Abstract
PTPN22 encodes a tyrosine phosphatase that inhibits Src-family kinases responsible for antigen receptor signaling in lymphocytes, and is strongly linked with susceptibility to a number of autoimmune diseases. As strength of TCR signal is critical to the thymic selection of regulatory T cells (Tregs) we examined the effect of murine PTPN22 deficiency on Treg development and function. In the thymus, numbers of pre-Tregs and Tregs increased inversely with the level of PTPN22. This increase in Tregs persisted in the periphery and could play a key part in the reduced severity observed in the PTPN22 deficient mice of experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis. This could explain the lack of association of certain autoimmune conditions with PTPN22 risk alleles.
Introduction
PTPN22 encodes a phosphatase known as lymphoid tyrosine phosphatase (Lyp) in humans and PEST-enriched protein tyrosine phosphatase (Pep) in mice. PTPN22 is expressed in T, B, NK and dendritic cells (1). It has been most widely studied in T cells where it functions to dephosphorylate key members of the TCR signaling pathway including ZAP 70, Src family kinases such as Lck, and VAV (2, 3).
Interest has been generated in this gene due to the observation that a Lyp polymorphism, R620W, is associated with a number of human autoimmune conditions including type I diabetes (T1D), rheumatoid arthritis, systemic lupus erythematosus (SLE) and Graves disease (4-6). Of interest, no association or a negative association has been observed between this polymorphism and other autoimmune diseases including multiple sclerosis and Crohn’s disease (7, 8).
The functional significance of this polymorphism and the molecular basis underlying these autoimmune associations is unclear. Several studies have suggested the mutation results in a gain-of-function in the phosphatase that reduces TCR signaling, a paradoxical observation considering its association with autoimmunity (6, 9, 10). It has been hypothesized this decreased signaling may alter thymic selection thresholds leading to escape of autoreactive T cells into the periphery, or decrease Treg number and/or function. However, others have reported that the consequence of this polymorphism is a decrease in phosphatase activity and increased signaling, resulting in an altered signaling threshold in T and B lymphocytes and dendritic cells (11, 12). Recent studies in which the Pep phosphatase in mice was mutated to express the equivalent of the Lyp R620W disease associated polymorphism suggested this results in rapid degradation of the phosphatase, thereby resulting in increased responsiveness in T cells and dendritic cells (12). These same investigators also demonstrated reduced levels of Lyp in human T cells homozygous for the risk related allele, suggesting reduced levels of phosphatase may be associated with disease.
We hypothesized that alteration in PTPN22 activity should affect Treg development, function and homeostasis. Changes in TCR signaling may alter the number of Tregs generated in the thymus as thymic selection is dependent on the strength of signal received by the developing thymocyte and Tregs require greater strength of signal than conventional T cells during development (13-17). PTPN22 directly affects ZAP 70 signaling, a key molecule upstream of several different pathways which could affect Foxp3 expression (3, 18). Furthermore, Treg development and homeostasis is influenced by IL-2 and other related common γ chain cytokines(19-23). If PTPN22 expression were to influence genes upstream of these cytokines and their receptors this could lead to increased development and homeostasis of Tregs.
In this study we have used PTPN22 deficient mice to investigate the impact on Treg development and function of altered levels of PTPN22. We show that thymic numbers of Tregs are increased in the absence of PTPN22, a trend that continues in the periphery and results in a functional outcome in a mouse model of autoimmunity.
Materials and Methods
Mice
Experimental procedures were carried out according to the National Institutes of Health Guide for the care and Use of Laboratory Animals. PTPN22 −/− mice were obtained from Dr. Andrew Chan (Genentech, San Francisco, CA) and have previously been described (24). PTPN22+/− mice were obtained by interbreeding PTPN22 −/− mice with C57BL/6 (Jackson laboratories). FoxP3 GFP mice were provided by Dr. Mitch Kronenberg (La Jolla Institute of Allergy and Immunology) with permission from Dr. Alexander Rudensky (Memorial Sloan-Kettering Cancer Center).
EAE induction
Female mice, at least 9 weeks old, were injected s.c. with 200 μg of MOG 35-55 (Genscript, Piscataway, NJ) in complete freunds adjuvant (Difco, Detroit, MI), half injected behind the neck and the other half at the base of the tail. Two hours later mice were injected i.p. with 300 ng of Pertussis Toxin (List Biologicals, Campbell, CA), and another injection was given 24 hours later. Mice were assessed daily for 28 days for signs of disease using the following clinical scores: 0: no disease; 1: Limp tail; 2: hind limb weakness, wobbly walk; 3: hind limb paralysis; 4: front limb paralysis; 5:death/moribund.
For Treg depletion experiments, mice were also injected i.p. with 300 μg of anti-CD25 antibody (PC-61) (TSRI antibody core, La Jolla, CA) on day −1 and day 4.
Flow cytometry
Cells to be stained were resuspended in FACS buffer (HBSS containing 1% FCS) and incubated with the indicated antibodies for 15 minutes on ice. Cells were then washed in FACS buffer before acquisition on an LSR-II flow cytometer (BD Bioscience, Franklin Lakes, NJ) and analysis using Flowjo (Treestar). Antibodies used were anti-mouse CD4 FITC and PerCP (both BD), CD8 Pacific Blue/PerCP (both Biolegend, San Diego, CA), CD25 APC and FITC, GITR-Biotin, CD122-Biotin (all Biolegend). Biotinylated antibodies were detected with strepavidin-Fitc or PerCP (BD Bioscience). For intracellular staining of Treg markers, an intracellular staining kit (Fix/Perm, eBioscience, San Diego CA) was used together with anti-mouse Foxp3 PE (Ebioscience), Helios Pacific Blue (Biolegend) and CTLA-4 APC (Biolegend). For cell sorting, splenocyte and thymocyte single cell suspensions were antibody labeled as described above and the desired populations were sorted using FACS Aria (BD Bioscience) or Mo Flo XDP (Beckman Coulter, Indianapolis, IN) by TSRI Flow cytometry core facility.
