Abstract
1,4-Dioxan-2-one, 1, was synthesized and the equilibrium constant between it and the hydrolysis product 2-(2-hydroxyethoxy) acetic acid, 2, was determined as KO = 70 ± 3.5 in acidic aqueous media, 25 °C, ionic strength 1M, (KCl), 5% by volume acetonitrile. The carboxylic acid dissociation constant of 2 was determined under the same conditions to be pKa = 3.31 ± 0.02. On the basis of these two determinations, the equilibrium constant between 1 and carboxylic acid anion, 3, and the proton was calculated to be KOA = 0.034 ± 0.002 M. The stability of 1 was determined in the range of pH between 1 and 8.5 in buffered aqueous solutions under the conditions above by UV spectrophotometric methods and exhibited simple first order kinetics of decay. On the basis of buffer dilution plots, the values of ko, the rate constant for solvent mediated decomposition, were determined. The plot of log ko against pH is consistent with a three term rate law for solvolysis with a hydrogen ion catalyzed rate constant kH+ = 1.1 (± 0.1) M−1 min−1, a water catalyzed rate constant, kw = 9.9 (± 0.5) × 10−4 min−1, and a hydroxide ion catalyzed rate constant, kOH = 4.1 (± 0.3) × 104 M−1 min−1. The t1/2 for decay at pH = 7.0, at 25 °C, is ~2 h. Treatment of F344 rats with aflatoxin B1 (AFB1) (positive control) elicited the expected preneoplastic foci in the livers of each rat tested, while subsequent administration of 1 (a total of 1.32 g over 12 weeks) failed to statistically increase focal number or focal volume percent. In 8 rats administered 1 (1.32 g, 12 weeks) alone, no increase above background foci was detected. This study does not support compound 1 as a common carcinogen.
Introduction
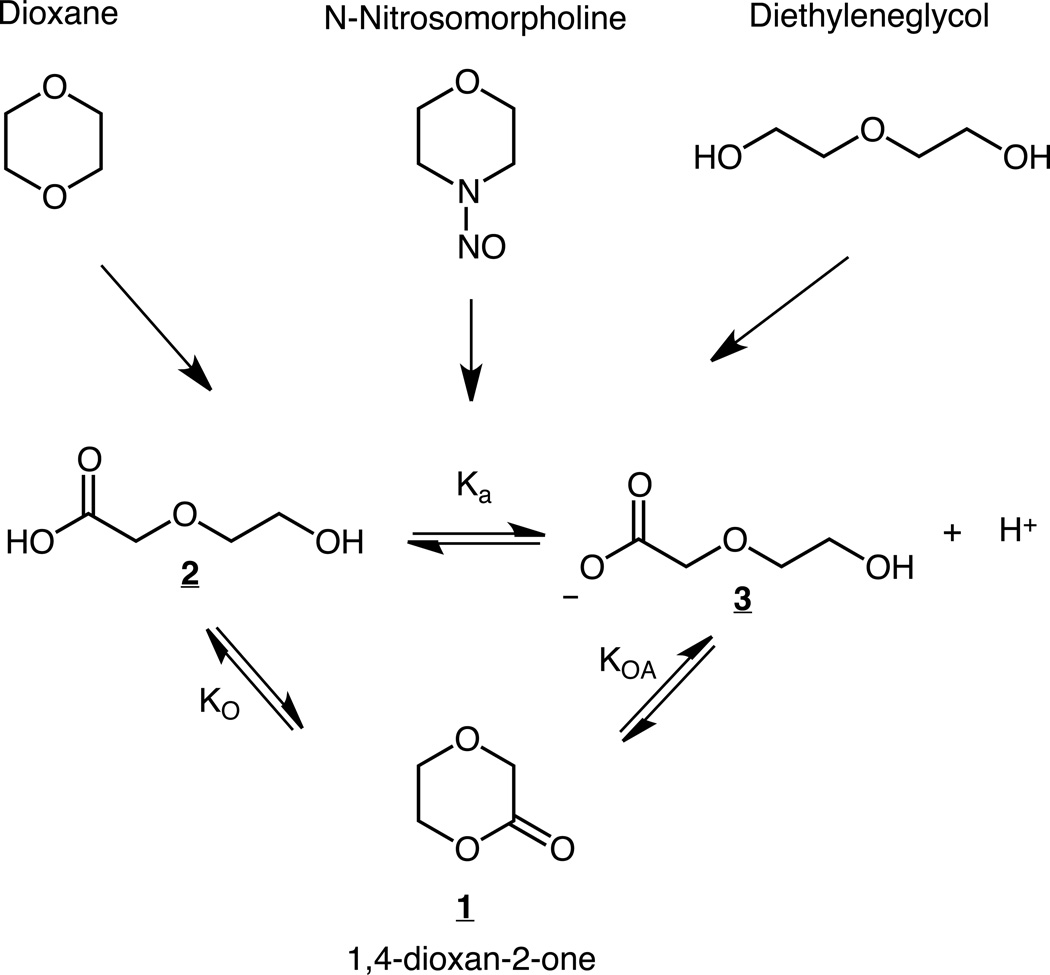
The powerful rodent liver carcinogen N-nitrosomorpholine,1–3 the much weaker rodent liver carcinogen dioxane4–6 and the weak rodent bladder/mammary carcinogen diethyleneglycol7, 8 are all to a significant degree metabolized in vivo to a common set of compounds, Fig. 1, at the carboxylic acid level of oxidation.9–11 Studies with these carcinogens did not identify all of the metabolites depicted in Fig. 1, but their existence and distribution is dictated by the thermodynamic cycle depicted within Fig. 1. The lactone 1, 1,4-dioxan-2-one, Fig. 1, was purported to be the major metabolite from dioxane in rats.11 The chemistry and biological activity of 1 is also of interest as monomeric 1 is used in the synthesis of polymers employed as resorbable sutures.12
Figure 1.
Three carcinogens share a common set of metabolites.
