Abstract
Spinal cord injury (SCI) results in immune depression. To better understand how injury inhibits humoral immunity, the effects of chronic thoracic SCI on B cell development and immune responses to thymus-independent (TI) type-2 and thymus-dependent (TD) antigens were determined. Mice received complete crush injury or control laminectomy at either thoracic level 3 (T3), which disrupts descending autonomic control of the spleen, or at T9, which conserves most splenic sympathetic activity. Although mature B cell numbers were only mildly reduced, bone marrow B cell production was transiently but profoundly depressed immediately after injury. Despite the return of normal B cell production four weeks after SCI, mice receiving T3-injury showed a significant reduction in their ability to mount primary TI-2 or TD immune responses. The latter were marked by decreases in germinal center B cells as well as class switched high-affinity antibody secreting cells. Importantly, injury did not affect affinity maturation per se, pre-existing B cell memory, or secondary humoral immune responses. Together, these findings show that chronic high thoracic SCI impairs the ability to mount optimal antibody responses to new antigenic challenges, but spares previously established humoral immunity.
Introduction
Bacterial infections are the leading cause of death among patients who survive spinal cord injury (SCI), reflecting generalized immune depression (1, 2). These observations suggest SCI impairs humoral immunity via multiple mechanisms, including dysregulation of both the hypothalamic-pituitary-adrenal (HPA)-axis and sympathetic nervous system (SNS). For example, corticosteroids secreted by the (HPA)-axis following stress or injury can diminish B cell lymphopoiesis (3). Further, norepinephrine secreted by SNS nerves, which innervate lymphoid organs, can bind to B cells and influence their responsiveness (4–8). Accordingly, assessment of how SCI per se, as well as accompanying dysregulation of the (HPA)-axis and/or SNS, contribute to these effects, is of particular clinical interest.
Studies using murine models of SCI have begun to dissect the relative roles played by loss of splenic sympathetic regulation versus increased injury-induced stress hormones in perturbations of B cell homeostasis and function. Acute injury at thoracic level T3, which disrupts autonomic control of the spleen, results in fewer total splenic B cells and impaired thymus-dependent (TD) antibody responses (9, 10). Dysregulation of the SNS was implicated in these alterations, as blocking of SNS derived norepinephrine signaling restored TD antibody responses in T3-injured mice, and was intact in both laminectomy controls and mice injured at T9, a level at which the majority of central sympathetic regulation to the spleen is conserved (9). While these findings show that acute SCI disrupts primary TD humoral responses, the question remains whether these effects persist during chronic injury. Moreover, it is unclear whether these findings reflect generalized shifts in the numbers or functional capacities of all B lineage cells, or instead differentially impact particular B cell subsets and their associated functions. Further, as patients are most often severely affected by pathogens that characteristically elicit thymus-independent (TI) humoral responses (2), it is essential to know how SCI affects primary TI responses. Finally, whether the processes required to generate high-affinity antibodies during primary TD responses are intact, as well as whether pre-existing memory B cell numbers and responses are retained, is unknown.
Accordingly, to further understand how SCI affects B cell maintenance, responsiveness, and memory, we have conducted detailed assessments of B cell subsets and function in mice receiving complete crush SCI at either T3 or T9. We show that previously observed reductions in splenic B cells during acute SCI reflect cessation of B lymphopoiesis, since developing bone marrow (BM) B cell subsets and transitional (TR) B cells were profoundly reduced 8 days post SCI. Blunted B cell genesis is transient, as developing BM subsets were completely restored to pre-injury levels after 28 days. Further, mature follicular (FO) B cells, but not marginal zone (MZ) B cells, were reduced following injury. Evaluation of antigen-specific B cell responses during chronic injury revealed that the magnitude of both TI and primary TD responses were reduced in T3 injured mice. Finally, we show that SCI impacts neither memory B cell numbers nor the ability to mount anamnestic responses to antigens encountered prior to injury. Together, our findings reveal that the humoral immune system is dynamically altered following SCI, and that time post-injury, as well as the injury level per se, are important considerations for future basic and translational investigation.
Materials and Methods
Mice and Injury
Age-matched 5- to 7-week-old female C57BL/6 mice were purchased from the National Cancer Institute, Bethesda, Maryland. All procedures were approved by the University of California at Irvine Institutional Animal Care and Use Committee. Mice were initially anesthetized with Avertin (0.5ml/20g); when supplemental anesthesia was required, one-fourth of the original dose was given. Body temperature was maintained by placing mice on a water-circulating jacketed heating pad at 37±0.5°C. The skin over the upper thoracic area was shaved and cleaned with a Betadyne solution. Using aseptic techniques, the skin was incised and connective and muscle tissues were bluntly dissected to expose the third (T3) or the ninth (T9) vertebral body. A laminectomy of a single vertebral lamina was performed at T2-T3 or T9-T11 to expose the dorsal spinal cord. Experimental bilateral crush injury was performed using forceps (Dumont #5) placed on either side of exposed spinal cord following laminectomy. The points of the forceps were then brought together, held for one second, and released. A complete bilateral crush injury results in loss of motor function caudal to the injury site. The crush injury produces complete paralysis of the hindlimbs and mice do not recover the ability to walk (11). Therefore, hindlimb motor recovery behavioral assessment was not conducted for our studies. Incomplete lesions were identified on days 0 and 27 post-injury (following recovery from surgical anesthesia and prior to immunization, respectively) in mice displaying any degree of ankle, knee, and/or hip non-reflexive movement, and were subsequently excluded from experimental analysis. After injury or laminectomy-only, the muscles and skin were sutured separately and mice given subcutaneous injections of lactated Ringer’s solution (1ml/20g) for hydration, Buprenex (Buprenorphine, 0.05mg/kg) for analgesia, and Baytril (Enroflaxacin, 2.5mg/kg) for prophylaxis against urinary tract infections. Un-injured mice did not undergo any surgery, but were anesthetized. Mice were placed in cages with Alpha-Dri bedding (Newco Distributors Inc.), warmed directly on water-jacketed heating pads at 37°C, until recovered from anesthesia. Thereafter, half of each cage was place on heating jacket for up to 3 days post-surgery, until coat quality improved and mobility around the cage resumed. Post-operative care involved daily treatments of lactated Ringer’s solution and Baytril for the first six days post-surgery, and daily Buprenex treatments for the first three days post-surgery. Post-operative care of injured mice also included manual bladder expression 2x/day for the duration of experiments.
Injury induction, immunizations, mouse care, spinal column histology, and serum cort analyses were performed at the University of California, Irvine. All mice were euthanatized in a closed receptacle containing gauze soaked with isoflurane anesthetic, death occurred within 2 min. Following euthanasia and harvest, tissues were shipped to the University of Pennsylvania overnight on ice in DMEM containing 10% FBS for Flow cytometry and NP-specific ELISPOT analyses. Serum was frozen and shipped on dry ice for quantification of NP specific antibodies.
Antigens and immunizations
Primary immunizations with NP (4-Hydroxy-3-Nitrophenyl Acetyl) conjugated to protein or carbohydrate antigen were conducted after 28 days post-injury. For primary TI-2 responses, mice were immunized i.p. with 50ug NP50-Ficoll (Biosearch Technologies) in PBS and analyzed at 4.5 days post-immunization. For primary TD responses, mice were immunized i.p. with 50ug of NP-conjugated to chicken gamma globulin (NP15-CGG, Biosearch Technologies) precipitated in alum and analyzed at 14 days after immunization. For secondary immunizations, mice were immunized i.p. with 50ug of NP15-CGG 54 days prior to injury. 28 days post-injury, mice were either boosted with 50ug of NP15-CGG i.p. or received no treatment. Organs were harvested 7 days after secondary immunization.
