Abstract
Studies using animal models have shown that general anesthetics such as ketamine trigger widespread and robust apoptosis in the infant rodent brain. Recent clinical evidence suggests that the use of general anesthetics on young children (at ages equivalent to those used in rodent studies) can promote learning deficits as they mature. Thus, there is a growing need to develop strategies to prevent this injury. In this study, we describe a number of independent approaches to address therapeutic intervention. Postnatal day 7 (P7) rats were injected with vehicle (sterile PBS) or the NMDAR antagonist ketamine (20 mg/kg). At 8 hours after, we prepared brains for immunohistochemical detection of the pro-apoptotic enzyme activated caspase-3 (AC3). Focusing on the somatosensory cortex, AC3-positive cells were then counted in a non-biased stereological manner. We found AC3 levels were markedly increased in ketamine-treated animals. In one study, microarray analysis of the somatosensory cortex from ketamine-treated P7 pups revealed that expression of activity dependent neuroprotective protein (ADNP) was enhanced. Thus, we injected P7 animals with the ADNP peptide fragment NAP 15 min before ketamine administration and found we could dose-dependently reverse the injury. In separate studies, pretreatment of P6 animals with 20 mg/kg vitamin D3 or a non-toxic dose of ketamine (5 mg/kg) also prevented ketamine-induced apoptosis at P7. In contrast, pretreatment of P7 animals with aspirin (30 mg/kg) 15 min before ketamine administration actually increased AC3 counts in some regions. These data show that a number of unique approaches can be taken to address anesthesia-induced neurotoxicity in the infant brain, thus providing MDs with a variety of alternative strategies that enhance therapeutic flexibility.
Keywords: glutamate, rat, anesthesia, neonatal, cell death, prevention
Introduction
For the last 10 years or so, studies from our lab have shown that blockade of the NMDAR or activation of the GABAAR induces rapid, widespread and robust apoptotic injury in the CNS of P7 rats (Turner et al., 2002, Lema Tomé et al., 2006, Lema Tomé et al., 2006, Turner et al., 2007b, Turner et al., 2009a, Turner et al., 2009d). In the rodent brain, evidence suggests that injury is age-dependent: peaking at P7, diminishing by P14, and absent by P21 (Turner et al., 2002, Turner et al., 2007b, Turner et al., 2009d). As well as promoting apoptotic injury these agents can also trigger diverse molecular and behavioral changes (Turner et al., 2002, Lema Tomé et al., 2006, Lema Tomé et al., 2007, Turner et al., 2007a, Turner et al., 2007b, Ringler et al., 2008, Lyall et al., 2009, Turner, 2009, Turner et al., 2009a, Turner et al., 2009b, Turner et al., 2009d, Gutierrez et al., 2010). Agents that target these receptors (ketamine, nitrous oxide, isoflurane, propofol, or benzodiazepines) are used routinely as general anesthetics or sedatives on young children. Suggesting there is cause for clinical concern, increased learning deficits have been described in children (0–4 years old) previously exposed to these agents (Wilder et al., 2009).
During the last decade we have explored both the mechanism behind and consequences of this injury. For example, loss of calcium appears to be the primary event as: (1) loss of calcium leads to mitochondrial dysfunction and cytochrome C release (Turner et al., 2007b); (2) decreased calcium and increased activated caspase-3 (AC3) have been observed in the same neuron (Turner et al., 2007a); (3) ketamine-like brain damage can be either mimicked by calcium channel blockade or prevented by calcium channel activation (Turner et al., 2007b, Turner et al., 2009a); (4) loss of calcium leads to growth cone collapse, as well as reduced neurite length and complexity (Ringler et al., 2008) (5) MK801-induced AC3 is not observed in cells expressing calcium binding proteins (CaBPs) and the postnatal surge in CaBP expression overlaps well with age-dependent loss of sensitivity to NMDAR blockade (Lema Tomé et al., 2006, Lema Tomé et al., 2007). Downstream of this injury, changes in expression of proteins that have cytoskeletal, synaptic, neurotransmitter producing or calcium buffering roles have been observed (Turner et al., 2002, Lema Tomé et al., 2006, Turner et al., 2007b, Turner et al., 2009b, Turner et al., 2009c). This loss of calcium homeostasis in the developing CNS has long-term consequences as early stage injury is associated with auditory deficits when animals mature (Lyall et al., 2009).
Attempts to reduce neonatal brain injury vary both mechanistically and in approach. From caspase-3 inhibitors to preconditioning, to brain cooling, investigators have reported varying degrees of success (Rosomoff, 1959, Steen et al., 1979, Ikonomidou et al., 1989, Gidday et al., 1994, Ditsworth et al., 2003, Joly et al., 2004). More recently the neuroprotective protein ADNP (or its fragment peptide NAP) has shown great promise in other models (Zemlyak et al., 2000, Gozes and Divinski, 2007). Previously, we have examined how elevating intracellular calcium or manipulating core body temperature affects NMDAR blockade-induced injury (Turner et al., 2009a, Gutierrez et al., 2010).
In this article we explore a number of alternative strategies designed to prevent anesthesia-induced neurotoxicity. To optimize identification of novel targets we used a well-defined microarray approach to examine multiple genes simultaneously. If we are successful at demonstrating that new candidates can be therapeutically exploited in our model, at later times we can then explore the expression patterns of these candidate genes in much greater detail to determine their role in ketamine-induced neurotoxicity.
For other approaches described here, we know from our previous studies that loss of calcium homeostasis may be a critical first step in the sequence of events that lead to cell death following NMDAR blockade. Further, we have shown that cells that express CaBPs are spared injury following NMDAR blockade, suggesting that regulating calcium homeostasis could be pivotal to preventing anesthesia-induced injury (Lema Tomé et al., 2006, Turner et al., 2009a). Thus, if we can stabilize or enhance calcium homeostasis prior to ketamine exposure we may be able to offset injury. Vitamin D3 is known to induce expression of CaBPs and so pretreatment with vitamin D3 might attenuate ketamine-induced injury. Alternatively, studies from other groups have shown that prior exposure to a sub-lethal level of an insult (preconditioning; such as ischemia) can greatly attenuate neurotoxicity following exposure to a lethal level of the same insult. Thus, we hypothesized that prior exposure of neonatal rats to a ketamine dose that did not produce apoptosis would reduce injury from a ketamine dose that reliably produces damage. Work elsewhere has suggested that targeting arachidonic acid metabolites (or their receptors) might be another fruitful direction (Berger et al., 2004, Zheng et al., 2007) but the role of such substances in anesthesia-induced neonatal brain injury has yet to be explored. As a first step then, we hypothesized that prior injection of the COX1/COX2 inhibitor aspirin would reduce or prevent ketamine-induced injury in our model.
Whereas these approaches are mechanistically quite diverse, we feel it is imperative to get ahead of the problem of anesthesia-induced injury and offer MDs as many alternative therapeutic strategies as possible. Indeed, in the following article we show that there is more than one way to defeat ketamine-induced injury.
Experimental Procedures
Treatments groups
All in vivo procedures used in these studies were approved by the Wake Forest University Animal Care and Use Committee and in accordance with NIH guidelines. All efforts were made to reduce the numbers and suffering of animals used. Animals (Sprague-Dawley) were obtained from Harlan (Charlotte, NC). Pups were maintained in the cage with the mother until the day of the experiment (water and food were available ad libitum). For all studies, at P7 pups were divided in roughly equal male-female groups and injected with either saline (sterile PBS) or ketamine (20 mg/kg, 4 times over 3 hours; previously found to induce robust apoptotic injury (Gutierrez et al., 2010)). In one study, brain tissue was processed for microarray analysis (see below). In this microarray study, some animals were exposed to MK801 (1 mg/kg) to compare to ketamine-treated animals. In other studies, animals were pretreated with a variety of agents prior to injections on P7 (see below).