The role of lactone 1 in N-nitrosomorpholine carcinogenesis or its promotion has not been considered. DNA damage from a major metabolite of N-nitrosomopholine, 3-hydoxy-N-nitrosomorpholine, has been recently demonstrated via the formation of covalent adducts with DNA derived of diazonium ions intermediates.13
Lactone 1 has been speculated to be a genotoxic agent in dioxane and dietheylene glycol carcinogenesis, on the basis of structural analogy with other lactones that manifest DNA damage,11 but evidence of genotoxicity by either agent or their metabolites is weak. In 2007, the U.S. Agency for Toxic Substances and Disease Registry released the “Draft Toxicological Report for 1,4-Dioxane”14 that summarizes that only 2 of 17 in vitro studies, most employing xenobiotic metabolizing fractions, elicited positive indications of genotoxicity in the form of mutation, DNA damage, chromosomal aberrations or sister chromatid exchange (SCE). One of these two studies was considered weakly positive for SCE. A study not included in the Draft reiterates a lack of mutagenesis in the Ames assay with or without S9 fraction.15 As summarized,14 in vivo studies, involving doses with rats at, or above, the gram/kilogram body weight range, are somewhat more positive with respect to genotoxicity. Five of 15 such studies document mainly clastogenic activity.
The present report in part summarizes work carried out to determine the stability of lactone 1 preparatory to its in vivo testing, additionally reported here, regarding its ability to induce or promote liver carcinogenesis in the rat.
In the F344 rat, hepatic cancer and the process of cancer formation (i.e., liver carcinogenesis) can be initiated by a number of chemicals. The liver carcinogen aflatoxin B1 (AFB1) is a secondary metabolic product of the mold Aspergillus flavus and AFB1 is a potent hepatotoxin and carcinogen in the F344 rat and many other experimental animals, livestock and humans.16–19 The F344 rat is highly sensitive to AFB1 and the cancer process (i.e., liver carcinogenesis) has been extensively studied. The early putative preneoplastic foci that are induced by AFB1 can readily be quantified20, 21 and both foci size and focal number in the liver can be measured. Experiments with the F344 rat provide examples of the growth of AFB1-induced foci being enhanced22 or suppressed20, 21, 23–26 by feeding chemicals subsequent to damaging DNA with AFB1. There is additional evidence that these agents ultimately modify (increase or suppress) the occurrence of actual liver cancer and not just the precursor foci.27, 28 Lactone 1 was evaluated both for its ability to induce early putative preneoplastic lesions (henceforth termed foci) that ultimately give rise to frank hepatocellular cancers as well as the ability of lactone 1 to enhance or promote the growth of foci that have been induced by AFB1. In neither case did 1 exhibit measurable activity.
Experimental
Chemicals
Except as noted, chemicals, solvents and isotopically enriched reagents employed in synthetic and analytical procedures were obtained from readily available commercial sources. Deionized water was obtained in house and filtered for HPLC. Acetonitrile was dried and distilled from calcium hydride. Tetrahydrofuran was dried and distilled from sodium. HPLC solvents were purchased as ‘HPLC grade” and were not further purified.
Analytical determinations
Measurements of pH were performed on an Orion pH meter, model SA720, with a combination electrode. Two point calibrations were done before pH values were recorded. Calibrations were performed using commercially available standards or 0.10 M HCl, pH = 1.10, as appropriate. Elemental analyses were performed by Atlantic Microlabs, Inc., Norcross, GA.
The equilibrium constant between 1 and its open-chain acid form was determined by measuring, by means of HPLC, the amount of 1, calculated by interpolation from a standard curve, that remained after equilibration of an acidic aqueous solution of 1. The initial concentration was gravimetrically determined. The equilibrium constant was determined as a function of ionic strength, by addition of KCl and hydrogen ion concentration in aqueous solutions that were 5% by volume acetonitrile and initially contained 0.05 M lactone.
The pKa of (2-hydroxyethoxy)acetic acid was determined by measuring the pH of solutions of the carboxylic acid anion (Na+ salt) that were partially neutralized by HCl. The pKa measurement was made at various ionic strengths between 0.05 and 1M with a concentrated KCl stock. Final solutions contained 5% by volume acetonitrile. The resulting buffers varied in concentration between 0.07 and 0.08 M. Values of pH were recorded at two temperatures, the meter standardized at pH = 7.00 and 1.10 at the appropriate temperatures. Average values of pKa were calculated from the Henderson-Hasselbalch equation. No correction was made for the existence of the lactone, which would have necessitated changes that were within the standard deviation of the initial determinations.
Kinetics
The decay of 1 was initially monitored in D2O by observing, by 1H-NMR, the loss of intensity of the overlapping resonances (4.78 ppm) for the hydrogens attached to C-6 at 25 °C in 0.005 M DCl, 0.005 M 1 and 0.005 M acetonitrile as an internal standard. The kinetics of decay, under identical conditions, of absorbance at 225nm was monitored, for comparison, by UV spectrometry. The good agreement, see Results, of these two measurements validated an extended kinetic analysis by UV spectrometry monitoring absorbance at 220, 225, 230, or 235 nm. The reaction conditions for the extended study were 5% by volume acetonitrile in water (H2O), ionic strength 1 M (KCl), 25 °C and 1 at an initial concentration of 0.0013 M. Solutions were buffered with formic, acetic, cacodylic and ethylphosphonic acid buffers. Buffer dilution plots of kobsd against buffer concentration, containing at least 4 buffer concentrations, were constructed from first order decay curves monitored for at least 3 t1/2 of reaction. Such plots were linear and, from extrapolation to zero buffer concentration, the buffer independent rate constant, ko, was derived. The associated buffer pH was taken as the average pH value of the measured values, at reaction endpoint, of all the buffer concentrations in a given experiment. Occasional checks of the pH, at t ~ 0, indicated no significant change in pH in the course of the reaction.
Synthesis. 1,4-dioxane-2-one (1)
A modification of an earlier preparation was employed.12 A 2 L three-neck flask chilled in an ice bath and containing 134 g of 2-(2-methoxyethoxy)acetic acid (2 mol) was fitted with a reflux condenser and a stopper. To the remaining neck of the flask was fitted a chilled (0 °C) addition funnel containing 113 mL (3 mol eq) of chilled (0 °C) 62 % aqueous hydrobromic acid solution, which was immediately added dropwise to the stirred round bottom flask. The reaction mixture was maintained on ice for another 30 min. The reaction mixture was then stirred at room temperature for 1 hr, and subsequently heated with the temperature gradually rising to reach 150 °C over 2 hr. The temperature was then increased to 180 °C and the solution was refluxed overnight. The reaction was subsequently cooled to room temperature, and the pH was adjusted to 3 with NaHCO3. The lactone was purified by subsequent vacuum distillation at bp 70–75 °C at 0.8 mm Hg to yield a product of 90–95 % purity based on NMR. Further recrystallization from tetrahyrofuran yielded 60 % (61 g) of colorless needles (Tm = 26.7 °C). 1H NMR (400 MHz, CDCl3, ppm): δ = 4.49 (t, 2H), 4.37 (s, 2H), 3.87 (t, 2H). 13C NMR: 166.62, 68.63, 66.42, 62.70. Anal. Calcd for C4H6O3: C, 47.06; H, 5.92; Found: C, 46.90; H, 5.94.