Corticosterone EIA
All blood collection by cardiac puncture occurred following euthanasia with anesthetic, and we consistently performed these procedures at the same time of day throughout these experiments. Previously it has been shown that post-mortem blood collection does not influence CORT levels in other non-manipulated mice sharing the same environment (12), however, as a precautionary measure to avoid variable stress-responses, blood collection from individual experimental groups (beginning with un-injured control mice) was done in a procedure room separate to where animals were housed. Plasma was isolated using buffered citrate from blood collected from each mouse post-mortem, and corticosterone levels assessed by ELISA (Immunodiagnostic Systems).
Histological analysis
Spinal columns were dissected from experimentally injured mice and post-fixed in 4% paraformaldehyde overnight. Prior to spinal cord extraction from column, the thoracic vertebral location of the laminectomized region was recorded. Spinal cords were cryoprotected in 20% sucrose and embedded in O.C.T. (Tissue Tek). 8 μm thick sagittal sections were stained with Hematoxylin and eosin and visualized using a bright field microscope. Spleens were immersed in O.C.T. and flash frozen using 2-methylbutane cooled with liquid nitrogen. 7 μm sections were fixed with cold acetone and stored at −20 °C. Prior to staining, the sections were re-hydrated in 1× PBS, blocked with 10% goat serum, and stained with murine reactive antibodies: AF647-anti-IgD (eBioscience), Rhodamine-anti-IgM F(ab)2 (Southern Biotech), and AF647-anti-CD3ε (eBioscience). Sections were mounted using Biomeda Gel/Mount (Electron Microscopy Sciences) and were visualized on a LSM-510 Meta confocal microscope (Zeiss).
Flow Cytometry
Splenocytes and bone marrow (BM) were harvested and stained using the following murine reactive antibodies: APCCy7-anti-CD19 (BD Biosciences), FITC-anti-B220 (BD Biosciences), eFlour450-anti-CD21/35 (eBiosciences), PE-anti-CD23 (BD Biosciences), PECy7-anti-IgM (BD Biosciences), QDOT 705-streptavidin (Invitrogen), APC-anti-AA4.1 (eBioscience), AF700-anti-B220 (eBioscience), FITC-anti-Lambda (Southern biotechnology), PECy5-anti-CD4 (BD Biosciences), PE-Cy5-anti-CD8 (BD Biosciences), PE-Cy5-anti-Gr1 (eBioscience), PECy5-anti-F4/80 (eBioscience), peanut agglutinin (PNA) conjugated to FITC (Vector Laboratories), eFLour450-anti-IgD (eBiosciences), PE-Cy7-anti-Fas (BD Bioscience). PE-Cy5.5-anti-CD21/35 was donated by D. Allman (University of Pennsylvania). NP was conjugated to APC and Qdot 655 was conjugated to anti-Kappa (Southern Biotechnology) in-house. For identification of NP-specific responders, intracellular staining was conducted. Live cells were stained for T (CD4+, CD8+) and myeloid cells (F4/80+, Gr1+) to gate out non-B cells. Then, the cells were fixed and permeabilized using FIX & PERM® kit (Caltag) according to the manufacturer’s protocol and stained with reagents to detect NP, Igλ and Igκ. For identification of surface NP+ germinal center (GC) B cells, we conducted staining on live non-permeabilized splenocytes. Live/Dead discrimination was assessed using either DAPI (Invitrogen) or Live/dead Fixable Aqua™ (Invitrogen). Doublet discrimination was performed by FSC/SSC Width vs Height analysis. Total cell numbers were calculated by multiplying the frequency of gated cells among live singlets by the total number of live cells harvested. Data were collected on a BD LSR II flow cytometer and analyzed with FlowJo software (Tree Star).
NP-specific ELISA/ELISPOTs
Plates were coated with either 10ug/ml NP33-BSA or NP4-BSA in 100mM bicarbonate buffer and blocked with PBS containing 2% BSA. For ELISA, sera were incubated for 2 h at 37° C/5.5% CO2. NP-specific IgG1 or IgM standard was a gift of Dr. Garnett Kelsoe (Duke University, Durham, NC). Detection was conducted using HRP-conjugated goat anti-mouse IgG1 or IgM (Southern Biotechnology) with a TMB substrate kit (BD Biosciences) and color development was quantified using EMax (Molecular Devices). For ELISPOTs, cell suspensions containing 1 × 106 cells were incubated for 4 h at 37° C and developed with biotin-conjugated anti-mouse IgM or IgG1 (Southern Biotechnology) followed by ExtrAvidin-Alkaline Phosphatase using NBT/BCIP substrate (Sigma). Color development was terminated with 1 M NaH2PO4 and spots were enumerated on a CTL-ImmunoSpot reader (Cellular Technologies).
Data analyses and statistics
One way ANOVA with Dunnetts post-hoc analysis was performed with GraphPad Prism (GraphPad software Inc.). Comparisons were made between injured groups and un-injured control groups, between SCI injured groups and their respective laminectomy control groups, and between T9- and T3-SCI injured groups.
Results
Splenic architecture is preserved after spinal cord injury
The majority of splenic sympathetic innervation derives from mid-thoracic levels, thus injury at or above this region will result in loss of supraspinal control and decentralized sympathetic activity ((13, 14), depicted in Supplemental Fig. 1A). Accordingly, the effects of SCI on humoral immune function were assessed using a previously established model of injury above the site of splenic innervation, i.e. T3 crush injury (9, 10). As controls, we used either un-injured mice, mice that received a crush injury at T9, a level at which splenic innervation is largely intact, or mice that received laminectomy surgery without crush injury. Post-mortem histological analysis of thoracic vertebra from T3- and T9-injured mice was routinely performed to confirm injury location and complete crush. (Supplemental Fig. 1B, C). The lesions in injured mice at one week post-injury were qualitatively similar to previously described crush-SCI in C57BL/6 mice (15). Importantly, crush-injury results in no white matter sparing, produces complete paralysis of the hindlimbs, and loss of the ability to walk (11). In addition, T3-SCI was accompanied by a decrease in splenic weight as reported previously (9, 10). Immunohistologic evaluations at d28 post-injury revealed that splenic microanatomy was preserved, including the T-cell rich peri-arterial lymphoid sheaths and adjacent IgD+IgM+ B cell follicles (Supplemental Fig. 1D). Thus, the general organization of the splenic white pulp is preserved following SCI.
B cell genesis transiently ceases following SCI
Despite the maintenance of lymphoid architecture, prior reports showed that T3-SCI adversely affects total splenic B cell numbers (9, 10, 16). Such reductions may reflect reduced B cell genesis, a direct loss of all or some mature B cell subsets, or both. In order to assess the relative contributions of these mechanisms, we examined developmental and mature B cell subsets in the BM (Fig 1, Table I) and spleen (Fig 2, Table II) during acute and chronic SCI. The gating strategies employed (17) resolve developmental and mature B cell subsets whose dynamics and functional characteristics are well established (reviewed in (18–20)). Briefly, following Ig heavy and light chain gene rearrangements during the pro-B and pre-B cell stages, developing B cells in the BM enter the immature (IMM) B cell stage and then migrate to the periphery as transitional (TR) B cells. All of these developmental subsets bear the AA4.1 marker and are further resolved according to the criteria shown in Figs. 1 and 2. In the BM, Pre-B and Pro-B cells lack surface IgM, whereas IMM B cells are surface IgM+ (Fig 1A and 1B). Splenic TR cells are all AA4.1+, and are further resolved into the TR1, TR2, and TR3 subsets based on differential CD23 and IgM expression levels (Fig. 2). Developing B cells fully transit these BM and splenic developmental stages over 4–6 days (17, 21–24), so all of these subsets turnover quickly and therefore deplete rapidly if B lymphopoiesis stops. Following the TR differentiation stages, B cells complete maturation and enter either the FO or MZ pools. Both of these mature, pre-immune subsets lack the AA4.1 marker, and are distinguished by differential IgM and CD21/35 expression (Fig. 2) (17, 24, 25). Under normal conditions, most developing B cells adopt the FO fate, but differentiation skews to the MZ fate under conditions of B lymphopenia or reduced BM output (25). Unlike developing B cell subsets, MZ and FO B cells turn over slowly and persist for months to years (22, 26, 27), so their numbers are modestly affected by short-term perturbations in B cell genesis.
