Abstract
Purpose
To describe the development of the BrightArm upper extremity rehabilitation system, and to determine its clinical feasibility with older hemiplegic patients.
Method
The BrightArm adjusted arm gravity loading through table tilting. Patients wore an arm support that sensed grasp strength and communicated wirelessly with a personal computer. Games were written to improve cognitive, psychosocial and the upper extremity motor function and adapted automatically to each patient. The system underwent feasibility trials spanning 6 weeks. Participants were evaluated pre-therapy, post-therapy, and at 6 weeks follow-up using standardized clinical measures. Computerized measures of supported arm reach and game performance were stored on a remote server.
Results
Five participants had clinically significant improvements in their active range of shoulder movement, shoulder strength, grasp strength, and their ability to focus. Several participants demonstrated substantially higher arm function (measured with the Fugl-Meyer test) and two were less-depressed (measured with the Becks Depression Inventory, Second Edition). The BrightArm technology was well-accepted by the participants, who gave it an overall subjective rating of 4.1 on a 5 point Likert scale.
Conclusions
Given these preliminary findings, it will be beneficial to evaluate the BrightArm through controlled clinical trials and to investigate its application to other clinical populations.
Keywords: Stroke, elderly, virtual reality, BrightArm, cognition, shoulder strength, skilled nursing facility
Introduction
Stroke is a leading cause of serious long-term disability and can impact areas of cognitive, psychosocial and physical functioning [1]. Cognitive impairments post-stroke are largely dependent on lesion localization, and can impair executive, language, visuo-spatial/perceptual, learning and memory domains [2]. Depression is also common [3] and can further tax already vulnerable neuro-cognitive functions. Physically, post-stroke Hemiplegia may result in unilateral upper extremity (UE) weakness, reduced active range of movement and arm function, and consequently, diminished independence in performing activities of daily living (ADLs) [4]. The older adult population prospect of permanent disability or dementia is increased if age-related cognitive decline is present pre-stroke [5]. The combination of reduced mobility, reduced independence in ADLs, weakness, memory loss and depression may cause many older stroke survivors to be institutionalized in skilled nursing facilities (SNFs).
Long-term SNF residents who are stroke survivors, and who suffer from a combination of motor, cognitive and emotive disabilities could benefit from therapy which addresses all these domains. Current standard of care addresses the motor, cognitive and emotive deficits separately, with therapy provided by different clinicians (physical therapists, occupational therapists, neuro-psychologists, psychiatrists and others). By contrast, integrative rehabilitation, a concept introduced here, simultaneously addresses the motor, cognitive and emotive deficits in a single-point-of-care approach. Integrative virtual rehabilitation uses custom therapeutic games in which the patient is asked to solve cognitive problems (such as making decisions on object sequences or remembering the location of image pairs) through physical exertion (arm movement and grasping with the affected hand). The emotive domain is addressed by making the integrative rehabilitation custom games always winnable and by lavishly congratulating the patient for success. This approach is likely to increase self-esteem, provide a feeling of accomplishment, and thus reduce depression, without anti-depressant medication.
In an effort to normalize the residential experience, most SNFs offer an array of entertainment and hands-on activities to engage their residents, referred to as health maintenance programs. Among the most common are low-cost game consoles. However, off-the-shelf game consoles such as the Wii are not appropriate for many residents, especially those with physical limitation due to Hemiplegia. These residents are likely to experience difficulty lifting their impaired arm to play or holding the remote controller [6]. To further complicate matters, for residents post-stroke, the inability to win games designed for healthy young adults may lead to an increasing sense of failure or a heightened sense of disability. This may result in higher degree of depression and may lower exercise adherence rates [7].
The entertainment and motivational value of game consoles should be channeled towards new computerized systems that accommodate even the lowest functioning SNF residents. Key to such adaptation is the provision of gravity support to the plegic arm and game interfaces that do not require pressing buttons or holding a remote. Furthermore, such new systems should be wheelchair-accessible (as the vast majority of SNF long-term residents are wheelchair users). These custom rehabilitation games need to provide an approach combining sensory, motor and cognitive elements aimed at improved motor function, range of movement, strength, memory, focusing and reduce depression.
Rutgers University Telerehabilitation Institute (Piscataway, USA) developed the “Rutgers Arm” which is a virtual reality-based rehabilitation system that provides gravity support for the plegic arm via a low-friction custom table. Patients interacted with custom designed games by moving their arm on a low-friction arm support and by grasping a rubber pear in lieu of buttons [8]. A feasibility study of the Rutgers Arm on four chronic post-stroke participants showed clinical benefit in improved arm function, larger active range of motion, increased grasp strength and improved morale, following 6 weeks of training [9]. However, the participants were not residents of a SNF, and their pre-post evaluations did not include measures of neurocognitive functioning (i.e., memory, executive function, and/or depression).
Bright Cloud International Corp is a spinoff of Rutgers University that has been developing the BrightArm™, a follow-up to the Rutgers Arm. This article presents the design characteristics of the BrightArm system, as well as its evaluation protocol and first feasibility study on a group of older adult stroke survivors. These were residents of Roosevelt Care Center, a 420-bed SNF in Edison, USA.
Methods
The BrightArm rehabilitation system
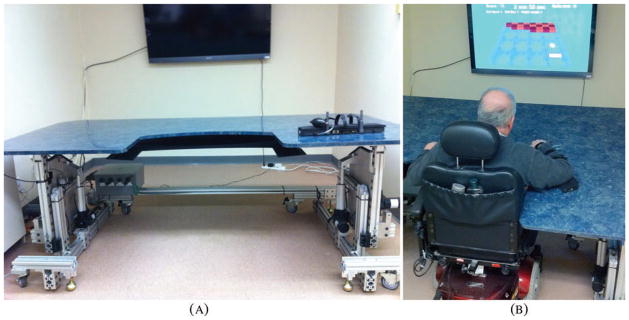
The BrightArm system, shown in Figure 1a [10], consisted of a motorized training table, an instrumented forearm support, two overhead digital cameras, a large high-definition TV, a multicore PC workstation, a sound system, a remote clinical server and a library of custom-designed rehabilitation games.
Figure 1.
The BrightArm™ integrative rehabilitation system: (a) general view; (b) participant training on the BrightArm tilted upwards. Copyright Bright Cloud International. Reprinted by permission.
Motorized table
The BrightArm table top has a rectangular shape with rounded corners and a U-shape cutout facing the patient. Unlike the Rutgers Arm, which had an asymmetrical shape requiring a change in the display position depending on which arm was trained, the symmetrical BrightArm table allowed patients to train either arm without the need for display re-orientation. The table top consisted of an engineered wood core covered on the top side with Formica. This material was chosen for its low surface friction and ease of cleaning. Along the distal edge of the table were embedded infrared (IR) light-emitting diodes (LED), which pointed upwards and were imaged by overhead cameras, part of the vision tracking system described later. The LEDs were positioned flush with the top surface so as not to hinder the movement of the patient’s forearm support. The table top was supported by a modular structure constructed from aluminum beams and fasteners, and sitting on wheels for ease of transport. The supporting structure housed four electrical actuators, two position sensors, a safety plate assembly and a custom electronic controller. The four actuators were identical, DC-powered linear motors placed symetrically about the table’s central axis. Two actuators raised or lowered the table to accommodate different body types from pediatric to the 90-percentile adult male, while the other two actuators tilted the table top down or up. A more difficult movement for lower functioning spastic patients was arm movement away from their trunk. Thus the table could be tilted up to 15 degrees down from horizontal, so arm movement away from the trunk was assisted by gravity. Conversely, the same two actuators could tilt the Table 20 degrees up from horizontal, so higher functioning patients had to move their affected arm against gravity.
