Abstract
Current therapies for systemic lupus erythematosus (SLE), a debilitating, potentially lethal, multifactorial systemic autoimmune disease, are limited to suppressing disease activity and are associated with multiple adverse effects. Recent advances in basic and translational sciences have elucidated a crucial role for the interferon-alpha (IFNα) pathway in the pathogenesis of this enigmatic disease. The so-called “type I interferon signature” has emerged as a major risk factor for disease activity of SLE. Multiple genes encoding for molecules within the type I interferon pathway have been associated with SLE in genome wide association studies. In addition, innate immune receptors are thought to be triggered by either endogenous and/or exogenous stimuli that lead to hypersecretion of IFNα. We review the multiple emerging treatment strategies targeting IFNα-related pathways. These include monoclonal antibodies against IFNα, anti-IFNα antibody-inducing vaccines, and inhibitors of toll-like receptors. We also summarize the current status of these pharmaceutical agents in early clinical trials.
Keywords: pDC, TLR, IRF, JAK/STAT, Pin1, interferonopathies, virome, proteasome
1. INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic multisystem autoimmune disease with a wide range of clinical manifestations and a pathogenesis whose details have remained relatively elusive. Dysregulation of adaptive immune responses in SLE leads to autoantibody production and immune complex deposition in various tissues [1–2]. Clinical manifestations commonly appear in the skin, kidney, musculoskeletal, and hematologic systems, but SLE can also affect the lungs, central nervous system, serous membranes and virtually every other organ system of the body [1, 3]. The disease is responsible for significant morbidity and mortality, with most recent studies showing a 10-year survival of approximately 70–90% [4–5]. Both genetic and environmental factors have been linked to SLE [2, 6]. The genetic risk of developing SLE is generally thought to result from the aggregate effects of multiple polymorphisms (although rare single gene mutations also cause SLE-like disease) [7]. Environmental triggers include smoking [8], UV light [9], various medications [10], and possibly certain viruses [2].
Current therapies for SLE are generally lacking in effectiveness and/or safety, and include primarily nonspecific immunomodulatory, immunosuppressive or cytotoxic agents. These therapies inhibit broadly inflammatory mediators or pathways, including those that are not particularly relevant to SLE pathogenesis. Antimalarial agents and nonsteroidal anti-inflammatory drugs (NSAIDs) remain the first-line drugs for mild disease. Corticosteroids are the primary therapy for more serious disease or one that is resistant to first-line agents, as well as during a lupus flare. Other systemic treatments targeting inflammation include cyclophosphamide, mycophenolate mofetil, and azathioprine. Less commonly used immunosuppressive agents include methotrexate, cyclosporine, tacrolimus, and leflunomide [11–12]. All of these therapies have a broad range of nonspecific effects, and are associated with considerable toxicities [11–12]. More recently developed biologic therapies have been studied in SLE patients and B cell targeted therapy appears to provide some benefit. Belilumab (an inhibitor of the molecule “B Lymphocyte Stimulator,” or BLyS) was recently given FDA-approval for use in treating SLE, the first drug in over 40 years to achieve this status [13]. The original FDA-approved disease-modifying drug for SLE, hydroxychloroquine, an antimalarial agent, has a lengthy track record in the treatment of lupus and has been shown to have an impact on survival [14]. Antimalarial agents have a variety of effects that may be relevant to their therapeutic benefit in SLE, including interference with Toll-like receptor (TLR) signaling pathways that induce interferon-alpha (IFNα) production [15]. Additional evidence has also implicated IFNα in SLE pathogenesis, heightening interest in development of novel pharmaceutical agents that specifically target the IFNα pathway. The role of IFNα in disease pathogenesis, and the current state of development of therapies targeting IFNα are discussed below.
2. PATHOGENESIS OF SLE
A poor understanding of the pathogenesis of SLE has hampered the development of new therapies directed at the underlying disease process. SLE involves immune dysregulation at the interface between the innate and adaptive immune systems with both endogenous and exogenous triggers contributing to evolution of disease and induction of disease flares, e.g. viral infections, UV light exposure and certain drugs. Basic research has led to the widely held view that defective clearance of apoptotic cellular debris in SLE patients causes a loss of self-tolerance, autoantibody generation, and the formation of immune complexes [16–19]. Several clinical manifestations of SLE are thought to be the result of autoantibody and immune-complex deposition in tissues leading to a secondary inflammatory response [20]. In addition, direct damage of tissues by T cells and maladaptive mechanisms of tissue injury might also be at play.
2.1 PHYSIOLOGIC ROLE OF INTERFERON-ALPHA
Interferon-alpha is a pleiotropic cytokine belonging to the type I cytokine family, and numerous studies over the past several years have provided increasing evidence for a central role of IFNα in the pathogenesis of SLE [21–26]. Type I interferons are normally produced by the innate immune system in response to viral infections [27–29]. They act via type I interferon receptors (INFARs) to trigger the JAK/STAT signaling cascade, leading to induction of interferon-stimulated genes (ISGs) that amplify interferon signaling, activate the adaptive immune system, and produce factors that directly inhibit viral replication [27–29]. IFNα can influence the function and activation of most types of adaptive immune cells [28, 29] after its secretion is stimulated via RNA or DNA sensing receptors [30, 31]. Its effects range from upregulation of major histocompatibility complex (MHC) and costimulatory molecules to increased survival and activation of dendritic cells, B cells, and T cells [27, 30, 33]. Plasmacytoid dendritic cells (pDCs) are the main source of serum IFNα [34]. They are normally present in lymphoid tissues, but have been found in large numbers in the skin of patients with SLE [35].
Viruses and their nucleic acids induce type I interferon expression specifically via activation of endosomal Toll-like receptors [30] and cytosolic nucleic acid sensors [30–32]. Ligand binding induces a conformational change in the receptors that permits association of an adaptor protein, which in TLR signaling is most commonly MyD88 [36]. After binding to the cytosolic domain of the TLR, MyD88 recruits interleukin-1 receptor-associated kinase (IRAK) 4 and IRAK1, which in turn form a complex with tumor necrosis factor receptor-associated factor (TRAF) 6 [37]. Transcription factors known as Interferon Regulatory Factors (IRF) 5 and 7 subsequently interact directly with the MyD88/IRAK/TRAF6 complex and are activated via phosphorylation and ubiquitination [38–42]. Activated IRFs translocate to the nucleus, bind to specific regulatory sequences in IFNα genes, and promote their transcription, leading to the broad range of effects described above [40, 42–46].
2.2 ASSOCIATIONS OF INTERFERON-ALPHA WITH SLE
While early studies demonstrated high levels of interferons in the sera of patients with SLE [21], the first convincing evidence linking IFNα to SLE came in 2003 when global gene expression profiling of peripheral blood mononuclear cells from SLE patients showed that a high number of IFNα-responsive genes were abnormally expressed [22–23]. Subsequent reports have shown that IFNα therapy for neoplasias or chronic viral infection can induce SLE with features almost identical to those seen in idiopathic disease [47–50]. High serum levels of IFNα have also been identified as a heritable risk factor for SLE, with clustering of high IFNα levels among family members of patients with the disease [24]. An increasing body of evidence suggests that the elevated levels of IFNα seen in SLE are likely to result from primary genetic variations in the type I interferon signaling pathway and that such variations could be central to the pathogenesis of SLE. Along these lines, rare genetic disorders with complex autoimmune phenotypes have been discovered and linked to the type I interferon pathway [51]. An example would be a disorder caused by mutations in Trex1, a 3' repair exonuclease that was elegantly shown to negatively regulate the response to IFN-stimulatory endogenous retroelements [52]. These syndromes have recently been termed appropriately “type I interferonopathies”. Importantly, many patients affected by them develop lupus-like features which are most likely due to excess IFNα or dysregulation of the type I interferon pathway ([51] and references herein).
While genetic alterations contribute clearly to classic SLE pathogenesis, the possibility that chronic viral infections or particular viruses within the endogenous virome add to elevations and/or fluctuations of type I interferons in lupus patients is intriguing and not mutually exclusive [53–54]. Interestingly, virus-like tubuloreticular inclusions within lymphocytes from SLE patients have been observed decades ago and correlated with higher IFNα [55]. Their relationship, however, to both an infectious agent and the pathogenesis of SLE remain unproven to date ([55] and references herein). On the other hand, the associations of IFNα with SLE in general have also been mechanistically supported by multiple murine studies, e.g. with the New Zealand Black (NZB) mouse model of systemic lupus; genetic deficiency of the type I interferon receptor in NZB mice led to significantly reduced disease activity [56]. A comprehensive summary of the various in vivo models that functionally link the type I interferon pathway with systemic autoimmunity and lupus goes beyond the scope of this review. The effects of elevated IFNα on the immune system in SLE patients is briefly reviewed in the next paragraph.