IL-2 stimulation and pSTAT 5 staining
FACS sorted CD4+ CD8− T cells from spleens were incubated in serum free RPMI (Invitrogen, Grand Island, NY) for 30 minutes at 37°C. Cells were then washed and resuspended in cRPMI with 20 ng/ml IL-2 (kindly provided by Dr. Charles Surh, TSRI) for 0, 2, 5 and 20 minutes at 37°C. Cells were immediately fixed using 2% v/v paraformaldehyde (Electron Micoscopy Sciences, Hatfield, PA) and incubated at room temperature for 10 minutes. Cells were then resuspended in 90% ice-cold methanol (Sigma Aldrich, St Louis, MO) and incubated at −20° for 30 minutes. Cells were then surface stained and pSTAT5 was stained using anti-human pSTAT5 (BD)
Calcium flux
Thymocytes were rested in cRPMI for 20 mins at 37°C, before staining. Thymocytes from one genotype (5×106 ) were labeled with 1μg Cy5 dye in cRPMI (GE healthcare, Chalfont, UK) for 5 mins at room temperature and the other genotype was left unstained. Cy5 labeled cells were washed and mixed with unlabeled cells. These cells were then loaded with INDO-1 AM (Invitrogen) in RPMI media for 30 minutes at 37°C. Following washing the cells were stained with CD4 PerCP (BD), CD8 PeCy7 (BD) and CD3 biotin (Ebioscience) and resuspended in HBSS (Gibco) and kept on ice. Cells were then warmed to 37°C prior to running the sample and ran for 30 seconds to establish a baseline, 10μg/ml Strepavidin-PE (Invitrogen) was added at 30 seconds to cross-link CD3. At 2 minutes, 10mM CaCl2 (Sigma Aldrich) was added and the cells run until 5 minutes. At 5 minutes 1μg/ml of ionomycin (EMD Biosciences, La Jolla, CA) was added and the sample run until 7 minutes.
Quantitative RT-PCR
Relative expression levels of PTPN22 were measured in each cell subpopulation by qPCR. Cell lysis, cDNA synthesis, and qPCR were performed using the Power SYBR® Green Cells-to-CT™ Kit (Ambion, Austin, TX) and a Roche Lightcycler 480 (Roche Applied Science, Indianapolis, IN). Primers were purchased from SABiosciences (Frederick, MD). PTPN22 expression levels were first normalized to the expression levels of the housekeeping gene RNA Polymerase II and then calculated relative to the PTPN22 expression in the CD4+ Teffs. For error analysis, the standard deviation of each sample was calculated by the square root of the sum of the squares of the standard deviations of the triplicate Cp values of the PTPN22 and RNA Polymerase II. The standard deviations of each sample were used to calculate the upper and lower ranges of the relative fold expression values.
T cell suppression assay
Tregs were isolated from splenocytes using the CD4+ CD25+ Regulatory T cell isolation kit according to manufacturers instructions (Miltenyi Biotec Inc. Auburn, CA). The remaining CD4+ CD25− cells were CFSE labeled and 3 × 104 cells were plated in a 96 well plate along with 1 × 105 irradiated splenocytes (30Gy). Tregs were titrated into the cultures at varying ratios of Teff:Treg and 1μg/ml soluble anti-CD3 antibody (Ebioscience) was added. The cells were cultured for 72 or 96 hours at 37°C with 5% CO2. Flow cytometry was carried out to assess proliferation of Teff cells.
Intracellular cytokine staining
Draining lymph nodes were harvested from mice that had been induced with EAE 10 days previously. Cells were counted and seeded in a 6 well plate at 1×106 cells/ml. Brefeldin A was added to the culture at 5μg/ml (Sigma Aldrich). Cells were stimulated with either MOG 35-55 peptide (30μg/ml), PMA and ionomycin (5ng/ml and 500ng/ml respectively) or left unstimulated for 5 hours at 37°C, 5% CO2. Cells were then harvested and fixed with Cytofix/Cytoperm buffer (BD bioscience) for 15 minutes at 4°C. Cells were then washed in Perm/wash solution (BD bioscience) and stained with anti-mouse IL-17A PE and IFN-γ APC antibodies (both Biolegend).
Statistical Analysis
All analyses were performed using a one way ANOVA test with Tukey post test unless. Exceptions being Figure 1E which is detailed in the Quantitative PCR method above, and Figues 7A and B which used a Mann Whitney U test. All tests were performed by Prism Graphpad software (Prism, La Jolla, CA).
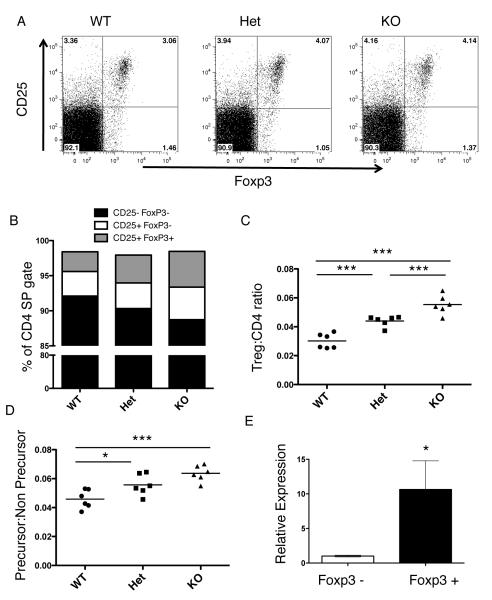
Figure 1.