Microarray Analysis
RNA was extracted using the Trizol protocol (Invitrogen; Carlsbad, CA): brain tissue was homogenized in Trizol (50 mg tissue/ml solution) and incubated for 5 min at room temperature. Chloroform (0.2 ml/ml Trizol solution) was added to the sample, shaken vigorously and left for 2–3 min at room temperature. Samples were centrifuged at 12,000 rpm at 8°C, after which the supernatant was removed and the RNA pellet washed 3 times with 75% ethanol. The sample was then centrifuged at 10,000 rpm for 5 min, and the pellet air-dried and dissolved in 50 μl of RNase-free water. Once total RNA was isolated, samples were assessed for RNA integrity using an Agilent RNA Bioanalyzer. RNA samples with an RNA integrity number (RIN) greater than 8.0 were carried forward for qPCR or microarray analysis.
For microarray studies, 2–5 micrograms of total RNA isolated from the somatosensory cortex were subjected to microarray analysis. Labeled cRNA was generated according to standard Affymetrix protocols and hybridized to Affymetrix Rat Genome 230 2.0 gene expression arrays. expression arrays. Microarrays (18 total) were scanned in two batches using the Affymetrix Gene ChipTM Command Console software (AGCC). Both of the batches contained 9 arrays of three groups, vehicle, MK801 and Ketamine and 3 replicates in each group. Log signal intensity distributions, pair-wise correlations between arrays and RNA degradation were examined to assess the quality of each hybridization. Raw expression data were normalized using Systematic Variation Normalization (SVN) algorithm (Chou et al., 2005). Normalized expression profiles were then batch corrected with Combat (Johnson et al., 2007) and baseline-adjusted with averaged expression of vehicle. For comparisons among the three groups, vehicle, MK801, and Ketamine, ANOVA were applied with the false discovery rate (FDR) at 0.05 in selecting differentially expressed genes. In addition, for gene expression profiles consisting of all 18 arrays and three groups, the extraction of expression patterns was performed using EPIG (Chou et al. 2007).
Pretreatment strategies
Following microarray analysis (see above), we found that activity dependent neuroprotective protein (ADNP) was up-regulated and so we targeted its fragment peptide, NAPVSIPQ (NAP) for further study. P7 pups were injected as above but in some ketamine-treated animals we injected NAP (5-20 mg/kg) 15 min before ketamine administration. NAP-injected or vehicle-injected animals served as controls. For vitamin D3 studies, we pretreated with 1–20 mg/kg vitamin D3 (1-α-2,5-dihydroxy-vitamin D3; calcitriol; Vit-D3) 24 hours earlier (to allow time for receptor internalization, translocation to the nucleus, transcription, translation and protein synthesis) and again at P7, 15 min prior to ketamine injection (to maintain Vit-D3 receptor activation for the duration of ketamine exposure). Vit-D3- or vehicle-injected animals served as controls. For the preconditioning study we pretreated with 5 mg/kg ketamine (which in our hands does not promote AC3 (see (Gutierrez et al., 2010)) 24 h before we administered 20 mg/kg ketamine. Ketamine (20 mg/kg) or vehicle-injected animals served as controls. For the aspirin study, 30 mg/kg aspirin (a dose found to protect the CNS from injury in other models; see (Zheng et al., 2007)) was injected 15 min before ketamine (20 mg/kg). Aspirin- or vehicle-injected animals served as controls. In all studies, animals were returned to their mothers and brains were processed 8 h later for AC3 (see below).
Tissues processing and Immunohistochemistry (IHC)
Eight hours after the first ketamine injection (see above), animals were anesthetized with 2% isoflurane, and then perfused with PBS followed by 4% paraformaldehyde in PBS (4% PFA). Brains were further fixed in 4% PFA for another 24 hr and placed in 10, 20, and 30% sucrose-PBS solutions for several 3 days. Brains were cut into 60 μm thick coronal sections and stored free-floating at 4°C until further processed.
Sections were processed for antigen retrieval (10 mM sodium citrate (pH 6.0) at 95°C for 3–5 min), rapidly cooled and washed (PBS, room temperature), and then incubated with rabbit cleaved Caspase-3 (Asp175; 1:2000; R&D Systems) in IHC buffer (1% BSA, 0.1% TX100, PBS; pH 7.4) at 4°C overnight. Sections were washed in PBS and reacted with biotinylated goat anti-rabbit secondary antibody (1:200 in IHC buffer; Vector Lab, Burlingame, CA) for 2 hours at room temperature. After further PBS washes, sections were then exposed to ABC Elite solution (1–2 hours; Vector Labs) and VIP chromagen solution (5 min; Vector Labs). Sections were then washed, mounted onto glass slides (Superfrost Plus; Fisher, Pittsburgh, PA), air-dried, dehydrated in ascending ethanol concentrations and coverslipped using Depex mounting media (Fisher). Assay controls were performed by omitting the primary antibody.
Stereological quantification
Numbers of AC3-positive neurons were estimated within lamina IV and V of the SSC (SSCIV-V), as previous studies have reported reliable and robust injury in these layers (Turner et al., 2007b, Turner et al., 2009a, Turner et al., 2009b). In some studies (see Fig 4 and Fig 5) we also evaluated AC3-positive profiles in the cingulate or retrosplenial cortex (all layers) for comparison to the SSCIV-V. Counts were performed using a standardized stereology procedure that employed an optical fractionator workflow (StereoInvestigator 7.0; MicroBrightField Inc, Williston, VT) supported by an Olympus BX51 microscope with a 100x oil immersion objective (PlanApo 1.40 NA, Nikon), an X-Y motorized stage (MicroBrightField Inc), and a digital camera (Microfire A/R, Optronics, Goleta, CA). Briefly, we determined that the average section thickness (after tissue processing) was 34 ± 0.8 μm. We therefore set guard zones of 3 μm and a disector height of 25–28 μm (depending on individual section thickness). The mean estimated number of AC3 positive cells (± SE) was then determined across all animals in a given treatment group. For a more detailed account of this approach the reader is referred to previous studies (Gutierrez et al., 2010).
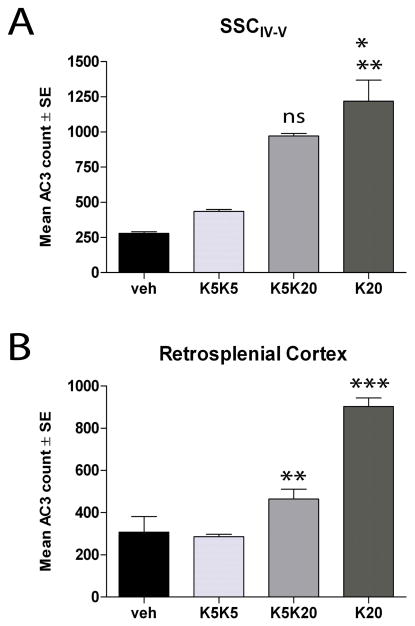
Fig. 4. Preconditioning protects against ketamine-induced injury.
