Abstract
Our objectives were to summarize literature on the association of amyotrophic lateral sclerosis (ALS) with pesticides as a group and to evaluate associations of ALS with specific pesticides. We conducted a meta-analysis of published studies of ALS and pesticides as a group and investigated the association of ALS with specific pesticides, using data from the Agricultural Health Study (AHS), a cohort including 84,739 private pesticide applicators and spouses. AHS participants provided information on pesticide use at enrollment in 1993-1997. In mortality data collected through February, 2010, ALS was recorded on death certificates of 41 individuals whom we compared to the remaining cohort (controls), using unconditional logistic regression adjusted for age and gender to calculate odds ratios (ORs) and 95% confidence intervals. In the meta-analysis, ALS was associated with use of pesticides as a group (1.9, 1.1-3.1). In the AHS, ALS was not associated with pesticides as a group, but was associated with use of organochlorine insecticides (OCs) (1.6, 0.8-3.5), pyrethroids (1.4, 0.6-3.4), herbicides (1.6, 0.7-3.7), and fumigants (1.8, 0.8-3.9). ORs were elevated for ever use of the specific OCs aldrin (2.1, 0.8-5.1), dieldrin (2.6, 0.9-7.3), DDT (2.1, 0.9-5.0), and toxaphene (2.0, 0.8-4.9). None of these associations was statistically significant. Similar results were observed in an analysis restricted to men. In conclusion, the meta-analysis suggests that ALS risk is associated with use of pesticides as a group, and our analysis of AHS data points to OC use in particular. The latter results are novel but based on a small number of cases and require replication in other populations.
Keywords: amyotrophic lateral sclerosis, motor neuron disease, cohort study, meta-analysis, pesticides, organochlorine insecticides
1. INTRODUCTION
ALS is a rapidly progressive neurodegenerative disease affecting motor neurons in the brain and spinal cord, with symptoms including muscular weakness, spasticity, and hyperreflexia. The condition is rare, with an annual incidence of 1 to 2 per 100,000; incidence is greater in men and increases with age.
The etiology of ALS is not well understood. Approximately 10% of ALS cases have a family history of ALS, likely involving genetic factors. Environmental factors including metals, organic solvents, and pesticides may also contribute to ALS (Sutedja et al, 2009). Exposure to pesticides as a group has been explicitly evaluated in seven case-control studies (Bonvicini et al, 2010; Chancellor et al, 1993; Deapen and Henderson, 1986; Gunnarsson et al, 1992; McGuire et al 1997; Morahan and Pamphlett, 2006; Savatierri et al, 1991) and one cohort study (Weisskopf et al, 2009b); exposure was generally associated with increased risk in the case-control studies although the relationship was not always statistically significant. None of these studies evaluated the role of specific pesticides in ALS etiology.
To summarize existing findings, we conducted a meta-analysis of published studies of ALS and exposure to pesticides as a group. We then evaluated the association of ALS with specific pesticides, using data from the Agricultural Health Study (AHS), a cohort of licensed pesticide applicators and their spouses. Our study provides the first analytic evaluation of the role of specific pesticides in ALS.
2. MATERIALS AND METHODS
2.1. Exposure to pesticides as a group: Meta-analysis of published studies
We searched for epidemiologic studies of ALS and pesticide exposure in Medline through December 31, 2011, using the MeSH terms “amyotrophic lateral sclerosis,” “motor neuron disease,” and “pesticides.” We also searched the bibliographies of retrieved articles. All identified case-control and cohort studies with information on pesticide use were included in the analysis (Bonvicini et al, 2010; Chancellor et al, 1993; Deapen and Henderson, 1986; Gunnarsson et al, 1992; McGuire et al 1997; Morahan and Pamphlett, 2006; Savatierri et al, 1991; Weisskopf et al, 2009b). We regarded studies as random effects and estimated summary ORs using mixed-model analysis of variance (Normand 1999) unless a preliminary test showed no evidence of study-to-study heterogeneity in relative risk estimates (i.e., p>0.40), whereupon we used fixed-effects analysis of variance.
2.2. Exposure to specific pesticides: Study of the AHS cohort
The cohort was enrolled in 1993 to 1997 in Iowa and North Carolina (Alavanja et al, 1996) and included 52,394 private pesticide applicators (mostly farmers) and 32,345 of their spouses. Most applicators were men (97%), most spouses were women (99%), and most of the cohort was white and not Hispanic (99%). Applicators enrolled by completing a questionnaire at pesticide licensing sites, and spouses enrolled by completing a questionnaire at home. Some applicators (~42%) completed a supplemental questionnaire at home. The questionnaires collected information on lifetime pesticide use as well as demographics, lifestyle, and medical history (http://aghealth.nci.nih.gov/questionnaires.html). Personally applying pesticides was reported by both applicators (99%) and spouses (56%). Mortality data were available for the cohort through February 7, 2010, from state mortality files and the National Death Index. The study was approved by IRBs at all participating institutions, and all cohort members provided informed consent.
