Abstract
Background
Liver dysfunction increases post-surgical morbidity and mortality. The Model of End-stage Liver Disease (MELD) estimates liver function but can be inaccurate in patients receiving oral anticoagulation. We evaluated the impact of liver dysfunction on outcomes following ventricular assist device (VAD) implantation and the dynamic changes in liver dysfunction that occur during VAD support.
Methods
We retrospectively analyzed 255 patients (147 patients with pulsatile devices and 108 patients with continuous flow devices) who received a long-term VAD between 2000 and 2010. Liver dysfunction was estimated by MELD and MELD-eXcluding INR (MELD-XI), with patients grouped by score ≥17 or <17. Primary outcomes were on-VAD, post-transplant and overall survival.
Results
MELD and MELD-XI correlated highly (R ≥ 0.901, p<0.0001) in patients not on oral anticoagulation. Patients with MELD or MELD-XI <17 had improved on-VAD and overall survival (p<0.05) with a higher predictive power for MELD-XI. During VAD support, cholestasis initially worsened but eventually improved. Patients with pre-VAD liver dysfunction who survived to transplant had lower post-transplant survival (p=0.0193). However, if MELD-XI normalized during VAD support, post-transplant survival improved and was similar to that of patients with low MELD-XI scores.
Conclusions
MELD-XI is a viable alternative for assessing liver dysfunction in heart failure patients on oral anticoagulation. Liver dysfunction is associated with worse survival. However, if MELD-XI improves during VAD support, post-transplant survival is similar to those without prior liver dysfunction, suggesting an important prognostic role. We also found evidence of a transient cholestatic state following LVAD implantation that deserves further examination.
Keywords: Transplantation, ventricular assist device, risk assessment, cardiomyopathy, liver dysfunction
INTRODUCTION
Liver dysfunction due to end-stage heart failure (HF) is often referred to as cardiac or congestive hepatopathy [1]. The underlying pathophysiology is related to poor end-organ perfusion leading to ischemic parenchymal changes with hepatocellular necrosis especially in cases of acute decompensation. Second, passive hepatic venous congestion develops in the setting of right heart dysfunction with increased right atrial pressures [1, 2]. Cholestatic changes are the hallmark of chronic congestive hepatopathy, with serum bilirubin and alkaline phosphatase often elevated [3]. While early stages are reversible, long-term congestive hepatopathy leads to irreversible damage to the liver parenchyma and cirrhosis with associated transaminitis [4]. The management is focused on treating the underlying cardiac abnormalities and hepatic function has been shown to benefit from cardiac transplantation with normalization of liver function assays by 6 months post-transplant [5].
Although individual laboratory assays can provide some insight on a patient’s liver function, the composite Model for End-Stage Liver Disease (MELD) is a more robust score of liver dysfunction. It was first developed to predict mortality in patients undergoing transjugular intrahepatic portosystemic shunt procedures [6][7] and has since been verified as a measure of liver dysfunction, providing an objective score based on a patient’s creatinine, total bilirubin, and international normalized ratio (INR). In 2002, the United Network for Organ Sharing adopted this system for prioritizing liver transplant candidates based on disease severity [8, 9]. Of note, elevated MELD scores also predict post-operative mortality in cirrhotic patients undergoing major digestive, orthopedic and cardiovascular surgery [10]. For patients with a MELD score <8, 30-day mortality was 5.7% compared to >50% for patients with MELD >20.
Left ventricular assist devices (LVADs) are increasingly utilized to treat patients with end-stage HF leading to improvements in both survival and quality of life as a bridge-to-transplantation (BTT) or destination therapy (DT) [11]. A recent study by Matthews et al. demonstrated that liver dysfunction (defined as a MELD >17) prior to LVAD implantation predicts both increased perioperative blood product use and six-month survival [12]. However, no study has analyzed the impact of dynamics in liver dysfunction on outcomes after LVAD insertion. Ventricular support should lead to improvements in cardiac hepatopathy yet no study has reported the specific factors associated with this potential relationship or its effect on survival. One reason for this has been the lack of a good measure of liver function in HF patients during LVAD support, which often requires oral anticoagulation with warfarin. Because warfarin increases INR, which is a major component of the MELD score, MELD becomes an inaccurate gauge of liver dysfunction.
As an alternative to the traditional MELD system, the MELD-XI (MELD eXcluding INR) score was developed by Heuman et al. [13]. It is calculated from creatinine and total bilirubin only. MELD-XI was validated in a population of over 7000 patients with liver cirrhosis and it highly correlated with MELD in patients not on oral anticoagulation both scores predicting survival similarly. Given that INR is not used in its calculation, MELD-XI will remain accurate even if a patient receives oral anticoagulation. Therefore, it is potentially a more effective method of estimating liver dysfunction in patients on LVAD support requiring concomitant oral anticoagulation.
In this study, we aimed to validate the MELD-XI and MELD scoring systems in HF patients undergoing LVAD placement. We also followed serum markers of cholestasis, hepatic injury, and other relevant conditions during LVAD support, and analyzed the role that changes in liver dysfunction assessed by MELD-XI may play in predicting post-transplant survival.
METHODS
Patient Selection
Approval for this study was obtained from the Institutional Review Board at Columbia University Medical Center. All patients receiving a long-term VAD between January 1, 2000, and September 7, 2010, at Columbia University Medical Center were included. Given that the vast majority of these patients (85%) received a pulsatile or continuous-flow HeartMate device, we restricted our patient population to those who received these devices. Sub-analysis was performed for patients who were supported by continuous-flow devices. Patients who were on temporary mechanical circulatory support prior to long-term VAD support were excluded. Patients were also excluded if pre-operative labs were unavailable.
Data Collection
Patient data was obtained from hospital medical records. Pre-operative laboratory values were defined as the last set of results immediately prior to VAD implantation. Primary outcomes included overall survival, on-VAD survival and post-OHT survival in those receiving a transplant. Laboratory values were assessed post-operatively at 30 days, 3 months, and 6 months for those who had a VAD for at least that length of time and immediately prior to transplant if they underwent OHT. Post-operative right heart failure (RHF) was defined as requirement of nitric oxide inhalation>48hr, inotropic support>14 days or/and RVAD after LVAD.