Neonatal rats were injected with vehicle (sterile PBS) or ketamine (5 mg/kg, at 0, 1, 2, 3h) on P6 and/or P7 as follows: P6 vehicle - P7 vehicle (vehicle control; veh); P6 5 mg/kg ketamine - P7 5 mg/kg ketamine (K5K5); P6 5 mg/kg ketamine - P7 20 mg/kg ketamine (K5K20); P7 20 mg/kg ketamine (K20). At 8h, the numbers of AC3 cells in the SSCIV-V were estimated by non-biased stereology. Data show means ± SE (N = 3 for each group). A. AC3 counts in the SSCIV-V. *** p<0.001, K20 vs veh; *p<0.05, K20 vs K5K5. Not indicated are non-significant differences for K5K5 vs veh, and significant differences for K5K20 vs veh (p<0.05). B. AC3 counts in the Retrosplenial cortex. ***p<0.001 K20 vs veh, **p<0.01 K20 vs K5K20. Not indicated are significant differences between K5K5 vs veh, K5K20 vs veh, and K5K20 vs K5K5 (p<0.05). Statistical analyses performed using one-way ANOVA with Bonferroni post-test comparison of means.
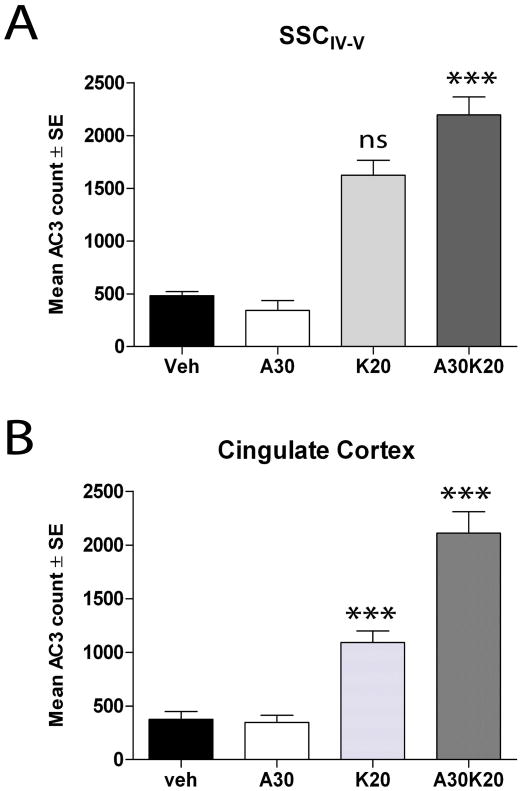
Fig. 5. Aspirin enhances ketamine-induced injury.
P7 rats were injected with vehicle (sterile PBS; Veh), aspirin (30 mg/kg; A30), 20 mg/kg ketamine (at 0, 1, 2, and 3h; K20) or aspirin plus ketamine (A30-K20). At 8h brains were fixed and sectioned for AC3 estimations. Data show means ± SE (N = 5 for each group). A. AC3 counts in the SSCIV-V .*** p<0.001, A30-K20 vs veh; ns not significant K20 vs A30-K20. Not indicated are significant differences between K20 vs veh (p<0.01) and K20 vs A30 (p<0.05) as well as non-significant differences between A30 vs veh. B. AC3 counts in the cingulate cortex. ***p<0.001 A30-K20 vs veh and A30-K20 vs K20. Not indicated are significant differences between K20 vs veh (p<0.01) and K20 vs A30 (p<0.05). Statistical analyses performed using one-way ANOVA with a Bonferroni post-test comparison of means.
Statistical Analysis
For AC3 counts, we compared means across all groups by ANOVA. Significant differences between the groups were determined using a Bonferroni post-test comparison of means. All statistical analyses were performed using GraphPad Prism 4.0 (GraphPad, San Diego, CA).
Results
Ketamine enhances or represses expression of multiple genes
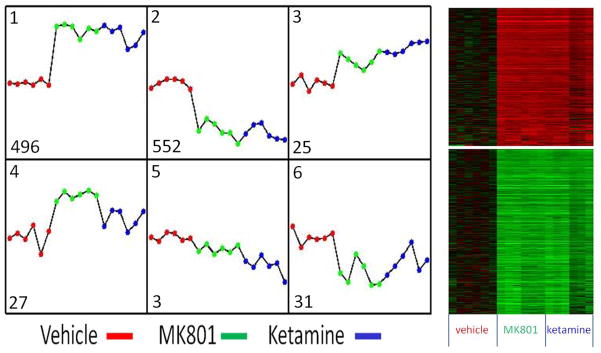
We have shown elsewhere (Lema Tomé et al., 2006, Turner et al., 2009c) that wholesale changes in gene expression are to be expected following NMDAR blockade. To monitor these changes in a more efficient and comprehensive manner, we turned to the powerful technique of microarray analysis. We compared gene expression in ketamine- or MK801-treated animals with that of vehicle-treated animals. We chose the time point of 8h because peak induction of the pro-apoptotic enzyme AC3 is observed at this time (Turner et al., 2007b). Following cRNA hybridization and raw data processing (see Methods), six distinct patterns of gene expression were extracted on the basis of the expression profile correlation values, the minimum cluster size for the patterns, and the cluster-partitioning resolution by EPIG as shown in Fig. 1 (Chou et al., 2007). With stringent conditions of signal-to-noise ratio of 3 (P < 0.006), magnitude of 0.4 (1.31-fold change), and a correlation r value of 0.64 (P < 0.01), applied in gene selection, a total of 650 probesets were assigned to the six patterns. We found most of the changes falling into Pattern 1 (enhanced; 496 probesets) and Pattern 2 (repressed; 552 probesets). Importantly, ketamine and MK801 enhanced and repressed genes in a similar manner, suggesting that these NMDAR antagonists promote identical molecular changes across a large number of functionally diverse genes, which may provide potential therapeutic value. Genes in Pattern 1 included activity dependent neuroprotective protein (ADNP) with a statistically significant p-value (ANOVA p = 3.6 × 10−5 and FDR adjusted p = 8.6 × 10−4, fold-change = 1.42). This was a particularly exciting discovery as both ADNP and the amino acid peptide motif within ADNP, NAPVSIPQ (NAP) have been shown to be highly neuroprotective elsewhere (Kumral et al., 2006, Gozes, 2007, Gozes and Divinski, 2007, Pascual and Guerri, 2007, Zemlyak et al., 2007, Jehle et al., 2008, Zemlyak et al., 2009c).
Fig. 1. Changes in gene expression following NMDAR blockade.
P7 animals were injected with vehicle, MK801 (1 mg/kg) or ketamine (20 mg/kg) and at 8h total RNA was isolated for microarray analysis (N = 6 per group). Global gene expression patterns were extracted by EPIG method and revealed 6 patterns of gene expression, with most of the changes falling into Pattern 1-enhanced or Pattern 2-repressed. The numbers at left corner in each pattern are the probe sets categorized to the corresponding pattern. The vertical axes with zero at the middle are the changes in gene expression (log2 intensity) relative to the vehicle. The heatmaps on right side correspond to 496 enhanced genes of pattern 1 (upper part) and 552 repressed genes of pattern 2 (lower part). Importantly, MK801 and ketamine enhanced and repressed genes in a similar manner, suggesting that these NMDAR antagonists promote essentially identical molecular changes across a large number of functionally diverse genes. From these data we identified activity dependent neuroprotective protein (ADNP) as a candidate gene for further study (see Fig. 2).