2.3 INTERFERON-ALPHA IN SLE IMMUNOPATHOGENESIS
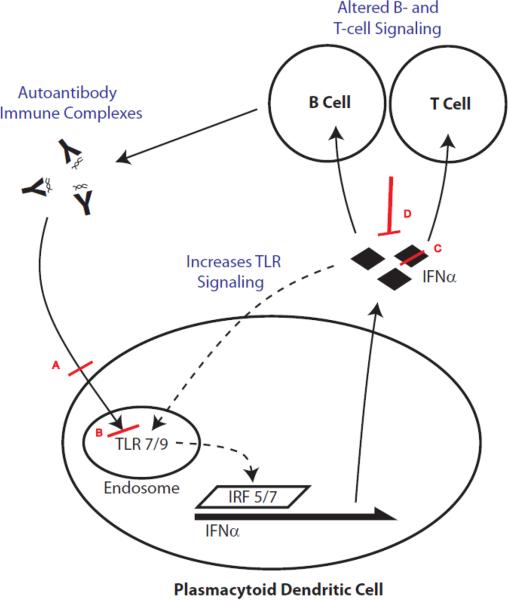
High levels of IFNα have been shown to contribute directly to immune dysregulation in a number of ways. IFNα, for example, causes dendritic cells to mature and become more likely to activate T cells. In lupus patients, myeloid dendritic cells are more likely to present self-antigens to T cells in a stimulatory manner, likely as a result of high IFNα levels [25]. IFNα has also been shown to decrease regulatory T cell activity in patients with lupus, further contributing to autoimmunity [25–26]. IFNα increases TLR7 signaling in dendritic cells, which in turn leads more IFNα production, forming a positive feedback loop [25, 57–58]. Figure 1 shows a simplified overview of the mechanism of IFNα production in SLE patients as well as emerging therapeutic targets as discussed in the next section.
Figure 1. Mechanism of IFNα production in SLE patients and potential targeting approaches.
Shown is a simplified model of how aberrant IFNα production can occur in SLE patients. Inherently abnormal T and B lymphocyte signaling in conjunction with innate immune stimulation via endogenous and/or exogenous triggers leads to a vicious cycle of autoantibody-immune complex signaling. The putative autoantigens in SLE form complexes with a myriad of autoantibodies produced in SLE. These complexes have been shown to trigger TLR signaling in antigen-presenting cells, in particular TLR7 and TLR9 in plasmacytoid dendritic cells (pDC). These endosomal ligands can also physiologically be triggered by viral or bacterial nucleic acids. The DNA/RNA molecules could be released from either pathogens or commensals which then amplify the IFNα circuit in SLE patients. Endosomal TLR7/9 signaling leads to interferon regulator factor (IRF) 5/7 activation and induction of IFNα gene production. Translated and secreted IFNα can in turn stimulate T and B cells as well as augment TLR signaling, closing the cycle that leads to ongoing IFNα pathway activation. Shown in red are some of the current approaches described in the main text that attenuate this vicious cycle. A, blockade of TLR signaling (at various levels) via novel TLR7/9 inhibitors; B, endosomal inhibition of TLR7/9 with antimalarials like hydroxychloroquine (which is only one of many modes of action of antimalarials); C, direct targeting of IFNα via monoclonal antibodies; D, indirect targeting of IFNα using a kinoid that leads to an endogenous T- and B-cell mediated anti-IFNα response after vaccination.
2.4 INTERFERON REGULATORY FACTORS
Recent genome-wide association studies (GWAS) and genetic studies using a candidate-gene approach have identified more than 25 SLE susceptibility genes involved in adaptive immunity, autoantibody production, innate immunity, and interferon signaling [59–75], including STAT4 variants sensitizing to increased IFNα production [62, 76–79] and polymorphisms in TNFAIP3, the gene encoding for the ubiquitin-modifying enzyme A20 that is required for termination of TLR responses [67, 69, 80–82]. An association of SLE-risk with polymorphisms of the transcription factor interferon regulatory factor (IRF) 5 was initially discovered by linkage analysis of genes in the type I interferon pathway [59–60], and was subsequently confirmed in several GWAS [65–67]. The major association of IRF5 with SLE risk has also been demonstrated across multiple populations [46, 60–61, 83–87]. Recent studies have identified up to five major haplotypes of IRF5, and have shown that certain haplotypes are associated with increased SLE risk while others may be protective [60–61, 88–89]. IRF5 haplotypes conferring SLE risk are also associated with increased serum IFNα activity [88–89]. For each of these haplotypes, however, increased IFNα activity was only seen in a subset of the patients who had specific autoantibodies [88–89]. For example, anti-double-stranded DNA and anti-Ro autoantibodies were each associated with a different IRF5 haplotype, and each haplotype-auto-antibody pair strongly predicted elevated serum IFNα in patients with SLE [89]. In addition to inducing several proinflammatory cytokines [90–91], IRF5 has been shown to promote transcription of IFNα in specific cell types, including precursor dendritic cells [40, 43–45]. Similar to IRF5, IRF7 variants have also been linked to SLE risk [92]. Furthermore, several studies have shown correlations between specific IRF7 variants and elevated IFNα levels only in a subset of the patients who had certain autoantibodies [46, 93]. IRF7 has also been shown to play a critical role in IFNα production [42]. Taken together, the above studies suggest that IRF gene polymorphisms combined with autoantibody activation of TLR receptors leads to higher IFNα levels in SLE patients [94–96].
3. THERAPEUTIC TARGETS FOR SLE
Evidence for the IFNα pathway in the pathogenesis of lupus has highlighted IFNα and many related signaling molecules as attractive therapeutic targets. Several clinical trials are now underway investigating monoclonal antibodies against IFNα (discussed below) [97–99]. Other therapeutic approaches under investigation include vaccination against IFNα using an IFNα-kinoid [100–101], inhibition of TLRs [102], blockade of INFARs [103], and inhibition of JAK/STAT signaling [104]. IFNα pathway-targeting approaches for SLE patients that are currently in development or early clinical trials are discussed in more detail below and are summarized in table I.
Table I.
SLE therapies in development targeting the IFNα pathway*.
| Therapeutic Approaches | Pharmaceutical Agents in Early Clinical Development |
|---|---|
| Anti-IFNα monoclonal antibodies | Sifalimumab (Medi-545, Medimmune, Inc.); fully human anti-IFNα monoclonal antibody |
| Rontalizumab (Genentech); recombinant humanized anti-IFNα monoclonal antibody | |
| AGS-009 (Argos Therapeutics); humanized IgG4 anti-IFNα monoclonal antibody | |
| Anti-INFα Vaccination | IFNα-Kinoid (NeoVacs); active immunotherapy inducing an anti-IFNα response |
| TLR inhibitors | IMO-3100 (Idera Pharmaceutials); oligonucleotide-based inhibitor of TLR7 and TLR9 |
| DV1179 (Dynavax); oligonucleotide-based inhibitor of TLR7 and TLR9 | |
Therapeutic approaches targeting the IFNα and TLR pathways are shown in the left column. Monoclonal antibodies, kinoid, or oligonucleotide-based inhibitors are listed on the right column. Companies involved in developing these agents are listed in parentheses.
3.1 BIOMARKERS FOR INTERFERON-ALPHA ACTIVITY
In order to evaluate the efficacy of IFNα-targeted therapies and to select suitable candidates for treatment, it has been necessary to identify appropriate biomarkers for disease-related IFNα-activity. Attempts to directly measure type I interferon proteins by enzyme-linked immunosorbent assays (ELISA) have yielded low reproducibility and poor correlation with functional assays [24, 105–107]. Traditional methods of measuring IFNα are generally indirect, and rely on the effects of IFNα on the survival or proliferation of cultured cells. These include the Daudi cell proliferation assay, which quantifies the antiproliferative effect of IFNα [108–109], and bioassays measuring the growth of virally infected cell cultures to quantify the antiviral effects of IFNα [105]. Initial reports of interferon-inducible genes [110] were followed by large-scale gene expression analyses that first characterized the “type I interferon signature” in SLE [22–23]. The identification of genes specifically induced by IFNα provided a proxy for directly measuring IFNα levels. In one functional assay, quantitative RT-PCR analysis of representative IFNα-induced genes in reporter cells is used to detect IFNα in patient sera [24, 106]. A more recent study used microarrays to identify a set of 21 genes that would serve as pharmacodynamic and diagnostic biomarkers in the evaluation of anti-IFNα monoclonal antibody therapies [107, 111]. These genes were selected because they met the study's predefined requirements of being IFNα/β-inducible, over-expressed in whole blood from SLE patients, induced by ex-vivo stimulation with SLE patient sera, and neutralized by an anti-IFN-α/β monoclonal antibody [111]. Interestingly, application of this assay to a population of 202 SLE patients yielded a bimodal distribution between patients that were negative and those that were positive for the type I interferon signature [107]. While the significance of this is unclear, it suggests that biomarkers are likely to become increasingly important in identifying subgroups of patients who may benefit from IFNα-targeted therapies under development [107]. Other possible biomarkers for IFNα-related lupus activity include IFNα-inducible cytokines. Recent studies demonstrated that serum levels of interferon-regulated chemokines correlate well with disease activity, in particular the Th1-related chemokine CXCL10 (IP-10), whose gene expression has been shown to be induced by type I IFNs in human peripheral blood mononuclear cells [112–114].
