Abstract
Enterococcus faecalis, a gram-positive opportunistic pathogen, has become one of the leading causes of nosocomial infections. Normally a resident of the gastrointestinal tract, extensive use of antibiotics has resulted in the rise of E. faecalis strains that are resistant to multiple antibiotics. This, compounded with the ability to easily exchange antibiotic determinants with other bacteria, has made certain E. faecalis infections difficult to treat medically. The genetic toolbox for the study of E. faecalis has expanded greatly in recent years, but has lacked methodology to stably introduce a gene in single copy in a non-disruptive manner for complementation or expression of non-native genes. In this study, we identified a specific site in the genome of E. faecalis OG1RF that can serve as an expression site for a gene of interest. This site is well conserved in most of the sequenced E. faecalis genomes. A vector has also been developed to integrate genes into this site by allelic exchange. Using this system, we complemented an in-frame deletion in eutV, demonstrating that the mutation does not cause polar effects. We also generated an E. faecalis OG1RF strain that stably expresses the green fluorescent protein and is comparable to the parent strain in terms of in vitro growth and pathogenicity in C. elegans and mice. Another major advantage of this new methodology is the ability to express integrated genes without the need for maintaining antibiotic selection, making this an ideal tool for functional studies of genes in infection models and co-culture systems.
Keywords: Enterococcus faecalis, genomic integration, complementation, green fluorescent protein
1. Introduction
Enterococcus faecalis is a gram-positive commensal inhabitant of the intestinal tract of humans, animals and insects and has been used as an ingredient in probiotics (Aarestrup et al., 2002; Tannock and Cook, 2002). However, it is also an opportunistic pathogen and has emerged as a leading cause of hospital-acquired extraintestinal infections, including urinary tract infections, bacteremia, wound infections and endocarditis (Malani et al., 2002). E. faecalis exhibits high levels of resistance to several antibiotics and is talented in horizontal exchange of antibiotic resistance determinants (Kak and Chow, 2002). Such features, compounded with the intrinsic ability of enterococci to thrive in various environmental conditions, have made it increasingly difficult for clinicians to combat this pathogen. Thus the need to understand this infectious agent at the genetic and physiological level is clearly important. An integral requirement of such inquiry and analysis is the availability of molecular and genetic tools. One important category of tools in functional studies is the means to complement genes-of-interest (GOI).
Several plasmids have been developed that allow complementation in E. faecalis. One is the pDL267 vector that contains a kanamycin cassette and a pUC origin (Dunny et al., 1991). Another set of vectors has been developed from the streptococcal plasmid pMV158, a natural replicon of Streptococcus agalactiae (Burdett, 1980). One example is pLS1, a broad-host-range vector that has tetracycline resistance (Lacks et al., 1986). A set of vectors, pMSP3535 and pMSP3535VA, were developed to allow nisin-controlled expression of selected genes in enterococci (Bryan et al., 2000). Kristich et. al. did further engineering and developed plasmid pCJK96 for rhamnose-inducible expression in E. faecalis by replacing the nisin system found in pMSP3535 with a rhamnose-inducible system (Kristich et al., 2007b). Both these plasmids use an erythromycin resistance determinant for selection in both E. coli and E. faecalis. However we have found erythromycin resistance difficult to select for in E. coli. To address this, we previously modified pCJK96 by adding an ampicillin resistance cassette for better selection in E. coli. The vector, pSD2, retained the erm cassette for selection in E. faecalis (Ramesh, 2012).
These vectors can be used for complementation of mutant strains to directly attribute a functional property to the absence of a specific gene. However, these plasmids are not stably transmitted, and maintenance requires that they carry a resistant determinant so that constant antibiotic selection can be applied. Because many strains of E. faecalis are resistant to many antibiotics that are routinely used in the laboratory, there are a limited number of markers that can be used. As a result, introducing certain plasmids into certain strains of this bacterium becomes problematic. Also, the need to maintain antibiotic selective pressure to retain a complementing gene is undesirable if the phenotype of the mutant being studied requires assessment in an infection model, where it can become difficult or impossible to maintain antibiotic exposure.
In the recent past, Kristich et. al. developed a markerless allelic exchange system that can efficiently insert fragments into a targeted site in the E. faecalis genome (Kristich et al., 2007a). In this study, we identified a site within the genome of E. faecalis OG1RF, flanked by two convergent reading frames that is a desirable site for insertion of a GOI. To take advantage of this site, we developed a vector system that allows the insertion of any GOI into the specific genomic location by allelic exchange. The system provides a useful new addition to the E. faecalis molecular toolbox. First, it allows complementation in scenarios that would otherwise require the maintenance of two different plasmids in the same bacterium. One such case is elaborated in this study. Second, it circumvents the need for antibiotic markers and selection to maintain a complemented phenotype, making it particularly suitable for demonstrating the contribution of a specific gene in an infection model or other host-related experiment. Third, it complements by introducing a gene in single rather than multiple copy. Finally, it allows the stable addition of genes that are not part of the native genome.
Using our new system, we successfully complimented a mutant phenotype to demonstrate the role of a response regulator, EutV, in the regulation of the ethanolamine utilization genes. We also generated another useful tool for the E. faecalis community, a strain of OG1RF that stably and evenly produces green fluorescent protein (GFP) in a population of cells, as determined by flow cytometry. Additionally, the strain’s properties are identical to wild type OG1RF, in terms of growth in culture and infectivity of the model nematode host C. elegans and mice.
2. Material and methods
2.1 Bacterial strains and media
All bacterial strains used in this study are listed in Table 1. Media was purchased from DIFCO and chemicals from Sigma, unless otherwise mentioned. E. coli strains were routinely cultured in Luria Bertani broth at 37°C. Antibiotics were added at the following concentrations: spectinomycin, 100μg/ml; erythromycin, 300μg/ml; and streptomycin, 25μg/ml. E. faecalis strains were cultured in Brain Heart Infusion (BHI) at 37°C. Antibiotics were added at the following concentrations : erythromycin, 50μg/ml; and rifampicin, 100μg/ml.
