Abstract
OBJECTIVE
Diaphragmatic weakness, due to both atrophy and contractile dysfunction, is a well-documented response following prolonged mechanical ventilation (MV). Evidence indicates that activation of the proteases calpain and caspase-3 are essential for MV-induced diaphragmatic weakness to occur. We tested the hypothesis that a regulatory cross-talk exists between calpain and caspase-3 in the diaphragm during prolonged MV. To test this prediction, we determined if selective pharmacological inhibition of calpain would prevent activation of caspase-3 and conversely, if selective inhibition of caspase-3 would abate calpain activation.
DESIGN
Animal study.
SETTING
University Research Laboratory
SUBJECTS
Female Sprague-Dawley rats
INTERVENTIONS
Animals were randomly divided into a control or one of three 12 hour MV groups that were treated with/without a selective pharmacological protease inhibitor: 1) control; 2) MV; 3) MV with a selective caspase-3 inhibitor; and 4) MV with a selective calpain inhibitor.
MEASUREMENTS AND MAIN RESULTS
Compared to control, MV resulted in calpain and caspase-3 activation in the diaphragm accompanied by atrophy of type I, type IIa, and type IIx/IIb fibers. Independent inhibition of either calpain or caspase-3 prevented this MV-induced atrophy. Pharmacological inhibition of calpain prevented MV-induced activation of diaphragmatic caspase-3 and inhibition of caspase-3 prevented activation of diaphragmatic calpain. Further, calpain inhibition also prevented the activation of caspase-9 and caspase-12, along with the cleavage of Bid to tBid, all upstream signals for caspase-3 activation. Lastly, caspase-3 inhibition prevented the MV-induced degradation of the endogenous calpain inhibitor, calpastatin.
CONCLUSIONS
Collectively, these results indicate that MV-induced diaphragmatic atrophy is dependent upon the activation of both calpain and caspase-3. Importantly, these findings provide the first experimental evidence in diaphragm muscle that calpain inhibition prevents the activation of caspase-3 and vice versa, caspase-3 inhibition prevents the activation of calpain. These findings support our hypothesis that a regulatory calpain/caspase-3 cross-talk exists whereby calpain can promote caspase-3 activation and active caspase-3 can enhance calpain activity in diaphragm muscle during prolonged MV.
Keywords: mechanical ventilation, diaphragm, calpain, caspase-3, skeletal muscle, atrophy
INTRODUCTION
Mechanical ventilation (MV) is used clinically to maintain adequate alveolar ventilation for patients unable to do so. Although MV is a life-saving intervention in patients suffering from respiratory failure, prolonged MV results in a rapid development of diaphragm weakness, due to both diaphragmatic atrophy and contractile dysfunction (1–3). MV-induced diaphragmatic weakness is potentially important because respiratory muscle weakness is predicted to contribute to the difficulties in weaning patients from the ventilator (4). At present, the signaling pathways responsible for this rapid onset of diaphragmatic weakness remain unclear. Therefore, insight into these pathways is essential to develop a therapeutic strategy for preventing MV-induced diaphragmatic weakness.
During prolonged MV, several key proteolytic enzymes are activated in diaphragm muscle, including calpain, caspase-3, and the proteasome (2, 5). Although all three proteases contribute to proteolysis, it appears that calpain and caspase-3 play a crucial role in MV-induced diaphragmatic weakness. Indeed, pharmacological inhibition of calpain can protect the diaphragm from MV-induced proteolysis, atrophy and contractile dysfunction (6). Further, inhibition of caspase-3 can also protect against MV-induced diaphragmatic atrophy (5). Together, these findings raise an intriguing question, why does selective inhibition of either protease protect the diaphragm from MV-induced dysfunction? A potential answer to this question is that a regulatory cross-talk exists between calpain and caspase-3 in the diaphragm during prolonged MV, whereby they can activate each other. It is currently unknown if a regulatory cross-talk exists in skeletal muscle, but it has been reported that in neurons during cerebral ischemia reperfusion injury, calpain can activate caspase-3 and conversely, caspase-3 can regulate calpain activation (7). Several potential mechanisms may explain this regulatory interaction in neurons. For example, it is feasible that active caspase-3 can promote calpain activation by degrading the endogenous calpain inhibitor, calpastatin (8). Moreover, calpain can facilitate caspase-3 activation via several potential upstream pathways (e.g. activation of Bid and/or Bax) (9–11). Based upon both published work and our preliminary experiments we formulated the hypothesis that during prolonged MV, a regulatory cross-talk occurs in the diaphragm between the calpain and caspase-3 proteolytic systems, whereby active calpain can activate caspase-3 and vice versa. Our findings support this hypothesis and reveal that during MV, inhibition of diaphragmatic calpain activity prevented activation of caspase-3 and inhibition of caspase-3 prevented activation of calpain. These data provide the first evidence that during prolonged MV, calpain and caspase-3 participate in regulatory cross-talk in diaphragm muscle.
METHODS
Experimental Design
Young adult female Sprague-Dawley rats were assigned to one of four experimental groups (n=8 per group), 1) control, 2) 12 hrs of MV 3) 12 hrs of MV with a specific caspase-3 inhibitor 4) 12 hrs of MV with a specific calpain inhibitor. The Institutional Animal Care and Use Committee of the University of Florida approved these experiments.
Control Animals and Mechanical Ventilation
Control animals were acutely anesthetized with sodium pentobarbital (60 mg/kg body weight IP). After reaching a surgical plane of anesthesia, the diaphragms were quickly removed and the costal diaphragm was divided into several segments. A strip of the medial costal diaphragm was immediately used for in vitro contractile measurements, a separate section was stored for histological measurements, and the remaining portions of the costal diaphragm were rapidly frozen in liquid nitrogen and stored at −80°C for subsequent biochemical analyses.
MV animals were tracheostomized and mechanically ventilated with a pressure-controlled ventilator (Servo Ventilator 300, Siemens AG; Munich, Germany) for 12 hours as previously reported (12).
Calpain Inhibition
To prevent MV-induced diaphragmatic calpain activation, we administered 3 mg/kg body weight of SJA-6017 dissolved in 88% propylene, 10% ethyl alcohol, 2% benzyl alcohol and given intravenously as a bolus at the beginning of MV (Calpain Inhibitor VI, N-(4-fluorophenylsulfonyl)-L-valyl-L-leucinal, EMD Chemicals, Gibbstown, NJ).