MELD and MELD-XI Definition
We used the UNOS modification of the MELD score [8], which uses the formula MELD = 3.78*Ln (Bili) + 11.2*Ln (INR) + 9.57*Ln (Cr) + 6.43. Any variable with a value less than 1 is assigned a value of 1 to avoid negative scores; thus, the minimum possible MELD score is 6.43. MELD-XI is defined by the formula MELD-XI = 5.11*Ln (Bili) + 11.76*Ln (Cr) + 9.44 [13]. Again, variables with values less than 1 were given the value of 1, with a minimum possible MELD-XI score of 9.44 [13]. According to the MELD and MELD-XI score prior to VAD surgery, patients were dichotomized into those with values ≥17 and those with values<17 as previously described [12].
Statistical Analysis
Statistical analyses were performed using a statistical package (Stata 11, College Station, TX). Statistical significance was determined based on a pre-established alpha of 0.05. Associations between categorical data were tested using Chi-squared and Fisher’s Exact Tests. Continuous data was compared using Student’s T-tests. Survival was compared using Kaplan-Meier analysis and log-rank tests. A Cox-proportional hazards model was used to test the significance of the individual variables as predictors of survival. The relation between MELD and MELD-XI scores was investigated by Pearson’s correlation analysis.
RESULTS
Patient Cohort
We captured a total of 264 adult patients who underwent primary long-term VAD placement between January 2000 and September 2010, including 158 (60%) pulsatile HeartMate (HM) and 106 (40%) HMII recipients. Of these, the initial LVAD goal was BTT in 215 (81.44%), DT in 46 (17.42%), and bridge to decision in 3 (1.14%) (Table 1). Due to incomplete medical records that prevented calculation of pre-VAD MELD, 9 patients were excluded, leaving 255 study subjects. Sub-analysis of survival after continuous flow LVAD implantation included 104 patients.
Table 1.
Clinical characteristics of patients undergoing VAD placement
| MELD <17 | MELD ≥ 17 | p-value | MELD-XI <17 | MELD-XI ≥ 17 | p-value | |
|---|---|---|---|---|---|---|
| n | 176 | 79 | 157 | 98 | ||
| Age (year-old) | 53.1±14.8 | 57.3±11.2 | 0.028 | 52.6±15.0 | 57.4±11.3 | 0.006 |
| Female (%) | 37 (21.0%) | 12 (15.2%) | 0.274 | 38 (24.2%) | 11 (11.2%) | 0.011 |
| Male (%) | 139 (79.0%) | 67 (84.8%) | 119 (75.8%) | 87 (88.8%) | ||
| BMI (kg/m2) | 27.2±6.0 | 26.4±4.3 | 0.293 | 27.1±5.9 | 26.6±4.9 | 0.504 |
| Diabetes (%) | 55/165 (33.3%) | 28/72 (38.9%) | 0.410 | 42/146 (28.8%) | 41/91 (45.1%) | 0.011 |
| CAD (%) | 73/165 (44.2%) | 31/71 (43.7%) | 0.934 | 62/146 (42.5%) | 42/90 (46.7%) | 0.528 |
| COPD (%) | 9/162 (5.6%) | 5/71 (7.0%) | 0.765 | 7/144 (4.9%) | 7/89 (7.9%) | 0.348 |
| Pre-op LVEF (%) | 17.6%±6.4% | 18.6%±8.3% | 0.359 | 18.2%±6.7% | 17.5%±7.5% | 0.544 |
| Ventilation (%) | 22/140 (15.7%) | 11/60 (18.3%) | 0.647 | 22/123 (17.9%) | 11/77 (14.3%) | 0.504 |
| h/o Hepatits (%) | 1/102 (1.0%) | 1/48 (2.1%) | 0.539 | 2/95 (2.1%) | 0/55 (0%) | 0.532 |
| h/o Cancer (%) | 20/161 (12.4%) | 6/70 (8.6%) | 0.395 | 17/142 (12.0%) | 9/89 (10.1%) | 0.663 |
| BTT (vs DT) (%) | 140/176 (79.6%) | 67/77 (87.0%) | 0.156 | 127/156 (81.4%) | 80/97 (82.5%) | 0.831 |
Baseline Characteristics
Most pre-operative characteristics were similar between the two groups, except that patients with MELD ≥ 17 (n=79) or MELD-XI ≥ 17 (n=98) were older (Table 1). Also, patients with elevated MELD-XI were more likely to be male (88.8% v 75.8%, p=0.011) and diabetic (45.1% v 28.8%, p=0.011). As expected, pre-operative creatinine and bilirubin levels were significantly greater for patients with elevated scores. INR was similar between MELD-XI groups but significantly higher (1.62 v 1.25, p<0.0001) in the MELD ≥ 17 group, as was expected. In addition, AST and ALT levels were generally higher and albumin levels generally lower in the elevated MELD and MELD-XI groups (Table 2).
Table 2.