3.2 MONOCLONAL ANTIBODIES DIRECTED AT INTERFERON-ALPHA
Sifalimumab (MedImmune LLC, Gaithersburg, MD), also known as Medi-545, is a fully human anti-IFNα monoclonal antibody studied in a phase I randomized, double-blind, dose-escalation study in adults with moderately active SLE [109]. By quantifying the type I interferon signature as described above, the authors identified a dose dependent inhibition of the IFNα pathway by sifalimumab. Exploratory data from the phase I trial showed that sifalimumab-treated subjects required fewer new or increased immunosuppressive treatments and had fewer SLE Disease Activity Index flares [109]. Rontalizumab (Genentech, Inc., South San Francisco, CA), a recombinant humanized monoclonal anti-IFNα antibody, was also shown to inhibit the IFNα/β-inducible gene signature in a phase I clinical trial [115]. Both sifalimumab and rontalizumab are currently being tested in phase II clinical trials [97–98, 116–117]. AGS-009, under development by Argos Therapeutics Inc. (Durham, NC), is a humanized immunoglobulin G4 (IgG4) monoclonal antibody that recently completed a phase 1 clinical [99, 118].
3.3 INTERFERON-ALPHA VACCINE
Further evidence for the potential benefit of anti-IFNα-antibodies in SLE comes from a recent study of SLE patients with endogenous anti-IFNα autoantibodies (AIAAs) [119]. The study reported that endogenous AIAAs were found in the serum of approximately 25% of the SLE patients studied, and that the presence of these autoantibodies was associated with lower levels of serum type I IFN bioactivity, reduced downstream IFN-pathway activity, and lower disease activity. They also showed that sera from AIAA-positive patients were able to neutralize the activity of type I IFN in vitro [119]. These studies suggest that an IFNα vaccine designed to induce AIAAs could potentially benefit SLE patients. Neovacs S.A. (Paris, France) is currently developing an IFNα kinoid vaccine for this purpose. This vaccine was recently shown to induce polyclonal antibodies in mice, which neutralize all subtypes of human IFNα as well as IFNα in sera from patients with SLE [100–101]. Results were recently reported of a phase I–II double-blind, placebo-controlled, dose-escalation study on twenty-eight patients with mild to moderate seropositive lupus [120–121]. The authors demonstrated a dose-related anti-IFNα response in all immunized patients. This was associated with a significant down-regulation of SLE over-expressed genes among a subset of patients who were found to have a positive IFNα signature at baseline.
3.4 TOLL-LIKE RECEPTOR INHIBITORS
TLR inhibition is another intriguing possibility for treatment of lupus, and could reduce the production of IFNα by pDCs in SLE patients. There is also evidence that TLR inhibitors could improve glucocorticoid activity in patients with SLE. A recent study demonstrated that chronic stimulation of pDCs via activation of TLR receptors by self nucleic acid-containing immune complexes contributes to the decreased activity of glucocorticoids in SLE [122]. Antimalarials (e.g. hydroxychloroquine), long used for treatment of lupus, are thought to work at least partially via inhibition of intracellular TLRs as mentioned above [123]. Idera Pharmaceuticals (Cambridge, MA) has recently developed a synthetic oligonucleotide-based inhibitor of TLR7 and TLR9 referred to as IMO-3100. Although there are currently no peer-reviewed studies, Idera reports that the drug inhibited disease development in lupus prone mice [124]. They also report that the drug suppressed TLR7- and TLR9-mediated induction of cytokines, including IFNα, in a recent four-week placebo-controlled multiple-dose phase I clinical trial [125]. Dynavax Technologies Corp. (Berkeley, CA) has also been developing oligonucleotide-based TLR inhibitors for use in autoimmune diseases such as SLE [126]. They reported that such inhibitors prevented and reversed disease in a mouse model of cutaneous lupus [127] and that phase I clinical trials of a bifunctional TLR7- and TLR9-inhbitor called DV1179 have begun [102, 128]. Pfizer Inc. (New York, NY) has also developed a TLR inhibitor that is in phase I clinical trials [129]. Known as CpG-52364, this inhibitor has activity against TLR7/8/9 and was previously reported to be effective in a murine model of lupus [130].
3.5 OTHER POTENTIAL TARGETS
Other potential therapeutic targets along the IFNα pathway include the type I interferon receptors (INFARs) and the JAK/STAT pathway. Medi-546 (MedImmune LLC, Gaithersburg, MD), formerly known as MDX-1333, is a fully human monoclonal antibody directed against subunit 1 of the type I interferon receptor, and is currently in clinical trials for treatment of lupus [103]. Recent research has suggested that inhibitors of JAK1 or JAK2 may also provide potential benefit in SLE [131–132] and a Jak1/Jak2 inhibitor, INCBO18424, recently underwent clinical trials for myeloproliferative disease [104].
Several studies have shown that proteasome inhibitors, such as bortezomib, carfilzomib, and the immunoproteasome-specific inhibitor ONX 0914 are effective therapies in murine models of lupus [133–135]. While proteasome inhibitors have previously been shown to block plasma cell proliferation and reduce autoantibody formation, a recent study indicates these drugs also efficiently suppress IFNα production by TLR-activated pDCs [135]. Other work has shown that bortezomib inhibits pDCs by targeting intracellular trafficking of TLRs [136]. While proteasome inhibition is a decidedly less targeted approach than those described above, the combined effects on both plasma cells and pDCs could prove beneficial in some patients.
Finally, the molecular biology of the IFN- and TLR-related pathways is rapidly progressing and more rational targeting approaches could emerge quickly from discoveries in the basic sciences. Based on a recent publication, it would be, for instance, a logical approach to modify an isoprolyl isomerase called Pin1 [137–138]. Pin1 specifically facilitates IRAK1 activation and release from the TLR receptor complex (in particular TLR7/9) [138]. Importantly, deletion of Pin1 completely abrogated the production of type I interferon by pDCs while affecting only relatively little the production of proinflammatory cytokines [138]. Targeting specifically Pin1 or IRAK1 might lead to novel selective inhibitors for SLE patients that potentially leave a broader proinflammatory response intact in vivo. Broad inhibition of the type I interferon pathway might be associated with unacceptable risks that are discussed in the last section below.
3.5 POTENTIAL RISKS OF INHIBITING THE INTERFERON-ALPHA PATHWAY
Inhibiting the IFNα pathway theoretically has the potential to significantly increase risk of new or reactivated infections, particularly with viruses. In phase I clinical trials of sifalimumab (discussed above), placebo and treatment groups had similar rates of infection. One case of sinusitis and one upper respiratory tract infection were the only infections that were considered treatment-related among the patients receiving sifalimumab during the blinded phase of the study. Rates of conversion to positive viral surveillance test results were similar among placebo- and sifalimumab-treated subjects [109]. The IFNα vaccine also had an apparently favorable safety profile, with reports of only minor and transient infections. Concerns about the potential for irreversible long-term effects of the vaccine were eased by reports that serum antibody concentrations declined after the last vaccine dose [120]. For most of the drugs discussed above, we must wait until larger phase III clinical trials have been completed before we will know what the side effect profile of IFNα-pathway inhibition will be, and whether viral or other infections will pose a serious problem. Finally, in light of the fact that at least 20% of human cancers are linked to infectious agents including viruses [139–140], a word of caution should also be said about the theoretical potential of inducing malignancies that are otherwise prevented by an intact type I interferon pathway.
4. SUMMARY
In summary, evidence from gene expression profiling in SLE patients, as well as from animal and in vitro models, has stimulated the development of anti-cytokine therapy for this disease. Given the remarkable success of targeting cytokines with biologic drugs to treat other autoimmune disease, especially TNF in rheumatoid arthritis, this approach seems reasonable, and we may soon see the emergence of a new class of effective drugs for SLE. Limited early data using sifalimumab has shown reduced SLE disease activity and a decreased need for immunosuppressive agents among patients receiving the drug. Most of the preliminary data however has focused on IFNα/β-inducible gene signatures as a measurement of treatment effectiveness, and it is still unclear how well this will correlate with clinical improvement in SLE symptoms. It is also unclear how significant the increased risk of infections and malignancies will be in patients receiving drugs targeting the IFNα pathway. Despite these potential roadblocks, it seems likely that we will see the emergence of additional SLE therapies in the near future, and inhibition of the “IFNα signature” appears to be a promising approach.
Highlights
Several anti-IFNα monoclonal antibodies are being tested in early clinical trials.
Active immunotherapy with a kinoid leads to a sustained anti-IFNα immune response.
Novel TLR7/TLR9 inhibitors are expected to attenuate stimulation of IFNα secretion.