Caspase-3 Inhibition
To prevent MV-induced diaphragmatic caspase-3 activation we administered 3 mg/kg body weight of AC-DEVD-CHO dissolved in 0.9% sterile saline and given intravenously as a bolus at the beginning of MV (AC-DEVD-CHO [Asp-Glu-Val-Asp-CHO] Enzo Life Sciences, Farmingdale, NY).
Western Blot Analysis
Diaphragmatic protein extracts were assayed as previously described (12). Membranes were probed for 4-HNE (Abcam, Cambridge, MA), (active) calpain-1, cleaved caspase-3, cleaved caspase-9, cleaved caspase-8 (Cell Signaling Technology, Danvers, MA), Bid/tBid (Imgenex, San Diego, CA), total calpain, calpastatin, α-II spectrin and cleaved caspase-12 (Santa Cruz Biotechnology, Santa Cruz, CA). To control for protein loading and transfer differences, membranes were stained with Ponceau S (see online supplement). Ponceau S stained membranes were scanned and the lanes were quantified (440CF imaging system, Kodak, New Haven, CT) to normalize Western blots to protein loading.
Measurement of In Vitro Diaphragmatic Contractile Properties
Upon sacrifice, a muscle strip, including the tendinous attachments at the central tendon and rib cage was dissected from the mid-costal region. The strip was suspended vertically with one end connected to an isometric force transducer (model FT-03, Grass Instruments, Quincy, MA) within a jacketed tissue bath and diaphragm skeletal muscle contractile properties were measured as previously reported (3).
Myofiber Cross-Sectional Area
Sections from frozen diaphragm samples were cut at 10 µm using a cryotome (Shandon Inc., Pittsburgh, PA) and immunohistochemically stained as described previously (5). CSA was determined using Scion software (NIH, Bethesda, MD).
Statistical Analysis
Comparisons between groups for each dependent variable were made by a one-way analysis of variance (ANOVA) and, when appropriate, a Tukey HSD (honestly significant difference) test was performed post-hoc. Significance was established at p < 0.05. Data are presented as means ± SEM.
RESULTS
Physiological responses to prolonged MV
To ensure that our MV protocol was successful in maintaining homeostasis, we measured arterial blood pressures, arterial PCO2, arterial PO2 and arterial pH in all animals at the beginning of the experiments and at various time intervals during MV. Our results confirm that arterial blood pressure (mean 102 ± 4 mmHg), arterial PO2 (mean 87 ± 5 torr [11.6 ± 0.6 kPa]), arterial PCO2 (mean 39 ± 2 torr [5.2 ± 0.3 kPa]) and pH (range 7.3–7.5) were maintained during MV. Furthermore, during the course of MV, no significant (p<0.05) changes occurred in the body weights of our animals.
Pharmacological inhibitors selectively inhibited their respective proteases and did not exhibit antioxidant properties
We evaluated the selectivity of our calpain and caspase-3 inhibitors with independent in vitro activity assays using the predicted peak in vivo concentrations of each inhibitor. Our results reveal that calpain proteolytic activity in vitro was not diminished when incubated in the presence of the casapse-3 inhibitor. Furthermore, caspase-3 proteolytic activity was not reduced when incubated in the presence of the calpain inhibitor. Finally, our results also reveal that caspase-9 enzymatic activity was not blunted when incubated in the presence of the calpain inhibitor (see online supplement). Note, however, that caspase-9 activity was diminished in the presence of the caspase-3 inhibitor. Due to the lack of a commercially available purified caspase-12 enzyme, we were not able to determine the effects of the calpain or caspase-3 inhibitor on caspase-12 activity. Collectively, these results indicate that our primary experimental findings are not influenced by off-target effects of our pharmacological inhibitors.
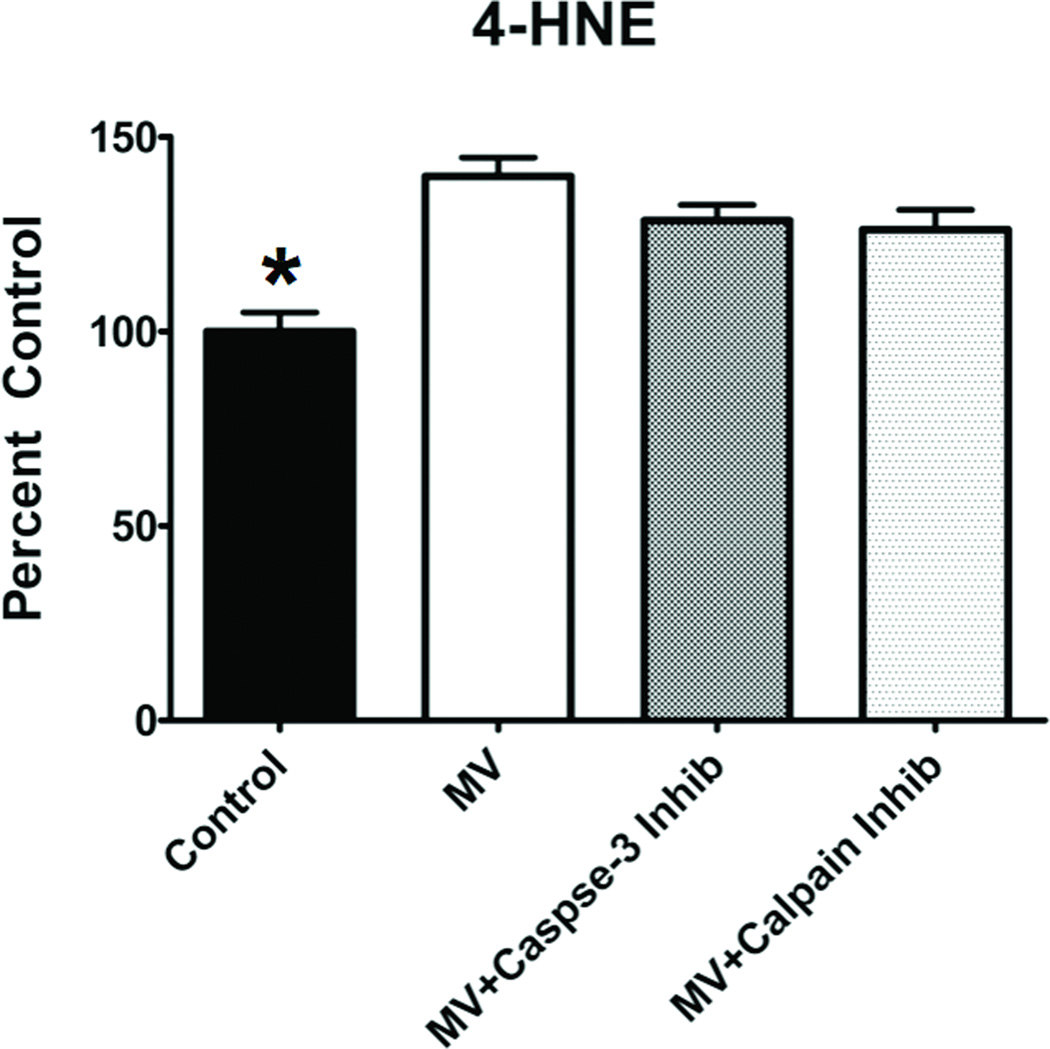
Furthermore, to determine if our protease inhibitors exhibited antioxidant properties and protected against MV-induced oxidative damage in the diaphragm we measured a reliable biomarker of oxidative damage (i.e., 4-HNE conjugated proteins). Compared to control, diaphragmatic levels of 4-HNE were higher in all of the MV groups (figure 1). Importantly, no differences existed in diaphragmatic levels of 4-HNE between the MV groups, indicating that the proteolytic inhibitors did not exhibit antioxidant properties.
