiments, the authors also incorporated results from quail-to-chick proepicardial chimera studies, stating that in the AV valve leaflets of the developed chimera heart no quail derived cells could be found. They inferred from these observations that the EPDCs that can be observed to populate the valves in early stages of development do not survive (or disappear) from these tissues at later stages. Combined, these observations raised the question of what the role of the epicardium is in the development of the valvuloseptal complex in the murine heart.
In this study we report that EPDCs, migrate through the AV junction, to gradually populate the lateral AV cushions and, to a significantly lesser extent, the major AV cushions, a process that begins around ED12. Eventually, the EPDCs make up the vast majority of the mesenchyme of the lateral leaflets. While the mechanisms that drive the population of the lateral cushions by the EPDCs have yet to be elucidated, it is tempting to speculate that they involve molecular cues unique to the tissues that are found in the lateral AV junction and/or cushions. The development of the lateral AV cushions has historically received very little attention, notwithstanding the significant contribution they make to the mature leaflets of the AV valves (Lamers and Moorman, 2002) (Snarr et al., 2008) and the involvement of these leaflets in pathologies of the heart, such as mitral valve prolapse and Ebstein’s anomaly (Ho et al., 2000; Paranon and Acar, 2008). The left lateral cushion gives rise to the mural (or posterior) leaflet of the mitral valve, a leaflet that is commonly observed to undergo myxomatous degeneration in the pathology of mitral valve prolapse. The right lateral cushion gives rise to the parietal leaflet of the right AV valve (mouse) or the antero-superior leaflet of the tricuspid valve in the human heart (Lamers et al., 1995). Little is known regarding why the lateral AV cushions, when compared to the major AV cushions, develop relatively late in development (de Lange et al., 2004; Wessels et al., 1996) and it is yet to be determined whether the well-studied molecular mechanisms that drive the formation of the major AV cushions (Eisenberg and Markwald, 1995; MacGrogan et al., 2011; Sugi et al., 2004) are also responsible for the formation of the lateral cushions. In this context it is important to note that in several respects their development does not mimic that of the major AV cushions. For instance, the development of the lateral cushions does not involve an initial stage in which they are seen as acellular puffy subendocardial spaces filled with significant amounts of extracellular matrix components. In fact, it appears that endocardial EMT is the driving force in the development of the lateral cushions. However, the lateral cushions never accumulate large amounts of endocardially derived cells. As demonstrated in this paper, the growth of the leaflets that derive from the lateral cushions, and hence the development of the mesenchyme/valvular fibroblasts therein, seems to rely heavily on the influx of EPDCs. Our current observations regarding the preferential migration of EPDCs into the lateral AV cushions and the importance of these cushions to valvuloseptal morphogenesis emphasizes that more research is needed to elucidate the molecular mechanisms underlying their development in both health and disease. While at present there is no evidence that EPDCs play a role in the valve pathologies that affect specific components of the valvular apparatus more than others, e.g. Ebstein’s Anomaly or Myxomatous Valve Disease, the differential contribution of EPDCs to the respective leaflets underscores the importance of understanding the spatiotemporal contribution of EPDCs to the heart.