PTPN22 deficiency results in increased Tregs in the thymus. The thymus from wild type (WT), PTPN22+/− (Het) and PTPN22−/− (KO) mice were removed and stained for CD8, CD4, Foxp3 and CD25. A. Representative dot plots of the CD4 SP subset gating based on CD25 and Foxp3 expression. B. Subsets found within the CD4+ CD8− (CD4 SP) population in the thymus based on Foxp3 and CD25 expression. C. The Treg:CD4 ratio in the thymus, comparing CD4+ Foxp3+ to CD4+ Foxp3− cells. D. Ratio of Treg precursors (CD4+ Foxp3− CD25+) compared to Treg non-precursors (CD4+ Foxp3− CD25−) for each of the three genotypes. E. CD4+ Teff (CD4+ CD8− GFP−) and Treg (CD4+ CD8− GFP+) from the thymus of B6 mice expressing EGFP under the control of the mouse Foxp3 promoter were FACS sorted. Relative expression levels of PTPN22 were measured in each cell subpopulation by qPCR. Graphs show relative fold expression of PTPN22 with 95% confidence intervals shown. Each graph is representative of 3 experiments using 3 littermate pairs of mice.***, p< 0.001; **, p<0.01; *, p< 0.05
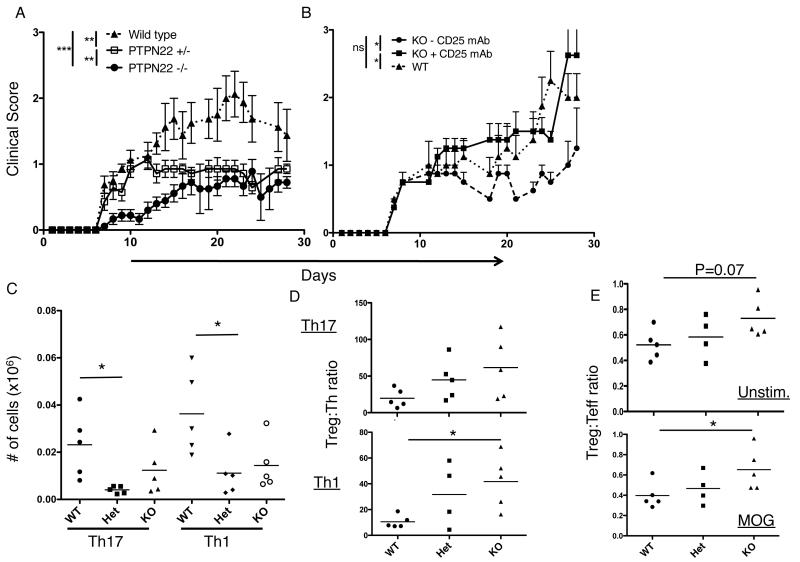
Figure 7.
PTPN22 deficient mice are protected from EAE. A. EAE was induced in female mice at 9 weeks of age by s.c. injection of MOG35-55 peptide in CFA followed by i.p. injection of pertussis toxin on days 1 and 2. Mice were scored each day for 28 days (n= WT 8, Het 7, KO 9). This experiment was performed twice with WT and KO mice n=4 . B. EAE was induced in WT and KO mice on day 1 as described and mice received an i.p. injection of anti-CD25 depleting antibody on days −1 and +4 (n= WT, 5; KO, 4; KO + CD25mAb, 4). C. EAE was induced as described in previously and draining lymph nodes were harvested at day 10 then restimulated with MOG for 5 hours then stained for IL-17 and IFN-γ. D. Harvested LN cells were restimulated with MOG peptide and stained for IL-17, IFN-γ and FoxP3, the Treg:Th ratio is plotted for both Th17 and Th1 cells. E. Harvested LN cells were stained immediately ex vivo (unstimulated, top panel) or restimulated with MOG peptide (bottom panel) as in part D. Treg/Teff ratio is plotted (effector CD4 cells are defined as CD4+ CD44hi Foxp3−). ***, p< 0.001; **p<0.01; *, p< 0.05
Results
Tregs are increased in the thymus of PTPN22 deficient mice
As previously reported (24), we observed that PTPN22 deficiency does not affect the numbers and proportions of the major developing T cell subsets in the thymus (data not shown). In order to identify Tregs and their precursors among thymic CD4+ populations from wild type (WT), PTPN22+/− (Het) and the PTPN22−/− (KO) mice, thymocytes were analyzed for expression of Foxp3 and CD25 (Figure 1A). Comparing the numbers of Tregs (CD4+ Foxp3+), a significantly higher ratio of Tregs to CD4 cells was observed in both the Het and the KO mice as compared with WT (Figure 1B and C). Foxp3 and CD25 staining was used to distinguish Tregs (CD4+ Foxp3+ CD25+) from Treg precursor populations (CD4+ Foxp3− CD25+) (22). An increase was observed in the proportion of both Tregs and their precursors in the CD4 compartment of the Het and KO mice as compared to WT (Fig 1B and C). This can be seen as a significant difference when comparing the ratio of precursors to non-precursors (CD4+Foxp3−CD25−) (Figure 1D). Overall these data demonstrate increased numbers of Tregs and their precursor population in the thymus of PTPN22 deficient mice. Furthermore, this occurs in a dose dependent manner with the KO mice exhibiting higher percentages of Treg populations than the Het.
Based on these results, we would anticipate that PTPN22 should be expressed in thymic Tregs. Examination by quantitative real-time PCR confirmed higher levels of expression of PTPN22 in CD4+Foxp3+ thymocytes relative to conventional CD4 single positive thymocytes (Fig. 1E).
In addition to increased numbers of Tregs, we observed a slight but non-significant increase in the level of expression of CD25 on thymic Tregs in Het and KO mice (Figure 2A and B). This difference is not apparent at the precursor stage (Figure 2C) but appears after Foxp3 is expressed. We also analyzed CD122 expression, the β chain of the IL-2 receptor, but found no difference in its expression by PTPN22 KO, Het, or WT (Supplementary Figure 1).
Figure 2.
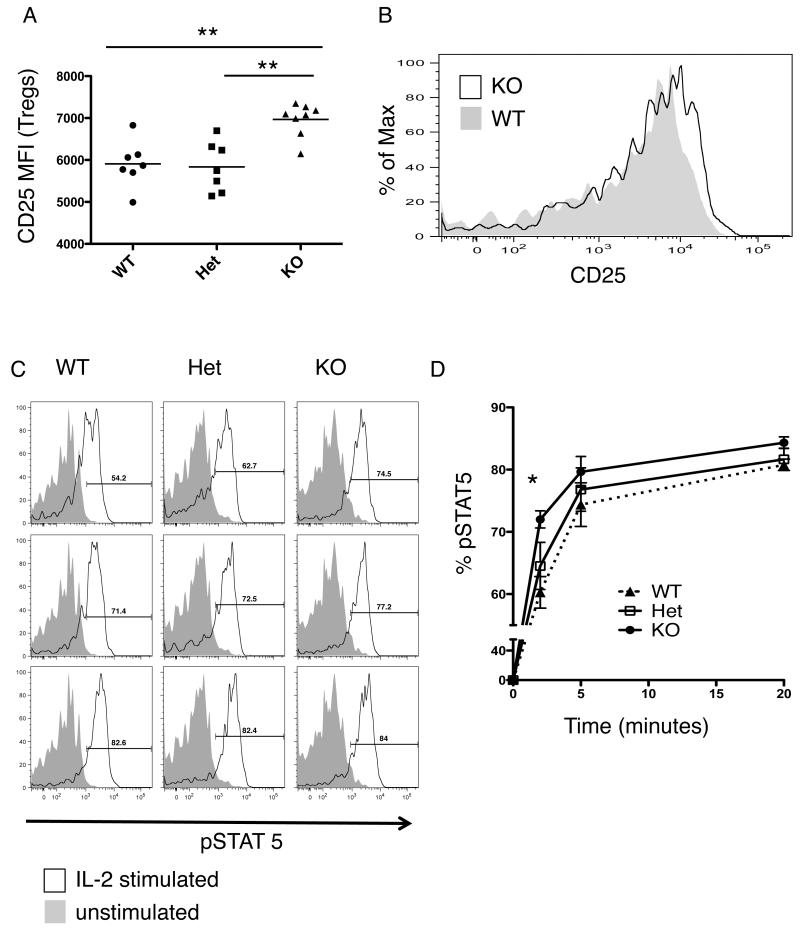
CD25 expression on thymic Tregs and Treg precursors. A. MFI of CD25 on Tregs (CD4+ Foxp3+ CD25+) in the thymus of WT, Het and KO mice. B. Representative histogram of CD25 expression on Tregs in the thymus of WT (filled plot) and KO mice (unfilled plot) (Het plot was not included for clarity). C. MFI of CD25 on Treg precursors (CD4+ Foxp3− CD25+).
