Abstract
The eggs of the mosquito Aedes aegypti possess the ability to undergo an extended quiescence hosting a fully developed 1st instar larvae within the chorion. As a result of this life history traitpharate larvae can withstand months of quiescence inside the egg where they depend on stored maternal reserves. A. aegypti mosquitoes are frequently associated with urban habitats that may contain significant metal pollution. Therefore, the duration of quiescence and extent of nutritional depletion may affect the physiology and survival of larvae that hatch in a suboptimal habitat. The aim of this study was to determine the effect of an extended quiescence on larval nutrient reserves and the subsequent effects of metal exposure on larval fitness, survival and development. We hypothesized that an extended quiescence would reduce nutritional reserves and alter the molecular response to metal exposure thereby reducing larval survival and altering larval development. As a molecular marker for metal stress responses, we evaluated transcriptional changes in the metallothionein gene (AaMtn) in response to quiescence and metal exposure. Extended 1st instar quiescence resulted in a significant decrease in lipid reserves and negatively affected larval fitness and development. AaMtn transcription and metal tolerance were compromised in first instars emerged from eggs that had undergone an extended quiescence. These findings suggest that newly emerged mosquito larvae that had survived a relatively long pharate 1st instar quiescence (as might occur during a dry season) are more vulnerable to environmental stress. Pharate 1st instar quiescence could have implications for vector control strategies. Newly emerged mosquito larvae at the end of the dry season or start of the wet season are physiologically compromised, and therefore potentially more susceptible to vector control strategies than mosquito larvae hatched subsequently throughout the wet season.
Keywords: Aedes aegypti, quiescence, larvae, metal tolerance, metallothionein, vector ecology
1. Introduction
Container breeding mosquitoes such as Aedes aegypti possess the ability to undergo a seasonal dormancy as an unhatched 1st instar. Embryonic development is completed within ~3 days after oviposition and a fully developed 1st instar larva resides within the chorion of the egg in a dormant state referred to as quiescence. This unique quiescent stage does not have a clear homologue in other insects and has been referred in previous work as a pharate 1st instar diapause (Alekseev, 2007; Clements, 1992). Pharate 1st instar quiescent larvae will hatch out immediately upon exposure to the appropriate stimulus; in this way quiescence differs from diapause, which is a hormonally controlled and pre-programmed state of developmental arrest in which the larvae are refractory to hatching stimuli for an extended period of time. As a result of this life history trait, Aedesaegypti produce eggs that, in addition to being desiccation resistant, can withstand months of dormancy. A wide variety of studies have shown that energetic costs of long-term diapause are reflected in lower post-hatching survival and reduced fecundity (Hahn and Denlinger 2007; 2011). However, relatively little work has been conducted examining the energetic costs and fitness consequences of quiescence.
Aedes aegypti, the primary vector of dengue fever, is well adapted to survive and develop in the anthropogenic bodies of water characteristic of many urban environments (Crovello and Hacker, 1972; Huber, 2008; Trpis and Hausermann, 1975). These breeding sites can be characterized by heterogeneous and dynamic conditions such as varying water volume and/or nutrient content and the presence of toxic metals and/or other physiological stressors. Metals, in particular, are common in urban environments where there exist many sources of metal pollution such as run-off, atmospheric fall out, and the indiscriminant use of metal-containing herbicides. (Mireji, et al., 2008; Sarkar et al., 2004). In addition, the breeding sites themselves may be metallic (cans, dumpsters, cisterns, etc.). The ability to adaptto metal-polluted urban environments may provide distinct advantages to larval mosquitoes. However, larval mosquitoes may also incur fitness costs. Previous studies have suggested that metal stress during mosquito larval development might affect a mosquito’s fitness and ability to transmit diseases (Rayms-Keller, 1998; Sarkar et al., 2004; Mireji et al., 2010a).
Although excess metal ions in the aquatic environment are generally toxic, mechanisms exist in larval mosquitoes to chelate, sequester or otherwise eliminate or detoxify such toxins. One such mechanism is the expression of metallothioneins (Mtn). Metallothioneins are a group of low molecular weight cysteine-rich proteins essential for metal homeostasis, sequestration and detoxification; they have a high affinity for transition metals and are expressed in response to metal exposure in a wide variety of organisms (Amiard, et al., 2006; Andrews, 2000; Balamurugan, et al., 2007; Egli, et al., 2006; Kagi, 1991; Postuma, and van Straalen, 1993; Roesijadi, 1996). Mtn transcription has been validated as a sensitive indicator of metal exposure and an appropriate molecular marker to assess the physiological response to metal stress (Dallinger, 1994; Egli, et al., 2006; Mireji, et al., 2010b; Sarkar, et al., 2004).
The aim of this study was to determine if the duration of pharate 1st instar quiescence(newly oviposited eggs vs. eggs having undergone an extended quiescence period) would reduce larval fitness and decrease the ability of newly hatched larvae to tolerate metal stress. To test this hypothesis the effect of quiescence on the physiological and molecular responses to metal stress in newly hatched larval mosquitoes was assessed in three ways: 1) by determining the duration of the development period, tolerance to starvation and quantifying neutral lipids in 1st instar larvae hatched from new and old eggs, 2) by characterizing the response of larvae hatched from new or old eggs to acute and chronic exposures to copper (Cu) and 3) by describing the molecular response to metal exposure through quantification of metallothionein mRNA in larvae hatched from new or old eggs exposed to Cu. Extended pharate 1st instar quiescence results in a significant decrease in lipid reserves and negatively affects larval fitness and development. In addition, metallothionein transcription and metal tolerance were compromised in first instars emerged from eggs that have been subjected to extended quiescence. These findings suggest that newly emerged mosquito larvae that had survived a long pharate 1st instar quiescence (as might occur during the dry season)are more vulnerable to environmental stress.