Wt1cre-mG expression in the myocardium
The potential of EPDCs to differentiate into cardiomyocytes also continues to be a matter of controversy. In the early quail-to-chick proepicardial transplant studies, (pro) epicardially derived cardiomyocytes were never observed (Manner, 1999; Perez-Pomares et al., 2002). In vitro studies show, however, that under standard culture conditions, (pro) epicardial cells will readily differentiate into cardiomyocytes (Kruithof et al., 2006). From these observations it was inferred that, while EPDCs have the potential to undergo myocardial differentiation, during normal avian cardiac morphogenesis proepicardial cells do not differentiate into cardiomyocytes (van Wijk and van den Hoff). A few years ago, this paradigm was challenged in the work by two different groups. Using a Tbx18:cre mouse model, Cai and colleagues presented data that suggested that a large population of myocytes in the interventricular septum, as well as more isolated patches of cardiomyocytes within the walls of the heart, have an epicardial origin (Cai et al., 2008). An epicardial origin for a cardiomyocyte subpopulation was also suggested by Zhou and colleagues (Zhou et al., 2008), who used a Wt1GFPCre and an inducible Wt1CreERT2 mouse to perform epicardial cell trace experiments. While the distribution of the Wt1-cre labeled cardiomyocytes in the reporter mouse used by Zhou and co-workers differed from the one reported with the Tbx18-cre mouse, the general pattern of cre-induced lacZ expression was rather similar. The interpretation of the data in the Tbx18-cre mouse was challenged by Christoffels and colleagues who showed that the observed myocardial staining could be explained by the endogenous expression of Tbx18 in the myocardial cell lineage (Christoffels et al., 2009). Recently, the Tabin laboratory published an epicardial cell fate study using two new epicardial-cre models; the Sema3DeGFPcre and the ScxGFPCre mouse. Although the studies in this paper were focused on investigating the epicardial origin of coronary endothelium, they also reported that, specifically when using the ScxGFPCre mouse in combination with the ROSA26- lacZ, a significant amount of right ventricular myocardium in the right ventricular wall gets labeled. It is noteworthy that even though the use of every single epicardial-cre in combination with ROSA26 reporter mice does lead to labeling of subsets of myocardial cells, the distribution of labeled myocardial cells significantly differs from model to model. As has been discussed by others (Christoffels et al., 2009; Katz et al., 2012) the use of “tissue-specific” cre mice carries the inherent risk that the cre-recombinase might be leaky and will recombine in other cell types than the one which is specifically being targeted and/or that low levels of the factors that drive the cre-constructs are being expressed in these cells. The labeling in our Wt1cre-mG hearts does not show the intense and extensive staining observed in the above mentioned studies in which the ROSA26-lacZ reporter mouse was used. The relatively low number of Wt1cre-EGFP positive myocytes in the interventricular septum seems to correlate with the distribution of Wt1 mRNA expressing cells. we have not observed co-expression of Wt1 protein with Wt1cre-EGFP and MF20 in triple immunofluorescent labeling studies. Thus, while our epicardial cell-fate experiments certainly do not show the extensive myocardial staining seen in previous studies (Cai et al., 2008; Zhou et al., 2008), we cannot exclude the possibility that a subset of myocardial cells may share a common progenitor with the epicardium or may actually be epicardially derived.
Conclusions
Using an mWt1/IRES/GFP-Cre (Wt1Cre) mouse we have studied the contribution of epicardially-derived cells (EPDCs) to the developing mouse heart with emphasis on the emerging populations of fibroblasts and fibroblast-like cells. In concordance with previous reports, we found that EPDCs contribute to the majority, if not all, of the interstitial fibroblasts found in the embryonic myocardium. Remarkably, while epicardially-derived fibroblasts start to migrate into the compact ventricular myocardium around ED12, and have populated this myocardium by ED14, they do not migrate into the trabecular myocardium until after ED17. Migration of EPDCs through the atrioventricular myocardium and into the endocardially-derived lateral atrioventricular cushions begins around ED12. As development progresses the epicardially-derived fibroblasts eventually become the predominant cell type in the leaflets that derive from these lateral cushions. Importantly, the contribution of EPDCs to the leaflets derived from the major AV cushions is very limited. This new paradigm in valve development has significant pragmatic consequences for experimental studies and the interpretation of experimental results when using endocardial and/or epicardial specific cre-mice. More importantly, this new insight into the origins of the valvular mesenchyme of the respective leaflets of the AV valves may lead to the development of new testable hypotheses regarding the pathogenesis of cardiac abnormalities and diseases that preferentially affect individual leaflets of the AV valvuloseptal complex.