Increased signaling in PTPN22 deficient cells
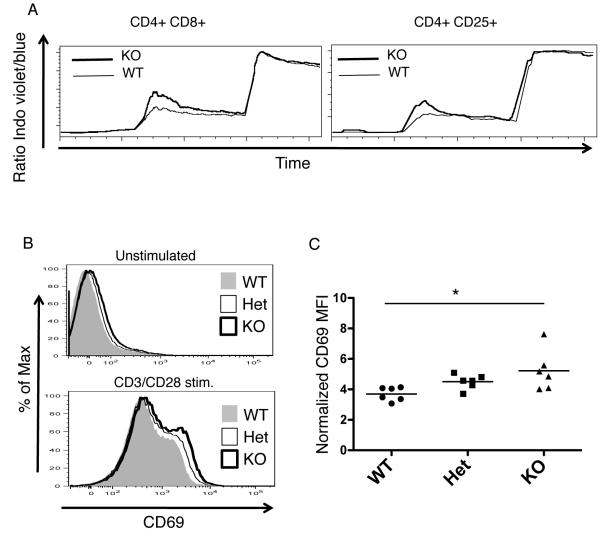
As both TCR and IL-2 receptor signals are believed to be major factors in thymic Treg development (20-22, 25), we next addressed whether loss of PTPN22 expression would alter TCR signaling in thymocytes. Ca2+ flux is a major early consequence of TCR signaling initiated by PLCγ1 and results in NFAT translocation into the nucleus (26). CD3 stimulation resulted in a greater increase in Ca2+ flux in the double positive thymocytes of PTPN22 KO mice as compared to WT mice (Fig 3A) and also in the CD4+ CD25+ population of thymocytes, which contains both Tregs and their precursors. CD69 expression, another indicator of strength of TCR signaling (27, 28), was slightly higher on double positive thymocytes from KO and Het mice than on this same population from WT mice both in ex vivo, unstimulated thymocytes and those stimulated overnight with anti-CD3 and anti-CD28 (Figure 3B and C).
Figure 3.
Signaling differences in the thymus of PTPN22 deficient vs. WT mice. A, Calcium flux in double positive (DP) and CD4+CD25+ thymocytes derived from wild type (thin line) or KO (thick line) mice. Cells were pre-labeled with anti-CD3 biotin antibody and stimulated at 30 seconds with strepavidin. Exogenous calcium was added at 120 seconds and ionomycin at 300 seconds. For the experiment shown, WT cells were labeled with Cy5 and mixed with unlabeled KO cells to stimulate under identical conditions. During the same experiment the Cy5 labeling was reversed to ensure it did not interfere with Ca Flux. This is an example of one of 4 separate experiments (WT n=8 KO n=8). B. CD69 expression on DP thymocytes from WT (filled plot), Het (unfilled, thin line) and KO (unfilled, thick line) mice either from unstimulated thymocytes or CD3/CD28 stimulated thymocytes (overnight stimulation). C, Normalized MFI of CD69 on CD3/CD28 stimulated DP thymocytes. *, p< 0.05
Overall, these data suggest there is increased signaling downstream of the TCR in developing thymocytes due to a loss of PTPN22 expression.
Tregs are increased in the periphery of PTPN22 deficient mice
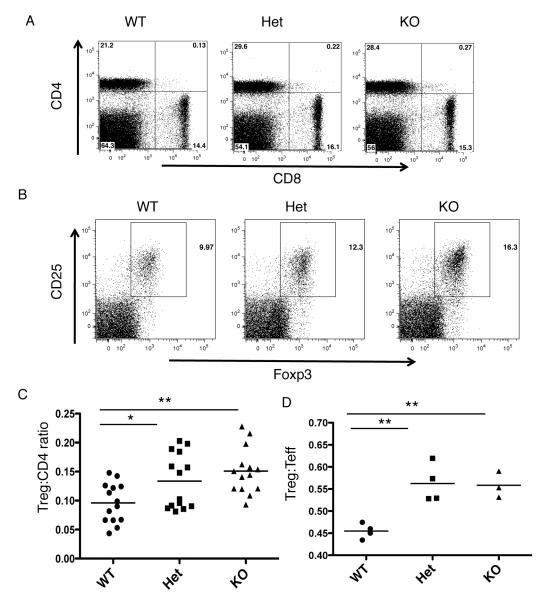
We next sought to determine whether the increased Treg numbers were maintained in the periphery of PTPN22 deficient mice. Examination of the splenic composition revealed that there may be a increase in the proportions of CD4 T cells in the spleen (Figure 4A), and interestingly the percentage of Tregs increased resulting in a significantly higher ratio of Tregs:CD4 cells in the PTPN22 Het and KO mice compared to the WT (Figure 4B and C). Comparison of Tregs:effector CD4 cells (CD4+ CD44hi Foxp3−) revealed significantly higher ratios in the PTPN22 Het and KO as compared to the WT mice (Figure 4D).
Figure 4.