Figure 1.
Levels of 4-hydroxyl-nonenal-conjugated (4-HNE) proteins in the diaphragm of the four experimental groups. Values are means ± SEM. * = different (p<0.05) from MV, MV+Calpain Inhibitor and MV+Caspase-3 Inhibitor groups.
Inhibition of calpain and caspase-3 prevents MV-induced contractile dysfunction and atrophy
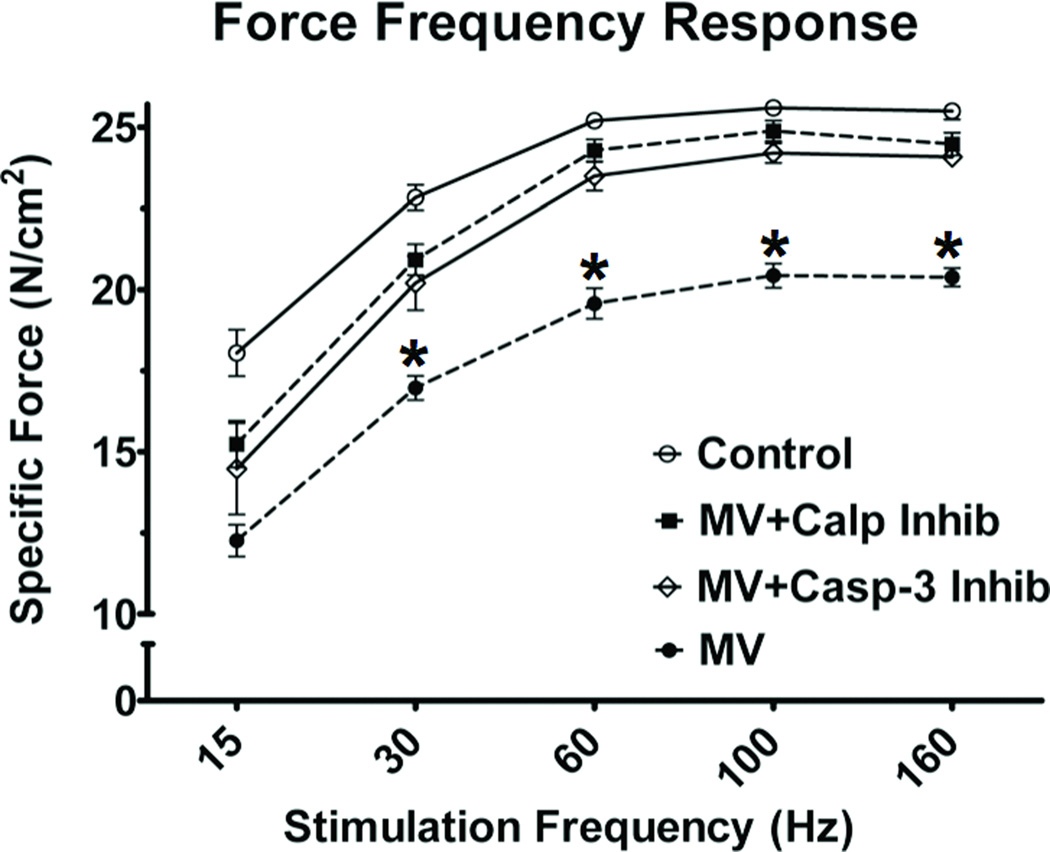
To assess the impact of 12 hours of MV on diaphragm contractile dysfunction, we measured diaphragm contractile performance in vitro using strips of diaphragm muscle. Similar to previous studies (3, 12–16), 12 hours of MV resulted in a significant decrease in diaphragmatic force production at both sub-maximal and maximal stimulation frequencies (figure 2). Selective inhibition of calpain and caspase-3 rescued the diaphragm from MV-induced contractile dysfunction at all stimulation frequencies above 15 Hz. Importantly, this is the first report indicating that inhibition of caspase-3 can protect the diaphragm from MV-induced contractile dysfunction.
Figure 2.
Effects of prolonged MV on the diaphragmatic force-frequency response (in vitro) in all four experimental groups. Values are means ± SEM. * = different (p<0.05) from all other groups.