Table 1.
Strains, plasmids and primers used in this study
| Description | Source | |
|---|---|---|
| Strains | ||
| E. faecalis | ||
| OG1RF | Wild type strain, FaR, RfR | Lab stock |
| AR2 | ΔeutVW, FaR, RfR | Fox et.al. |
| ΔeutV | Markerless deletion of eutV in OG1RF, FaR, RfR | This work |
| CK111 | Conjugative donor strain | Kristich et.al. (2007) |
| SD48 | OG1RF pSD3, FaR, RfR, EmR | Ramesh et. al. (2012) |
| SD49 | ΔeutVW pSD3, FaR, RfR, EmR | Ramesh et. al. (2012) |
| SD218 | ΔeutVW [ef2238::PCeutV::ef2239] pSD3, FaR, RfR, EmR | This work |
| SD219 | ΔeutV [ef2238::PCeutV::ef2239] pSD3, FaR, RfR, EmR | This work |
| SD234 | OG1RF [ef2238::Pmalgfp::ef2239] | This work |
| E. coli | ||
| TOP10 | Strain used for construction of pSD2-based plasmids | Lab stock |
| EC1000 | Host for pCJK47-based plasmids; provides RepA in trans | Lab stock |
| OP50 | Strain used as food source for C. elegans | Lab stock |
| C. elegans | ||
| N2 | Wild type Bristol strain | Lab stock |
| Plasmids | ||
| pCJK47 | Plasmid for markerless exchange | Kristich et.al. (2007) |
| pCR8/GW/TOPO | TA cloning vector from Invitrogen | Lab stock |
| pMV158gfp | Source of gfp | Nieto & Espinosa (2003) |
| pRV1 | pCJK47 (ef2238 - ef2239) | This work |
| pTOPOIC | pTOPO (ef2238 - ef2239) | This work |
| pSD15 | pTOPOIC MCS | This work |
| pSD16 | pSD15 PCeutV | This work |
| pSD17 | pRV1 PCeutV | This work |
| pSD3 | pSD2 eutP 5′ UTR | This work |
| pSD18 | pSD15 gfp | This work |
| pSD19 | pRV1 gfp | This work |
| pHFZ2 | pSD2 eutSΔT | Ramesh et. al. (2012) |
| Primers | ||
| Ef2238 F | 5′CCCATGGCTATAATAGTACTTGAGAAGGAGGC3′ | This work |
| Ef2238R | 5′AAAAAATAAGGACGGTTCCTTTATAGGAGCCCCGGGGGT CCTTATTTTTTATTTCTGGCGTGG3′ | This work |
| Ef2239F | 5′GCTCCTATAAAGGAACCGTCCTTATTTTTT3′ | This work |
| Ef2239R | 5′AACTGCAGGGTAAAACTAGGAGGGAAGCATATG3′ | This work |
| SD100 | 5′PhosgggGGTACCACGCATGCTGCAGACGCGTTACGTATCGGATCCAGAATTCGTGATATCTGAATTCGTCGACAAGCTTCTCGAGCCTAGGCTAGCTCTAGACCACACGTGTGGGGGCC CGAGCTCGCGGCCGCccc3′ | This work |
| SD101 | 5′PhosgggGCGGCCGCGAGCTCGGGCCCCCACACGTGTGGTCTAGAGCTAGCCTAGGCTCGAGAAGCTTGTCGACGAATTCAGATATCACGAATTCTGGATCCGATACGTAACGCGTCTG CAGCATGCGTGGTACCccc3′ | This work |
| SD143 | 5′GCCTGCAGCGAGCTCGGTACCCAGCTTGATTTGATAGC3′ | This work |
| SD144 | 5′GGCGGCCGCCTATTTGTATAGTTCATCCATGCCATGTG3′ | This work |
| SD42 | 5′ GCGTCGACCGACAAAT CAAGCAACTCCGTCAA3′ | |
| SD106 | 5′CGTAATAGGTTCATCATCGACTATTACAATTCGTCCATCC ATCCAGACTCGCTCCTTTAATTTAGC3′ | This work |
| SD107 | 5′ATGGATGGACGAATTGTAATAGTCG3′ | This work |
| SD110 | 5′GCGCGGCCGCTTATTCATCATCCATTACAATCAATTCTGC3′ | This work |
2.2 DNA manipulations, primers and sequencing
All primers used in this study are listed in Table 1. Primers were synthesized by Integrated DNA Technologies (Coralville, IA). Sequencing was done by GeneWiz (South Plainfield, NJ). High Fidelity Taq polymerase (Invitrogen, Carlsbad, CA) was used for all PCR reactions. Restriction enzymes (NEB) and T4 DNA ligase (Promega, WI) were used as per manufacturer’s instructions. Plasmid extraction (Qiagen) and transformation were performed as per standard protocols.
2.3 Plasmid construction and allelic exchange
All plasmids used in this study are listed in Table 1. The primers ef2238f/r and ef2239f/r were used to amplify the ORFs of ef2238 and ef2239 respectively from E. faecalis OG1RF genomic DNA. The ef2238 reverse primer was designed to incorporate a single SmaI site in the intergenic sequence between the two genes. These two amplicons were then used as templates with outer primers to generate a second PCR product that consisted of the amplicons fused together with a unique SmaI site between them. This cassette was digested with PstI and NcoI and ligated into a similarly digested pCJK47 vector to yield pRV1. The same undigested cassette was also inserted into pCR8/GW/TOPO to generate pTOPOIC. The multiple cloning site of the vector pSTBlue was synthesized as oligomers with terminal SmaI sites. These oligomers were annealed and ligated into the unique SmaI site between ef2238 and ef2239 in pTOPOIC to generate pSD15. To generate pSD16, the constitutive promoter was amplified using SD42 and SD106 from pHFZ2. The eutV gene was amplified with SD107 and SD110. The promoter was then fused to the eutV gene using crossover PCR and digested with SalI and NotI. Both fragments were ligated into similarly digested pSD15 to generate pSD16. The inserted fragments along with the remaining MCS was excised by SmaI digestion and ligated into SmaI-digested pRV1, resulting in pSD17. These constructs were introduced into E. faecalis CK111 by electropration and introduced into either the AR2 or the ΔeutV strain by conjugation. Allelic exchange was performed as described in Kristich et. al (Kristich et al., 2007a). The reporter plasmid pSD3 was introduced into these strains by electroporation.
