Abstract
β adrenergic receptors are a class of G protein-coupled receptors that have essential roles in regulating heart rate, blood pressure, and other cardiorespiratory functions. Although the role of β adrenergic receptors in the peripheral nervous system is well characterized, very little is known about their role in the central nervous system despite being localized in many brain regions involved in autonomic activity and regulation. Since parasympathetic activity to the heart is dominated by cardiac vagal neurons (CVNs) originating in the Nucleus Ambiguus (NA), β adrenergic receptors localized in the NA represent a potential target for modulating cardiac vagal activity and heart rate. This study tests the hypothesis that activation of β adrenergic receptors alters the membrane properties and synaptic neurotransmission to CVNs. CVNs were identified in brainstem slices and membrane properties and synaptic events were recorded using the whole-cell-voltage-clamp technique. The non-selective β agonist isoproterenol significantly decreased inhibitory GABAergic and glycinergic, as well as excitatory glutamatergic neurotransmission to cardiac vagal neurons. In addition, the β1 selective receptor agonist dobutamine, but not β2 or β3 receptor agonists, significantly decreased inhibitory GABAergic and glycinergic and excitatory glutamatergic neurotransmission to CVNs. These decreases in neurotransmission to CVNs persisted in the presence of tetrodotoxin (TTX). These results provide a mechanism by which activation of adrenergic receptors in the brainstem can alter parasympathetic activity to the heart. Likely physiological roles for this adrenergic receptor activation are coordination of parasympathetic-sympathetic activity and β receptor mediated increases in heart rate upon arousal.
1. Introduction
Adrenergic receptors (AR) are a diverse class of G protein-coupled receptors involved in the regulation of heart rate, blood pressure as well as metabolic function (Zheng et al., 2004; Zheng et al., 2005). Three subclasses of βARs have been identified, each having distinct and often opposing effects dependent upon the targeted tissue and receptor activated. For example, activation of β1 receptors in the heart by endogenous catecholamine release from postganglionic sympathetic neurons leads to increased heart rate, contractility and cardiac output making these receptors an excellent clinical target for treating arrhythmia, tachycardia and hypertension (Esler, 2000). Activation of β2 receptors causes dilation of blood vessels and relaxation of the respiratory tract, advantageous for increasing blood flow to exercising muscle and enhancing ventilation (Nials et al., 1993). Although the β3 subclass has traditionally been considered to regulate metabolic functions such as lipolysis, recent work has suggested β3 receptor activation in the heart also causes a decrease in contractility by altering Na+-K+ pumps (Bundgaard et al., 2010).
Despite the powerful and endogenous activation of βARs in the peripheral nervous system and the frequent use of βAR antagonists used to treat cardiovascular diseases such as cardiac arrhythmias and hypertension, very little is known about the role of βARs within the central nervous system, and, in particular, the modulation of parasympathetic activity which dominates the control of heart rate in the adult. Parasympathetic activity originates from cardiac vagal neurons (CVNs) located in the Nucleus Ambiguus (NA) (Mendelowitz and Kunze, 1991; Mendelowitz, 1999). Although the activity of αARs have been well characterized in CVNs, the binding of catecholamines to either α or β ARs can elicit very different physiological responses (Philbin et al., 2010; Boychuk et al., 2011). Additionally, previous studies have identified βARs both in close proximity to, and in populations of neurons known to project to CVNs, therefore making βARs a potential brainstem target likely to alter neurotransmission to CVNs. For example, immunohistochemical studies have identified dense β1AR localization in the NA, nucleus of the solitary tract, ventrolateral medulla, as well as other brainstem structures involved in cardiorespiratory function (Paschalis et al., 2009). Additionally, autoradiographical and immunohistochemical techniques indicate the presence of β2 in close proximity to the NA (Ampatzis and Dermon, 2010). Furthermore, specific hybridization studies of brain mRNA indicate the presence of β3 receptors in the brainstem, although at much lower levels than in areas such as the hippocampus and cortex (Summers et al., 1995). The aim of this study was to test whether βARs are endogenously active and can modulate three essential inputs to CVNs: the inhibitory GABAergic and glycinergic and excitatory glutamatergic neurotransmission to CVNs in the NA, as well as evoke direct postsynaptic changes in CVNs.
2. Methods
Experimental Procedures
All animal procedures were performed in compliance with the institutional guidelines at The George Washington University and were in accordance with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association and the NIH publication (85–23, revised 1996) ‘Guide for the Care and Use of Laboratory Animals’. The minimal number of animals was used and attention was given to minimize any possible discomfort.
In an initial surgery, 2–5 day old Sprague-Dawley rats were anesthetized with hypothermia to slow the heart and aid in recovery. A right thoracotomy was performed to expose the heart and the retrograde tracer, rhodamine (XRITC, Molecular Probes, 2% solution, 20–50μL), was then injected into the pericardial sac to retrogradely label CVNs.
On the day of the experiment, one to three days after the injection of the fluorescent tracer, the animal was anesthetized with isoflurane and sacrificed by cervical dislocation. The brain was rapidly removed and immersed in a cold HEPES buffer (4°C) with the following composition: NaCl (140 mM), KCl (5 mM), CaCl2 (2 mM), glucose (5 mM), HEPES (10 mM). The buffer was continuously oxygenated with 100% O2. Using a dissection microscope, the hindbrain was isolated. The brain was glued to a stage and placed in the slicing chamber of a vibratome filled with the above buffer solution. Slices 500–600 μm in thickness were cut. The slices were then mounted in a perfusion chamber and submerged in a perfusate with the following composition: NaCl (125 mM), KCl (3 mM), CaCl2 (2 mM), NaHCO3 (26 mM), glucose (5 mM), HEPES (5 mM) and oxygenated with 95% O2/5% CO2 gas mixture. The osmolarity of all solutions was 285–290 mosM, and the pH was maintained between 7.35 and 7.4.