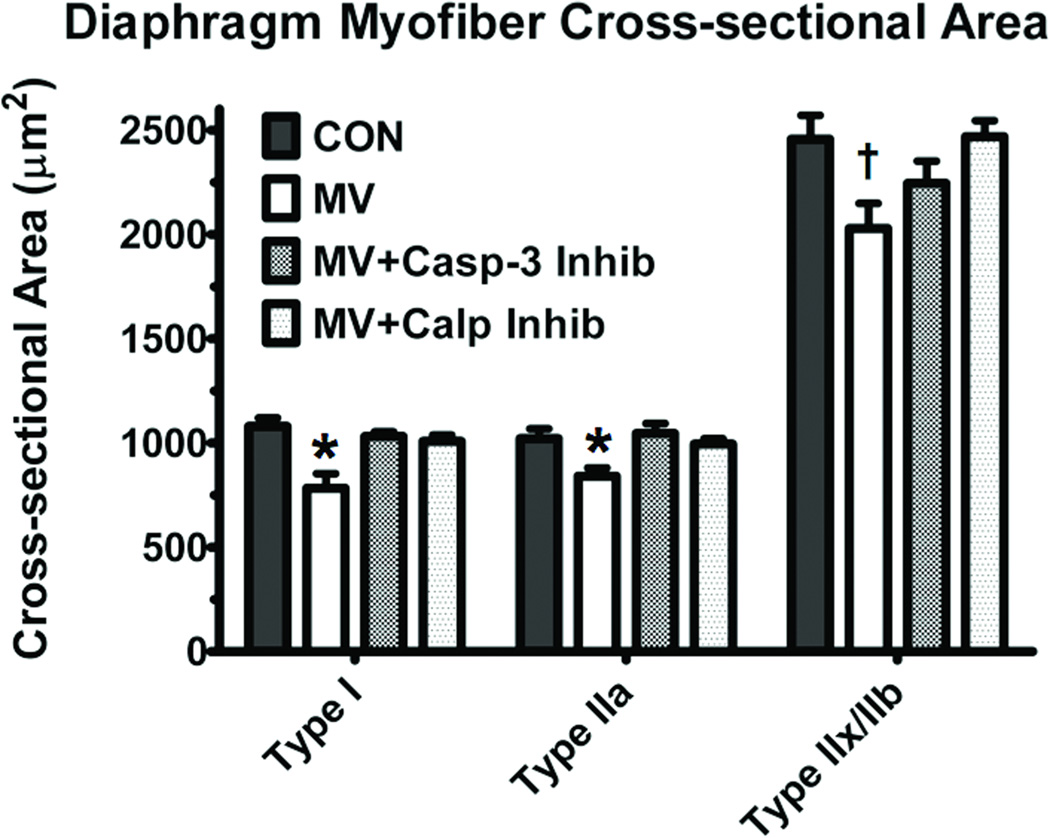
Previous work shows that both calpain and caspase-3 activation is sufficient to result in MV-induced diaphragmatic atrophy (5, 6). Indeed, compared to control animals, MV induced a ~27% decrease in fiber cross-sectional area in type I fibers, a ~18% decrease in type IIa fibers and a ~18% decrease in type IIx/IIb fibers. However, both calpain and caspase-3 inhibition prevented the MV-induced decreases in cross-sectional area of type I and IIa fiber types (figure 3). Additionally, calpain inhibition also prevented the MV-induced atrophy in type IIx/IIb fibers.
Figure 3.
Fiber cross-sectional area (CSA) in diaphragm muscle myofibers from all four experimental groups. Values are means ± SEM. * = different (p<0.05) from Control, MV+Calpain Inhibitor and MV+Caspase-3 Inhibitor groups. †= different (p<0.05) from both Control and MV+Calpain Inhibitor groups.
Calpain and caspase-3 activity increased during 12 hours of MV
Consistent with previous reports (5, 12), 12 hours of MV increased both calpain-1 and caspase-3 activity in the diaphragm (figures 4A and 4C). Further, MV increased diaphragmatic levels of the calpain and caspase-3 specific α-II spectrin degradation products; these independent degradation products are excellent biomarkers of in vivo protease activity (figure 4B and 4D).
Figure 4.
Calpain and Caspase-3 activity in the diaphragm of all four experimental groups. A) The active form of calpain 1 in diaphragm muscle at the completion of 12 hours of MV. B) Levels of the 145 kDa α-II-spectrin breakdown product (SBPD) in diaphragm muscle following 12 hours of MV. Note that the SBDP 145 kDa is an α-II-spectrin breakdown product that is specific to calpain cleavage of intact α-II-spectrin. C) The cleaved and active band of caspase-3 in diaphragm muscle at the completion of 12 hours of MV. D) Levels of the 120 kDa α-II-spectrin breakdown product (SBPD) in diaphragm muscle following 12 hours of MV. Note that the SBDP 120 kDa is an α-II-spectrin breakdown product that is specific to caspase-3 cleavage of intact α-II-spectrin. The images above the histograms in Figure 5 are representative western blots of data from the four experimental groups. Values are means ± SEM. * = different (p<0.05) from both Control and MV+Calpain Inhibitor groups. † = different (p<0.05) from both Control and MV+Caspase-3 Inhibitor groups. ‡ = different (p<0.05) from Control group.
The calpain inhibitor prevented calpain activity and the caspse-3 inhibitor prevented caspase-3 activity
To evaluate the effectiveness of our protease inhibitors, we measured markers of their respective protease activities after 12 hours of MV. As expected, pharmacological inhibition of calpain activity prevented MV-induced increases in active calpain-1 and the accumulation of the calpain specific α-II spectrin degradation product in the diaphragm (figure 4A and 4B). Similarly, pharmacological inhibition of caspase-3 prevented the MV-induced increases in active caspase-3 and the caspase-3 specific α-II spectrin degradation product within the diaphragm (figure 4C and 4D).
Calpain inhibition prevented caspase-3 activation and conversely, caspase-3 inhibition prevented calpain activation
Importantly, when animals were treated with the calpain inhibitor there was an attenuation in the MV-induced increase in active caspase-3 (figure 4C) and the caspase-3 specific α-II spectrin degradation product in the diaphragm (figure 4D). Similarly, 12 hours of MV with the caspase-3 inhibitor reduced the MV-induced increases in active calpain-1 (figure 4A) and the calpain-1 specific α-II spectrin cleavage product in diaphragm fibers (figure 4B). Collectively, these results indicate that inhibition of calpain prevents the activation of caspase-3 and vice versa. These findings are important and clearly support the hypothesis that regulatory cross-talk exists between calpain and caspase-3 in the diaphragm during prolonged MV.