Here we summarize these novel approaches to target the IFNα pathway in SLE patients.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Institutes of Health (K08 AI095318 to M.A.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
REFERENCES
- [1].Lahita RG. Systemic lupus erythematosus. 3rd ed. Academic Press; San Diego: 1999. [Google Scholar]
- [2].Tsokos GC. Systemic lupus erythematosus. The New England journal of medicine. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- [3].Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, Mejia JC, Aydintug AO, Chwalinska-Sadowska H, de Ramon E, Fernandez-Nebro A, Galeazzi M, Valen M, Mathieu A, Houssiau F, Caro N, Alba P, Ramos-Casals M, Ingelmo M, Hughes GR. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine. 2003;82:299–308. doi: 10.1097/01.md.0000091181.93122.55. [DOI] [PubMed] [Google Scholar]
- [4].Pons-Estel GJ, Alarcon GS, Scofield L, Reinlib L, Cooper GS. Understanding the epidemiology and progression of systemic lupus erythematosus. Seminars in arthritis and rheumatism. 2010;39:257–268. doi: 10.1016/j.semarthrit.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Trager J, Ward MM. Mortality and causes of death in systemic lupus erythematosus. Current opinion in rheumatology. 2001;13:345–351. doi: 10.1097/00002281-200109000-00002. [DOI] [PubMed] [Google Scholar]
- [6].Jonsen A, Bengtsson AA, Nived O, Truedsson L, Sturfelt G. Gene-environment interactions in the aetiology of systemic lupus erythematosus. Autoimmunity. 2007;40:613–617. doi: 10.1080/08916930701511051. [DOI] [PubMed] [Google Scholar]
- [7].Moser KL, Kelly JA, Lessard CJ, Harley JB. Recent insights into the genetic basis of systemic lupus erythematosus. Genes and immunity. 2009;10:373–379. doi: 10.1038/gene.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Simard JF, Costenbader KH, Liang MH, Karlson EW, Mittleman MA. Exposure to maternal smoking and incident SLE in a prospective cohort study. Lupus. 2009;18:431–435. doi: 10.1177/0961203308098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bijl M, Kallenberg CG. Ultraviolet light and cutaneous lupus. Lupus. 2006;15:724–727. doi: 10.1177/0961203306071705. [DOI] [PubMed] [Google Scholar]
- [10.Ballestar E, Esteller M, Richardson BC. The epigenetic face of systemic lupus erythematosus. J Immunol. 2006;176:7143–7147. doi: 10.4049/jimmunol.176.12.7143. [DOI] [PubMed] [Google Scholar]
- [11].Yildirim-Toruner C, Diamond B. Current and novel therapeutics in the treatment of systemic lupus erythematosus. The Journal of allergy and clinical immunology. 2011;127:303–312. doi: 10.1016/j.jaci.2010.12.1087. quiz 313-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wong M, La Cava A. Lupus, the current therapeutic approaches. Drugs Today (Barc) 2011;47:289–302. doi: 10.1358/dot.2011.47.4.1583186. [DOI] [PubMed] [Google Scholar]
- [13].Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, Leon MG, Tanasescu C, Nasonov E, Lan JL, Pineda L, Zhong ZJ, Freimuth W, Petri MA. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–731. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- [14].Alarcon GS, McGwin G, Bertoli AM, Fessler BJ, Calvo-Alen J, Bastian HM, Vila LM, Reveille JD. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L) Annals of the rheumatic diseases. 2007;66:1168–1172. doi: 10.1136/ard.2006.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sun S, Rao NL, Venable J, Thurmond R, Karlsson L. TLR7/9 antagonists as therapeutics for immune-mediated inflammatory disorders. Inflammation & allergy drug targets. 2007;6:223–235. doi: 10.2174/187152807783334300. [DOI] [PubMed] [Google Scholar]
- [16].Munoz LE, Gaipl US, Franz S, Sheriff A, Voll RE, Kalden JR, Herrmann M. SLE--a disease of clearance deficiency? Rheumatology (Oxford) 2005;44:1101–1107. doi: 10.1093/rheumatology/keh693. [DOI] [PubMed] [Google Scholar]
- [17].Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis and rheumatism. 1998;41:1241–1250. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- [18].Janko C, Schorn C, Grossmayer GE, Frey B, Herrmann M, Gaipl US, Munoz LE. Inflammatory clearance of apoptotic remnants in systemic lupus erythematosus (SLE) Autoimmunity reviews. 2008;8:9–12. doi: 10.1016/j.autrev.2008.07.015. [DOI] [PubMed] [Google Scholar]
- [19].Rahman A, Isenberg DA. Systemic lupus erythematosus. The New England journal of medicine. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- [20].Koffler D, Schur PH, Kunkel HG. Immunological studies concerning the nephritis of systemic lupus erythematosus. The Journal of experimental medicine. 1967;126:607–624. doi: 10.1084/jem.126.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. The New England journal of medicine. 1979;301:5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- [22].Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. Interferoninducible gene expression signature in peripheral blood cells of patients with severe lupus. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. The Journal of experimental medicine. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes and immunity. 2007;8:492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Niewold TB, Clark DN, Salloum R, Poole BD. Interferon alpha in systemic lupus erythematosus. Journal of biomedicine & biotechnology. 2010;2010:948364. doi: 10.1155/2010/948364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yan B, Ye S, Chen G, Kuang M, Shen N, Chen S. Dysfunctional CD4+,CD25+ regulatory T cells in untreated active systemic lupus erythematosus secondary to interferon-alpha-producing antigen-presenting cells. Arthritis and rheumatism. 2008;58:801–812. doi: 10.1002/art.23268. [DOI] [PubMed] [Google Scholar]
- [27].Elkon KB, Stone VV. Type I interferon and systemic lupus erythematosus. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2011;31:803–812. doi: 10.1089/jir.2011.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fitzgerald-Bocarsly P, Dai J, Singh S. Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine & growth factor reviews. 2008;19:3–19. doi: 10.1016/j.cytogfr.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Liu SY, Sanchez DJ, Cheng G. New developments in the induction and antiviral effectors of type I interferon. Current opinion in immunology. 2011;23:57–64. doi: 10.1016/j.coi.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- [31].Ranjan P, Bowzard JB, Schwerzmann JW, Jeisy-Scott V, Fujita T, Sambhara S. Cytoplasmic nucleic acid sensors in antiviral immunity. Trends in molecular medicine. 2009;15:359–368. doi: 10.1016/j.molmed.2009.06.003. [DOI] [PubMed] [Google Scholar]
- [32].Keating SE, Baran M, Bowie AG. Cytosolic DNA sensors regulating type I interferon induction. Trends in immunology. 2011;32:574–581. doi: 10.1016/j.it.2011.08.004. [DOI] [PubMed] [Google Scholar]
- [33].Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nature medicine. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- [34].Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunological reviews. 2010;234:142–162. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL. Plasmacytoid dendritic cells (natural interferon- alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. The American journal of pathology. 2001;159:237–243. doi: 10.1016/s0002-9440(10)61689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Takeda K, Kaisho T, Akira S. Toll-like receptors. Annual review of immunology. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- [37].Horton CG, Pan ZJ, Farris AD. Targeting Toll-like receptors for treatment of SLE. Mediators of inflammation. 2010;2010 doi: 10.1155/2010/498980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Annals of the New York Academy of Sciences. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- [39].Kawai T, Sato S, Ishii KJ, Coban C, Hemmi H, Yamamoto M, Terai K, Matsuda M, Inoue J, Uematsu S, Takeuchi O, Akira S. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nature immunology. 2004;5:1061–1068. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- [40].Balkhi MY, Fitzgerald KA, Pitha PM. Functional regulation of MyD88-activated interferon regulatory factor 5 by K63-linked polyubiquitination. Molecular and cellular biology. 2008;28:7296–7308. doi: 10.1128/MCB.00662-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Honda K, Yanai H, Mizutani T, Negishi H, Shimada N, Suzuki N, Ohba Y, Takaoka A, Yeh WC, Taniguchi T. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15416–15421. doi: 10.1073/pnas.0406933101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- [43].Barnes B, Lubyova B, Pitha PM. On the role of IRF in host defense. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2002;22:59–71. doi: 10.1089/107999002753452665. [DOI] [PubMed] [Google Scholar]