NAP protects against ketamine-induced injury in a dose-dependent manner
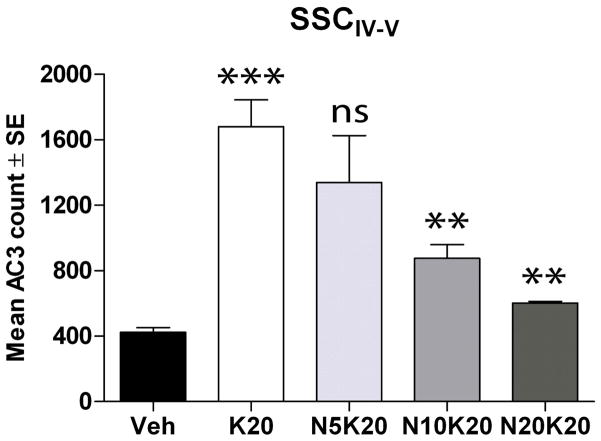
To examine the neuroprotective potential of NAP in our model, P7 rats were injected with vehicle, ketamine or ketamine plus NAP (5, 10 or 20 mg/kg). At 8 hr, we estimated the mean AC3-positive cells in layer IV-V of the somatosensory cortex (SSCIV-V; this region was chosen because robust apoptotic injury is consistently observed in this territory). Ketamine promoted a robust increase in AC3 compared to controls (Fig. 2), similar to that observed in many other studies (Turner et al., 2002, Lema Tomé et al., 2006, Turner et al., 2007b, Turner et al., 2009a, Turner et al., 2009d, Gutierrez et al., 2010). NAP was found to dose-dependently block this ketamine-induced injury. Indeed, at the 20 mg/kg dose (N20K20), AC3 levels appeared close to that found in vehicle controls. These data suggest NAP has strong translational potential for this model.
Fig. 2. NAP prevents ketamine-induced injury.
Our microarray study (see Fig. 1) suggested that we target the ADNP fragment peptide NAP for its neuroprotective potential. P7 rat pups were injected with vehicle (Veh), 20 mg/kg ketamine (K20; 4 doses over 3 hours) or ketamine plus NAP (5, 10, or 20 mg/kg; N5K20, N10K20, N20K20, respectively; NAP injected 15 min prior to ketamine). After 8h, animals were anesthetized, perfused with 4% PFA, brains removed and 60 μm coronal sections were processed for AC3-ir. AC3-positive cells in layers IV and V of the somatosensory cortex (SSCIV-V) were counted using non-biased stereology. Data expressed as means ± SE (N = 6 for all groups). Significant differences were observed between K20 and vehicle (p<0.001***) and between N10K20 vs K20 and N20K20 and K20 (p<0.01**; ANOVA; Bonferroni post-test). No significant difference in the means was found when N5K20 was compared to K20.
Other genes identified by microarray analysis
Although we chose to focus on ADNP, the array data indicated that there were many more genes whose expression was altered by ketamine exposure. Whereas it would be beyond the scope of this manuscript to review the entire array, a number of candidate genes were identified that would merit further study. For example, genes in Pattern 1 (see Fig. 1) such as neuronal pentraxin 1 (ranked 10) and calcium/calmodulin-dependent protein kinase II alpha (ranked 282) as well as genes in Pattern 2 such as the GABAAR (487), Nerve Growth Factor inducible VGF (ranked 29) and BDNF (ranked 31), all may play some role in regulating brain injury (Hajimohammadreza et al., 1995, Jevtovic-Todorovic et al., 2003, Hossain et al., 2004, Lu et al., 2006, Shimazawa et al., 2010). Given that ADNP ranked 356 in the array, some of the genes mentioned above may be at least as efficacious as ADNP at influencing ketamine-induced injury.
Vitamin D3 prevents NMDAR blockade-induced injury
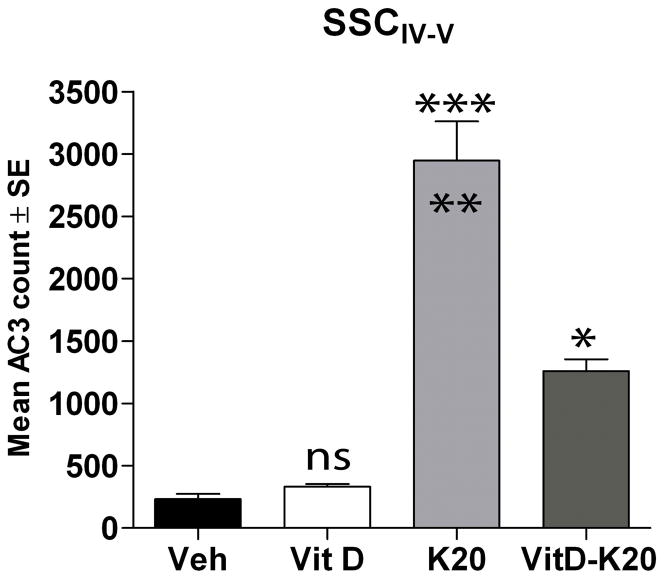
We know from previous studies that NMDAR blockade-induced apoptotic injury does not occur in cells that express CaBPs (Lema Tomé et al., 2006, Lema Tomé et al., 2007, Lema Tomé et al., 2008). Vitamin D3 (1-α-2,5-dihydroxy-vitamin D3; calcitriol; Vit-D3) can induce CaBP expression or enhance trophic factor action (Neveu et al., 1994, Naveilhan et al., 1996), both of which can stabilize intracellular calcium. Thus, by enhancing calcium buffering, we hypothesized that prior injection with Vit-D3 would protect the neonatal brain from NMDAR blockade-induced injury. Rat pups were exposed to vehicle, 20 mg/kg Vit-D3, ketamine or ketamine plus Vit-D3 and AC3 expression in the SSCIV-V was estimated 8 hours later. Ketamine induced a robust increase in AC3 compared to vehicle (Fig. 3). In animals injected with Vit-D3 prior to exposure to ketamine, we found greater than 50% reduction in AC3 levels. Finally, Vit-D3-only treated animals displayed AC3 levels almost identical to vehicle-treated animals. Thus, we show for the first time that Vit-D3 is effective in reducing brain injury following ketamine exposure in P7 animals. Because Vit-D3 is already in clinical use (Soliman et al., 2010), we feel these studies will have high translational value.
Fig. 3. Vitamin D3 protects against ketamine-induced injury.
P7 rat pups were injected with vehicle (sterile PBS), vitamin D3 (0.5 μg/kg; Vit D), 20 mg/kg ketamine (4 doses over 3 hours, K20) or ketamine plus vitamin D3 (K20-Vit D). Vitamin D3 was injected 15 min prior to ketamine exposure at P7 as well 24 hours earlier to allow time for receptor internalization, translocation to the nucleus, transcription, translation and protein synthesis. After 8h, animals were anesthetized, perfused with 4% PFA, brains removed and 60 μm coronal sections were processed for AC3-ir. AC3-positive cells in the SSCIV-V were counted using non-biased stereology. Data expressed as means ± SE (N = 5 for all groups); *** p<0.001 when comparing K20 to Veh or Vit D-K20 groups; * p<0.05, when comparing Vit D-K20 to vehicle or Vit D groups (ANOVA, Bonferroni post-test comparison). A significant difference (p<0.001) was also found between K20 and Vit D groups (not indicated).