Electrophysiological Recording
Individual CVNs located in the NA were identified by the presence of the fluorescent tracer rhodamine, and differential interference contrast optics along with infrared illumination and infrared-sensitive video detection cameras were used to gain better spatial resolution. The pipettes were filled with a solution consisting of KCl (150 mM), MgCl2 (4 mM), EGTA (10 mM), Na-ATP (2 mM), HEPES (10mM) at a pH of 7.3 for recording inhibitory GABAergic and glycinergic events, and K-gluconic acid (150 mM), HEPES (10 mM), EGTA (10 mM), MgCl2 (1 mM), and CaCl2 (1 mM) at a pH of 7.3 for recording excitatory glutamatergic events. CVNs were studied by whole-cell patch-clamp techniques and were voltage clamped at a holding potential of −80 mV.
GABAergic inhibitory postsynaptic currents (IPSCs) were isolated by adding strychnine (1μM), a glycinergic receptor antagonist, to the perfusate. To isolate glycinergic IPSCs, gabazine (25μM), a GABAA receptor antagonist, was included in the perfusate. Glutamatergic excitatory postsynaptic currents (EPSCs) were isolated by adding gabazine (25μM) and strychnine (1μM) to the perfusate.
The following pharmacological agents were applied by inclusion in the perfusate after a 5–10 min control period: the non-selective β agonist isoproterenol (100 μM), the β1 selective agonist dobutamine (10 μM), the β2 selective agonist albuterol (10 μM), and the β3 selective agonist BRL 37344 (1 μM). Each agent was applied exclusively to a slice and no slice was used for more than one experiment. In another set of experiments, atenolol (100 μM), a β1 selective antagonist, was applied in the perfusate for 5 mins prior to and during application of dobutamine (10 μM) for 5 min while isolating for inhibitory or excitatory synaptic events. In a different set of experiments, increasing concentrations of dobutamine were applied in the perfusate for 5 min intervals after isolating for GABAergic or glycinergic inhibitory events or excitatory glutamatergic events. The concentrations of dobutamine applied in order were 0.01 μM, 0.1 μM, 1 μM, 10 μM, and 100 μM with each concentration being applied immediately after 5 min application of the previous concentration. In another set of experiments, TTX, a voltage-gated sodium channel blocker, was applied in the perfusate 5 mins prior to and during application of dobutamine (10 μM) for 5 minutes while isolating for inhibitory and excitatory synaptic events. All drug concentrations were selected based on selective activation of the targeted receptor based on previous work in the literature.
The effect of isoproterenol (100 μM) on membrane resistance was also examined. A voltage-step from −80mV to −85mV was applied to assess changes in the post-synaptic membrane resistance in CVNs.
Isoproterenol was purchased from Tocris Bioscience (Ellisville, MO). TTX was purchased from Ascent Scientific (Princeton, NJ). All other drugs were purchased from Sigma (St. Louis, MO). MiniAnalysis (Synaptosoft version 4.3.1) was used to analyze experimental traces. The threshold for the GABAergic, glycinergic and glutamatergic events was 5 times the root mean square of noise. Membrane resistance was determined using ClampFit (Molecular Devices). The last three minutes of recorded synaptic events prior to drug application were averaged for control values. A two minute transition period was then allowed after application of drugs before averaging the next three minutes of synaptic events for changes during receptor activation. Results are presented as mean ± S.E. and statistically compared with a paired Student’s t-test except for the experiments with atenolol and repeated dobutamine applications in which an ANOVA with repeated measures was performed (for a significant difference of *P<0.05).