Calpain inhibition alters upstream caspase-3 signaling
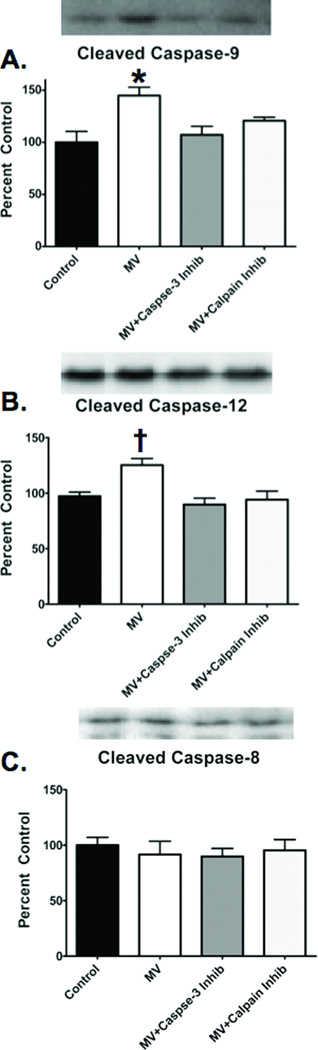
To investigate the basis for the regulatory cross-talk between calpain and caspase-3, we performed additional measurements to gain insight into how calpain can activate caspase-3. We measured the activities of both caspase-9 and 12, as these caspases are upstream activators of caspase-3 and both proteases can be activated either directly by calpain (e.g., caspase-12) or indirectly as a downstream by-product of calpain action (e.g., caspase-9) (17). Our results reveal that 12 hours of MV results in activation of both caspase-9 and caspase-12 in the diaphragm. However, pharmacological inhibition of calpain prevented the MV-induced activation of both caspase-9 and caspase-12 in the diaphragm (figure 5A and 5B). Further, to determine whether the death receptor caspase-8 pathway contributed to MV-induced caspase-3 activation, we measured caspase-8 activity in the diaphragm. Our results revealed that caspase-8 is not activated in the diaphragm during prolonged MV (figure 5C). These findings indicate that MV-induced caspase-3 activation in the diaphragm is regulated via an intrinsic pathway and not through an extracellular mechanism.
Figure 5.
Cleaved caspase-9, cleaved caspase-12 and cleaved caspase-8. A) Cleaved caspase-9 levels in the diaphragm of the four experimental groups. B) Cleaved caspase-12 levels in the diaphragm of the four experimental groups. C) Cleaved Casepase-8 levels in the diaphragm of the four experimental groups. The images above the histograms in Figures 6 are representative western blots from the four experimental groups. Values are means ± SEM. * = different (p<0.05) from Control and Caspase-3 Inhibitor groups. † = different (p<0.05) from all other groups.
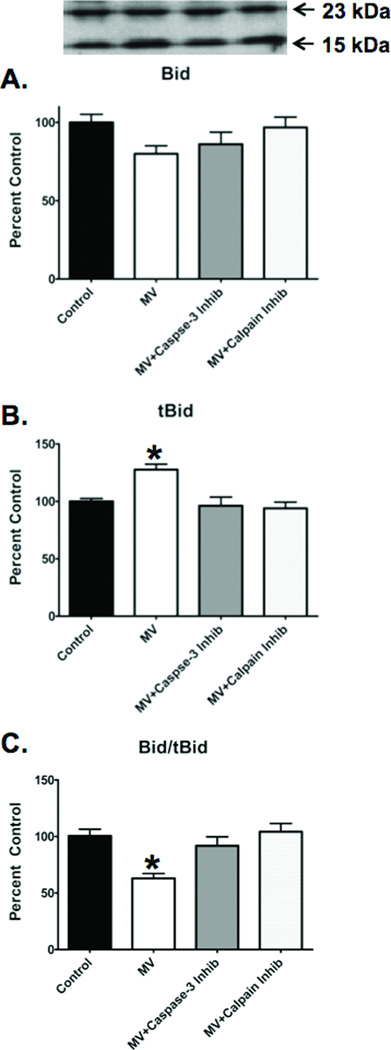
To determine if calpain activates upstream pro-apoptotic factors that promote caspase-9 and subsequent caspase-3 activation in the diaphragm, we measured the calpain-mediated cleavage of Bid into truncated Bid (tBid). tBid is a pro-apoptotic molecule that translocates to the mitochondria and interacts with other pro-apoptotic molecules (e.g., Bak and Bax) to initiate mitochondrial release of cytochrome C, leading to caspase-3 activation. Compared to control, the diaphragmatic Bid/tBid ratio was significantly lower in diaphragms from mechanically ventilated animals (figure 6C), indicating increased proteolytic cleavage of Bid into tBid. Importantly, the inhibition of calpain prevented this decrease in the Bid/tBid ratio, demonstrating that calpain is required for the formation of tBid in the diaphragm during prolonged MV (figure 6C).
Figure 6.
Bid, tBid and the Bid/tBid ratio in the diaphragm of the four experimental groups. A) Bid levels in the diaphragm at the completion of 12 hours of MV. Note that the 23-kDa band represents Bid. B) tBid levels in the diaphragm at the completion of 12 hours of MV. Note that the 15-kDa band represents the cleaved form of Bid, tBid. C) Bid/tBid ratio in the diaphragm at the completion of 12 hours of MV. The image above the histograms in Figure 7 is a representative western blot of data from the four experimental groups. Values are means ± SEM. * = different (p<0.05) from all other groups.
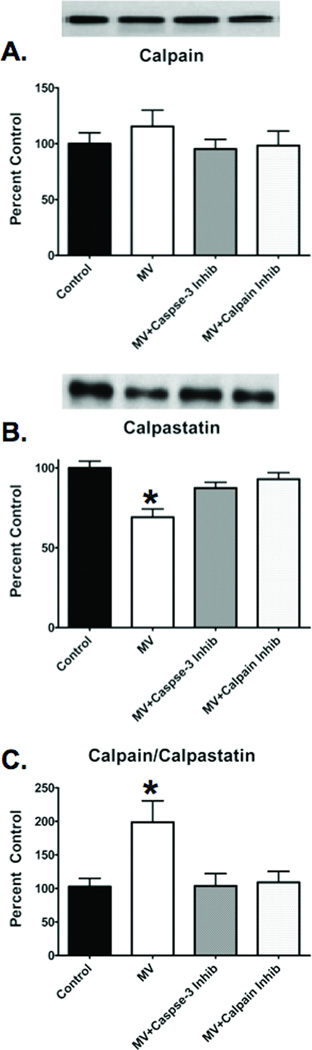
Caspase-3 inhibition prevents degradation of calpastatin
To investigate the mechanism for caspase-3 to activate calpain, we measured diaphragmatic levels of total calpain and calpastatin. Calpastatin is an endogenous inhibitor of calpain activation and also a substrate of caspase-3 (8). The ratio of total calpain to calpastatin is an indicator of calpain activity and when the calpain/calpastatin ratio increases, it reflects a degradation of calpastatin and a subsequent likely increase in calpain activity. Compared to control, prolonged MV resulted in a significant increase in the diaphragmatic calpain/calpastatin ratio (figure 7C). However, inhibition of caspase-3 in the diaphragm during prolonged MV preserved the levels of calpastatin and the calpain/calpastatin ratio remained low, similar to control (figure 7C).