Sodium 2-hydroxyethoxy acetate (3-Na+)
The procedure for synthesis of 1, above, was initiated. Subsequent to the overnight reflux, the solution was cooled to room temperature and neutralized with 50 % aqueous NaOH. The mixture was diluted with 150 mL absolute ethanol and heated at 80 °C for 15 min. The solution was allowed to cool to room temperature with stirring. Stirring was continued until a white precipitate formed. Crystallization was allowed to proceed at 4 °C. The white needles were collected and rinsed with cold absolute ethanol to yield the desired product in 66% final yield. Mp = 206.5 °C. 1H NMR (400 MHz, D2O, ppm): δ = 3.83 (s, 2H), 3.60 (t, 2H), 3.50 (t, 2H). 13C NMR: 178.19, 71.80, 60.50. Anal. Calcd for C4H7O4Na: C, 33.81; H, 4.97; Found: C, 33.84; H, 5.00.
Animal Studies
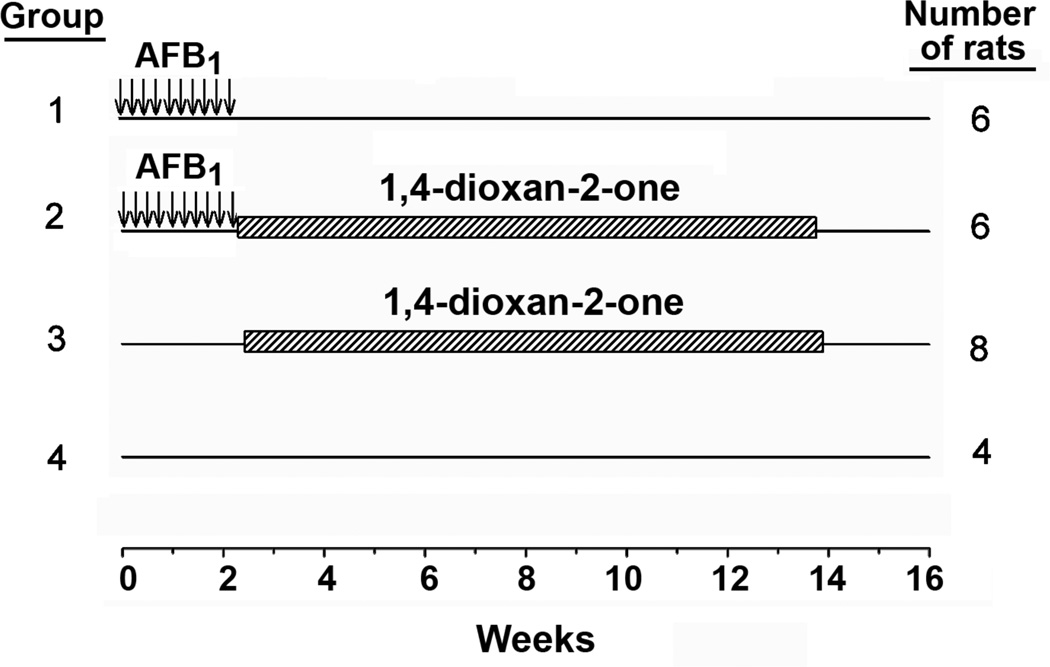
Male F344 rats (90–100g) were purchased from Charles River Laboratories (Wilmington, MA) and allowed 2 weeks to acclimatize to the animal facility prior to treatment. Rats were fed the highly purified diet AIN-76A (American Institute of Nutrition) without the added antioxidant ethoxyquin (Harlan, Madison, WI). Throughout the experiments, both food and water were available ad libitum. The experimental protocol is schematically illustrated in Figure 2. At 5 weeks of age (about 125g body weight), all rats were gavaged with either AFB1 (25 µg/rat) or vehicle (tricaprylin) 5 days per week for 2 successive weeks for a cumulative dose of 250 µg AFB1 per rat. Commencing the week immediately following AFB1 treatment, rats were gavaged with lactone 1, dissolved in water immediately prior to use, three days (MWF) per week for 12 weeks. In the first week, the doses of dioxane were each 0.02g/rat for each of the three days. This dose was equal to or less than 15% of the reported LD50 dose.29 In the subsequent 11 weeks, all doses were 0.04g/rat and they were equal to or less than 26% of the reported LD50 dose.
Figure 2.
Carcinogenesis treatment protocol. Aflatoxin B1 (AFB1) treatment is indicated by the arrows (groups 1 and 2) and 1,4-dioxane-2-one treatment is indicated by the hatched bars (groups 2 and 3). Group 4 is a vehicle treatment group.
At 12 hours prior to autopsy, food was withdrawn to reduce the glycogen accumulation in the livers. From the left lateral hepatic lobe, multiple 2 mm thick sections were cut by hand, fixed in acetone at 4°C, embedded in paraffin, and processed by routine histological methods. Hepatic sections (5 µm thick) were stained by standard immunohistochemical methods for expression of glutathione S-transferase-placental isoform P (GST-P) foci and the foci were identified and analyzed by light microscopy. As with previous analyses21, 24, 26, the observed focal data of number of foci per unit area of tissue examined and their individual focal transactional areas were subjected to morphometric transformation resulting in foci per cm3 liver tissue, mean focal diameter and the volume percent of liver occupied by GST-P positive foci, a parameter analogous to tumor burden. Extensive details of these procedures have been published previously.20, 26, 30
Statistical analyses
Focal data (foci per cubic cm, mean diameter, and volume %) were statistically analyzed using a t-test (Stata Corp., College Station, TX).
Results
Equilibria
Values for the equilibrium constant KO, Fig. 1, at 25 °C under a variety of ionic strength and acid conditions, are listed in Table 1. The data were obtained by allowing 1 and 2 to equilibrate, the initial concentration of 1 having been gravimetrically determined, and measuring the amount of 1 remaining by reverse phase HPLC on the basis of interpolation from a standard curve for 1.
Table 1.