We identified 41 individuals with ALS on their death certificates, 37 as the underlying and 4 as a contributing cause of death. Medical records of seven of the 41 were available for review by the study neurologist (RSB) using published criteria (Brooks et al, 2000); five were diagnosed with ALS (one definite, two probable, and two possible) and one with progressive bulbar palsy; one was indeterminate. Based on this validation, and because death certificates are a reliable source of information on ALS (Kamel et al, 2008), we defined cases as individuals with ALS on their death certificates. Remaining cohort members without ALS served as controls.
Cohort members provided information on ever use and on years and days per year of use of any pesticide. We used this information to calculate total lifetime days of use of any pesticide, dichotomized as minimal use (≤25 lifetime days) or greater than minimal use (>25 days). Cohort members also provided information on ever use of 50 specific pesticides which we used to construct variables for ever use of pesticides in functional and chemical classes. We also evaluated 29 specific pesticides used by at least 5 cases. For each exposure variable, we compared individuals who were or were not exposed.
We compared the 41 ALS cases to the remaining 84,698 AHS cohort members without ALS (controls), using unconditional logistic regression to calculate odds ratios (ORs) and 95% confidence intervals (CIs). We adjusted all models for age and gender. Some models were also adjusted for cigarette smoking (ever/never), education (≤high school vs >high school), state (Iowa vs North Carolina), or head injury (ever/never). We conducted an additional analysis excluding two cases who died within two years of enrollment in the AHS, as they may have already had ALS, and another analysis restricted to individuals at least 60 years old, to address the possibility of uncontrolled confounding by age. To adjust for correlated use of organochlorine insecticides (OCs), for each specific OC we created a variable representing ever use of any other OC and ran models including both this variable and the specific OC. All analyses were conducted with SAS 9.2 (Cary, NC) using AHS data releases P1REL20100501, P2REL20100700.02, and P3REL1000_00.
3. RESULTS
Studies included in the meta-analysis (Table 1, Figure 1) evaluated occupational exposure to pesticides as a group, usually with some minimal criterion for ever use, eg, regular use and/or use for some minimal period. Relative risks for pesticide use ranged from 1.1 to 4.7. We saw little evidence of heterogeneity among the seven case-control studies (p>0.8); the summary OR for pesticide use was 2.2 (95% CI, 1.5-3.3). Inclusion of the cohort study, which introduced some heterogeneity (p=0.17), yielded a slightly lower summary OR of 1.9 (1.1-3.1).
Table 1.
ALS and use of pesticides as a group, studies published through December 31, 2011.
| Study | Design | N Cases | N Controls | Control Matching | Exposure | OR/HR | 95% CI | ||
|---|---|---|---|---|---|---|---|---|---|
| Total | Exposed | Total | Exposed | ||||||
| Deapen 1986(2) | case-control | 518 | 16 | 518 | 8 | age, gender | “long-term” occupational exposure up to 3 y prior to dx | 2.0 | 0.8-5.4 |
| Savatierri 1991(3) | case-control | 46 | 5 | 92 | 4 | age, gender, urban/rural residence, SES | “continual exposure to agricultural chemicals” | 3.0 | 0.4-20 |
| Gunnarsson 1992(4) | case-control | 58 men | 3 men | 189 men | not given | population sample, no matching | occupational exposure | 1.1 | 0.2-5.3 |
| Chancellor 1993(5) | case-control | 103 | 28 | 103 | 23 | physician practice, age, gender | “regular” occupational contact over at least 12 months | 1.4 | 0.6-3.1 |
| McGuire 1997(6) | case-control | 94 men | 21 men | 190 men | 21 men | age | industrial hygienist evaluation of lifetime occupational history | 2.4 | 1.2-4.8 |
| Morahan 2006(7) | case-control | 179 | 21 | 179 | 6 | age, gender, ethnicity | regular exposure (weekly for at least 6 months) | 1.6 | 1.0-2.4 |
| Bonvicini 2010(8) | case-control | 41 | 13 | 82 | 11 | age, gender | agricultural or other pesticide-related professional activities for at least 6 months | 3.6 | 1.2-11 |
| Weisskopf 2009(9) | cohort | 1097 | 59 | 12,917,000 person-years | 661,000 person-years | not applicable | current or past regular exposure | 1.1 | 0.8-1.4 |
Figure 1.