The BrightArm was designed so that it’s tilting axis passed through the patient’s trunk along the coronal plane when the patient was seated at the table. This design feature made it unnecessary to lift or lower the patient’s trunk as a function of table tilt, allowing the use of regular wheelchairs. The momentary table height and tilt angle were measured by cabled analog potentiometers connected to a digital data collection unit. This data unit converted the analog potentiometer output to digital words which were sent to the PC. This allowed the table height and tilt angle to be precisely calculated based on prior calibration and displayed in real-time. The therapist could adjust the table height and tilt manually using a custom hand-held pendant connected to the BrightArm custom electrical controller. Depending on how much adjustment was needed, the therapist could choose to move the BrightArm table top either fast or slow while monitoring the tilt and height values displayed on the TV. The controller also monitored a safety plate that was placed just under the table top. This safety plate was made of Delrin plastic and was suspended underneath the table top. If the safety plate made contact with the patient’s body while the table was being lowered or tilted, the controller immediately overrode the therapist’s command and stopped the table from moving further (and emitted an audible alert). This arrangement prevented patient injury due to human error, while at the same time assuring maximum work envelope corresponding to a particular patient’s anatomy.
Computerized forearm support
Patients sitting at the table placed their hemiplegic arm on a forearm support (shown on top of the BrightArm table in Figure 1a). This support consisted of a rectangular plastic shell housing a custom electronics assembly, rechargeable batteries and a wireless transmitter. The forearm instrumentation included a solid-state, differential pressure sensor used to measure the pressure exerted by patients when grasping a rubber pear connected to the forearm support, and non-contact, optical proximity detectors. These proximity sensors were located on the underside at each end of the forearm support shell and detected unwanted lifting of the forearm off the table. Grip strength was measured directly in psi for correlation with classical dynamometer measurements [11]. The wireless transmitter inside the forearm support was used to send unidirectional packets to a wireless receiver at the PC at a data rate of no less than 40 packets/second. Each packet contained information on the patient’s grasp strength, lift sensor status, and remaining battery charge level. When not in use, the forearm support was charged using a standard 9–12V wall charger, providing at least 8 hours of continuous use per charge. Attached to the rectangular plastic shell were two cylindrical plastic “towers” of identical shape and height, each housing an upwards-pointing IR LED at its tip. These LEDs were used by the vision system to track the position and orientation of the forearm support in the plane of the table. The forearm support top was covered with a removable memory foam pad, designed to ensure comfort. The foam pad was coded, so that each patient had a personalized pad, so to reduce the risk of skin disease transmission. The front area under the patient’s fingers was covered with a sheet of Teflon, a feature which prevented the patient’s fingernails from digging into the foam pad. This made grasp strength sensing more accurate, especially for weak hands. The forearm support was secured to the patient’s arm by three Velcro loops of such dimensions and spacing to accommodate the placement of wrist weights, commonly used in arm rehabilitation. The underside of the forearm support was equipped with a number of Teflon beads meant to lower contact friction when the patient moved the affected arm while supported by the Formica surface.
Overhead digital cameras
A pair of Edmund Optics CMOS machine vision cameras with IR filters were mounted on the ceiling above the BrightArm, facing downwards. The cameras were located so that each imaged more than half of the BrightArm surface, corresponding to the arm being trained. These cameras had a frame rate of 50 frames per second at a maximum resolution of 1280 × 1024 pixels and were connected via gigabit Ethernet. A router was used to create a local network, allowing communication between the two overhead cameras and the PC running the rehabilitation games. While both cameras were connected to the router, a manual switch selected which camera output was being used, corresponding to the arm being tracked. A custom program, written in C++, initialized the camera data, calibrated it based on the position of the table LED’s, determined the position of the forearm support, and passed this information to the rehabilitation games.
PC workstation
An HP Z200 workstation, running Windows 7 (64 bit) operating system, processed the IR camera images to track the patient’s arm movement, rendered the real-time game graphics and interactive sound, and automatically stored game data during each rehabilitation session. In order to achieve real-time processing of the camera images, game rendering, file management and internet communication, the workstation used a quad-core Xeon X3460 CPU. The real-time graphics were rendered by a mid-range NVIDIA Quadro FX1800 768MB graphics card.
Remote clinical server
After the completion of each rehabilitation session, the data were uploaded via a dedicated Internet connection to a remote clinical server, for permanent storage and further analysis. Data were stored in an Oracle MySQL database via a custom-designed Java application which read and parsed the games’ output files. Authorized users could remotely access the data for graphing and analysis of session configuration data, session baselines and game performance.
Custom rehabilitation games
A major advantage over off-the-shelf games was BrightArm’s ability to adapt to each patient’s capabilities each day, thus making games winnable and improving morale. This adaptation was done automatically, based on supported arm reach and grasp strength baselines performed at the start of each rehabilitation session.
During supported arm reach baseline the patient saw an avatar of the rehabilitation table and was instructed to move the affected arm as far as possible, but without lifting it off the table and without trunk leaning. The surface reached by the patient was visualized by a change in table avatar surface color, providing easily understood feedback on performance. Arm reach depended on the table tilt, thus baseline data stored on the PC included the reach zone shape and area (as measured by the overhead cameras), the table tilt angle, wrist weight (if any), and session date. The arm reach baseline in turn determined the placement of virtual objects in the games, such that they were reachable by the patient, no matter how small his/her achievable physical movement.
Subsequent to supported arm reach baseline the patient was instructed to move the arm to a comfortable location on the table and then to exert maximum grasp on the arm support rubber pear. The grasp pressure was sensed by the arm support instrumentation, transmitted to the PC, and displayed on the TV screen as an easily recognizable, virtual thermometer gauge. The gauge consisted of a color bar whose height was proportional to patient’s grasp strength and provided easily interpretable feedback. The maximum voluntary grasp strength measurement was repeated three times interspersed with short rest periods, and the readings were averaged to provide the grasp strength baseline. Each of the custom games described below had some settings that required reach and grasp dual-tasking. For momentary grasp, used only when a virtual object was picked up, thresholds were set at 25% of the baselined grasp strength of that day. For settings which required the patient to sustain the grasp once the virtual object had been picked up, the threshold was set as only 10% of maximum. These values were in-line with human-factors literature comparing maximum and sustained grasp levels [12], and were intended to prevent arm pain and discomfort observed in earlier trials with chronic post-stroke participants [8].
In the current study, the BrightArm therapy sessions consisted of five custom games written in Java3D [13]. Pick-and-place was designed to improve motor control and shoulder/grasp strength, by asking the patient to pick up a ball with a hand avatar and follow a prescribed ideal path to a target, while the actual arm movement was traced in real time. Dual-tasking was implemented by requiring the maintenance of grasp strength en route to the target. Difficulty was further increased by making the target area progressively smaller.
Breakout 3D game trained visual focusing, executive function (anticipation), arm speed and shoulder/grasp strength. In BrightArm’s version of the well-known Breakout arcade game [14], the paddle avatar was controlled by the patient to bounce virtual balls towards an array of bricks. Furthermore, arm movement was maximized by allowing only one brick to be destroyed for every ball bounce off the paddle. In dual-task settings the patient was asked to grasp just before the ball had to be bounced, lest the ball passed through the paddle avatar and was lost. Further difficulty levels were set by varying the speed of the ball and the size of the paddle.
Treasure Hunt trained arm endurance, motor speed and shoulder/grasp strengthening. The patient was told to dig out treasure chests buried in the sand on a desert island, using a shovel avatar. Game difficulty was increased by sand storms that covered some of the already dug out treasures requiring more arm movement. In a dual-task condition the patient had to grasp to pick up the shovel and keep grasping to make it move. The sound of waves could be heard and had a relaxing effect on patients.