3. Results
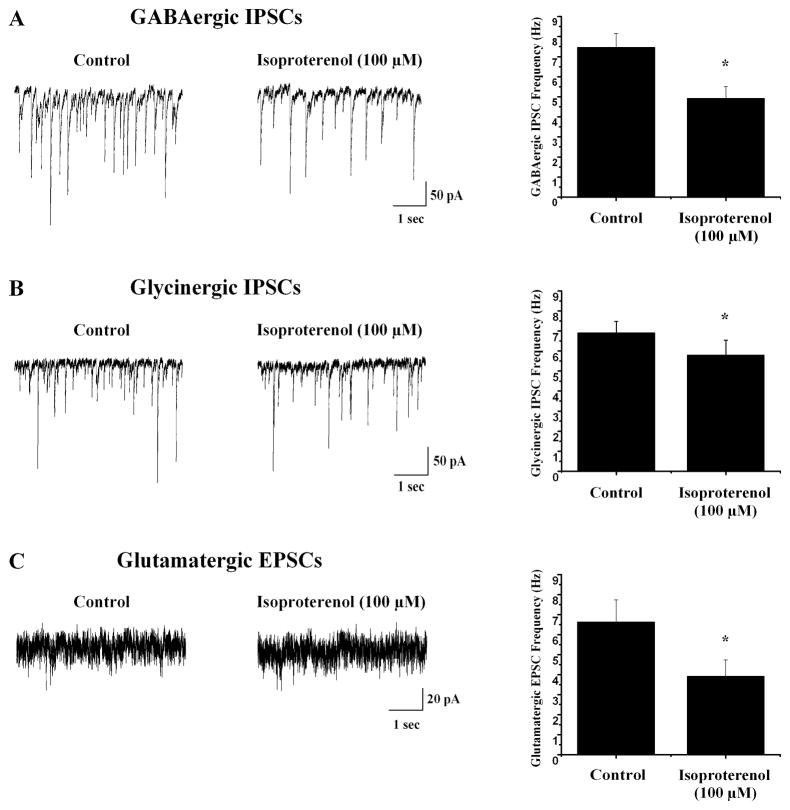
Application of the non-selective β agonist isoproterenol (100 μM) evoked a significant decrease in both inhibitory GABAergic and glycinergic neurotransmission to CVNs as well as excitatory glutamatergic neurotransmission to CVNs, see figure 1. GABAergic inhibitory postsynaptic current (IPSC) frequency significantly decreased from 7.5±0.7 Hz to 4.9±0.6 Hz (n=7, *p<0.05) as did glycinergic IPSC frequency which decreased from 6.9±0.6 Hz to 5.8±0.8 Hz (n=10, *p<0.05) in the presence of isoproterenol. Similarly glutamatergic excitatory postsynaptic current (EPSC) frequency to CVNs decreased from 6.5±1.1 Hz to 3.6±0.8 Hz in the presence of isoproterenol, see figure 1 (n=8, *p<0.05). To test if isoproterenol was affecting membrane resistance in postsynaptic neurons a series of voltage steps were applied. Isoproterenol did not elicit any significant change in resistance in CVNs (control=605±124 MΩ, isoproterenol= 633±283 MΩ; n=6, p>0.05).
Fig. 1.
Application of the non-selective β agonist isoproterenol (100μM) significantly decreased GABAergic and glycinergic IPSC frequency as well as glutamatergic EPSC frequency to CVNs. A Representative traces from a typical experiment isolating for GABAergic events is shown on the left with summary data shown on the right (n=7). B On the left, typical traces are shown from an experiment isolating for glycinergic IPSCs with summary results on the right (n=10). C Raw data from an experiment isolating for glutamatergic EPSCs is shown on the left with summary data on the right (n=8).
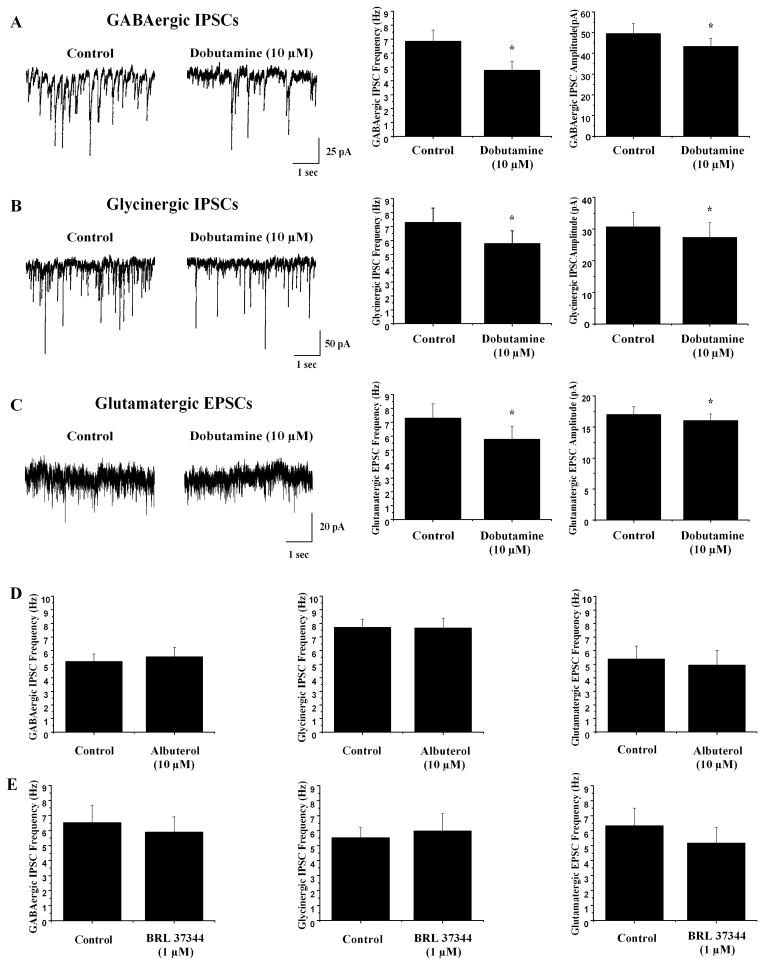
To determine which subtype of βARs was responsible for inhibiting neurotransmission to CVNs, βAR subtype selective agonists were applied in additional experiments. The β1 selective agonist dobutamine (10 μM) elicited a significant decrease in both inhibitory GABAergic and glycinergic neurotransmission to CVNs and excitatory glutamatergic neurotransmission to CVNs, see figure 2. In the presence of dobutamine, GABAergic IPSC frequency decreased from 6.8±0.8 Hz to 4.7±0.6 Hz (n=8, *p<0.05) and glycinergic IPSC frequency decreased from 7.3±1.0 Hz to 5.8±0.9 Hz (n=8, *p<0.05). Glutamatergic EPSC frequency decreased from 6.1±1.1 Hz to 4.3±0.9 Hz (n=7, *p<0.05). In addition, dobutamine significantly decreased the amplitude of GABAergic and glycinergic IPSCs as well as glutamatergic EPSCs. GABAergic IPSCs decreased in amplitude from 49.5±4.9 pA to 43.3±4.0 pA(n=8, *p<0.05) while glycinergic IPSC amplitude decreased from 30.6±4.7 pA to 27.3±4.7 pA (n=8, *p<0.05). Similarly, glutamatergic EPSC amplitude decreased from 17.0±1.3 pA to 16.0±1.1 pA (n=8, *p<0.05).