PTPN22 deficiency results in increased peripheral Tregs. Spleens were removed from WT, Het and KO mice and stained for CD4, CD8, Foxp3, CD25. A. Representative CD4 vs. CD8 plots for the three genotypes of mice. B. Representative dot plots showing CD25 and Foxp3 expression of the CD4 gate on WT, Het and KO splenocytes C. Treg:CD4 ratio comparing the CD4+ Foxp3+ cells to CD4+ Foxp3− cells in the spleen of the three groups. D. Treg: effector CD4 ratio in the spleen of the three genotypes. Effector CD4 cells are defined as CD4+ CD44hi Foxp3− **p<0.01; *, p< 0.05
CD25 is increased on peripheral Tregs from PTPN22 KO mice
CD25 expression was also found to be increased in peripheral Tregs from PTPN22 KO mice as compared to WT and Het mice (Figures 5A and 5B). Following stimulation of splenocytes with IL-2, we measured pSTAT5 levels by flow cytometry as a downstream readout of signaling through CD25/IL-2 receptor. CD4+ CD25+ cells from PTPN22 KO mice show higher pSTAT5 and significantly more rapid kinetics of phosphorylation of the STAT 5 molecule compared to WT mice (Figure 5C and D). Overall these data show that the increased CD25 levels on Tregs from PTPN22 KO mice observed in the thymus are maintained in the periphery. This results in a greater ability to phosphorylate STAT5, an important inducer of transcription and stabilization of Foxp3 (20).
Figure 5.
CD25 expression on peripheral Tregs of PTPN22 deficient mice. A. MFI of CD25 on Tregs (CD4+ Foxp3+) in the spleen of WT, Het and KO mice. B. Representative histogram showing CD25 expression on CD4+ Foxp3+ Tregs from WT (filled plot) and KO (unfilled plot) mice. C. CD4+ cells were purified from spleens of WT, Het and KO mice and serum starved for 20 minutes. IL-2 was then added in complete culture medium and the cells were incubated for the designated time points. Cells were then fixed and stained for pSTAT 5 by flow cytometry. CD4+ CD25+ cells were gated and the unstimulated plot (filled plot) for each genotype is overlayed with the stimulated plot (unfilled plot) (this is representative 3 separate experiments n= 6 mice per group). D, line graph representing the histograms shown in part C showing the kinetics of pSTAT5, there is a statistical significance at 2mins between WT and KO groups. **p<0.01; *, p< 0.05
Increased Tregs can lead to protection from experimental autoimmune encephalomyelitis (EAE) in PTPN22 deficient mice
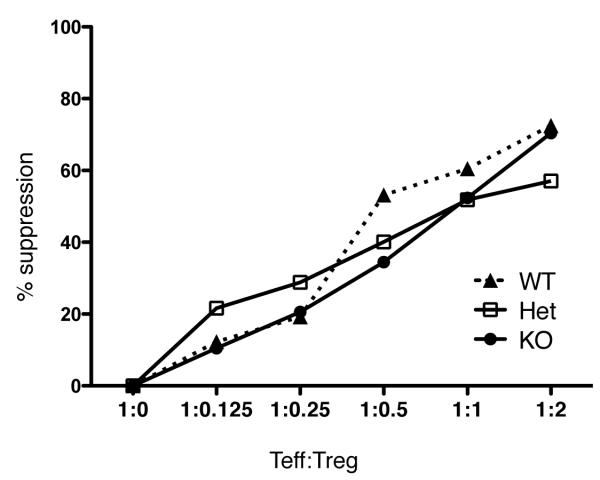
As the percentage of Tregs is increased in the periphery of PTPN22 deficient mice, we wished to determine whether this resulted in a functional effect in vivo. In vitro Treg assays using fixed numbers of Tregs to suppress the proliferation of CD4 effector cells revealed no difference in the suppressive capability of Tregs from the three genotypes (Figure 6) and analysis of the levels of expression of functional markers utilized by Tregs, such as CTLA-4, revealed no distinct differences in expression among Tregs from PTPN22 KO, Het or WT (Supplementary Figure 2). GITR showed a slight increase in expression on splenic Tregs from KO compared to WT mice (Supplementary Figure 2). However, it is often the case that changes in the ratio of Treg:Teff have profound consequences with respect to protection from autoimmune disease (29, 30).
Figure 6.
Suppressive capability of Tregs from PTPN22 deficient mice. CD4+ CD25+ Tregs were isolated from splenocytes from WT, Het and KO mice using magnetic separation. 3 × 104 CFSE labeled Teff (CD4+ CD25−) T cells were cultured in the presence of 1 × 105 irradiated splenocytes and soluble anti-CD3 antibody (1μg/ml). Tregs were titrated into the cultures at the ratios indicated. The cells were cultured for 72 hours and then proliferation was assessed by flow cytometry. This experiment is representative of 3 separate experiments. The 96 hour timepoint shows similar results from 2 separate experiments (data not shown).
In order to investigate the role played in vivo by the increased the Treg:Teff cell ratio observed in PTPN22 deficient mice, we induced EAE using myelin oligodendrocyte protein (MOG) peptide and pertussis toxin (Figure 7). This particular model was selected as it is well documented that Tregs can ameliorate EAE in vivo (29, 30). We observed a protective effect by the loss of PTPN22, as wild type mice exhibited significantly higher clinical scores than the Het and KO mice. Furthermore, reduction of the number of Tregs (~50%) using a CD25 depleting antibody caused an increase in the severity of EAE in the KO mice approaching wild type levels (Figure 7B). Analysis of T helper subsets and Treg numbers in the draining lymph nodes revealed that both Th1 and Th17 numbers were lower in KO and Het mice compared to wild type (Figure 7C). Furthermore the Treg:Th ratio was higher in KO mice compared to WT mice for both Th1 (significantly) and Th17 subsets, and the overall Treg:Teff ratio was higher both immediately ex vivo and significantly higher following MOG restimulation (Figure 7D and E). These data suggest that the increased number of Tregs observed in PTPN22 deficient mice has a functional consequence in an autoimmune setting.