Card Island game trained short-term visual memory and focusing. Now the island had an array of cards arranged face down on the sand, and the patient was asked to pair them. BrightArm customized the card deck for each participant, showing family members, pets, and sports images, which motivated the patient and facilitated memory training. Game difficulty was increased with the number of cards to be paired and dual-tasking required that the patient move a hand avatar over a chosen card and then grasp to turn it face up. Once a card had been seen by the patient, when it turned face down again, its back pattern color changed to provide a memory cue. If two subsequent cards matched they disappeared from the island.
Finally, Tower of Hanoi 3D trained executive function, motor control and shoulder/grasp strength by asking patients to stack disks of different diameters on one of three poles. The disks were initially on one pole, stacked in decreasing size from bottom to top and the patient had to re-stack the disks on another pole in the same order of sizes, while using the third pole as a way point. The number of disks was one element in gradating cognitive load, since a larger number of disks corresponded to a longer sequence of moves. In BrightArm’s version of the classic logic game, which is normally played with a computer mouse, the game presented a 3-D scene showing 3-D disks, 3-D poles and a hand avatar controlled by the participant’s arm movement and grasping. In the dual-task setting, the patient had to grasp first to pick a disk up, and then keep grasping en route to the desired pole.
Each of the five games included a summary of performance feedback once completed, and rewards (fireworks and applause), which provided positive reinforcement and were meant as morale boosters.
Feasibility study design
In order to gauge the clinical effect and the patients’ acceptance of the BrightArm system and therapy, it was necessary to conduct a feasibility evaluation. The inclusion criteria for this study were residency in a SNF, Hemiplegia due to stroke, time since stroke longer than 12 months and being older than 60 years. Good mental awareness was needed to be able to comprehend the consent form and the simulation exercise demands. Exclusion criteria were total lack of active movement in the hemiplegic arm, blindness, severe cognitive delay, or dementia. All participants were residents of Roosevelt Care Center (Edison, USA) and received medical clearance from their treating physicians for participation. They were subsequently consented using a form approved by the Western Institutional Review Board (an independent board overseeing research involving human subjects) which reviewed and approved this feasibility study in accordance with Federal Guidelines. The BrightArm system was subsequently installed at the Roosevelt Care Center in a dedicated room and then pre-tested with four older healthy volunteers. Subsequently, six residents chronic post-stroke underwent rehabilitation on the BrightArm in fall 2010. Of these participants, one dropped out due to illness unrelated to the study and five completed the experimental therapy.
Participants characteristics
The vital statistics, motor impairment, depression level, cognitive state, education, language primarily spoken, as well as co-morbidity characteristics of the five participants are summarized in Table 1. The one female and four males, who ranged in age from 62 to 81, started the experimental therapy between 19 and 119 months after their stroke. Four participants were affected on the left side of their body, had severe motor impairments and were initially very low functioning (an initial UE score for the Fugl-Meyer Assessment (FMA) [15] of 4 to 11 points out a maximum of 66 points). One participant was affected on the right side of his body, and initially had marked motor impairment with a FMA score of 28. All participants presented with some degree of spasticity at the elbow and fingers, and were not using their affected arm in ADLs. Depression levels (according to Beck’s Depression Inventory [16]) varied among participants, one presenting with moderate depression (initial score 26), three being minimally depressed (initial scores 1, 6, 11), and one participant had no depression (initial score 0). The participants’ education levels varied, with three completing high school, one having attended some college and one having a post-secondary degree. Three were native English speakers, and two were not. Cognitively, four participants initially exhibited severe impairments in attention or memory or both, while one participant had less severe cognitive impairments. All participants had multiple medical problems, two having a history of epilepsy and one of severe anxiety. All participants ambulated in wheelchairs within the SNF.
Table 1.
Participants characteristics and medical history pre-intervention. Copyright Bright Cloud International. Reprinted by permission.
| Participant | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age (years) | 65 | 69 | 62 | 81 | 67 |
| Gender | Female | Male | Male | Male | Male |
| Race | White | White | White | Black | White |
| Months since stroke | 119 | 21 | 73 | 73 | 19 |
| Affected side | Left | Left | Right | Left | Left |
| Motor impairment level | Severe | Severe | Marked | Severe | Severe |
| FMA UE score | 5 | 4 | 28 | 10 | 11 |
| Spasticity | Elbow, fingers | Elbow, fingers | Fingers | Elbow, fingers | Elbow, fingers |
| Depression level | Moderate | Minimal | Minimal | Minimal | Normal |
| Cognitive state | Severely impaired visual and verbal attention, Severely impaired visual memory | Severely impaired visual memory | Severely impaired verbal memory | Severely impaired visual and verbal attention, Severely impaired visual and verbal memory | Moderately impaired verbal attention, Mildly impaired visual attention |
| Co-morbidities | Hypertension, Leg amputation, Anemia, Aneurism heart, Tobacco use | Epilepsy, Ischemic Heart, Hypothiroidism, Asthma, Chronic airway obstruction, Chronic pain | Aphasia, Convulsion, Hypertension, Epilepsy | Malignant kidney, Nocturia, Hypertension, Abnormal gait, Cataracts | Anxiety, Macular degeneration, Hypertension |
| Ambulation | Wheelchair | Wheelchair | Wheelchair | Wheelchair | Wheelchair |
| Language | Non-English | English | English | Non-English | English |
| Education (yrs) | 12 | 12 | 18 | 15 | 12 |
Data collection instruments
The feasibility study used an ABAA protocol, data being collected pre- (A), during training (B), post- (A), and at 6 weeks after the completion of training (A). Therapy consisted of 18 sessions over 6 weeks, with each participant attending 3 therapy sessions per week. Therapy session data consisted of arm reach and grasp strength baselines, as well as game performance data collected during play. Every 2 weeks of training, the participants rated their experience on a subjective evaluation paper questionnaire with nine questions. Ratings ranged from 1 (least desirable outcome) to 5 (most desirable one).
The pre-, post-, and follow-up evaluation sessions involved data collection of standardized UE motor and functional measures (by a senior OT), and of neuropsychological measures of attention/concentration, processing speed, learning, memory, and executive functioning (by a neuropsychologist). Both clinicians were blinded to the therapy methodology and scope, however the neuropsychologist subsequently became co-author of this paper (JSH).
The UE motor impairment evaluation measured affected shoulder strength (using calibrated weights placed at the wrist), grasp strength (using a mechanical Jamar dynamometer), finger pinch strength (using a mechanical pinch gauge), as well as shoulder and elbow active range of motion (using a mechanical goniometer). The arm and hand function were measured with the Jebsen test of hand function [17], the UE subset of the FMA, and through independence in ADLs reported on a modified standardized questionnaire [18]. The ADL questionnaire modification consisted of removing tasks that SNF residents do not perform (driving, preparing food, vacuuming, or tying laces). To improve ADL report accuracy, post-training questionnaires were completed by the nurse or certified nurse aide who cared for each resident, while at follow-up the SNF director (also a co-author of this article) evaluated each participant.
The Beck Depression Inventory, Second Edition (BDI-II) was used as a standardized measure in the emotive evaluations. On this measure, raw scores range from 0–13 (“minimal depression”), to 29–63 (“severe depression).” Verbal and visual attention were assessed with sub-tests of the Neuropsychological Assessment Battery [19] (NAB) Attention Module (Digits and Dots sub-tests, respectively). Verbal and visual learning and memory were evaluated with the Hopkins Verbal Learning Test, Revised (HVLT-R) [20] and the Brief Visuo-spatial Memory Test, Revised (BVMT-R) [21], respectively.
Neuropsychological test scores are reported as T-scores accompanied by labeled degree of perceived impairment. The T-scores range varied from 28 and below (“severely impaired”) to 64 and above (“superior”).