Baseline laboratory values in patients undergoing VAD placement
| MELD <17 | MELD ≥ 17 | p-value | MELD-XI <17 | MELD-XI ≥ 17 | p-value | |
|---|---|---|---|---|---|---|
| Albumin (mg/dL) | 3.56±0.54 | 3.38±0.52 | 0.016 | 3.53±0.55 | 3.45±0.53 | 0.242 |
| Total Protein (mg/dL) | 6.5±1.2 | 6. 4±1.2 | 0.608 | 6.4±1.2 | 6.5±1.2 | 0.445 |
| AST (IU/L) | 48±78 | 135±318 | 0.0007 | 61±160 | 97±234 | 0.156 |
| ALT (IU/L) | 65±177 | 143±350 | 0.018 | 70±182 | 121±321 | 0.106 |
| Alk. Phosphatase (IU/L) | 92±52 | 101±55 | 0.183 | 92±52 | 100±55 | 0.219 |
| Total Bilirubin (mg/dL) | 1.42±0.98 | 2.36±1.34 | <0.0001 | 1.31±0.73 | 2.36±1.46 | <0.0001 |
| Direct Bilirubin (mg/dL) | 0.48±0.52 | 0.94±0.67 | <0.0001 | 0.43±0.35 | 0.93±0.78 | <0.0001 |
| White Blood Count (103/μL) | 8.9±3.3 | 10.2±4.2 | 0.011 | 8.9±3.3 | 10.0±4.1 | 0.026 |
| Hemoglobin (g/dL) | 11.3±1.9 | 10.9±1.8 | 0.136 | 11.2±1.9 | 11.0±1.9 | 0.442 |
| Hematocrit (%) | 34.2±5.3 | 33.0±5.4 | 0.081 | 34.1±5.2 | 33.5±5.6 | 0.371 |
| Platelet Count (103/μL) | 200±77 | 191±97 | 0.422 | 202±80 | 191±89 | 0.304 |
| INR | 1.25±0.20 | 1.62±0.57 | <0.0001 | 1.34±0.41 | 1.42±0.38 | 0.141 |
| Blood Urea Nitrogen (mg/dL) | 33±16 | 50±23 | <0.0001 | 30±13 | 51±22 | <0.0001 |
| Creatinine (mg/dL) | 1.34±0.40 | 2.14±0.70 | <0.0001 | 1.26±0.33 | 2.12±0.64 | <0.0001 |
| Sodium (mg/dL) | 134±5 | 131±6 | 0.0005 | 134±5 | 131±6 | <0.0001 |
| Potassium (mg/dL) | 4.1±0.5 | 4.2±0.5 | 0.321 | 4.1±0.5 | 4.2±0.5 | 0.195 |
MELD and MELD-XI values highly correlated, especially after excluding patients on oral anticoagulation within 5 days before VAD placement (R=0.901, p<0.0001).
Survival differences based on pre-VAD liver dysfunction scores
In total, 48 patients died on LVAD support, with six-month and one-year survival of 82.4% and 75.8%, respectively. On-VAD survival was defined as survival to heart transplantation, VAD explant (eg, for ventricular recovery) with survival for at least 30 days, or survival with device in place to the last date of follow-up. Patients with lower MELD or MELD-XI scores had better survival after VAD implantation. When dichotomized by MELD, patients with pre-VAD scores <17 had higher 18-month (Figure 1a, 73.5% v 63.2%, p=0.0050) and long-term on-VAD survival (p = 0.0069). Patients with a MELD-XI <17 also had higher 18-month (Figure 1b, 77.2% v 59.8%, p=0.0220) and long-term (p=0.0437) on-VAD survival. The 30-day post-VAD mortality of patients with high MELD-XI scores was 8.0%. Most of these deaths occurred during the early post-operative period due to multi-organ system failure, typically associated with sepsis or pre-existing organ failure such as post-cardiotomy shock as indication for VAD.
Figure 1. Survival based on the degree of liver dysfunction assessed by MELD and MELD-XI scores at the time of VAD implantation.
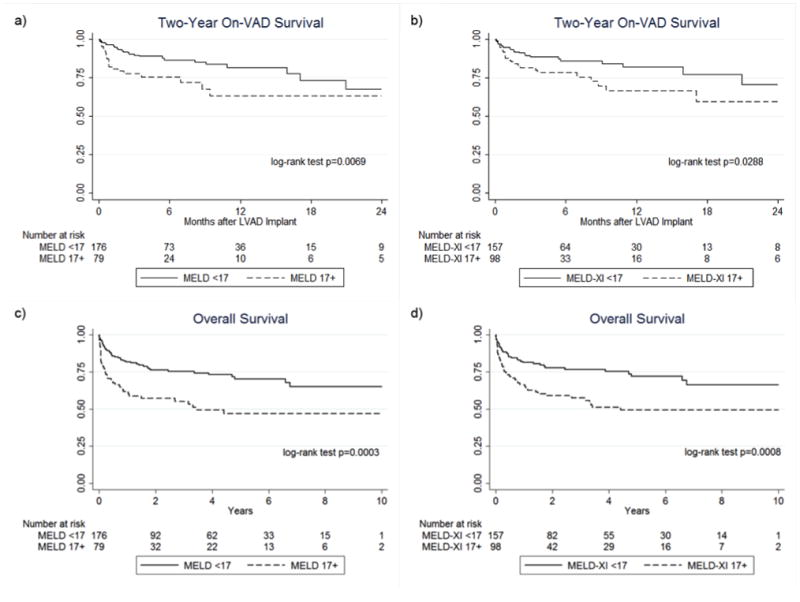
2-year on-VAD survival based on MELD (a) and MELD-XI (b) scores show significantly worse outcomes for patients with a score ≥17 compared to patients with scores <17. Overall survival based on MELD (c) and MELD-XI (c) scores demonstrate a more pronounced survival difference between patients with a score <17 vs ≥17.
In addition to an on-VAD survival advantage, patients with a MELD or MELD-XI <17 prior to LVAD implantation had an overall survival advantage as well (Figure 1c and 1d). Transplantation rates were similar between groups and thus did not account for this survival difference (Table 3). The duration of BTT VAD support was also similar across groups.
Table 3.
Initial therapeutic goal of LVAD implantation and subsequent rate of heart transplantation by MELD and MELD-XI groups in BTT patients and the entire cohort.
| MELD <17 | MELD ≥ 17 | p-value | MELD-XI <17 | MELD-XI ≥ 17 | p-value | |
|---|---|---|---|---|---|---|
| BTT | 79.4% | 85.1% | 0.293 | 80.9% | 81.6% | 0.883 |
| All patients | 65.7% | 55.4% | 0.124 | 64.3% | 59.2% | 0.409 |
In a subset of 104 patients with continuous flow devices, 71 patients had a MELD-XI<17 prior to LVAD surgery and the remaining 33 patients showed a MELD XI ≥17. The analysis of patients who were supported by continuous flow devices also revealed that on-VAD survival and overall survival were better in patients with MELD XI<17 compared to those in patients with MELD-XI ≥17 (log-rank p=0.0279 and p=0.0398, respectively, Figure 2a and 2b).