Preconditioning can block ketamine-induced AC3
In past studies we have found that 20 mg/kg ketamine leads to robust apoptosis 8h later (increased AC3 in SSCIV-V) (Gutierrez et al., 2010). However, we wondered if (as in other brain injury models) prior exposure to a non-toxic challenge would protect the brain from a well-defined toxic challenge. Thus, P6 rats were divided into 4 groups: (1) inject at P6 with vehicle, inject at P7 with vehicle; (2) inject at P6 with 5 mg/kg ketamine, inject at P7 with 5 mg/kg ketamine (K5K5); (3) inject at P6 with 5 mg/kg ketamine, inject at P7 with 20 mg/kg ketamine (K5K20); (4) inject at P7 with 20 mg/kg ketamine (K20).
As expected, the K20 animals displayed robust apoptosis in the SSCIV-V that was about 5-fold greater than vehicle controls (Fig. 4A). Although K5K20 animals had less AC3 than that found in the K20 group it was not significant. However, when we expanded our study to other brain regions, significant differences between the K20 and K5K20 were observed, for example the retrosplenial cortex (Fig. 4B). These data suggest that despite regional variability, prior exposure to low-dose ketamine at P6 can attenuate injury from high dose ketamine at P7 and that anesthetics can be used against themselves to prevent anesthesia-induced apoptosis.
Aspirin enhances ketamine-induced injury
Aspirin is a non-steroidal anti-inflammatory that blocks both COX1 (thromboxane) and COX2 (prostaglandin) activity. COX2 activity in particular has been shown to regulate brain injury in other models (Strauss, 2010). Thus, we examined if aspirin could influence ketamine-induced neonatal brain injury. P7 rats were injected with vehicle, 30 mg/kg aspirin (A30), 20 mg/kg ketamine (K20) or aspirin plus ketamine (A30K20). Aspirin was injected 15 min prior to ketamine. At 8h, AC3 expression in the SSCIV-V was estimated.
Whereas mean AC3 counts were low in the vehicle and the A30 groups, mean AC3 counts was significantly increased in the K20 group (Fig. 5A). Although AC3 counts were higher still in the A30K20 group, they did not reach significance. However, when we examined other brain regions (such as the cingulate cortex) we found that mean AC3 values were significantly increased in the A30K20 group compared to the K20 group (Fig 5B). Based on work elsewhere, we had hoped that aspirin would provide some degree of protection but the data presented in this study are intriguing as they suggest that blockade of arachidonic acid metabolism prevents the generation of substances that may be neuroprotective.
Discussion
With respect to anesthesia-induced brain injury in neonates, NIH, the FDA as well as the International Anesthesiology Research Society (IARS) have urged researchers to not only define the scope of the problem but develop therapeutic strategies that target prevention. Thus, whereas past studies from our lab have focused on describing NMDAR blockade-induced injury as well as target mechanisms, more recent work has focused on developing ways to prevent this injury. In this article, we have described a number of approaches designed to defeat injury and report on some exciting or surprising outcomes.
We used a microarray approach to determine changes in gene expression following ketamine exposure. Of the 30,000 genes sampled there was enhancement or repression of over 1000 genes. One gene in particular (activity dependent neuroprotective peptide; ADNP) got our attention immediately because both ADNP and the amino acid peptide motif within ADNP, NAPVSIPQ (NAP) have been shown to be highly neuroprotective elsewhere (Zamostiano et al., 2001, Kumral et al., 2006, Gozes, 2007, Gozes and Divinski, 2007, Pascual and Guerri, 2007, Zemlyak et al., 2007, Jehle et al., 2008, Zemlyak et al., 2009c). This may indicate that (in our own model) the CNS may be mounting a compensatory response to NMDAR blockade-induced injury. Indeed, we show that NAP was able to dose-dependently block ketamine-induced injury when administered 15 min prior to ketamine (Fig. 2). Given that NAP is undergoing clinical trials to defeat other types of brain injury (Gozes et al., 2008), we believe this discovery represents a breakthrough for the field of anesthesia-induced neurotoxicity.
Xenon (an inhalation anesthetic) has been shown to induce ADNP in P7 rats (Cattano et al., 2008), suggesting that (as in our study) that the brain is triggered into self-protection mode. Xenon is thought to act by directly blocking glutamate action at the NMDAR as well as decreasing channel pore size (Liu et al., 2010), suggesting some mechanistic overlap with ketamine. Because xenon (and other anesthetics) has been used as a preconditioning treatment to prevent other injuries (such as hypoxia and or ischemia), Cattano et al correctly argue that xenon-induced elevation of ADNP expression is neuroprotective in nature. However, in our model, anesthesia itself is used to trigger injury and so by the time ADNP expression is enhanced it may be too late to prevent ketamine-induced apoptosis. Indeed, we show that injection of the ADNP peptide fragment NAP 15 min prior to ketamine administration was neuroprotective, suggesting there is a critical window of efficacy.
The potential of NAP in defeating brain injury is certainly not limited to anesthesia-induced damage. For example, NAP was able to block a variety of neuronal injuries (induced by electrical blockade, beta-amyloid peptide, or Apo-E deficiency) at the femtomolar level (Bassan et al., 1999). Further, NAP was effective at preventing diabetes-induced brain damage (Idan-Feldman et al., 2011) as well as ibotenic acid-induced excitotoxic injury in newborn mice (Sokolowska et al., 2011). The mechanism of action of NAP has not been fully determined but blocking the early stages of apoptosis from being engaged (Zemlyak et al., 2009c) and/or protecting the cytoskeleton from injury-induced breakdown are possible candidates (Zemlyak et al., 2009a, Zemlyak et al., 2009b).
Although we focused on ADNP, there were other candidate genes that came to our attention. For example, from Pattern 1 (enhanced), neuronal pentraxin 1 and calcium/ calmodulin-dependent protein kinase II alpha have been implicated in regulating hypoxia-ischemia (HI)-induced injury in the brain (Hajimohammadreza et al., 1995, Hossain et al., 2004). From Pattern 2 (repressed), we identified the beta 1 and alpha 4 subunits of the GABAAR, activation of which is known promote injury similar to NMDAR blockade (Jevtovic-Todorovic et al., 2003). Further, again from Pattern 2, Nerve Growth Factor-inducible VGF has been proposed to mediate neuroprotection against ALS (Shimazawa et al., 2010) whereas BDNF may regulate anesthesia-induced neonatal brain injury (Lu et al., 2006). Clearly, there are plenty of other candidate genes to exploit for their therapeutic potential and our success with ADNP (and NAP) suggest this will be a fruitful direction to take for other gene candidates. Indeed, future studies will focus on determining expression of these genes at the molecular and cellular level as well as monitoring regional and temporal variations in their expression following NMDAR blockade.
In a separate study we used our well-developed Calcium Set Point (CSP) hypothesis (Turner et al., 2007a, Turner et al., 2007b) to guide us in developing strategies to defeat ketamine-induced injury. Earlier studies have shown that NMDAR blockade-induced AC3 in P7 animals occurs in cells that lack CaBPs and cells that express CaBPs at this age do not co-express AC3 (Lema Tomé et al., 2006). Further, between P10-P14, injury from NMDAR blockade diminishes rapidly, and at this time in development CaBP expression rapidly increases (Lema Tomé et al., 2008). Vitamin D3 can induce CaBP expression or enhance trophic factor action (Neveu et al., 1994, Naveilhan et al., 1996), both of which can stabilize intracellular calcium. Thus, although CaBP expression is lower at P7 than at later ages, we reasoned that if we could enhance CaBP expression at P7 then perhaps we could stabilize calcium homeostasis and offset injury. In our model, vitamin D3 was indeed effective at preventing ketamine-induced injury. As proof-of-concept that regulating calcium homeostasis may be key to protecting the brain from anesthesia-induced injury, we have previously shown that the calcium channel agonist BAYK 8644 can reverse NMDAR-induced neonatal brain injury (Turner et al., 2009a). However, because BAYK 8644 directly increases calcium flow across the cell membrane, it is likely it will also reverse the action of most anesthetics. In contrast, vitamin D3 acts indirectly on calcium levels and so is unlikely to interfere with anesthetic action (indeed, in the studies described above we found vitamin D3 did not alter ketamine-induced sedation). Thus, vitamin D3 administration would appear to be a more practical option when addressing the prevention of ketamine-induced brain injury.