Values of Ko, the equilibrium constant for lactone 1 and its open-chain acid form, determined at 25 °C.
| [HCl], M | Total Ionic Strengtha, M | Kob |
|---|---|---|
| 0.05 | 0.05 | 71 |
| 0.05 | 0.15 | 72 |
| 0.05 | 0.25 | 71 |
| 0.05 | 0.45 | 71 |
| 0.05 | 0.55 | 70 |
| 0.10 | 1.0 | 70 |
| 0.20 | 1.0 | 70 |
Beyond [HCl], the balance of the ionic strength was maintained with KCl.
The standard deviation of each determination is <5% on the basis of triplicate injections.
Values for the pKa of 2 under a variety of conditions of ionic strength and temperature are listed in Table 2. The values of pKa were determined by measuring values of pH of partially neutralized solutions of the sodium salt of 3 the concentration of which was gravimetrically determined. On the basis of the observed pH, the ratio HA/A- was adjusted for the fractional ionization and the pKa was calculated therefrom by use of the Henderson-Hasselbalch equation. No correction was made for the presence of 1 (see Discussion).
Table 2.
Determination of pKa values for 2 under varying conditions of ionic strength and temperature.
| Ionic Strength, M a | Observed pH b | Calculated pKac |
|---|---|---|
| 0.05 | 3.44 ± 0.01 | 3.43 |
| 0.25 | 3.37 ± 0.01 | 3.36 |
| 0.35 | 3.37 ± 0.01 | 3.36 |
| 0.45 | 3.37 ± 0.01 | 3.36 |
| 0.55 | 3.39 ± 0.01 | 3.38 |
| 1.0 d | 3.31, 3.58, 3.81 e | 3.31 ± 0.02 f |
| 1.0 d | 3.27, 3.54, 3.76 g | 3.27 ± 0.01 h |
Unless otherwise stated, the total buffer concentration was 0.05 M, at a prepared buffer ratio of AH/A- = 1.0. The balance of the ionic strength was maintained with KCl.
Unless otherwise stated the value is the mean and standard deviation of duplicate determinations at 25 °C.
Unless otherwise stated, the value is the pKa calculated, for T= 25 °C, from the observed pH, the buffer ratio, corrected for the fractional dissociation of the acid form, and the Henderson-Hasselbalch equation.
For buffer concentrations between 0.070 and 0.080 M. The balance of ionic strength was maintained with KCl.
The pH values refer to measured values, at 25 °C, for buffers prepared at a buffer ratio of HA/A- = 1.0, 35/65 and 1/3, respectively.
The value of pKa, at 25 °C, is the mean and standard deviation of the pKa values calculated from the observed pH values at the three buffer ratios, corrected for the fractional dissociation of the acid form, using the Henderson-Hasselbalch equation.
The pH values refer to measured values, at 37 °C, for buffers prepared at a buffer ratio of HA/A- = 1.0, 35/65 and 1/3, respectively.
The value of pKa, at 37 °C, is the mean and standard deviation of the pKa values calculated from the observed pH values at the three buffer ratios, corrected for the fractional dissociation of the acid form, using the Henderson-Hasselbalch equation.
Kinetics
The kinetics of decay of 1 were initially determined by 1H-NMR by monitoring the disappearance of hydrogen resonances at position 6 of the lactone in D2O solutions containing 0.005 M DCl at 25 °C. In addition, decay of a shoulder of absorbance between 220 and 240 nm was monitored by UV spectrophotometry under identical conditions. Both processes exhibited good first order decay for 2.5 t1/2 of decay, data not shown, and rate constants that were the same within experimental error: 0.017 ± 0.002 min−1 (NMR) and 0.016 ± 0.001 min−1 (UV). The good agreement validated a more extensive study using the more facile UV method with reactions in H2O solutions containing 5% by volume acetonitrile.
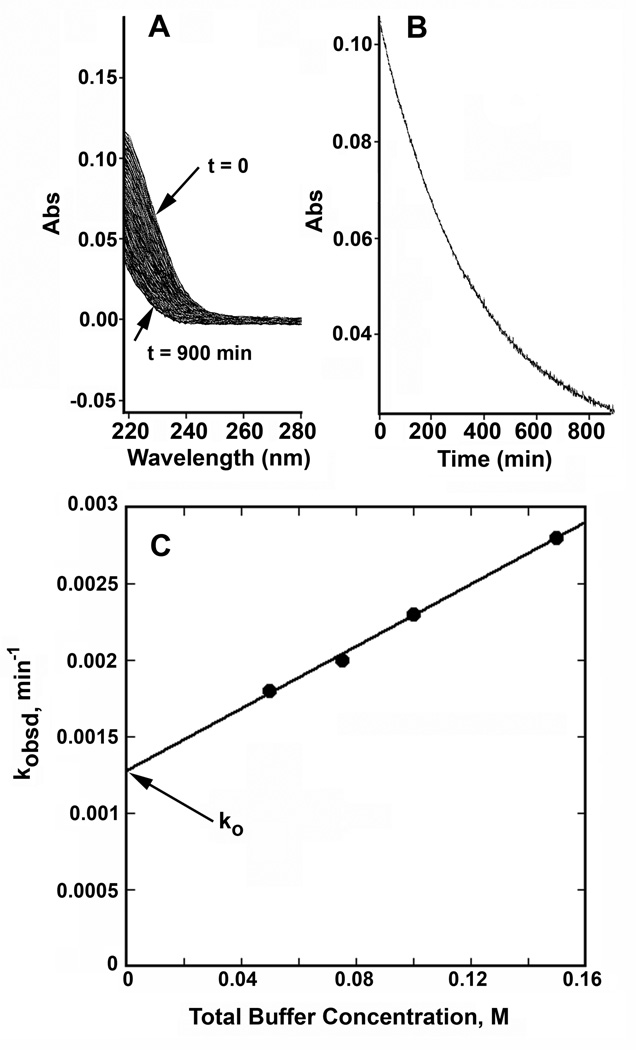
Figure 3A depicts the decrease in the UV absorbance spectrum as a function of time in a cacodylic acid buffer, 90% acid form, spanning more than three t1/2 of decay. The change in absorbance at 225 nm against time for this experiment is indicated in Fig. 3B and includes an overlaid fit to a simple firstorder decay process. Plots of kobsd as function cacodylic acid concentration are plotted in Fig. 3C. Generally these plots contained a minimum of four points at buffer concentrations of buffer of less than 0.2 M total buffer concentration. Such plots were typically linear and permitted assignment, by extrapolation of a value of ko, the buffer independent rate constant of hydrolysis. For any given plot, the largest value of kobsd generally did not exceed the value of ko by more than a factor of 7.
Figure 3.