Figure 2. Sub-analysis of survival in patients with continuous flow devices based on the degree of liver dysfunction assessed by MELD-XI score at the time of VAD implantation.
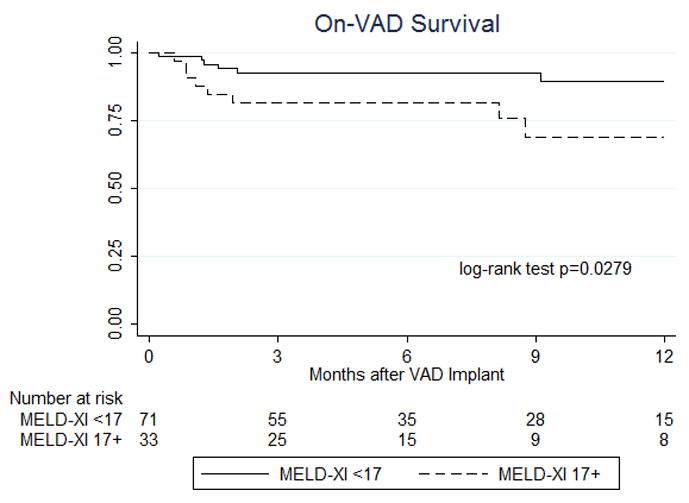
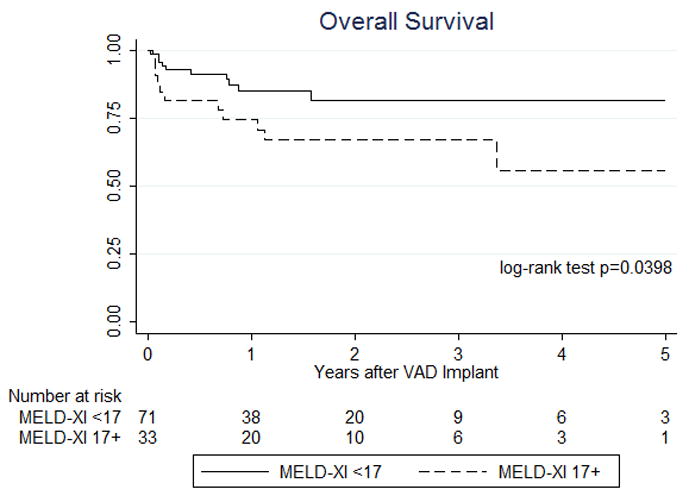
12-month on-VAD survival based on MELD-XI scores shows significantly worse outcomes for patients with a score ≥17 compared to patients with scores <17 (a). Overall survival up to 5 year after VAD implantation based on MELD-XI scores demonstrate a significantly worse outcome for patients with a score ≥17 compared to patients with scores <17 (b).
Impact of MELD-XI on survival
Given that creatinine levels showed a significant difference between those with MELD-XI < and ≥17, we performed a Cox-proportional hazard analysis on both creatinine alone and MELD-XI as predictors of survival. Cox regression showed both creatinine and MELD-XI predictive of survival, but multivariable analysis is not feasible due to colinearity. After grouping patients by high preoperative creatinine (>1.5 mg/dL) and high MELD-XI (≥17), multivariable analysis confirmed high MELD-XI as a predictive variable (HR 1.84, 95% CI 1.081–3.135, p=0.025) but not high creatinine levels (HR 1.2, 95% CI 0.716–2.081, p=0.464).
In addition, we performed a Cox proportional hazard ratio analysis based on MELD and MELD-XI scores using the values as continuous variables (Table 4). Both MELD and MELD-XI scores were significantly associated with on-VAD survival and overall survival; however, the MELD score showed only a trend of association with 2-year on VAD survival.
Table 4.
Cox-proportional hazard models of MELD and MELD-XI as predictors for survival
| HR (95% CI) | p-value | |
|---|---|---|
| 1 year on-VAD survival | ||
| MELD | 1.058 (1.003–1.117) | 0.039 |
| MELD-XI | 1.060 (1.003–1.119) | 0.038 |
| 2 year on-VAD survival | ||
| MELD | 1.053 (1.000–1.110) | 0.051 |
| MELD-XI | 1.058 (1.004–1.116) | 0.036 |
| Overall survival | ||
| MELD | 1.067(1.026–1.1.8) | 0.001 |
| MELD-XI | 1.064(1.024–1.105) | 0.001 |
Abbreviations not defined in the text; HR, hazard ratio; CI, confidential interval. MELD and MELD-XI were analyzed as continuous variables.
Improvement of Laboratory Values on VAD Support
Laboratory values generally improved during VAD support. We followed the trends of these values from pre-VAD implantation to 30 days of VAD support to late VAD support (Table 5). “Late VAD” values were defined as at time of heart transplant or, for those who did not undergo transplant, at 6 months of VAD support. The mean duration to transplant for patients after VAD implantation was 180 days (median 124.5 days). The mean duration to transplant was not significantly different between those with MELD-XI≥17 and those with MELD XI<17 (162.6 vs. 183.6 days, p=0.35). Renal function improved overall, with BUN, Cr, and sodium levels all normalizing. Transaminases, albumin, and total protein improved on average as well.
Table 5.
