Abstract
Burkitt lymphoma (BL) in the general population and immunosuppressed persons with AIDS in United States was characterized by three age-specific incidence peaks near 10, 40 and 70 years. We hypothesized that BL from different geographical areas may exhibit pediatric, adult, and elderly age incidence peaks. We investigated this hypothesis using data on 3403 cases obtained from the International Agency for Research on Cancer (1978–2002). Data from Africa were sparse or incomplete, and thus were excluded. Age-standardized rates (ASR) and age-specific incidence rates were calculated, supplemented with calculations performed using age-period-cohort models. The ASR rose 5.3% (95% confidence interval (CI), 5.0–5.6) per year in males and 4.6% (95% CI, 4.5–4.8) in females. The ASR increased gradually in children and steeply in adults and most rapidly in the elderly both in males and females. Overall, BL male/female ASR ratio was 2.5, but it declined from 3.1 (95% CI, 3.0–3.3) for pediatric BL to 2.3 (95% CI 2.2–2.4) for adult BL and 1.5 (95% CI, 1.4–1.6) for elderly BL. Age-specific incidence peaks occurred near 10 years and 70 years in all regions and periods. A peak near 40 years of age emerged in the mid-1990s, particularly in men. Findings using APC models confirmed those based on standard analyses. Our findings, based on international BL cases, support our hypothesis that BL is multimodal and that BL peaks at different ages may be clues to differences in the etiology and/or biology of BL at those ages.
Keywords: Burkitt lymphoma, epidemiology, multimodal cancer, non-Hodgkin lymphoma, HIV/AIDS
Introduction
Burkitt lymphoma (BL) is an aggressive B cell non-Hodgkin lymphoma (NHL) broadly divided into three established but pathologically indistinguishable epidemiological subtypes: endemic, sporadic, and immunodeficiency-related BL(1). At the molecular level, BL is characterized by deregulation of MYC(1), a master regulator of cellular differentiation, growth and apoptosis(2). In 80–90% of cases, MYC is deregulated by translocation of MYC coding sequences on chromosome 8 to chromosome 14 adjacent to promoter sequences of immunoglobulin heavy chains on (14q32) (1). In 10–15% of tumors, variant translocations to chromosome 2 (2p12) adjacent to promoter sequences of kappa (κ) light chain genes or chromosome 22 adjacent to lambda (λ) light chain genes (22q11) are found (1). Interestingly, chromosomal translocations have not been demonstrated in at least 10% of BL tumors(3). These cases indicate further heterogeneity in the molecular basis of BL (4, 5).
We recently reported trimodal age-specific incidence peaks of BL near 10, 40, and 70 years, respectively, in males, and two peaks near 10 and 70 years in females in the U.S. using data from the Surveillance Epidemiology, and End Results (SEER) program (6). We attributed the adult peak, particularly in males, to AIDS-related BL, given the higher HIV prevalence in men (7, 8). However, trimodal patterns were noted in AIDS-related BL in the U.S. using data from the HIV/AIDS Cancer Match Study (9). Multimodal peaks of BL suggested that BL diagnosed at different ages may have different etiology and/or biology(10). This is reminiscent of Hodgkin lymphoma, whose multimodal peaks are considered clues to different etiology and/or biology (11, 12). Whether BL diagnosed at different age varies at a molecular level is unknown. No differences in molecular abnormalities with age were observed in one relatively small study conducted in German (13). We hypothesized that BL from different geographical areas may exhibit pediatric, adult, and elderly age incidence peaks. We investigated this hypothesis using data from the International Agency for Research on Cancer (IARC). Demonstration of multimodal BL in other geographical areas would strengthen the justification to investigate molecular abnormalities of BL with age.
Methods
Study population
BL case and population data were obtained from the IARC for cases published in Cancer Incidence in Five Continents Volume I to IX diagnosed during 1963 to 2002 (14). Although the data included cases from all five continents, cases from sub-Saharan Africa, the Middle East and North Africa, and the Caribbean were sparse and/or incomplete population data, and, thus, excluded from the analyses. Because the study spans calendar periods and regions when BL was coded differently, we defined BL according to registry codes used in the period when cases were registered. These changed as follows: 200.2 in the International Classification of Diseases, 9th edition (ICD-9) published in 1975 (15); C83.7 in ICD-10 published in 1992 (16); morphology code 9753 in the Manual of Tumor Nomenclature and Coding (MOTNAC) published in 1968 (17); 9750/3 in the International Classification of Diseases for Oncology (ICD-O) published in 1976 (18); and 9687/3 in ICD-O-2 and ICD-O-3 published in 1990(19) and 2000 (20).