2. Materials and Methods
2.1 Mosquito rearing and maintenance
A. aegypti of the Rockefeller strain were reared in 28 °C and 80% relative humidity under a photoperiod of 16 h light: 8 h dark. Mated adults were offered a cotton pad soaked in 3% sucrose solution. Four-day-old female mosquitoes were fed porcine blood equilibrated to 37 °C, and ATP was added to the blood meal to a final concentration of 1 mM immediately before use. Eggs were oviposited on papers and stored under insectary conditions in zip lock bags inside Tupperware containers (adapted from Munstermann, 1997). Biological replication was achieved by generating eggs and or larvae as needed from independent and separate hatches of mosquito eggs across numerous generations.
2.2 Reagents
Reconstituted soft water (RSW) was used in all bioassays. RSW was prepared by adding reagent-grade NaHCO3 (48mg), CaSO4 2H2O (30mg), MgSO4 (30mg) and KCl (2mg) to 1 liter of deionized water (pH~7.4). All reagents were purchased from Fisher Scientific, Pittsburgh, PA.
2.3 Copper measurement
Reagent grade copper sulfate pentahydrate was dissolved in RSW. Nominal Cu concentrations were confirmed by inductively coupled plasma mass spectrometric analysis (ICP-MS) using an HP 4500 plus IPC-MS instrument (Hewlett-Packard Co., Wilmington, DE), equipped with a Babington-type nebulizer and an ASX-500 auto sampler (Cetac Technologies Inc., Omaha, NE).
2.4. Fitness assays
In the manuscript, “new eggs” are pharate 1st instars that remained dormant for less than one week. “Old eggs” are pharate 1st instars that have undergone extended quiescence for over 2 months. Experiments were performed under the temperature, humidity and light cycle conditions described in section 2.1 (“Insectary conditions”). All assays were started at ~12:00 pm.
2.4.1 Time to pupation (length of larval stage)
Pharate 1st instars (eggs) were hatched in deoxygenated water. Upon emergence, three independent replicates of groups of 200 1st instar larvae were reared to adulthood in 23×40×15 cm polypropylene plastic pans containing 1L of RSW for the reared clean treatment and 1 ppm Cu for the metal stressed treatment. Larvae reared in copper (Cu) are referred to as “metal stressed”. Larval diet consisted of 750 μl of 10% liver powder suspension (MP Biomedicals Inc., Aurora, OH.) added to the pan every day except day 2. Pupae were counted each day (at noon) throughout the pupation period. The time to pupation was calculated using the following equation: T = Σ (DxN)/Σ (N)(Kosalwat and Knight, 1987), where: T = the average time to pupation (days), D = number of days from hatching to pupation (age in days) and N = number of insects pupated at that day.
2.4.2 Larval survival under starvation conditions
Three independent replicates of groups of 25 newly emerged 1st instar larvae from new and old eggs were assayed in 15 cm diameter glass petri dishes containing 100 ml of RSW in the absence of food. The number of larvae found not responsive to mechanical stimulation every 24 h were counted as “dead” and removed.
2.5 Acute toxicity stress assays
Three independent replicates of groups of 45 newly emerged 1st instar larvae from new and old eggs were assayed in 15 cm diameter glass petri dishes holding 100 ml of RSW containing 1.25 ppm of Cu (from CuSO4) for 48 h. A concentration of 1.25 ppm Cu was chosen based on preliminary 24 h acute toxicity assays (~LC30). Experiments were performed under insectary conditions and in the presence of food (75ul 10% liver powder suspension). Larvae found not responsive were counted as “dead”. Negative controls were reared in the same conditions in the absence of Cu.
2.6 Lipid and protein analysis
2.6.1 Staining of lipids
Newly emerged 1st instars hatched from new and old eggs were fixed in 4% formaldehyde for 2h, washed with cold PBS, stained with Oil Red-O for 30 min., washed with cold PBS and imaged with a Leica Model MZ6 stereomicroscope at 40x magnification(adapted from Nishiura et. al., 2007).
2.6.2 Quantification of total larval neutral lipids
Neutral lipids were quantified using a triglyceride quantification kit (Biovision, Mountain View, CA; cat# K622-100). Ten newly emerged 1st instars from each treatment (new vs. old) were assayed in triplicate for three biological replicates.
2.6.3 Quantification of total larval proteins
Total protein contents of larvae were quantified using a Pierce 660nm Protein Assay from Fisher Scientific (prod. #322662). Fifty newly emerged 1st instars from each treatment (new vs. old) were assayed in triplicate for three biological replicates.