Figure 7.
Total calpain, calpastatin and total calpain/calpastatin ratio in the diaphragm of the four experimental groups. A). Total calpain in the diaphragm after 12 hours of MV. B). Calpastatin in the diaphragm after 12 hours of MV. C). Total calpain/calpastatin ratio in the diaphragm after 12 hours of MV. The images above the histogram in Figures 8 are representative western blots of data from the four experimental groups. Values are means ± SEM. * = different (p<0.05) from Control, MV+Calpain Inhibitor and MV+Caspase-3 Inhibitor groups.
DISCUSSION
Overview of the major findings
Collectively, these results indicate that MV-induced diaphragmatic atrophy is dependent upon the activation of both calpain and caspase-3. Importantly, these experiments provide the first experimental evidence that a regulatory cross-talk exists between calpain and caspase-3 in diaphragm muscle during prolonged MV. A detailed discussion of these results follows.
Biochemical Basis of Calpain/Caspase-3 Cross-talk
The first evidence that a regulatory cross-talk exists between calpain and caspase-3 originated from in vitro experiments in non-muscle cells (7, 9, 18–20). Although calpain activity is regulated by several factors, it is clear that increased free calcium in the cytosol is essential for calpain activation. Calpain activity is also regulated by the endogenous calpain inhibitor calpastatin and a decrease in cellular levels of calpastatin is a requirement for full activation of calpain (21, 22). Interestingly, calpastatin is a substrate for both calpain and caspase-3 (8). Indeed, active caspase-3 has been shown to rapidly degrade calpastatin and promote calpain activation in cells (8).
Evidence also suggests that calpain can increase caspase-3 activation through several signaling pathways. Specifically, active calpain can cleave procaspase-12 to generate active caspase-12 (17, 23), which can then activate caspase-3 with or without caspase-9 as an intermediate (23, 24). Alternatively, calpain can act on pro-apoptotic proteins such as Bid and Bax (9–11). Once activated, these pro-apoptotic factors can form a pore in the mitochondrial outer membrane, promoting the release of cytochrome C from the mitochondria into the cytosol (9, 25, 26). Following release from the mitochondria, cytochrome C can then bind to the cytosolic protein Apaf-1 to form the apoptosome, which activates caspase-3 via a caspase-9, mediated pathway (27, 28). Therefore, a sound theoretical basis for a calpain/casapase-3 cross-talk exists and a regulatory interaction between these proteases has been reported in non-muscle cells during neural ischemia-reperfusion (7, 9), in vitro apoptosis (18, 19) and photoreceptor degeneration (20).
Regulatory Cross-talk Between Calpain/Caspase-3 Exists in Diaphragm Muscle During Mechanical Ventilation
Our prior work and the current study clearly demonstrates that prolonged MV activates both calpain and caspase-3 in the diaphragm and that activation of these proteases play a key role in MV-induced diaphragmatic atrophy and contractile dysfunction (5, 15). Importantly, the present study provides the first evidence that a regulatory cross-talk exists between the calpain and caspase-3 proteolytic systems in diaphragm muscle during MV whereby, active calpain promotes caspase-3 activation and vice versa.
To investigate potential mechanisms responsible for the calpain-mediated activation of capase-3, we measured the activities of two upstream activators of caspase-3 (i.e., capase-9 and caspase-12) in the diaphragm. Our results reveal that during prolonged MV, the activities of both caspase-9 and caspase-12 are increased in the diaphragm. Furthermore, pharmacological inhibition of calpain prevented the MV-induced activation of both caspase-9 and 12 and also averted the activation of caspase-3. These observations support previous in vitro findings that calpain activation plays an important role in the activity of caspases-3, 9 and 12 (17).
Our data also suggest that calpain can activate caspase-3, at least in part, by activating pro-apoptotic factors and activation of caspase-9 via a mitochondrial-mediated pathway. Indeed, previous work using an in vivo model of cardiac ischemia reperfusion injury indicates that calpain can cleave Bid into the pro-apoptotic factor tBid (9). In the current experiments, compared to control, the Bid/tBid ratio decreased in diaphragms from mechanically ventilated animals, indicating that prolonged MV initiates cleavage of Bid to form tBid. However, when MV animals were treated with a calpain inhibitor, Bid cleavage was prevented and diaphragm tBid levels remained similar to control.
Interestingly, treatment of animals with the caspase-3 inhibitor also protected against MV-induced tBid formation in the diaphragm. There are two possible explanations for this observation. First, since inhibition of caspase-3 also prevents calpain activation, it is feasible that this finding was due to the failure to activate calpain in the diaphragm. A second possibility is that, during prolonged MV, active caspase-3 can cleave Bid to form tBid. In this regard, evidence from in vitro experiments indicate that purified active caspase-3 can cleave recombinant Bid (29). However, it remains unknown if active caspase-3 can cleave Bid in myofibers in vivo.
We also investigated the mechanism responsible for the finding that caspase-3 activation is a requirement for calpain activation in the diaphragm during MV. Based upon previous work indicating that caspase-3 can degrade calpain’s endogenous inhibitor calpastatin (8), we predicted that inhibition of caspase-3 would preserve diaphragmatic levels of calpastatin and therefore, retard the MV-induced action of calpain. Our findings support this postulate and reveal that when caspase-3 activity was inhibited in the diaphragm during MV, the calpain/calpastatin ratio remained at control levels and calpain was not activated. Collectively, these results are consistent with the concept that caspase-3 can regulate calpain activity, at least in part, via the degradation of calpastatin.