- [44].Barnes BJ, Moore PA, Pitha PM. Virus-specific activation of a novel interferon regulatory factor, IRF-5, results in the induction of distinct interferon alpha genes. The Journal of biological chemistry. 2001;276:23382–23390. doi: 10.1074/jbc.M101216200. [DOI] [PubMed] [Google Scholar]
- [45].Barnes BJ, Richards J, Mancl M, Hanash S, Beretta L, Pitha PM. Global and distinct targets of IRF-5 and IRF-7 during innate response to viral infection. The Journal of biological chemistry. 2004;279:45194–45207. doi: 10.1074/jbc.M400726200. [DOI] [PubMed] [Google Scholar]
- [46].Salloum R, Niewold TB. Interferon regulatory factors in human lupus pathogenesis. Translational research : the journal of laboratory and clinical medicine. 2011;157:326–331. doi: 10.1016/j.trsl.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ronnblom LE, Alm GV, Oberg KE. Possible induction of systemic lupus erythematosus by interferon-alpha treatment in a patient with a malignant carcinoid tumour. Journal of internal medicine. 1990;227:207–210. doi: 10.1111/j.1365-2796.1990.tb00144.x. [DOI] [PubMed] [Google Scholar]
- [48].Gota C, Calabrese L. Induction of clinical autoimmune disease by therapeutic interferon-alpha. Autoimmunity. 2003;36:511–518. doi: 10.1080/08916930310001605873. [DOI] [PubMed] [Google Scholar]
- [49].Ioannou Y, Isenberg DA. Current evidence for the induction of autoimmune rheumatic manifestations by cytokine therapy. Arthritis and rheumatism. 2000;43:1431–1442. doi: 10.1002/1529-0131(200007)43:7<1431::AID-ANR3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- [50].Niewold TB, Swedler WI. Systemic lupus erythematosus arising during interferon-alpha therapy for cryoglobulinemic vasculitis associated with hepatitis C. Clinical rheumatology. 2005;24:178–181. doi: 10.1007/s10067-004-1024-2. [DOI] [PubMed] [Google Scholar]
- [51].Crow YJ. Type I interferonopathies: a novel set of inborn errors of immunity. Annals of the New York Academy of Sciences. 2011;1238:91–98. doi: 10.1111/j.1749-6632.2011.06220.x. [DOI] [PubMed] [Google Scholar]
- [52].Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Blank M, Shoenfeld Y, Perl A. Cross-talk of the environment with the host genome and the immune system through endogenous retroviruses in systemic lupus erythematosus. Lupus. 2009;18:1136–1143. doi: 10.1177/0961203309345728. [DOI] [PubMed] [Google Scholar]
- [54].Ramos-Casals M. Viruses and lupus: the viral hypothesis. Lupus. 2008;17:163–165. doi: 10.1177/0961203307086268. [DOI] [PubMed] [Google Scholar]
- [55].Klippel JH, Carette S, Preble OT, Friedman RM, Grimley PM. Serum alpha interferon and lymphocyte inclusions in systemic lupus erythematosus. Annals of the rheumatic diseases. 1985;44:104–108. doi: 10.1136/ard.44.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Santiago-Raber ML, Baccala R, Haraldsson KM, Choubey D, Stewart TA, Kono DH, Theofilopoulos AN. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. The Journal of experimental medicine. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Asselin-Paturel C, Brizard G, Chemin K, Boonstra A, O'Garra A, Vicari A, Trinchieri G. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. The Journal of experimental medicine. 2005;201:1157–1167. doi: 10.1084/jem.20041930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Rajagopal D, Paturel C, Morel Y, Uematsu S, Akira S, Diebold SS. Plasmacytoid dendritic cell-derived type I interferon is crucial for the adjuvant activity of Toll-like receptor 7 agonists. Blood. 2010;115:1949–1957. doi: 10.1182/blood-2009-08-238543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sigurdsson S, Nordmark G, Goring HH, Lindroos K, Wiman AC, Sturfelt G, Jonsen A, Rantapaa-Dahlqvist S, Moller B, Kere J, Koskenmies S, Widen E, Eloranta ML, Julkunen H, Kristjansdottir H, Steinsson K, Alm G, Ronnblom L, Syvanen AC. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. American journal of human genetics. 2005;76:528–537. doi: 10.1086/428480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Graham RR, Kozyrev SV, Baechler EC, Reddy MV, Plenge RM, Bauer JW, Ortmann WA, Koeuth T, Gonzalez Escribano MF, Pons-Estel B, Petri M, Daly M, Gregersen PK, Martin J, Altshuler D, Behrens TW, Alarcon-Riquelme ME. A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nature genetics. 2006;38:550–555. doi: 10.1038/ng1782. [DOI] [PubMed] [Google Scholar]
- [61].Graham RR, Kyogoku C, Sigurdsson S, Vlasova IA, Davies LR, Baechler EC, Plenge RM, Koeuth T, Ortmann WA, Hom G, Bauer JW, Gillett C, Burtt N, Cunninghame Graham DS, Onofrio R, Petri M, Gunnarsson I, Svenungsson E, Ronnblom L, Nordmark G, Gregersen PK, Moser K, Gaffney PM, Criswell LA, Vyse TJ, Syvanen AC, Bohjanen PR, Daly MJ, Behrens TW, Altshuler D. Three functional variants of IFN regulatory factor 5 (IRF5) define risk and protective haplotypes for human lupus. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6758–6763. doi: 10.1073/pnas.0701266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Remmers EF, Plenge RM, Lee AT, Graham RR, Hom G, Behrens TW, de Bakker PI, Le JM, Lee HS, Batliwalla F, Li W, Masters SL, Booty MG, Carulli JP, Padyukov L, Alfredsson L, Klareskog L, Chen WV, Amos CI, Criswell LA, Seldin MF, Kastner DL, Gregersen PK. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. The New England journal of medicine. 2007;357:977–986. doi: 10.1056/NEJMoa073003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cunninghame Graham DS, Graham RR, Manku H, Wong AK, Whittaker JC, Gaffney PM, Moser KL, Rioux JD, Altshuler D, Behrens TW, Vyse TJ. Polymorphism at the TNF superfamily gene TNFSF4 confers susceptibility to systemic lupus erythematosus. Nature genetics. 2008;40:83–89. doi: 10.1038/ng.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Nath SK, Han S, Kim-Howard X, Kelly JA, Viswanathan P, Gilkeson GS, Chen W, Zhu C, McEver RP, Kimberly RP, Alarcon-Riquelme ME, Vyse TJ, Li QZ, Wakeland EK, Merrill JT, James JA, Kaufman KM, Guthridge JM, Harley JB. A nonsynonymous functional variant in integrin-alpha(M) (encoded by ITGAM) is associated with systemic lupus erythematosus. Nature genetics. 2008;40:152–154. doi: 10.1038/ng.71. [DOI] [PubMed] [Google Scholar]
- [65].Harley JB, Alarcon-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL, Tsao BP, Vyse TJ, Langefeld CD, Nath SK, Guthridge JM, Cobb BL, Mirel DB, Marion MC, Williams AH, Divers J, Wang W, Frank SG, Namjou B, Gabriel SB, Lee AT, Gregersen PK, Behrens TW, Taylor KE, Fernando M, Zidovetzki R, Gaffney PM, Edberg JC, Rioux JD, Ojwang JO, James JA, Merrill JT, Gilkeson GS, Seldin MF, Yin H, Baechler EC, Li QZ, Wakeland EK, Bruner GR, Kaufman KM, Kelly JA. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nature genetics. 2008;40:204–210. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hom G, Graham RR, Modrek B, Taylor KE, Ortmann W, Garnier S, Lee AT, Chung SA, Ferreira RC, Pant PV, Ballinger DG, Kosoy R, Demirci FY, Kamboh MI, Kao AH, Tian C, Gunnarsson I, Bengtsson AA, Rantapaa-Dahlqvist S, Petri M, Manzi S, Seldin MF, Ronnblom L, Syvanen AC, Criswell LA, Gregersen PK, Behrens TW. Association of systemic lupus erythematosus with C8orf13-BLK and ITGAM-ITGAX. The New England journal of medicine. 2008;358:900–909. doi: 10.1056/NEJMoa0707865. [DOI] [PubMed] [Google Scholar]
- [67].Graham RR, Cotsapas C, Davies L, Hackett R, Lessard CJ, Leon JM, Burtt NP, Guiducci C, Parkin M, Gates C, Plenge RM, Behrens TW, Wither JE, Rioux JD, Fortin PR, Graham DC, Wong AK, Vyse TJ, Daly MJ, Altshuler D, Moser KL, Gaffney PM. Genetic variants near TNFAIP3 on 6q23 are associated with systemic lupus erythematosus. Nature genetics. 2008;40:1059–1061. doi: 10.1038/ng.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kozyrev SV, Abelson AK, Wojcik J, Zaghlool A, Linga Reddy MV, Sanchez E, Gunnarsson I, Svenungsson E, Sturfelt G, Jonsen A, Truedsson L, Pons-Estel BA, Witte T, D'Alfonso S, Barizzone N, Danieli MG, Gutierrez C, Suarez A, Junker P, Laustrup H, Gonzalez-Escribano MF, Martin J, Abderrahim H, Alarcon-Riquelme ME. Functional variants in the B-cell gene BANK1 are associated with systemic lupus erythematosus. Nature genetics. 2008;40:211–216. doi: 10.1038/ng.79. [DOI] [PubMed] [Google Scholar]