Fig. 2.
Application of the β1 selective agonist dobutamine (10 μM) significantly inhibited GABAergic and glycinergic IPSC frequency and amplitude in addition to glutamatergic EPSC frequency and amplitude to CVNs while application of the β2 selective agonist albuterol (10 μM) and β3 selective agonist BRL 37344 (1 μM) did not significantly effect neurotransmission to CVNs. A On the left, representative traces from a typical experiment isolating for GABAergic IPSCs with summary data shown on the right (n=7). B Raw data from a typical experiment isolating for glycinergic IPSCs is shown on the left with summary data on the right (n=7). C Representative traces from an experiment isolating for glutamatergic EPSCs are shown on the left with summary data on the right (n=7). D Albuterol had no significant effect on GABAergic or glycinergic IPSC frequency or glutamatergic EPSC frequency to CVNs. E Application of BRL 37344 had no significant effect on inhibitory or excitatory neurotransmission to CVNs.
To test if β2 receptor activation also had an effect in modulating neurotransmission to CVNs, the β2 subtype selective agonist albuterol (10 μM) was applied. Application of albuterol (10 μM) caused no significant change in either GABAergic IPSC frequency (control=5.2±0.6 Hz, albuterol=5.5±0.7 Hz; see Fig. 2, n=7, p>0.05) or glycinergic IPSC frequency (control=7.7±0.6 Hz, albuterol=7.7±0.7 Hz; see Fig. 2, n=11, p>0.05). Similarly, albuterol did not elicit a significant change in glutamatergic EPSC frequency (control=5.4±0.4 Hz, albuterol=4.9±0.4 Hz; see Fig. 2, n=7, p>0.05). Additionally, albuterol had no significant effect on the amplitude of either IPSCs or EPSCs. To determine if β3 receptor activation could modulate neurotransmission to CVNs, the β3 selective agonist BRL 37344 (1 μM) was applied. There was no significant change in GABAergic IPSC frequency, glycinergic IPSC frequency, or glutamatergic EPSC frequency upon application of BRL 37344 (see Fig. 2, p>0.05). While BRL 37344 had no effect on glycinergic or glutamatergic neurotransmission to CVNs, it did significantly decrease the amplitude of GABAergic IPSCs from 54.5±6.8 pA to 47.9±6.3 pA (n=7, *p<0.05).
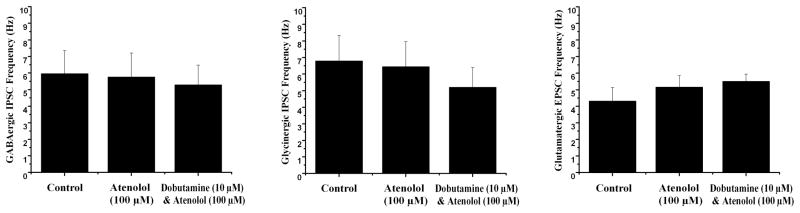
To further test if the inhibition of neurotransmission to CVNs upon application of dobutamine was mediated solely by activation of β1 receptors, the β1 selective antagonist atenolol (100 μM) was applied prior to and during application of dobutamine. Atenolol by itself increased EPSC frequency, see figure 3, but these changes were not significant (p>0.05). Atenolol also did not evoke any significant changes in inhibitory GABAergic or glycinergic IPSCs. Subsequent application of dobutamine in the presence of atenolol did not elicit any change in frequency or amplitude of neurotransmission to CVNs (see Fig. 3, p>0.05).
Fig. 3.
Application of the β1 selective antagonist atenolol (100 μM) prior to and during application of dobutamine prevented the significant decrease in GABAergic IPSCs (n=8) and glycinergic IPSCs (n=8) as well as glutamatergic EPSCs to CVNs (n=7) seen when dobutamine is applied by itself. While application of atenolol increased glutamatergic EPSCs, this change was not significant.