The gfp used in this study was amplified from pMV158gfp with primers SD143 and SD144 and digested with PstI and NotI. Plasmids pSD18 and pSD19 was generated using similar cloning procedures as described above and SD234 was obtained by standard allelic exchange methods as described (Kristich et al., 2007a).
2.4 β-galactosidase assays
Beta-galactosidase assays were done as described previously (Ramesh, 2012). Briefly, overnight cultures grown in BHI broth with 50μg/mL erythromycin were diluted 1:25 into MM9HY medium or MM9HY supplemented with 33mM ethanolamine and 40μg/mL AdoCbl. Strains were then grown anaerobically, without agitation, for 3.5hrs in the dark. Cells were resuspended in 1:10 Z buffer and lysed using 0.1mm glass disruption beads (Research Products International) for 2 minutes in a mini bead beater. Samples were cleared and incubated with o-nitrophenyl β-D-galactoside (ONPG). Color development was measured at 414 nm using a plate reader. Total protein in samples was estimated using a Pierce BCA kit (Thermo Scientific, Rockwood, IL) as per manufacturer’s instructions. Reporter activity was reported in arbitrary units that indicate absorbance per μg/μl total protein. Samples were assayed in triplicate. Mean and standard deviations were calculated from experimental replicates. The experiment was repeated on three independent occasions.
2.5 C. elegans
C. elegans strains were grown and maintained as previously described (Hope, 1999). The following bacterial strains were used in this study: E. coli OP50, E. faecalis OG1RF and E. faecalis SD234. C. elegans strains used in this study are indicated in Table 1. The killing assays and statistical analysis, by the Kaplan-Meier method, were done as described previously (Garsin et al., 2001). The experiment was replicated on three independent occasions.
2.6 Fluorescence microscopy
A suspension of E. faecalis SD234 in PBS was mounted on glass slides for microscopy. L4 C. elegans worms were exposed to E. faecalis strains for 24 hours at 25°C. Worms were collected, paralyzed with 1mM levamisole and mounted on 2% agarose pads. Bacterial and worm samples were imaged at 100X and 10X magnification respectively, using an Olympus IX81 automated inverted microscope. Data was processed using Slidebook (version 5.0) software.
2.7 Flow cytometry analysis
Overnight cultured cells were inoculated in BHI medium to a starting OD600 of 0.1 and incubated at 37°C without shaking. Aliquots of one OD600 equivalents of E. faecalis cells in BHI medium were harvested at exponential and post-exponential phase and washed twice with PBS, fixed with 1% paraformaldehyde and analyzed with a BD FACS calibur flow cytometer (BD Bioscience, CA). Mean fluorescent intensity was measured using the FL1 (fluorescence at 530 nm) from 10,000 events with triplicates.
2.8 Murine peritonitis infections
For monoinfections, E. faecalis strains OG1RF and SD234 were tested following our previously published method (Singh et al., 1998). In brief, mice were injected intraperitoneally with bacteria (grown in BHI medium), premixed with sterile rat fecal extract (SRFE) and were observed for 4 days for survival. Inocula of (1.2 × 107, 1.2 × 108, 1.2 × 109 CFU/ml) for OG1RF and (8 × 106, 8 × 107, 8 × 108 CFU/ml) for SD234 were used to compare animal survival/mortality using five mice per inoculum. For mixed infection competition assays, OG1RFand SD234 at approximately the same OD600, i.e. 2.190 and 2.165 respectively, were mixed in equal amounts (~1:1) and inoculated using the same method as described above for monoinfection. Bacterial CFUs determined for OG1RF and SD234 from the inoculum were 5 × 108/mouse and 4 × 108/mouse, respectively. After 24 hrs of infection, kidney pairs and spleen were aseptically removed from dead animals, homogenized in 5 ml of saline and dilutions were plated on to Enterococcosel agar (EA) supplemented with 100μg/ml rifampicin. Bacteria were examined and enumerated by fluorescence microscopy using a Leica MZ16F stereoscope. Comparison of the survival curves at similar inocula was performed using a log-rank test and the percentages of SD234 or OG1RF strains in the inoculum versus in kidney pair and spleen for individual mice were analyzed for significance by the paired t test. Graph Pad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA) was used for graphs and statistical analysis.
2.9 Ethics statement
The mouse peritonitis model and procedures were performed in accordance with the institutional policies and the guidelines stipulated by the animal welfare committee, University of Texas Health Science Center at Houston (AWC, UTHSC), protocol # HSC-AWC-10-135.
3. Results and discussion
3.1. The E. faecalis genomic insertion site for expression (GISE)
To identify a site appropriate for inserting genes, we looked for an intergenic region flanking two convergently expressed genes. Our rationale was that such a site would be unlikely to cause polar effects, i.e. changes in gene expression in the surrounding region, due to insertion of a gene that is expressed. We also tried to choose a region shared by most strains of E. faecalis. The region between ef2238 and ef2239 fit these criteria and became our genomic insertion site for expression (GISE). The region containing the genes ef2238 and ef2239 and the intergenic sequence will henceforth be referred to as the insertion cassette (IC).
The GISE is present in both E. faecalis OG1RF and V583 strains (Bourgogne et al., 2008). BLAST searches also revealed that the GISE is nearly 100% conserved in all 16 of the E. faecalis strains sequenced by the Broad Institute (Palmer et al., 2010), with the exception of E. faecalis X98. In addition, the GISE is also conserved in the Enterococcus sp. 7L76 and E. faecalis strain ATCC29200 and TX1322. No regions of similarity were found in a number of clinical isolates. Hence, this site can be utilized for expression of GOI, not only in OG1RF, but in many other E. faecalis strains. This region had no homology to any of the sequenced E. faecium, E. casseliflavus and E. gallinarum strains, making it an E. faecalis – specific site. The utility of the GISE is at least two-fold. It can be utilized for complementation studies and for the expression of non-native genes, such as those that encode a fluorescent protein. In this study, we demonstrated both uses of the GISE.