BL data published in Volumes I–IV were quite sparse and were excluded from incidence-based analysis (cases diagnosed during 1963–1977). Case data for each registry were provided for males and females in 18 5-year age periods of diagnosis (0–4 to 80–84, then 85+ years) and in five 5-year calendar diagnosis periods (1978–1982 to 1998–2002, corresponding to Volumes V–IX). The completeness of the BL cases in our study cannot be verified. In addition, the accuracy of pathology diagnosis for the cases was not verified by centralized pathology review because registries do not routinely collect tissues of cases they register. Thus, the impact of refinements in the methods used to diagnose BL, including use of immune stains and cytogenetic studies cannot be determined from our data.
Incidence rates
Age-specific incidence rates were calculated for cases diagnosed during 1978 to 2002 when BL data probably were reasonably complete and diagnosis based on consistent criteria. Age-standardized rates (ASR) were calculated adjusted by the direct method to the World Standard Population(21). ASR (cases per million person-years) were plotted by calendar period on a log-rate and linear-time scale(22) such that a slope (or angle) of 10 degrees on the graph approximates a 1% per year change in the ASR. Estimated annual percentage change (EAPC) in the rates and 95% confidence intervals (CI) were calculated for all ages combined and for each age group for males and females (23). Age-specific male/female incidence rate ratios were calculated and graphed.
Although it would have been preferable to consider five 5-year time periods for age-specific trends analyses, the earlier data were somewhat sparse; we thus we grouped the data into two 10-year periods (1978–1987 and 1988–1997) and one 5-year calendar period (1998–2002) to improve statistical stability. Following the same practice in previous study in the U.S. (6), these standard analyses were supplemented with age-period-cohort (APC) models. APC models provide a novel method to calculate descriptive statistics that investigate period, cohort and age effects. These statistics included the overall and local (age-specific) fitted net drifts and deviations. The net drift is defined as the linear trend in the logarithm of the age-specific rates over time and is calculated as the sum of the period and cohort slopes. It is conceptually similar but not numerically equivalent to the EAPC for the cross-sectional rates because of adjustment for cohort effects. The overall and local net drifts for males and females were graphed to explore variation by age. Deviations are parameters that describe non-linear departures from linear trends in the age, period, and cohort effects. Age, period, and cohort deviations were calculated using APC models following the method of Holford (24, 25). The mean age, period, and cohort deviations between males and females was compared using the Wald test for independent samples (26). Age, period and cohort deviations permit fine exploration of acceleration or deceleration changes in the incidence with age, calendar time, and cohort by examining for significant inflexion points, i.e., points where the incidence of BL changes significantly upwards or downwards. This was done by comparing the slope of the rate over three time intervals before a defined time point with the slope of the rate in three intervals after that defined time point in successive “sliding windows” across age, period, or cohort intervals (27). Results were considered to be statistically significant when the two-sided P value was ≤ .05. Analysis of inflexion points was exploratory, so the P values for these analyses were not adjusted for multiple comparisons. Statistical analyses were implemented using Matlab version 2010b (MathWorks, Inc., Natick, MA).
Heterogeneity of BL incidence patterns by region
The risk for BL is influenced by malaria, Epstein-Barr virus (EBV), and human immunodeficiency virus (HIV) (7, 8), which were not measured in this study. We investigated the correlations between the BL rates and regional HIV prevalence as estimated by the Joint United Nations Program on AIDS (UNAIDS (28). The UNAIDS regions were divisible into two broad groups: South and Southeast Asia, East Asia, and Latin America, which have high HIV and malaria burden; and Western and Central Europe, the Caribbean, North America and Canada, and Australia, which have a low HIV burden and no malaria. We assessed variation of BL age-adjusted rates overall and for three truncated age groups (0–19, 25–49, and 55–84 years, excluding 20–24 and 50–54 years to more clearly define the separate pediatric, adult, and elderly age groups) and 95% CI across UNAIDS regions (28).
Results
Demographic characteristics of the BL cases
There were 3,403 cases diagnosed during 1963–2002 (40 years) and recorded in 60 cancer registries. Most cases (69.3%) were male (Table 1). The overall mean age at BL diagnosis was 33.5 years, but it was younger in males than in females (30.1 versus 41.0 years, P ≈ 0 for the null hypothesis of equality of means). The age distribution of the BL cases was 41.7%, 37.4%, and 20.9% for the age groups 0–19, 20–59, 60+ years, respectively.
Table I.