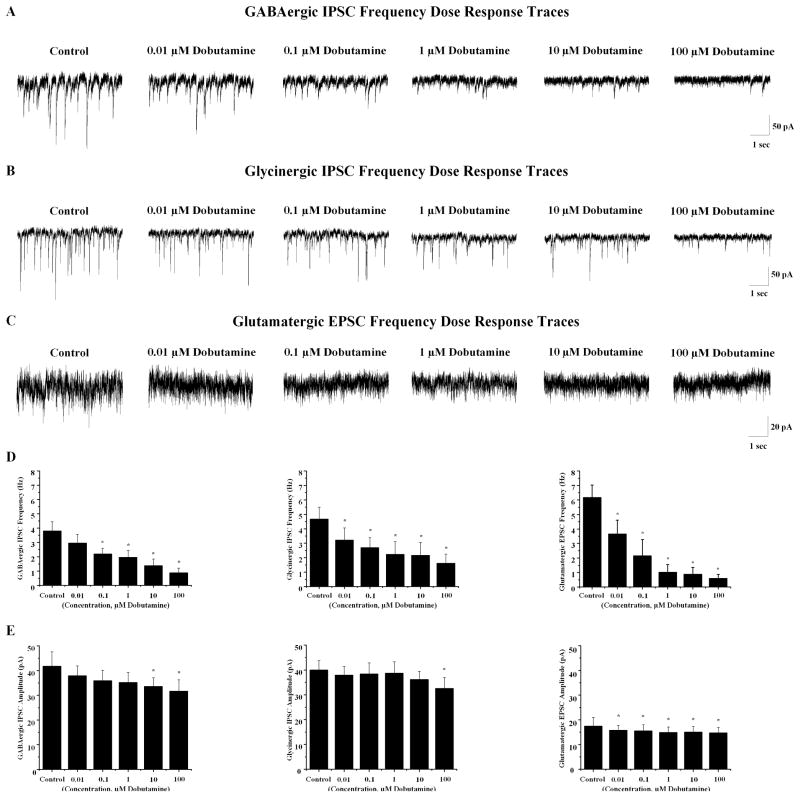
To determine the sensitivity of β1 receptor activation a range of doses of dobutamine were utilized. GABAergic IPSC frequency in CVNs did not significantly decrease in the presence of 0.01 μM dobutamine (control= 3.8±0.65 Hz, 0.01 μM dobutamine=3.0±0.61 Hz, n=7, p>0.05, see Fig. 4). However in the presence of 0.1 μM dobutamine, GABAergic IPSC frequency in CVNs was significantly decreased to 2.2±0.4 Hz (n=7, *p<0.05). GABAergic IPSC frequency continued to decrease significantly as dobutamine concentrations were increased; 1 μM dobutamine elicited a decrease in GABAergic IPSC frequency to 2.0±0.5 Hz (n=7, *p<0.05). Furthermore, 10 μM dobutamine decreased GABAergic IPSC frequency to 1.4±0.5 Hz (n=7, *p<0.05) while 100 μM dobutamine decreased GABAergic IPSC frequency to 0.9±0.3 Hz (n=7, *p<0.05). GABAergic IPSC amplitude did not significantly decrease in the presence of 0.01 μM, 0.1 μM, or 1 μM. However, in the presence of 10 μM and 100 μM dobutamine, GABAergic IPSC amplitude significantly decreased from 41.8±5.8 pA to 33.6±3.5 pA (n=7, *p<0.05) and from 41.8±5.8 pA to 31.6±4.6 pA (n=7, *p<0.05), respecitively. Glycinergic neurotransmission to CVNs was more sensitive to dobutamine as 0.01 μM dobutamine significantly decreased glycinergic IPSC frequency to CVNs from 4.7±0.8 Hz to 3.2±0.9 Hz (n=7, *p<0.05), see Fig. 4. Glycinergic neurotransmission to CVNs further decreased to 2.7±0.7 Hz at a dobutamine concentration of 0.1 μM (n=7, *p<0.05). At 1 μM dobutamine, glycinergic IPSC frequency decreased to 2.2±0.9 Hz (n=7, *p<0.05). Furthermore, 10 μM dobutamine decreased glycinergic IPSC frequency to 2.1±0.9 Hz (n=7, *p<0.05) while 100 μM dobutamine decreased glycinergic IPSC frequency to 1.6±0.6 Hz (n=7, *p<0.05). The amplitude of glycinergic IPSCs was significantly decreased with application 100 μM dobutamine (control: 40.0±3.2 pA, 100 μM dobutamine: 32.6±4.2 pA, n=7, *p<0.05, see Fig. 4) but not with the lower concentrations of dobutamine. Like glycinergic IPSCs, glutamatergic EPSC frequency to CVNs significantly decreased at all concentrations of dobutamine tested. Glutamatergic EPSC frequency significantly decreased in the presence of 0.01 μM dobutamine from 5.7±0.9 Hz to 3.4±0.9 Hz (n=7, *p<0.05), see figure 4. As the concentration of dobutamine was increased, glutamatergic neurotransmission to CVNs was further inhibited; 0.1 μM dobutamine decreased glutamatergic EPSC frequency to 1.9±1.1 Hz (n=7, *p<0.05). 1 μM dobutamine further decreased glutamatergic EPSC frequency to 0.9±0.5 Hz (n=7, *p<0.05) and 10 μM dobutamine decreased glutamatergic EPSC frequency to 0.7±0.4 Hz (n=7, *p<0.05) while 100 μM dobutamine decreased glutamatergic EPSC frequency to 0.5±0.2 Hz (n=7, *p<0.05). All concentrations of dobutamine tested significantly decreased glutamatergic EPSC amplitude (control: 18.1±3.3 pA, .01 μM dobutamine: 16.1±2.0 pA, .1 μM dobutamine: 16.0±2.3 pA, 1 μM dobutamine: 15.3±2.1 pA, 10 μM dobutamine: 15.4±2.1 pA, 100 μM dobutamine: 15.2.±2.1 pA, n=7, *p<0.05, see Fig. 4).
Fig. 4.
Increasing concentrations of the β1 selective agonist dobutamine significantly inhibited GABAergic and glycinergic IPSCs as well as glutamatergic EPSCs to CVNs. A GABAergic IPSC frequency was significantly inhibited from the control frequency starting at 0.1 μM with further inhibition as the dobutamine concentration was increased. The amplitude of GABAergic IPSCs were significantly inhibited at 10 and 100 μM B Glycinergic IPSC frequency was significantly inhibited by 0.01 μM dobutamine with higher concentrations further inhibiting neurotransmission to CVNs. The amplitude of glycinergic IPSCs were significantly decreased at 100 μM. C Glutamatergic EPSC frequency and amplitude to CVNs were significantly inhibited by 0.01 μM dobutamine with increased inhibition as dobutamine concentrations were increased. D Summary data isolating for GABAergic, glycinergic and glutamatergic neurotransmission are shown (n=7) E The summary results for amplitude data is shown for GABAergic, glycinergic, and glutamatergic neurotransmission to CVNs (n=7).