It is evident from our results that the loss of either calpain or caspase-3 activity is sufficient to prevent diaphragmatic atrophy and weakness. At the core of this observation is the finding that calpain can activate caspase-3 and vice versa. Clearly, either calpain or caspase-3 must be activated at some level to initiate the regulatory cross-talk between these proteases. Hence, this raises two important questions, which protease is activated first and how is it activated? During prolonged MV, an increased production of reactive oxygen species (ROS) is a requirement for increased diaphragmatic proteolysis and atrophy (12, 13). In experimental models of ROS-mediated apoptosis in cell culture, it appears that calpain is activated first and in turn activates caspase-3 (18, 30, 31). This is a plausible scenario in our experimental model, given that skeletal muscle inactivity results in both an increase in cellular ROS production and elevated levels of cytosolic calcium which can ultimately lead to calpain activation (32, 33). Nonetheless, our current data cannot resolve which protease (i.e., calpain or caspase-3) is activated first in the diaphragm during prolonged MV.
In contrast to the current results, Supinski et al. reported that while both calpain and caspase-3 contribute to sepsis-induced diaphragmatic muscle wasting, there was no direct evidence of a regulatory cross talk between these proteases (34). In sepsis-induced diaphragm wasting, the investigators propose that calpain and caspase-3 are activated in parallel and remain independently active, downstream of a common inflammatory signal (i.e., cytokines). The disparity between the current experiments and the work of Supinski et al. may be due to the differences between the two experimental models. In the sepsis model of muscle wasting there is an inflammatory response which results in caspase-3 activation via the extrinsic apoptotic caspase-8 pathway (35). Conversely, in healthy rats, prolonged MV is not associated with an increase in circulating cytokines (36) and our results show that caspase-8 is not activated in the diaphragm. Therefore, the divergent results between the current study and the work of Supinski et al. could indicate that the signaling pathways regulating caspase-3 activation in skeletal muscle vary between different conditions of muscle wasting.
Critique of the experimental model
Because of the invasive nature of obtaining diaphragm muscle samples from humans, an animal model is required to perform mechanistic experiments to explore the signaling pathways that promote MV-induced diaphragmatic weakness. We selected the rat as the experimental animal in these experiments for several reasons. First, the anatomical features and function of the rat diaphragm are both similar to the human diaphragm (37, 38). Second, the fiber type composition of the rat and human diaphragm are comparable (1, 39). Finally, the time course of MV-induced atrophy in the rat and human diaphragm are also similar (1, 2).
The protease inhibitors utilized in these experiments were chosen due to their ability to effectively inhibit calpain and caspase-3 in vivo and because of their high level of specificity. As discussed in the methods and results sections, to confirm that these inhibitors are highly selective in our experimental model, we performed in vitro experiments using the estimated peak in vivo concentrations of the protease inhibitors used in these experiments. Our results confirm that the calpain inhibitor (SJA-6017) does not inhibit caspase-3 activity and likewise, the caspase-3 inhibitor (AC-DEVD-CHO) does not inhibit calpain. Also, note that SJA-6017 does not inhibit caspase-9. Collectively, these findings confirm the high level of selectivity of our pharmacological probes and demonstrate that our results are not due to off-target pharmacological effects.
Supplementary Material
Acknowledgments
Funded by NIH RO1 HL087839 awarded to SKP
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Levine S, Nguyen T, Taylor N, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358(13):1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- 2.Shanely RA, Zergeroglu AM, Lennon SL, et al. Mechanical ventilation-induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. Am J Respir Crit Care Med. 2002;166(10):1369–1374. doi: 10.1164/rccm.200202-088OC. [DOI] [PubMed] [Google Scholar]
- 3.Powers SK, Shanely RA, Coombes JS, et al. Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. J Appl Physiol. 2002;92(5):1851–1858. doi: 10.1152/japplphysiol.00881.2001. [DOI] [PubMed] [Google Scholar]
- 4.Laghi F, Cattapan SE, Jubran A, et al. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med. 2003;167(2):120–127. doi: 10.1164/rccm.200210-1246OC. [DOI] [PubMed] [Google Scholar]
- 5.McClung JM, Kavazis AN, DeRuisseau KC, et al. Caspase-3 regulation of diaphragm myonuclear domain during mechanical ventilation-induced atrophy. Am J Respir Crit Care Med. 2007;175(2):150–159. doi: 10.1164/rccm.200601-142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maes K, Testelmans D, Powers S, et al. Leupeptin inhibits ventilator-induced diaphragm dysfunction in rats. Am J Respir Crit Care Med. 2007;175(11):1134–1138. doi: 10.1164/rccm.200609-1342OC. [DOI] [PubMed] [Google Scholar]
- 7.Sun M, Zhao Y, Xu C. Cross-talk between calpain and caspase-3 in penumbra and core during focal cerebral ischemia-reperfusion. Cell Mol Neurobiol. 2008;28(1):71–85. doi: 10.1007/s10571-007-9250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang K, Posmantur R, Nadimpalli R, et al. Caspase-mediated fragmentation of calpain inhibitor protein calpastatin during apoptosis. Arch Biochem Biophys. 1998;356(2):187–196. doi: 10.1006/abbi.1998.0748. [DOI] [PubMed] [Google Scholar]
- 9.Chen M, He H, Zhan S, et al. Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J Biol Chem. 2001;276(33):30724–30728. doi: 10.1074/jbc.M103701200. [DOI] [PubMed] [Google Scholar]