- [69].Musone SL, Taylor KE, Lu TT, Nititham J, Ferreira RC, Ortmann W, Shifrin N, Petri MA, Kamboh MI, Manzi S, Seldin MF, Gregersen PK, Behrens TW, Ma A, Kwok PY, Criswell LA. Multiple polymorphisms in the TNFAIP3 region are independently associated with systemic lupus erythematosus. Nature genetics. 2008;40:1062–1064. doi: 10.1038/ng.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Han JW, Zheng HF, Cui Y, Sun LD, Ye DQ, Hu Z, Xu JH, Cai ZM, Huang W, Zhao GP, Xie HF, Fang H, Lu QJ, Xu JH, Li XP, Pan YF, Deng DQ, Zeng FQ, Ye ZZ, Zhang XY, Wang QW, Hao F, Ma L, Zuo XB, Zhou FS, Du WH, Cheng YL, Yang JQ, Shen SK, Li J, Sheng YJ, Zuo XX, Zhu WF, Gao F, Zhang PL, Guo Q, Li B, Gao M, Xiao FL, Quan C, Zhang C, Zhang Z, Zhu KJ, Li Y, Hu DY, Lu WS, Huang JL, Liu SX, Li H, Ren YQ, Wang ZX, Yang CJ, Wang PG, Zhou WM, Lv YM, Zhang AP, Zhang SQ, Lin D, Li Y, Low HQ, Shen M, Zhai ZF, Wang Y, Zhang FY, Yang S, Liu JJ, Zhang XJ. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nature genetics. 2009;41:1234–1237. doi: 10.1038/ng.472. [DOI] [PubMed] [Google Scholar]
- [71].Gateva V, Sandling JK, Hom G, Taylor KE, Chung SA, Sun X, Ortmann W, Kosoy R, Ferreira RC, Nordmark G, Gunnarsson I, Svenungsson E, Padyukov L, Sturfelt G, Jonsen A, Bengtsson AA, Rantapaa-Dahlqvist S, Baechler EC, Brown EE, Alarcon GS, Edberg JC, Ramsey-Goldman R, McGwin G, Jr., Reveille JD, Vila LM, Kimberly RP, Manzi S, Petri MA, Lee A, Gregersen PK, Seldin MF, Ronnblom L, Criswell LA, Syvanen AC, Behrens TW, Graham RR. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nature genetics. 2009;41:1228–1233. doi: 10.1038/ng.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Yang W, Shen N, Ye DQ, Liu Q, Zhang Y, Qian XX, Hirankarn N, Ying D, Pan HF, Mok CC, Chan TM, Wong RW, Lee KW, Mok MY, Wong SN, Leung AM, Li XP, Avihingsanon Y, Wong CM, Lee TL, Ho MH, Lee PP, Chang YK, Li PH, Li RJ, Zhang L, Wong WH, Ng IO, Lau CS, Sham PC, Lau YL. Genome-wide association study in Asian populations identifies variants in ETS1 and WDFY4 associated with systemic lupus erythematosus. PLoS genetics. 2010;6:e1000841. doi: 10.1371/journal.pgen.1000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lessard CJ, Adrianto I, Kelly JA, Kaufman KM, Grundahl KM, Adler A, Williams AH, Gallant CJ, Anaya JM, Bae SC, Boackle SA, Brown EE, Chang DM, Criswell LA, Edberg JC, Freedman BI, Gregersen PK, Gilkeson GS, Jacob CO, James JA, Kamen DL, Kimberly RP, Martin J, Merrill JT, Niewold TB, Park SY, Petri MA, Pons-Estel BA, Ramsey-Goldman R, Reveille JD, Song YW, Stevens AM, Tsao BP, Vila LM, Vyse TJ, Yu CY, Guthridge JM, Bruner GR, Langefeld CD, Montgomery C, Harley JB, Scofield RH, Gaffney PM, Moser KL. Identification of a systemic lupus erythematosus susceptibility locus at 11p13 between PDHX and CD44 in a multiethnic study. American journal of human genetics. 2011;88:83–91. doi: 10.1016/j.ajhg.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Yang J, Yang W, Hirankarn N, Ye DQ, Zhang Y, Pan HF, Mok CC, Chan TM, Wong RW, Mok MY, Lee KW, Wong SN, Leung AM, Li XP, Avihingsanon Y, Rianthavorn P, Deekajorndej T, Suphapeetiporn K, Shotelersuk V, Baum L, Kwan P, Lee TL, Ho MH, Lee PP, Wong WH, Zeng S, Zhang J, Wong CM, Ng IO, Garcia-Barcelo MM, Cherny SS, Tam PK, Sham PC, Lau CS, Lau YL. ELF1 is associated with systemic lupus erythematosus in Asian populations. Human molecular genetics. 2011;20:601–607. doi: 10.1093/hmg/ddq474. [DOI] [PubMed] [Google Scholar]
- [75].Okada Y, Shimane K, Kochi Y, Tahira T, Suzuki A, Higasa K, Takahashi A, Horita T, Atsumi T, Ishii T, Okamoto A, Fujio K, Hirakata M, Amano H, Kondo Y, Ito S, Takada K, Mimori A, Saito K, Kamachi M, Kawaguchi Y, Ikari K, Mohammed OW, Matsuda K, Terao C, Ohmura K, Myouzen K, Hosono N, Tsunoda T, Nishimoto N, Mimori T, Matsuda F, Tanaka Y, Sumida T, Yamanaka H, Takasaki Y, Koike T, Horiuchi T, Hayashi K, Kubo M, Kamatani N, Yamada R, Nakamura Y, Yamamoto K. A Genome-Wide Association Study Identified AFF1 as a Susceptibility Locus for Systemic Lupus Eyrthematosus in Japanese. PLoS genetics. 2012;8:e1002455. doi: 10.1371/journal.pgen.1002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kariuki SN, Kirou KA, MacDermott EJ, Barillas-Arias L, Crow MK, Niewold TB. Cutting edge: autoimmune disease risk variant of STAT4 confers increased sensitivity to IFN-alpha in lupus patients in vivo. J Immunol. 2009;182:34–38. doi: 10.4049/jimmunol.182.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Sigurdsson S, Nordmark G, Garnier S, Grundberg E, Kwan T, Nilsson O, Eloranta ML, Gunnarsson I, Svenungsson E, Sturfelt G, Bengtsson AA, Jonsen A, Truedsson L, Rantapaa-Dahlqvist S, Eriksson C, Alm G, Goring HH, Pastinen T, Syvanen AC, Ronnblom L. A risk haplotype of STAT4 for systemic lupus erythematosus is over-expressed, correlates with anti-dsDNA and shows additive effects with two risk alleles of IRF5. Human molecular genetics. 2008;17:2868–2876. doi: 10.1093/hmg/ddn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Svenungsson E, Gustafsson J, Leonard D, Sandling J, Gunnarsson I, Nordmark G, Jonsen A, Bengtsson AA, Sturfelt G, Rantapaa-Dahlqvist S, Elvin K, Sundin U, Garnier S, Simard JF, Sigurdsson S, Padyukov L, Syvanen AC, Ronnblom L. A STAT4 risk allele is associated with ischaemic cerebrovascular events and anti-phospholipid antibodies in systemic lupus erythematosus. Annals of the rheumatic diseases. 2010;69:834–840. doi: 10.1136/ard.2009.115535. [DOI] [PubMed] [Google Scholar]
- [79].Yin H, Borghi MO, Delgado-Vega AM, Tincani A, Meroni PL, Alarcon-Riquelme ME. Association of STAT4 and BLK, but not BANK1 or IRF5, with primary antiphospholipid syndrome. Arthritis and rheumatism. 2009;60:2468–2471. doi: 10.1002/art.24701. [DOI] [PubMed] [Google Scholar]
- [80].Adrianto I, Wen F, Templeton A, Wiley G, King JB, Lessard CJ, Bates JS, Hu Y, Kelly JA, Kaufman KM, Guthridge JM, Alarcon-Riquelme ME, Anaya JM, Bae SC, Bang SY, Boackle SA, Brown EE, Petri MA, Gallant C, Ramsey-Goldman R, Reveille JD, Vila LM, Criswell LA, Edberg JC, Freedman BI, Gregersen PK, Gilkeson GS, Jacob CO, James JA, Kamen DL, Kimberly RP, Martin J, Merrill JT, Niewold TB, Park SY, Pons-Estel BA, Scofield RH, Stevens AM, Tsao BP, Vyse TJ, Langefeld CD, Harley JB, Moser KL, Webb CF, Humphrey MB, Montgomery CG, Gaffney PM. Association of a functional variant downstream of TNFAIP3 with systemic lupus erythematosus. Nature genetics. 2011;43:253–258. doi: 10.1038/ng.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, McNally E, Pickart C, Ma A. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nature immunology. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- [82].Lodolce JP, Kolodziej LE, Rhee L, Kariuki SN, Franek BS, McGreal NM, Logsdon MF, Bartulis SJ, Perera MA, Ellis NA, Adams EJ, Hanauer SB, Jolly M, Niewold TB, Boone DL. African-derived genetic polymorphisms in TNFAIP3 mediate risk for autoimmunity. J Immunol. 2010;184:7001–7009. doi: 10.4049/jimmunol.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Reddy MV, Velazquez-Cruz R, Baca V, Lima G, Granados J, Orozco L, Alarcon-Riquelme ME. Genetic association of IRF5 with SLE in Mexicans: higher frequency of the risk haplotype and its homozygozity than Europeans. Human genetics. 2007;121:721–727. doi: 10.1007/s00439-007-0367-6. [DOI] [PubMed] [Google Scholar]
- [84].Shin HD, Kim I, Choi CB, Lee SO, Lee HW, Bae SC. Different genetic effects of interferon regulatory factor 5 (IRF5) polymorphisms on systemic lupus erythematosus in a Korean population. The Journal of rheumatology. 2008;35:2148–2151. doi: 10.3899/jrheum.080124. [DOI] [PubMed] [Google Scholar]
- [85].Kawasaki A, Kyogoku C, Ohashi J, Miyashita R, Hikami K, Kusaoi M, Tokunaga K, Takasaki Y, Hashimoto H, Behrens TW, Tsuchiya N. Association of IRF5 polymorphisms with systemic lupus erythematosus in a Japanese population: support for a crucial role of intron 1 polymorphisms. Arthritis and rheumatism. 2008;58:826–834. doi: 10.1002/art.23216. [DOI] [PubMed] [Google Scholar]
- [86].Kelly JA, Kelley JM, Kaufman KM, Kilpatrick J, Bruner GR, Merrill JT, James JA, Frank SG, Reams E, Brown EE, Gibson AW, Marion MC, Langefeld CD, Li QZ, Karp DR, Wakeland EK, Petri M, Ramsey-Goldman R, Reveille JD, Vila LM, Alarcon GS, Kimberly RP, Harley JB, Edberg JC. Interferon regulatory factor-5 is genetically associated with systemic lupus erythematosus in African Americans. Genes and immunity. 2008;9:187–194. doi: 10.1038/gene.2008.4. [DOI] [PubMed] [Google Scholar]