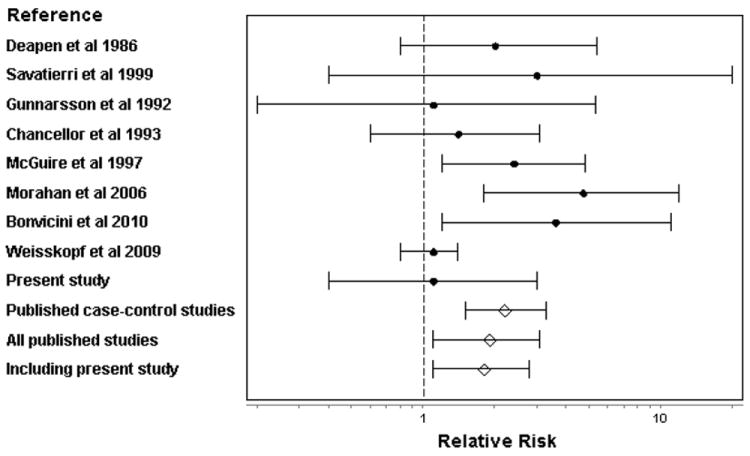
Meta-analysis of ALS risk and occupational use of pesticides as a group. Details of included studies are presented in Table 1. The filled circles represent relative risks from individual studies and the open diamonds represent meta-analytic results; the error bars show 95% confidence intervals in both cases.
In the AHS, gender, state, education and smoking history were similar in ALS cases and controls; as expected, cases were older than controls (Table 2). Race/ethnicity and alcohol use were similar in cases and controls (data not shown). Similar proportions of cases and controls had ever personally used pesticides as a group or had used pesticides as a group more than minimally (>25 lifetime days) (Table 2). Inclusion of the present study in the meta-analysis slightly reduced the ORs for use of pesticides as a group: the summary ORs were 2.0 (1.4-3.1) without and 1.8 (1.1-2.8) with the published cohort study.
Table 2.
Characteristics of ALS cases and controls, AHS cohort 1993-2010
| Characteristic | Level | Case | Control | OR1 | 95% CI | |||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | |||||
| Age Group | ≤ 50 | 11 | 27 | 51881 | 61 | 0.3 | 0.1 | 0.7 |
| 51-60 | 13 | 32 | 18464 | 22 | 1.0 | reference | ||
| 61-70 | 12 | 29 | 11201 | 13 | 1.5 | 0.7 | 3.4 | |
| ≥ 70 | 5 | 12 | 3152 | 4 | 2.3 | 0.8 | 6.5 | |
| Gender | Women | 18 | 44 | 33466 | 40 | 1.0 | reference | |
| Men | 23 | 56 | 51232 | 60 | 0.8 | 0.4 | 1.5 | |
| State | Iowa | 27 | 66 | 53620 | 63 | 1.0 | reference | |
| North Carolina | 14 | 34 | 31078 | 37 | 0.7 | 0.4 | 1.4 | |
| Education | ≤ High school | 21 | 60 | 42181 | 54 | 1.0 | reference | |
| > High school | 14 | 40 | 35871 | 46 | 1.1 | 0.5 | 2.3 | |
| Missing | 6 | 6646 | ||||||
| Smoking (lifetime) | Never | 25 | 63 | 48909 | 60 | 1.0 | reference | |
| Ever | 15 | 38 | 32376 | 40 | 0.8 | 0.4 | 1.6 | |
| Missing | 1 | 3413 | ||||||
| Personally mixed or applied pesticides | No | 7 | 18 | 14296 | 17 | 1.0 | reference | |
| Yes | 33 | 83 | 68772 | 83 | 1.1 | 0.4 | 3.0 | |
| Missing | 1 | 1630 | ||||||
| Cumulative days mix/apply pesticides | ≤ 25 days | 18 | 44 | 33289 | 39 | 1.0 | reference | |
| > 25 days | 23 | 56 | 51409 | 61 | 0.8 | 0.4 | 1.8 | |
All models include age and gender
Cases were more likely than controls to have ever used grouped OCs, pyrethroids, herbicides, or fumigants (Table 3). Among 29 specific pesticides used by at least five cases (Table 4), ORs were ≥1.0 for all OCs while ORs for pesticides in other chemical or functional groups were more variable. In particular, ORs were ≥2.0 for the OCs aldrin, dieldrin, DDT, and toxaphene. None of these associations was statistically significant. Further adjustment for state, education, or cigarette smoking, or exclusion of two cases who died within two years of enrollment did not materially alter associations with OCs as a group, aldrin, dieldrin, DDT or toxaphene. In analyses restricted to individuals at least 60 years old, ORs were elevated for aldrin (1.8), dieldrin (2.6), and toxaphene (3.9), but not for DDT (0.8) or OCs as a group (1.3). In analyses restricted to men, ORs were elevated for OCs as a group (2.0), aldrin (2.4), dieldrin (3.0), DDT (2.4), and toxaphene (2.2); too few female cases had used pesticides to analyze separately.