Kinetics of decay of 1,4-dioxan-2-one in aqueous solutions, 5% by volume acetonitrile, ionic strength 1 M, KCl, cacodylic acid buffer, 90% acid form. A. Change in UV spectrum as a function of time, total buffer concentration = 0.08 M. B. Semi-logarithmic plot of absorbance change in A as a function of time, fit to a first order process is overlaid on the data. C. Change in kobsd as a function buffer concentration extrapolated to the intercept to derive the value of ko, the buffer independent rate constant.
Carcinogenesis
The results of the animal studies are shown in Table 3. Large numbers of GST-P positive foci were observed in all rats treated with AFB1 (groups 1 (AFB1) and 2 (AFB1 + 1)) and the average size of these observed foci (focal transactional areas) was similar for the two groups treated with AFB1 (>200µm - data not shown). Only one of 8 rats in Group 3 (1 only) had a single focus and that individual focus was small (65 µm diameter) compared to the average foci induced by AFB1 (>200µm). Rats treated with vehicle only, Group 4, had no foci. Observed focal data are inherently biased as large foci are over represented; thus, these data were subjected to morphological transformation. For number of foci per volume of liver or the mean focal diameter of the foci, there was no statistically significant difference between groups 1 and 2. Livers of rats not receiving AFB1 (groups 3 and 4) had few if any foci. The volume % of liver occupied by GST-P positive foci (i.e., focal volume %) is the most robust measure and is analogous to tumor burden. There was no statistically significant difference in foci number, size or volume % between groups 1 and 2 and groups 3 and 4.
Table 3.
Observed and morphometric data for F344 rats dosed with AFB1 (group 1), AFB1 followed by 1 (group 2), 1 only (group 3) and vehicle (group 4).
| Observed | Morphometric Data | ||||
|---|---|---|---|---|---|
| Group | Number of Rats |
Mean Foci Counteda |
Foci per cubic cma |
Mean Diametera (microns) |
Volume %a |
| 1 | 6 | 44 ± 8 | 1230 ± 410 | 227 ± 35 | 3.1 ± 1.0 |
| 2 | 6 | 40 ± 3 | 1190 ± 230 | 210 ± 32 | 2.1 ± 0.7 |
| 3 | 8 | 0.1 ± 0.1 | 6.1 ± 6.1 | 65 | 0.001 |
| 4 | 4 | 0 | 0 | --- | 0 |
Values reported are mean ± standard error of the mean.
Discussion
Equilibria
The equilibrium constant Ko, Table 1, is comparatively large and in favor of the open chain carboxylic acid, relative to the lactone. A lower limit value of KO > 50, not inconsistent with the values in Table 1, was obtained by monitoring the disappearance of the lactone multiplets in D2O, 1 M DClO4, to equilibrium. At endpoint the lactone multiplets were barely visible above background preventing the determination of an actual value. The values determined under the varying conditions establish that there is a negligible effect of varying ionic strength on the equilibrium constant KO, as was to have been expected given the absence of charged species on either side. The values of KO, Table 1, are slightly to significantly larger than those reported previously for a set of permethylated hexose- δ- lactones31 and glucono- δ -lactone32 which range from 54 to 1.0 in aqueous media. In contrast, smaller ring lactones can be more thermodynamically favorable than their open chain forms.33, 34 The large value of KO means that even in the most favorable, acidic, environment, were the acid and lactone to reach equilibrium, the concentration of the lactone would be just 1.4% of the sum of both forms.
The value of the apparent pKa for ionization of the open chain acid was determined under a differing conditions of ionic strength and/or temperature on the basis of the measured pH of partially neutralized solutions of acid; and, inspection of the values in Table 2 suggests expected values and small changes in pKa with conditions. In calculating the value, inclusion of the lactone concentration was neglected because the small concentration value affected the absolute value of pKa by less than the experimental error in the determinations. By comparison, the value of the apparent pKa of methoxyacetic acid has been reported as 3.31 at 25 °C, ionic strength 1M (KCl). Further, the apparent pKa values at 25 °C at ionic strength 1 M (NaClO4 or NaCl) for the permethylated hexose-δ-lactones and glucono-δ-lactone range from 3.31 to 4.20.31, 32, 35 The effect of changes in ionic strength and temperature are small, consistent with expectations on the basis of what is known for the effects upon acetic acid ionization.36, 37
The determination of KO and the pK a permits the calculation of KOA, Fig.1, as KOA = Ka × KO = 0.034 ±0.002 M, in which the uncertainty is calculated as the error propagated in the multiplication. Thus, at equilibrium, at pH = 7, the lactone represents ~3 ×10−4 percent of the total species represented in Fig 1.
Kinetics
The kinetics of decay of lactone 1 were monitored in parallel by UV spectrophotometry and 1HNMR in solutions of D2O and the values of kobsd were the same within the error of the determinations. This validated a more extended investigation in buffered H2O solutions using the more facile UV method.
Generally the value of kobsd varied as a function of buffer concentration according to equation 1, in which kbuf is the second order rate constant for
| (1) |
acceleration of the decay by total buffer species, while ko is the buffer-independent rate constant, obtained by linear extrapolation of plots such as in Fig. 3c. While stimulation by the oxygen acid buffers studied was significant, relative to the buffer independent rate constants, in the range of buffer concentrations studied, a more detailed study and analysis of these constants was not attempted.