Discussion
Although still somewhat controversial, recent evidence favors a model in which the autoimmune disease associated R620W polymorphism in the Pep/Lyp phosphatase results in a reduced level of the phosphatase (12). Thus, mice deficient in PTPN22 are excellent models in which to study the consequences of this polymorphism on the immune system. Previous work on PTPN22 KO mice focused mainly on its effects on thymic selection of conventional CD4 and CD8 T cells, and on peripheral naïve and effector/memory T cells. No effect on thymic deletion was observed, although positive selection was enhanced (24). Accumulating evidence suggests that Treg development requires affinity for self-antigens and some mutations that reduce the strength of TCR signaling during thymic selection, such as the mutation in the SH2 domain of ZAP 70 that occurs in SKG mice, result in autoimmune disease due to a reduction in the production of Tregs (31). Here we have examined the effect of altered levels of PTPN22 expression on Treg development, homeostasis and function. We observed that PTPN22 deficiency enhances Treg numbers in both the thymus and periphery.
Interestingly when analyzing the expression of PTPN22 in different cell types we observed an increase in PTPN22 mRNA in CD4+ Foxp3+ cells in the thymus compared to CD4+ Foxp3− cells. This is in contrast to the periphery where we have confirmed results published by Marson et al. showing higher PTPN22 in Foxp3− cells compared to Foxp3+ cells (Supplementary Figure 3)(32). They suggested Foxp3 has a negative regulatory role on expression of PTPN22. We hypothesize that this difference between the thymus and periphery may be due to stronger signaling in the thymus resulting in upregulation of PTPN22 as a feedback mechanism, which is eventually reduced by Foxp3 expression in the periphery. Overall, the expression of PTPN22 in Foxp3+ cells in the thymus suggested this gene might be playing an important role in Treg development.
Work by Lio and Hsieh has suggested that Treg development is a 2-step process (22). First, TCR signaling by self-antigens results in upregulation of CD25. Subsequent signaling through IL-2 activates STAT5 and contributes to Foxp3 transcription and the acquisition of a Treg phenotype (20, 22). As reported previously, we observed that PTPN22 deficiency results in increased Ca2+ flux among double positive thymocytes, which may be responsible for the increase in Treg precursors in PTPN22 KO and Het mice (11). In addition, we show Ca2+ flux is also increased in the CD4+ CD25+ population of thymocytes in KO compared to WT mice and may account for the larger number of Tregs. Unlike conventional CD4+CD25−Foxp3− thymocytes, the CD4+ CD25+ Foxp3− Treg precursors have the ability to upregulate Foxp3 in vitro upon treatment with IL-2 (22). Therefore it is likely the increased numbers of CD4+ CD25+ Foxp3− cells leads to increased numbers of Foxp3 expressing Tregs in the thymus.
Foxp3 regulation has been shown to be dependent on a number of transcription factors. Of particular relevance to this work is the observation that there are NFAT binding sites in the Foxp3 promoter, as well as the CD25 promoter (33, 34). We show here that Ca2+signaling, an important event upstream of activation of NFAT, is higher in Treg precursors in the KO mice. Based on the current literature we hypothesize that this leads to increased expression of Foxp3 and CD25. Thymic Tregs in PTPN22 KO mice demonstrated increased expression of CD25 compared to wild type mice. We did not observe an increase in another common γ-chain cytokine receptor, CD122 (Supplementary Figure 1). However, CD25 signaling can initiate STAT5 signals, which lead to transcription of the Foxp3 gene (35). Based on this data we propose that increased thymocyte signaling at the double positive stage of development can lead to an increased number of Tregs which are stabilized and maintained through CD25 dependent signals both in the thymus and periphery.
Peripheral Treg numbers were also increased in PTPN22 KO mice. This increase could be due to increased thymic output of nTregs or alternatively, a change in the iTreg compartment. We used the Helios marker to differentiate between the 2 subsets and found an increase in both types (Supplementary Figure 4). However recent reports in the literature suggest that Helios may not be as specific for thymically developed Tregs as first thought (36). Therefore, additional experiments will need to be performed to assess the effect of PTPN22 expression on peripheral Tregs. Despite increased CD25 on peripheral Tregs in KO mice, and their ability to rapidly phosphorylate STAT5 in response to IL-2 stimulation, we cannot say that homeostasis of peripheral Tregs is altered. Thymic output of Tregs is increased during PTPN22 deficiency, and this is maintained in the periphery at a ratio similar to that in WT mice. This suggests that the difference is generated in the thymus and maintained in the periphery. A possible mechanism, based on these data is that following increased thymic output of Tregs, peripheral signals through CD25 signaling can maintain and stabilize FoxP3 in these cells. The data suggest this is true for KO Tregs as these have more CD25 and can phosphorylate more STAT 5 in response to IL-2. However, Het Tregs and WT Tregs in the periphery look similar in terms of CD25 and pSTAT 5 and therefore the major difference between these mice is that thymic production of Tregs has been increased in the Het. Of interest, studies in which the numbers of Foxp3+ cells in the periphery of normal donors homozygous for the susceptible 620W allele and the protective allele were compared found significantly higher percentages of Tregs in donors expressing the 620W allele (unpublished observations by X. Castro Dopico, J. Todd and L.Wicker). This further supports the hypothesis that the susceptibility allele results in reduced levels of PTPN22.
In vitro Treg assays showed no increase in the suppressive ability of Tregs from KO mice compared to wild type mice. However, in such assays Treg numbers were kept constant amongst the genotypes tested and did not reflect the true situation in vivo where we find increased numbers of Tregs. To address the functional consequence of increased Tregs we used an in vivo model of autoimmunity. EAE is a mouse model of multiple sclerosis induced, in this case, by injection of MOG peptide with pertussis toxin to activate MOG specific T cells. Previous studies in this model have shown that disease can be inhibited by Tregs (29, 30). After inducing the disease in WT, Het and KO mice we observed that Het and KO mice developed significantly less disease than the wild type mice. We have shown that increased T cell signals through loss of PTPN22 can lead to increased Tregs. EAE protection in Het and KO mice could be dependent on this higher number of Tregs as depletion of these Tregs by anti-CD25 antibody increased disease severity in the KO mouse.
Our analysis demonstrates that PTPN22 can affect Treg development and as a result alter the balance of Teff cells to Tregs in the periphery. The reduction in PTPN22 does not appear to result in higher suppressive activity by these Tregs, but the increased ratio of Tregs to effector T cells can suppress the onset of EAE. The data presented here show that although there are more Tregs in the Het compared to the WT mice, the CD25 levels are similar and the kinetics of pSTAT5 upregulation is similar. This could explain the result in Figure 7A showing the Het is not as protective in the initial stages of EAE as the KO mouse which could in part result from the less stable nature of the Treg phenotype in these mice. However the Het does show a protective phenotype over the course of the disease, future experiments will explore whether a loss of PTPN22 could be playing a role in Th1/Th17 differentiation and/or activity.