Dynamics in laboratory values before and after VAD placement
| Pre-VAD | 30 days on-VAD | p-value1 | Late On-VAD | p-value2 | |
|---|---|---|---|---|---|
| Albumin (mg/dL) | 3.5±0.6 | 3.4±0.5 | 0.0001 | 3.9±0.7 | <0.0001 |
| Total Protein (mg/dL) | 6.4±1.3 | 6.7±1.0 | 0.0759 | 7.3±1.1 | <0.0001 |
| AST (IU/L) | 75±191 | 34±35 | 0.0021 | 40±98 | 0.0742 |
| ALT (IU/L) | 89±245 | 27±24 | 0.0003 | 34±67 | 0.0057 |
| Alk. Phosphatase (IU/L) | 94.6±52.9 | 156.9±217.9 | <0.0001 | 120.5±88.3 | <0.0001 |
| Total Bilirubin (mg/dL) | 1.7±1.2 | 2.2±6.1 | 0.4472 | 1.0±0.9 | <0.0001 |
| Direct Bilirubin (mg/dL) | 0.6±0.6 | 1.0±3.2 | 0.3631 | 0.3±0.5 | <0.0001 |
| White Blood Count (103/μL) | 9.4±3.7 | 10.1±4.2 | 0.0254 | 8.5±3.5 | 0.0029 |
| Hemoglobin (g/dL) | 11.2±1.9 | 10.2±1.5 | <0.0001 | 11.3±1.9 | 0.5760 |
| Hematocrit (%) | 33.9±5.4 | 32.3±4.5 | <0.0001 | 35.1±5.6 | 0.0508 |
| Platelet Count (103/μL) | 197±84 | 285±113 | <0.0001 | 230±88 | 0.0027 |
| INR | 1.36±0.40 | 1.67±0.69 | <0.0001 | 1.53±0.56 | <0.0001 |
| INR (Pulsatile HM) | 1.42±0.46 | 1.38±0.54 | 0.7254 | 1.34±0.46 | 0.2401 |
| INR (HM II) | 1.28±0.30 | 1.98±0.70 | <0.0001 | 1.82±0.58 | <0.0001 |
| Blood Urea Nitrogen (mg/dL) | 38±20 | 23±15 | <0.0001 | 24±13 | <0.0001 |
| Creatinine (mg/dL) | 1.6±0.6 | 1.3±0.7 | <0.0001 | 1.3±0.6 | <0.0001 |
| Sodium (mg/dL) | 133±5 | 137±3 | <0.0001 | 137±3 | <0.0001 |
| Potassium (mg/dL) | 4.2±0.5 | 4.2±0.5 | 0.1203 | 4.3±0.7 | 0.0547 |
| MELD | 14.7±5.4 | 14.7±5.4 | 0.7615 | 13.5±4.9 | 0.0294 |
| MELD-XI | 15.8±5.6 | 14.0±5.4 | <0.0001 | 13.3±3.9 | <0.0001 |
p-value for comparison pre-VAD vs. on-VAD 30 days;
p-value for comparison pre-VAD vs. late on-VAD.
INR was difficult to use as a gauge of synthetic function given that some patients received oral anticoagulation during VAD support. Given differences in post-operative anticoagulation requirements, the trend in INR differed based on device. For pulsatile HM recipients, post-operative long-term anticoagulation was generally not required and there was no significant change in INR (1.42±0.46 v 1.34±0.46, p=0.256). For HMII recipients, who typically received oral anticoagulation, INR rose (1.28±0.30 v 1.82±0.6, p<0.0001).
Cholestasis during VAD Support
There was evidence that cholestasis worsened during early VAD support. Mean alkaline phosphatase levels increased significantly during the first 30 days of VAD support (94.6 to 156.9 IU/L, p<0.0001), during which 85.7% of patients had an increased level. Although levels decreased during further VAD support (Late VAD mean: 120.5 IU/L, p=0.022), they did not return to pre-VAD levels. Hyperbilirubinemia also worsened during the first 30 days of VAD support, with mean total bilirubin levels increasing from 1.7 to 2.2 mg/dL, although the mean value did normalize subsequently (Late VAD mean: 1.0 mg/dL, p<0.0001). Direct bilirubin had a similar trend. This data suggests that LVAD insertion not only fails to rapidly resolve cholestatic disease but may in fact exacerbate it initially.
When we performed a separate analysis for patients with pulsatile devices and those with continuous flow devices, mean alkaline phosphatase levels increased significantly during the first 30 days of VAD support in both groups (88.6 to 159.2 IU/L, p=0.0010 for pulsatile group; 100.0 to 146.5 IU/L, p<0.0001 for continuous flow group, respectively). However, the decrease in alkaline phosphatase levels during long-term VAD support was only significant in patients with pulsatile devices (late VAD mean: 1290.0 IU/L, p<0.0001) but not in patients with continuous flow devices (late VAD mean: 107.9 IU/L, p=0.1652). Hyperbilirubinemia also showed a trend towards worsening during the first 30 days of VAD support in both groups with mean total bilirubin levels increasing from 1.8 to 2.2 mg/dL in the pulsatile VAD group (p=0.4885) and 1.5 to 2.1 mg/dL in the continuous flow VAD group (p=0.2854), but this normalized subsequently in both groups (late VAD mean: 1.1 mg/dL, p<0.0001 for pulsatile VADs; 1.0 mg/dL, p<0.0001 for continuous flow VADs, p<0.0001, respectively). This data suggests that LVAD insertion is associated with an early development of hepatic cholestasis which is evident even after a prolonged time interval of VAD support while other parameters of liver dysfunction improve significantly (Table 5).
Right heart failure after VAD surgery
Because of the incomplete data regarding the duration of nitric oxide inhalation after VAD support, the data regarding RHF development after VAD surgery was obtained from a total of 230 patients in the present study. Among those, 72 patients (31.3%) developed RHF after VAD surgery. RVAD implantation was required in 36 patients (14.1%). The proportion of patients who developed RHF was not significantly different between patients with MELD score ≥17 and those with MELD score <17 (33.9% vs. 29.4%, p=0.510) or MELD-XI ≥17 and those with MELD-XI <17 (33.7% vs. 28.9%, p=0.445). However, when we compared the proportion of patients who eventually underwent cardiac transplantation, patients who developed RHF on-VAD support were less likely to reach transplantation (56.9% vs. 72.8%, p=0.017) even when only analyzing patients with VAD for BTT (64.9% vs. 79.6%, p=0.033).