Table 3.
ALS and ever use of pesticides grouped by function or chemistry, AHS cohort 1993-2010
| Pesticide group1 | Cases | Controls | OR2 | 95% CI | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | ||||
| Insecticides3 | 29 | 73 | 58476 | 70 | 1.3 | 0.6 | 2.9 |
| Organochlorines | 18 | 46 | 26136 | 32 | 1.6 | 0.8 | 3.5 |
| Organophosphates | 22 | 55 | 51713 | 62 | 0.8 | 0.4 | 1.7 |
| Carbamates | 18 | 45 | 41721 | 50 | 0.8 | 0.4 | 1.5 |
| Pyrethroids | 6 | 15 | 12471 | 15 | 1.4 | 0.6 | 3.4 |
| Herbicides | 30 | 75 | 60709 | 73 | 1.6 | 0.7 | 3.7 |
| Fungicides | 9 | 23 | 19544 | 24 | 1.0 | 0.4 | 2.2 |
| Fumigants | 9 | 23 | 12111 | 15 | 1.8 | 0.8 | 3.9 |
Organochlorines: aldrin, chlordane, DDT, dieldrin, heptachlor, lindane, toxaphene; organophosphates: chlorpyrifos, coumaphos, diazinon, dichlorvos, fonofos, malathion, parathion, phorate, terbufos, trichlorfon; carbamates: aldicarb, carbaryl, carbofuran; pyrethroids: permethrin used on crops or animals (asked separately); herbicides: 2,4-D, 2,4,5-T, 2,4,5-TP, alachlor, atrazine, butylate, chloromuronethyl, cyanazine, dicamba, EPTC, glyphosate, imazethapyr, metolachlor, metrobuzin, paraquat, pendamethylin, petroleum oil, trifluralin; fungicides: benomyl, captan, chlorothalonil, metalaxyl, maneb/mancozeb, ziram; fumigants: aluminum phosphide, carbon disulfide/carbon tetrachloride, ethylene dibromide; methyl bromide.
All models include age and gender
Some participants reported use of more than one type of insecticide so numbers for the chemical subgroups do not sum to the total
Table 4.
ALS and ever use of specific pesticides, AHS cohort 1993-2010
| Pesticide | Case | Control | OR1 | 95% CI | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | ||||
| Organochlorine insecticides | |||||||
| Aldrin | 9 | 26 | 9024 | 12 | 2.1 | 0.8 | 5.1 |
| Chlordane | 8 | 23 | 13057 | 17 | 1.1 | 0.4 | 2.5 |
| Dieldrin | 5 | 15 | 3210 | 4 | 2.6 | 0.9 | 7.3 |
| DDT | 13 | 38 | 13092 | 17 | 2.1 | 0.9 | 5.0 |
| Heptachlor | 6 | 18 | 7286 | 10 | 1.4 | 0.5 | 3.7 |
| Lindane | 5 | 14 | 9034 | 12 | 1.2 | 0.5 | 3.4 |
| Toxaphene | 7 | 20 | 6937 | 9 | 2.0 | 0.8 | 4.9 |
| Organophosphate insecticides | |||||||
| ChlorpyrIfos | 8 | 21 | 22305 | 28 | 0.8 | 0.3 | 1.7 |
| Malathion | 14 | 39 | 39200 | 50 | 0.6 | 0.3 | 1.3 |
| Parathion | 5 | 14 | 7638 | 10 | 1.4 | 0.5 | 3.8 |
| Phorate | 6 | 17 | 15791 | 21 | 0.7 | 0.3 | 1.9 |
| Terbufos | 6 | 17 | 19220 | 25 | 0.7 | 0.3 | 1.7 |
| Carbamate insecticides | |||||||
| Carbaryl | 18 | 47 | 36252 | 46 | 1.0 | 0.5 | 1.8 |
| Herbicides | |||||||
| 2 4-D | 20 | 50 | 42134 | 52 | 1.0 | 0.5 | 2.1 |
| 2,4,5,T | 7 | 19 | 10066 | 13 | 1.3 | 0.5 | 3.2 |
| Alachlor | 12 | 32 | 26302 | 34 | 1.0 | 0.4 | 2.2 |
| Atrazine | 15 | 38 | 36898 | 45 | 0.7 | 0.3 | 1.6 |
| Chlorimuron Ethyl | 5 | 14 | 17880 | 23 | 0.7 | 0.2 | 1.9 |
| Cyanazine | 11 | 28 | 20158 | 26 | 1.3 | 0.6 | 2.9 |
| Dicamba | 12 | 32 | 24332 | 31 | 1.4 | 0.6 | 3.1 |
| Glyphosate | 25 | 61 | 48847 | 60 | 1.2 | 0.6 | 2.5 |
| Imazethapyr | 5 | 13 | 20811 | 27 | 0.5 | 0.2 | 1.3 |
| Metolachlor | 10 | 26 | 22737 | 29 | 1.0 | 0.4 | 2.2 |
| Metribuzin | 9 | 25 | 21248 | 28 | 1.1 | 0.5 | 2.6 |
| Pendimethalin | 7 | 19 | 21906 | 29 | 0.7 | 0.3 | 1.7 |
| Petroleum Oil | 7 | 21 | 22627 | 30 | 0.7 | 0.3 | 1.8 |
| TrIfluralin | 15 | 38 | 26376 | 34 | 1.5 | 0.7 | 3.2 |
| Fungicides | |||||||
| Metalaxyl | 7 | 18 | 11955 | 15 | 1.3 | 0.5 | 3.1 |
| Fumigants | |||||||
| Methyl bromide | 5 | 13 | 8648 | 11 | 1.2 | 0.5 | 3.2 |
All models include age and gender
Information on cumulative lifetime use of specific pesticides was limited. We evaluated exposure-response trends for nine herbicides and one insecticide for which there were at least 10 exposed cases; this category did not include any of the OC insecticides. We found no significant trends (data not shown).