Experimental protocol
At the start of each session the participants’ spastic elbow and fingers were stretched by an occupational therapist (OT), followed by baseline measurements. The duration of the BrightArm therapy increased from 20 minutes of actual play per session in week 1, to 30 minutes in week 2, 40 minutes in week 3 and 50 minutes in weeks 4 through 6.
Session training intensity was similarly increased, primarily by gradating the BrightArm table tilt angle, which was 0 degrees (horizontal) in weeks 1 through 3, then 10 degrees in week 4 and 20 degrees in weeks 5 and 6. During each session the participants played a sequence of the five games described earlier, which was repeated as needed to achieve the prescribed session duration for that week. The difficulty of each exercise was progressively increased from easier games with no required grasping in weeks 1 and 2, to most difficult ones requiring sustained grasping in weeks 5 and 6. During the last 2 weeks of therapy difficulty was further increase by asking participants to attempt game play while wearing wrist weights. An OT or certified occupational therapist assistant (COTA) was present in the room and guided or assisted the participants when needed. In the last 2 weeks of therapy, once the participants finished the game sequence for that day, they were asked to perform actual ADLs under supervision. These activities included squeezing a rubber ball, using silverware in simulated feeding, picking up objects with the plegic hand and in the case of Participant 3 (who started with the highest arm function of the group), writing. At the end of the therapy, participants were told to continue using their plegic arm including squeezing a rubber ball daily so as not to loose the gains they had achieved.
Statistical methods
Comparisons of continuous variables pre- to post-, post- to follow-up were done by two paired t-tests. The sample was not large enough to test the assumptions necessary for a repeated measures ANOVA. Results were transformed so that the larger the mean difference the more positive the finding. P-values less than 0.05 were deemed statistically significant and p-values between 0.05 and 0.10 were deemed to be “trend-level”; no multiple-testing adjustment was done. Results are expressed as 95% confidence intervals to document the precision of all statistical estimates. Though low power makes negative statement less reliable, any positive statistically significant findings imply the findings are robust and not obscured by the small sample size. All analyses were conducted using SAS 9.2 (SAS Institute, Inc, Cary, NC) [22].
Results
Participants’ UE motor impairment
Shoulder and hand strength
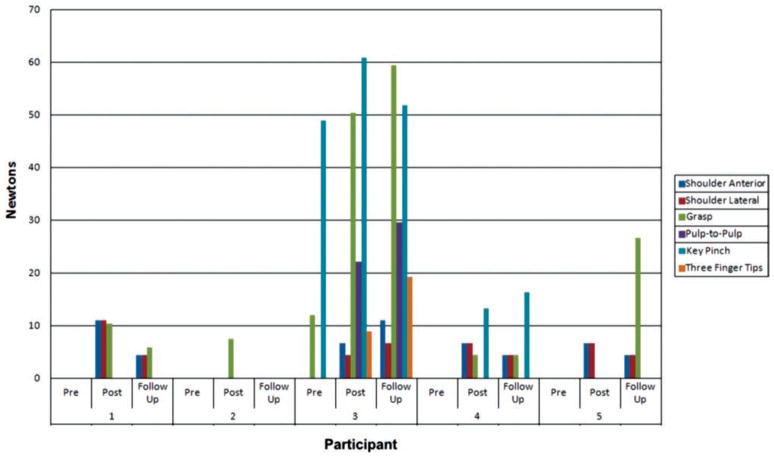
All five participants made progress in the strength of their affected UE (see Figure 2). While pre-training, none could lift weights placed at their affected wrist, post-training four participants could lift weights of 0.68 kg to 1.13 kg when lifting their affected arm in front or laterally. This is substantially more than the 15% threshold of clinical significance in shoulder strengthening [23]. Grasp strength increased in four participants, three of whom had been unable to grasp pre-training. Participant 3 had a notable 419% increase in grasp strength and became able to pinch pulp-to-pulp or with three fingertips post-training. While Participant 3 grasp strength increased from 12 N pre to 50 N post, this falls below the 45 N clinically significant value given by Lang and colleagues [24]. Six weeks after the end of therapy, the gains in strength were largely maintained compared to pre-training, except for Participant 2 who started with the highest impairment level of the group. The pre-post group mean difference in shoulder (anterior) strength was 6.2 (95% C.I.: 1.3, 11.2); p = 0.02. The mean difference in shoulder (lateral) strength was 4.9 (95% C.I.: 0.0, 9.82); p = 0.05. These statistically significant shoulder strength differences persisted for the group as a whole through follow-up: pre-follow-up differences had p-values of 0.02 (lateral) and 0.05 (anterior).
Figure 2.
Shoulder, grasp and pinch strength improvement in study participants. shoulder strength measured with wrist weights, grasp strength with jamar dynamometer and pinch strength with pinch gauge. values given in newtons. Copyright Bright Cloud International. Reprinted by permission.
Shoulder and elbow active range of motion
Dean [25] in a randomized control study of shoulder positioning after stroke, argues that a range increase larger than 10 degrees has clinical significance. Training on the BrightArm resulted in an increase in the active range of motion of the affected arm in all participants, as shown in Table 2. All had clinically significant improvements in shoulder abduction and 4 participants had a clinically significant increase in shoulder flexion. All but the highest functioning participant increased their active elbow flexion (by 5 to 20 degrees), and three saw increases in their elbow extension of 5 to 15 degrees. Post-therapy Participants 4 and 5 had substantial increases in their range of elbow pronation, which increased by 20 degrees and 30 degrees, respectively. At 6 week follow-up some of these gains were lost, however shoulder flexion and shoulder extension ranges remained larger than pre-training in four participants. Shoulder abduction range was larger at follow-up in all participants compared to pre-training, with Participant 5 having a remarkable increase of 40 degrees in his active shoulder abduction 6 weeks after therapy ended. The same participant had a remarkable 25 degrees increase in his elbow flexion and 30 degrees increase in elbow pronation at follow-up, compared to his pre-training. The pre-post mean difference in shoulder (flexion) was 11.0 (95% C.I.: 0.8, 21.2); p = 0.04.
Table 2.
Changes in active range of motion (degrees) in five nursing home residents chronic post-stroke after 6 weeks of training. Copyright Bright Cloud International. Reprinted by permission.
| Session | Participant 1
|
Participant 2
|
Participant 3
|
Participant 4
|
Participant 5
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR | PO | FU | PR | PO | FU | PR | PO | FU | PR | PO | FU | PR | PO | FU | |
|
Shoulder |
|||||||||||||||
| Flexion | 0–20 | 0–45 | 0–40 | 0 | 0 | 0 | 0–35 | 0–50 | 0–60 | 0–30 | 0–50 | 0–35 | 0–50 | 0–65 | 0–65 |
| Extension | 0–40 | 0–45 | 0–45 | 0 | 0–30 | 0–20 | 0–40 | 0–50 | 0–50 | 0–20 | 0–35 | 0–30 | 0–30 | 0–35 | 0–30 |
| Abduction | 0–40 | 0–60 | 0–50 | 0–15 | 0–30 | 0–20 | 0–30 | 0–40 | 0–50 | 0–30 | 0–50 | 0–45 | 0–20 | 0–50 | 0–60 |
| Internal rotation | 0–20 | 0–25 | 0–25 | 0 | 0–30 | 0–10 | 0–40 | 0–50 | 0–50 | 0–70 | 0–70 | 0–50 | 0–30 | 0–30 | 0–40 |
|
Elbow |
|||||||||||||||
| Flexion | 95–120 | 95–125 | 95–125 | 70–85 | 50–85 | 70–85 | 70–110 | 70–110 | 70–110 | 92–110 | 87–120 | 85–110 | 70–85 | 55–85 | 50–90 |
| Extension | 95 | 90 | 95 | 70 | 70 | 70 | 70 | 70 | 70 | 92 | 87 | 85 | 70 | 55 | 50 |
| Pronation | 0–90 | 0–90 | 0–90 | 0 | 0 | 0 | 0–50 | 0–55 | 0–50 | 0–30 | 0–50 | 0–50 | 0–20 | 0–50 | 0–50 |
| Supination | 0–5 | 0–5 | 0–5 | 0 | 0 | 0 | 0–10 | 0–15 | 0–15 | 0–10 | 0–10 | 0–10 | 0 | 0–10 | 0–10 |
PR, pre-training; PO, post-training; FU, 6 week follow-up.