2.7 Quantitative real-time PCR (qPCR)
Newly emerged 1st instar larvae from new and old eggs were exposed to 1.25 ppm of Cu (from CuSO4) in RSW under insectary conditions and in the absence of food for 6 h. Total RNA was isolated from three independent biological replicates of ~50 larvae using RNA-binding glass powder as previously described (Noriega and Wells, 1993). Contaminating genomic DNA was removed using the DNA-free™ kit (Ambion, Austin, TX). Reverse transcription was carried out using the Verso cDNA Kit (Fisher Scientific). Real time qPCR was performed with the 7300 Real Time PCR System using TaqMan® Gene Expression Assays together with TaqMan® Universal PCR Master Mix (Applied Biosystems, Foster City, CA). The primer probes for housekeeping gene 60S ribosomal protein L32 (rpL32) (Accession number: AAEL003396) and for metallothionein (Mtn)(Accession number:AAEL008176) were as follows(exon borders in bold):
rpL32 Forward: 5′ CCAAGCCAGATTCCTAACATTCAAA 3′
rpL32 Reverse: 5′ TGCCCTGTCCGCAACC 3′
rpL32 Probe: 5′ CTTGCAATCATTACCACAGCAC 3′
Mtn Forward: 5′ GCCAAGCCAGATTCCTAACATTCAA 3′
Mtn Reverse: 5′ GCAACCGGAGGTGCACTT 3′
Mtn Probe: 5′ AAGTGCTGTGGTAATGATTGC 3′
qPCR reactions were carried in a 20 μl volume according to manufacturer’s recommendations for Custom TaqMan® Gene Expression Assays. Reactions were run in triplicate using 1 μl of cDNA per reaction. Standard curves to quantify relative gene copy number were made from serial dilutions of plasmids containing rpL32 or Mtn gene (300,000, 30,000, 3000, 300 and 30 copies of a plasmid per reaction). Real time data were collected by 7300 System SDS Software and analyzed in Microsoft Excel. Mtn transcript levels were normalized with rpL32 transcript levels in the same sample. Relative Mtn transcript levels are expressed as a number of copies of Mtn transcript per 10,000 copies of rpL32 transcript. Each qPCR data point is average of three independent biological replicates.
2.8 Data analysis
Graphical representations were performed with GraphPad Prism Software (san Diego, CA) version 3.00 for Windows. Statistical analysis was performed with Excel 2007. The results were expressed as mean and SEM and considered significantly different at P < 0.05 by a one tailed students t test (P<0.05). Significant differences in variance were determined by F test.
3. Results
3.1 Effect of quiescence on 1st instar larval survivorship and development
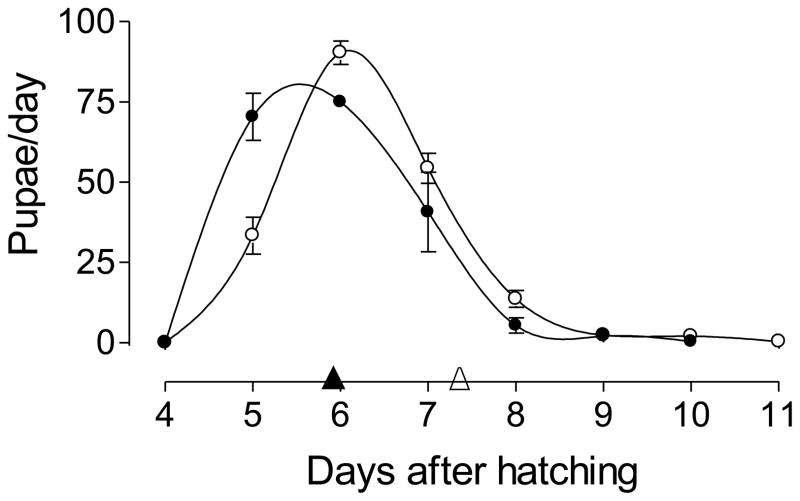
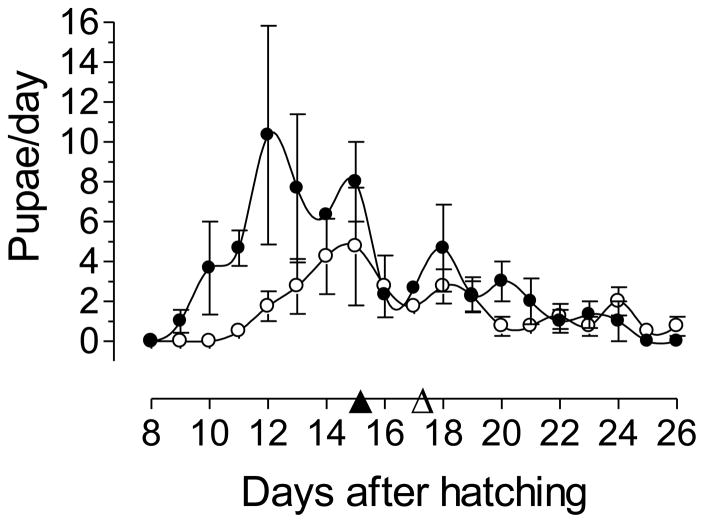
When reared in a “clean” environment (without metal), larvae hatched from older eggs exhibit a significantly longer larval development period than larvae hatched from new eggs as evidenced by a mean 7.33 vs. 5.95 days to pupation (Fig. 1). There was no observed mortality in either treatment.
Fig. 1. Larval development period in a clean environment.
Larval development period was determined as the mean time to reach pupation for larvae reared in a clean environment. Mean larval development periods were: 5.95 days for larvae from new eggs (▲) and 7.33 days for larvae from old eggs (△). Each point is the mean ± SEM of three independent replicates of groups of 200 larvae hatched from new eggs (●) or old eggs (○).