3.2 Construction of pRV1
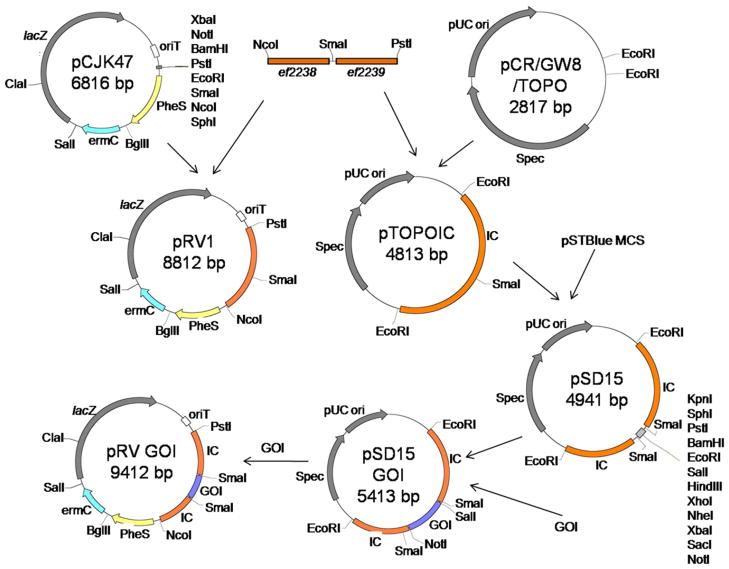
We developed the vector, pRV1, for insertion of GOIs into the GISE using the allelic exchange method (Kristich et al., 2007a). We engineered a single SmaI site between ef2238 and ef2239 by crossover PCR. Ligation of this IC into pCJK47 eliminated the existing SmaI site in the multiple cloning site (MCS), resulting in a unique site for insertion of the GOI. This vector was named pRV1 (Fig. 1). Since pRV1 was limited to a single blunt site for ligation of the GOI, we decided to introduce a more elaborate MCS at this SmaI site for greater ease of GOI introduction. We chose the MCS of the pSTBlue vector because it has a large number of usable restriction sites. Because we found the pRV1 plasmid difficult to manipulate in E. coli compared to other plasmids (see below), we decided to perform further manipulations in the pCR8/GW/TOPO vector. Therefore, the IC was introduced into pCR8/GW/TOPO and the pSTBlue MCS cloned into the SmaI site to generate pSD15 (Fig 1). GOIs were first cloned into the IC in pSD15, and then excised and ligated into pRV1, as shown in Fig. 1. Though cloning directly into pRV1 is theoretically possible, we found this two-step cloning process preferable for the following reasons. In comparison to pRV1, the pCR8/GW/TOPO vector is much smaller. Unlike pRV1, it is a high copy number vector and can be easily retrieved in large quantities from small culture volumes by mini prep. Using of pCR8/GW/TOPO also facilitates transformation into common E. coli host strains such as DH5α, compared to EC1000, which is required for working with pCJK47 (Kristich et al., 2007a) and is less efficiently transformed by electroporation. Thus, subcloning into the pSD15 intermediate alleviates several inherent technical difficulties.
Figure 1.
Schematic representation of the construction of clones in pRV1. See Methods section for details. The gene-of-interest represented is the PCeutV gene described in the text. IC-1 and IC-2 represent ef2238 and ef2239 respectively. Plasmids are not drawn to scale.
3.3. Complementation of the E. faecalis EutVW two-component system
E. faecalis has a very flexible metabolism that allows it to utilize a wide range of nutritional sources. Ethanolamine, which is present in abundance in the intestine, can be used by E. faecalis as a source of both carbon and nitrogen. Utilization of this compound requires the upregulation of the ethanolamine utilization (eut) genes (reviewed by (Garsin, 2010)). The E. faecalis eut genes are positively regulated by an adenosylcobalamin-binding riboswitch and a two-component regulatory system (TCS). The presence of both ethanolamine and adenosylcobalamin is required for the induction of the eut genes (Baker and Perego, 2011; Del Papa and Perego, 2008; Fox et al., 2009; Ramesh, 2012). The eut TCS consists of a sensor histidine kinase, EutW, which autophosphorylates in the presence of ethanolamine and then transfers the phosphate to its cognate response regulator, EutV (Del Papa and Perego, 2008; Fox et al., 2009). EutV is a member of the ANTAR (AmiR and NasR transcriptional anti-terminator regulators) family of proteins that regulates its target genes by interacting with their nascent transcripts and preventing the formation of transcriptional terminators (Shu and Zhulin, 2002). Antitermination by EutV occurs at each of four terminators that are present in the eut locus (Baker and Perego, 2011; Fox et al., 2009; Ramesh, 2012). Deletion of either eutW, eutV, or both, rendered the eut genes uninducible under conditions that normally favor expression. The eutV and eutW genes are under the control of a promoter that precedes eutS. A transcriptional terminator follows the eutS promoter, which is the target of antitermination by EutV when the system is induced. Deletion of the terminator renders the promoter constitutively active (Ramesh, 2012).