Because ALS was associated primarily with OCs, we compared characteristics of individuals who did or did not use these pesticides. Users of OCs as a group, compared to nonusers, were older (mean age 52 vs 45), more likely to be male (90% vs 49%), and more likely to have ever smoked (50% vs 35%); education levels did not differ. Similar differences were observed for aldrin, dieldrin, DDT, and toxaphene. Individuals who did or did not use OCs as a group were equally likely to live in Iowa (64% vs 62%). However, distributions by state differed for specific OCs: for aldrin and dieldrin, higher proportions of users than nonusers lived in Iowa (79% vs 65-66%), while for DDT and toxaphene lower proportions of users than nonusers lived in Iowa (48-55% vs 68-69%). None of these differences explained the association of ALS with OC use.
Head injury, reported by only two cases, was not related to ALS. Adjustment for head injury did not alter associations of OCs with ALS. No case reported a physician diagnosis of pesticide, lead, or solvent poisoning or use of herbicides in the military.
4. DISCUSSION
We found that ALS risk was associated with use of OCs, pyrethroids, herbicides, and fumigants but not other pesticide classes. Four specific OCs were also associated with ALS risk: aldrin, dieldrin, DDT, and toxaphene. Although not statistically significant, these associations provide leads for further investigation. Results were not materially changed by adjustment for potential confounders including smoking or head injury, which may be risk factors for ALS (Chen et al, 2007; Schmidt et al, 2010; Weisskopf et al, 2009a), and results were similar in an analysis restricted to men.
Previous case-control studies have evaluated the role of exposure to pesticides as a group, most finding ORs that were elevated although not always statistically significant (Table 1). Exposure assessment in some of these studies was limited, consisting of a single question (Bonvicini et al, 2010; Chancellor et al, 1993; Deapen and Henderson, 1986; Gunnarsson et al, 1992; Savattieri et al, 1991). Although recall bias is a concern in small case-control studies, particularly those with limited exposure assessment, studies with more detailed exposure assessment also had positive findings. For example, a study based on industrial hygienist evaluation of a complete occupational history (McGuire et al, 1997) found an overall association (OR 2.0) that was apparent in men (OR 2.4) but not in women (OR 0.9). The association was stronger among men with greater exposure (OR 2.8) and appeared to be confined to insecticides, but exposure to other pesticide classes was uncommon. Another study (Morahan and Pamphlett, 2006) found that regular exposure was more strongly associated with increased risk than ever exposure.
Only one previous cohort study (Weisskopf et al 2009b) has evaluated the relationship of ALS to use of pesticides as a group, finding a weak association (OR 1.1) similar to that reported here. Results from these two cohort studies may suggest that the associations reported by the case-control studies due to bias, but alternative explanations exist. Weisskopf et al (2009b) adjusted for all neurotoxic exposures, a strategy that may have resulted in over-control, while the absence of associations of ALS risk with use of pesticides as a group in the AHS is potentially related to the fact that in this agricultural cohort essentially all men and more than half of the women had used at least one pesticide, thus limiting variability for this general measure. Future studies may help to resolve differences related to study design.