3.2 Effect of quiescence on larval nutrient reserves
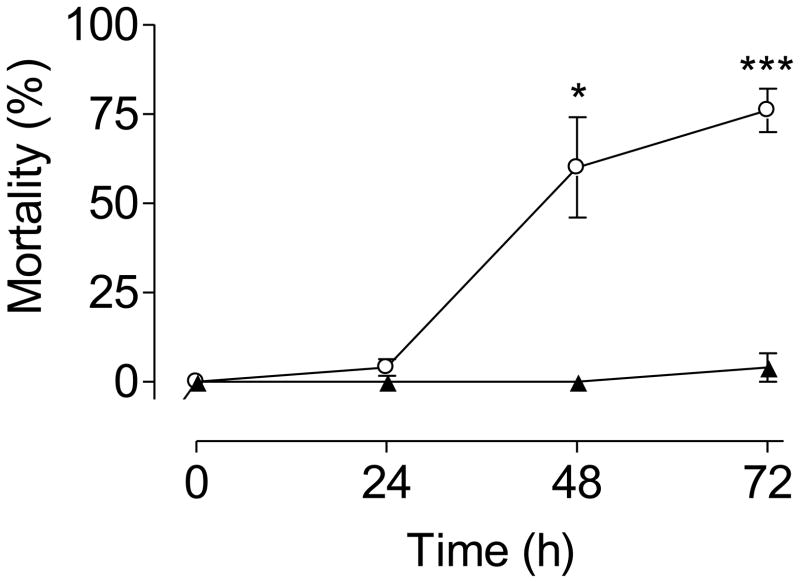
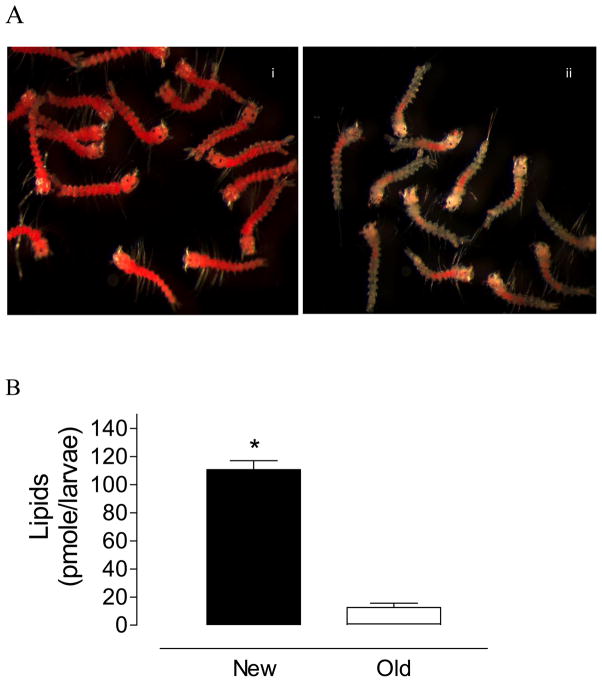
1st instar larvae hatched from older eggs exhibited a significantly higher mean mortality in response to starvation (76 vs. 4%) in a 72 h acute assay (Fig. 2). Neutral lipid reserves decreased ~9 fold in pharate 1st instars larvae that had been subjected to 3 month quiescence(old eggs) (Fig. 3). Quiescence had no effect on the total amount of protein measured in newly hatched larvae. Both groups had approximately 150 ng protein/larva (n=50).
Fig. 2. Tolerance to starvation.
Mortality of newly hatched larvae in the absence of food was assessed in a 72 hour starvation assay. Each point is the mean ± SEM of three independent replicates of groups of 25 larvae hatched from new eggs (▲) or old eggs (○)(* p<0.05, t test, unpaired, one tailed).
Fig. 3. Lipid reserves.
A) Oil Red O staining of lipid reserves in newly hatched larvae emerging from new eggs (i) or old eggs (ii). B) Total neutral lipids measured in newly emerged 1st instars. Results are means ± SEM of three independent replicates of groups of 90 larvae hatched from new eggs (filled bars) or old eggs (empty bars)(* p<0.01, t test, paired, one tailed).
3.3 Effect of quiescence on 1st instar larvae response to metal stress
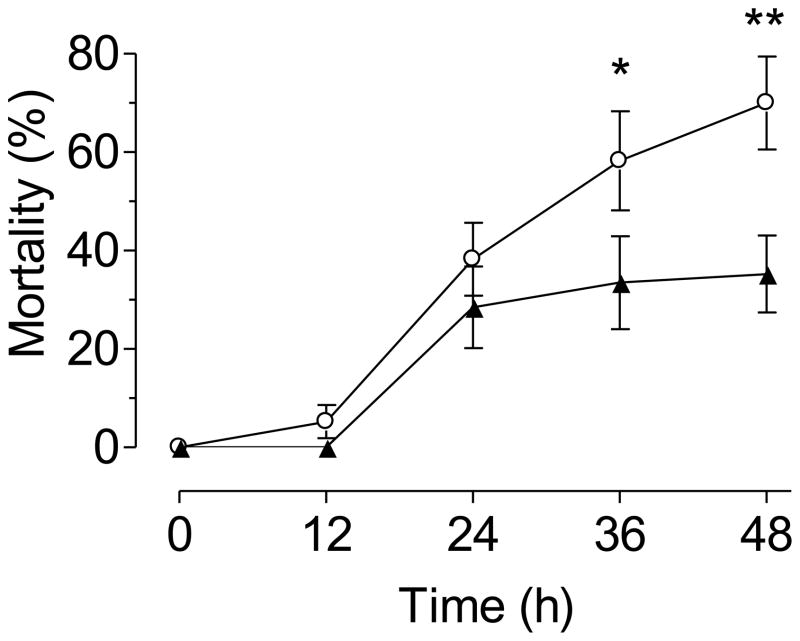
Newly emerged 1st instar larvae hatched from old eggs exhibited a 2-fold increase in mortality (70% vs. 35% in new eggs) in a 48 h acute toxicity assay (Fig. 4). When larvae are reared to adulthood in the presence of metal stress, larvae hatched from older eggs exhibited a significantly higher mortality than larvae emerged from new eggs (85% vs. 53% respectively) and a significantly longer larval development period than larvae hatched from new eggs (17.38 vs. 15.02 days)(Fig. 5).