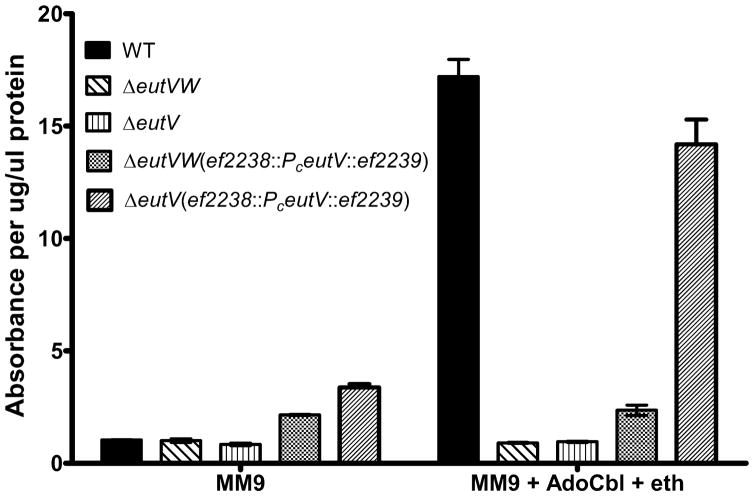
To demonstrate that we could compliment a eutV mutation in the eut locus by replacing the gene at the GISE, we generated constructs where the eutV gene was under the control of a constitutive eutS promoter (PC eutV). This PC eutV was inserted into the GISE in an OG1RF host background that had a deletion in eutV, or in both eutV and eutW. In all instances we used a PCR verification step to ensure that the insertion event had occurred at the correct site in the genome. In our previous studies, we have used the reporter pSD3 to measure the activity of the eut TCS (Ramesh, 2012). This reporter contains a translational fusion of lacZ to the 5′UTR of eutP, the first gene of the eut locus. The 5′UTR of eutP is also a target for antitermination by EutV upon activation and hence functions as a reporter of positive regulation by the EutVW TCS (Fox et al., 2009). Since EutV is activated by phosphorylation by EutW upon sensing ethanolamine (Del Papa and Perego, 2008; Fox et al., 2009), expression of EutV from the remote site was expected to activate the reporter only in the eutV background. Our results indicate that the pSD3 reporter is activated only under inducing conditions in the eutV host background but not in the eutVW double mutant (Fig. 2). This provides evidence that our previously generated eutV in-frame deletion does not disrupt the downstream expression of eutW. We can express eutV in a remote location and recover the ability to induce gene expression, which has been shown to require eutW. We were unable to complement this mutation previously by expressing eutV on a plasmid because that would have required the strain to carry two plasmids – the complementing plasmid, and the lacZ reporter. This was not possible due to the lack of a plasmid with different origin of replication that could co-exist with pSD3 in the same bacterial cell. Thus, expression of eutV from this remote site makes complementation of our deletion in the eut locus possible.
Figure 2.
Complementation of the EutVW two-component system in E. faecalis. Expression of the eutP:lacZ reporter, as measured by β-galactosidase assays, is shown in different host backgrounds. Expression from the reporter is observed in wild type OG1RF in the presence of adenosylcobalamin and ethanolamine, but not in mutants lacking either the entire EutVW two-component system or just the EutV regulator. Expression of the integrated PceutV is unable to induce reporter activity in a eutVW deletion mutant but shows near wild type levels of reporter activity in a eutV deletion background. Data for each culture condition was collected in triplicates. Error bars indicate standard deviation.
3.4. Stable expression of gfp
To test the utility of the GISE for expression of a foreign gene, we chose the gene that encodes green fluorescent protein (gfp). The gfp gene was amplified from a Streptococcal plasmid vector pMV158gfp where the gfp gene is under the control of the inducible promoter of the pneumococcal malM gene, Pmal (Nieto and Espinosa, 2003). This promoter is negatively regulated by the MalR transcriptional repressor in Streptococcus pneumoniae, and repression is released in the presence of maltose (Nieto et al., 1997). However, the interaction between the MalR/Pmal repressor/promoter pair is not functional in E. faecalis. The expression of gfp from the pMV158gfp plasmid has been shown to be constitutive in both E. faecalis OG1RF and V583 strains (Lorenzo-Diaz and Espinosa, 2009; Thomas et al., 2008). The gfp reporter under the control of the malM promoter was integrated into the GISE, resulting in the SD234 strain.
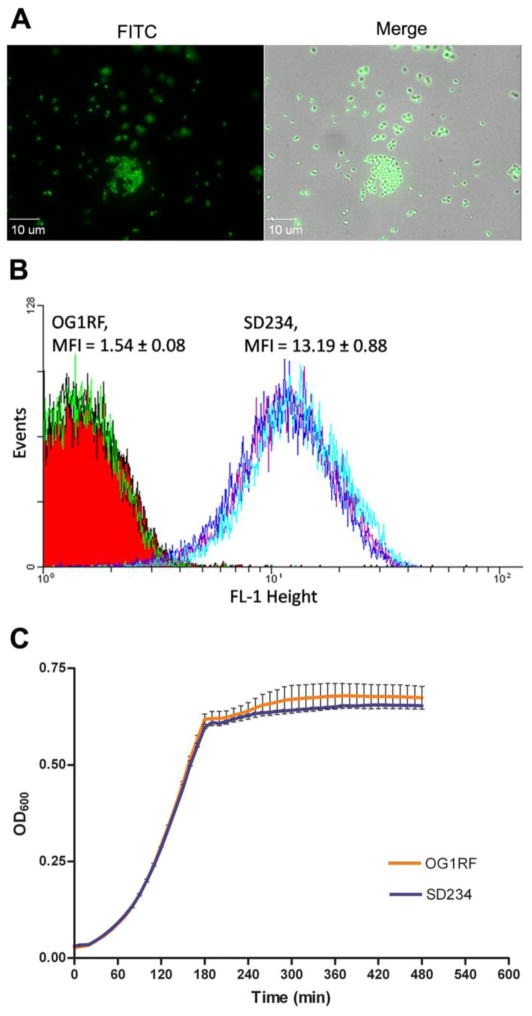
Observation of dilute suspensions of the E. faecalis SD234 strain showed strong production of GFP based on color (Fig. 3A). We employed FACS methodology to analyze what percent of the cell population at exponential and post-exponential stages were producing GFP. In previous attempts to generate gfp-expressing strains of E. faecalis, we had observed that GFP was not present uniformly throughout the population (Garsin lab, data not shown). However, as shown in Fig. 3B, the SD234 cells exhibited a mean fluorescence intensity (MFI) of 13.19, 7-fold higher than that of for the wild type OG1RF cells (MFI = 1.5). No minor peak was observed in the histogram of the SD234 cells, indicating the ability of SD234 to stably produce GFP. No major change in morphology of SD234 was observed based on the forward and side scatter profile of the population. Analysis of post-exponential phase cells showed similar results. The method of growth, static or shaking, also did not impact the production of GFP in these cells (data not shown). Analysis of the growth curve of the gfp-expressing SD234 strain showed no difference from that of wild type OG1RF (Fig 3C), suggesting that expression of gfp from the GISE did not have any gross deleterious effect on the laboratory fitness of the strain.