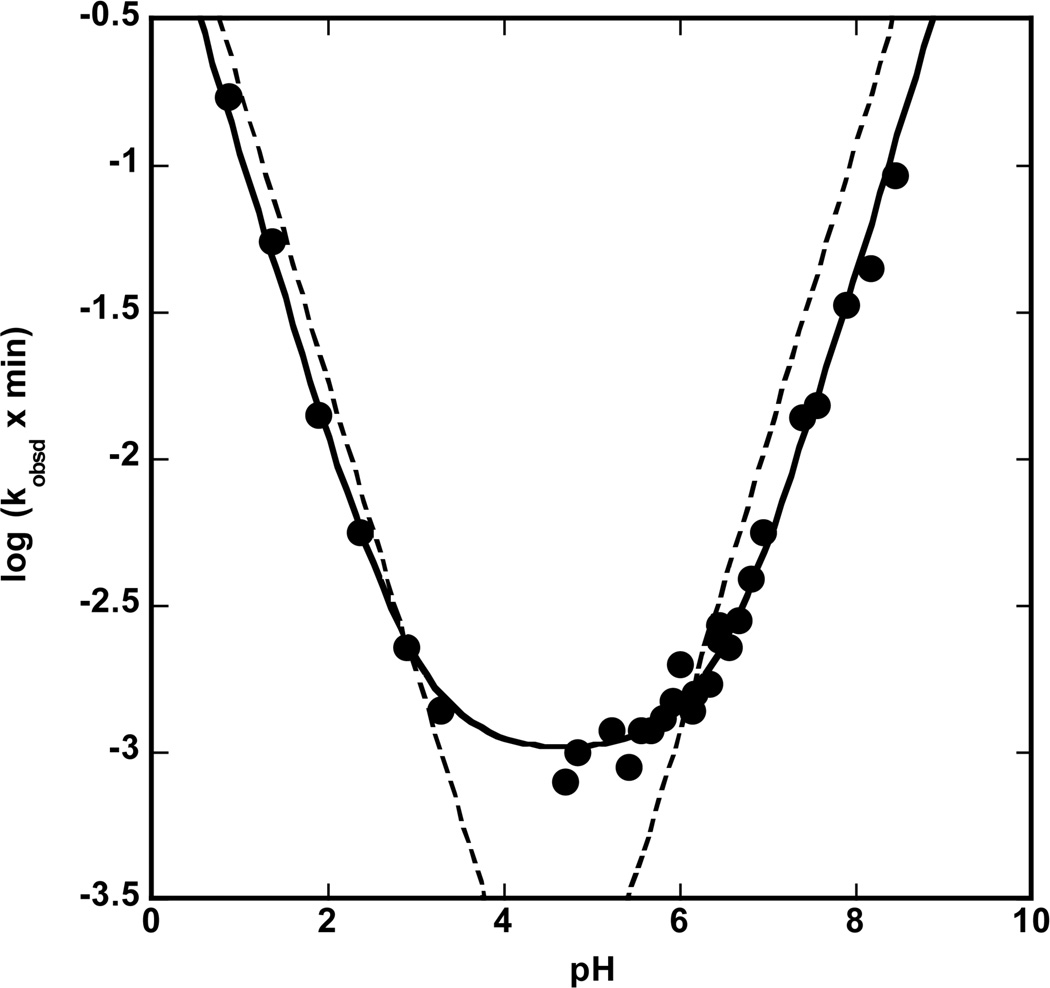
The variation of the value of log ko as a function of solution pH is presented in Fig. 4 in which the solid circles represent the actual values of ko determined in this study. The data in Fig. 4 are best described by a rate law for the variation of log ko with pH that contains three kinetic terms, as indicated in equation 2, one involving the hydrogen ion, kH+, a pH-independent term, kw,
| (2) |
and one involving hydroxide ion, kOH. The best fit to the data of the rate law of equation 2 is indicated by the solid line in Fig. 4 with rate constant of kH+ = 1.1 (± 0.1) M−1 min−1, kw = 9.9 (± 0.5) × 10−4 min−1 and kOH = 4.1 (± 0.3) × 104 M−1 min−1. The best fit to an alternative two-term rate law containing only terms for the hydrogen and hydroxide ions is indicated by the dashed line in Fig. 4 and underestimates the rate constants observed in pH range from 3.5 – 6 by rate factors as large ~4, well outside the experimental error of the determinations.
Figure 4.
Plot of the log of ko as function of pH. The solid line is a best fit to a three-term rate law, eq 2, for the solvent mediated hydrolysis. The dashed line is the best fit to a two-term rate law for solvent mediated hydrolysis.
The reactivity of lactone 1 with solvent species is comparable with what has been observed for other lactones.31, 32 D-Glucono-δ-lactone is more reactive than 1 by less than a factor of 3 in the hydrogen ion, hydroxide ion and water-catalyzed hydrolyses. Similarly, in all but one case, the rate constants for the hydrolysis reactions of permethylated glycono-δ-lactones are within a factor of five of those determined here for 1. In the case of tetra-O-methylmannono-δ-lactone, the rate constant for hydrogen ion catalyzed hydrolysis of 1 is faster by a factor of 12, though a reason for this is not clear. Surprisingly, 1 is ~1500 more reactive toward hydroxide ion than valerolactone,38 perhaps reflecting greater electrophilicity and better leaving group ability in the former, and possibly a difference in rate limiting step on the reaction coordinate.
Though the equilibria determined above substantially disfavor the lactone 1, the kinetics indicate a chemical stability that is sufficient to transit the extremes of the digestive tract. The t1/2 for decay of 1 at pH 2 and 7 is 57 min and 127 min, respectively. The t1/2 at pH = 7 is within a factor of three of those for decay of N-nitroso-N-methylurea39 and N-methyl-N’-nitro-N-nitrosoguanidine40 while the t1/2 at pH = 2 is within a factor of 3 of trimethyl-N-nitrosourea.39 Each of these N-nitroso compounds is a documented rodent carcinogen when orally administered.1, 41–43 These solvolysis studies further established the feasibility for designing protocols for the animal studies described below.
Carcinogenesis in the rat
As expected with this rat model of liver carcinogenesis, the small total dose and short dosing schedule of AFB1 (see Fig 2) resulted in large numbers of hepatic foci (Table 3, group1); however, subsequent exposure to lactone 1 did not modify the number of foci (group 1 vs group 2) that developed nor were their average focal size altered. Additionally, exposure to only lactone 1 resulted in no significant initiation of hepatic foci. One very small focus was encountered in the tissue sections of 1 of 8 rats receiving the cumulative total dose of 1.32 g of lactone 1. Small spontaneous foci are occasionally observed in control rat livers.30 For example, in a group of control rats exposed to no known carcinogen, but similar in age and sex to rats of this study, one spontaneous focus was observed and it was 2.5 times as large as the one observed in this study.
It is certainly possible that larger doses of lactone 1 treated over a more lengthy study period with examination of rats living to much older ages might have provided evidence of hepatic carcinogenesis, but this study does not presage such an outcome.
Alternative genotoxins by which diethylene glycol, dioxane and N-nitrosomorpholine might manifest their carcinogenicity are the aldehyde preceding formation of 1, aldehydes from the further metabolism of 1–3 and, in the case of N-nitrosomorpholine, the diazonium ion that has been proven to alkylate DNA and other aldehydes derived from diazonium ion fragmentation.44
Acknowledgments
Funding Support
This work was supported in part by NIH grant R01 CA52881 (JCF), Aberdeen Proving Ground contract W91ZLK-08-P-1038 (JCF) and NIH grant R01 CA 39416 (BDR).