It is of interest that multiple sclerosis is one autoimmune disease in which the risk allele of PTPN22 is not associated with increased disease (7, 8). It has been speculated that one difference between PTPN22 associated diseases (rheumatoid arthritis, type 1 diabetes, systemic lupus erythematosus and Hashimotos thyroiditis) and non-associated diseases (MS, Crohn’s and colitis) is the importance of a humoral component in the former that appears to be much less prominent in the latter (7). In a recent study the risk allele of PTPN22 has been associated with incomplete deletion of autoreactive B cells (12). It may be speculated that autoimmune diseases that are strongly associated with the presence of autoantibodies may be less susceptible to inhibition by endogenous Tregs. In fact, there is evidence that Treg numbers are not deficient during development of T1D and may in fact be increased, although their ability to survive may be impaired, in part due to defects in the CD25R-IL-2 pathway (37-39).
These data further our understanding of PTPN22 function in cell types that have not been explored previously and suggest that if risk alleles in PTPN22 result in reduced levels of phosphatase, this does not jeopardize Treg production or survival and are likely to contribute to autoimmunity through a different mechanism, such as enhanced responsiveness by effector cells, autoreactive B cells, and dendritic cells. Future studies will examine the consequence of PTPN22 deficiency in these cells.
Supplementary Material
Acknowledgements
We would like to acknowledge Kristi Marquardt for breeding and technical assistance. Also TSRI FACS core facility for assistance and sorting.
1. This research was supported by grants AI070351 and AI050864 from the National Institutes of Health. LSW is supported by grants from the JDRF and the Wellcome Trust. The Cambridge Institute for Medical Research is the recipient of a Wellcome Trust Strategic Award (079895).
Footnotes
The authors have no conflict of interest.
References
- 1.Matthews RJ, Bowne DB, Flores E, Thomas ML. Characterization of hematopoietic intracellular protein tyrosine phosphatases: description of a phosphatase containing an SH2 domain and another enriched in proline-, glutamic acid-, serine-, and threonine-rich sequences. Mol Cell Biol. 1992;12:2396–2405. doi: 10.1128/mcb.12.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cloutier JF, Veillette A. Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase. J Exp Med. 1999;189:111–121. doi: 10.1084/jem.189.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu J, Katrekar A, Honigberg LA, Smith AM, Conn MT, Tang J, Jeffery D, Mortara K, Sampang J, Williams SR, Buggy J, Clark JM. Identification of substrates of human protein-tyrosine phosphatase PTPN22. J Biol Chem. 2006;281:11002–11010. doi: 10.1074/jbc.M600498200. [DOI] [PubMed] [Google Scholar]
- 4.Todd JA, Walker NM, Cooper JD, Smyth DJ, Downes K, Plagnol V, Bailey R, Nejentsev S, Field SF, Payne F, Lowe CE, Szeszko JS, Hafler JP, Zeitels L, Yang JH, Vella A, Nutland S, Stevens HE, Schuilenburg H, Coleman G, Maisuria M, Meadows W, Smink LJ, Healy B, Burren OS, Lam AA, Ovington NR, Allen J, Adlem E, Leung HT, Wallace C, Howson JM, Guja C, Ionescu-Tirgoviste C, Simmonds MJ, Heward JM, Gough SC, Dunger DB, Wicker LS, Clayton DG. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39:857–864. doi: 10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Criswell LA, Pfeiffer KA, Lum RF, Gonzales B, Novitzke J, Kern M, Moser KL, Begovich AB, Carlton VE, Li W, Lee AT, Ortmann W, Behrens TW, Gregersen PK. Analysis of families in the multiple autoimmune disease genetics consortium (MADGC) collection: the PTPN22 620W allele associates with multiple autoimmune phenotypes. Am J Hum Genet. 2005;76:561–571. doi: 10.1086/429096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, MacMurray J, Meloni GF, Lucarelli P, Pellecchia M, Eisenbarth GS, Comings D, Mustelin T. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004;36:337–338. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- 7.De Jager PL, Sawcer S, Waliszewska A, Farwell L, Wild G, Cohen A, Langelier D, Bitton A, Compston A, Hafler DA, Rioux JD. Evaluating the role of the 620W allele of protein tyrosine phosphatase PTPN22 in Crohn’s disease and multiple sclerosis. Eur J Hum Genet. 2006;14:317–321. doi: 10.1038/sj.ejhg.5201548. [DOI] [PubMed] [Google Scholar]
- 8.Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R, Anderson CA, Bis JC, Bumpstead S, Ellinghaus D, Festen EM, Georges M, Green T, Haritunians T, Jostins L, Latiano A, Mathew CG, Montgomery GW, Prescott NJ, Raychaudhuri S, Rotter JI, Schumm P, Sharma Y, Simms LA, Taylor KD, Whiteman D, Wijmenga C, Baldassano RN, Barclay M, Bayless TM, Brand S, Buning C, Cohen A, Colombel JF, Cottone M, Stronati L, Denson T, De Vos M, D’Inca R, Dubinsky M, Edwards C, Florin T, Franchimont D, Gearry R, Glas J, Van Gossum A, Guthery SL, Halfvarson J, Verspaget HW, Hugot JP, Karban A, Laukens D, Lawrance I, Lemann M, Levine A, Libioulle C, Louis E, Mowat C, Newman W, Panes J, Phillips A, Proctor DD, Regueiro M, Russell R, Rutgeerts P, Sanderson J, Sans M, Seibold F, Steinhart AH, Stokkers PC, Torkvist L, Kullak-Ublick G, Wilson D, Walters T, Targan SR, Brant SR, Rioux JD, D’Amato M, Weersma RK, Kugathasan S, Griffiths AM, Mansfield JC, Vermeire S, Duerr RH, Silverberg MS, Satsangi J, Schreiber S, Cho JH, Annese V, Hakonarson H, Daly MJ, Parkes M. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiorillo E, Orru V, Stanford SM, Liu Y, Salek M, Rapini N, Schenone AD, Saccucci P, Delogu LG, Angelini F, Bitti M. L. Manca, Schmedt C, Chan AC, Acuto O, Bottini N. Autoimmune-associated PTPN22 R620W variation reduces phosphorylation of lymphoid phosphatase on an inhibitory tyrosine residue. J Biol Chem. 2010;285:26506–26518. doi: 10.1074/jbc.M110.111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arechiga AF, Habib T, He Y, Zhang X, Zhang ZY, Funk A, Buckner JH. Cutting edge: the PTPN22 allelic variant associated with autoimmunity impairs B cell signaling. J Immunol. 2009;182:3343–3347. doi: 10.4049/jimmunol.0713370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zikherman J, Hermiston M, Steiner D, Hasegawa K, Chan A, Weiss A. PTPN22 deficiency cooperates with the CD45 E613R allele to break tolerance on a non-autoimmune background. J Immunol. 2009;182:4093–4106. doi: 10.4049/jimmunol.0803317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Zahir N, Jiang Q, Miliotis H, Heyraud S, Meng X, Dong B, Xie G, Qiu F, Hao Z, McCulloch CA, Keystone EC, Peterson AC, Siminovitch KA. The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness. Nat Genet. 2011;43:902–907. doi: 10.1038/ng.904. [DOI] [PubMed] [Google Scholar]
- 13.Carter JD, Calabrese GM, Naganuma M, Lorenz U. Deficiency of the Src homology region 2 domain-containing phosphatase 1 (SHP-1) causes enrichment of CD4+CD25+ regulatory T cells. J Immunol. 2005;174:6627–6638. doi: 10.4049/jimmunol.174.11.6627. [DOI] [PubMed] [Google Scholar]
- 14.Wong P, Barton GM, Forbush KA, Rudensky AY. Dynamic tuning of T cell reactivity by self-peptide-major histocompatibility complex ligands. J Exp Med. 2001;193:1179–1187. doi: 10.1084/jem.193.10.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 16.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 17.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3−expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 20.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 21.Vang KB, Yang J, Mahmud SA, Burchill MA, Vegoe AL, Farrar MA. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J Immunol. 2008;181:3285–3290. doi: 10.4049/jimmunol.181.5.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayer AL, Yu A, Malek TR. Function of the IL-2R for thymic and peripheral CD4+CD25+ Foxp3+ T regulatory cells. J Immunol. 2007;178:4062–4071. doi: 10.4049/jimmunol.178.7.4062. [DOI] [PubMed] [Google Scholar]
- 24.Hasegawa K, Martin F, Huang G, Tumas D, Diehl L, Chan AC. PEST domain-enriched tyrosine phosphatase (PEP) regulation of effector/memory T cells. Science. 2004;303:685–689. doi: 10.1126/science.1092138. [DOI] [PubMed] [Google Scholar]
- 25.Soper DM, Kasprowicz DJ, Ziegler SF. IL-2Rbeta links IL-2R signaling with Foxp3 expression. Eur J Immunol. 2007;37:1817–1826. doi: 10.1002/eji.200737101. [DOI] [PubMed] [Google Scholar]
- 26.Masuda ES, Imamura R, Amasaki Y, Arai K, Arai N. Signalling into the T-cell nucleus: NFAT regulation. Cell Signal. 1998;10:599–611. doi: 10.1016/s0898-6568(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 27.Brandle D, Muller S, Muller C, Hengartner H, Pircher H. Regulation of RAG-1 and CD69 expression in the thymus during positive and negative selection. Eur J Immunol. 1994;24:145–151. doi: 10.1002/eji.1830240122. [DOI] [PubMed] [Google Scholar]
- 28.Jung LK, Haynes BF, Nakamura S, Pahwa S, Fu SM. Expression of early activation antigen (CD69) during human thymic development. Clin Exp Immunol. 1990;81:466–474. doi: 10.1111/j.1365-2249.1990.tb05357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Teige I, Birnir B, Issazadeh-Navikas S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat Med. 2006;12:518–525. doi: 10.1038/nm1402. [DOI] [PubMed] [Google Scholar]
- 30.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka S, Maeda S, Hashimoto M, Fujimori C, Ito Y, Teradaira S, Hirota K, Yoshitomi H, Katakai T, Shimizu A, Nomura T, Sakaguchi N, Sakaguchi S. Graded attenuation of TCR signaling elicits distinct autoimmune diseases by altering thymic T cell selection and regulatory T cell function. J Immunol. 2010;185:2295–2305. doi: 10.4049/jimmunol.1000848. [DOI] [PubMed] [Google Scholar]
- 32.Marson A, Kretschmer K, Frampton GM, Jacobsen ES, Polansky JK, MacIsaac KD, Levine SS, Fraenkel E, von Boehmer H, Young RA. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 2007;445:931–935. doi: 10.1038/nature05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mantel PY, Ouaked N, Ruckert B, Karagiannidis C, Welz R, Blaser K, Schmidt-Weber CB. Molecular mechanisms underlying FOXP3 induction in human T cells. J Immunol. 2006;176:3593–3602. doi: 10.4049/jimmunol.176.6.3593. [DOI] [PubMed] [Google Scholar]
- 34.Schuh K, Twardzik T, Kneitz B, Heyer J, Schimpl A, Serfling E. The interleukin 2 receptor alpha chain/CD25 promoter is a target for nuclear factor of activated T cells. J Exp Med. 1998;188:1369–1373. doi: 10.1084/jem.188.7.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW, Vegoe AL, Hsieh CS, Jenkins MK, Farrar MA. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28:112–121. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, Ziegelbauer J, Yassai M, Li SH, Relland LM, Wise PM, Chen A, Zheng YQ, Simpson PM, Gorski J, Salzman NH, Hessner MJ, Chatila TA, Williams CB. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35:109–122. doi: 10.1016/j.immuni.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brusko T, Wasserfall C, McGrail K, Schatz R, Viener HL, Schatz D, Haller M, Rockell J, Gottlieb P, Clare-Salzler M, Atkinson M. No alterations in the frequency of FOXP3+ regulatory T-cells in type 1 diabetes. Diabetes. 2007;56:604–612. doi: 10.2337/db06-1248. [DOI] [PubMed] [Google Scholar]
- 38.Putnam AL, Vendrame F, Dotta F, Gottlieb PA. CD4+CD25high regulatory T cells in human autoimmune diabetes. J Autoimmun. 2005;24:55–62. doi: 10.1016/j.jaut.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.