In another study, we employed a preconditioning approach similar to that used in other models of brain injury (Matsushima and Hakim, 1995). By using a low (non-toxic) dose of ketamine we had hoped to trigger a mild ketamine response in the infant rat CNS that would offset toxicity from a higher dose of ketamine, and our data indicate we were at least partially successful. Of all the approaches used by our lab, this strategy may be both the simplest and most cost effective as it suggests children can be pre-treated with the same anesthesia that will be used when they undergo general surgery. This may not be feasible in an emergency situation however.
Finally, to simplify therapeutic choices for physicians, we felt that we should examine drugs readily available and already well-defined. In this respect, aspirin has been used successfully in other brain injury models (Berger et al., 2004). However, we report here that ketamine-induced injury was enhanced rather than blocked by pretreatment with aspirin. Although disappointing with respect to defeating anesthesia-induced injury, this outcome gives us new insight and suggests that future studies should target prostaglandins or prostaglandin receptors. Indeed, the COX2 metabolite prostaglandin D2 (PGD2) is thought to be neuroprotective, acting through the DP1 receptor (Liang et al., 2005).
Regional variability in the efficacy of some of the strategies employed here was observed. This has been described previously by this lab in other studies (Turner et al., 2009a), suggesting there may be limits to the protective effects of a given agent. At present we are unsure why this was so but likely variations in regional and layer-specific distribution of ion channels or receptors are factors. Further, because brain regions vary in the time-course of their developmental programs, the neuroprotective potential of each agent at P7 may depend on the relative maturity of intracellular pathways and/or expression of cell surface proteins in any given region.
Recent evidence from the Mayo Clinic suggests that, in children under the age of 2 years, multiple (but perhaps not single) exposures to anesthetics is a cause for concern (Flick et al., 2011). Although earlier clinical studies had shown the window of vulnerability may extend to 4 years old (Wilder et al., 2009), the more recent report from this group (Flick et al., 2011) suggests this window may be narrower than animal studies imply. Indeed, greater effort may be required to more thoroughly examine the equivalency of the perinatal period in rodents and non-human primate with that of humans, as indicated elsewhere (Clancy et al., 2001, Todd, 2004, Quinn, 2005, de Graaf-Peters and Hadders-Algra, 2006, Clancy et al., 2007a, Clancy et al., 2007b).
In some respects the studies described here represent a survey of approaches and a lot of work remains to be done in the future. For example, for our ADNP/NAP studies, it would be vital to know when, where and in which cells ADNP up-regulation takes place after ketamine exposure. It would also be clinically very useful to know if post-treating with NAP is effective at preventing injury. For our vitamin D3 studies, we would need to show that CaBP expression is indeed up-regulated as we suspect. Alternatively, vitamin D3 could affect calcium homeostasis by up-regulating BDNF expression or enhancing trkB-mediated signaling. For our preconditioning study, we need to determine the shortest time and the lowest dose that are effective at stopping injury from high dose anesthesia. Just as importantly, we will need to know if there is a threshold dose above which preconditioning is either no longer observed or acts synergistically to amplify rather than reduce injury. Finally, for our aspirin study, we cannot discount the potent vascular effects of this drug and perhaps changes in local blood flow are responsible for enhanced injury.
There are now many labs that address the prevention of anesthesia-induced injury with each offering a unique approach. For example the Jetrovic-Todorovic lab has identified melatonin or BDNF as agents that could be used to block anesthesia-induced injury (Yon et al., 2006), whereas Bittigau, Ikonomiodou and colleagues suggest estradiol might be clinically useful (Asimiadou et al., 2005). Importantly, we are all working as a community to define the scope of the problem and develop ways to prevent injury. Should ongoing clinical studies bear out what is found in animal models, MDs will need as many options as possible to defeat anesthesia-induced neurotoxicity. We feel the studies we describe here and elsewhere will go a long way to achieving this goal.
There is a growing need to develop strategies to prevent anesthesia-induced injury
We describe a number of independent approaches to address therapeutic intervention
Ketamine induced apoptotic injury in P7 rats
NAP, vitamin D3 and low-dose ketamine all attenuated or prevented this injury
Aspirin enhanced the injury
Acknowledgments
This work was supported by NIH RO1 NS051632, Wake Forest Intramural Research Support and Mr and Mrs Tab Williams Family Neuroscience Endowment Fund. Animal handling and tissue processing performed by SG, CL and CPT. Stereological cell counting and statistical analysis performed by all authors (except LM, JC). RNA isolation performed by CL and microarray procedure and analysis performed by LM and JC. We are also indebted to Lou Craddock for her assistance with the microarray work. Manuscript construction by SG, JC and CPT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asimiadou S, Bittigau P, Felderhoff-Mueser U, Manthey D, Sifringer M, Pesditschek S, Dzietko M, Kaindl AM, Pytel M, Studniarczyk D, Mozrzymas JW, Ikonomidou C. Protection with estradiol in developmental models of apoptotic neurodegeneration. Ann Neurol. 2005;58:266–276. doi: 10.1002/ana.20553. [DOI] [PubMed] [Google Scholar]
- Bassan M, Zamostiano R, Davidson A, Pinhasov A, Giladi E, Perl O, Bassan H, Blat C, Gibney G, Glazner G, Brenneman DE, Gozes I. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem. 1999;72:1283–1293. doi: 10.1046/j.1471-4159.1999.0721283.x. [DOI] [PubMed] [Google Scholar]
- Berger C, Xia F, Schabitz WR, Schwab S, Grau A. High-dose aspirin is neuroprotective in a rat focal ischemia model. Brain Res. 2004;998:237–242. doi: 10.1016/j.brainres.2003.11.049. [DOI] [PubMed] [Google Scholar]
- Cattano D, Valleggi S, Ma D, Kastsiuchenka O, Abramo A, Sun P, Cavazzana AO, Natale G, Maze M, Giunta F. Xenon induces transcription of ADNP in neonatal rat brain. Neurosci Lett. 2008;440:217–221. doi: 10.1016/j.neulet.2008.05.086. [DOI] [PubMed] [Google Scholar]
