Abstract
The higher prevalence of autoimmune diseases in women compared to men could be due to effects of ovarian hormones, pregnancy and/or the presence of a 2nd X chromosome. To elucidate the role of these factors, we investigated the prevalence and spectrum of autoimmune diagnoses in women with primary ovarian insufficiency associated with X chromosome monosomy (Turner syndrome, TS, n=244) and women with karyotypically normal (46,XX) primary ovarian insufficiency (POI, n=457) in a prospective study, conducted at the National Institutes of Health. We compared the study group prevalence to normative data for the U.S. population of women. Chronic lymphocytic (Hashimoto’s) thyroiditis (HT) occurred in 37% of women with TS vs. 15% with POI (P<0.0001); HT prevalence in both ovarian insufficiency groups significantly exceeded that in U.S. population of women (5.8%). Inflammatory bowel (IBD, 4%) and celiac disease (CD, 2.7%) were significantly increased in TS, but not in POI. No other autoimmune diagnosis, including Graves’ disease or Type 1 diabetes appears to be significantly increased in either group. Women with TS had higher pro-inflammatory IL6 and TGF β1 levels (p<0.0001 for both), and lower anti-inflammatory IL10 and TGF β2 levels (p<0.005 for both) compared to POI and to normal volunteers. Lifetime estrogen exposure and parity were significantly lower in TS compared to POI, which were in turn lower than the general population of women. The finding that lymphocytic thyroiditis is greatly increased in both women with TS and POI suggests that factors associated with ovarian insufficiency per se promote this form of autoimmunity. The absence of a normal second X-chromosome further contributes to increased autoimmunity in TS.
Keywords: Autoimmune diseases, Hashimoto’s thyroiditis gender specific, Turner syndrome, Primary Ovarian Insufficiency, X chromosome, TGFβ, IL6
1. Introduction
Turner syndrome (TS) results from a sex-chromosomal anomaly characterized by a presence of one normal X chromosome and a missing or structurally abnormal second sex chromosome1. It affects 50 per 100 000 live born girls2. The phenotype includes female gender, short stature, primary ovarian failure, some physical features resulting from consequences of fetal lymphedema and skeletal abnormalities1. Congenital cardiovascular defects, atherosclerosis, osteoporosis and fractures, endocrine and metabolic disorders, hearing loss and specific cognitive deficits are recognized contributors for increased morbidity and mortality and decreased life expectancy in this syndrome1, 3–4.
Autoimmunity has been recognized as one of the more prominent characteristics of TS1 with thyroid autoimmunity, and specifically Hashimoto’s thyroiditis (HT) being by far the most common diagnosis3. Over the years several studies have described increased incidence of variety of autoimmune conditions besides HT, such as Type 1 diabetes3, 5–6, celiac disease6, and ulcerative colitis3. However, except for the thyroid autoimmunity there seems to be no clear agreement about the actual risk of other autoimmune diseases, neither of the overall burden of autoimmunity in TS. In addition, the reason why TS predisposes to autoimmunity remains unclear.
Autoimmune diseases are typically more common in women than men7–10 and explanation(s) for this sex dimorphism remain uncertain11–13. Much attention has been given to a potential role for estrogen in divergent immunological responses and disease susceptibility. While estrogens seem to impact the course of human autoimmune diseases, effects are often inconsistent12, 14–15. Pregnancy has also been suspected of contributing to excess autoimmunity in women, with plausible mechanisms including retention of allogenic16 fetal cells. Another factor implicated in excess autoimmunity in women involves the process of X chromosome inactivation, wherein one of the two X chromosomes undergoes inactivation or transcriptional silencing during early embryonic development. This typically results in tissue mosaicism in which approximately 50% of cells express the maternally-derived (XMat) and 50% express the paternally-derived (XPat) X chromosome. It has been proposed that X chromosome inactivation may be skewed during thymic development resulting in predominant expression of only one set of X chromosome encoded self-antigens. This may lead to inadequate thymic deletion of autoreactive T-lymphocytes, which in turn leads to impaired “self” antigen recognition and tolerance17. The risk of initiation of an autoimmune reaction would be enhanced if such autoreactive T cells encounter XPat or XMat specific antigens in peripheral tissues17.
In the present study we investigate the prevalence of autoimmune disorders in large groups of community dwelling, generally healthy women with TS and women with karyotypically normal, spontaneous POI. The goal was to illuminate possible effects of reduced ovarian function, low pregnancy experience and the presence or absence of a normal 2nd X chromosome on risk for such disorders.
2. Methods
2.1 Study Subjects
We collected data from 224 consecutive participants in the “Turner Syndrome: Genotype and Phenotype” study (CH-00-0291, NCT 00006334) and 457 consecutive participants in the “Ovarian Follicle Function in Patients with Premature Ovarian Failure” study (CH-91-0127, NCT 00001275) between January 2000 and March 2009. Both studies were conducted at the Clinical Center of the National Institutes of Health (NIH). Both groups were recruited primarily through notices on the internet and NIH home page. Study protocols were approved by the National Institute of Child Health and Human Development (NICHD) institutional review board, and all participants gave written informed consent.
Subjects with TS had their diagnoses confirmed by a 50-cell peripheral white blood cell karyotype demonstrating loss of all or part of the 2nd sex chromosome in > 70% of the cells. Women with POI had a history of at least four months of non-iatrogenic oligo/amenorrhea occurring before age 40, with at least two follicle stimulating hormone (FSH) levels in the menopausal range. Only individuals with a normal 46,XX 50-cell peripheral karyotype were included in the POI group.