Burkitt lymphoma cases diagnosed during 1963 to 2002 and reported to the International Agency for Research on Cancer
| All Cases | Males | Females | M/F Ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | N | Rate | 95% CI | N | Rate | 95% CI | N | Rate | 95% CI | IRR(mf) | 95% CI |
| All cases | 3,403 | 1.09 | 1.05–1.13 | 2,360 | 1.55 | 1.49–1.62 | 1,043 | 0.62 | 0.58–0.66 | 2.51 | 2.43–2.60 |
| UNAIDS Region | |||||||||||
| East Asia | 87 | 0.26 | 0.20–0.32 | 69 | 0.43 | 0.32–0.54 | 18 | 0.10 | 0.05–0.14 | 4.46 | 3.52–5.40 |
| Canada | 780 | 1.43 | 1.32–1.53 | 557 | 2.12 | 1.94–2.30 | 223 | 0.72 | 0.62–0.82 | 2.95 | 2.74–3.16 |
| Western and Central Europe | 1,693 | 1.40 | 1.33–1.47 | 1,148 | 1.98 | 1.86–2.10 | 545 | 0.81 | 0.74–0.89 | 2.44 | 2.32–2.56 |
| South and Southeast Asia | 198 | 0.41 | 0.35–0.47 | 137 | 0.56 | 0.46–0.65 | 61 | 0.26 | 0.19–0.33 | 2.11 | 1.82–2.41 |
| Australia | 472 | 1.63 | 1.48–1.79 | 337 | 2.41 | 2.15–2.68 | 135 | 0.83 | 0.68–0.98 | 2.91 | 2.65–3.17 |
| Latin America | 162 | 1.21 | 1.00–1.41 | 106 | 1.59 | 1.22–1.95 | 56 | 0.85 | 0.62–1.08 | 1.88 | 1.59–2.17 |
| Age group | |||||||||||
| <20 years (Pediatric) | 1,418 | 1.44 | 1.37–1.52 | 1,093 | 2.16 | 2.03–2.28 | 325 | 0.69 | 0.61–0.76 | 3.13 | 2.97–3.29 |
| 20–59 years (Adult) | 1,273 | 0.72 | 0.68–0.76 | 893 | 1.00 | 0.94–1.07 | 380 | 0.43 | 0.39–0.48 | 2.31 | 2.18–2.44 |
| >60 years (Elderly) | 712 | 1.46 | 1.34–1.57 | 374 | 1.80 | 1.61–1.99 | 338 | 1.18 | 1.04–1.31 | 1.53 | 1.42–1.64 |
| Calendar period | |||||||||||
| 1978–1982 | 233 | 0.64 | 0.56–0.73 | 161 | 0.90 | 0.76–1.05 | 72 | 0.38 | 0.28–0.47 | 2.41 | 2.10–2.72 |
| 1983–1987 | 410 | 0.79 | 0.71–0.87 | 287 | 1.11 | 0.98–1.24 | 123 | 0.47 | 0.38–0.55 | 2.37 | 2.14–2.60 |
| 1988–1992 | 622 | 1.06 | 0.98–1.15 | 436 | 1.51 | 1.37–1.66 | 186 | 0.60 | 0.51–0.69 | 2.54 | 2.34–2.74 |
| 1993–1997 | 862 | 1.35 | 1.25–1.44 | 594 | 1.91 | 1.76–2.07 | 268 | 0.76 | 0.66–0.86 | 2.52 | 2.36–2.69 |
| 1998–2002 | 1,218 | 1.72 | 1.62–1.83 | 844 | 2.49 | 2.31–2.67 | 374 | 0.94 | 0.83–1.04 | 2.65 | 2.51–2.80 |
N, number of cases; Rate per million person-years, age-adjusted (world standard population); 95% CI, 95% confidence intervals; IRR(mf), Male/female incidence rate ratio; UNAIDS Joint United Nations Program on AIDS.
Regions ordered according to HIV prevalence, based on UNAIDS data for 2008, from lowest to highest; regions with sparse Burkitt lymphoma data (Eastern Europe, Central Asia, sub-Saharan Africa, Middle East and North Africa, and Caribbean) were excluded.
Age-standardized rates
The overall ASR was 1.09 per million person-years (Table 1). The ASRs were low (0.26–0.41 per million person-years) in East Asia and South and Southeast Asia, and relatively high (1.40–1.63 per million person-years) in Western and Central Europe, Canada, and Australia. The overall ASR rose almost 170% during the study period from 0.64 per million person-years during 1978–1982 to 1.72 during 1998–2002. The EAPC of the ASR increased more steeply among males (5.3%, 95% CI 5.0–5.6) in males than among females (4.6%, 95% CI 4.5–4.8) (Figure 1A). These increases were notable for pediatric, adult, and elderly cases among both males (Figure 1B) and females (Figure 1C), but with notable differences across groups. The ASR for pediatric BL increased gradually (EAPC 3.1% for males, 1.6% in females), more steeply both for adult BL (8.2% for males and 6.3% for females), and most rapidly for elderly BL (9.7% for males and 10.0% for females).
Figure 1.