EPDCs do not invade trabecular myocardium until after ED17
EPDCs preferentially populate the lateral atrioventricular cushions
EPDCs do not significantly contribute to the major atrioventricular cushions
EPDCs in valves express characteristic fibroblast markers
EPDCs significantly contribute to the VICs of postnatal AV valves
Acknowledgements
The authors would like to thank Dr. Brian Snarr for helpful discussions during preparation of the manuscript. The authors also would like to acknowledge the financial support by the following grants: NCRR C06 RR018823, NCRR C06 RR015455, 3P20RR016434-10S1 (A.W., R.A.N.), NIH-NHLBI R01HL084285 (A.W.), NIH-NHLBI R01-HL055373 (J.B.E.B.), NIH 5T32 HL007260 (M.M.L), NIH 5T32-GM008716-12 (L.E.B.), AHA 09GRNT2060075 (A.W.), AHA GIA 0455612Z (R.D.), AHA 0855946G (R.D), AHA 11PRE7310036 (L.E.B.), AHA 11SDG5270006 (R.A.N.), The Foundation Leducq (Paris, France) Transatlantic Mitral Network of Excellence grant 07CVD04 (R.A.N.), European Community’s Sixth Framework Program Grant LSHM-CT-2005-018630 (MJBvdH and BvW), Netherlands Heart Foundation Grant 1996M002 (MJBvdH and BvW), and BFU-2009-07929 and TERCEL Cooperative Research Network (J.M.P-P)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aanhaanen WT, Moorman AF, Christoffels VM. Origin and development of the atrioventricular myocardial lineage: insight into the development of accessory pathways. Birth Defects Res A Clin Mol Teratol. 2011;91:565–577. doi: 10.1002/bdra.20826. [DOI] [PubMed] [Google Scholar]
- Acharya A, Baek ST, Banfi S, Eskiocak B, Tallquist MD. Efficient inducible Cre-mediated recombination in Tcf21cell lineages in the heart and kidney. Genesis. 2011;49:870–877. doi: 10.1002/dvg.20750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Chaboissier MC, Behringer RR, Rowitch DH, Schedl A, Epstein JA, de Crombrugghe B. Essential role of Sox9 in the pathway that controls formation of cardiac valves and septa. Proc Natl Acad Sci U S A. 2004;101:6502–6507. doi: 10.1073/pnas.0401711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker ML, Christoffels VM, Moorman AF. The cardiac pacemaker and conduction system develops from embryonic myocardium that retains its primitive phenotype. J Cardiovasc Pharmacol. 2010;56:6–15. doi: 10.1097/FJC.0b013e3181e775d3. [DOI] [PubMed] [Google Scholar]
- Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol. 2007;293:H1883–H1891. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J, Evans SM. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffels VM, Grieskamp T, Norden J, Mommersteeg MT, Rudat C, Kispert A. Tbx18 and the fate of epicardial progenitors. Nature. 2009;458:E8–E9. doi: 10.1038/nature07916. discussion E9–10. [DOI] [PubMed] [Google Scholar]
- de Jong F, Opthof T, Wilde AA, Janse MJ, Charles R, Lamers WH, Moorman AF. Persisting zones of slow impulse conduction in developing chicken hearts. Circ Res. 1992;71:240–250. doi: 10.1161/01.res.71.2.240. [DOI] [PubMed] [Google Scholar]
- de Lange FJ, Moorman AF, Anderson RH, Manner J, Soufan AT, de Gier-de Vries C, Schneider MD, Webb S, van den Hoff MJ, Christoffels VM. Lineage and morphogenetic analysis of the cardiac valves. Circ Res. 2004;95:645–654. doi: 10.1161/01.RES.0000141429.13560.cb. [DOI] [PubMed] [Google Scholar]
- del Monte G, Casanova JC, Guadix JA, MacGrogan D, Burch JB, Perez-Pomares JM, de la Pompa JL. Differential Notch signaling in the epicardium is required for cardiac inflow development and coronary vessel morphogenesis. Circ Res. 2011;108:824–836. doi: 10.1161/CIRCRESAHA.110.229062. [DOI] [PubMed] [Google Scholar]
- Dettman RW, Denetclaw W, Jr, Ordahl CP, Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Developmental biology. 1998;193:169–181. doi: 10.1006/dbio.1997.8801. [DOI] [PubMed] [Google Scholar]