- Chou JW, Zhou T, Kaufmann WK, Paules RS, Bushel PR. Extracting gene expression patterns and identifying co-expressed genes from microarray data reveals biologically responsive processes. BMC Bioinformatics. 2007;8:427. doi: 10.1186/1471-2105-8-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007a;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007b;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: what is happening when? Early Hum Dev. 2006;82:257–266. doi: 10.1016/j.earlhumdev.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Ditsworth D, Priestley MA, Loepke AW, Ramamoorthy C, McCann J, Staple L, Kurth CD. Apoptotic neuronal death following deep hypothermic circulatory arrest in piglets. Anesthesiology. 2003;98:1119–1127. doi: 10.1097/00000542-200305000-00014. [DOI] [PubMed] [Google Scholar]
- Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, Sprung J, Weaver AL, Schroeder DR, Warner DO. Cognitive and Behavioral Outcomes After Early Exposure to Anesthesia and Surgery. Pediatrics. 2011 doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidday JM, Fitzgibbons JC, Shah AR, Park TS. Neuroprotection from ischemic brain injury by hypoxic preconditioning in the neonatal rat. Neurosci Lett. 1994;168:221–224. doi: 10.1016/0304-3940(94)90455-3. [DOI] [PubMed] [Google Scholar]
- Gozes I. Activity-dependent neuroprotective protein: from gene to drug candidate. Pharmacol Ther. 2007;114:146–154. doi: 10.1016/j.pharmthera.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Gozes I, Divinski I. NAP, a neuroprotective drug candidate in clinical trials, stimulates microtubule assembly in the living cell. Curr Alzheimer Res. 2007;4:507–509. doi: 10.2174/156720507783018208. [DOI] [PubMed] [Google Scholar]
- Gozes I, Divinski I, Piltzer I. NAP and D-SAL: neuroprotection against the beta amyloid peptide (1–42) BMC Neurosci. 2008;9 (Suppl 3):S3. doi: 10.1186/1471-2202-9-S3-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez S, Carnes A, Finucane B, Musci G, Oelsner W, Hicks L, Russell GB, Liu C, Turner CP. Is age-dependent, ketamine-induced apoptosis in the rat somatosensory cortex influenced by temperature? Neuroscience. 2010;168:253–262. doi: 10.1016/j.neuroscience.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajimohammadreza I, Probert AW, Coughenour LL, Borosky SA, Marcoux FW, Boxer PA, Wang KK. A specific inhibitor of calcium/calmodulin-dependent protein kinase-II provides neuroprotection against NMDA- and hypoxia/hypoglycemia-induced cell death. J Neurosci. 1995;15:4093–4101. doi: 10.1523/JNEUROSCI.15-05-04093.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MA, Russell JC, O'Brien R, Laterra J. Neuronal pentraxin 1: a novel mediator of hypoxic-ischemic injury in neonatal brain. J Neurosci. 2004;24:4187–4196. doi: 10.1523/JNEUROSCI.0347-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idan-Feldman A, Schirer Y, Polyzoidou E, Touloumi O, Lagoudaki R, Grigoriadis NC, Gozes I. Davunetide (NAP) as a preventative treatment for central nervous system complications in a diabetes rat model. Neurobiol Dis. 2011;44:327–339. doi: 10.1016/j.nbd.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Mosinger JL, Olney JW. Hypothermia enhances protective effect of MK-801 against hypoxic/ischemic brain damage in infant rats. Brain Res. 1989;487:184–187. doi: 10.1016/0006-8993(89)90956-6. [DOI] [PubMed] [Google Scholar]
- Jehle T, Dimitriu C, Auer S, Knoth R, Vidal-Sanz M, Gozes I, Lagreze WA. The neuropeptide NAP provides neuroprotection against retinal ganglion cell damage after retinal ischemia and optic nerve crush. Graefes Arch Clin Exp Ophthalmol. 2008;246:1255–1263. doi: 10.1007/s00417-007-0746-7. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly LM, Mucignat V, Mariani J, Plotkine M, Charriaut-Marlangue C. Caspase inhibition after neonatal ischemia in the rat brain. J Cereb Blood Flow Metab. 2004;24:124–131. doi: 10.1097/01.WCB.0000100061.36077.5F. [DOI] [PubMed] [Google Scholar]
- Kumral A, Yesilirmak DC, Sonmez U, Baskin H, Tugyan K, Yilmaz O, Genc S, Gokmen N, Genc K, Duman N, Ozkan H. Neuroprotective effect of the peptides ADNF-9 and NAP on hypoxic-ischemic brain injury in neonatal rats. Brain Res. 2006;1115:169–178. doi: 10.1016/j.brainres.2006.07.114. [DOI] [PubMed] [Google Scholar]
- Lema Tomé CM, Bauer C, Nottingham C, Smith C, Blackstone K, Brown L, Hlavaty C, Nelson C, Daker R, Sola R, Miller R, Bryan R, Turner CP. MK801-induced caspase-3 in the postnatal brain: inverse relationship with calcium binding proteins. Neuroscience. 2006;141:1351–1363. doi: 10.1016/j.neuroscience.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Lema Tomé CM, Miller R, Bauer C, Nottingham C, Smith C, Blackstone K, Brown L, Bryan R, Leigh A, Brady M, Busch J, Turner CP. Decline in age-dependent, MK801-induced injury coincides with developmental switch in parvalbumin expression: cingulate and retrosplenial cortex. Dev Psychobiol. 2007;49:606–618. doi: 10.1002/dev.20246. [DOI] [PubMed] [Google Scholar]
- Lema Tomé CM, Miller R, Bauer C, Smith C, Blackstone K, Leigh A, Busch J, Turner CP. Decline in age-dependent, MK801-induced injury coincides with developmental switch in parvalbumin expression: somatosensory and motor cortex. Dev Psychobiol. 2008;50:665–679. doi: 10.1002/dev.20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lema Tomé CM, Nottingham CU, Smith CM, Beauchamp AS, Leung PW, Turner CP. Neonatal exposure to MK801 induces structural reorganization of the central nervous system. Neuroreport. 2006;17:779–783. doi: 10.1097/01.wnr.0000220133.32091.d6. [DOI] [PubMed] [Google Scholar]
- Liang X, Wu L, Hand T, Andreasson K. Prostaglandin D2 mediates neuronal protection via the DP1 receptor. J Neurochem. 2005;92:477–486. doi: 10.1111/j.1471-4159.2004.02870.x. [DOI] [PubMed] [Google Scholar]
- Liu LT, Xu Y, Tang P. Mechanistic insights into xenon inhibition of NMDA receptors from MD simulations. J Phys Chem B. 2010;114:9010–9016. doi: 10.1021/jp101687j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LX, Yon JH, Carter LB, Jevtovic-Todorovic V. General anesthesia activates BDNF-dependent neuroapoptosis in the developing rat brain. Apoptosis. 2006;11:1603–1615. doi: 10.1007/s10495-006-8762-3. [DOI] [PubMed] [Google Scholar]
- Lyall A, Swanson J, Liu C, Blumenthal TD, Turner CP. Neonatal exposure to MK801 promotes prepulse-induced delay in startle response time in adult rats. Exp Brain Res. 2009;197:215–222. doi: 10.1007/s00221-009-1906-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K, Hakim AM. Transient forebrain ischemia protects against subsequent focal cerebral ischemia without changing cerebral perfusion. Stroke. 1995;26:1047–1052. doi: 10.1161/01.str.26.6.1047. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Neveu I, Baudet C, Funakoshi H, Wion D, Brachet P, Metsis M. 1,25-Dihydroxyvitamin D3 regulates the expression of the low-affinity neurotrophin receptor. Brain Res Mol Brain Res. 1996;41:259–268. doi: 10.1016/0169-328x(96)00103-9. [DOI] [PubMed] [Google Scholar]