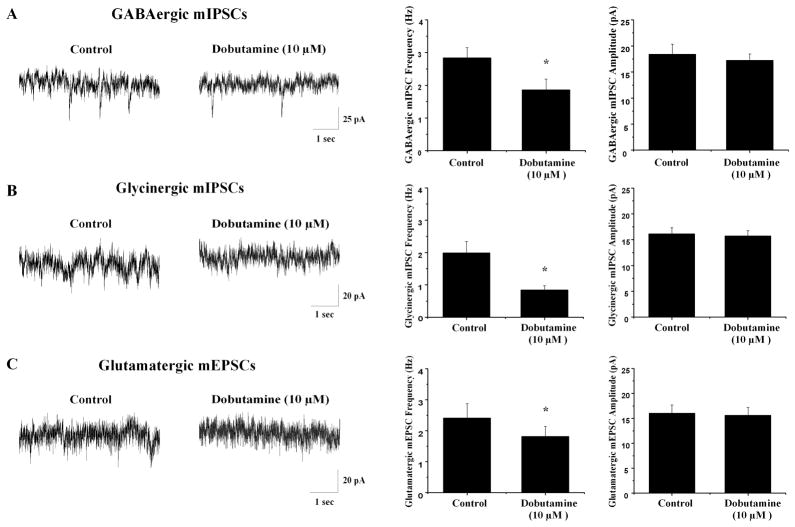
The voltage-gated sodium channel blocker tetrodotoxin (TTX, 1 μM) was included in the perfusate to eliminate action potential dependent events and isolate miniature inhibitory post synaptic currents (mIPSCs) and miniature excitatory post synaptic currents (mEPSCs). In the presence of TTX, dobutamine (10 μM) significantly inhibited GABAergic mIPSCs frequency from 2.4±0.5 Hz to 1.8±0.3 Hz (n=7, *p<0.05) and significantly depressed glycinergic mIPSC frequency from 2.8±0.3 Hz to 1.9±0.3 Hz (n=7, *p<0.05), see figure 5. Similarly, dobutamine elicited a significant decrease in mEPSC frequency from 2.0±0.3 Hz to 0.8±0.1 Hz (n=7, *p<0.05), see figure 5. In the presence of TTX dobutamine did not have any significant effect on mIPSC or mEPSC amplitude.
Fig. 5.
Miniature IPSCs and miniature EPSCs were isolated by inclusion of TTX in the perfusate prior to and during application of dobutamine (10 μM). A Dobutamine significantly inhibited GABAergic mIPSC frequency but not amplitude with representative traces shown on the left and summary data on the right (n=7). B Glycinergic mIPSC frequency but not amplitude was significantly decreased in the presence of dobutamine. Representative traces from a typical experiment are depicted on the left with summary data on the right (n=7). C Dobutamine significantly decreased glutamatergic mEPSC frequency but not amplitude to CVNs. On the left, representative traces from a typical experiment are shown with summary data on the right (n=7).
4. Discussion
There are three major findings from this study. First, the non-selective β agonist isoproterenol evoked a significant decrease in GABAergic, glycinergic and glutamatergic neurotransmission to CVNs. Second, this overall decrease in synaptic activity can be mimicked by the β1-selective receptor agonist dobutamine but not the β2-selective receptor agonist albuterol or β3-selective receptor agonist BRL 37344, indicating the decrease in neurotransmission is a β1 receptor selective response that could be abolished by the β1-selective receptor antagonist atenolol. The third major finding of this study is the decrease in GABAergic, glycinergic and glutamatergic neurotransmission to CVNs by β1 receptor activation persists in the presents of TTX, indicating the site of action of β1 receptor activation is most likely on the presynaptic nerve terminals surrounding CVNs.
In addition to decreasing the frequency of inhibitory and excitatory neurotransmission to CVNs, dobutamine also significantly decreased the amplitude of IPSCs and EPSCs. One possible explanation for this decrease in amplitude is activation of post-synaptic βARs by dobutamine, however this seems unlikely as there was no change in membrane resistance with isoproterenol. A second possibility is that as dobutamine also significantly decreases the frequency of synaptic events, a decrease in amplitude could be the result of a decrease in summation of action potential generated release of transmitters from multiple synaptic terminals. Consistent with this interpretation when action potential transmission is blocked with TTX and the release of transmitters are less frequent and represent spontaneous vesicular release there is no significant change in the amplitude of mIPSCs or mEPSCs upon application of dobutamine.
Prior work has investigated the effects of activation of βAR within the CNS on neurons involved in cardiovascular control. While injection of a βAR agonist into the NA has not been reported, regional perfusion of isoproterenol into the lateral or fourth ventricle of cats results in a significant increase in both heart rate and blood pressure (Bhargava et al., 1978). Furthermore, this increase was blocked by application of either the non-selective β-antagonists propranolol or sotalol. However the site of action that evoked these responses in vivo and the origin of adrenergic neurons or neuronal targets were not identified.