- Eisenberg LM, Markwald RR. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ Res. 1995;77:1–6. doi: 10.1161/01.res.77.1.1. [DOI] [PubMed] [Google Scholar]
- Gaussin V, Van de Putte T, Mishina Y, Hanks MC, Zwijsen A, Huylebroeck D, Behringer RR, Schneider MD. Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3. Proc Natl Acad Sci U S A. 2002;99:2878–2883. doi: 10.1073/pnas.042390499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittenberger-de Groot AC, Vrancken Peeters MP, Bergwerff M, Mentink MM, Poelmann RE. Epicardial outgrowth inhibition leads to compensatory mesothelial outflow tract collar and abnormal cardiac septation and coronary formation. Circ Res. 2000;87:969–971. doi: 10.1161/01.res.87.11.969. [DOI] [PubMed] [Google Scholar]
- Gittenberger-de Groot AC, Vrancken Peeters MP, Mentink MM, Gourdie RG, Poelmann RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ Res. 1998;82:1043–1052. doi: 10.1161/01.res.82.10.1043. [DOI] [PubMed] [Google Scholar]
- Hajdu Z, Romeo SJ, Fleming PA, Markwald RR, Visconti RP, Drake CJ. Recruitment of bone marrow-derived valve interstitial cells is a normal homeostatic process. J Mol Cell Cardiol. 2011;51:955–965. doi: 10.1016/j.yjmcc.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SY, Goltz D, McCarthy K, Cook AC, Connell MG, Smith A, Anderson RH. The atrioventricular junctions in Ebstein malformation. Heart. 2000;83:444–449. doi: 10.1136/heart.83.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icardo JM, Arrechedera H, Colvee E. The atrioventricular valves of the mouse. I. A scanning electron microscope study. J Anat. 1993;182(Pt 1):87–94. [PMC free article] [PubMed] [Google Scholar]
- Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, Srivastava D. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell. 2009;16:233–244. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz TC, Singh MK, Degenhardt K, Rivera-Feliciano J, Johnson RL, Epstein JA, Tabin CJ. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev Cell. 2012;22:639–650. doi: 10.1016/j.devcel.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Nakamura F, Lee W, Shifrin Y, Arora P, McCulloch CA. Filamin A is required for vimentin-mediated cell adhesion and spreading. Am J Physiol Cell Physiol. 2010;298:C221–C236. doi: 10.1152/ajpcell.00323.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Kolditz DP, Wijffels MC, Blom NA, van der Laarse A, Hahurij ND, Lie-Venema H, Markwald RR, Poelmann RE, Schalij MJ, Gittenberger-de Groot AC. Epicardium-derived cells in development of annulus fibrosis and persistence of accessory pathways. Circulation. 2008;117:1508–1517. doi: 10.1161/CIRCULATIONAHA.107.726315. [DOI] [PubMed] [Google Scholar]
- Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol. 2010;225:631–637. doi: 10.1002/jcp.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruithof BP, van Wijk B, Somi S, Kruithof-de Julio M, Perez Pomares JM, Weesie F, Wessels A, Moorman AF, van den Hoff MJ. BMP and FGF regulate the differentiation of multipotential pericardial mesoderm into the myocardial or epicardial lineage. Dev Biol. 2006;295:507–522. doi: 10.1016/j.ydbio.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Lamers WH, Moorman AF. Cardiac septation: a late contribution of the embryonic primary myocardium to heart morphogenesis. Circ Res. 2002;91:93–103. doi: 10.1161/01.res.0000027135.63141.89. [DOI] [PubMed] [Google Scholar]
- Lamers WH, Viragh S, Wessels A, Moorman AF, Anderson RH. Formation of the tricuspid valve in the human heart. Circulation. 1995;91:111–121. doi: 10.1161/01.cir.91.1.111. [DOI] [PubMed] [Google Scholar]