Figure 3.
Expression of gfp in E. faecalis. A) Fluorescent micrographs of SD234 expressing gfp. B) FACS analysis of exponential cultures of OG1RF wild type and SD234 strains. SD234 forms a tight peak representing a single population of fluorescent cells suggesting ubiquitous expression of gfp. C) In vitro growth of OG1RF and SD234 in BHI medium at 37°C. Each data point represents triplicate samples and error bars indicate standard error.
3.5. Stable in vivo expression of gfp in strain SD234 results in no loss of virulence in animal infection models
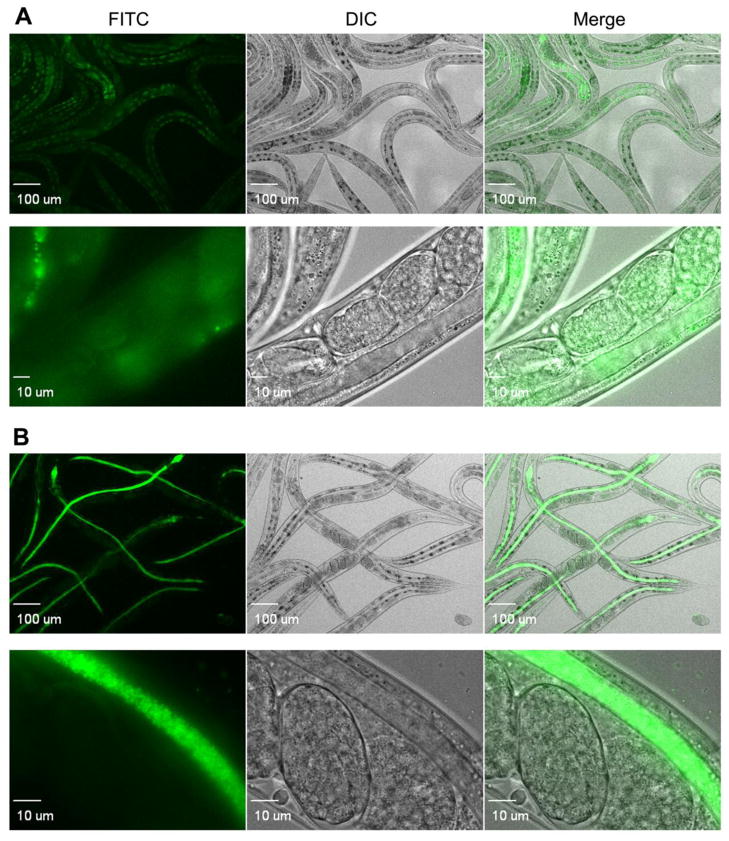
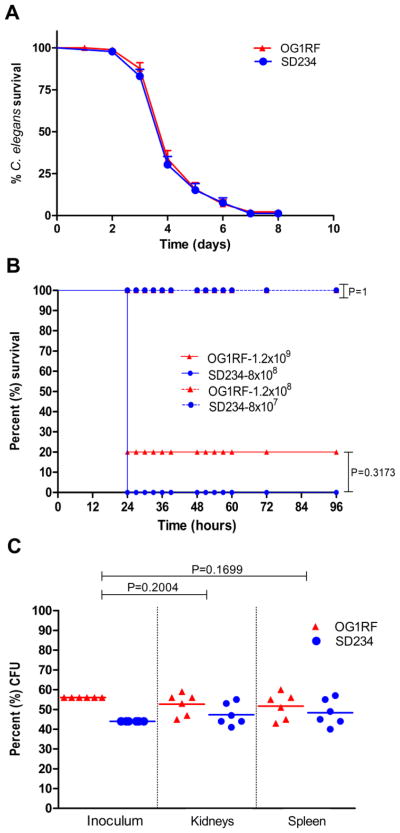
We next tested the ability to track the gfp-expressing E. faecalis strain in an infection model. We used the model nematode host Caenorhabditis elegans, which is transparent and has successfully been used as a model host for many human bacterial pathogens, including E. faecalis (Garsin et al., 2001). C. elegans worms were fed either wild type OG1RF or the gfp-expressing SD234 strain for a duration of 24 hours at which point they were collected, washed and anesthetized before being analyzed by fluorescence microscopy. As shown in Fig. 4, the SD234 strain stably and strongly produced the fluorophore in the host. In comparison, the pictures of the worms infected with the wild type strain, OG1RF, manifested only weak, background autofluorescence from the worm, which is a normal occurrence when they are infected (Chavez et al., 2007). In the images captured at higher magnification, single bacterial colonies of SD234 are discreetly visible and expression of gfp appeared uniform. We also compared the wild type OG1RF and the gfp-expressing SD234 strain for their ability to kill the C. elegans host. As shown in Fig. 5Aa, SD234 mirrors OG1RF in the kinetics of killing C. elegans.
Figure 4.
Production of GFP by SD234 inside C. elegans. Fluorescent micrographs of C. elegans nematodes infected with E. faecalis A) OG1RF and B) SD234 after 24 hours of feeding. Diffuse fluorescence observed in OG1RF-fed C. elegans is a result of autofluorescence that accumulates in the intestine of the worm. Fluoresent SD234 is distinctly seen within the nematode gut.
Figure 5.