In the meta-analysis we found statistically significant ORs between 1.8 and 2.2 depending on which studies were included. The meta-analysis was limited by the small number of studies, and by differences among studies in matching criteria, exposure metrics, and data analytic methods, but there was little evidence of heterogeneity among the case-control studies, and the meta-analytic association was statistically significant in all analyses. It is possible that this finding is a result of publication bias, since studies may not publish negative results if pesticide use was not a major focus, but the small number of published studies precluded statistical evaluation of this possibility.
Pesticides differ in chemical structure and vary widely in neurotoxicity, so evaluating pesticides as a group may obscure associations with specific pesticides. Fortunately, AHS cohort members varied considerably in their use of particular pesticides, allowing us to evaluate these specific exposures. Our results suggest that ALS is primarily related to use of OCs. OC use has been largely discontinued in the United States and is therefore associated with age, as is ALS. Although our models were adjusted for age, we cannot exclude the possibility that the observed associations are related to uncontrolled confounding by age. In individuals over the age of 60, ALS was no longer associated with DDT or with OCs as a group, suggesting that the relationship observed in the entire cohort may be due to residual confounding by age. However, even in this older subgroup ALS was associated with use of aldrin, dieldrin, and toxaphene. Interestingly, Parkinson’s disease is also associated with exposure to OCs in general (Hancock et al 2008; Elbaz et al 2009) as well as two specific OCs, beta-hexachlorocyclohexane (Richardson et al, 2009) and dieldrin (Tanner et al, 2011; Weisskopf et al, 2010).
Rural residence or aspects of agricultural activity other than pesticide use may be associated with increased ALS risk, but AHS cohort members share these characteristics regardless of exposure to particular pesticides. Thus these more general associations should not affect the association of ALS with use of a particular pesticide.
Previous reports concerning specific pesticides consist of case reports describing ALS occurring after exposure to a dithiocarbamate (Hoogenraad, 1988), OCs (Foncesca et al, 1993), pyrethroids (Doi et al, 2006), a mixture of an OC and a pyrethroid (Pall et al, 1987), or the fumigant methyl bromide (Shaw, 2010). A cohort study found an excess of ALS cases among manufacturing workers exposed to 2,4-D compared to other company employees, based on 3 cases (Burns et al, 2001). Less direct evidence has implicated organophosphate insecticides. For example, US veterans deployed to the first Persian Gulf war, reported to have increased risk of ALS (Horner et al, 2003), may have been highly exposed to organophosphate insecticides or other cholinesterase inhibitors (Golumb, 2008). Some studies have reported that ALS is associated with polymorphisms in the gene for paraoxonase 1, which detoxifies organothiophosphates (Costa et al, 2005). A recent meta-analysis found no relationship of ALS to coding polymorphisms in paraoxonase 1 (Wills et al, 2009), but the role of polymorphisms in the promoter region remains an open question (Landers et al, 2008). Increased ALS risk may also result from an interaction of paraoxonase 1 polymorphisms with pesticide exposure (Morahan et al, 2007; Kwee et al, 2009). Interestingly, an upper motor neuron syndrome with some similarities to ALS was found in individuals poisoned by triorthocresyl phosphate, a neurotoxic organophosphate contaminating Jamaica ginger, an alcoholic drink used in the US in the 1930s during Prohibition that caused “ginger jake” paralysis in thousands of US residents (Morgan and Penovich, 1978). Nevertheless, we did not find an association of ALS risk with OP use in the AHS cohort.
Because we conducted a mortality study, it is possible that the observed associations reflect survival as well as etiology. The factors most consistently associated with ALS survival are age, site of onset, and diagnostic delay [Ganesalingam et al, 2009]. We found little evidence that age confounded or modified our results. No published information suggests that pesticides affect site of onset or diagnostic delay, suggesting that these factors are not likely to confound our results. It is of course possible that there is no association with pesticide use in ALS cases with spinal onset or more slowly progressing disease, but we are unable to address this possibility with our data.
The present study has important strengths, including its prospective design, its basis in a farming population able to report exposure accurately, and its information on specific pesticides and confounders. Its principal limitation is the small number of ALS cases, which may have contributed to the lack of statistical significance for most associations. A larger case group would also have permitted more detailed evaluation of exposure determinants such as duration or intensity of use, application methods, or use of personal protective equipment. However, given the rarity of ALS, few prospective studies are likely to have both larger case groups and the detailed exposure information available for the AHS cohort.