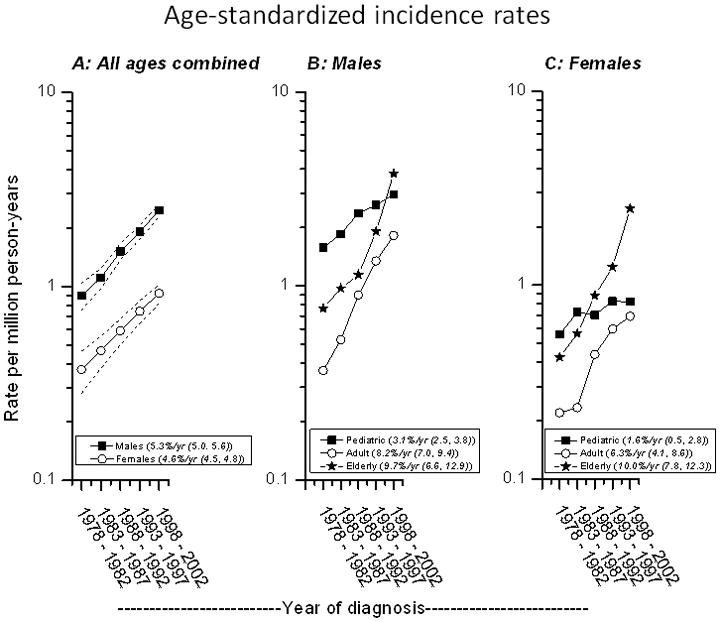
Burkitt lymphoma age-adjusted (world standard) incidence trends. Incidence from 1978–1982 through 1998–2002 for males (solid squares) and females (open circles) of all ages (Panel A); for pediatric (0–19 years), adult (20–59 years), and elderly (60+ years) BL in males (Panel B) and in females (Panel C), based on data reported in Cancer Incidence in Five Continents Volumes V to IX. Numbers in parentheses in the panel legends are estimated annual percentage change and 95% CI.
The ASR for pediatric and elderly BL were comparable and were twice as high as the ASR for adult BL (1.44 and 1.46, respectively, versus 0.72) (Table 1). Gender differences were noted. Among the males, ASR for pediatric BL was significantly higher than for the elderly (2.16, 95% CI, 2.03–2.28 versus 1.80, 95% CI 1.61–1.99). Among the females, the ASR for the elderly ASR was significantly higher than for the pediatric (1.18, 95% CI, 1.04–1.31 versus 0.69, 95% CI 0.61–0.76). The ASR among males was 2.5 times that among females (1.55 versus 0.62), with notable differences by age and regions but not by calendar year periods. The male/female ASR ratio decreased from 3.1 in pediatric, to 2.3 in adult and to 1.5 in elderly BL. Compared with Latin America, the male/female ratio was significantly higher for all regions except South and Southeast Asia (Table I).
Pediatric and elderly BL incidence rate peaks were present in all regions (Table 2). The rates were lower (consistent with a valley or low peak) and at ages 25–49 years. The age-specific ASR peaks varied significantly between regions (all P < .05). The incidence of pediatric BL was highest in Western and Central Europe regions (2.11), intermediate in Latin America (1.78) and lowest in East Asia and South and Southeast Asia (0.59–0.64). Regional BL ASR for all ages combined were unrelated to regional HIV prevalence among males (r = 0.18, P = .7) and among females (r = 0.45, P = .4). No correlations with region were apparent in the pediatric, adult, or elderly age groups.
Table II.
Burkitt lymphoma incidence for three age groups of cases diagnosed during 1978–2002 and reported to the
| Age Group, years | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–19 | 25–49 | 55–84 | |||||||||||
| Region | N | Rate | 95% CI | N | Rate | 95% CI | N | Rate | 95% CI | HIV Prevalence (%) | |||
| All Regions | 1372 | 1.57 | 1.48 | 1.65 | 829 | 0.79 | 0.73 | 0.84 | 817 | 1.45 | 1.34 | 1.55 | |
| East Asia | 42 | 0.64 | 0.44 | 0.83 | 10 | 0.10 | 0.04 | 0.16 | 28 | 0.48 | 0.30 | 0.66 | 0.10 |
| Canada | 268 | 1.82 | 1.60 | 2.04 | 200 | 1.02 | 0.88 | 1.17 | 216 | 2.07 | 1.78 | 2.35 | 0.15 |
| Western and Central Europe | 684 | 2.11 | 1.95 | 2.27 | 425 | 1.04 | 0.94 | 1.13 | 384 | 1.38 | 1.23 | 1.52 | 0.30 |
| South and Southeast Asia | 114 | 0.59 | 0.48 | 0.7 | 36 | 0.19 | 0.13 | 0.25 | 33 | 0.73 | 0.47 | 0.98 | 0.30 |
| Australia | 161 | 1.92 | 1.62 | 2.22 | 125 | 1.16 | 0.96 | 1.36 | 139 | 2.33 | 1.92 | 2.73 | 0.38 |
| Latin America | 103 | 1.78 | 1.44 | 2.13 | 33 | 0.65 | 0.42 | 0.87 | 17 | 1.28 | 0.64 | 1.92 | 0.60 |
International Agency for Research on Cancer
Rate per million person-years, age-adjusted (world standard population); HIV prevalence based on UNAIDS estimates for 2008; age groups 20–24 years and 50–54 years were excluded.