Participants’ UE function and independence in ADLs
Due to participants’ elbow and finger spasticity only Participant 3 was able to perform the timed tasks of the Jebsen test. At the end of therapy he was faster in writing, simulated page turning and lifting small common objects. Overall, he was 18% faster post-training compared to the time it took pre-training. This is close to the 20% overall improvement in Jebsen test total time cited as clinically meaningful in a recent study of chronic stroke patients using robotic UE therapy [26]. At follow-up this participant showed continued improvement, as his Jebsen test took 25% less time to complete vs. pre-training.
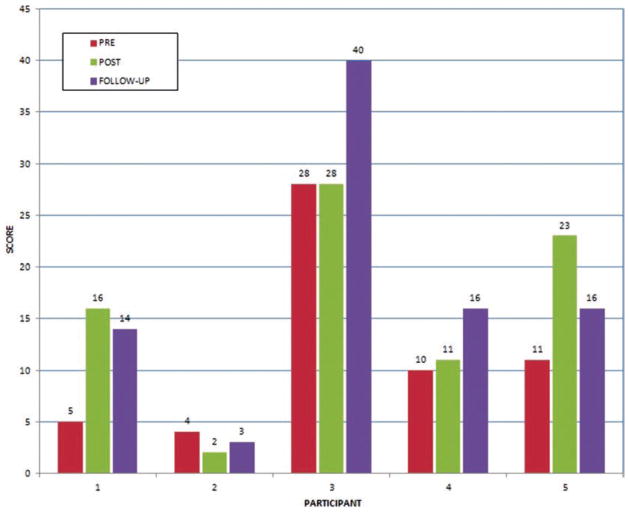
Pre-therapy, Participants 1, 2, 4 and 5 were severely impaired, with initial FMA UE scores of 5, 4, 10, and 11, respectively (Figure 3). Post-therapy, three participants had essentially no change in their arm function; however Participants 1 and 5 progressed into the marked motor impairment range, with FMA score increases of 11 and 12, respectively. At follow-up 4 participants had higher FMA scores than pre-therapy, with Participant 3 having a remarkable increase of 12 points compared to pre-training. While Participants 4 and 5 were severely impaired pre-training, they both progressed to the marked impairment range at follow-up. Improvement in the group mean FMA UE score from pre-training to follow-up was a statistically significant 6.2 points (95% C.I.: 0.2, 12.2); p = 0.05.
Figure 3.
Impaired arm functional changes measured by the Fugl-Meyer upper extremity scores (66 represents maximum possible score). PR, pre; PO, post; FU, follow-up. Copyright Bright Cloud International. Reprinted by permission.
Improved shoulder strength and increased affected arm use resulted in more independence in 12 standardized ADLs in four of the five participants. Post-therapy, the number of tasks the participants were unable to perform dropped for three participants (see Table 3). These gains were either maintained or improved upon at follow-up. The pre–post mean decrease in the number of activities patients were unable to perform was 3.6 (95% C.I.: 0.0, 7.6); p = 0.07. These (trend-level) differences increased for the group as a whole through follow-up: pre-follow-up mean decrease was 5.8 (95% C.I.: 3.8, 7.8); p = 0.001.
Table 3.
Changes in independence in 12 activities of daily living in five nursing home residents chronic post-stroke after 6 weeks of training. Copyright Bright Cloud International. Reprinted by permission.
| Participant
|
1
|
2
|
3
|
4
|
5
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Session | PR | PO | FU | PR | PO | FU | PR | PO | FU | PR | PO | FU | PR | PO | FU |
| Unable to perform | 11 | 3 | 4 | 11 | 11 | 4 | 6 | 2 | 2 | 11 | 10 | 4 | 11 | 6 | 7 |
| Some difficulty | 0 | 4 | 7 | 0 | 0 | 7 | 6 | 3 | 9 | 1 | 1 | 8 | 1 | 5 | 4 |
| No difficulty | 1 | 5 | 1 | 1 | 1 | 1 | 0 | 7 | 1 | 0 | 1 | 0 | 0 | 1 | 1 |
PR, pre-training; PO, post-training; FU- six week follow-up.
Participants’ emotive and cognitive outcomes
Mood generally improved post-training, as seen in Table 4. Notably, Participant 1’s depression level dropped to “mild”, and Participant 4 showed a substantially reduced score (2 vs. 11) within the “minimal” range. At follow-up, Participant 1’s mood continued to improve to minimal depression, and three other participants maintained. Only Participant 4 lost his emotive gains at follow-up, presenting with “mild” depression vs. “minimal” level at pre-training.
Table 4.
Emotive and cognitive outcomes in five nursing home residents chronic post-stroke after 6 weeks of training, and at 6 weeks follow-up. Copyright Bright Cloud International. Reprinted by permission.
| Participant 1
|
Participant 2
|
Participant 3
|
Participant 4
|
Participant 5
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR | PO | FU | PR | PO | FU | PR | PO | FU | PR | PO | FU | PR | PO | FU | |
| Session | Emotive
|
||||||||||||||
| Beck depression inventory | Mod 26 | Mild 18 | Min 7 | Min 1 | Min 1 | Min 2 | Min 6 | Min 5 | Min 4 | Min 11 | Min 2 | Mild 14 | Norm 0 | Min 5 | Norm 0 |
| Cognitive
|
|||||||||||||||
| Verbal attention (digits forward) | Sev T = 25 | Mild T = 35 | Sev T = 25 | Hi A T = 58 | Avg T = 56 | Hi A T = 58 | Mod T = 30 | Mild T = 36 | Mod T = 30 | Mod T = 30 | Sev T = 28 | Mild T = 35 | Mod T = 30 | Avg T = 46 | Lo A T = 41 |
| Verbal working memory (digits backward) | Mild T = 32 | Mild T = 31 | Lo A T = 39 | Avg T = 46 | Avg T = 55 | Lo A T = 39 | Lo A T = 39 | Avg T = 44 | Lo A T = 39 | Sev T = 25 | Mild T = 31 | Mild T = 31 | Avg T = 46 | Hi A T = 60 | Lo A T = 39 |
| Visual attention (Dots) | Sev T = 27 | Lo A T = 39 | Sev T = 27 | Avg T = 50 | Avg T = 53 | Mild T = 34 | Lo A T = 41 | Avg T = 44 | Avg T = 56 | Sev T = 24 | Avg T = 40 | Sev T = 24 | Mild T = 34 | Avg T = 44 | Lo A T = 42 |
| Verbal memory HVLT-R Trials 1–3 |
Mild T = 35 | Sev T = 26 | Lo A T = 42 | Avg T = 46 | Avg T = 50 | Avg T = 44 | Sev T = 27 | Mod T = 30 | Avg T = 48 | Sev T = 24 | Sev T = 27 | Sev T = 22 | Lo A T = 37 | Mild T = 36 | Sev T = 26 |
| Visual memory BVMT-R Trials 1–3 |
Sev T = 22 | Sev T = 23 | Sev T<20 | Sev T = 23 | Lo A T = 37 | Avg T = 45 | Lo A T = 42 | Avg T = 55 | Avg T = 51 | Sev T <20 | Sev T<20 | Sev T <20 | Avg T = 47 | Sup T= 67 | Mild T = 34 |
Avg, average; Hi A, high average; Lo A, Low average; Mod, moderately depressed or moderately impaired; Mild, mildly impaired or mildly depressed; Mini, minimally depressed; Norm, normal (no depression); Sev, severely impaired; Sup, superior; PR, pre-training; PO, post-training, FU, six week follow-up; T, T-score.