4.1 Conclusions
Our meta-analysis suggests that ALS risk may be associated with use of pesticides as a group, and our analysis of AHS data points to OC use in particular. The latter results are novel but are based on a small number of cases and require replication in other populations.
Highlights.
In a meta-analysis of 8 studies, ALS was associated with general pesticide exposure
In an agricultural cohort, ALS was associated with organochlorine insecticides
Specific pesticides associated with ALS were aldrin, dieldrin, DDT, and toxaphene
Acknowledgments
The AHS was conducted by the University of Iowa (Iowa Field Station: Dr C Lynch and E Heywood) and Battelle Inc (North Carolina Field Station: C Knott and M Hayslip); central data coordination was provided by Westat (K Torres, S Legum, and M Dunn). The study was supported by the intramural research program of the NIH, NIEHS (Z01-ES049005 and Z01-ES049030) and NCI (Z01-CP010119).
Footnotes
CONFLICTS OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alavanja M, Sandler D, McMaster S, et al. The Agricultural Health Study. Environ Health Perspect. 1996;104:362–369. doi: 10.1289/ehp.96104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvicini F, Marcello N, Mandrioli J, et al. Exposure to pesticides and risk of amyotrophic lateral sclerosis: a population-based case-control study. Ann Ist Super Sanita. 2010;46:284–287. doi: 10.4415/ANN_10_03_10. [DOI] [PubMed] [Google Scholar]
- Brooks BR, Miller RG, Swash M, et al. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Burns CJ, Beard KK, Cartmill JB. Mortality in chemical workers potentially exposed to 2,4-dichlorophenoxyacetic acid (2,4-D) 1945-94: an update. Occup Environ Med. 2001;58:24–30. doi: 10.1136/oem.58.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb BA. Acetylcholinesterase inhibitors and Gulf War illnesses. Proc Natl Acad Sci U S A. 2008;105:4295–300. doi: 10.1073/pnas.0711986105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chancellor AM, Slattery JM, Fraser H, Warlow CP. Risk factors for motor neuron disease -- A case-control study based on patients from the Scottish motor neuron disease register. J Neurol Neurosurg Psychiatry. 1993;56:1200–1206. doi: 10.1136/jnnp.56.11.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Richard M, Sandler DP, et al. Head Injury and Amyotrophic Lateral Sclerosis. Am J Epidemiol. 2007;166:810–816. doi: 10.1093/aje/kwm153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Vitalone A, Cole TB, et al. Modulation of paraoxonase (PON1) activity. Biochem Pharmacol. 2005;69:541–50. doi: 10.1016/j.bcp.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Deapen D, Henderson B. A case-control study of amyotrophic lateral sclerosis. Am J Epidemiol. 1986;123:790–799. doi: 10.1093/oxfordjournals.aje.a114308. [DOI] [PubMed] [Google Scholar]
- Doi H, Kikuchi H, Murai H, et al. Motor neuron disorder simulating ALS induced by chronic inhalation of pyrethroid insecticides. Neurology. 2006;67:1894–1895. doi: 10.1212/01.wnl.0000244489.65670.9f. [DOI] [PubMed] [Google Scholar]
- Elbaz A, Clavel J, Rathouz PJ, et al. Professional exposure to pesticides and Parkinson disease. Ann Neurol. 2009;66:494–504. doi: 10.1002/ana.21717. [DOI] [PubMed] [Google Scholar]
- Fonseca RG, Resende LAL, Silva MD, et al. Chronic motor neuron disease possibly related to intoxication with organochlorine insecticides. Acta Neurol Scand. 1993;88:56–58. doi: 10.1111/j.1600-0404.1993.tb04187.x. [DOI] [PubMed] [Google Scholar]
- Ganesalingam J, Stahl D, Wijesekera L, Galtrey C, Shaw CE, Leigh PN, Al-Chalabi A. Latent cluster analysis of ALS phenotypes identifies prognostically differing groups. PloS One. 2009;4:e7107. doi: 10.1371/journal.pone.0007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnarsson LG, Bodin L, Soderfeldt B, Axelson O. A case-control study of motor neurone disease - Its relation to heritability, and occupational exposures, particularly to solvents. Br J Ind Med. 1992;49:791–798. doi: 10.1136/oem.49.11.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock DB, Martin ER, Mayhew GM, et al. Pesticide exposure and risk of Parkinson’s disease: a family-based case-control study. BMC Neurol. 2008;8:6. doi: 10.1186/1471-2377-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenraad T. Dithiocarbamates and Parkinson’s disease. Lancet. 1988;1:767. doi: 10.1016/s0140-6736(88)91573-5. [DOI] [PubMed] [Google Scholar]