2.2 Medical history
We used questionnaires, personal interviews and review of available medical records to gathered detailed medical histories. Diagnoses of autoimmune/inflammatory diseases/conditions were recorded only if they were documented in previous medical records or the study participant was being treated for a particular disease/condition at the time of admission into the study, and this disease/condition corresponded to the listing of autoimmune and autoimmune related diseases published by the American Autoimmune Related Diseases Association: http://www.aarda.org/research_display.php?ID=47. For purposes of this study, participants with spontaneous hypothyroidism were given the diagnosis of Hashimoto’s thyroiditis. Estimation of the overall burden of autoimmune disorders and comparison to the US population was based on the following 30 relatively common autoimmune diseases, including 22 found in our study populations: Hashimoto’s thyroiditis; Graves’ disease, Celiac disease, Ulcerative colitis, Crohn’s disease, Pernicious anemia, Primary biliary cirrhosis/Autoimmune hepatitis, Type-1 Diabetes, Vitiligo, Myasthenia gravis, Alopecia totalis, Addison’s disease, Autoimmune Oophoritis, Idiopathic thrombocytopenic purpura, Autoimmune hemolytic anemia, Psoriasis, Iridocyclitis, Pemphigoid, Multiple sclerosis, Glomerulonephritis, Lupus, Rheumatoid arthritis, Ankylosing spondylitis, Dermatomyositis, Behcet syndrome, Rheumatic fever, Primary Sjogren Syndrome, Scleroderma/Systemic sclerosis, Systemic vasculitides.
2.3 Genotyping
Karyotype was determined by G-banding on 50 peripheral white blood cells for all study participants. FISH using X and Y specific alpha satellite DNA probes was employed to characterize marker and ring chromosomes in women with TS.
The major karyotypes for the TS study population were: 45,X =108 (48.2%); 46,Xi(X)(q) or mosaic for 45,X/46,Xi(X)(q) = 52 (23.3%); 46,Xdel(Xp) or 46,Xdel(Xp)/45,X = 11 (4.9%); 46,XdelXq or 46,Xdel(Xq)/45,X= 11 (4.9%); mosaics for 45,X/46,XX or 45,X/47,XXX or 45,X/46,XY = 19 (8.5%) with the 45,X cell line representing >70% of the 50-metaphase score in all cases. The remaining 23 (10.3%) were mosaics for 45,X and a 2nd abnormal cell line including a structurally abnormal X or Y chromosome (ring, isodicentric, pseudo-isodicentric, recombinant). The parental origin of the single normal X-chromosome was determined as previously reported18. All women with POI had normal 46, XX karyotypes.
2.4 Cytokine measurements
Serum for cytokine measurements was collected from TS and POI study participants and healthy female volunteers as described previously19. Stored samples from women aged 20–40 yrs with body mass index 18–30 kg/m2 were selected for use in this study. Transforming growth factor beta-1 and beta-2 (TGF β1, TGF β2), and interleukin 6 and 10 (IL6 & IL10) concentrations were measured with Enzyme Linked Immunosorbent Assays (Quantikine® Immunoassay kits manufactured by R&D systems; catalog numbers DB100B, DB250, D6050, D1000B) according to manufacturer protocol. Samples were activated before TGFβ assay by acidification using 1 M HCl. All measurements were performed in duplicate. The intra-assay coefficients of variation were 5.6% for TGF β1, 5.2% for TGF β2, 4.2% for IL6, and 5.0% for IL10.
2.5 Statistics
Continuous data are expressed as means ± standard deviation. Nominal variables are expressed as number, percent or per 1000 as needed. Continuous variables were compared by ANCOVA with t-test. Generalized linear model with binominal distribution was used to analyze associations and to compare nominal variables. P-value of <0.05 was the cutoff for rejecting the nil hypothesis with Bonferroni correction used for multiple comparisons. Our study sample of n=224 (TS) and n=457 (POI) provides ≥80% power to detect the following magnitudes of increased risk compared to the general population (allowing ≤0.05 probability of type 1 error): for disease with 5% prevalence in the general population, our minimal detectable increase is 1.7 fold for TS and 1.5-fold for POI; for disease with 1% prevalence – 2.7 fold for TS and 2.2 fold for POI, and for 0.5% prevalence – 3.6 fold for TS and 2.6 fold increase for POI. JMPR 8.0 (SAS Institute Inc., Cary, NC) statistical software was used.
3. Results
3.1 Study groups
There were 224 TS participants with average age of 35.7 (18–67 yrs) and 451 POI participants, with average age of 31.9 (18–42 yrs). The age difference reflects the inclusion criteria for age for the respective study: ≥18 years for TS, and 18–42 years for POI. The racial composition was similar in the two groups, with 80–90% self-identified as White, and the remainder equally divided between Black, Asian and Hispanic.
3.2 Spectrum of Autoimmune Diagnoses in TS and POI
Women with TS had over 2-fold higher cumulative incidence of autoimmune diseases compared to those with POI: 15.3 versus 7.1 cases /1000 patient-years, p<0.0001. Almost 50% of the TS study group (102/224) carried at least one autoimmune diagnosis, compared to 20% of the POI group (91/457; p<0.0001). Fourteen women with TS (6.3%) compared to 11 women with POI (2.4%) had more than one autoimmune diagnosis at the time of study, p=0.022.
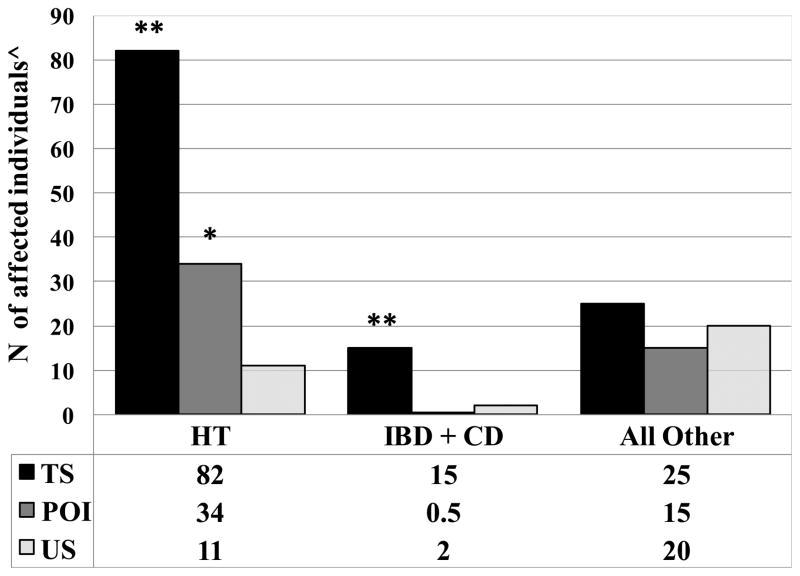
Hashimoto’s thyroiditis (HT) was the leading autoimmune diagnosis encountered in our study, affecting 37% of women with TS and 15% of women with POI (P<0.0001; Table 1). This is compared to the 5% prevalence in the general US female population (relative risk in TS =7.2, 95% CI: 5.8–8.7, and in POI 3.0, 95% CI: 2.3–3.7). Since HT typically increases in prevalence with age, we also compared the rate in age-stratified TS and POI groups. HT was present in 24/76 or 31.6% of TS and 17/137 or 12.4% of the POI groups aged 18–29 (P=0.001), and in 38/99 or 38.4% of the TS group and 52/32 or 16.2% of the POI group aged 30–45 (P<0.0001).