- Lie-Venema H, Gittenberger-de Groot AC, van Empel LJ, Boot MJ, Kerkdijk H, de Kant E, DeRuiter MC. Ets-1 and Ets-2 transcription factors are essential for normal coronary and myocardial development in chicken embryos. Circ Res. 2003;92:749–756. doi: 10.1161/01.RES.0000066662.70010.DB. [DOI] [PubMed] [Google Scholar]
- Lie-Venema H, van den Akker NM, Bax NA, Winter EM, Maas S, Kekarainen T, Hoeben RC, deRuiter MC, Poelmann RE, Gittenberger-de Groot AC. Origin, fate, and function of epicardium-derived cells (EPDCs) in normal and abnormal cardiac development. Scientific World Journal. 2007;7:1777–1798. doi: 10.1100/tsw.2007.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln J, Kist R, Scherer G, Yutzey KE. Sox9 is required for precursor cell expansion and extracellular matrix organization during mouse heart valve development. Dev Biol. 2007;305:120–132. doi: 10.1016/j.ydbio.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGrogan D, Luna-Zurita L, de la Pompa JL. Notch signaling in cardiac valve development and disease. Birth Defects Res A Clin Mol Teratol. 2011;91:449–459. doi: 10.1002/bdra.20815. [DOI] [PubMed] [Google Scholar]
- Manner J. Does the subepicardial mesenchyme contribute myocardioblasts to the myocardium of the chick embryo heart? A quail-chick chimera study tracing the fate of the epicardial primordium. Anat Rec. 1999;255:212–226. doi: 10.1002/(sici)1097-0185(19990601)255:2<212::aid-ar11>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- Markwald RR, Fitzharris TP, Manasek FJ. Structural development of endocardial cushions. Am J Anat. 1977;148:85–119. doi: 10.1002/aja.1001480108. [DOI] [PubMed] [Google Scholar]
- Mellgren AM, Smith CL, Olsen GS, Eskiocak B, Zhou B, Kazi MN, Ruiz FR, Pu WT, Tallquist MD. Platelet-derived growth factor receptor beta signaling is required for efficient epicardial cell migration and development of two distinct coronary vascular smooth muscle cell populations. Circ Res. 2008;103:1393–1401. doi: 10.1161/CIRCRESAHA.108.176768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merki E, Zamora M, Raya A, Kawakami Y, Wang J, Zhang X, Burch J, Kubalak SW, Kaliman P, Belmonte JC, Chien KR, Ruiz-Lozano P. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc Natl Acad Sci U S A. 2005;102:18455–18460. doi: 10.1073/pnas.0504343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AW, McInnes L, Kreidberg J, Hastie ND, Schedl A. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development. 1999;126:1845–1857. doi: 10.1242/dev.126.9.1845. [DOI] [PubMed] [Google Scholar]
- Moorman AF, Christoffels VM. Cardiac chamber formation: development, genes, and evolution. Physiol Rev. 2003;83:1223–1267. doi: 10.1152/physrev.00006.2003. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Norden J, Grieskamp T, Lausch E, van Wijk B, van den Hoff MJ, Englert C, Petry M, Mommersteeg MT, Christoffels VM, Niederreither K, Kispert A. Wt1 and retinoic acid signaling in the subcoelomic mesenchyme control the development of the pleuropericardial membranes and the sinus horns. Circ Res. 2010;106:1212–1220. doi: 10.1161/CIRCRESAHA.110.217455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RA, Moreno-Rodriguez R, Wessels A, Merot J, Bruneval P, Chester AH, Yacoub MH, Hagege A, Slaugenhaupt SA, Aikawa E, Schott JJ, Lardeux A, Harris BS, Williams LK, Richards A, Levine RA, Markwald RR. Expression of the familial cardiac valvular dystrophy gene, filamin-A, during heart morphogenesis. Dev Dyn. 2010;239:2118–2127. doi: 10.1002/dvdy.22346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranon S, Acar P. Ebstein's anomaly of the tricuspid valve: from fetus to adult: congenital heart disease. Heart. 2008;94:237–243. doi: 10.1136/hrt.2006.105262. [DOI] [PubMed] [Google Scholar]