Age-specific incidence rates
Fifty-eight BL cases diagnosed during 1963–1977 were excluded from age-specific analyses. Age-specific incidence rates peaked near the age of 10 years (pediatric BL) and around 70 years (elderly BL) during all calendar periods among males and females (Figure 2). A third peak emerged around the age of 40 years (adult BL) in 1998–2002, particularly in males. For both males and females, the incidence rates rose slightly in successive calendar-year periods for pediatric BL and steeply for the adult and elderly BL, reminiscent of the temporal trends noted in Figure 1.
Figure 2.
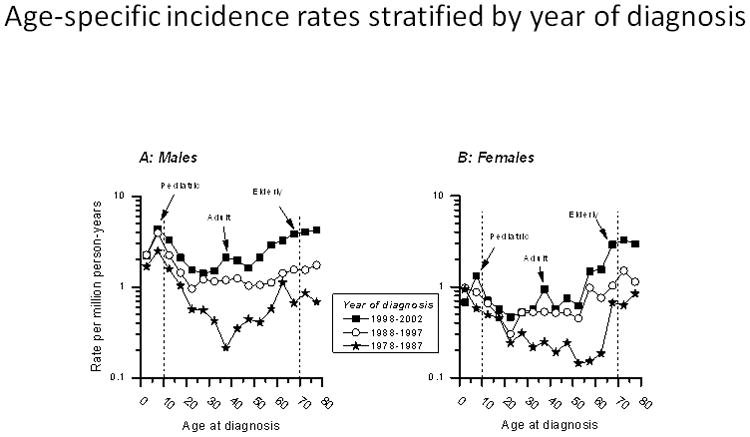
Burkitt lymphoma age-specific incidence rates. Rates for males (Panel A) and females (Panel B) during two 10-year calendar periods (1978–1987, 1988–1997) and one 5-year calendar period (1998–2002), based on data reported in Cancer Incidence in Five Continents Volumes V to IX.
The APC fitted global net drifts rose 8.1 % per year in males (Figure 3A) and 6.3% per year in females (Figure 3B). The fitted local net drifts showed substantial variation in age-specific drifts from <5% per year for pediatric BL to 10% or higher per year for adult and elderly BL in both males and females. The male/female age-specific male/female incidence rate ratio (IRRMF) was highest at 4 among children aged 5–9, then decreased progressively with age to >3 at ages 10–24 years, 2–3 at ages 25–64 years, and <2 at ages 65 years and older (Figure 4).
Figure 3.
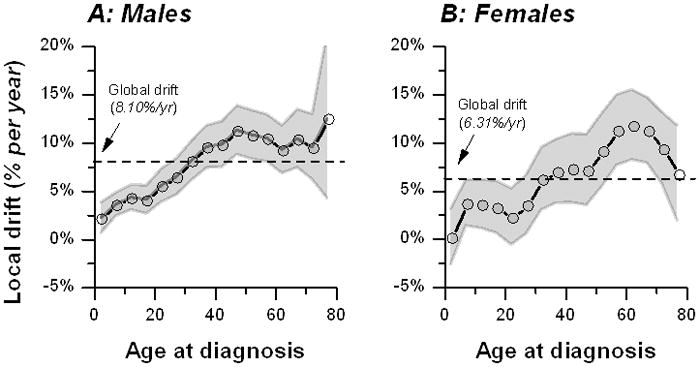
Fitted local drifts (circles connected by a dotted line) for males (Panel A) and females (Panel B) during 1978 through 2002, based on data reported in Cancer Incidence in Five Continents Volumes V to IX. The local drifts are bounded by 95% confidence bands. The dotted line depicts the global drift for all ages combined. Conceptually, local drifts are similar, but not numerically equivalent, to the estimated age-specific annual percentage change of the ASR (see methods).
Figure 4. Burkitt lymphoma age-specific male-to-female rate ratios during 1978–2002.
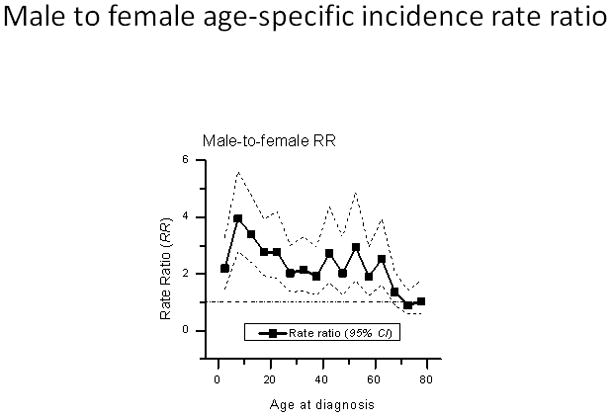
Ratios based on data reported in Cancer Incidence in Five Continents Volumes V to IX.
There was evidence of statistically significant inflexion points in the age deviations around the age of 22 and 50 years. These points ages when the age-specific incidence rates changes between pediatric and adult years and between adult and elderly years. Period deviations were flat. This indicates the temporal rate changes in BL incidence remained constant during the study period. Cohort deviations showed a statistically significant inflexion at the 1958 birth-cohort. This cohort divides those who were exposed or not to AIDS in the early 1980s.