Table 1.
Prevalence of AID among women with TS compared to women with POI and US female population
| Diagnosis | TS (n=224) | POI (n=457) | P (TS v POI) | US female population* | RR in TS (95%CI) |
|---|---|---|---|---|---|
| Prevalence % (N) | Prevalence % | ||||
| Hashimoto’s thyroiditis | 36.6 %(82) | 15.1% (69) | < 0.0001 | 5.8%25 | 7.2 (5.85–8.7) |
| IBD^ | 4.0% (9) | 0.2%(1) | <0.0001 | 0.46%38 | 17.2 (8.5–33.2) |
| Celiac Disease | 2.7% (6) | 0 | 0.002 | 0.063%39† | 42.5 (12.4–144.8) |
| Graves disease | 2.7% (6) | 0.9% (4) | 0.354 | 1.9%9 | 1.4 (0.57–3.22) |
| Type-1 DM | 0.9% (2) | 0.7% (3) | 0.552 | 0.3840 | 2.37 (0.40–9.9) |
| Psoriasis | 3.1% (7) | 1.3%(6) | 0.114 | 3.15%41 | 0.99 (0.3–3.1) |
| Rheumatoid arthritis | 0.9% (2) | 1.5% (7) | 0.642 | 1.06%42 | 0.84 (0.14–3.40) |
| Lupus | 0.9% (2) | 0.2% (1) | 0.34%43 | 2.6 (0.44–11.0) | |
| Vitiligo | 0.4% (1) | 0.4% (2) | 0.4%9 | ||
| Ankylosing spondylitis | 0.4% (1) | 0 | 0.07%42 | ||
| Iridocyclitis | 0.4% (1) | 0 | 0.06%44 | ||
| Alopecia totalis | 0.4% (1) | 0 | 0.024%45 | ||
| Myasthenia gravis | 0.4% (1) | 0 | 0.0074%46 | ||
| Sarcoidosis | 0.4% (1) | 0 | 0.005%47 | ||
| Rheumatic fever | 0 | 0.4% (2) | <0.3%48 | ||
| Addison’s disease | 0 | 0.2% (1) | 0.026%46 | ||
| Pemphigoid | 0 | 0.2% (1) | 0.008%49 | ||
| Dermatomyositis | 0 | 0.2% (1) | 0.0068%9 | ||
| Behcet syndrome | 0 | 0.2% (1) | 0.0064%50 | ||
| AI Oophoritis | 0 | 0.2% (1) | No data | ||
The prevalence data for the U.S. adult female population, obtained from the sources cited in the Table “column 5” includes all women age ≥20 and thus many older women relative to the TS and POI groups.
IBD = Inflammatory Bowel Disease: Crohn’s disease n=5 women with TS and 1 wit POI and Ulcerative colitis, n=4 women with TS).
Diagnosis of celiac disease was based on clinical grounds.
Statistical comparison of prevalence of a specific AID between TS and POI groups was performed only if there were ≥ 5 cases by using a generalized linear model with correction for age; the Bonferroni correction for multiple comparisons specifies P<0.007 as significant. The risk of a specific autoimmune disease relative to the US adult female population (RR) was calculated only for TS group, and only for diagnoses with ≥2 cases in the TS group.
Interestingly, immunity directed at the thyroid stimulating hormone receptor (Graves’ disease) was not significantly increased in TS or POI groups (Table 1). Given our study sample sizes, we could detect a ≥2.1-fold increase in RR for Graves disease in TS and a 1.8-fold increase in POI.
Inflammatory bowel disease (IBD) and celiac disease were both increased in TS but not POI (IBD 4.0% TS vs 0.2% POI, p<0.0001; celiac disease 2.7% TS vs 0% POI, p=0.002; prevalence in the US female population is 0.46% and 0.063% respectively; Table 1; Fig. 1).
Fig. 1. High prevalence of Hashimoto’s thyroiditis and inflammatory bowel disease/celiac disease in Turner Syndrome.
TS - Turner Syndrome; POI – Primary Ovarian Insufficiency; US – United States adult population of women; IBD – Inflammatory Bowel Disease; CD-Celiac Disease.
^For TS the numbers of diagnoses are actual count; For POI and US female population the numbers represent expected diagnoses for a sample equal to TS group, i.e. 224. For the POI group the counts are extrapolated from the actual diagnoses in 457 individual. For the general US population of women the numbers were derived from published epidemiological data, based on 30 relatively common autoimmune diseases, including 22 found in our study populations (see text) and adjusted for a sample of 224 patients. **P <0.0001 for comparisons TS v POI and TS v US population; *P <0.0001 for comparison of POI v US population. ( 2 test with Yates correction)
Type 1 diabetes was not significantly increased in our TS or POI groups compared to the US population of women (Table 1). The estimated RR was 2.4 among women with TS (p=0.5) and 1.7 in POI (p=0.59), with statistical power of our sample size to detect RR > 4.7 in TS and RR >2.9 in POI. There were no cases of liver autoimmune disease, i.e. chronic active hepatitis or biliary cirrhosis in our 681 patients. Among subjects with TS, there was no statistical association between the diagnoses of HT and IBD or CD (data not shown). While HT and pooled IBD/CD are clearly increased in the TS population, the combined all other autoimmune diagnoses are not significantly increased in TS or POI compared to the U.S. population of women (Fig 1).