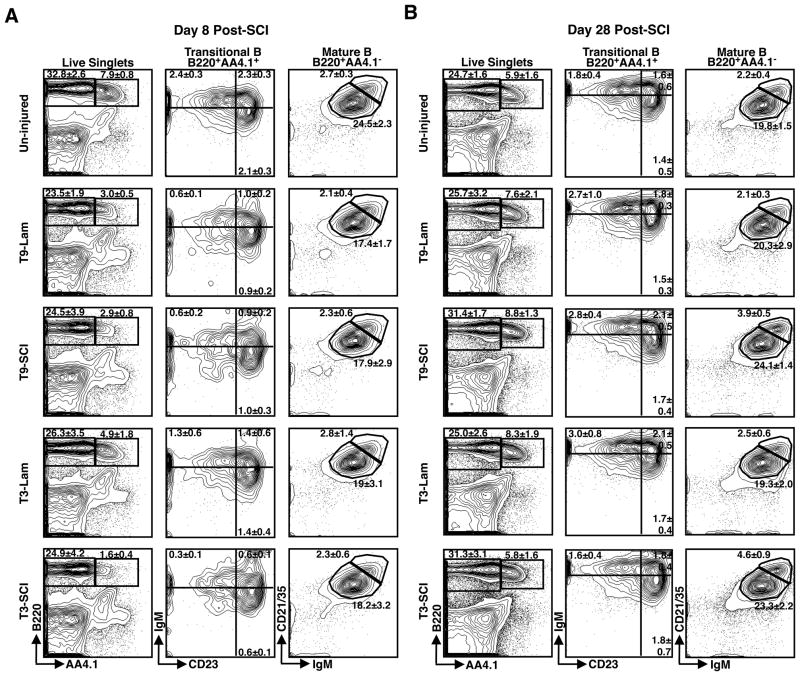
Figure 1. The frequency of B cell progenitors in BM is reduced following acute SCI.
Representative FACS gating strategy to identify B cell subsets in the BM at day 8 (A) and day 28 (B) after T3-SCI, T9-SCI, T3-Lam, T9-Lam or in un-injured control mice. Contour plots show the mean frequency and standard deviation of B220+AA4.1+ Developing B cells in the BM (left panels) and further subsetting of IgM+CD19+ immature (IMM) and IgM−CD19+ Pro/Pre B cell populations (right panels) among live singlets. At day 8 post-SCI, n=5 for un-injured, n=8 for T9-Lam, n=6 for T9-SCI, n=8 for T3-Lam, and n=5 for T3-SCI. At day 28 post-SCI, n=5 for uninjured, n=7 for T9-Lam, n=5 for T9-SCI, n=7 for T3-Lam, and n=6 for T3-SCI.
Table I.
Numbers of Bone Marrow B cell subsets during acute and chronic SCIa
| Total Cells | Pro/Pre B | Immature B | ||
|---|---|---|---|---|
| Un-injuredb | 37.3±7.6 | 2.47±1.23 | 0.76±0.43 | |
| Day 8 Post Injuryc | T9-Lam | 38.03±8.7 | 1.06±0.29* | 0.18±0.05* |
| T9-SCI | 46.32±8.88* | 1.07±0.95* | 0.28±0.37* | |
| T3-Lam | 46.05±5.6* | 1.85±0.35 | 0.43±0.13 | |
| T3-SCI | 47.97±9.49* | 0.68±0.83*,# | 0.17±0.17* | |
| Day 28 Post Injuryd | T9-Lam | 34.61±4.42 | 2.32±0.6 | 0.84±0.25 |
| T9-SCI | 47.39±9.56*,# | 2.78±0.92 | 0.88±0.32 | |
| T3-Lam | 36.5±5.47 | 2.54±0.44 | 0.95±0.14 | |
| T3-SCI | 52.83±9.7*,# | 2.51±0.98 | 0.81±0.23 | |
Mean ± SD of cells (×106) per hind limb, gated according to representative FACS plots shown in Fig. 1. Data has been pooled across 6 experiments for un-injured controls, 1 for laminectomies, and 3 for SCI at each level.
n=25 for Un-injured.
n=8 for T9-Lam, n=13 for T9-SCI, n=8 for T3-Lam and n=12 for T3-SCI.
n=6 for T9-Lam, n=13 for T9-SCI, n=7 for T3-Lam and n=13 for T3-SCI.
Denotes significant difference from un-injured control at p<0.05
Denotes significant difference from respective laminectomy control at p<0.05
Figure 2. The frequency of B cell progenitors in spleen is reduced following acute SCI.
Representative FACS gating strategy to identify B cell subsets in the spleen at day 8 (A) and day 28 (B) after T3-SCI, T9-SCI, T3-Lam, T9-Lam or in un-injured control mice. Contour plots show the mean frequency and standard deviation of developing B220+AA4.1+ and mature B220+AA4.1− splenic B cells (left panels), and further subsetting of IgMhiCD23− transitional type 1 (TR1), IgMhiCD23+ transitional type 2 (TR2), and IgMloCD23+ transitional type 3 (TR3) developing B cells (middle panels, gates configured clockwise) as well as IgMhiCD21/35hi MZ B and IgMloCD21/35lo FO B cells (right panels) among live singlets. At day 8 post-SCI, n=5 for un-injured, n=8 for T9-Lam, n=6 for T9-SCI, n=8 for T3-Lam, and n=5 for T3-SCI. At day 28 post-SCI, n=5 for un-injured, n=7 for T9-Lam, n=5 for T9-SCI, n=7 for T3-Lam, and n=6 for T3-SCI.
Table II.
Numbers of splenic B cell subsets during acute and chronic SCIa
| Total Cells | TR1 | TR2 | TR3 | FO | MZ | ||
|---|---|---|---|---|---|---|---|
| Un-injuredb | 131.6±26.2 | 2.7±0.6 | 2.5±0.8 | 2.3±0.7 | 28.9±5.4 | 3.2±0.5 | |
| Day 8 Post Injuryc | T9-Lam | 138.0±9.2 | 0.9±0.1* | 1.3±0.2* | 1.2±0.2* | 24.1±3.0 | 2.9±0.6 |
| T9-SCI | 104.3±15.6*,# | 0.6±0.2* | 0.9±0.2* | 1.0±0.3* | 18.3±2.1* | 2.3±0.5 | |
| T3-Lam | 111.1±22.1 | 1.5±0.9* | 1.6±0.7* | 1.6±0.5 | 21.2±6.5* | 3.1±1.9 | |
| T3-SCI | 111.3±18.5 | 0.3±0.1*,# | 0.7±0.2*,# | 0.7±0.2*,# | 20.2±3.9* | 2.5±0.5 | |
| Day 28 Post Injuryd | T9-Lam | 149.7±32.9 | 3.8±0.8* | 2.6±0.4 | 2.2±0.4 | 30.4±8.2 | 3.2±0.7 |
| T9-SCI | 86.8±12.5*,# | 2.5±0.6# | 2.2±0.6 | 2.0±0.7 | 21.1±4.0# | 3.4±0.4 | |
| T3-Lam | 119±22.2 | 3.5±0.7 | 2.5±0.5 | 2.0±0.4 | 22.9±4.6 | 2.9±0.5 | |
| T3-SCI | 93.6±24.1* | 1.6±0.4*,# | 1.8±0.5 | 1.8±1.0 | 22.3±5.9 | 4.3±1.3*,# | |
Number ± SD of cells (×106) per spleen, representative FACS plots shown in Fig. 2. Figures shown are representative of 3 experiments at each post-injury time point. Data shown has been pooled across 2 experiments for un-injured controls, 1 for laminectomies, and 1 for SCI at each level.