- Perez-Pomares JM, Phelps A, Sedmerova M, Carmona R, Gonzalez-Iriarte M, Munoz-Chapuli R, Wessels A. Experimental studies on the spatiotemporal expression of WT1 and RALDH2 in the embryonic avian heart: a model for the regulation of myocardial and valvuloseptal development by epicardially derived cells (EPDCs) Dev Biol. 2002;247:307–326. doi: 10.1006/dbio.2002.0706. [DOI] [PubMed] [Google Scholar]
- Prunotto M, Caimmi PP, Bongiovanni M. Cellular pathology of mitral valve prolapse. Cardiovasc Pathol. 19:e113–e117. doi: 10.1016/j.carpath.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Runyan RB, Markwald RR. Invasion of mesenchyme into three-dimensional collagen gels: a regional and temporal analysis of interaction in embryonic heart tissue. Dev Biol. 1983;95:108–114. doi: 10.1016/0012-1606(83)90010-6. [DOI] [PubMed] [Google Scholar]
- Smart N, Bollini S, Dube KN, Vieira JM, Zhou B, Davidson S, Yellon D, Riegler J, Price AN, Lythgoe MF, Pu WT, Riley PR. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–644. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CL, Baek ST, Sung CY, Tallquist MD. Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circ Res. 2011;108:e15–e26. doi: 10.1161/CIRCRESAHA.110.235531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snarr BS, Kern CB, Wessels A. Origin and fate of cardiac mesenchyme. Dev Dyn. 2008;237:2804–2819. doi: 10.1002/dvdy.21725. [DOI] [PubMed] [Google Scholar]
- Snarr BS, O'Neal JL, Chintalapudi MR, Wirrig EE, Phelps AL, Kubalak SW, Wessels A. Isl1 expression at the venous pole identifies a novel role for the second heart field in cardiac development. Circ Res. 2007a;101:971–974. doi: 10.1161/CIRCRESAHA.107.162206. [DOI] [PubMed] [Google Scholar]
- Snarr BS, Wirrig EE, Phelps AL, Trusk TC, Wessels A. A spatiotemporal evaluation of the contribution of the dorsal mesenchymal protrusion to cardiac development. Dev Dyn. 2007b;236:1287–1294. doi: 10.1002/dvdy.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider P, Olaopa M, Firulli AB, Conway SJ. Cardiovascular development and the colonizing cardiac neural crest lineage. Scientific World Journal. 2007;7:1090–1113. doi: 10.1100/tsw.2007.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider P, Standley KN, Wang J, Azhar M, Doetschman T, Conway SJ. Origin of cardiac fibroblasts and the role of periostin. Circ Res. 2009;105:934–947. doi: 10.1161/CIRCRESAHA.109.201400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi Y, Yamamura H, Okagawa H, Markwald RR. Bone morphogenetic protein-2 can mediate myocardial regulation of atrioventricular cushion mesenchymal cell formation in mice. Dev Biol. 2004;269:505–518. doi: 10.1016/j.ydbio.2004.01.045. [DOI] [PubMed] [Google Scholar]
- van Wijk B, van den Hoff M. Epicardium and myocardium originate from a common cardiogenic precursor pool. Trends Cardiovasc Med. 20:1–7. doi: 10.1016/j.tcm.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Vasquez C, Mohandas P, Louie KL, Benamer N, Bapat AC, Morley GE. Enhanced fibroblast-myocyte interactions in response to cardiac injury. Circ Res. 107:1011–1020. doi: 10.1161/CIRCRESAHA.110.227421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Hernandez M, Kovacs A, De Langhe S, Ornitz DM. FGF10/FGFR2b signaling is essential for cardiac fibroblast development and growth of the myocardium. Development. 2011;138:3331–3340. doi: 10.1242/dev.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viragh S, Challice CE. The origin of the epicardium and the embryonic myocardial circulation in the mouse. Anat Rec. 1981;201:157–168. doi: 10.1002/ar.1092010117. [DOI] [PubMed] [Google Scholar]