Discussion
Availability of a large dataset of BL cases obtained from IARC allowed us to investigate the hypothesis that multimodal patterns, previously reported in the U.S. (6, 9), were apparent among BL cases from different geographical regions as well. In both studies BL incidence peaks were observed among males near 10, 40, and 70 years for age groups 0–19 years, 20–59 years, and 60+ years, respectively, but with some differences. While the adult peak was apparent among males in all years in the U.S., this peak only emerged in the late 1990s in the international data.
Multimodality is generally accepted as an epidemiological clue that a condition considered one is comprised of a mixture of two or more distinct biological entities (11, 29). Our results strengthen support for our hypothesis that BL may be comprised of a mixture of tumors with distinct biologic properties. For BL, these properties may include greater propensity for clinical onset at pediatric, adult, and/or elderly ages. In support, the temporal trends for pediatric, adult, and elderly BL tumors were different. The implications of the trimodal peaks on treatment, prognosis, and outcome are unclear. We found that the 5-year relative mortality of BL in the U.S. was lowest (<25%) for pediatric BL, intermediate (50%) for adult BL, and highest (75%) for elderly BL(6). Similar patterns have been reported from clinical studies (30, 31). For example, the outcome of BL was inferior among cases over 40 years of age and worse among those over 60 years of age (32), when compared to pediatric BL cases. Although still preliminary, our epidemiological and clinical results are consistent with the idea that the biology and/or etiology of BL diagnosed at different ages may have implications for treatment, prognosis, and outcome of BL.
BL is generally accepted as a pediatric disease. However, a substantial proportion of BL were adult. The proportion was 50% in the U.S. (6) and 37% in the current study. Because BL is an AIDS defining condition (33), this pattern highlights HIV as a risk factor for BL and its particular impact on adult BL. However, our finding that adult BL emerged in the 1990s in the international cases, while it was noted almost immediately in the early 1980s in the U.S, shortly after the onset of the AIDS epidemic, is puzzling. Possibly, cases were or are incompletely registered outside the U.S., because relatively incomplete cancer registration (34), particularly in those countries with high HIV prevalence. Alternatively, the lag might be a clue to differences in survival with HIV, or to differences in the biological traits of BL in different populations.
Our study has some limitations. We had limited data (about 15%) from developing countries, mostly Brazil and India, which constrains the generalizability of results to countries in sub-Saharan Africa, where BL is endemic. Second, central review of the pathology of cases to confirm the diagnoses was not done. This may have resulted in misclassification of cases and non-cases. Lack of a single criterion to use as a gold standard for BL diagnosis and the continuing evolution of the criteria for diagnosis of BL (35, 36) would complicate plans for central pathology review. Expert hematopathologists disagree about the diagnosis of BL in 10–20% in children and 52–74% in adult and elderly BL (37). Although misclassification cannot be dismissed (1, 38), the agreement between the main epidemiological patterns in the current study and our previous study suggest the findings are likely valid. The strengths of our study include large sample size, having cases from different geographic, socioeconomic and genetic backgrounds.
To summarize, our results suggest that recent international BL incidence may have trimodal incidence peaks. These results support our hypothesis, based on U.S. data, that BL peaking at different ages may have different etiology and/or biology.
Acknowledgments
This work was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services. We thank Ruth Parsons and Michael Curry of Information Management Systems (Rockville, Maryland) for preparing the files for analysis.
Footnotes
Authors’ contributions
S M Mbulaiteye conceived the study, guided the analysis, interpreted the data and drafted the manuscript. W F Anderson analyzed the data, interpreted the data, and helped to draft the report. J Ferlay assembled data for analysis, interpreted the data and edited the manuscript. C Chang reviewed the analysis and interpreted the data. PS Rosenberg interpreted the data, and edited the paper. K Bhatia interpreted the data. SS Devesa analyzed the data, interpreted the data and edited the paper. DM Parkin interpreted the data and edited the paper. All authors commented on and contributed to the final draft of the manuscript.
Conflict of interest
None declared.