n=10 for Un-injured
n=8 for T9-Lam, n=6 for T9-SCI, n=8 for T3-Lam, and n=5 for T3, SCI.
n=7 for T9-Lam, n=5 for T9-SCI, n=7 for T3-Lam, and n=6 for T3-SCI.
Denotes significant difference from un-injured control at p<0.05
Denotes significant difference from respective laminectomy control at p<0.05
At day 8 post-SCI, the proportions of all developmental B cell subsets were profoundly reduced in both T3- and T9-SCI compared to un-injured mice (Fig. 1A and 2A). These declines reflect significant decreases in the numbers of BM Pro-B, Pre-B and IMM B cells, as well as all splenic TR subsets (Figs. 1A and 2A, Tables I and II). In contrast to acute injury, BM developmental subsets were fully restored during chronic injury (Fig. 1B, Table I). The splenic TR subsets also recovered, but were sometimes still mildly reduced at 28 days post injury (Fig. 2B, Table II). Rapid depletion and subsequent reconstitution of B cell progenitor pools indicates that SCI engenders transient cessation of B cell genesis that is largely re-established within 28 days after injury.
Because B cell genesis resumes within 28 days after SCI, it is unlikely that sustained dysregulation of sympathetic activity underlies blunted B cell genesis during acute injury. Instead, they likely reflect injury-related stress or inflammation, both of which can yield depression of BM B cell genesis via corticosteroid hormones or inflammatory cytokines, respectively (3, 28, 29). Consistent with this possibility, similar transient reductions in developing B cells were observed in mice receiving laminectomy injuries (Figs. 1 and 2, Tables I and II), and serum corticosterone (CORT) levels were elevated following SCI or laminectomy, regardless of injury location (Supplemental Table I). However, serum CORT returned to uninjured levels 28 days following laminectomy, yet remained elevated 28 days post T9- or T3-SCI, despite recovery of B cell genesis in all treatment groups. Accordingly, while elevated CORT may contribute to blocked B cell production during acute SCI, it is alone insufficient to mediate this effect, and probably acts in concert with additional inhibitors of B cell genesis that are temporarily present following injury.
Follicular, but not marginal zone, B cells are reduced during SCI
We next addressed how acute and chronic SCI affect mature splenic B cell pools. For all experiments, FO B cell numbers were modestly reduced following acute or chronic SCI. These consistent reductions frequently, but not always, achieved statistical significance (Table II). In contrast, MZ B cell numbers were always either preserved or increased during both acute and chronic SCI (Table II). Variability in the degrees to which FO B cell numbers decreased and MZ B cell numbers expanded likely reflects a dynamic interplay of several factors. Foremost among these are the extent to which B lymphopoiesis is blunted and how quickly it is re-established, as these parameters will determine the degree of impact on FO B cell numbers and the associated skewing of cells into the MZ pool (25). In toto, our findings extend prior observations demonstrating that reduced numbers of splenic B cells during acute SCI reflect a severe truncation of TR subsets, combined with a consistent but less profound depletion of FO, but not MZ, B cells (9). Moreover, we find that during chronic SCI, B lymphopoiesis has resumed, but mild reductions in TR and FO B cell numbers persist. Nonetheless, a large proportion of the mature B cell pool remains intact and, if functional, should be able to respond to antigenic challenge during chronic stages of SCI.
The TI-2 immune response is profoundly decreased after chronic T3-injury
Despite the persistence of substantial mature B cell numbers, prior studies showed that acute SCI yields blunted primary TD antibody responses (9). Whether these defects extend to TI responses, as well as whether they continue during chronic injury, has not been determined. Accordingly, we examined NP-specific responses during chronic SCI. The NP-specific response in C57BL/6 mice is dominated by Igλ+ B cells, thus responding cells can be tracked based upon both NP-binding and Igλ expression (30). Additionally, numbers of NP-specific antibody secreting cells (ASCs) and antibody concentrations can be assessed by ELISPOT and ELISA, respectively.
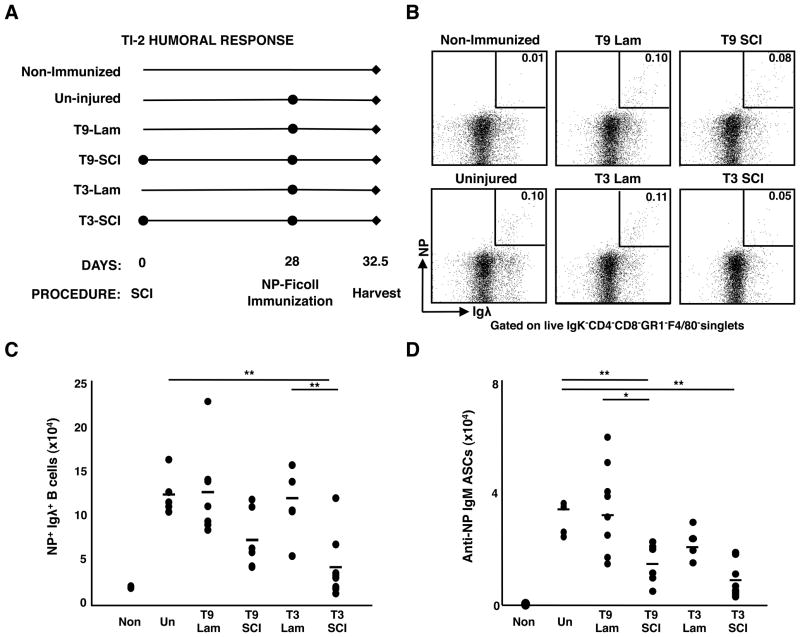
Using this model, we first tested whether chronic SCI affects TI-2 responses using NP-conjugated to the carbohydrate polymer Ficoll (NP-Ficoll). MZ B cells are responsible for the majority of the TI-2 response and, as their numbers are not reduced following SCI, any differences in response magnitude likely reflects altered functionality (31). Mice were challenged with NP-Ficoll i.p. 28 days post-injury and responses were assessed 4.5 days later, at the peak of the response (32) (Fig. 3A). Compared to un-injured controls, mice with chronic T3-SCI, but not T3-Lam, had profoundly diminished NP-specific responses. This was evidenced by significantly decreased numbers of responding splenic NP+Igλ+ B cells and NP-specific IgM-producing ASCs (Fig. 3B–D). In multiple experiments, serum NP-specific antibody concentrations were correspondingly reduced by 3-to 4-fold (data not shown). Though often mildly reduced compared to uninjured controls, TI-2 responses in mice receiving T9-SCI were highly variable and less severely affected than those in mice receiving T3-SCI. Thus chronic T3 SCI severely impacts responsiveness to TI-2 antigens despite the presence of mature B cells, including normal to elevated numbers of MZ B cells.