There was substantial improvement in verbal and visual attention post-therapy, with Participant 5 progressing from moderately impaired verbal attention (T = 30) to the average range (T = 46), and from mildly impaired visual attention (T = 34) to the average range (T = 44). The same participant had a remarkable improvement from average (T = 47) to the superior range (T = 67) visual memory post-intervention. At follow-up Participants 4 and 5 continued to improve in their verbal attention, compared to pre-training, while the other three participants’ gains had extinguished. The visual attention of Participants 3 and 5 was better at follow-up compared to pre-training, while the other three participants lost their gains in this cognitive domain. Participant 5’s remarkable improvement in visual memory post-therapy was not maintained and at follow-up he was in the mildly impaired range (T = 34). The pre-post mean difference in visual attention was 8.8 (95% C.I.: 1.7, 15.9); p = 0.03, which is statistically significant. The pre-post mean difference in visual memory was 9.6 (95% C.I.: −1.2, 20.4); p = 0.07 (trend level).
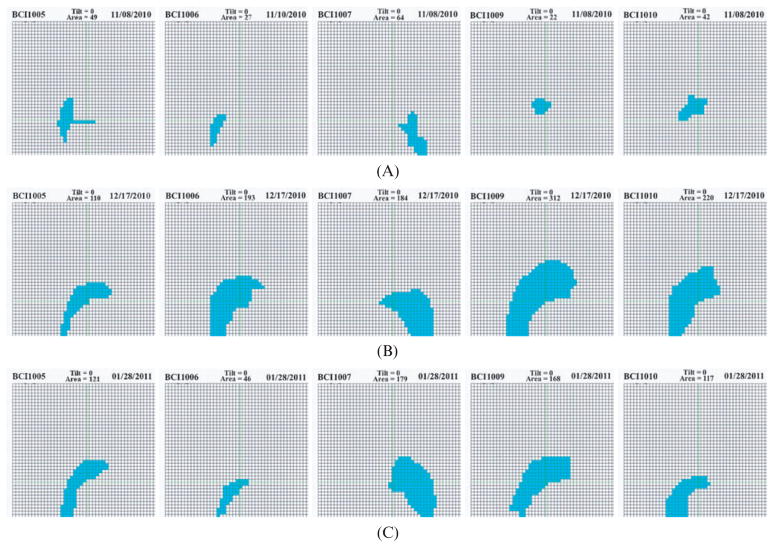
Baseline measures of supported arm reach
The overhead cameras were used to measure the affected arm reach pre-training, post-training and at 6 week follow-up, with the BrightArm table kept horizontal. As seen in Figure 4, all participants had substantial increases in their arm reach post-therapy, which ranged from 224% for Participant 1 to 1,418% for Participant 4. Furthermore, the shape of the arm reach area for all participants became closer to that of an unimpaired person. At follow-up participants maintained some of these gains.
Figure 4.
Supported arm reach baseline in five nursing home residents chronic post-stroke: (a) before training (top); (b) after training (middle); (c) at 6 weeks follow-up (bottom). Copyright Bright Cloud International. Reprinted by permission.
Participants’ subjective evaluation of the BrightArm
Participants gave the BrightArm prototype an overall score of 4.1 out of 5 maximum. They scored lowest (3.2) for the frequency of technical problems, followed by the degree of muscle pain/discomfort (3.4). Participants liked the system, giving it the highest score (4.7), and rated their interest in the therapy games with the second-highest score (4.6). Even though the therapy sessions were intensive, and lasted up to 50 minutes, the participants practiced without complaining, or leaving the room. Nurses and CNA’s felt that the participants were excited about the trials and were disappointed when the trials ended.
Discussion
Motor and function improvements
Participants 3 and 5 had significant grasp strength increases of 11.6 and 13.8 Newtons, above the repeatability of the Jamar dynamometer [27]. Participant 3, the only one who could exert a force in grasping pre-training, could grasp four times stronger post-straining, and continued to improve at follow-up. This participant was the one who most consistently followed the research team request that participants repeatedly squeeze a rubber ball daily after formal rehabilitation ended.
Improvements in grasp strength were also observed in an earlier study involving four participants chronic post-stroke who trained on the Rutgers Arm precursor system to the BrightArm [9]. Two of these participants had been unable to grasp pre-therapy, but could do so following 6 weeks of training. Furthermore, all four participants who trained on the Rutgers Arm improved in pinch strength. Another study using a gravity supported training device and virtual environments investigated possible effect on muscle strength and arm function in patients with paresis due to multiple sclerosis [28]. Ten subjects used the Armeo Spring (Hocoma AG, Zürich, Switzerland) to play games for half-hour sessions, three times/week for 8 weeks. Results showed that participants improved in arm function, but that there was no significant increase in muscle strength. Another study looked at grip strength in 832 elders with activity limitations, age 60–80+ [29]. Results showed that there was a general weakening with age, as expected. Moreover, the participants who had motor impairments and those with cognitive impairments were significantly weaker than age-matched participants with minimal impairments or with vision impairments. The fact that cognitive impairments in the elderly led to less powerful grasp is interesting, and could potentially open another area of application for the BrightArm integrative approach.
In the current study FMA scores improved by 11 and 12 points in three of our participants, well above the chance FMA score differences of ±2 points [30]. Furthermore, these increases exceed the 10% FMA score increase threshold of clinical significance [31]. These results are better than those obtained at Rutgers University by two older stroke survivors who had progressed 8 and 9 points, respectively [9]. The FMA score increases in the BrightArm study are about twice as large as what has been reported for other UE virtual rehabilitation studies of patients chronic post-stroke [32,33]. These FMA score increases are about four times as large as has been reported for robotic therapy with the MIT MANUS [34].
Reduction in impairment levels and improved function transferred to some degree to more independence in ADLs. However, despite using a standardized questionnaire, data was collected from three sources at the pre- (OT), post- (nurse) and follow-up (investigator) evaluations, which constitutes a confounding factor.
Emotive and cognitive gains
There was general improvement in the participants’ emotive state following the BrightArm therapy, with Participant 1 showing the greatest reduction in depression severity. Our results are in-line with a recent study on 19 geriatric participants who completed a 12 week study playing Wii games for half an hour, three times/week [35]. More than one-third of the participants had a 50-percent or greater reduction of depressive symptoms.
Simple auditory attention improved in four of our participants post-intervention, however these gains had generally extinguished at follow-up. Four participants improved substantially in their visual attention/focusing, a positive change that may be explained by the game interaction which involved fast graphics, something that trained primarily the visual cortex. Other studies on young video game players (those playing at least 4 hours/week in the preceding 6 months) found that they were significantly better in terms of attentional resources compared to those that played little or no video games [36]. The decline observed at follow-up after 6 weeks of no games in our study supports the assumption that interactive game graphics had been the primary factor in the participants’ improved focusing.