References
- 1.Leoncini L, Raphael M, Stein H, Harris NL, Jaffe ES, Kluin PM, editors. Burkitt lymphoma. Lyon: International Agency for Research on Cancer (IARC); 2008. pp. 262–264. [Google Scholar]
- 2.Klein G. Burkitt lymphoma-A stalking horse for cancer research? Semin Cancer Biol. 2009 doi: 10.1016/j.semcancer.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Leucci E, Cocco M, Onnis A, De Falco G, van Cleef P, Bellan C, van Rijk A, Nyagol J, Byakika B, Lazzi S, Tosi P, van Krieken H, Leoncini L. MYC translocation-negative classical Burkitt lymphoma cases: an alternative pathogenetic mechanism involving miRNA deregulation. J Pathol. 2008;216:440–450. doi: 10.1002/path.2410. [DOI] [PubMed] [Google Scholar]
- 4.Hummel M, Bentink S, Berger H, Klapper W, Wessendorf S, Barth TF, Bernd HW, Cogliatti SB, Dierlamm J, Feller AC, Hansmann ML, Haralambieva E, Harder L, Hasenclever D, Kuhn M, Lenze D, Lichter P, Martin-Subero JI, Moller P, Muller-Hermelink HK, Ott G, Parwaresch RM, Pott C, Rosenwald A, Rosolowski M, Schwaenen C, Sturzenhofecker B, Szczepanowski M, Trautmann H, Wacker HH, Spang R, Loeffler M, Trumper L, Stein H, Siebert R. A biologic definition of Burkitt's lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006;354:2419–2430. doi: 10.1056/NEJMoa055351. [DOI] [PubMed] [Google Scholar]
- 5.Dave SS, Fu K, Wright GW, Lam LT, Kluin P, Boerma EJ, Greiner TC, Weisenburger DD, Rosenwald A, Ott G, Muller-Hermelink HK, Gascoyne RD, Delabie J, Rimsza LM, Braziel RM, Grogan TM, Campo E, Jaffe ES, Dave BJ, Sanger W, Bast M, Vose JM, Armitage JO, Connors JM, Smeland EB, Kvaloy S, Holte H, Fisher RI, Miller TP, Montserrat E, Wilson WH, Bahl M, Zhao H, Yang L, Powell J, Simon R, Chan WC, Staudt LM. Molecular diagnosis of Burkitt's lymphoma. N Engl J Med. 2006;354:2431–2442. doi: 10.1056/NEJMoa055759. [DOI] [PubMed] [Google Scholar]
- 6.Mbulaiteye SM, Anderson WF, Bhatia K, Rosenberg PS, Linet MS, Devesa SS. Trimodal age-specific incidence patterns for Burkitt lymphoma in the United States, 1973–2005. Int J Cancer. 2010;126:1732–1739. doi: 10.1002/ijc.24934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mbulaiteye SM, Biggar RJ, Bhatia K, Linet MS, Devesa SS. Sporadic childhood Burkitt lymphoma incidence in the United States during 1992–2005. Pediatr Blood Cancer. 2009;53:366–370. doi: 10.1002/pbc.22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogwang MD, Bhatia K, Biggar RJ, Mbulaiteye SM. Incidence and geographic distribution of endemic Burkitt lymphoma in northern Uganda revisited. Int J Cancer. 2008;123:2658–2663. doi: 10.1002/ijc.23800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guech-Ongey M, Simard EP, Anderson WF, Engels EA, Bhatia K, Devesa SS, Mbulaiteye SM. AIDS-related Burkitt lymphoma in the United States: what do age and CD4 lymphocyte patterns tell us about etiology and/or biology? Blood. doi: 10.1182/blood-2010-03-275917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith SM. AIDS-related BL and CD4 count: a clue? Blood. 116:5435–5436. doi: 10.1182/blood-2010-09-306407. [DOI] [PubMed] [Google Scholar]
- 11.Macmahon B. Epidemiological evidence of the nature of Hodgkin's disease. Cancer. 1957;10:1045–1054. doi: 10.1002/1097-0142(195709/10)10:5<1045::aid-cncr2820100527>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Jarrett RF. Viruses and Hodgkin's lymphoma. Ann Oncol. 2002;13 (Suppl 1):23–29. doi: 10.1093/annonc/13.s1.23. [DOI] [PubMed] [Google Scholar]
- 13.Klapper W, Szczepanowski M, Burkhardt B, Berger H, Rosolowski M, Bentink S, Schwaenen C, Wessendorf S, Spang R, Moller P, Hansmann ML, Bernd HW, Ott G, Hummel M, Stein H, Loeffler M, Trumper L, Zimmermann M, Reiter A, Siebert R. Molecular profiling of pediatric mature B-cell lymphoma treated in population-based prospective clinical trials. Blood. 2008;112:1374–1381. doi: 10.1182/blood-2008-01-136465. [DOI] [PubMed] [Google Scholar]
- 14.Ferlay J, Parkin DM, Curado MP, Bray F, Edwards B, Shin HR, Forman D. Cancer Incidence in Five Continents, Volumes I to IX: IARC CancerBase No 9. Lyon, France: International Agency for Research on Cancer; 2010. [Google Scholar]
- 15.Manual of the international statistical classification of diseases, injuries, and causes of death. Geneva: World Health Organization; 1977. [Google Scholar]
- 16.International Statistical Classification of Diseases and Related Health Problem. Tenth Revision. Geneva: World Health Organization; 1992. [PubMed] [Google Scholar]