Non-standard Abbreviations
- AFB1
aflatoxin B1
- HPLC
high-performance liquid chromatography
- MWF
Monday-Wednesday-Friday
- GST-P
glutathione S-transferase-placental isoform P
References
- 1.Druckrey H, Preussmann R, Ivankovic S, Schmahl D. Organotropic carcinogenic effects of 65 various N-nitroso- compounds on BD rats. Z. Krebsforsch. 1967;69:103–201. [PubMed] [Google Scholar]
- 2.Lijinsky W. Chemistry and biology of Nnitroso compounds. Cambridge, UK: Cambridge University Press; 1992. [Google Scholar]
- 3.Mohr U. Carcinogenesis of N-nitroso-morpholine and derivatives in Syrian golden hamsters. Prog. Exp. Tumor Res. 1979;24:235–244. doi: 10.1159/000402100. [DOI] [PubMed] [Google Scholar]
- 4.Argus MF, Sohal RS, Bryant GM, Hoch-Ligeti C, Arcos JC. Dose-response and ultrastructural alterations in dioxane carcinogenesis. Influence of methylcholanthrene on acute toxicity. Eur. J. Cancer. 1973;9:237–243. doi: 10.1016/0014-2964(73)90088-1. [DOI] [PubMed] [Google Scholar]
- 5.Kociba RJ, McCollister SB, Park C, Torkelson TR, Gehring PJ. 1,4-Dioxane. Results of a 2-year ingestion study in rats. Toxicology and Applied Pharmacology. 1974;30:275–286. [Google Scholar]
- 6.Argus MF, Arcos JC, Hochligeti C. Studies on the carcinogenic activity of protein-denaturing agents: hepatocarcinogenicity of dioxane. J. Natl. Cancer Inst. 1965;35:949–958. [PubMed] [Google Scholar]
- 7.Fitzhugh OG, Nelson AA. Comparison of the chronic toxicity of triethylene glycol with that of diethylene glycol. J. Ind. Hyg. Toxicol. 1946;28:40–43. [PubMed] [Google Scholar]
- 8.Weil CS, Carpenter CP, Smyth HF., Jr Urinary bladder calculus and tumor response following either repeated feeding of diethylene glycol or calcium oxalate stone implantation. Ind. Med. Surg. 1967;36:55–57. [PubMed] [Google Scholar]
- 9.Braun WH, Young JD. Identification of beta-hydroxyethoxyacetic acid as the major urinary metabolite of 1,4-dioxane in the rat. Toxicol. Appl. Pharmacol. 1977;39:33–38. doi: 10.1016/0041-008x(77)90174-0. [DOI] [PubMed] [Google Scholar]
- 10.Hecht SS, Young R. Metabolic alpha-hydroxylation of N-nitrosomorpholine and 3,3,5,5- tetradeutero-N-nitrosomorpholine in the F344 rat. Cancer Res. 1981;41:5039–5043. [PubMed] [Google Scholar]
- 11.Woo YT, Arcos JC, Argus MF. Metabolism in vivo of dioxane: identification of p-dioxane-2-one as the major urinary metabolite. Biochem. Pharmacol. 1977;26:1535–1538. doi: 10.1016/0006-2952(77)90430-0. [DOI] [PubMed] [Google Scholar]
- 12.Grablowitz H, Lendlein A. Synthesis and characterization of a,w-dihydroxy-telechelic oligo(p-dioxanone) J. Mater. Chem. 2007;17:4050–4056. [Google Scholar]
- 13.Zink CN, Soissons N, Fishbein JC. Products of the direct reaction of the diazonium ion of a metabolite of the carcinogen N-nitrosomorpholine with purines of nucleosides and DNA. Chemical research in toxicology. 2010;23:1223–1233. doi: 10.1021/tx100093a. [DOI] [PubMed] [Google Scholar]
- 14.Frumkin H, Gerberding JL. U.S.DHHS, ATSDR; 2007. Draft toxicological profile for 1,4-dioxane; pp. 1–208. http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=955&tid=199. [Google Scholar]
- 15.Kubo T, Urano K, Utsumi H. Mutagenicity characteristics of 255 environmental chemicals. Journal of Health Science. 2002;48:545–554. [Google Scholar]
- 16.Busby WF, Wogan GN. Aflatoxins. In: Searle CE, editor. Chemical Carcinogens. Washington, D.C.: Am. Chem. Soc.; 1984. pp. 945–1136. [Google Scholar]
- 17.Eaton WF, Groopman JD. The Toxicology of Aflatoxins: Human Health, Veterinary and Agricultural Significance. New York: Academic Press; 1994. [Google Scholar]
- 18.Kensler TW, Groopman JD, Sutter TR, Curphey TJ, Roebuck BD. Development of cancer chemopreventive agents: Oltipraz as a paradigm. Chem. Res. Tox. 1999;12:113–126. doi: 10.1021/tx980185b. [DOI] [PubMed] [Google Scholar]
- 19.Kensler TW, Roebuck BD, Wogan GN, Groopman JD. Aflatoxin: a 50-year odyssey of mechanistic and translational toxicology. Toxicol. Sci. 2011;120(Suppl 1):S28–S48. doi: 10.1093/toxsci/kfq283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kensler TW, Groopman JD, Eaton DL, Curphey TJ, Roebuck BD. Potent inhibition of aflatoxin-induced hepatic tumorigenesis by the monofunctional enzyme inducer 1,2-dithiole-3-thione. Carcinogenesis. 1992;13:95–100. doi: 10.1093/carcin/13.1.95. [DOI] [PubMed] [Google Scholar]
- 21.Roebuck BD, Curphey TJ, Li Y, Baumgartner KJ, Bodreddigari S, Yan J, Gange SJ, Kensler TW, Sutter TR. Evaluation of the cancer chemopreventive potency of dithiolethione analogs of oltipraz. Carcinogenesis. 2003;24:1919–1928. doi: 10.1093/carcin/bgg173. [DOI] [PubMed] [Google Scholar]
- 22.Maxuitenko YY, MacMillan DL, Kensler TW, Roebuck BD. Evaluation of the post-initiation effects of oltipraz on aflatoxin B1-induced preneoplastic foci in a rat model of hepatic tumorigenesis. Carcinogenesis. 1993;14:2423–2425. doi: 10.1093/carcin/14.11.2423. [DOI] [PubMed] [Google Scholar]
- 23.Kensler TW, Egner PA, Dolan PM, Groopman JD, Roebuck BD. Mechanism of protection against aflatoxin tumorigenicity in rats fed 5-(2-pyrazinyl)-4-methyl-1,2-dithiol-3-thione (oltipraz) and related 1,2-dithiol-3-thiones and 1,2-dithiol-3-ones. Cancer Res. 1987;47:4271–4277. [PubMed] [Google Scholar]