Playing interactive physical games also improved visual memory in three of our participants. Other studies have shown that playing video games results in improved visual memory. A study on 72 undergraduate students found that time spent playing video games was a predictor of improved visual memory [37]. A randomized study of 36 elderly nursing home residents consisted of 3 months of intensive virtual reality training followed by 3 months of periodic booster sessions [38]. The control group underwent equivalent face-to-face training sessions using music therapy. Results showed significant improvements in memory tests, especially in long-term recall, in the experimental group, and continued cognitive decline in the control group. This finding, if replicated, could indicate that long-term memory gains (or at least arresting age-related decline) will be possible with periodic virtual reality boosters. Overcoming barriers for such booster therapy within a SNF care system remains a challenge.
Integrating motor and cognitive training with multi-sensory feedback
Interaction with our surroundings is multi-modal, utilizing sight, sound, touch, smell and taste feedback. This multi-modal interaction with real-time feedback is the reason users feel immersed in virtual environments [39]. Within the rehabilitation domain this multi-modal interaction with avatars allows for realistic, thus ecological training. Furthermore, such multimodal interaction promotes cross-modal reinforcement, where an unaffected sensorial modality supplements a weak one. An example is the role sound plays in supplementing an imperfect visual channel [40, 41]. Several of our study participants had vision deficits, typically found in the elderly. Nonetheless, they were able to play the BrightArm games, which were interactive and required good hand-eye coordination. This may be due to our use of interactive sound and touch feedback to supplement their diminished visual feedback.
Virtual rehabilitation research has shown that the addition of visual feedback in the form of games to robotic rehabilitation of the lower extremity resulted in more clinical benefit than training the same amount of time on the same robot in the absence of virtual reality graphics [42]. A more recent article looking at UE robotic rehabilitation also supports the benefit of added sensory feedback through video games and haptics [43]. While the BrightArm is not truly a rehabilitation robot, it does include the elements of assisted/resisted motor training through repetitions, similar to robotic training. In addition the system uses rich multi-modal feedback which seems to have benefited the study participants. Unlike robotic systems with virtual reality graphics feedback, BrightArm is the first system to integrate physical movement into cognitive task solving. This results in the novel integrative rehabilitation approach possible on a system that is safer than robots.
Conclusions
BrightArm is a computerized training table designed to provide integrative rehabilitation using custom virtual rehabilitation games. It supports weak arms, gradates gravity loading through tilting, is wheelchair-accessible and stores performance data on a remote server. The system uses unobtrusive vision tracking to measure arm movement on the table and incorporates real-time grasp sensing for dual-task training. Its games are automatically adaptable to each patient, and winnable regardless of level of function. The first BrightArm feasibility trial, on five older post-stroke long-term residents of a SNF showed good acceptance as well as clinical benefits in the motor, emotive and cognitive domains. Given these promising preliminary findings, it will be beneficial to continue the BrightArm clinical evaluation through controlled studies on larger post-stroke populations, as well as investigating other clinical populations, such as individuals in the acute and subacute phase post-stroke.
Implications for Rehabilitation.
It is possible to improve arm function in older hemiplegic patients many years after stroke.
Integrative rehabilitation through games combining cognitive (memory, focusing, executive function) and physical (arm movement, hand-eye coordination, grasping, dual-tasking) elements is enjoyable for this population.
The severity of depression in the elderly can be reduced through virtual reality games, as long as games adapt to the patient, are winnable and provide rewards for success.
Acknowledgments
Research reported here was made possible by grant 1R43NS070613-01 from the National Institutes of Health. A. Nair OT performed the motor evaluations. M. Jagtap OT, P. Joshi OT, and Y. Lockhart COTA trained the participants.
Footnotes
Declaration of interest
Grigore Burdea, Bryan Rabin and Doru Roll are co-inventors on patents related to the technology described in this article. They are shareholders, as well as part-time or contractor employees of Bright Cloud International Corp. Jasdeep Hundal has been a contractor for the same company. Frank Damiani is Administrator and Director of Medical Care for the SNF where the project study took place. Research reported here was supported by grant 1R43NS070613-01 from the National Institutes of Health/NINDS. BrightArm is a trademark of Bright Cloud International Corp.
References
- 1.Mercier L, Audet T, Hébert R, Rochette A, Dubois MF. Impact of motor, cognitive, and perceptual disorders on ability to perform activities of daily living after stroke. Stroke. 2001;32:2602–2608. doi: 10.1161/hs1101.098154. [DOI] [PubMed] [Google Scholar]
- 2.Cavanagh SJ, Gordon VL. Grading scales used in the management of aneurysmal subarachnoid hemorrhage: a critical review. [Accessed 26 February, 2001];J Neurosci Nurs. 2002 34:288–295. doi: 10.1097/01376517-200212000-00002. Available at: http://findarticles.com/p/articles/mi_hb6374/is_2_34/ai_n28949077/ [DOI] [PubMed] [Google Scholar]
- 3.van de Weg FB, Kuik DJ, Lankhorst GJ. Post-stroke depression and functional outcome: a cohort study investigating the influence of depression on functional recovery from stroke. Clin Rehabil. 1999;13:268–272. doi: 10.1191/026921599672495022. [DOI] [PubMed] [Google Scholar]
- 4.Hellström K, Lindmark B, Wahlberg B, Fugl-Meyer AR. Self-efficacy in relation to impairments and activities of daily living disability in elderly patients with stroke: a prospective investigation. J Rehabil Med. 2003;35:202–207. doi: 10.1080/16501970310000836. [DOI] [PubMed] [Google Scholar]
- 5.Nys GM, van Zandvoort MJ, de Kort PL, Jansen BP, de Haan EH, Kappelle LJ. Cognitive disorders in acute stroke: prevalence and clinical determinants. Cerebrovasc Dis. 2007;23:408–416. doi: 10.1159/000101464. [DOI] [PubMed] [Google Scholar]
- 6.Lee JH, Ku J, Cho W, Hahn WY, Kim IY, Lee SM, Kang Y, et al. A virtual reality system for the assessment and rehabilitation of the activities of daily living. Cyberpsychol Behav. 2003;6:383–388. doi: 10.1089/109493103322278763. [DOI] [PubMed] [Google Scholar]
- 7.Patten CA, Vickers KS, Martin JE, Williams CD. Exercise interventions for smokers with a history of alcoholism: exercise adherence rates and effect of depression on adherence. Addict Behav. 2003;28:657–667. doi: 10.1016/s0306-4603(01)00280-5. [DOI] [PubMed] [Google Scholar]
- 8.Burdea G, Cioi D, Martin J, Fensterheim D, Holenski M. The Rutgers Arm II Rehabilitation System – A feasibility study. IEEE Trans Neur Sys Rehab Eng. 2010;18(5):505–514. doi: 10.1109/TNSRE.2010.2052128. [DOI] [PubMed] [Google Scholar]
- 9.Burdea G, Cioi D, Martin J, Rabin B, Kale S, DiSanto A. Motor Retraining in virtual reality: A feasiblity study of upper-extremity rehabilitation in individuals with chronic stroke. Phys Ther Educ. 2011;25(1):20–29. [Google Scholar]
- 10.Burdea G, Arezki A, Bouzit M, Cioi D, Kuttuva M, Fensterheim D. Rehabilitation Systems and Methods. 12/192818. US Patent Appl. 2008 Aug 15;
- 11.Hamilton GF, McDonald C, Chenier TC. Measurement of grip strength: validity and reliability of the sphygmomanometer and jamar grip dynamometer. J Orthop Sports Phys Ther. 1992;16:215–219. doi: 10.2519/jospt.1992.16.5.215. [DOI] [PubMed] [Google Scholar]
- 12.Yamauchi J, Hargens A. Effects of dynamic and static handgrip exercises on hand and wrist volume. Eur J Appl Physiol. 2008;103:41–45. doi: 10.1007/s00421-008-0672-3. [DOI] [PubMed] [Google Scholar]
- 13.Palmer I. Essential Java 3D fast. Springer-Verlag London Ltd; 2001. [Google Scholar]
- 14.Arcade History. [Accessed 27 February 2011];Breakout Atari. 1976 Available at http://www.arcade-history.com/?n=breakout&page=detail&id=3397.