Infection of C. elegans and mice with E. faecalis OG1RF and SD234. A) Killing assay of C. elegans worms infected with E. faecalis OG1RF and SD234. Each data point reflects the average of three groups of thirty worms each (P = 0.5949). Intraperitoneal infection of mice with B) monocultures or C) mixed cultures of OG1RF and SD234. B) Kaplan-Meier survival plots of wild type OG1RF (red) and SD234 (blue) are shown. Inoculum is indicated. C) Mice were infected with a 1:1 mix of OG1RF (red) and SD234 (blue). Percentage CFU of OG1RF and SD234 present in the inoculum, and recovered from the kidneys and spleens of mice are shown.
We next analyzed the expression and infectivity of SD234 in an intraperitoneal mouse infection model. Groups of mice were injected with either OG1RF or SD234 and survival was monitored. Both groups of mice showed similar mortality rates (Fig 5B). SD234 colonies recovered from these mice were fluorescent when plated on solid media. As in the C. elegans model, no significant difference was observed between the virulence of wild type OG1RF and SD234 in mice. We also performed competition assays in which mice were inoculated with an equal mix of OG1RF and SD234. After 24 hours of infection, the numbers of OG1RF and SD234 in the kidneys and spleen of the mice were estimated. Dilutions of organ homogenates were plated, and the two bacterial species were differentiated visually on the basis of fluorescence. As shown in Figure 5C, both strains were recovered in similar numbers suggesting that SD234 was as fit as the wild type strain in causing infection and dissemination in the mouse model.
Thus, production of GFP from gene insertion into the GISE does not cause any gross physiological shortcomings and is comparable to the wild type strain in terms of in vivo virulence in the animals tested. This makes SD234 a potentially useful tool for tracking E. faecalis cells in infection models, co-cultures and biofilm formation. Generation of mutants in the SD234 background will allow easy and efficient enumeration of the wild type and mutant populations visually. Although gfp-expressing E. faecalis strains have been generated in the past, these strains have suffered from lack of robust and uniform expression. The added advantage of the SD234 strain is its independence from selection by antibiotic pressure. Expressing gfp from the chromosome rather than an extrachromosomal plasmid circumvents the need to maintain its presence by antibiotic selection.
4. Conclusions
In this study we have identified a site in the E. faecalis OG1RF genome that allows the introduction of a gene of interest by markerless allelic exchange. We have designed a new vector for the insertion of a GOI and demonstrated two different utilities of this GISE. Complementation of a mutation in the E. faecalis EutVW TCS was demonstrated successfully, by inserting the gene encoding the response regulator into the GISE. Since, integration of genes into the GISE is not accompanied by addition of an antibiotic marker, one of the advantages of this method is its independence from maintaining selective pressure to ensure stable expression. This is particularly useful, since E. faecalis is resistant to several routinely used antibiotics. Strains created using this method are ideal for assessment in an infection model where maintenance of antibiotic selection is challenging. It also is a viable alternative for studies that would otherwise require the presence of two plasmids in E. faecalis. Due to the presence of multiple copies of plasmids, expression of genes from plasmids is normally higher and can have deleterious effects on the normal physiology of the bacterium. Such a situation is avoided by insertion into the GISE, as expression occurs from a single copy of the gene. We also showed that the GISE can be used to express a foreign gene such as gfp. The GFP-labeled E. faecalis strain showed no defects in growth in media, and production of the fluorophore was stable and even throughout the population of cells, including within the C. elegans host. The GFP-labeled strain also retained its fluorescence when recovered from infected mice. Additionally, the GFP-labeled strain was as efficient as wild type in killing both C. elegans and mice, suggesting that expression from the GISE does not have any gross physiological effects on bacterial virulence. It was also shown to survive in numbers similar to that of the wild type when inoculated in mice as an equal mixture. Therefore, the GFP producing strain, SD234, could provide an ideal background for the development of mutants that can be followed in infection models or co-cultures in an antibiotic-independent manner. The GISE is well conserved in most E. faecalis strains and this technique could potentially be used in strains other than OG1RF. Finally, as the GISE allows expression of foreign genes, this technology could lead to the development of OG1RF as a heterologous host for expression and analysis of genes present in other less genetically amenable enterococcal strains and species.
Highlights.
A vector was designed to allow integration of DNA into a specific site in the E. faecalis genome.
The method bypasses the need to maintain antibiotic pressure for expression of genes of interest.
Using this system, we complimented a mutant in the EutVW two component system.
An E. faecalis strain that stably and evenly produces green fluorescent protein (GFP) was created.
The gfp-expressing strain was identical to wild type for in vitro growth and killing of C. elegans and mice.