- 24.Liby K, Yore MM, Roebuck BD, Baumgartner KJ, Honda T, Sundararajan C, Yoshizawa H, Gribble GW, Williams CR, Risingsong R, Royce DB, Dinkova-Kostova AT, Stephenson KK, Egner PA, Yates MS, Groopman JD, Kensler TW, Sporn MB. A novel acetylenic tricyclic bis-(cyano enone) potently induces phase 2 cytoprotective pathways and blocks liver carcinogenesis induced by aflatoxin. Cancer Res. 2008;68:6727–6733. doi: 10.1158/0008-5472.CAN-08-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Primiano T, Egner PA, Sutter TR, Kelloff GJ, Roebuck BD, Kensler TW. Intermittent dosing with oltipraz: relationship between chemoprevention of aflatoxin-induced tumorigenesis and induction of glutathione S-transferases. Cancer Res. 1995;55:4319–4324. [PubMed] [Google Scholar]
- 26.Yates MS, Kwak MK, Egner PA, Groopman JD, Bodreddigari S, Sutter TR, Baumgartner KJ, Roebuck BD, Liby KT, Yore MM, Honda T, Gribble GW, Sporn MB, Kensler TW. Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole. Cancer Res. 2006;66:2488–2494. doi: 10.1158/0008-5472.CAN-05-3823. [DOI] [PubMed] [Google Scholar]
- 27.Kensler TW, Gange SJ, Egner PA, Dolan PM, Munoz A, Groopman JD, Rogers AE, Roebuck BD. Predictive value of molecular dosimetry: individual versus group effects of oltipraz on aflatoxin-albumin adducts and risk of liver cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:603–610. [PubMed] [Google Scholar]
- 28.Roebuck BD, Liu YL, Rogers AE, Groopman JD, Kensler TW. Protection against aflatoxin B1-induced hepatocarcinogenesis in F344 rats by 5-(2-pyrazinyl)-4-methyl-1,2-dithiole-3-thione (oltipraz): predictive role for short-term molecular dosimetry. Cancer Res. 1991;51:5501–5506. [PubMed] [Google Scholar]
- 29.Woo YT, Argus MF, Arcos JC. Effect of mixed-function oxidase modifiers on metabolism and toxicity of the oncogen dioxane. Cancer Res. 1978;38:1621–1625. [PubMed] [Google Scholar]
- 30.Maxuitenko YY, Curphey TJ, Kensler TW, Roebuck BD. Protection against aflatoxin B1-induced hepatic toxicity as short-term screen of cancer chemopreventive dithiolethiones. Fundam. Appl. Toxicol. 1996;32:250–259. doi: 10.1006/faat.1996.0128. [DOI] [PubMed] [Google Scholar]
- 31.Pocker Y, Green E. Hydrolysis of D-glucono-d-lactone. II. Comparative studies of general acid-base catalyzed hydrolysis of methylated derivatives. J. Am. Chem. Soc. 1974;96:166–173. [Google Scholar]
- 32.Pocker Y, Green E. Hydrolysis of D-glucono-d-lactone. I. General acid-base catalysis, solvent deuterium isotope effects, and transition state characterization. J. Am. Chem. Soc. 1973;95:113–119. doi: 10.1021/ja00782a019. [DOI] [PubMed] [Google Scholar]
- 33.Coffin FD, Long FA. Arrhenius parameters for acid hydrolysis of gamma-butyrolactone; search for a reaction with water. J. Am. Chem. Soc. 1952;74:5767–5768. [Google Scholar]
- 34.Perez-Prior MT, Manso JA, Garcia-Santos MdP, Calle E, Casado J. Reactivity of lactones and GHB formation. J. Org. Chem. 2005;70:420–426. doi: 10.1021/jo040271i. [DOI] [PubMed] [Google Scholar]
- 35.Sawyer DT, Bagger JB. The Lactone-acid-salt equilibria for D-glucono-d-lactone and the hydrolysis kinetics for this lactone. J. Am. Chem. Soc. 1959;81:5302–5306. [Google Scholar]
- 36.Perrin DD, Dempsey B, Sergeant EP. pKa Prediction for Organic Acids and Bases. Cambridge, UK: Chapman and Hall; 1981. [Google Scholar]
- 37.Robinson RA, Stokes RH. Electrolyte Solutions. London: Butterworths Scientific Publications; 1955. [Google Scholar]
- 38.Hegan DS, Woldenden JH. The kinetics of the alkaline hydrolysis of some gamma-lactones. J. Chem. Soc. 1939:508–510. [Google Scholar]
- 39.Snyder JK, Stock LM. Reaction of alkylnitrosoureas in aqueous solution. J. Org. Chem. 1980;45:1990–1999. [Google Scholar]
- 40.Galtress CL, Morrow PR, Nag S, Smalley TL, Tschantz MF, Vaughn JS, Wichems DN, Ziglar SK, Fishbein JC. Mechanisms for the solvolytic decomposition of the carcinogen N-methyl-N'-nitro-N-nitrosoguanidine In aqueous solutions. J. Am. Chem. Soc. 1992;114:1406–1411. [Google Scholar]
- 41.Lijinsky W, Reuber MD, Blackwell BN. Carcinogenicity of nitrosotrialkylureas in Fischer 344 rats. J. Natl. Cancer Inst. 1980;65:451–453. [PubMed] [Google Scholar]
- 42.Schoental R, Bensted JP. Gastro-intestinal tumours in rats and mice following various routes of administration of N-methyl-N-nitroso-N'-nitroguanidine and N-ethyl-N-nitroso-N'-nitroguanidine. British journal of cancer. 1969;23:757–764. doi: 10.1038/bjc.1969.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sugimura T, Fujimura S. Tumour production in glandular stomach of rat by N-methyl-N'-nitro-N-nitrosoguanidine. Nature. 1967;216:943–944. doi: 10.1038/216943a0. [DOI] [PubMed] [Google Scholar]
- 44.Kim HJ, Fishbein JC. Reexamination of the aqueous chemistry of N-Nitroso-3-hydroxymorpholine, a metabolite of the carcinogen N-nitrosomorpholine. Chem Res Toxicol. 2003;16:715–720. doi: 10.1021/tx020114j. [DOI] [PubMed] [Google Scholar]