- 10.Chen M, Won D-J, Krajewski S, et al. Calpain and mitochondria in ischemia/reperfusion injury. J Biol Chem. 2002;277(32):29181–29186. doi: 10.1074/jbc.M204951200. [DOI] [PubMed] [Google Scholar]
- 11.Wood D, Thomas A, Devi L, et al. Bax cleavage is mediated by calpain during drug-induced apoptosis. Oncogene. 1998;17(9):1069–1078. doi: 10.1038/sj.onc.1202034. [DOI] [PubMed] [Google Scholar]
- 12.Whidden MA, Smuder AJ, Wu M, et al. Oxidative stress is required for mechanical ventilation-induced protease activation in the diaphragm. J Appl Physiol. 2010;108(5):1376–1382. doi: 10.1152/japplphysiol.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Betters JL, Criswell DS, Shanely RA, et al. Trolox attenuates mechanical ventilation-induced diaphragmatic dysfunction and proteolysis. Am J Respir Crit Care Med. 2004;170(11):1179–1184. doi: 10.1164/rccm.200407-939OC. [DOI] [PubMed] [Google Scholar]
- 14.Van Gammeren D, Falk DJ, Deruisseau KC, et al. Reloading the diaphragm following mechanical ventilation does not promote injury. Chest. 2005;127(6):2204–2210. doi: 10.1378/chest.127.6.2204. [DOI] [PubMed] [Google Scholar]
- 15.Maes K, Testelmans D, Powers SK, et al. Leupeptin inhibits ventilator-induced diaphragm dysfunction in rats. Am J Respir Crit Care Med. 2007;175(11):1134–1138. doi: 10.1164/rccm.200609-1342OC. [DOI] [PubMed] [Google Scholar]
- 16.Whidden MA, McClung JM, Falk DJ, et al. Xanthine oxidase contributes to mechanical ventilation-induced diaphragmatic oxidative stress and contractile dysfunction. J Appl Physiol. 2009;106(2):385–394. doi: 10.1152/japplphysiol.91106.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan Y, Dourdin N, Wu C, et al. Ubiquitous calpains promote caspase-12 and JNK activation during endoplasmic reticulum stress-induced apoptosis. J Biol Chem. 2006;281(23):16016–16024. doi: 10.1074/jbc.M601299200. [DOI] [PubMed] [Google Scholar]
- 18.Del Bello B, Moretti D, Gamberucci A, et al. Cross-talk between calpain and caspase-3/-7 in cisplatin-induced apoptosis of melanoma cells: a major role of calpain inhibition in cell death protection and p53 status. Oncogene. 2006;26(19):2717–2726. doi: 10.1038/sj.onc.1210079. [DOI] [PubMed] [Google Scholar]
- 19.Altznauer F, Conus S, Cavalli A, et al. Calpain-1 regulates Bax and subsequent Smac-dependent caspase-3 activation in neutrophil apoptosis. J Biol Chem. 2004;279(7):5947–5957. doi: 10.1074/jbc.M308576200. [DOI] [PubMed] [Google Scholar]
- 20.Sharma A, Rohrer B. Calcium-induced calpain mediates apoptosis via caspase-3 in a mouse photoreceptor cell line. J Biol Chem. 2004;279(34):35564–35572. doi: 10.1074/jbc.M401037200. [DOI] [PubMed] [Google Scholar]
- 21.Murachi T. Calpain and Calpastatin. Trends Biochem Sci. 1983;8:167–169. [Google Scholar]
- 22.Goll DE, Thompson VF, Li H, et al. The calpain system. Physiol Rev. 2003;83(3):731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150(4):887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morishima N, Nakanishi K, Takenouchi H. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. J Biol Chem. 2002;277(37):34287–34294. doi: 10.1074/jbc.M204973200. [DOI] [PubMed] [Google Scholar]
- 25.Cai J, Yang J, Jones DP. Mitochondrial control of apoptosis: the role of cytochrome c. Biochim Biophys Acta. 1998;1366(1–2):139–149. doi: 10.1016/s0005-2728(98)00109-1. [DOI] [PubMed] [Google Scholar]
- 26.Rossé T, Olivier R, Monney L, et al. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391(6666):496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- 27.Zhai D, Huang X, Han X, et al. Characterization of tBid-induced cytochrome c release from mitochondria and liposomes. FEBS Lett. 2000;472(2–3):293–296. doi: 10.1016/s0014-5793(00)01471-x. [DOI] [PubMed] [Google Scholar]
- 28.Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91(4):479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 29.Hayakawa A, Kawamoto Y, Nakajima H, et al. Bid truncation mediated by caspases-3 and -9 in vinorelbine-induced apoptosis. Apoptosis. 2008;13(4):523–530. doi: 10.1007/s10495-008-0184-y. [DOI] [PubMed] [Google Scholar]
- 30.Waterhouse N, Finucane D. Calpain activation is upstream of caspases in radiation-induced apoptosis. Cell Death Differ. 1998;5(12):1051–1061. doi: 10.1038/sj.cdd.4400425. [DOI] [PubMed] [Google Scholar]
- 31.Wu J, Liu T, Xie J, et al. Mitochondria and calpains mediate caspase-dependent apoptosis induced by doxycycline in HeLa cells. Cell Mol Life Sci. 2006;63(7–8):949–957. doi: 10.1007/s00018-005-5565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ingalls CP, Wenke JC, Armstrong RB. Time course changes in [Ca2+]i, force, and protein content in hindlimb-suspended mouse soleus muscles. Aviat Space Environ Med. 2001;72(5):471–476. [PubMed] [Google Scholar]
- 33.Kandarian SC, Stevenson EJ. Molecular events in skeletal muscle during disuse atrophy. Exerc Sport Sci Rev. 2002;30(3):111–116. doi: 10.1097/00003677-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Supinski GS, Wang W, Callahan LA. Caspase and calpain activation both contribute to sepsis-induced diaphragmatic weakness. J Appl Physiol. 2009;107(5):1389–1396. doi: 10.1152/japplphysiol.00341.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Supinski GS, Ji X, Wang W, et al. The extrinsic caspase pathway modulates endotoxin-induced diaphragm contractile dysfunction. J Appl Physiol. 2007;102(4):1649–1657. doi: 10.1152/japplphysiol.00377.2006. [DOI] [PubMed] [Google Scholar]
- 36.Hudson MB, Smuder AJ, Nelson WB, et al. Pressure Support Ventilation Promotes Diaphragmatic Atrophy and Weakness. Crit Care Med. 2011 IN PRESS. [Google Scholar]
- 37.De Troyer A. Respiratory muscles. In: Crystal RaJW., editor. The lung: scientific foundations. New York: Raven press; 1991. pp. 869–883. [Google Scholar]
- 38.Greene E. Anatomy of the rat. New York: Hafner Publishing company; 1963. [Google Scholar]
- 39.Powers SK, Demirel HA, Coombes JS, et al. Myosin phenotype and bioenergetic characteristics of rat respiratory muscles. Med Sci Sports Exerc. 1997;29(12):1573–1579. doi: 10.1097/00005768-199712000-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.