Acknowledgments
We would like to express our thanks to M. Espinosa for his kind gift of the pMV158gfp plasmid. The research was supported by grants R01AI076406 and R56AI093699 to DAG and R01AI047923 to BEM from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarestrup FM, Butaye P, Witte W. Nonhuman reservoirs of enterococci. In: Gilmore MS, editor. The enterococci: pathogenesis, molecular biology, and anibiotic resistance. ASM Press; Washington, DC: 2002. pp. 55–99. [Google Scholar]
- Baker KA, Perego M. Transcription antitermination by a phosphorylated response regulator and cobalamin-dependent termination at a B riboswitch contribute to ethanolamine utilization in Enterococcus faecalis. J Bacteriol. 2011;193:2575–2586. doi: 10.1128/JB.00217-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgogne A, Garsin DA, Qin X, Singh KV, Sillanpaa J, Yerrapragada S, Ding Y, Dugan-Rocha S, Buhay C, Shen H, Chen G, Williams G, Muzny D, Maadani A, Fox KA, Gioia J, Chen L, Shang Y, Arias CA, Nallapareddy SR, Zhao M, Prakash VP, Chowdhury S, Jiang H, Gibbs RA, Murray BE, Highlander SK, Weinstock GM. Large scale variation in Enterococcus faecalis illustrated by the genome analysis of strain OG1RF. Genome Biol. 2008;9:R110. doi: 10.1186/gb-2008-9-7-r110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan EM, Bae T, Kleerebezem M, Dunny GM. Improved vectors for nisin-controlled expression in gram-positive bacteria. Plasmid. 2000;44:183–190. doi: 10.1006/plas.2000.1484. [DOI] [PubMed] [Google Scholar]
- Burdett V. Identification of tetracycline-resistant R-plasmids in Streptococcus agalactiae (group B) Antimicrob Agents Chemother. 1980;18:753–760. doi: 10.1128/aac.18.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez V, Mohri-Shiomi A, Maadani A, Vega LA, Garsin DA. Oxidative stress enzymes are required for DAF-16-mediated immunity due to generation of reactive oxygen species by Caenorhabditis elegans. Genetics. 2007;176:1567–1577. doi: 10.1534/genetics.107.072587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Papa MF, Perego M. Ethanolamine activates a sensor histidine kinase regulating its utilization in Enterococcus faecalis. J Bacteriol. 2008;190:7147–7156. doi: 10.1128/JB.00952-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny GM, Lee LN, LeBlanc DJ. Improved electroporation and cloning vector system for gram-positive bacteria. Appl Environ Microbiol. 1991;57:1194–1201. doi: 10.1128/aem.57.4.1194-1201.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KA, Ramesh A, Stearns JE, Bourgogne A, Reyes-Jara A, Winkler WC, Garsin DA. Multiple posttranscriptional regulatory mechanisms partner to control ethanolamine utilization in Enterococcus faecalis. Proc Natl Acad Sci U S A. 2009;106:4435–4440. doi: 10.1073/pnas.0812194106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garsin DA. Ethanolamine utilization in bacterial pathogens: roles and regulation. Nat Rev Microbiol. 2010;8:290–295. doi: 10.1038/nrmicro2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garsin DA, Sifri CD, Mylonakis E, Qin X, Singh KV, Murray BE, Calderwood SB, Ausubel FM. A simple model host for identifying Gram-positive virulence factors. Proc Natl Acad Sci U S A. 2001;98:10892–10897. doi: 10.1073/pnas.191378698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope IA. C. elegans. A Practical Approach. Oxford University Press Inc; United States: 1999. [Google Scholar]
- Kak V, Chow JW. Acquired antibioic resistances in entetrococci. In: Gilmore MS, editor. The Enterococci: pathogenesis, molecular biology, and antibiotic resistance. ASM Press; Washington, DC: 2002. pp. 355–384. [Google Scholar]
- Kristich CJ, Chandler JR, Dunny GM. Development of a host-genotype-independent counterselectable marker and a high-frequency conjugative delivery system and their use in genetic analysis of Enterococcus faecalis. Plasmid. 2007a;57:131–144. doi: 10.1016/j.plasmid.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristich CJ, Wells CL, Dunny GM. A eukaryotic-type Ser/Thr kinase in Enterococcus faecalis mediates antimicrobial resistance and intestinal persistence. Proc Natl Acad Sci U S A. 2007b;104:3508–3513. doi: 10.1073/pnas.0608742104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks SA, Lopez P, Greenberg B, Espinosa M. Identification and analysis of genes for tetracycline resistance and replication functions in the broad-host-range plasmid pLS1. J Mol Biol. 1986;192:753–765. doi: 10.1016/0022-2836(86)90026-4. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Diaz F, Espinosa M. Large-scale filter mating assay for intra- and inter-specific conjugal transfer of the promiscuous plasmid pMV158 in Gram-positive bacteria. Plasmid. 2009;61:65–70. doi: 10.1016/j.plasmid.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Malani PN, Kauffman CA, Zervos MJ. Enterococcal disease, epidemiology, and treatment. In: Gilmore MS, editor. The enterococci: pathogenesis, molecular biology, and antibiotic resistance. ASM Press; Washington, DC: 2002. pp. 385–408. [Google Scholar]
- Nieto C, Espinosa M. Construction of the mobilizable plasmid pMV158GFP, a derivative of pMV158 that carries the gene encoding the green fluorescent protein. Plasmid. 2003;49:281–285. doi: 10.1016/s0147-619x(03)00020-9. [DOI] [PubMed] [Google Scholar]
- Nieto C, Espinosa M, Puyet A. The maltose/maltodextrin regulon of Streptococcus pneumoniae. Differential promoter regulation by the transcriptional repressor MalR. J Biol Chem. 1997;272:30860–30865. doi: 10.1074/jbc.272.49.30860. [DOI] [PubMed] [Google Scholar]
- Palmer KL, Carniol K, Manson JM, Heiman D, Shea T, Young S, Zeng Q, Gevers D, Feldgarden M, Birren B, Gilmore MS. High-quality draft genome sequences of 28 Enterococcus sp. isolates. J Bacteriol. 2010;192:2469–2470. doi: 10.1128/JB.00153-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh A, DebRoy S, Goodson JR, Fox KA, Garsin DA, Winkler WC. The Mechanism for RNA Recognition by ANTAR Regulators of Gene Expression. 2012 doi: 10.1371/journal.pgen.1002666. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu CJ, Zhulin IB. ANTAR: an RNA-binding domain in transcription antitermination regulatory proteins. Trends Biochem Sci. 2002;27:3–5. doi: 10.1016/s0968-0004(01)02036-9. [DOI] [PubMed] [Google Scholar]
- Singh KV, Qin X, Weinstock GM, Murray BE. Generation and testing of mutants of Enterococcus faecalis in a mouse peritonitis model. J Infect Dis. 1998;178:1416–1420. doi: 10.1086/314453. [DOI] [PubMed] [Google Scholar]
- Tannock GW, Cook G. Enterococci as members of the intestinal microflora of humans. In: Gilmore MS, editor. The enterococci: pathogenesis, molecular biology, and antibiotic resistance. ASm Press; Washinton, DC: 2002. pp. 101–132. [Google Scholar]
- Thomas VC, Thurlow LR, Boyle D, Hancock LE. Regulation of autolysis-dependent extracellular DNA release by Enterococcus faecalis extracellular proteases influences biofilm development. J Bacteriol. 2008;190:5690–5698. doi: 10.1128/JB.00314-08. [DOI] [PMC free article] [PubMed] [Google Scholar]