Fig. 4. Tolerance to metal stress.
Mortality of newly hatched 1st instar larvae exposed to 1.25 ppm Cu in a 48 hour acute toxicity assay. Each point is the mean ± SEM of three independent replicates of groups of 45 larvae hatched from new eggs (▲) or old eggs (○) (* p<0.05, t test, unpaired, one tailed).
Fig. 5. Larval development periodin a metal environment.
Larval development periodas determined by mean time to reach pupation for larvae reared in 1.00 ppm Cu. Mean larval development period for larvae from new eggs = 15.02 days (▲) and 17.38 days for larvae from old eggs (△). Each point is the mean ± SEM of three independent replicates of groups of 200 larvae hatched from new eggs (●) or old eggs (○).
3.4 Effect of quiescence on metallothionein mRNA levels
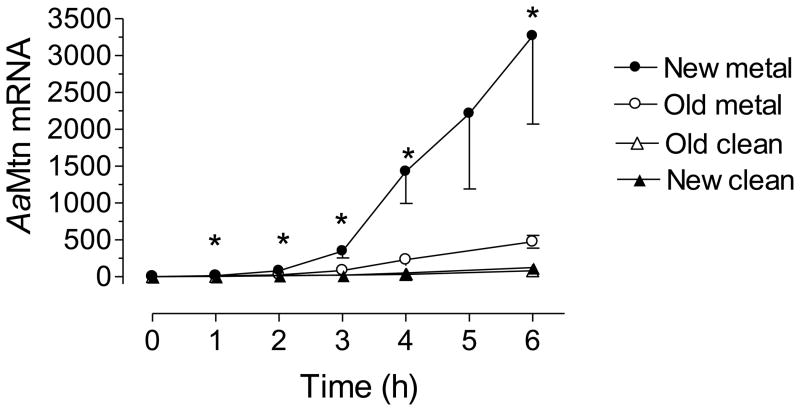
Newly emerged 1st instar larvae from new eggs exposed to Cu for 6 h exhibited a 26-fold increase in AaMtn mRNA levels, whereas newly emerged 1st instar larvae from old eggs exposed to Cu for 6 h exhibited only a 4-fold increase (Fig. 6). The Mtn response to metal stress of 1st instars hatched from old eggs was significantly less robust throughout the time course. In addition, AaMtn mRNA levels from 1st instars from old eggs did not exhibit the magnitude in variance that 1st instars from new eggs did. Variances in Mtn response are significantly different at all-time points except at 3 h (F test, p<0.05). There was a moderate but significant increase on Mtn mRNA levels in larvae held in clean RSW for 6 h, regardless of the eggs’ age. This increase is most likely related with the developmental and homeostatic roles of Mtn in mosquito larvae.
Fig. 6. Metallothionein mRNA levels.
Newly hatched larvae were exposed to clean RSW or RSW containing 1.25 pm Cu for six hours. AaMtn mRNA is expressed as copy number of AaMtn mRNA/10,000 copies of rpL32 mRNA. Each RT-PCR data point is a mean ± SEM of three independent biological replicates of 50 larvae (* p<0.05, t test, paired, one tailed) (Filled bars are larvae from new eggs; empty bars are larvae from old eggs).
4. Discussion
4.1 The duration of quiescence alters the nutritional status, developmental rate and starvation tolerance of newly hatched larvae
The fertilized mosquito egg is a closed system, bound by an impermeable chorion and dependent on maternally-derived lipid reserves to complete embryogenesis and maintain a quiescent metabolism. Over 90% of stored egg lipids are triglycerides (van Handel, 1993) and are likely critical for energy production during quiescence in 1st instar larvae (Hahn and Denlinger, 2007). It would seem there was a simple relationship between the duration of quiescence and the nutritional and physiological status of newly hatched larvae. 1st instars hatched from eggs subjected to an extended quiescence emerged with less lipid reserves (Fig. 3), were less tolerant to starvation(Fig. 2) and had a longer larval developmental period (Fig. 1). The observed reduction of lipidsin larvae hatched from old eggs is probably the result of the oxidation of lipids to support basal metabolism (Hahn and Denlinger, 2007). Although oxygen consumption and metabolism are depressed after embryogenesis is completed,(Weissman-Strum and Kindler, 1962), an extended 1st instar larvae quiescence would eventually deplete egg reserves.