3.3 Estrogen exposure, pregnancy and risk for AID
The excess of certain AID, including HT and IBD/CD, in women compared to men has led to the consideration of a role for estrogens and/or pregnancy as risk factors for these diseases. However, both TS and POI groups have less estrogen exposure and a very low rate of pregnancy compared to the general population of women. To quantify the relative degree of estrogen exposure in our study groups, we used the estrogen exposure index (EExp %), which measures the percent of time between the usual age of menarche and menopause that each subject had estrogen exposure (either endogenous production or pharmacologic estrogen treatment). As shown in Table 2, the average EExp% was slightly higher in TS and POI individuals with a diagnosis of HT, but this increase was not statistically significant. Estrogen exposure appears to have no association with diagnoses of IBD/CD in TS (Table 2). There was only one case of IBD in the POI group. There was no association between history of pregnancy and risk for HT or IBD/CD in either TS or POI groups (Table 2).
Table 2.
Parity, lifetime estrogen exposure and the risk for AID
| AID | TS (224) | POI (457) | ||||
|---|---|---|---|---|---|---|
| Gn (n=8) | G0 (n=216) | EExp % | G+ (169) | G0 (288) | EExp % | |
| HT + | 5 (62.5%) | 77 (35.6%) | 69±33 (n=82) | 24 (14.2%) | 45 (15.6%) | 87±13 (n=69) |
| HT − | 3 (37.5%) | 139 (64.4%) | 64±33 (n=142) | 145 (85.8%) | 243 (84.4%) | 82±22 (n=388) |
| P | X2=2.27; P=0.13 | P=0.28 | X2=0.33; p=0.56 | P=0.07 | ||
| IBD/CD + | 0 | 15 (6.9%) | 64±36 (n=15) | |||
| IBD/CD − | 8 (100%) | 201 (93.1%) | 64±32 (n=209) | |||
| P | X2=1.38; P=0.24 | P=0.86 | ||||
Gn denotes a history of at least one pregnancy; G0 – no history of pregnancy; EExp%-estrogen exposure (calculation is explained in methods).
3.4 Smoking
Smoking has been identified as a risk for thyroid autoimmunity so we reviewed the smoking history in our two groups. The smoking rate was low for both study groups: 15/224 (6.7%) current or former smokers for TS and 65/457 (14.2%) for POI. Smoking was not associated with increased risk for HT (data not shown).
3.5 Cytokine Profiles
We compared serum levels of cytokines relevant for inflammation/immunity (TGF β1, TGF β2, IL6 and IL10) in women with TS (sex-chromosome haploinsufficiency and primary ovarian insufficiency), versus 20 women with POI (normal sex-chromosomes, and primary ovarian insufficiency), versus 20 age matched NV women (normal sex-chromosomes, and normal ovarian function), Fig. 2.
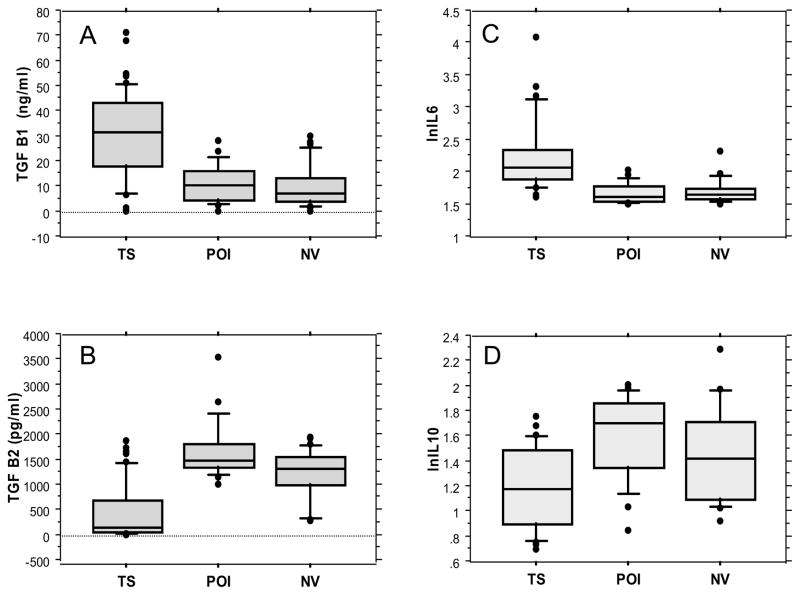
Fig. 2. Serum cytokines in women with TS, POI and women with normal ovarian function.
The box plot graphs show interquartile range and median within the box and 10–90 centile range within the whiskers. There were 20 women in the POI and NV assays, and 49 in the TS group. Each group included individuals between ages 20–40 yrs and BMI 18–30 kg/m2. This data is further compared in Table 3. TS - Turner syndrome; POI - primary ovarian insufficiency; NV- women with normal ovarian function
Serum levels of pro-inflammatory cytokines IL6 and TGF β1 were approximately 2-fold higher in TS compared to POI and NV women, while serum levels of anti-inflammatory cytokines TGF β2 and IL10 were significantly decreased (Fig. 2 and Table 3). Sub analysis did not show association of current cytokine levels with presence or absence of autoimmune diseases.
Table 3.
Disruption of cytokine balance in women with TS
| Control (40) | TS (49) | P | |
|---|---|---|---|
| Age (yr) | 34±8 | 31±12 | 0.12 |
| Body mass (kg/m2) | 25.8±6.5 | 28.2±8.9 | 0.17 |
| TGF β1 (ng/ml) | 11.9±1.3 | 31.6±2.5 | <0.0001 |
| TGF β2 (pg/ml) | 1607±71 | 486±80 | <0.0001 |
| IL6 (pg/ml) | 5.6±0.2 | 10.3±0.9 | 0.0004 |
| IL10 (pg/ml) | 4.9±0.3 | 3.5±0.1 | <0.0001 |
Control group included 20 women with POI and 20 female volunteers with normal ovarian function. Cytokine levels were equal for POI and normal volunteer groups (shown in Fig. 2) so here the data were combined as ‘Controls’. Cytokine levels were compared by ANCOVA with age and BMI as covariates.