- [87].Siu HO, Yang W, Lau CS, Chan TM, Wong RW, Wong WH, Lau YL, Alarcon-Riquelme ME. Association of a haplotype of IRF5 gene with systemic lupus erythematosus in Chinese. The Journal of rheumatology. 2008;35:360–362. [PubMed] [Google Scholar]
- [88].Niewold TB, Kelly JA, Flesch MH, Espinoza LR, Harley JB, Crow MK. Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients. Arthritis and rheumatism. 2008;58:2481–2487. doi: 10.1002/art.23613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Niewold TB, Kelly JA, Kariuki SN, Franek BS, Kumar AA, Kaufman KM, Thomas K, Walker D, Kamp S, Frost JM, Wong AK, Merrill JT, Alarcon-Riquelme ME, Tikly M, Ramsey-Goldman R, Reveille JD, Petri MA, Edberg JC, Kimberly RP, Alarcon GS, Kamen DL, Gilkeson GS, Vyse TJ, James JA, Gaffney PM, Moser KL, Crow MK, Harley JB. IRF5 haplotypes demonstrate diverse serological associations which predict serum interferon alpha activity and explain the majority of the genetic association with systemic lupus erythematosus. Annals of the rheumatic diseases. 2012;71:463–469. doi: 10.1136/annrheumdis-2011-200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Takaoka A, Yanai H, Kondo S, Duncan G, Negishi H, Mizutani T, Kano S, Honda K, Ohba Y, Mak TW, Taniguchi T. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243–249. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- [91].Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nature reviews. Immunology. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- [92].Fu Q, Zhao J, Qian X, Wong JL, Kaufman KM, Yu CY, Mok MY, Harley JB, Guthridge JM, Song YW, Cho SK, Bae SC, Grossman JM, Hahn BH, Arnett FC, Shen N, Tsao BP. Association of a functional IRF7 variant with systemic lupus erythematosus. Arthritis and rheumatism. 2011;63:749–754. doi: 10.1002/art.30193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Salloum R, Franek BS, Kariuki SN, Rhee L, Mikolaitis RA, Jolly M, Utset TO, Niewold TB. Genetic variation at the IRF7/PHRF1 locus is associated with autoantibody profile and serum interferon-alpha activity in lupus patients. Arthritis and rheumatism. 2010;62:553–561. doi: 10.1002/art.27182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Batteux F, Palmer P, Daeron M, Weill B, Lebon P. FCgammaRII (CD32)-dependent induction of interferon-alpha by serum from patients with lupus erythematosus. European cytokine network. 1999;10:509–514. [PubMed] [Google Scholar]
- [95].Lovgren T, Eloranta ML, Kastner B, Wahren-Herlenius M, Alm GV, Ronnblom L. Induction of interferon-alpha by immune complexes or liposomes containing systemic lupus erythematosus autoantigen- and Sjogren's syndrome autoantigen-associated RNA. Arthritis and rheumatism. 2006;54:1917–1927. doi: 10.1002/art.21893. [DOI] [PubMed] [Google Scholar]
- [96].Niewold TB. Interferon alpha as a primary pathogenic factor in human lupus. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2011;31:887–892. doi: 10.1089/jir.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Greth W. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): A Study to Evaluate the Efficacy and Safety of Sifalimumab in Adults.ClinicalTrials.gov 2000- [cited 2011 Dec 17], Available from: http://clinicaltrials.gov/ct2/show/NCT01283139 NLM Identifier: NCT01283139. [Google Scholar]
- [98].Kennedy W. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): A Study to Evaluate the Efficacy and Safety of Rontalizumab in Patients With Moderately to Severely Active Systemic Lupus Erythematosus.ClinicalTrials.gov 2000- [cited 2011 Dec 17]. Available from: http://clinicaltrials.gov/ct2/show/NCT00962832 NLM Identifier: NCT00962832. [Google Scholar]
- [99].Miesowicz F. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): An Investigation of AGS-009 in Patients With Systemic Lupus Erythematosus (SLE)ClinicalTrials.gov 2000- [cited 2012 Mar 5]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00960362 NLM Identifier: NCT00960362. [Google Scholar]
- [100].Mathian A, Amoura Z, Adam E, Colaone F, Hoekman MF, Dhellin O, Vandepapeliere P, Haroche J, Piette JC, Lebon P, Grouard-Vogel G. Active immunisation of human interferon alpha transgenic mice with a human interferon alpha Kinoid induces antibodies that neutralise interferon alpha in sera from patients with systemic lupus erythematosus. Annals of the rheumatic diseases. 2011;70:1138–1143. doi: 10.1136/ard.2010.141101. [DOI] [PubMed] [Google Scholar]
- [101].Zagury D, Le Buanec H, Mathian A, Larcier P, Burnett R, Amoura Z, Emilie D, Peltre G, Bensussan A, Bizzini B, Gallo RC, Koutouzov S. IFNalpha kinoid vaccine-induced neutralizing antibodies prevent clinical manifestations in a lupus flare murine model. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5294–5299. doi: 10.1073/pnas.0900615106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Dynavax Technologies, Pipeline ∷ Autoimmunity / Inflammation [Internet] [cited 2011 Dec 17]. Available from: http://www.dynavax.com/autoimmunity.html, in.
- [103].Yoo S, LLC M. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): A Study of the Efficacy and Safety of MEDI-546 in Systemic Lupus Erythematosus.ClinicalTrials.gov 2000- [cited 2012 Mar 5]. Available from: http://clinicaltrials.gov/ct2/show/NCT01438489 NLM Identifier: NCT01438489. [Google Scholar]
- [104].Quintas-Cardama A, Vaddi K, Liu P, Manshouri T, Li J, Scherle PA, Caulder E, Wen X, Li Y, Waeltz P, Rupar M, Burn T, Lo Y, Kelley J, Covington M, Shepard S, Rodgers JD, Haley P, Kantarjian H, Fridman JS, Verstovsek S. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115:3109–3117. doi: 10.1182/blood-2009-04-214957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Jabs WJ, Hennig C, Zawatzky R, Kirchner H. Failure to detect antiviral activity in serum and plasma of healthy individuals displaying high activity in ELISA for IFN-alpha and IFN-beta. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 1999;19:463–469. doi: 10.1089/107999099313901. [DOI] [PubMed] [Google Scholar]
- [106].Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis and rheumatism. 2006;54:1906–1916. doi: 10.1002/art.21890. [DOI] [PubMed] [Google Scholar]
- [107].Yao Y, Higgs BW, Richman L, White B, Jallal B. Use of type I interferon-inducible mRNAs as pharmacodynamic markers and potential diagnostic markers in trials with sifalimumab, an anti-IFNalpha antibody, in systemic lupus erythematosus. Arthritis research & therapy. 2010;12(Suppl 1):S6. doi: 10.1186/ar2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Nederman T, Karlstrom E, Sjodin L. An in vitro bioassay for quantitation of human interferons by measurements of antiproliferative activity on a continuous human lymphoma cell line. Biologicals : journal of the International Association of Biological Standardization. 1990;18:29–34. doi: 10.1016/1045-1056(90)90066-9. [DOI] [PubMed] [Google Scholar]
- [109].Merrill JT, Wallace DJ, Petri M, Kirou KA, Yao Y, White WI, Robbie G, Levin R, Berney SM, Chindalore V, Olsen N, Richman L, Le C, Jallal B, White B. Safety profile and clinical activity of sifalimumab, a fully human anti-interferon alpha monoclonal antibody, in systemic lupus erythematosus: a phase I, multicentre, double-blind randomised study. Annals of the rheumatic diseases. 2011;70:1905–1913. doi: 10.1136/ard.2010.144485. [DOI] [PubMed] [Google Scholar]
- [110].Rozzo SJ, Allard JD, Choubey D, Vyse TJ, Izui S, Peltz G, Kotzin BL. Evidence for an interferon-inducible gene, Ifi202, in the susceptibility to systemic lupus. Immunity. 2001;15:435–443. doi: 10.1016/s1074-7613(01)00196-0. [DOI] [PubMed] [Google Scholar]