Figure 3. Humoral responses to TI-2 antigens are decreased during chronic T3-SCI.
(A) Experimental design for NP-Ficoll immunization: 28 days post-SCI or post-laminectomy, mice were immunized with NP-Ficoll and the splenic tissue was analyzed at day 4.5. (B) Representative FACS gating strategy to identify NP-specific B cells. Dot plots show the frequency of Aqua−CD4−CD8−F4/80−Gr1−Igκ−Igλ+NP+ responding B cells among live singlets. (C) Total number of NP-specific B cells. (D) Total number of NP-specific IgM ASCs. For all data sets, n=5 for un-injured, n=7 for T9-Lam, n=6 for T9-SCI, n=7 for T3-Lam, and n=8 for T3-SCI. ** denotes significance at p<0.01.
Primary TD responses are decreased during chronic T3-SCI
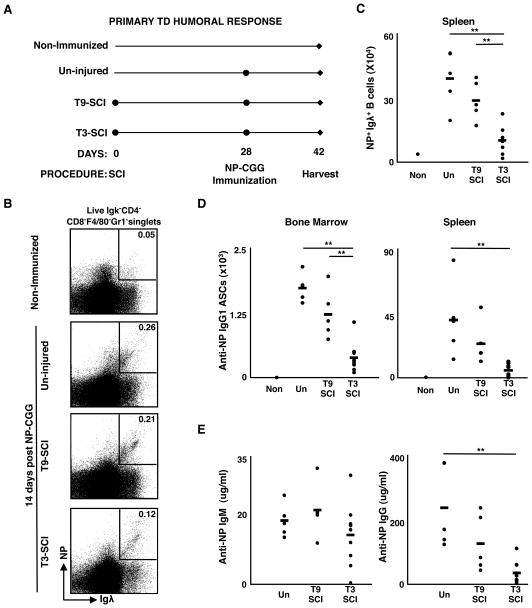
To extend studies examining acute SCI, we asked whether defects in TD responses would persist during chronic SCI. Therefore, mice were challenged 28 days post-SCI with NP-CGG, and responding cells in the spleen and bone marrow were evaluated (Fig. 4A). NP-specific TD responses peak between 10–14 days post-immunization (33), thus, we assessed the responses at day 14. Compared to uninjured and T9-injured controls, there was a significant reduction in the frequency and number of splenic NP+Igλ+ B cells in T3-injured mice (representative staining Figs. 4B and 4C, Supp. Fig 2A). Furthermore, both the number and frequency of NP-specific IgG1 ASCs were significantly reduced in the BM and spleen after T3, but not T9, injury when compared to un-injured mice (Fig. 4D, Supp. Fig 2B). Consistent with a defect in the generation of these cells, serum levels of anti-NP-specific IgG1, but not IgM, were also significantly reduced in T3-injured mice compared to un-injured controls (Fig. 4E). In sum, chronic T3-injury severely diminishes the magnitude of primary TD responses.
Figure 4. The magnitude of TD responses is reduced during chronic SCI.
(A) Experimental design for NP-CGG immunization: 28 days post-SCI, mice were immunized with NP-CGG, and at day 14 post-immunization, spleen and bone marrow from 2 hind limbs were analyzed. (B) Representative FACS gating strategy to identify NP-specific B cells. Dot plots show the frequency of Aqua−CD4−CD8−F4/80−Gr1−Igκ−Igλ+NP+ responding B cells among live singlets. (C) The total number of splenic NP-specific cells. (D) Total number of NP-specific IgG1 ASCs present in BM and spleen. (E) Serum levels of NP-specific IgM and IgG1 antibody above non-immunized controls. For all data sets, n=5 for un-injured, n=5 for T9-SCI, and n=9 for T3-SCI. Data are representative of two experiments. ** denotes significance at p<0.01 and * denotes significance at 0.01≤p<0.05.
Affinity maturation is intact during chronic SCI despite reductions in germinal center (GC) B cells and total high-affinity antibody
Effective TD responses require the formation of GCs, where B cells undergo class switch recombination, somatic hypermutation, and selection to yield the high-affinity, isotype-switched B cell clones that populate the memory and long lived ASC compartments (34). As the magnitude of TD responses was severely reduced during chronic SCI, we assessed whether the GC reaction was also compromised. NP-specific GC B cells can be identified cytoflourimetrically by gating on activated B cells (IgD− CD19+) that bind NP and peanut agglutinin (PNA), and express high levels of Fas (representative gating strategy shown in Fig. 5A) (35–37). At 14 days post immunization, T3-injured mice had significantly reduced numbers of NP-specific splenic GC B cells compared to un-injured mice (Fig. 5B).
Figure 5. Germinal center B cells and high-affinity ASCs are reduced in T3-injured mice after TD immunization.
(A) Representative FACS plots for identification of NP-specific GC B cells in un-injured mice (CD19+CD4−CD8−F4/80−Gr1−IgD−PNA+NP+Fas+). Numbers shown in each dot plot are percentages of each population within the parent gate. (B) The absolute number of GC B cells in the spleen (n=4–5 mice per group). (C) Total number of high-affinity NP-specific IgG1 ASCs in BM and spleen. (D) Serum concentrations of NP-specific high-affinity IgG1 antibody above non-immunized controls. (E) Affinity maturation within the spleen as determined by the ratio of high-affinity to total IgG1 ASCs (Figure 4D); For C–E, n=5 for uninjured, n=5 for T9-SCI, and n=9 for T3-SCI. Data are representative of two experiments. ** denotes significance at p<0.01 and * denotes significance at 0.01≤p<0.05.
Diminished GC B cell numbers could reflect either the loss of total responding cells, and therefore a loss of B cells initiating the GC fate, or an alteration to GC function resulting in the premature termination of GC B cell differentiation. Previous studies have established that while cells with both low and high-affinity for NP bind to highly-substituted NP-BSA (NP33), only those with high-affinity for NP bind to lowly-substituted NP-BSA (NP4) (33, 38). In this way, both total and high-affinity NP-specific antibody can be detected via ELISPOT and ELISA. Therefore, to examine whether reductions in GC B cells resulted in a loss of GC function, we assessed the relative affinity of responding ASCs and antibody from injured mice. Although the numbers and frequencies of high-affinity IgG1 ASCs in the spleen and BM were significantly reduced in T3-injured mice compared to un-injured mice, they were nonetheless detectable (Fig. 5C, Supp. Fig 2C). Similarly, ELISA revealed that both chronic T3- and T9-SCI injured mice had high-affinity NP-specific IgG1, albeit at significantly reduced levels compared to un-injured mice (Fig. 5D). The presence of high-affinity NP-specific ASCs and antibody suggests that the somatic hypermutation and selection processes required for affinity maturation are indeed intact following SCI. To confirm that the loss in total GC B cells and high-affinity NP-specific ASCs was due primarily to a paucity of responding B cells, we compared the ratio of high-affinity/total IgG1 ASCs and found that they were comparable to un-injured mice (Fig. 5E). Together, these observations show that the affinity maturation process per se is intact during chronic SCI, but diminished numbers of responding cells yield fewer high-affinity effectors.