Although βAR typically play a facilatory role in synaptic transmission, βAR mediated inhibition has been previously reported. Recent work has identified β1ARs acting as autoreceptors in rat sympathetic neurons to suppress axonal outgrowth (Clarke et al., 2010). Additionally, the application of norepinephrine (in combination with α1 and β2 antagonists to isolate for β1 receptors) in rat periaqueductal gray neurons resulted in decreased GABAergic IPSCs (Xiao et al., 2008). In addition, β1 antagonists increased GABAergic IPSCs in these neurons further supporting an inhibitory role of β1AR activation on GABAergic IPSCs. It has also been reported that application of isoproterenol to lumbar motoneurons located in the spinal cord of neonatal rats resulted in significant inhibition of glutamatergic mEPSC frequency (Tartas et al., 2010). These results in periaqueductal gray neurons as well as lumbar motorneurons are similar to the inhibition of synaptic inputs to CVNs in this study.
The source of the adrenergic neurons that project to CVNs and activate β1 adrenergic receptors on presynaptic terminals surrounding CVNs are unknown. Based on the literature one likely source of the adrenergic pathway to CVNs are adrenergic neurons located in the rostral ventrolateral medulla (RVLM). Catecholaminergic neurons originating in the RVLM have widespread projections within the brainstem, in particular the NA (Card et al., 2006; Ondicova and Mravec, 2010). This connection represents a potential pathway for mediating sympathetic - parasympathetic coordination, whether it is antagonism or co-activation, depending on the challenges and whether the target is presynaptic inhibitory GABAergic and glycinergic, or excitatory glutamatergic synaptic terminals, respectively. While possible, it seems unlikely adrenergic pathways would simultaneously inhibit both excitatory and inhibitory inputs to CVNs. It is more likely the innervation of excitatory and inhibitory synapses on CVNs are selectively and preferentially activated and different pathways, activated by different challenges or conditions, likely selectively diminish only inhibitory or excitatory synapses on CVNs. As examples, co-activation of both sympathetic activity and parasympathetic activity to the heart occur in response to activation of the diving reflex, as well as chemoreceptor activation (Foster and Sheel, 2005; Alboni et al., 2011). In response to these challenges it might be anticipated that increased activity of C1 neurons in the RVLM would evoke sympathetic activation and adrenergic-mediated dis-inhibition (inhibition of inhibitory GABAergic and glycinergic neurotransmission) to increase the activity of CVNs (Abbott et al., 2009). In response to other challenges, such as changes in blood pressure, opposite changes in sympathetic and parasympathetic activity occur. In response to hypotension, for example, the anticipated increase in activity of C1 neurons in the RVLM would have increased effectiveness to increase heart rate and cardiac output if parasympathetic activity was diminished, which could occur via adrenergic activation of β1 receptors inhibiting glutamatergic neurotransmission to CVNs.
Another adrenergic population of neurons likely to modulate CVN activity includes neurons in the locus coeruleus (LC). The LC is the largest group of adrenergic neurons in the central nervous system and has widespread projections throughout the brain. Specifically, it has been shown to have connections to brainstem areas involved in autonomic function, including the NA and the dorsal motor nucleus of the vagus (Samuels and Szabadi, 2008). Moreover, the LC has been shown to play a central role in behavioral arousal which is accompanied by an increase in heart rate (Kuo et al., 2008; Carter et al., 2010). One possible pathway through which this increase in heart rate could occur is by increased activity of adrenergic LC neurons projecting to the NA and β1 receptor mediated diminished glutamatergic neurotransmission to CVNs. This would provide a mechanism for coordinating arousal with decreased parasympathetic activity and increased heart rate.
While additional work is necessary to identify the source and conditions that activate the adrenergic modulatory inputs to CVNs, this study has provided a foundation for understanding the receptors, mechanisms and sites of action of adrenergic receptor modulation of parasympathetic activity to the heart within the brainstem. This study has demonstrated β1, but not β2 or β3 receptor activation, acting at presynaptic nerve terminals, decreases neurotransmission to CVNs.
Adrenergic modulation of brainstem parasympathetic cardiac neurons
β1 selective agonists decrease neurotransmission via presynaptic sites of action
Arousal and heart rate
Acknowledgments
This work was supported by the National Institutes of Health Grants HL49965, HL59895, HL72006 awarded to DM.