Dynamics in MELD and MELD-XI during VAD Support
MELD-XI scores improved on average (15.8±5.6 v 13.3±3.9, p<0.0001) following LVAD support, with 67% of patients having a decreased MELD-XI score at time of OHT or after 6 months of LVAD support, including 92% of the patients with elevated pre-operative MELD-XI. MELD showed a similar but less dramatic change, improving from 14.7±5.4 to 13.5±4.9 (p=0.027), due to the effect of warfarin treatment on INR. Thus, MELD and MELD-XI were no longer highly correlated (R=0.6887, p<0.0001). We decided to use MELD-XI as our measure of liver dysfunction in subsequent analyses to avoid the influence of oral anticoagulation.
Survival Impact of MELD-XI During VAD Support
Given the significant improvement seen in MELD-XI score following one month of VAD support, the impact of these MELD-XI scores on overall and on-VAD survival was assessed. Among patients who had their VAD in place for at least 30 days, on-VAD and overall survival was significantly improved for those who had a MELD-XI <17 after 30 days of VAD support (Figure 3a–b, p<0.0001 and p=0.0275, respectively). Patients with a MELD-XI ≥17 after 30 days of LVAD support had a similar rate of heart transplantation as those with a lower score (57.5% v 68.7%, p=0.174), so rates of transplantation did not explain this overall survival difference.
Figure 3. One year on-VAD and long-term survival based on MELD-XI score at postoperative day 30.
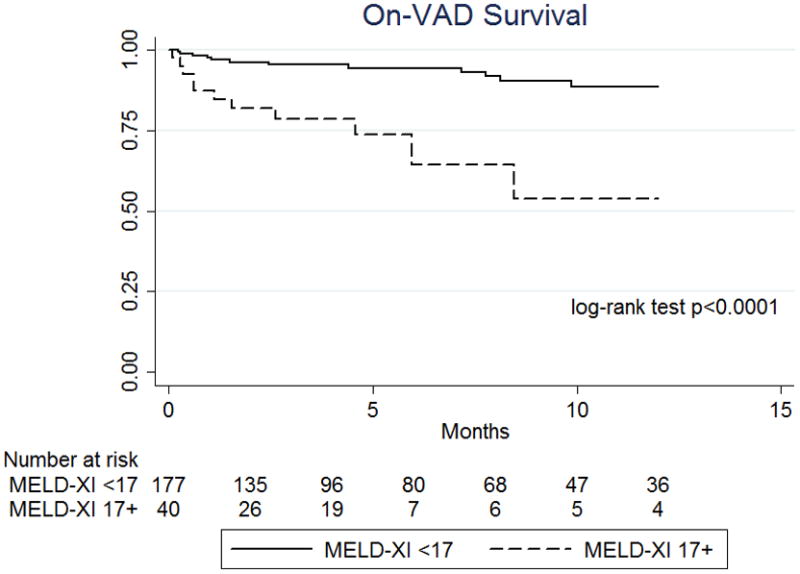
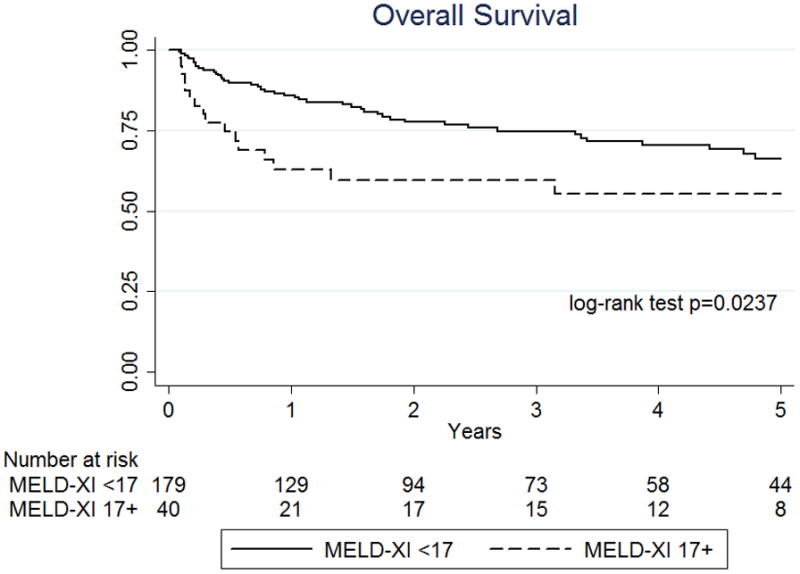
(a) One year on-VAD survival and (b) overall survival based on MELD-XI score on post-operative day (POD) 30 after LVAD implantation. This comparison involved only patients who received LVAD support for at least 30 days omitting patients who died or were transplanted prior to POD 30. Time point zero reflects POD 30.
Survival Impact of Improvement in MELD-XI During VAD Support
For patients successfully bridged to OHT, the effect of improving MELD-XI during VAD support was assessed. Of the 255 total patients in our study, 164 eventually received an OHT (64.3%). Among patients receiving an OHT, those with a pre-VAD MELD-XI ≥ 17 had worse post-OHT survival (log-rank test p = 0.0193). Subgroup analysis demonstrated that this was largely due to patients whose MELD-XI remained elevated during VAD support (“High/High” group), who had worse short-term and long-term post-OHT survival compared to patients with a low MELD-XI throughout (“Low/Low” group; p=0.0182 and 0.0295, respectively). However, patients who had an elevated MELD-XI pre-VAD that improved to <17 by the time of OHT (“High/Low” group) had near-identical short-term post-OHT survival compared to Low/Low patients (Figure 4, p=0.5217), with similar 10-year post-OHT survival as well (67.2% vs 73.5%, p=0.1164).
Figure 4. Post-transplant Survival of patients based on dynamics in MELD-XI score during VAD support.