3.6 Genetic factors and risk of autoimmune disorder in TS
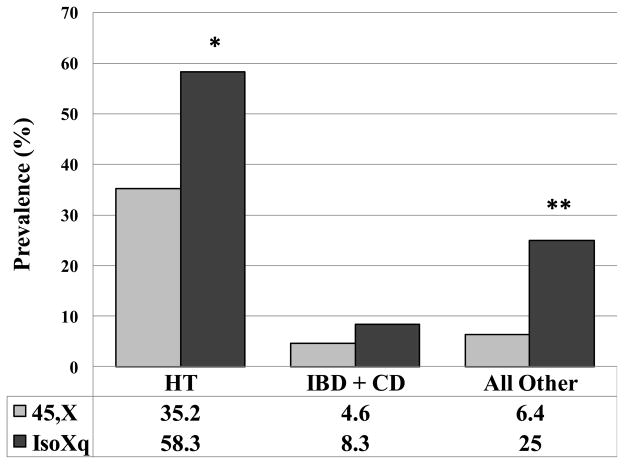
We compared the prevalence of HT, IBD/CD and all other AID in women with Turner syndrome with pure 45,X karyotype to those with karyotype containing an isochromosome Xq (IsoXq). IsoXq results from deletion of the short arm of the X-chromosome (Xp), and anti-parallel fusion of two long arms (Xq). The risk for HT was significantly higher in the IsoXq group (P<0.03). There seems to be a trend for increased risk for IBD/CD in the IsoXq group although statistical significance was not attained due to small number of cases. However, the IsoXq group had significantly more cases of “other” AID (P<0.02; fig 3).
Fig. 3. Isochromosome Xq is associated with higher risk for autoimmunity.
45, X – women with TS and 45,X karyotype in all 50 peripheral white blood cells (n=108); IsoXq – women with karyotype that contains isochromosome Xq in 50–100% of the tested cells, and 45,X in the rest (n=24). IsoXq results from a deletion of the short arm (Xp) and anti-parallel fusion of duplicated long arm Xq. Data represents prevalence (%). The comparison was made by using generalized linear model with age adjustment. *P=0.032; **P=0.018
The parental origin of the single normal X chromosome was conclusively determined for 156 women with TS; it was maternally derived (XMat) in 73%; and paternally-derived (XPat) in 27%. The prevalence of HT was similar in XMat and XPat groups (P=0.7). There were too few cases in other AID categories to allow XMat versus XPat analyses.
4. Discussion
We found a high prevalence of autoimmune diseases in two groups of women with primary ovarian insufficiency. Almost half of women with TS and 20% of women with idiopathic POI were affected by at least one autoimmune disease. Similar to the general US population, Hashimoto’s thyroiditis was by far the dominant autoimmunity in our study groups but with significantly higher prevalence: 37% in TS, 15% in POI and 4.8% in the general population of women. In addition to the high rate of thyroid autoimmunity, women with TS had significantly increased risk of intestinal autoimmunity, i.e. inflammatory bowel and celiac diseases, compared to both women with POI and the US population of women. Consistent with their high rate of autoimmune/inflammatory disorders, women with TS exhibited significant increase in pro-inflammatory cytokines (IL6 and TGF β2) and decreases in anti-inflammatory cytokines (IL10 and TGF β1) compared to karyotypically normal women with or without ovarian insufficiency. An elevated IL6 level has been noted in women with TS previously20.
The clear finding that common autoimmune disorders are most notably increased in study groups with lower estrogen exposure and low pregnancy rates compared to the general population of women suggests that estrogen exposure and pregnancy do not explain the greater risk for autoimmunity in women compared to men. The fact that the highest prevalence of autoimmunity is seen in women with TS that have more extreme estrogen deficiency and lowest pregnancy rate strongly supports the view that factors associated with ovarian failure increase the rate of autoimmunity.
The present study confirms earlier observations indicating high prevalence of thyroid autoimmunity in TS3, 21–23, and in POI24(an early report from the NIH POI study that did not include current participants). Importantly, we were able to show that the increased thyroid autoimmunity in both these groups is specifically Hashimoto’s thyroiditis. Graves’ disease does not appear increased in either group; however, a larger sample size might empower detection of a small increase in TS of this less common type of thyroid autoimmunity.
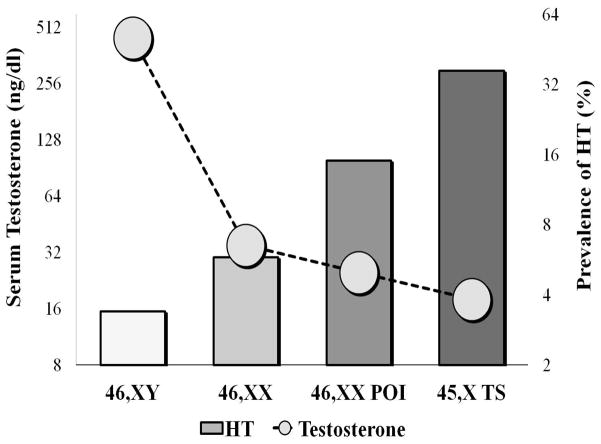
HT is the most common autoimmune disease, affecting 3.4% of men and 5.8% of women in the US 25. It is characterized by chronic lymphocytic infiltration of the thyroid gland and gradual destruction of the follicles resulting in thyroid dysfunction and frank hypothyroidism. Lymphocytic cytokines induce thyreocyte apoptosis involving fas and fas ligand pathways 26. Thyroid peroxidase and thyroglobulin antibodies are often used as diagnostic marker although their pathogenetic role is uncertain. Risk factors for HT include female gender, family history, age, thyroid injuries, and smoking27. Our observation (illustrated on Fig. 4) shows a stepwise increase in the prevalence of HT from men (3.4%) to women (5.8%), to women with idiopathic 46,XX POI (15%) to women with TS (37%) thus leading to the hypothesis that androgen deficit may be involved in the pathogenesis of this autoimmune condition. Androgens, such as testosterone and androstenedione are produced in greatest abundance by normal testes, but are also produced by normally functioning ovaries. Average circulating testosterone levels and predicted lifetime androgen exposure are highest in normal men, followed by women with normal ovarian function, and are significantly reduced in women with 46,XX POI28 and even lower in women with TS29 (Fig. 4). Thus androgen exposure exhibits an inverse correlation with risk for HT. Consistent with a protective role for androgen effects, androgens are shown to suppress autoimmunity in experimental systems, including reduced development of HT in mice7, 30. Thus, higher androgen levels might exert a protective role in men, while low androgen levels seem to be one plausible explanation for the increased risk of HT in women with ovarian insufficiency.