Abreviations
- AR
adrenergic receptor
- CVN
cardiac vagal neuron
- NA
Nucleus Ambiguus
- IPSCs
inhibitory postsynaptic currents
- EPSCs
excitatory postsynaptic currents
- mIPSCs
miniature IPSCs
- mEPSCs
miniature EPSCs
- TTX
tetrodotoxin
- RVLM
rostral ventrolateral medulla
- LC
locus coeruleus
Footnotes
Authorship Contributions
Participated in research design: Bateman, Boychuk, and Mendelowitz
Conducted experiments: Bateman, Boychuk, and Philbin
Performed data analysis: Bateman, Boychuk, Philbin, and Mendelowitz
Wrote or contributed to writing of the manuscript: Bateman, Boychuk, and Mendelowitz
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott SB, Stornetta RL, Socolovsky CS, West GH, Guyenet PG. Photostimulation of channelrhodopsin-2 expressing ventrolateral medullary neurons increases sympathetic nerve activity and blood pressure in rats. J Physiol. 2009;587:5613–5631. doi: 10.1113/jphysiol.2009.177535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alboni P, Alboni M, Gianfranchi L. Diving bradycardia: a mechanism of defence against hypoxic damage. J Cardiovasc Med (Hagerstown) 2011;12:422–427. doi: 10.2459/JCM.0b013e328344bcdc. [DOI] [PubMed] [Google Scholar]
- Ampatzis K, Dermon CR. Regional distribution and cellular localization of beta2-adrenoceptors in the adult zebrafish brain (Danio rerio) J Comp Neurol. 2010;518:1418–1441. doi: 10.1002/cne.22278. [DOI] [PubMed] [Google Scholar]
- Bhargava KP, Jain IP, Saxena AK, Sinha JN, Tangri KK. Central adrenoceptors and cholinoceptors in cardiovascular control. Br J Pharmacol. 1978;63:7–15. doi: 10.1111/j.1476-5381.1978.tb07768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boychuk CR, Bateman RJ, Philbin KE, Mendelowitz D. alpha(1)-adrenergic receptors facilitate inhibitory neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Neuroscience. 2011;193:154–161. doi: 10.1016/j.neuroscience.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundgaard H, Liu CC, Garcia A, Hamilton EJ, Huang Y, Chia KK, Hunyor SN, Figtree GA, Rasmussen HH. beta(3) adrenergic stimulation of the cardiac Na+-K+ pump by reversal of an inhibitory oxidative modification. Circulation. 2010;122:2699–2708. doi: 10.1161/CIRCULATIONAHA.110.964619. [DOI] [PubMed] [Google Scholar]
- Card JP, Sved JC, Craig B, Raizada M, Vazquez J, Sved AF. Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: Implications for the central control of cardiovascular regulation. J Comp Neurol. 2006;499:840–859. doi: 10.1002/cne.21140. [DOI] [PubMed] [Google Scholar]
- Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke GL, Bhattacherjee A, Tague SE, Hasan W, Smith PG. β-adrenoceptor blockers increase cardiac sympathetic innervation by inhibiting autoreceptor suppression of axon growth. J Neurosci. 2010;30:12446–12454. doi: 10.1523/JNEUROSCI.1667-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler M. The sympathetic system and hypertension. American journal of hypertension. 2000;13:99S–105S. doi: 10.1016/s0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- Foster GE, Sheel AW. The human diving response, its function, and its control. Scandinavian journal of medicine & science in sports. 2005;15:3–12. doi: 10.1111/j.1600-0838.2005.00440.x. [DOI] [PubMed] [Google Scholar]
- Kuo TB, Shaw FZ, Lai CJ, Yang CC. Asymmetry in sympathetic and vagal activities during sleep-wake transitions. Sleep. 2008;31:311–320. doi: 10.1093/sleep/31.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelowitz D. Advances in Parasympathetic Control of Heart Rate and Cardiac Function. News Physiol Sci. 1999;14:155–161. doi: 10.1152/physiologyonline.1999.14.4.155. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D, Kunze DL. Identification and dissociation of cardiovascular neurons from the medulla for patch clamp analysis. Neurosci Lett. 1991;132:217–221. doi: 10.1016/0304-3940(91)90305-d. [DOI] [PubMed] [Google Scholar]
- Nials AT, Coleman RA, Johnson M, Magnussen H, Rabe KF, Vardey CJ. Effects of beta-adrenoceptor agonists in human bronchial smooth muscle. Br J Pharmacol. 1993;110:1112–1116. doi: 10.1111/j.1476-5381.1993.tb13929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondicova K, Mravec B. Multilevel interactions between the sympathetic and parasympathetic nervous systems: a minireview. Endocrine regulations. 2010;44:69–75. doi: 10.4149/endo_2010_02_69. [DOI] [PubMed] [Google Scholar]
- Paschalis A, Churchill L, Marina N, Kasymov V, Gourine A, Ackland G. beta1-Adrenoceptor distribution in the rat brain: an immunohistochemical study. Neurosci Lett. 2009;458:84–88. doi: 10.1016/j.neulet.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Philbin KE, Bateman RJ, Mendelowitz D. Clonidine, an alpha2-receptor agonist, diminishes GABAergic neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Brain research. 2010;1347:65–70. doi: 10.1016/j.brainres.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels ER, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organisation. Curr Neuropharmacol. 2008;6:235–253. doi: 10.2174/157015908785777229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers RJ, Papaioannou M, Harris S, Evans BA. Expression of beta 3-adrenoceptor mRNA in rat brain. Br J Pharmacol. 1995;116:2547–2548. doi: 10.1111/j.1476-5381.1995.tb17205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartas M, Morin F, Barriere G, Goillandeau M, Lacaille JC, Cazalets JR, Bertrand SS. Noradrenergic modulation of intrinsic and synaptic properties of lumbar motoneurons in the neonatal rat spinal cord. Frontiers in neural circuits. 2010;4:4. doi: 10.3389/neuro.04.004.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Zhou C, Atlas G, Delphin E, Ye JH. Labetalol facilitates GABAergic transmission to rat periaqueductal gray neurons via antagonizing beta1-adrenergic receptors--a possible mechanism underlying labetalol-induced analgesia. Brain research. 2008;1198:34–43. doi: 10.1016/j.brainres.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Han QD, Xiao RP. Distinct beta-adrenergic receptor subtype signaling in the heart and their pathophysiological relevance. Sheng li xue bao: [Acta physiologica Sinica] 2004;56:1–15. [PubMed] [Google Scholar]
- Zheng M, Zhu W, Han Q, Xiao RP. Emerging concepts and therapeutic implications of beta-adrenergic receptor subtype signaling. Pharmacology & therapeutics. 2005;108:257–268. doi: 10.1016/j.pharmthera.2005.04.006. [DOI] [PubMed] [Google Scholar]