Pre-existing B cell memory in the spleen is unaffected by chronic SCI
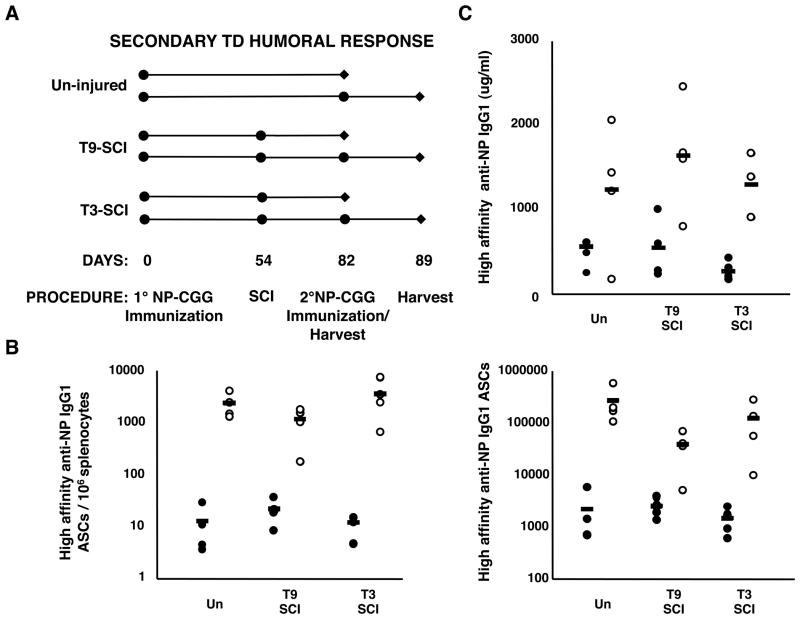
Memory B cells and long lived plasma cells formed during primary humoral responses provide protective immunity to subsequent antigen exposures, and are the mechanistic basis for most current vaccines (39). Accordingly, a potential risk facing those with SCI is loss of acquired immunity from prior natural immunizations or vaccinations. Thus, we addressed the affects of chronic SCI on established B cell memory and the ability to mount secondary responses. We immunized mice with NP-CGG, waited 54 days for the primary response to wane while allowing memory pools to establish (33, 40), then induced SCI (Fig. 6A). 28 days post-injury, one cohort of mice was analyzed without further treatment (designated as resting memory) and the second cohort was boosted with NP-CGG (designated as boosted memory). In the latter group, high-affinity ASCs were assessed 7 days post-immunization, at a time point well before new responders have undergone affinity maturation (33). Strikingly, we found no differences between the treatment groups in either the frequency or total number of high-affinity splenic ASCs (Fig. 6B) or the concentration of high-affinity antibody (Fig. 6C) in either the resting or the boosted memory responses. Thus, we conclude that despite the impaired capacity to mount primary TD responses following SCI, previously established humoral memory and the ability to respond to secondary challenge remain intact.
Figure 6. Secondary humoral responses are intact following chronic SCI.
(A) Experimental design for assessment of secondary TD responses in injured and un-injured mice. Mice were immunized 54 days prior to SCI, then 28 days post-injury resting (closed circles), and boosted (open circles) memory responses were assessed. (B) The frequency/million and total number of splenic high-affinity IgG1 ASCs, and (C) high-affinity IgG1 anti-NP antibody above non-immunized controls both before and after secondary challenge is shown (n=4–5 mice per group). Data are representative of two experiments.
Discussion
These studies probe the impact of acute and chronic SCI on B lymphopoiesis, pre-immune B cell homeostasis, and the capacity to mount primary or memory humoral responses. Our results reveal a transient cessation of BM B lymphopoiesis during acute T3- or T9-SCI, that resolves within 4 weeks of injury. Consistent with this decreased BM output, splenic TR B cell numbers show a corresponding transient decline and resurgence. FO B cell numbers were consistently but mildly reduced following injury, and were most severely affected following T3 injury. In contrast, MZ B cell numbers were either unaffected or increased. Despite the presence of these mature pre-immune B cells, both TI-2 and primary TD humoral immune responses were profoundly reduced during chronic T3-injury. Finally, we find that SCI affects neither existing memory B cell numbers nor their ability to respond upon re-challenge. In toto, these findings reveal previously unappreciated temporal and functional complexity in the impact of SCI on B cell compartments and humoral immunity.
The transient cessation of B lymphopoiesis following SCI likely reflects a combination of mechanisms. Stress hormones can both inhibit B lymphopoiesis and favor myelopoiesis, and are elevated during acute injury (3, 9). However, B lymphopoiesis recovered within 4 weeks despite continued elevation of corticosterone in chronically injured mice. These observations are consistent with those in chronic SCI patients, who have normal numbers of bone marrow lymphocytes despite elevated stress hormones (41, 42). Accordingly, transiently reduced B lymphopoiesis likely reflects additional or alternative mechanisms. Similar temporary reductions in B lymphopoiesis occur during inflammatory responses following immunization or infection (28, 29), and are associated with increases in pro-inflammatory cytokines such as TNFα, IL-1b, or IFN-α/β (43–45). Inasmuch as these cytokines transiently increase at the site of spinal injury (46), it is tempting to speculate that both inflammatory cytokines and stress hormones impact B lymphopoiesis during acute injury. B cell progenitors from T3-, but not T9-, SCI injured mice were significantly decreased when compared to laminectomy controls, thus it remains possible that injury level impacts the extent to which B cell genesis is inhibited. However, we favor the notion that it this reflects different levels of stress and inflammation, rather than a loss of sympathetic regulation, for several reasons. First, B cell genesis was significantly reduced in both T9 and T3 injured mice compared to un-injured controls, and both showed restoration after 4 weeks – something that would not be predicted to occur if it were due to the disruption of supraspinal sympathetic regulation. Moreover, we also observed reductions in B cell genesis following laminectomy, further supporting the notion that stress and inflammation associated with injury per se, rather than supra-spinal regulation, causes the blunting of B lymphopoiesis.
While consistent with prior studies demonstrating reductions in total CD19+ splenic B cells 3 days post T3 SCI (9), the detailed subsetting herein reveals that multiple factors contribute to this overall phenomenon. Thus, reductions in the TR pools contribute substantially to initial splenic B cell losses, reflecting truncated BM output. Likewise, there is a reduction in the number of mature FO B cells following acute injury. Despite blunted B cell genesis, a large fraction of FO B cells and all MZ B cells remain, consistent with their comparatively slow turnover rates (22). Surprisingly, though BM output resumes, steady state TR and FO B cell numbers are variably reduced during chronic injury (Table 2). These continued reductions in splenic B cell populations 28 days post injury may reflect the severity of initial loss of B cell genesis and consequently differing rates of its restoration. Alternatively, reduced numbers of splenic B cell subsets are consistent with prior findings that acute T3-SCI increases the proportion of apoptotic cells found among splenic lymphocytes (9, 16). As the ability to bind the cytokine B lymphocyte stimulator (BLyS, also termed BAFF) through BLyS receptor 3 (BR3) is essential for FO B cell survival (47–49), we assayed BR3 levels on FO B cells, and found no difference between un-injured and injured mice (data not shown). The preservation of MZ B cell numbers under all conditions is consistent with the selective preservation of this subset even under B lymphopenic conditions (25). This likely reflects both a larger proportion of TR B cells assuming an MZ B fate as they mature, as well as the acquisition of MZ B characteristics by FO B cells driven by B lymphopenia. Together, these findings strongly suggest that SCI has a direct affect on peripheral B cell homeostasis.