Larval growth and development depends on maternally inherited reserves in addition to nutrients acquired by the larvae. These results indicate that a 1st instar can undergoan extended quiescence, but with specific repercussions for doing so. The specific costs of 1st instar quiescence on the development and starvation tolerance of larvae is ecologically and epidemiologically relevant as mosquito populations in the field often encounter environments with sub-optimal nutritional conditions rendering adult mosquitoes with diverse fitness, reproductive potential and vector capacity(Alto et. al., 2005, 2008, Caroci, 2004). Based on the results discussed here, nutrition carried over from maternal reserves into the larval stages is very important for development and likely acts as a buffer against environmental stochasticity or suboptimal conditions.
4.2. The duration of quiescence alters the physiological response to metal stress in newly hatched larvae
The profound effect of an extended quiescence on newly hatched larvae suggested that other physiological and ecological implications were likely to exist. To explore this possibility newly hatched larvae were exposed to ecological relevant concentrations of copper in order to mimic the metal exposure larvae are likely to experience in urban environments. The reductions in nutritional reserves caused by an extended quiescence affected the response to metal exposure; after 48 hours of an acute exposure, significantly more larvae hatched from old eggs died than those hatched from new eggs (Fig. 4). In addition, larvae hatched from old eggs and reared to adulthood incopper exhibited a delayed developmental profile compared to larvae hatched from new eggs(Fig. 5).
The mortality of the larvae reared to adulthood in the metal stress experiment was not uniform among the four instar developmental stages. Most mortality occurred during the 1st instar stage. Larvae that reached the 2nd instar were generally able to reach the pupal stage. This would suggest that 1st instar mosquitoes are especially sensitive to copper exposure and that rapid tolerance to copper exposure develops in the survivors. A variety of other aquatic and terrestrial insects have been shown to be more sensitive to metals during the early instars rather than the late instars further suggesting tolerance develops (Williams et al. 1986; Pascoe et al. 1989; Timmermans et al., 1992).
4.3 The duration of quiescence alters the molecular response to metal stress in newly hatched larvae
While copper is a critical component of enzymes involved in respiration, oxidative stress protection, pigmentation and iron metabolism, it may also be toxic in excessive concentrations (Egli, et. al., 2006). Metallothionein has been well established as an active participant in the response to metal stress (Amiard, et al., 2006; Postuma, and van Straalen, 1993; Roesijadi, 1996). The sequestration of copper involves chelation by Mtn to reduce Cu toxicity following a copper shock. In Ae. aegypti 1st instar larvae there was a moderate but highly significant increase of AaMtn in the absence of metal, an indication of the critical role of this gene in metabolic pathways during early larval development. On the other hand, when exposed to copper, mosquitoes hatched from new eggs showed significant mRNA increases in less than one hour, and transcripts exhibited a dramatic 26-fold increase over 6 hrs (Fig. 6). An extended quiescence significantly reduced this homeostatic protective response to metal stress. A moderate 1.5 fold increase in the levels of Mtn mRNA was also described for A. gambiae larvae reared in a Cu environment (Mireji, et al., 2010b).
Detoxification, sequestration and elimination of metals and the neutralization of the oxidative stress associated with metal exposure are nutrient dependent processes (Calow, 1991; Servia, et al., 2006). The exponential increase of AaMtn mRNA observed in larvae from new eggs was replaced by a modest linear increase in larvae that experienced a long quiescence. The compromised nutritional status of larvae from old eggs possibly contributes to this decrease in phenotypic plasticity. While the connection between nutritional reserves, copper exposure and mortality is not fully explained by this study, it is likely that the energetic costs of metal homeostasis in an environment with excessive Cu cannot be met by those mosquitoes with reduced nutritional reserves.
Although the reduced metal tolerance and larval development evidenced by these experiments could be the result of a lack of sufficient nutritional reserves, alternative scenarios do exist that can explain reduced developmental rates and higher mortality, such as a direct effect of metals on nutrient availability or storage, and/or through additional physiological demands that metal acclimatization might induce. Metal ions have been found to chelate with amino acids and proteins and affect their bioavailability in insect; specifically metals interfere with enzymatic processes and peroxidize poly unsaturated fatty acids (Sivapalan and Guanapragasam, 1980), produce specific reductions in whole body lipids (Ortel, 1995) and cause fat body and midgut “injury” in some insects (Martoja et al., 1983; Rams-Keller, 1998).
4.4 Implications for Vector Ecology and Vector Control
The presence of mosquitoes has been confirmed in polluted habitats such as drains containing domestic wastewater and other man-made aquatic habitats (Barrera et al., 2008; Chinery, 1995; Mireji, et al 2008,). A. aegypti, being a container breeder, may be subject to greater physiological stress from metals in the environment than other species of mosquitoes. We would like to propose that newly emerged 1st instars of Aedine mosquitoes hatched from eggs that have been subjected to an extended quiescence, such as those experienced during the dry season, are more vulnerable to metal stress after hatching. During the wet season, subsequent generations that are not experiencing extended egg quiescence, regain their metal tolerance competence. Similar behavior extends to the responses to other forms of stress such as pollution stress, oxidative stress, insecticide metabolism and larval competition (Alto, et al., 2005; Poupardin et. al., 2008). As a consequence, extended quiescence could a) contribute to increased vector capacity via increased dengue viral load of adult females derived from quiescent eggs (Alto, et al., 2008) and b) have implications for vector control strategies, i.e., newly emerged mosquito larvae at the end of the dry season/start of the wet season are physiologically compromised, and therefore potentially more susceptible to vector control strategies than mosquito larvae hatched subsequently throughout the wet season.