- Horner R, Kamins K, Feussner J, et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology. 2003;61:742–9. doi: 10.1212/01.wnl.0000069922.32557.ca. [DOI] [PubMed] [Google Scholar]
- Kamel F, Umbach DM, Stallone L, et al. Association of lead exposure with survival in amyotrophic lateral sclerosis. Environ Health Perspec. 2008;116:943–947. doi: 10.1289/ehp.11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwee L, Allen K, Oddone E, et al. Examination of paraoxonase gene cluster and VietNam deployment in amyotrophic lateral sclerosis[abstract] Neuroepidemiology. 2009;33:68–78. [Google Scholar]
- Landers JE, Shi L, Cho TJ, et al. A common haplotype within the PON1 promoter region is associated with sporadic ALS. Amyotroph Lateral Scler. 2008;9:306–14. doi: 10.1080/17482960802233177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire V, Longstreth W, Jr, Nelson L, et al. Occupational exposures and amyotrophic lateral sclerosis: A population-based case-control study. Am J Epidemiol. 1997;145:1076–1088. doi: 10.1093/oxfordjournals.aje.a009070. [DOI] [PubMed] [Google Scholar]
- Morahan JM, Pamphlett R. Amyotrophic lateral sclerosis and exposure to environmental toxins: an Australian case-control study. Neuroepidemiology. 2006;27:130–135. doi: 10.1159/000095552. [DOI] [PubMed] [Google Scholar]
- Morahan JM, Yu B, Trent RJ, et al. A gene-environment study of the paraoxonase 1 gene and pesticides in amyotrophic lateral sclerosis. Neurotoxicology. 2007;28:532–40. doi: 10.1016/j.neuro.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Morgan JP, Penovich P. Jamaica ginger jake paralysis: 47 year follow-up. Arch Neurol. 1978;35:530–2. doi: 10.1001/archneur.1978.00500320050011. [DOI] [PubMed] [Google Scholar]
- Normand ST. Tutorial in biostatistics meta-analysis: formulating, evaluating, combining, and reporting. Statist Med. 1999;18:321–359. doi: 10.1002/(sici)1097-0258(19990215)18:3<321::aid-sim28>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Pall HA, Williams AC, Waring R, et al. Motor neurone disease as manifestation of pesticide toxicity. Lancet. 1987;ii:685. doi: 10.1016/s0140-6736(87)92468-8. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Shalat SL, Buckley B, et al. Elevated serum pesticide levels and risk of Parkinson disease. Arch Neurol. 2009;66:870–875. doi: 10.1001/archneurol.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savettieri G, Salemi G, Arcara A, Cassata M, Castiglione MG, Fierro B. A case-control study of amyotrophic lateral sclerosis. Neuroepidemiology. 1991;10:242–245. doi: 10.1159/000110279. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Kwee LC, Allen KD, et al. Association of ALS with head injury, cigarette smoking and APOE genotypes. J Neurol Sci. 2010;291:22–29. doi: 10.1016/j.jns.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw I. Motor neurone disease - a methyl bromide exposure cluster points to a causal mechanism. Hum Exp Toxicol. 2010;29:241–242. doi: 10.1177/0960327109359462. [DOI] [PubMed] [Google Scholar]
- Sutedja NA, Veldink JH, Fischer K, et al. Exposure to chemicals and metals and risk of amyotrophic lateral sclerosis: a systematic review. Amyotroph Lateral Scler. 2009;10:302–309. doi: 10.3109/17482960802455416. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Kamel F, Ross GW, et al. Rotenone, paraquat and Parkinson’s disease. Environ Health Perspect. 2011;119:866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Ascherio A. Cigarettes and amyotrophic lateral sclerosis: only smoke or also fire? Ann Neurol. 2009a;65:361–362. doi: 10.1002/ana.21700. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Morozova N, O’Reilly EJ, et al. Prospective study of chemical exposures and amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2009b;80:558–561. doi: 10.1136/jnnp.2008.156976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Knekt P, O’Reilly EJ, et al. Persistent organochlorine pesticides in serum and risk of Parkinson disease. Neurology. 2010;74:1055–1061. doi: 10.1212/WNL.0b013e3181d76a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills AM, Cronin S, Slowik A, et al. A large-scale international meta-analysis of paraoxonase gene polymorphisms in sporadic ALS. Neurology. 2009;73:16–24. doi: 10.1212/WNL.0b013e3181a18674. [DOI] [PMC free article] [PubMed] [Google Scholar]