Despite differential effects on mature, quiescent B cell pools, chronic T3 injury clearly impacts the magnitude of both TI and TD primary responses, which are dominated by MZ or FO B cells, respectively (31). The number of MZ B cells was not reduced after T3 injury, thus simple reductions in numbers cannot explain the diminished TI responses. Whether SCI also negatively affects B1 B cells, which can contribute to TI immunity (50), or other cell types such as bystander T cells, which contribute to the magnitude of TI-2 humoral responses, will be an important avenue for future exploration. Consistent with observations made during the acute phase of T3-SCI (9), the inability to mount optimal TD immune responses persisted during chronic injury, likely in part due to the reduced numbers of FO B cells observed. Alternatively, decreased FO B cell responsiveness may be due to SCI induced impairments to T cell responses as reported (9, 10). Nevertheless, affinity maturation is unaffected by SCI indicating that the process of somatic hypermutation and selection of high-affinity B cells within the GC reaction are largely independent of neuronal regulation. Since these key features of TD responses remain intact, strategies to induce protective humoral immunity after SCI might best be focused on increasing the numbers of initially responding B cells. The mechanism by which SCI results in such a dramatic loss of B cell function is likely multifaceted. Laminectomy did not hinder responses to TI challenge, suggesting that SNS innervation in part regulates these responses. However, administration of a β2AR blocker, Butoxamine (5 mg/kg per day, administered i.p.), which inhibits signaling downstream of SNS derived Norepinephrine, did not restore B cell function during chronic SCI when administered days 0–4 post-NP-Ficoll immunization (data not shown). Further, SCI injured mice, but not laminectomy controls, had elevated levels of CORT during chronic injury, implicating the HPA-axis. In toto, these findings suggest that a combination of dysregulated SNS signaling and HPA-axis stress hormones contributes to decreased B cell responsiveness following SCI.
Strikingly, SCI does not ablate previously established B cell memory, nor does it blunt secondary humoral responses, implying that memory B cells are unimpaired by injury. It is thus tempting to speculate that memory B cells are refractory to the immediate effects of SCI, and are independent of neuronal regulation. Regardless of underlying factors, this finding suggests that protective immunity established by prior vaccination or pathogen will remain intact following SCI. Whether protective immunity and memory can be established upon exposure to new TD antigens after SCI remains an open question. Indeed, primary TD responses are diminished during chronic SCI, but the ability to generate high-affinity B cells, and presumably the mechanisms to establish humoral memory, remain. Therefore, post-injury vaccination regimes might best focus on augmenting the frequency and expansion of initially responding cells to achieve protection. On the other hand, TI antigens will remain problematic, since prior memory for such antigens will not exist, and TI responses are persistently and severely compromised after T3 injury. Consequently, the use of conjugate vaccines, which couple TI epitopes to proteins to enable TD responses (51), might prove effective.
Supplementary Material
Acknowledgments
The authors thank the Reeve-Irvine Research Center and the Roman Reed Spinal Cord Research Program for facility and personnel support.
Funding was provided by the Craig H. Neilsen Foundation to T. E. L. and M. P. C., RR09-CY2009 (Roman Reed Spinal Cord Injury Research Program of the State of California) to T.E.L., NIH NS47718 to O.S., Research for Cure, and private donation to the Reeve-Irvine Research Center. K.S.H. was supported, in part, by NIH T32 NS045540-05 training grant. R.G. was supported by NIH Training grant T32 AI-055428.
Abbreviations used in the paper
- SCI
Spinal cord injury
- SNS
sympathetic nervous system
- HPA
hypothalamic-pituitary-adrenal
- TR
Transitional
- FO
Follicular
- MZ
Marginal zone
- GC
Germinal Center
- ASCs
antibody secreting cells
- TI
Thymus-Independent
- TD
Thymus-Dependent
- T9
Thoracic level 9
- T3
Thoracic level 3
- NP
4-Hydroxy-3-Nitrophenyl Acetyl
- BM
bone marrow
Footnotes
Author contributions: MAO, RG, KSH, PJO, and SAA performed the experiments; MPC, TEL, and OS helped design the experiments and provided critical reagents; and MAO, RG, KSH, MPC, and TEL wrote the manuscript.
References
- 1.Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6:775–786. doi: 10.1038/nrn1765. [DOI] [PubMed] [Google Scholar]
- 2.DeVivo MJ, Kartus PL, Stover SL, Rutt RD, Fine PR. Cause of death for patients with spinal cord injuries. Arch Intern Med. 1989;149:1761–1766. [PubMed] [Google Scholar]
- 3.Igarashi H, Medina KL, Yokota T, Rossi MI, Sakaguchi N, Comp PC, Kincade PW. Early lymphoid progenitors in mouse and man are highly sensitive to glucocorticoids. Int Immunol. 2005;17:501–511. doi: 10.1093/intimm/dxh230. [DOI] [PubMed] [Google Scholar]
- 4.Kohm AP, V, Sanders M. Suppression of antigen-specific Th2 cell-dependent IgM and IgG1 production following norepinephrine depletion in vivo. J Immunol. 1999;162:5299–5308. [PubMed] [Google Scholar]
- 5.Nance DM, V, Sanders M. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Podojil JR, V, Sanders M. Selective regulation of mature IgG1 transcription by CD86 and beta 2-adrenergic receptor stimulation. J Immunol. 2003;170:5143–5151. doi: 10.4049/jimmunol.170.10.5143. [DOI] [PubMed] [Google Scholar]
- 7.Felten SY, Olschowka J. Noradrenergic sympathetic innervation of the spleen: II. Tyrosine hydroxylase (TH)-positive nerve terminals form synapticlike contacts on lymphocytes in the splenic white pulp. J Neurosci Res. 1987;18:37–48. doi: 10.1002/jnr.490180108. [DOI] [PubMed] [Google Scholar]
- 8.Tabarowski Z, Gibson-Berry K, Felten SY. Noradrenergic and peptidergic innervation of the mouse femur bone marrow. Acta Histochem. 1996;98:453–457. doi: 10.1016/S0065-1281(96)80013-4. [DOI] [PubMed] [Google Scholar]
- 9.Lucin KM, V, Sanders M, Jones TB, Malarkey WB, Popovich PG. Impaired antibody synthesis after spinal cord injury is level dependent and is due to sympathetic nervous system dysregulation. Exp Neurol. 2007;207:75–84. doi: 10.1016/j.expneurol.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Held KS, Steward O, Blanc C, Lane TE. Impaired immune responses following spinal cord injury lead to reduced ability to control viral infection. Exp Neurol. 2010;226:242–253. doi: 10.1016/j.expneurol.2010.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inman DM, Steward O. Physical size does not determine the unique histopathological response seen in the injured mouse spinal cord. J Neurotrauma. 2003;20:33–42. doi: 10.1089/08977150360517164. [DOI] [PubMed] [Google Scholar]
- 12.Tuli JS, Smith JA, Morton DB. Corticosterone, adrenal and spleen weight in mice after tail bleeding, and its effect on nearby animals. Lab Anim. 1995;29:90–95. doi: 10.1258/002367795780740339. [DOI] [PubMed] [Google Scholar]
- 13.Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81:506–516. doi: 10.1053/mr.2000.3848. [DOI] [PubMed] [Google Scholar]
- 14.Cano G, Sved AF, Rinaman L, Rabin BS, Card JP. Characterization of the central nervous system innervation of the rat spleen using viral transneuronal tracing. J Comp Neurol. 2001;439:1–18. doi: 10.1002/cne.1331. [DOI] [PubMed] [Google Scholar]