Nutrient reserves decrease during pharate 1st instar quiescence in Aedes aegypti.
Duration of pharate 1st instar quiescence negatively affects subsequent larval fitness.
An extended quiescence period decreases metallothionein mRNA levels and compromises metal tolerance physiology
Acknowledgments
I would like to thank Dr. Marcela Nouzova, Dr. Jaime Mayoral, Dr. Crisalejandra Rivera and Mark Clifton for critical reading and feedback on the manuscript. This work was supported by NIH/NIGMS R25 GM061347 and the FIU MBRS RISE Biomedical Research Initiative to MHP
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alekseev VR. Monographiae biologicae Dordrecht. London: Springer; 2007. Diapause in aquatic invertebrates: theory and human use. [Google Scholar]
- Alto BW, Lounibos LP, Higgs S, Juliano SA. Larval competition differentially affects arbovirus infection in Aedes mosquitoes. Ecology. 2005;86:3279–3288. doi: 10.1890/05-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Lounibos LP, Mores CN, Reiskind MH. Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proceedings of the Royal Society B: Biological Sciences. 2008;275:463–471. doi: 10.1098/rspb.2007.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiard JC, Amiard-Triquet C, Barka S, Pellerin J, Rainbow PS. Metallothioneins in aquatic invertebrates: Their role in metal detoxification and their use as biomarkers. Aquatic Toxicology. 2006;76:160–202. doi: 10.1016/j.aquatox.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Andrews GK. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochemical Pharmacology. 2000;59:95–104. doi: 10.1016/s0006-2952(99)00301-9. [DOI] [PubMed] [Google Scholar]
- Balamurugan K, Egli D, Hua H, Rajaram R, Seisenbacher G, Georgiev O, Schaffner W. Copper homeostasis in Drosophila by complex interplay of import, storage and behavioral avoidance. The EMBO Journal. 2007;26:1035–1044. doi: 10.1038/sj.emboj.7601543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera R, Amador M, Diaz A, Smith J, Munoz-Jordan JL, Rosario Y. Unusual productivity of Aedes aegypti in septic tanks and its implications for dengue control. Medical and Veterinary Entomology. 2008:62–69. doi: 10.1111/j.1365-2915.2008.00720.x. [DOI] [PubMed] [Google Scholar]
- Calow P. Physiological costs of combating chemical toxicants: Ecological implications. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology. 1991;100:3–6. doi: 10.1016/0742-8413(91)90110-f. [DOI] [PubMed] [Google Scholar]
- Caroci AS, Li Y, Noriega FG. Reduced juvenile hormone synthesis in mosquitoes with low teneral reserves reduces ovarian previtellogenic development in Aedes aegypti. Journal of Experimental Biology. 2004:2685–2690. doi: 10.1242/jeb.01093. [DOI] [PubMed] [Google Scholar]
- Chinery WA. Impact of rapid urbanization on mosquitoes and their disease transmission potential in Accra and Tema, Ghana. African Journal Medical Science. 1995;24:179–188. [PubMed] [Google Scholar]
- Clements AN. The Biology of Mosquitoes. 1. Chapman and Hall; London: 1992. [Google Scholar]
- Crovello TJ, Hacker CS. Evolutionary strategies in life table characteristics among feral and urban strains of Aedes aegypti (L.) Evolution. 1972;26:185–196. doi: 10.1111/j.1558-5646.1972.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Dallinger R. Invertebrate organisms as biological indicators of heavy metal pollution. Applied Biochemistry and Biotechnology. 1994;48:27–31. doi: 10.1007/BF02825356. [DOI] [PubMed] [Google Scholar]
- Egli D, Domenech J, Selvaraj A, Balamurugan K, Hua H, Capdevila M, Georgiev O, Schaffner W, Atrian S. The four members of the Drosophila metallothionein family exhibit distinct yet overlapping roles in heavy metal homeostasis and detoxification. Genes to Cells. 2006;11:647–658. doi: 10.1111/j.1365-2443.2006.00971.x. [DOI] [PubMed] [Google Scholar]
- Hahn DA, Denlinger DL. Meeting the energetic demands of insect diapause: Nutrient storage and utilization. Journal of Insect Physiology. 2007;53:760–773. doi: 10.1016/j.jinsphys.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Hahn DA, Denlinger DL. Energetics of Insect Diapause. Annual Reviews of Entomology. 2011;56:103–121. doi: 10.1146/annurev-ento-112408-085436. [DOI] [PubMed] [Google Scholar]
- Huber K, Ba Y, Dia I, Mathiot C, Sall AA, Diallo M. Aedes aegypti in Senegal: genetic diversity and genetic structure of domestic and sylvatic populations. Am J Trop Med Hyg. 2008;79:218–229. [PubMed] [Google Scholar]
- Kagi JHR. Overview of metallothionein. Methods Enzymol. 1991;205:613–626. doi: 10.1016/0076-6879(91)05145-l. [DOI] [PubMed] [Google Scholar]
- Kosalwat P, Knight AW. Chronic toxicity of copper to a partial life cycle of the midge, Chironomus decorus. Archives of Environmental Contamination and Toxicology. 1987;16:283–290. [Google Scholar]
- Martoja R, Bouquegneau JM, Verthe C. Toxicological effects and storage of cadmium and mercury in an insect Locusta migratoria (Orthoptera) Journal of Invertebrate Pathology. 1983;42:17–32. [Google Scholar]