Fig. 4. Inverse relation of prevalence of Hashimoto’s thyroiditis and average serum testosterone.
Bars represent prevalence of Hashimoto’s thyroiditis express as % (right vertical axis). Circles represent average serum testosterone levels as ng/dl (left vertical axis) in men, women with normal ovarian function, women with 46,XX POI and women with TS. The values in both axes are on a logarithmic scale.
Estrogens are also produced by normal ovaries and have been implicated in modulation of some autoimmune diseases. In this study there was no correlation between estrogen exposure and thyroid autoimmunity or other autoimmune conditions. If estrogens have some role in autoimmunity modulation it should be suppressive rather than stimulating based on the fact that women with normal ovarian function and therefore higher lifetime estrogen exposure have much less thyroid and other autoimmunity compared to hypo-estrogenic women with POI and TS.
History of previous pregnancies was not associated with higher risk for HT in our study groups. The latter finding and the fact that thyroid autoimmunity is highly prevalent in two populations with low pregnancy rate do not support the hypothesis implicating fetal microchimerism as a major factor in the pathogenesis of thyroid autoimmunity. A recent population based study from Denmark also suggests that pregnancy has a very small role in the risk for autoimmune disorders in women31.
Women with TS have much higher prevalence of HT, IBD, CD and a variety of other autoimmune conditions than those with POI. Therefore, haploinsufficiency for X-chromosome related gene(s) may play a prominent role in pathogenesis of HT and other autoimmune conditions32. There are at least ten genes located on the X-chromosome that have possible immune regulatory functions33. One is FOXP3 that encodes a transcription factor critical for the function of natural regulatory T-cells (a subset of CD4 lymphocytes) that suppress auto-reactive T-cell in the periphery33. It maps to the short arm of the X-chromosome: Xp11.23 (http://omim.org/entry/300292). FOXP3 deletions cause immunodysregulation, polyendocrinopathy, and entheropathy, X-linked (IPEX). Polymorphisms of the FOXP3 gene are associated with thyroid autoimmunity in Caucasian men and women34, suggesting that FOXP3 haploinsufficiency could explain the increased risk of thyroid autoimmunity in women with TS.
Our study confirms the previously reported association of IsoXq karyotype among women with TS with excess thyroid autoimmunity22 and expands this risk to other AID diseases. The IsoXq is a relatively common rearrangement in which long arm (Xq) duplicates are fused together head to head, with deletion of the short arm. This creates a karyotype that is monosomic for Xp and trisomic for Xq. The full TS phenotype is mainly due to haploinsufficiency for pseudoautosomal genes located on the terminal Xp and is found in individuals with 46,XdelXp35. The mechanism by which IsoXq increases the risk of autoimmune diseases, especially HT, is unknown. Previously we have shown that IsoXq chromosome was associated with higher prevalence of type-2 DM36 which was explained by increased pro-inflammatory gene transcripts. Similar mechanism might be responsible for the higher risk of Hashimoto’s hypothyroidism in women with TS and IsoXq. We hypothesize that a missing Xp, as in 45,X or 46,Xi(Xq) or 46,Xdel(Xp) predisposes to thyroid autoimmunity (lymphocytic thyroiditis) possibly through FOXP3 haploinsufficiency, while having a triple dose of the Xq (as in IsoXq), in addition to missing Xp increases pro-inflammatory and apoptotic responses thus leading to higher prevalence of clinically evident target organ failure, i.e. hypothyroidism. We found no association of parental origin of the single normal X-chromosome and risk for HT. Also, the high prevalence of HT (35.2%) among individuals with a single X-chromosome (45,X) shown in this study is at odds with the hypothesis that skewed X-chromosome inactivation is a major mechanism for HT in women37.
To our knowledge this is the first prospective study to describe a spectrum of autoimmune diseases in a large groups of genetically well characterized, community dwelling (i.e., not clinic or hospital derived) adult women with TS and idiopathic POI. We were able to show that ovarian insufficiency is a risk factor for thyroid autoimmunity and thus women with TS and other forms of primary ovarian insufficiency should be screened for HT and hypothyroidism. Furthermore, we showed that X-chromosome gene haploinsufficiency is associated with pro-inflammatory cytokine phenotype and further increase in risk for HT, IBD/CD, and other autoimmune diseases.
Weakness of our study could be a positive ascertainment bias for thyroid diseases due to the characteristics of our study groups. Patients with POI and TS are more likely to have thyroid tests performed as part of their evaluation and follow up compared to their peers. This may lead to increased case finding of subclinical hypothyroidism in those two study groups creating the impression of high prevalence compared to the general population. While this possibility cannot be ruled out completely, it does not explain the magnitude of the increased prevalence of HT, especially taking into account the relatively young age of our study groups. Also, ascertainment bias cannot explain the observed difference between POI and TS. Furthermore, the epidemiological data for the US population is derived from NHANES study where thyroid tests were done for screening purposes. And finally, ascertainment bias cannot explain the increased number of IBD/CD and other autoimmune diagnoses in women with TS because these are not targets for screening. Our study might have a negative selection bias for participants who have autoimmune diseases that cause significant morbidity and thus the individuals affected by them are less likely to enroll in studies focusing on health issues unrelated to the severe condition. And finally, the number of patients with TS in this study is not large enough to allow detection of smaller but clinically relevant increases in the risk of relatively rare AID. This may explain why we did not find statistically significant increase in Graves disease or Type 1 DM, as reported by Scandinavian researchers3.