- 15.Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983;63:1606–1610. doi: 10.1093/ptj/63.10.1606. [DOI] [PubMed] [Google Scholar]
- 16.Beck AT, Steer RA, Brown GK. Manual for Beck depression inventory. 2. San Antonio, TX: Psychology Corporation; 1996. [Google Scholar]
- 17.Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Arch Phys Med Rehabil. 1969;50:311–319. [PubMed] [Google Scholar]
- 18.Stratford P, Binkley JM, Stratford POW. Development and initial validation of the upper extremity functional index. Physiotherapy Canada. 2001;281:259–266. [Google Scholar]
- 19.Hartman DE. The weight is over: A review of the R. A. Stern and T. White, Neuropsychological Assessment Battery (NAB) Applied Neuropsy. 2006;13(1):58–61. [Google Scholar]
- 20.Brandt J, Benedict RHB. Professional manual. Lutz, FL: Psychol Assessm Resources, Inc; 2001. Hopkins Verbal Learning Test – Revised. [Google Scholar]
- 21.Benedict RHB. Brief Visuospatial Memory Test – Revised. Odessa, FL: Psychological Assessment Resources, Inc; 1997. [Google Scholar]
- 22.SAS Institute Inc. SAS 9.2. Available online at http://www.sas.com/software/sas9/?gclid=CJa5gPudr6sCFYpM4AodUEGbIg.
- 23.Brosseau L, Wells GA, Finestone HM, Egan M, Dubouloz CJ, Graham I, Casimiro L, Robinson VA, Bilodeau M, McGowan J. Clinical practice guidelines for shoulder subluxation. Top Stroke Rehabil. 2006;13:51–54. [Google Scholar]
- 24.Lang CE, Edwards DF, Birkenmeier RL, Dromerick AW. Estimating minimal clinically important differences of upper extremity measures early after stroke. Arch Phys Med Rehabil. 2008;89:1693. doi: 10.1016/j.apmr.2008.02.022. Available online at http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2819021/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dean CM, Mackey FH, Katrak P. Examination of shoulder positioning after stroke: A randomised controlled pilot trial. Aust J Physiother. 2000;46:35–40. doi: 10.1016/s0004-9514(14)60312-3. [DOI] [PubMed] [Google Scholar]
- 26.Merians AS, Fluet GG, Qiu Q, Saleh S, Lafond I, Davidow A, Adamovich SV. Robotically facilitated virtual rehabilitation of arm transport integrated with finger movement in persons with hemiparesis. J Neuro Engineering and Rehab. 2011;8:27. doi: 10.1186/1743-0003-8-27. Available at http://www.jneuroengrehab.com/content/8/1/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Härkönen R, Harju R, Alaranta H. Accuracy of the Jamar dynamometer. J Hand Th er. 1993;6:259–262. doi: 10.1016/s0894-1130(12)80326-7. [DOI] [PubMed] [Google Scholar]
- 28.Gijbels D, Lamers I, Kerkhofs L, Alders G, Knippenberg E, Feys P. The Armeo Spring as training tool to improve upper limb functionality in multiple sclerosis: a pilot study. [Accessed 26 February, 2011];J Neuro Engineering Rehabil. 2011 :8. doi: 10.1186/1743-0003-8-5. Available at http://www.jneuroengrehab.com/content/8/1/5/ [DOI] [PMC free article] [PubMed]
- 29.Shechtman O, Mann WC, Justiss MD, Tomita M. Grip strength in the frail elderly. Am J Phys Med Rehabil. 2004;83:819–826. doi: 10.1097/01.phm.0000143398.00788.4e. [DOI] [PubMed] [Google Scholar]
- 30.Platz T, Pinkowski C, van Wijck F, Kim IH, di Bella P, Johnson G. Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: a multicentre study. Clin Rehabil. 2005;19:404–411. doi: 10.1191/0269215505cr832oa. [DOI] [PubMed] [Google Scholar]
- 31.Feys HM, De Weerdt WJ, Selz BE, Cox Steck GA, Spichiger R, Vereeck LE, Putman KD, Van Hoydonck GA. Effect of a therapeutic intervention for the hemiplegic upper limb in the acute phase after stroke: a single-blind, randomized, controlled multicenter trial. Stroke. 1998;29:785–792. doi: 10.1161/01.str.29.4.785. [DOI] [PubMed] [Google Scholar]
- 32.Piron L, Turolla A, Agostini M, Zucconi C, Cortese F, Zampolini M, Zannini M, et al. Exercises for paretic upper limb after stroke: a combined virtual-reality and telemedicine approach. J Rehabil Med. 2009;41:1016–1102. doi: 10.2340/16501977-0459. [DOI] [PubMed] [Google Scholar]
- 33.Housman SJ, Le V, Rahman T, Sanchez RJ, Reinkensmeyer DJ. Arm-training with T-WREX after chronic stroke: preliminary results of a randomized controlled trial. IEEE 10th International Conference on Rehabilitation Robotics; 2007; Noordwijk, Netherlands: IEEE; 2007. pp. 562–568. [Google Scholar]
- 34.Lo A, Guariho P, Richards LG, et al. Robot-assisted therapy for long-term upper limb impairement after stroke. NEJ Med. 2010;362:1772–1783. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg D, Depp CA, Vahia IV, Reichstadt J, Palmer BW, Kerr J, Norman G, Jeste DV. Exergames for subsyndromal depression in older adults: a pilot study of a novel intervention. Am J Geriatr Psychiatry. 2010;18:221–226. doi: 10.1097/JGP.0b013e3181c534b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green CS, Bavelier D. Action video game modifies visual selective attention. Nature. 2003;423:534–537. doi: 10.1038/nature01647. [DOI] [PubMed] [Google Scholar]
- 37.Ferguson CJ, Cruz A, Rueda S. Gender, video game playing habits and visual memory tasks. Sex Roles A J Research. 2008;58:279–286. [Google Scholar]
- 38.Optale G, Urgesi C, Busato V, Marin S, Piron L, Priftis K, Gamberini L, et al. Controlling memory impairment in elderly adults using virtual reality memory training: a randomized controlled pilot study. Neurorehabil Neural Repair. 2010;24:348–357. doi: 10.1177/1545968309353328. [DOI] [PubMed] [Google Scholar]
- 39.Burdea Grigore, Coiffet Philippe. Virtual Reality Technology. 2. Wiley; 2003. [Google Scholar]
- 40.Soto-Faraco S, Kingstone A, Spence C. Multisensory contributions to the perception of motion. Neuropsychologia. 2003;41:1847–1862. doi: 10.1016/s0028-3932(03)00185-4. [DOI] [PubMed] [Google Scholar]
- 41.Väljamäe A, Soto-Faraco S. Filling-in visual motion with sounds. Acta Psychol (Amst) 2008;129:249–254. doi: 10.1016/j.actpsy.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Mirelman A, Bonato P, Deutsch JE. Effects of training with a robot-virtual reality system compared with a robot alone on the gait of individuals after stroke. Stroke. 2009;40:169–174. doi: 10.1161/STROKEAHA.108.516328. [DOI] [PubMed] [Google Scholar]
- 43.Merians AS, Fluet GG, Qiu Q, Lafond I, Adamovich SV. Learning in a virtual environment using haptic systems for movement re-education: can this medium be used for remodeling other behaviors and actions? J Diabetes Sci Technol. 2011;5:301–308. doi: 10.1177/193229681100500215. [DOI] [PMC free article] [PubMed] [Google Scholar]