- [111].Yao Y, Higgs BW, Morehouse C, de Los Reyes M, Trigona W, Brohawn P, White W, Zhang J, White B, Coyle AJ, Kiener PA, Jallal B. Development of Potential Pharmacodynamic and Diagnostic Markers for Anti-IFN-alpha Monoclonal Antibody Trials in Systemic Lupus Erythematosus. Human genomics and proteomics : HGP. 2009;2009 doi: 10.4061/2009/374312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Bauer JW, Baechler EC, Petri M, Batliwalla FM, Crawford D, Ortmann WA, Espe KJ, Li W, Patel DD, Gregersen PK, Behrens TW. Elevated serum levels of interferon-regulated chemokines are biomarkers for active human systemic lupus erythematosus. PLoS Med. 2006;3:e491. doi: 10.1371/journal.pmed.0030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Bauer JW, Petri M, Batliwalla FM, Koeuth T, Wilson J, Slattery C, Panoskaltsis-Mortari A, Gregersen PK, Behrens TW, Baechler EC. Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: a validation study. Arthritis and rheumatism. 2009;60:3098–3107. doi: 10.1002/art.24803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Fu Q, Chen X, Cui H, Guo Y, Chen J, Shen N, Bao C. Association of elevated transcript levels of interferon-inducible chemokines with disease activity and organ damage in systemic lupus erythematosus patients. Arthritis research & therapy. 2008;10:R112. doi: 10.1186/ar2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].McBride JM, Wallace DJ, Yao Z, Morimoto A, Jiang J, Maciuca R, McLean I, Drappa J. Dose-dependent modulation of interferon regulated genes with administration of single and repeat doses of Rontalizumab in a phase I, placebo controlled, double blind, dose escalation study in SLE [abstract 2072] Arthritis and rheumatism. 2009;60:S775–S776. [Google Scholar]
- [116].Ethgen D. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): A Study to Evaluate Safety and Tolerability of Subcutaneous Doses of MEDI-545 in Subjects With Lupus.ClinicalTrials.gov 2000- [cited 2011 Dec 17]. Available from: http://clinicaltrials.gov/ct2/show/NCT00657189 NLM Identifier: NCT00657189. [Google Scholar]
- [117].Greth W. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): A Study to Evaluate the Long-Term Safety of MEDI-545 in Adult Subjects With Systemic Lupus Erythematosus or Myositis.ClinicalTrials.gov 2000- [cited 2011 Dec 17]. Available from: http://clinicaltrials.gov/ct2/show/NCT00979654 NLM Identifier: NCT00979654. [Google Scholar]
- [118].Abbey J. Argos Therapeutics News [Internet] 2010. Argos Therapeutics Initiates Dosing Of Patients In Phase 1 Clinical Trial Of Monoclonal Antibody-Based Therapy For Treatment Of Systemic Lupus Erythematosus. [cited 2011 Dec 17]. Available from: http://www.argostherapeutics.com/news/news_Phase_1-AGS-009-Final.html. [Google Scholar]
- [119].Morimoto AM, Flesher DT, Yang J, Wolslegel K, Wang X, Brady A, Abbas AR, Quarmby V, Wakshull E, Richardson B, Townsend MJ, Behrens TW. Association of endogenous anti-interferon-alpha autoantibodies with decreased interferon-pathway and disease activity in patients with systemic lupus erythematosus. Arthritis and rheumatism. 2011;63:2407–2415. doi: 10.1002/art.30399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Houssiau FA, Rashkov R, Hachulla E, Lazaro E, Jorgensen C, Spertini F, Mariette X, Grouard-Vogel G, Fanget B, Dhellin O, Lauwerys B, Vandepapeliere P. #2470. Active Immunization Against IFNα with IFN-Kinoid in SLE Patients Is Safe, Immunogenic and Induces Down-Regulation of IFN-Mediated Genes, ACR/ARHP scientific abstracts. Arthritis & Rheumatism. 2011;63:S963. [Google Scholar]
- [121].Vandepapeliere P. ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): Safety of IFNa Kinoid in Systemic Lupus Erythematosus.ClinicalTrials.gov 2000- [cited 2011 Dec 17]. Available from: http://clinicaltrials.gov/ct2/show/NCT01058343 NLM Identifier: NCT01058343. [Google Scholar]
- [122].Guiducci C, Gong M, Xu Z, Gill M, Chaussabel D, Meeker T, Chan JH, Wright T, Punaro M, Bolland S, Soumelis V, Banchereau J, Coffman RL, Pascual V, Barrat FJ. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465:937–941. doi: 10.1038/nature09102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Katz SJ, Russell AS. Re-evaluation of antimalarials in treating rheumatic diseases: re-appreciation and insights into new mechanisms of action. Current opinion in rheumatology. 2011;23:278–281. doi: 10.1097/BOR.0b013e32834456bf. [DOI] [PubMed] [Google Scholar]
- [124].Zhu FG, Yu D, Kandimalla ER, Monica NL, Agrawal S. Keystone Symposia: Dendritic Cells and the Initiation of Adaptive Immunity. 2011. Treatment with IMO-3100, a Novel TLR7 and TLR9 Antagonist, Inhibits Disease Development in Lupus Prone NZBW/F1 Mice. [Google Scholar]
- [125].Sullivan T, Atiee G, Bhagat L, Jiang W, Murphy J, Ostavnenko N, Precopio M, Putta M, Kandimalla E, Arbeit R. Keystone Symposia Meeting: Immunoregulatory Networks. 2011. A Novel Toll-like Receptor Antagonist for Autoimmune and Inflammatory Diseases: Safety and Pharmacodynamics in a Multiple-Dose Phase 1 Clinical Trial. [Google Scholar]
- [126].Barrat FJ, Coffman RL. Development of TLR inhibitors for the treatment of autoimmune diseases. Immunological reviews. 2008;223:271–283. doi: 10.1111/j.1600-065X.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- [127].Guiducci C, Tripodo C, Gong M, Sangaletti S, Colombo MP, Coffman RL, Barrat FJ. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. The Journal of experimental medicine. 2010;207:2931–2942. doi: 10.1084/jem.20101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Barrat FJ. Regulation of the Innate Immune Response Using Oligonucleotide-Based Inhibitors of Toll-Like Receptors. 7th Annual Meeting of the Oligonucleotide Therapeutics Society; Copenhagen, Denmark. 2011. [Google Scholar]
- [129].Pfizer . ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): First Safety Study in Humans of a Single Dose of CPG 52364.ClinicalTrials.gov 2000- [cited 2012 Mar 6]. Available from: http://clinicaltrials.gov/ct2/show/NCT00547014 NLM Identifier: NCT00547014. [Google Scholar]
- [130].Lipford G, Forsbach A, Zepp C, Nguyen T, Weeratna R, McCluskie M, Vollmer J, Davis H, Krieg AM. American College of Rheumatology 2007 Annual Scientific Meeting. 2007. Selective Toll-like receptor 7/8/9 antagonists for the oral treatment of autoimmune diseases. [Google Scholar]
- [131].Kawasaki M, Fujishiro M, Yamaguchi A, Nozawa K, Kaneko H, Takasaki Y, Takamori K, Ogawa H, Sekigawa I. Possible role of the JAK/STAT pathways in the regulation of T cell-interferon related genes in systemic lupus erythematosus. Lupus. 2011;20:1231–1239. doi: 10.1177/0961203311409963. [DOI] [PubMed] [Google Scholar]
- [132].Wang S, Yang N, Zhang L, Huang B, Tan H, Liang Y, Li Y, Yu X. Jak/STAT signaling is involved in the inflammatory infiltration of the kidneys in MRL/lpr mice. Lupus. 2010;19:1171–1180. doi: 10.1177/0961203310367660. [DOI] [PubMed] [Google Scholar]
- [133].Neubert K, Meister S, Moser K, Weisel F, Maseda D, Amann K, Wiethe C, Winkler TH, Kalden JR, Manz RA, Voll RE. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nature medicine. 2008;14:748–755. doi: 10.1038/nm1763. [DOI] [PubMed] [Google Scholar]
- [134].Hainz N, Thomas S, Neubert K, Meister S, Benz K, Rauh M, Daniel C, Wiesener M, Voll RE, Amann K. The Proteasome Inhibitor Bortezomib Prevents Lupus Nephritis in the NZB/W F1 Mouse Model by Preservation of Glomerular and Tubulointerstitial Architecture, Nephron. Experimental nephrology. 2012;120:e47–e58. doi: 10.1159/000334955. [DOI] [PubMed] [Google Scholar]
- [135].Ichikawa HT, Conley T, Muchamuel T, Jiang J, Lee S, Owen T, Barnard J, Nevarez S, Goldman BI, Kirk CJ, Looney RJ, Anolik JH. Beneficial effect of novel proteasome inhibitors in murine lupus via dual inhibition of type I interferon and autoantibody-secreting cells. Arthritis and rheumatism. 2012;64:493–503. doi: 10.1002/art.33333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Hirai M, Kadowaki N, Kitawaki T, Fujita H, Takaori-Kondo A, Fukui R, Miyake K, Maeda T, Kamihira S, Miyachi Y, Uchiyama T. Bortezomib suppresses function and survival of plasmacytoid dendritic cells by targeting intracellular trafficking of Toll-like receptors and endoplasmic reticulum homeostasis. Blood. 2011;117:500–509. doi: 10.1182/blood-2010-05-284737. [DOI] [PubMed] [Google Scholar]
- [137].Liou YC, Zhou XZ, Lu KP. Prolyl isomerase Pin1 as a molecular switch to determine the fate of phosphoproteins. Trends Biochem Sci. 2011;36:501–514. doi: 10.1016/j.tibs.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Tun-Kyi A, Finn G, Greenwood A, Nowak M, Lee TH, Asara JM, Tsokos GC, Fitzgerald K, Israel E, Li X, Exley M, Nicholson LK, Lu KP. Essential role for the prolyl isomerase Pin1 in Toll-like receptor signaling and type I interferon-mediated immunity. Nature immunology. 2011;12:733–741. doi: 10.1038/ni.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Stein RA. Epigenetics--the link between infectious diseases and cancer. JAMA. 2011;305:1484–1485. doi: 10.1001/jama.2011.446. [DOI] [PubMed] [Google Scholar]
- [140].Zur Hausen H. The search for infectious causes of human cancers: where and why. Virology. 2009;392:1–10. doi: 10.1016/j.virol.2009.06.001. [DOI] [PubMed] [Google Scholar]