5. Conclusion
In conclusion, our study demonstrates that ovarian insufficiency and absence of a second normal X chromosome are associated with an increased risk of development of Hashimoto’s thyroiditis and possibly other autoimmune disorders.
we studied autoimmunity in 46,XX and 45,X primary ovarian failure
we studied autoimmunity in context of estrogen, pregnancy and X chromosome number
ovarian failure is associated with risk for thyroid autoimmunity
missing an X-chromosome causes more thyroid and other autoimmunity
estrogen exposure does not increase autoimmunity risk
Acknowledgments
This work was supported by the intramural research program of the NICHD, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bondy CA. Care of girls and women with Turner syndrome: a guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab. 2007 Jan;92(1):10–25. doi: 10.1210/jc.2006-1374. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen J, Wohlert M. Chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Hum Genet. 1991 May;87(1):81–83. doi: 10.1007/BF01213097. [DOI] [PubMed] [Google Scholar]
- 3.Gravholt CH, Juul S, Naeraa RW, Hansen J. Morbidity in Turner syndrome. J Clin Epidemiol. 1998 Feb;51(2):147–158. doi: 10.1016/s0895-4356(97)00237-0. [DOI] [PubMed] [Google Scholar]
- 4.Schoemaker MJ, Swerdlow AJ, Higgins CD, Wright AF, Jacobs PA. Mortality in women with turner syndrome in Great Britain: a national cohort study. J Clin Endocrinol Metab. 2008 Dec;93(12):4735–4742. doi: 10.1210/jc.2008-1049. [DOI] [PubMed] [Google Scholar]
- 5.Jorgensen KT, Rostgaard K, Bache I, et al. Autoimmune diseases in women with Turner’s syndrome. Arthritis Rheum. 2010 Mar;62(3):658–666. doi: 10.1002/art.27270. [DOI] [PubMed] [Google Scholar]
- 6.Bonamico M, Pasquino AM, Mariani P, et al. Prevalence and clinical picture of celiac disease in Turner syndrome. J Clin Endocrinol Metab. 2002 Dec;87(12):5495–5498. doi: 10.1210/jc.2002-020855. [DOI] [PubMed] [Google Scholar]
- 7.McCombe PA, Greer JM, Mackay IR. Sexual dimorphism in autoimmune disease. Curr Mol Med. 2009 Dec;9(9):1058–1079. doi: 10.2174/156652409789839116. [DOI] [PubMed] [Google Scholar]
- 8.Beeson PB. Age and sex associations of 40 autoimmune diseases. Am J Med. 1994 May;96(5):457–462. doi: 10.1016/0002-9343(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997 Sep;84(3):223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 10.Shapira Y, Agmon-Levin N, Shoenfeld Y. Defining and analyzing geoepidemiology and human autoimmunity. J Autoimmun. 2010 May;34(3):J168–177. doi: 10.1016/j.jaut.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Selmi C. The X in sex: how autoimmune diseases revolve around sex chromosomes. Best Pract Res Clin Rheumatol. 2008 Oct;22(5):913–922. doi: 10.1016/j.berh.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Rubtsov AV, Rubtsova K, Kappler JW, Marrack P. Genetic and hormonal factors in female-biased autoimmunity. Autoimmun Rev. 2010 May;9(7):494–498. doi: 10.1016/j.autrev.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pietro I. Future directions in genetic for autoimmune diseases. Journal of Autoimmunity. 2009;33(1):1–2. doi: 10.1016/j.jaut.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Gilmore W, Weiner LP, Correale J. Effect of estradiol on cytokine secretion by proteolipid protein-specific T cell clones isolated from multiple sclerosis patients and normal control subjects. J Immunol. 1997 Jan 1;158(1):446–451. [PubMed] [Google Scholar]
- 15.Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol. 2010 Aug;10(8):594–604. doi: 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- 16.Stewart JJ. Theory and treatment of the X-inactivation chimera in female-prevalent autoimmune disease. Arch Immunol Ther Exp (Warsz) 1999;47(6):355–359. [PubMed] [Google Scholar]
- 17.Chitnis S, Monteiro J, Glass D, et al. The role of X-chromosome inactivation in female predisposition to autoimmunity. Arthritis Res. 2000;2(5):399–406. doi: 10.1186/ar118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van PL, Bakalov VK, Zinn AR, Bondy CA. Maternal X chromosome, visceral adiposity, and lipid profile. JAMA. 2006 Mar 22;295(12):1373–1374. doi: 10.1001/jama.295.12.1373. [DOI] [PubMed] [Google Scholar]
- 19.Corrigan E, Nelson L, Bakalov V, et al. Effects of ovarian failure and X-chromosome deletion on body composition and insulin sensitivity in young women. Menopause. 2006;13(6):911–916. doi: 10.1097/01.gme.0000248702.25259.00. [DOI] [PubMed] [Google Scholar]
- 20.Ostberg JE, Attar MJH, Mohamed-Ali V, Conway GS. Adipokine Dysregulation in Turner Syndrome: Comparison of Circulating Interleukin-6 and Leptin Concentrations with Measures of Adiposity and C-Reactive Protein. Journal of Clinical Endocrinology & Metabolism. 2005 May 1;90(5):2948–2953. doi: 10.1210/jc.2004-1966. [DOI] [PubMed] [Google Scholar]
- 21.Larizza D, Calcaterra V, Martinetti M. Autoimmune stigmata in Turner syndrome: when lacks an X chromosome. J Autoimmun. 2009 Aug;33(1):25–30. doi: 10.1016/j.jaut.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Elsheikh M, Wass JA, Conway GS. Autoimmune thyroid syndrome in women with Turner’s syndrome--the association with karyotype. Clin Endocrinol (Oxf) 2001 Aug;55(2):223–226. doi: 10.1046/j.1365-2265.2001.01296.x. [DOI] [PubMed] [Google Scholar]
- 23.Radetti G, Mazzanti L, Paganini C, et al. Frequency, clinical and laboratory features of thyroiditis in girls with Turner’s syndrome. Acta Pædiatrica. 1995;84(8):909–912. doi: 10.1111/j.1651-2227.1995.tb13791.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim TJ, Anasti JN, Flack MR, Kimzey LM, Defensor RA, Nelson LM. Routine endocrine screening for patients with karyotypically normal spontaneous premature ovarian failure. Obstet Gynecol. 1997 May;89(5 Pt 1):777–779. doi: 10.1016/s0029-7844(97)00077-x. [DOI] [PubMed] [Google Scholar]