- 17.Manual of tumor nomenclature. New York: American Cancer Society; 1951. [Google Scholar]
- 18.International Classification of Diseases for Oncology. Geneva: World Health Organization; 1976. [Google Scholar]
- 19.International Classification of Diseases for Oncology. Geneva: World Health Organization; 1990. [Google Scholar]
- 20.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin LH, Parkin DM, Whelan S, editors. International Classification of Diseases for Oncology (ICD-O-3) Geneva (Switzerland): World Health Organization; 2000. [Google Scholar]
- 21.Doll R, Payne P, Waterhouse JAH, editors. Cancer Incidence In Five Continents. Geneva: Union Internationale Contre le Cancer; 1966. [Google Scholar]
- 22.Devesa SS, Donaldson J, Fears T. Graphical presentation of trends in rates. Am J Epidemiol. 1995;141:300–304. doi: 10.1093/aje/141.4.300. [DOI] [PubMed] [Google Scholar]
- 23.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 24.Holford TR. Understanding the effects of age, period, and cohort on incidence and mortality rates. Annu Rev Public Health. 1991;12:425–457. doi: 10.1146/annurev.pu.12.050191.002233. [DOI] [PubMed] [Google Scholar]
- 25.Holford TR. The estimation of age, period and cohort effects for vital rates. Biometrics. 1983;39:311–324. [PubMed] [Google Scholar]
- 26.Rosenberg PS, Anderson WF. Proportional hazards models and age- period-cohort analysis of cancer rates. Stat Med. 29:1228–1238. doi: 10.1002/sim.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarone RE, Chu KC. Evaluation of birth cohort patterns in population disease rates. Am J Epidemiol. 1996;143:85–91. doi: 10.1093/oxfordjournals.aje.a008661. [DOI] [PubMed] [Google Scholar]
- 28.AIDS Epidemic Update: November 2009. Geneva: Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO); 2009. [Google Scholar]
- 29.Ries LAG, Devesa SS. Cancer incidence, mortality, and patient survival in the United States. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. New York: Oxford University Press; 2006. pp. 139–167. [Google Scholar]
- 30.Perkins AS, Friedberg JW. Burkitt lymphoma in adults. Hematology Am Soc Hematol Educ Program. 2008;2008:341–348. doi: 10.1182/asheducation-2008.1.341. [DOI] [PubMed] [Google Scholar]
- 31.Thomas DA, Faderl S, O'Brien S, Bueso-Ramos C, Cortes J, Garcia-Manero G, Giles FJ, Verstovsek S, Wierda WG, Pierce SA, Shan J, Brandt M, Hagemeister FB, Keating MJ, Cabanillas F, Kantarjian H. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106:1569–1580. doi: 10.1002/cncr.21776. [DOI] [PubMed] [Google Scholar]
- 32.Kelly JL, Toothaker SR, Ciminello L, Hoelzer D, Holte H, LaCasce AS, Mead G, Thomas D, Van Imhoff GW, Kahl BS, Cheson BD, Magrath IT, Fisher RI, Friedberg JW. Outcomes of patients with Burkitt lymphoma older than age 40 treated with intensive chemotherapeutic regimens. Clin Lymphoma Myeloma. 2009;9:307–310. doi: 10.3816/CLM.2009.n.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mbulaiteye SM, Parkin DM, Rabkin CS. Epidemiology of AIDS-related malignancies an international perspective. Hematol Oncol Clin North Am. 2003;17:673–696. v. doi: 10.1016/s0889-8588(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 34.Parkin DM. The evolution of the population-based cancer registry. Nat Rev Cancer. 2006;6:603–612. doi: 10.1038/nrc1948. [DOI] [PubMed] [Google Scholar]
- 35.Naresh KN, Ibrahim HA, Lazzi S, Rince P, Onorati M, Ambrosio MR, Bilhou-Nabera C, Amen F, Reid A, Mawanda M, Calbi V, Ogwang M, Rogena E, Byakika B, Sayed S, Moshi E, Mwakigonja A, Raphael M, Magrath I, Leoncini L. Diagnosis of Burkitt lymphoma using an algorithmic approach - applicable in both resource-poor and resource-rich countries. Br J Haematol. doi: 10.1111/j.1365-2141.2011.08771.x. [DOI] [PubMed] [Google Scholar]
- 36.Wright DH. What is Burkitt's lymphoma and when is it endemic? Blood. 1999;93:758. [PubMed] [Google Scholar]
- 37.Haralambieva E, Boerma EJ, van Imhoff GW, Rosati S, Schuuring E, Muller-Hermelink HK, Kluin PM, Ott G. Clinical, immunophenotypic, and genetic analysis of adult lymphomas with morphologic features of Burkitt lymphoma. Am J Surg Pathol. 2005;29:1086–1094. [PubMed] [Google Scholar]
- 38.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J. Lymphoma classification--from controversy to consensus: the R.E.A.L and WHO Classification of lymphoid neoplasms. Ann Oncol. 2000;11 (Suppl 1):3–10. [PubMed] [Google Scholar]