- Waller BR, 3rd, Wessels A. Cardiac morphogenesis and dysmorphogenesis. An immunohistochemical approach. Methods Mol Biol. 2000;135:151–161. doi: 10.1385/1-59259-685-1:151. [DOI] [PubMed] [Google Scholar]
- Wessels A, Markman MW, Vermeulen JL, Anderson RH, Moorman AF, Lamers WH. The development of the atrioventricular junction in the human heart. Circ Res. 1996;78:110–117. doi: 10.1161/01.res.78.1.110. [DOI] [PubMed] [Google Scholar]
- Wessels A, Markwald R. Cardiac morphogenesis and dysmorphogenesis. I. Normal development. Methods Mol Biol. 2000;136:239–259. doi: 10.1385/1-59259-065-9:239. [DOI] [PubMed] [Google Scholar]
- Wessels A, Perez-Pomares JM. The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells. The anatomical record. 2004;276:43–57. doi: 10.1002/ar.a.10129. [DOI] [PubMed] [Google Scholar]
- Wessels A, Sedmera D. Developmental anatomy of the heart: a tale of mice and man. Physiol Genomics. 2003;15:165–176. doi: 10.1152/physiolgenomics.00033.2003. [DOI] [PubMed] [Google Scholar]
- Wessels A, Vermeulen JL, Verbeek FJ, Viragh S, Kalman F, Lamers WH, Moorman AF. Spatial distribution of "tissue-specific" antigens in the developing human heart and skeletal muscle. III. An immunohistochemical analysis of the distribution of the neural tissue antigen G1N2 in the embryonic heart; implications for the development of the atrioventricular conduction system. Anat Rec. 1992;232:97–111. doi: 10.1002/ar.1092320111. [DOI] [PubMed] [Google Scholar]
- Wessels A, Vermeulen JL, Viragh S, Kalman F, Lamers WH, Moorman AF. Spatial distribution of "tissue-specific" antigens in the developing human heart and skeletal muscle. II. An immunohistochemical analysis of myosin heavy chain isoform expression patterns in the embryonic heart. Anat Rec. 1991;229:355–368. doi: 10.1002/ar.1092290309. [DOI] [PubMed] [Google Scholar]
- Wessels A, Vermeulen JL, Viragh S, Kalman F, Morris GE, Man NT, Lamers WH, Moorman AF. Spatial distribution of "tissue-specific" antigens in the developing human heart and skeletal muscle. I. An immunohistochemical analysis of creatine kinase isoenzyme expression patterns. Anat Rec. 1990;228:163–176. doi: 10.1002/ar.1092280208. [DOI] [PubMed] [Google Scholar]
- Winter EM, Gittenberger-de Groot AC. Epicardium-derived cells in cardiogenesis and cardiac regeneration. Cell Mol Life Sci. 2007;64:692–703. doi: 10.1007/s00018-007-6522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter EM, van Oorschot AA, Hogers B, van der Graaf LM, Doevendans PA, Poelmann RE, Atsma DE, Gittenberger-de Groot AC, Goumans MJ. A new direction for cardiac regeneration therapy: application of synergistically acting epicardium-derived cells and cardiomyocyte progenitor cells. Circ Heart Fail. 2009;2:643–653. doi: 10.1161/CIRCHEARTFAILURE.108.843722. [DOI] [PubMed] [Google Scholar]
- Wu M, Smith CL, Hall JA, Lee I, Luby-Phelps K, Tallquist MD. Epicardial spindle orientation controls cell entry into the myocardium. Dev Cell. 2010;19:114–125. doi: 10.1016/j.devcel.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg EM, Kalluri R. Origins of cardiac fibroblasts. Circ Res. 2010;107:1304–1312. doi: 10.1161/CIRCRESAHA.110.231910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng B, Ren XF, Cao F, Zhou XY, Zhang J. Developmental patterns and characteristics of epicardial cell markers Tbx18 and Wt1 in murine embryonic heart. J Biomed Sci. 2011;18:67. doi: 10.1186/1423-0127-18-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, von Gise A, Ma Q, Hu YW, Pu WT. Genetic fate mapping demonstrates contribution of epicardium-derived cells to the annulus fibrosis of the mammalian heart. Dev Biol. 2010;338:251–261. doi: 10.1016/j.ydbio.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]