- Mireji PO, Keating J, Hassanali A, Mbogo CM, Nyambaka H, Kahindi S, Beier JC. Heavy metals in mosquito larval habitats in urban Kisumu and Malindi, Kenya, and their impact. Ecotoxicology and Environmental Safety. 2008;70:147–153. doi: 10.1016/j.ecoenv.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mireji PO, Keating J, Hassanali A, Mbogo C, Muturi MN, Githure J, Beier J. Biological cost of tolerance to heavy metals in the mosquito Anopheles gambiae. Medical and Veterinary Entomology. 2010a:24. doi: 10.1111/j.1365-2915.2010.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mireji PO, Keating J, Hassanali A, Impoinvil DE, Mbogo CM, Muturi MN, Nyambaka H, Kenya EU, Githure JI, Beier JC. Expression of metallothionein and [alpha]-tubulin in heavy metal-tolerant Anopheles gambiae sensu stricto (Diptera: Culicidae) Ecotoxicology and Environmental Safety. 2010b;73:46–50. doi: 10.1016/j.ecoenv.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munstermann LE, Wasmuth LM. In: Handbook of Insect Rearing. Singh P, Moore RF, editors. Elsevier; Amsterdam: 1985. [Google Scholar]
- Nishiura JT, Burgos C, Aya S, Goryacheva Y, Lo W. Modulation of larval nutrition affects midgut neutral lipid storage and temporal pattern of transcription factor expression during mosquito metamorphosis. Journal of Insect Physiology. 2007;53:47–58. doi: 10.1016/j.jinsphys.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Wells MA. A comparison of three methods for isolating RNA from mosquitoes. Insect Molecular Biology. 1993;2:21–24. doi: 10.1111/j.1365-2583.1993.tb00121.x. [DOI] [PubMed] [Google Scholar]
- Ortel J. (Lymantria dispar, Lymantriidae, Lepid.) and its hemolymph. Bulletin of Environmental Contamination and Toxicology. 1995;55:216–221. doi: 10.1007/BF00203012. [DOI] [PubMed] [Google Scholar]
- Pascoe D, Williams KA, Green DWJ. Chronic toxicity of cadmium to Chironomus riparius Meigen — effects upon larval development and adult emergence. Hydrobiologia. 1989;175:109–115. [Google Scholar]
- Posthuma L, Van Straalen NM. Heavy-metal adaptation in terrestrial invertebrates : a review of occurrence, genetics, physiology and ecological consequences. Comparative biochemistry and physiology. C Comparative pharmacology and toxicology. 1993;106:11–38. [Google Scholar]
- Poupardin R, Reynaud S, Strode C, Ranson H, Vontas J, David JP. Cross-induction of detoxification genes by environmental xenobiotics and insecticides in the mosquito Aedes aegypti: Impact on larval tolerance to chemical insecticides. Insect Biochemistry and Molecular Biology. 2008;38:540–551. doi: 10.1016/j.ibmb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Rayms-Keller A, Olson KE, McGaw M, Oray C, Carlson JO, Beaty BJ. Effect of Heavy Metals on Aedes aegypti (Diptera:Culicidae) Larvae. Ecotoxicology and Environmental Safety. 1998;39:41–47. doi: 10.1006/eesa.1997.1605. [DOI] [PubMed] [Google Scholar]
- Roesijadi G. Metallothionein and its role in toxic metal regulation. Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology. 1996;113:117–123. doi: 10.1016/0742-8413(95)02079-9. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Duttagupta AK, Mal TK. Effects of heavy metals on population growth and metallothionein gene expression in the mosquito Culex quinquefasciatus, from Calcutta, India. Environmental Pollution. 2004;127:183–193. doi: 10.1016/j.envpol.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Servia M, Péry A, Heydorff M, Garric J, Lagadic L. Effects of copper on energy metabolism and larval development in the midge Chironomus riparius. Ecotoxicology. 2006;15:229–240. doi: 10.1007/s10646-005-0054-0. [DOI] [PubMed] [Google Scholar]
- Sivapalan P, Gnanapragasam NC. Influence of copper on the development and adult emergence of Homona coffearia; (Lepidoptera: Tortricidae) reared in vitro. Entomologia Experimentalis et Applicata. 1980;28:59–63. [Google Scholar]
- Timmermans KR, Peeters W, Tonkes M. Cadmium, zinc, lead and copper in Chironomus riparius (Meigen) larvae (Diptera, Chironomidae): uptake and effects. Hydrobiologia. 1992;241:119–134. [Google Scholar]
- Trpis M, Hausermann W. Demonstration of differential domesticity of Aedes aegypti (L.) (Diptera, Culicidae) in Africa by mark-release-recapture. Bulletin of Entomological Research. 1975:199–208. [Google Scholar]
- Van Handel E. Fuel metabolism of the mosquito (Culex quinquefasciatus) embryo. Journal of Insect Physiology. 1993;39:831–833. [Google Scholar]
- Weissman-Strum A, Kindler SH. Effect of Low Temperatures on Development, Hatching and Survival of the Eggs of Aedes aegypti (L) Nature. 1962;196:1231–1232. [Google Scholar]
- Williams KA, Green DWJ, Pascoe D, Gower DE. Cadmium, zinc, lead and copper in Chironomus riparius (Meigen) larvae (Diptera, Chironomidae): uptake and effects. Oecologia. 1986;70:362–366. doi: 10.1007/BF00379498. [DOI] [PubMed] [Google Scholar]