- 25.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002 Feb;87(2):489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 26.Brent GADT. Hypothyroidism and Thyroiditis. In: Melmed SPK, Reed Larsen P, Kronenberg HM, editors. Williams Textbook of Endocrinology. 12. Elsevier Saunders; 2011. pp. 406–439. [Google Scholar]
- 27.Tomer Y, Huber A. The etiology of autoimmune thyroid disease: A story of genes and environment. Journal of Autoimmunity. 32(3–4):231–239. doi: 10.1016/j.jaut.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalantaridou SN, Calis KA, Vanderhoof VH, et al. Testosterone deficiency in young women with 46,XX spontaneous premature ovarian failure. Fertil Steril. 2006 Nov;86(5):1475–1482. doi: 10.1016/j.fertnstert.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 29.Gravholt CH, Svenstrup B, Bennett P, Sandahl Christiansen J. Reduced androgen levels in adult turner syndrome: influence of female sex steroids and growth hormone status. Clin Endocrinol (Oxf) 1999 Jun;50(6):791–800. doi: 10.1046/j.1365-2265.1999.00720.x. [DOI] [PubMed] [Google Scholar]
- 30.Quintero OL, Amador-Patarroyo MJ, Montoya-Ortiz G, Rojas-Villarraga A, Anaya J-M. Autoimmune disease and gender: Plausible mechanisms for the female predominance of autoimmunity. Journal of Autoimmunity. (0) doi: 10.1016/j.jaut.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Jørgensen KT, Pedersen BV, Nielsen NM, Jacobsen S, Frisch M. Childbirths and risk of female predominant and other autoimmune diseases in a population-based Danish cohort. Journal of Autoimmunity. (0) doi: 10.1016/j.jaut.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Invernizzi P, Miozzo M, Selmi C, et al. X chromosome monosomy: a common mechanism for autoimmune diseases. The journal of immunology. 2005;175(1):575–578. doi: 10.4049/jimmunol.175.1.575. [DOI] [PubMed] [Google Scholar]
- 33.Pessach IM, Notarangelo LD. X-linked primary immunodeficiencies as a bridge to better understanding X-chromosome related autoimmunity. J Autoimmun. 2009 Aug;33(1):17–24. doi: 10.1016/j.jaut.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Ban Y, Tozaki T, Tobe T, et al. The regulatory T cell gene FOXP3 and genetic susceptibility to thyroid autoimmunity: An association analysis in Caucasian and Japanese cohorts. Journal of Autoimmunity. 2007;28(4):201–207. doi: 10.1016/j.jaut.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Bondy C, Cheng C. Monosomy for the X chromosome. Chromosome research. 2009;17(5):649–658. doi: 10.1007/s10577-009-9052-z. [DOI] [PubMed] [Google Scholar]
- 36.Bakalov VK, Cheng C, Zhou J, Bondy CA. X-chromosome gene dosage and the risk of diabetes in Turner syndrome. J Clin Endocrinol Metab. 2009 Sep;94(9):3289–3296. doi: 10.1210/jc.2009-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Invernizzi P, Pasini S, Selmi C, Miozzo M, Podda M. Skewing of X chromosome inactivation in autoimmunity. Autoimmunity. 2008;41(4):272–277. doi: 10.1080/08916930802024574. [DOI] [PubMed] [Google Scholar]
- 38.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007 Dec;5(12):1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Murray JA, Van Dyke C, Plevak MF, Dierkhising RA, Zinsmeister AR, Melton LJ., 3rd Trends in the identification and clinical features of celiac disease in a North American community, 1950–2001. Clin Gastroenterol Hepatol. 2003 Jan;1(1):19–27. doi: 10.1053/jcgh.2003.50004. [DOI] [PubMed] [Google Scholar]
- 40.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care. 2010 Mar;33(3):562–568. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003–2004. J Am Acad Dermatol. 2009 Feb;60(2):218–224. doi: 10.1016/j.jaad.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008 Jan;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 43.Ward MM. Prevalence of physician-diagnosed systemic lupus erythematosus in the United States: results from the third national health and nutrition examination survey. J Womens Health (Larchmt) 2004 Jul-Aug;13(6):713–718. doi: 10.1089/jwh.2004.13.713. [DOI] [PubMed] [Google Scholar]
- 44.Suhler EB, Lloyd MJ, Choi D, Rosenbaum JT, Austin DF. Incidence and prevalence of uveitis in Veterans Affairs Medical Centers of the Pacific Northwest. Am J Ophthalmol. 2008 Dec;146(6):890–896. e898. doi: 10.1016/j.ajo.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 45.Cooper GS, Bynum ML, Somers EC. Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun. 2009 Nov-Dec;33(3–4):197–207. doi: 10.1016/j.jaut.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003 May;2(3):119–125. doi: 10.1016/s1568-9972(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 47.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999 Aug;160(2):736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 48.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005 Nov;5(11):685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 49.Yeh SW, Ahmed B, Sami N, Razzaque Ahmed A. Blistering disorders: diagnosis and treatment. Dermatol Ther. 2003;16(3):214–223. doi: 10.1046/j.1529-8019.2003.01631.x. [DOI] [PubMed] [Google Scholar]
- 50.Calamia KT, Wilson FC, Icen M, Crowson CS, Gabriel SE, Kremers HM. Epidemiology and clinical characteristics of Behcet’s disease in the US: a population-based study. Arthritis Rheum. 2009 May 15;61(5):600–604. doi: 10.1002/art.24423. [DOI] [PMC free article] [PubMed] [Google Scholar]