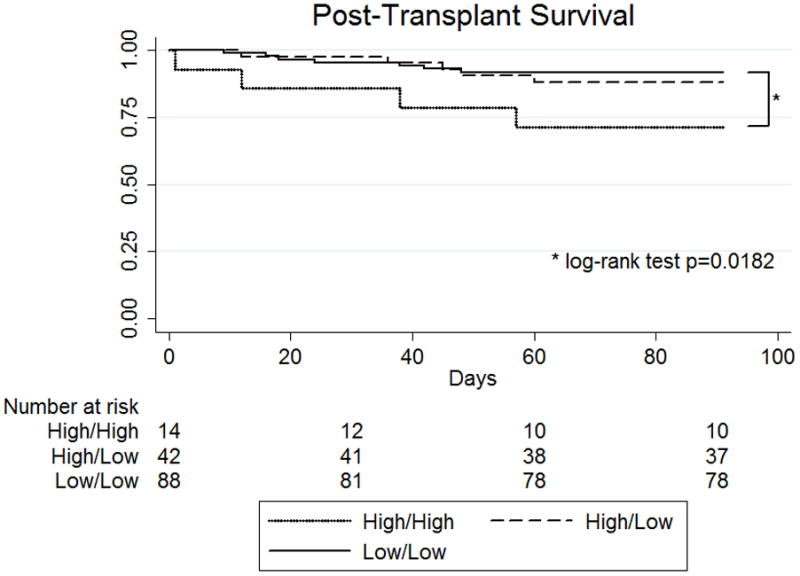
Low/Low patients had a MELD-XI <17 both before and during VAD support. High/Low patients had a MELD-XI ≥ 17 prior to VAD support that improved to <17 with VAD support. High/High patients had an elevated MELD-XI both before and during VAD support. Short-term survival following OHT was near identical between Low/Low and High/Low groups but significantly worse for High/High patients (p=0.0182). Longer term post-OHT survival analysis demonstrated a similar pattern in survival between the three groups; however, the small numbers of patients at risk prevented robust comparisons between all three groups.
DISCUSSION
This study assessed the validity of using MELD-XI as a reliable gauge of liver dysfunction in HF patients undergoing VAD implantation regardless of oral anticoagulation, which can augment their INR and MELD score. The MELD-XI score has been previously validated with MELD on two cirrhotic cohorts that encompassed over 7000 patients. Similarly, in our cohort of HF patients, we demonstrated a high level of correlation between MELD-XI and MELD in patients who were not receiving oral anticoagulation in the days preceding their VAD implantation.
This study confirms that pre-VAD MELD is a predictor of survival following VAD implantation as previously reported by Matthews et al. [12]. Patients with MELD scores below 17 had both an overall and on-VAD survival advantage over those with MELD scores of 17 or above. We also demonstrated MELD-XI as a similar predictor of both on-VAD and overall survival in this cohort. This provided evidence that MELD-XI not only correlates with MELD but is also a similar predictor of survival. Sub-analysis of patients who received continuous-flow devices alone also revealed that MELD-XI was significantly associated with on-VAD survival as well as overall survival.
We showed that MELD and MELD-XI scores correlated highly prior to VAD surgery; however, the correlation became weaker during VAD support. This is in part explained by the fact that we enrolled both patients with pulsatile and continuous flow VADs and that patients with continuous flow VADs were often treated with oral anticoagulation which resulted in increased INR that in turn affected the MELD score but not the MELD-XI score. Therefore, in the modern era with primary use of continuous flow VADs, the use of the MELD-XI scoring system is more appropriate to assess liver dysfunction of patients during VAD support.
Creatinine represents a major determinant of the MELD-XI score. Indeed, creatinine levels were significantly different between those with high MELD-XI score and low MELD-XI score in our cohort. However, we found that MELD-XI was the only factor highly associated with survival on multivariable analysis; therefore, we speculate that the MELD-XI score is not simply serving as a surrogate for renal dysfunction in this setting. In addition, it is possible that patients with high MELD scores were in more advanced HF considering their decreased baseline sodium concentrations which might have contributed to their higher mortality. Nevertheless, no differences in right ventricular failure were found between the various groups.
Both MELD and MELD-XI improved during LVAD support in our study group, suggesting that left ventricular support helped to reverse cardiac hepatopathy when present in our cohort. However, the improvement in MELD-XI was more clinically and statistically significant than MELD, largely due to increased INR in patients taking oral anticoagulation while on HMII support and the effect this had on their on-VAD MELD scores. Therefore, we followed MELD-XI scores as a more accurate measure of liver dysfunction in patients on VAD support. Mean MELD-XI dropped by nearly two points after only 30 days of VAD support and continued to decrease subsequently. In those who received an OHT, a pre-VAD MELD-XI ≥ 17 predicted worse post-transplant survival. However, post-transplant survival improved for those whose MELD-XI decreased below 17 by the time of transplant. Over the first three months following OHT, the survival of these “High/Low” MELD-XI patients was nearly identical to that of patients who had a MELD-XI <17 prior to VAD implantation. Long-term survival for the “High/Low” group was likewise similar to that of the “Low/Low” group. This is in contrast to the post-transplant survival curves of the “High/High” patients, which was significantly worse than the “Low/Low” group.
These findings suggest that the MELD-XI score can help identify appropriate candidates for OHT. End-stage HF patients with evidence of hepatopathy are reasonable candidates for VAD implantation, especially given that many will improve their liver function on VAD support. In fact, if liver function, as defined by MELD-XI, improves sufficiently on VAD support, our results suggest that post-transplant survival is generally similar to patients without liver dysfunction. However, if a patient’s MELD-XI remains persistently elevated before and during VAD support, their post-transplant survival is significantly decreased. Thus, MELD-XI before and during VAD support should be considered when evaluating heart transplant candidates. A high MELD-XI score post-LVAD implantation alone is probably not sufficient to recommend elimination of these patients from the transplant list. However, our findings could be used as a tool for the review of the individual transplant candidacy of these patients post-LVAD implantation based on the increased risk associated with cardiac transplantation. Ideally, we would have monitored hemodynamic data continuously in patients who had persistently high MELD-XI post-LVAD; however, due to risk for subsequent infection and other complications, we did not perform prolonged and repeated pressure monitoring post-operatively in the majority of patients. Therefore, we could not include more detailed post-operative hemodynamic data for these patients in the present study.