- Neveu I, Naveilhan P, Baudet C, Brachet P, Metsis M. 1,25-dihydroxyvitamin D3 regulates NT-3, NT-4 but not BDNF mRNA in astrocytes. Neuroreport. 1994;6:124–126. doi: 10.1097/00001756-199412300-00032. [DOI] [PubMed] [Google Scholar]
- Pascual M, Guerri C. The peptide NAP promotes neuronal growth and differentiation through extracellular signal-regulated protein kinase and Akt pathways, and protects neurons co-cultured with astrocytes damaged by ethanol. J Neurochem. 2007;103:557–568. doi: 10.1111/j.1471-4159.2007.04761.x. [DOI] [PubMed] [Google Scholar]
- Quinn R. Comparing rat's to human's age: how old is my rat in people years? Nutrition. 2005;21:775–777. doi: 10.1016/j.nut.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Ringler SL, Aye J, Byrne E, Anderson M, Turner CP. Effects of disrupting calcium homeostasis on neuronal maturation: early inhibition and later recovery. Cell Mol Neurobiol. 2008;28:389–409. doi: 10.1007/s10571-007-9255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosomoff HL. Protective effects of hypothermia against pathological processes of the nervous system. Ann N Y Acad Sci. 1959;80:475–486. doi: 10.1111/j.1749-6632.1959.tb49225.x. [DOI] [PubMed] [Google Scholar]
- Shimazawa M, Tanaka H, Ito Y, Morimoto N, Tsuruma K, Kadokura M, Tamura S, Inoue T, Yamada M, Takahashi H, Warita H, Aoki M, Hara H. An inducer of VGF protects cells against ER stress-induced cell death and prolongs survival in the mutant SOD1 animal models of familial ALS. PLoS One. 2010;5:e15307. doi: 10.1371/journal.pone.0015307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowska P, Passemard S, Mok A, Schwendimann L, Gozes I, Gressens P. Neuroprotective effects of NAP against excitotoxic brain damage in the newborn mice: implications for cerebral palsy. Neuroscience. 2011;173:156–168. doi: 10.1016/j.neuroscience.2010.10.074. [DOI] [PubMed] [Google Scholar]
- Soliman AT, El-Dabbagh M, Adel A, Al Ali M, Aziz Bedair EM, Elalaily RK. Clinical responses to a mega-dose of vitamin D3 in infants and toddlers with vitamin D deficiency rickets. J Trop Pediatr. 2010;56:19–26. doi: 10.1093/tropej/fmp040. [DOI] [PubMed] [Google Scholar]
- Steen PA, Soule EH, Michenfelder JD. Deterimental effect of prolonged hypothermia in cats and monkeys with and without regional cerebral ischemia. Stroke. 1979;10:522–529. doi: 10.1161/01.str.10.5.522. [DOI] [PubMed] [Google Scholar]
- Strauss KI. COX2 inhibitors for acquired brain injuries: is the time ripe? Crit Care Med. 38:723–724. doi: 10.1097/CCM.0b013e3181bc80b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss KI. COX2 inhibitors for acquired brain injuries: is the time ripe? Crit Care Med. 2010;38:723–724. doi: 10.1097/CCM.0b013e3181bc80b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd MM. Anesthetic neurotoxicity: the collision between laboratory neuroscience and clinical medicine. Anesthesiology. 2004;101:272–273. doi: 10.1097/00000542-200408000-00003. [DOI] [PubMed] [Google Scholar]
- Turner CP. Perfect storm in a baby's brain. Annual Society for Neurosscience Meeting; Chicago. 2009. p. 413.10. [Google Scholar]
- Turner CP, Connell J, Blackstone K, Ringler SL. Loss of calcium and increased apoptosis within the same neuron. Brain Res. 2007a;1128:50–60. doi: 10.1016/j.brainres.2006.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CP, Debenedetto D, Liu C. NMDAR blockade-induced neonatal brain injury: Reversal by the calcium channel agonist BayK 8644. Neurosci Lett. 2009a;450:292–295. doi: 10.1016/j.neulet.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CP, Debenedetto D, Walburg C, Ware E, Lambert A, Lee A, Swanson J, Stowe R, Lyle M, Desai P, Johnson R, Liu C. MK801-induced activated caspase-3 exhibits selective co-localization with GAD67. Neurosci Lett. 2009b;462:152–156. doi: 10.1016/j.neulet.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CP, Debenedetto D, Ware E, Stowe R, Lee A, Swanson J, Walburg C, Lambert A, Lyle M, Desai P, Liu C. Postnatal exposure to MK801 induces selective changes in GAD67 or parvalbumin. Exp Brain Res. 2009c doi: 10.1007/s00221-009-2059-z. [DOI] [PubMed] [Google Scholar]
- Turner CP, Debenedetto D, Ware E, Walburg C, Lee A, Stowe R, Swanson J, Lambert A, Lyle M, Desai P, Johnson R, Liu C. MK801-induced activated caspase-3 exhibits selective co-localization with GAD67. Neurosci Lett. 2009d;462:152–156. doi: 10.1016/j.neulet.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CP, Miller R, Smith C, Brown L, Blackstone K, Dunham SR, Strehlow R, Manfredi M, Slocum P, Iverson K, West M, Ringler SL, Berry ZC. Widespread neonatal brain damage following calcium channel blockade. Dev Neurosci. 2007b;29:213–231. doi: 10.1159/000095221. [DOI] [PubMed] [Google Scholar]
- Turner CP, Pulciani D, Rivkees SA. Reduction in intracellular calcium levels induces injury in developing neurons. Exp Neurol. 2002;178:21–32. doi: 10.1006/exnr.2002.8027. [DOI] [PubMed] [Google Scholar]
- Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yon JH, Carter LB, Reiter RJ, Jevtovic-Todorovic V. Melatonin reduces the severity of anesthesia-induced apoptotic neurodegeneration in the developing rat brain. Neurobiol Dis. 2006;21:522–530. doi: 10.1016/j.nbd.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Zamostiano R, Pinhasov A, Gelber E, Steingart RA, Seroussi E, Giladi E, Bassan M, Wollman Y, Eyre HJ, Mulley JC, Brenneman DE, Gozes I. Cloning and characterization of the human activity-dependent neuroprotective protein. J Biol Chem. 2001;276:708–714. doi: 10.1074/jbc.M007416200. [DOI] [PubMed] [Google Scholar]
- Zemlyak I, Furman S, Brenneman DE, Gozes I. A novel peptide prevents death in enriched neuronal cultures. Regul Pept. 2000;96:39–43. doi: 10.1016/s0167-0115(00)00198-1. [DOI] [PubMed] [Google Scholar]
- Zemlyak I, Manley N, Sapolsky R, Gozes I. NAP protects hippocampal neurons against multiple toxins. Peptides. 2007;28:2004–2008. doi: 10.1016/j.peptides.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Zemlyak I, Manley N, Vulih-Shultzman I, Cutler AB, Graber K, Sapolsky RM, Gozes I. The microtubule interacting drug candidate NAP protects against kainic acid toxicity in a rat model of epilepsy. J Neurochem. 2009a;111:1252–1263. doi: 10.1111/j.1471-4159.2009.06415.x. [DOI] [PubMed] [Google Scholar]
- Zemlyak I, Sapolsky R, Gozes I. NAP protects against cyanide-related microtubule destruction. J Neural Transm. 2009b;116:1411–1416. doi: 10.1007/s00702-009-0252-7. [DOI] [PubMed] [Google Scholar]
- Zemlyak I, Sapolsky R, Gozes I. NAP protects against cytochrome c release: inhibition of the initiation of apoptosis. Eur J Pharmacol. 2009c;618:9–14. doi: 10.1016/j.ejphar.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Schwab S, Grau A, Berger C. Neuroprotection by early and delayed treatment of acute stroke with high dose aspirin. Brain Res. 2007;1186:275–280. doi: 10.1016/j.brainres.2007.10.029. [DOI] [PubMed] [Google Scholar]