- 15.Inman D, Guth L, Steward O. Genetic influences on secondary degeneration and wound healing following spinal cord injury in various strains of mice. J Comp Neurol. 2002;451:225–235. doi: 10.1002/cne.10340. [DOI] [PubMed] [Google Scholar]
- 16.Lucin KM, V, Sanders M, Popovich PG. Stress hormones collaborate to induce lymphocyte apoptosis after high level spinal cord injury. J Neurochem. 2009;110:1409–1421. doi: 10.1111/j.1471-4159.2009.06232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 18.Cancro MP. Peripheral B-cell maturation: the intersection of selection and homeostasis. Immunol Rev. 2004;197:89–101. doi: 10.1111/j.0105-2896.2004.0099.x. [DOI] [PubMed] [Google Scholar]
- 19.Hardy RR, Li YS, Allman D, Asano M, Gui M, Hayakawa K. B-cell commitment, development and selection. Immunol Rev. 2000;175:23–32. [PubMed] [Google Scholar]
- 20.Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 21.Osmond DG. Population dynamics of bone marrow B lymphocytes. Immunol Rev. 1986;93:103–124. doi: 10.1111/j.1600-065x.1986.tb01504.x. [DOI] [PubMed] [Google Scholar]
- 22.Allman DM, Ferguson SE, Lentz VM, Cancro MP. Peripheral B cell maturation. II. Heat-stable antigen(hi) splenic B cells are an immature developmental intermediate in the production of long-lived marrow-derived B cells. J Immunol. 1993;151:4431–4444. [PubMed] [Google Scholar]
- 23.Rolink AG, Andersson J, Melchers F. Characterization of immature B cells by a novel monoclonal antibody, by turnover and by mitogen reactivity. Eur J Immunol. 1998;28:3738–3748. doi: 10.1002/(SICI)1521-4141(199811)28:11<3738::AID-IMMU3738>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 24.Lindsley RC, Thomas M, Srivastava B, Allman D. Generation of peripheral B cells occurs via two spatially and temporally distinct pathways. Blood. 2007;109:2521–2528. doi: 10.1182/blood-2006-04-018085. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava B, Quinn WJ, 3rd, Hazard K, Erikson J, Allman D. Characterization of marginal zone B cell precursors. J Exp Med. 2005;202:1225–1234. doi: 10.1084/jem.20051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sprent J, Basten A. Circulating T and B lymphocytes of the mouse. II. Lifespan. Cell Immunol. 1973;7:40–59. doi: 10.1016/0008-8749(73)90181-0. [DOI] [PubMed] [Google Scholar]
- 27.Forster I, Rajewsky K. The bulk of the peripheral B-cell pool in mice is stable and not rapidly renewed from the bone marrow. Proc Natl Acad Sci U S A. 1990;87:4781–4784. doi: 10.1073/pnas.87.12.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagaoka H, Gonzalez-Aseguinolaza G, Tsuji M, Nussenzweig MC. Immunization and infection change the number of recombination activating gene (RAG)-expressing B cells in the periphery by altering immature lymphocyte production. J Exp Med. 2000;191:2113–2120. doi: 10.1084/jem.191.12.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riley RL, Klinman NR. Differences in antibody repertoires for (4-hydroxy-3-nitrophenyl)acetyl (NP) in splenic vs immature bone marrow precursor cells. J Immunol. 1985;135:3050–3055. [PubMed] [Google Scholar]
- 31.Guinamard R, Okigaki M, Schlessinger J, Ravetch JV. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat Immunol. 2000;1:31–36. doi: 10.1038/76882. [DOI] [PubMed] [Google Scholar]
- 32.Garcia de Vinuesa C, O’Leary P, Sze DM, Toellner KM, MacLennan IC. T-independent type 2 antigens induce B cell proliferation in multiple splenic sites, but exponential growth is confined to extrafollicular foci. Eur J Immunol. 1999;29:1314–1323. doi: 10.1002/(SICI)1521-4141(199904)29:04<1314::AID-IMMU1314>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi Y, Dutta PR, Cerasoli DM, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. J Exp Med. 1998;187:885–895. doi: 10.1084/jem.187.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarlinton D. Germinal centers: form and function. Curr Opin Immunol. 1998;10:245–251. doi: 10.1016/s0952-7915(98)80161-1. [DOI] [PubMed] [Google Scholar]
- 35.Kraal G, I, Weissman L, Butcher EC. Germinal centre B cells: antigen specificity and changes in heavy chain class expression. Nature. 1982;298:377–379. doi: 10.1038/298377a0. [DOI] [PubMed] [Google Scholar]
- 36.Lagresle C, Bella C, Daniel PT, Krammer PH, Defrance T. Regulation of germinal center B cell differentiation. Role of the human APO-1/Fas (CD95) molecule. J Immunol. 1995;154:5746–5756. [PubMed] [Google Scholar]
- 37.Rose ML, Birbeck MS, Wallis VJ, Forrester JA, Davies AJ. Peanut lectin binding properties of germinal centres of mouse lymphoid tissue. Nature. 1980;284:364–366. doi: 10.1038/284364a0. [DOI] [PubMed] [Google Scholar]
- 38.Herzenberg LA, Black SJ, Tokuhisa T. Memory B cells at successive stages of differentiation. Affinity maturation and the role of IgD receptors. J Exp Med. 1980;151:1071–1087. doi: 10.1084/jem.151.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elgueta R, V, de Vries C, Noelle RJ. The immortality of humoral immunity. Immunol Rev. 2010;236:139–150. doi: 10.1111/j.1600-065X.2010.00924.x. [DOI] [PubMed] [Google Scholar]
- 40.Weiss U, Rajewsky K. The repertoire of somatic antibody mutants accumulating in the memory compartment after primary immunization is restricted through affinity maturation and mirrors that expressed in the secondary response. J Exp Med. 1990;172:1681–1689. doi: 10.1084/jem.172.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iversen PO, Nicolaysen A, Hjeltnes N, Nja A, Benestad HB. Preserved granulocyte formation and function, as well as bone marrow innervation, in subjects with complete spinal cord injury. Br J Haematol. 2004;126:870–877. doi: 10.1111/j.1365-2141.2004.05085.x. [DOI] [PubMed] [Google Scholar]
- 42.Campagnolo DI, Bartlett JA, Chatterton R, Jr, Keller SE. Adrenal and pituitary hormone patterns after spinal cord injury. Am J Phys Med Rehabil. 1999;78:361–366. doi: 10.1097/00002060-199907000-00013. [DOI] [PubMed] [Google Scholar]
- 43.Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J Exp Med. 2004;199:47–58. doi: 10.1084/jem.20031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dorshkind K. IL-1 inhibits B cell differentiation in long term bone marrow cultures. J Immunol. 1988;141:531–538. [PubMed] [Google Scholar]
- 45.Lin Q, Dong C, Cooper MD. Impairment of T and B cell development by treatment with a type I interferon. J Exp Med. 1998;187:79–87. doi: 10.1084/jem.187.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. 2007;500:267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- 47.Harless SM, V, Lentz M, Sah AP, Hsu BL, Clise-Dwyer K, Hilbert DM, Hayes CE, Cancro MP. Competition for BLyS-mediated signaling through Bcmd/BR3 regulates peripheral B lymphocyte numbers. Curr Biol. 2001;11:1986–1989. doi: 10.1016/s0960-9822(01)00598-x. [DOI] [PubMed] [Google Scholar]
- 48.Miller DJ, Hayes CE. Phenotypic and genetic characterization of a unique B lymphocyte deficiency in strain A/WySnJ mice. Eur J Immunol. 1991;21:1123–1130. doi: 10.1002/eji.1830210506. [DOI] [PubMed] [Google Scholar]
- 49.Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, Valmori D, Romero P, Werner-Favre C, Zubler RH, Browning JL, Tschopp J. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 51.Lesinski GB, Westerink MA. Novel vaccine strategies to T-independent antigens. J Microbiol Methods. 2001;47:135–149. doi: 10.1016/s0167-7012(01)00290-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.