One additional novel finding relates to cholestasis in cardiac hepatopathy patients undergoing VAD implantation. Our results demonstrated a general improvement in most laboratory values, even after only 30 days of VAD support. However, alkaline phosphatase, total bilirubin, and direct bilirubin all increased initially before decreasing. This suggests that the cholestatic picture of cardiac hepatopathy may be exacerbated by an LVAD, likely when there is concomitant right ventricular (RV) dysfunction. An LVAD would increase hepatic perfusion. However, with RV dysfunction, this increase in perfusion could actually worsen hepatic congestion, at least temporarily, and lead to an increase in cholestasis. Over time, this effect appears to dissipate, possibly due to the gradual improvement in RV function caused by improved left ventricular unloading. Of note, when we performed a separate analysis for comparison of patients with pulsatile versus continuous-flow devices, the trend of changes in alkaline phosphatase and bilirubin levels over time was similar to the overall population; however, the normalization of alkaline phosphatase levels at the late on-VAD stage was not significant in patients supported by continuous-flow VADs. We speculate that blood cell injury with subsequent hemolysis and potentially differences in intrahepatic blood flow are specifically associated with hemodynamic support through continuous flow VADs. Future studies focusing on this entity are necessary to elucidate the underlying pathophysiology and its specific impact on clinical outcomes.
Limitations
Several limitations of this study exist and stem largely from its retrospective nature. Clinical decisions were made in a non-blinded, non-protocoled fashion, possibly instilling bias. Our patients received LVADs over a span of more than 10 years, during which time criteria for selecting patients for a long-term LVAD and for OHT have evolved. Technology has also changed over this time. These changes, in addition to individual clinician variation, add variability to our findings that are difficult to adjust for. We also did not have complete data on all patients, requiring us to omit some patients. We could not obtain information on blood transfusions in all enrolled patients. Therefore, we could not evaluate the relationship between cholestasis and blood transfusions and possible hemolysis. Another limitation is the lack of pre- and post-operative hemodynamic data in the present study. Finally, we could not investigate the impact of MELD scores in patients who could not undergo heart transplantation since a number of coexisting factors determined the inability to undergo cardiac transplantation in these patients including infection and subsequent neurological adverse events. Therefore, we could not analyze the specific impact of MELD-XI, and liver dysfunction in general affected the decision to withhold cardiac transplantation in these patients. However, we anticipate that these omissions were made randomly with no significant impact on our results.
Conclusion
MELD-XI is a valid measure of liver dysfunction that does not rely on INR values and is thus more accurate than standard MELD scores in patients on oral anticoagulation. Both MELD and MELD-XI scores, dichotomized as <17 or ≥17, prior to LVAD insertion were predictive of on-VAD, overall, and post-transplant survival. A worsening picture of cholestasis was seen shortly after LVAD insertion but improved over time. In VAD patients with an elevated pre-VAD MELD-XI who receive an OHT, a decrease in score during VAD support to <17 improved post-transplant survival and can be used to help identify optimal transplant candidates.
Acknowledgments
This work was supported by grants from the NHLBI (K23 HL095742-01, P30 HL101272-01, UL1 RR 024156, HL073029) and the Herbert and Florence Irving Scholar Award to Dr. Schulze. The authors had full control over the study design, methods used, outcome parameters and results, analysis of data, and production of the written report. This study was funded without assistance from industry sources.
Footnotes
DISCLOSURES
Authors with individual financial disclosures to make include Dr. Yoshifumi Naka, who is a consultant for Terumo and Thoratec, and Dr. Ulrich Jorde, who is a consultant for Thoratec. Neither of these authors received additional funds or aid related to or as a consequence of this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Giallourakis CC, Rosenberg PM, Friedman LS. The liver in heart failure. Clin Liver Dis. 2002;6(4):947–67. viii–ix. doi: 10.1016/s1089-3261(02)00056-9. [DOI] [PubMed] [Google Scholar]
- 2.Bayraktar UD, Seren S, Bayraktar Y. Hepatic venous outflow obstruction: three similar syndromes. World J Gastroenterol. 2007;13(13):1912–27. doi: 10.3748/wjg.v13.i13.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau GT, Tan HC, Kritharides L. Type of liver dysfunction in heart failure and its relation to the severity of tricuspid regurgitation. Am J Cardiol. 2002;90(12):1405–9. doi: 10.1016/s0002-9149(02)02886-2. [DOI] [PubMed] [Google Scholar]
- 4.Myers RP, et al. Cardiac hepatopathy: clinical, hemodynamic, and histologic characteristics and correlations. Hepatology. 2003;37(2):393–400. doi: 10.1053/jhep.2003.50062. [DOI] [PubMed] [Google Scholar]
- 5.Dichtl W, et al. Cardiac hepatopathy before and after heart transplantation. Transpl Int. 2005;18(6):697–702. doi: 10.1111/j.1432-2277.2005.00122.x. [DOI] [PubMed] [Google Scholar]
- 6.Malinchoc M, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864–71. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 7.Kamath PS, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–70. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 8.UNOS. MELD/PELD calculator documentation. 2009 http://www.unos.org/SharedContentDocuments/MELD_PELD_Calculator_Documentation.pdf. (Retrieved 2010-10-21)
- 9.Freeman RB, et al. Results of the first year of the new liver allocation plan. Liver Transpl. 2004;10(1):7–15. doi: 10.1002/lt.20024. [DOI] [PubMed] [Google Scholar]
- 10.Teh SH, et al. Risk factors for mortality after surgery in patients with cirrhosis. Gastroenterology. 2007;132(4):1261–9. doi: 10.1053/j.gastro.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 11.Rose EA, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 12.Matthews JC, et al. Model for end-stage liver disease score predicts left ventricular assist device operative transfusion requirements, morbidity, and mortality. Circulation. 2010;121(2):214–20. doi: 10.1161/CIRCULATIONAHA.108.838656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heuman DM, et al. MELD-XI: a rational approach to “sickest first” liver transplantation in cirrhotic patients requiring anticoagulant therapy. Liver Transpl. 2007;13(1):30–7. doi: 10.1002/lt.20906. [DOI] [PubMed] [Google Scholar]


