Abstract
Functional and neuroanatomical asymmetries are an important characteristic of the human brain. The evolution of such specializations in the human cortex has provoked great interest in primate brain evolution. Most research on cortical sulci has revolved around linear measurements, which represent only one dimension of sulci organization. Here, we used a software program (BrainVISA) to quantify asymmetries in cortical depth and surface area from magnetic resonance images in a sample of 127 chimpanzees and 49 macaques. Population brain asymmetries were determined from 11 sulci in chimpanzees and seven sulci in macaques. Sulci were taken from the frontal, temporal, parietal, and occipital lobes. Population-level asymmetries were evident in chimpanzees for several sulci, including the fronto-orbital, superior precentral, and sylvian fissure sulci. The macaque population did not reveal significant population-level asymmetries, except for surface area of the superior temporal sulcus. The overall results are discussed within the context of the evolution of higher order cognition and motor functions.
Keywords: Chimpanzee, Brain asymmetry, Sulci morphology
1.1 Introduction
Functional and neuroanatomical asymmetries are a prominent feature of the human brain, most notably in regions associated with perception and production of language and speech (Corballis, 2002; Davidson, 1995). For instance, clinical and functional imaging studies in humans have well established that the left inferior frontal gyrus (IFG) and posterior temporal lobe (PT) are involved in language production and comprehension skills, respectively (Beaton, 1997; Foundas et al., 1998; Keller et al., 2009a). There is also evidence of leftward anatomical asymmetries in the posterior temporal lobe (Shapleske et al., 1999) and, to a lesser degree, the IFG, particularly among right-handed individuals, which many believe underlies the functional asymmetries for language found in these brain regions (Keller et al., 2009a; Keller et al., 2007).
Given the importance of the IFG and PT in language functions, the question of whether population-level asymmetries occur in nonhuman animals, notably chimpanzees, is of considerable scientific interest to the study of human evolution (Binder et al., 1997; Bruner and Holloway, 2010; Keller et al., 2009a). Many scientists have argued that because language is unique to humans and strongly left hemisphere dominant, population-level asymmetries are an adaptation of the human brain that occurred after the split of the common ancestor with genus Pan (Crow, 2004; Crow, 2009; Williams et al., 2006). They argue that genetic differences between apes and humans were necessary for the development of language and the associated brain asymmetries found in human but not nonhuman primate brains (Williams et al., 2006). This proposed evolutionary model of behavioral and brain asymmetry (Annett, 1985, 2002; Crow, 2004; Crow, 2009; Williams et al., 2006) is considered saltational; that is, these theoretical perspectives suggest that a qualitative shift and unique change of the human central nervous system occurred after the split between humans and chimpanzees. Thus, there should be no continuity in homologous asymmetries between these species.
In contrast to the saltational views, others have suggested that the evolution of behavioral and brain asymmetries occurred along a continuum with homologies evident for more primitive or conserved brain systems and specializations unique to humans in more recently evolved and expanded regions (Balzeau and Gilissen, 2010; Hopkins and Cantalupo, 2008). Specifically, recent in vivo imaging and analysis of post-mortem brains suggest that chimpanzees, and possibly other nonhuman primate species, show population-level leftward asymmetries in the PT (Gannon et al., 1998; Gannon et al., 2008; Gilissen, 1992, 2001; Hopkins and Nir, 2010; Spocter et al., 2010), but less consistently for the IFG (Cantalupo and Hopkins, 2001; Hopkins et al., 2008; Keller et al., 2009b; Schenker et al., 2010; Uylings et al., 2006). For example, measurement of the PT from MRI images reveals significant population-level leftward asymmetries in chimpanzees (Hopkins and Nir, 2010), but not in Old World monkeys (Lyn et al., 2011). Cytoarchitectonic studies have also shown that the volume of BA22, a constituent part of the PT, is larger in the left compared to right hemisphere in chimpanzees (Spocter et al., 2010) and rhesus monkeys (Gannon et al., 2008).
In the present study, we examined whether population-level asymmetries exist in sulci surface area and mean depth for 11 sulci of the chimpanzee and seven sulci from the macaque brain. For both species, we selected sulci from the frontal, temporal, parietal and occipital lobes (see Methods). Our main objective was to test the saltational hypothesis. According to this hypothesis, if population-level brain asymmetries emerged uniquely in humans as a consequence of either language and speech evolution, or other functional asymmetries, such as handedness as suggested by others (Corballis, 2003; Crow, 2004; Marchant and McGrew, 1991; Warren, 1980), then chimpanzees should fail to show significant population-level asymmetries in either surface area or mean depth for any of the sulci. Based on previous evidence of population-level asymmetries in the PT and, to a lesser extent, the IFG, we did not expect that the saltational hypothesis would be supported. Rather, we hypothesized that significant population-level asymmetries would be found in chimpanzees, particularly for sulci that serve as landmarks in defining the PT and IFG, including the sylvian fissure, precentral inferior, inferior frontal and fronto-orbital sulci. Our predictions for macaques were less confident because the existing literature on asymmetries in this genus has produced inconsistent results. Notwithstanding, if population-level asymmetries evolved prior to the split between apes and Old World monkeys, then it would be predicted that macaques monkeys would show population-level asymmetries for one or more sulci.
A second hypothesis we tested was related to theories suggesting that brain asymmetries evolved in the context of decreasing ratios in the size of the corpus callosum (CC) relative to whole brain volume and neocortical surface area (Oliveras et al., 2001; Rilling and Insel, 1999a). Comparative studies of the size of the CC in mammals, as well as within primate species, have shown that as brain size increased during evolution, the CC did not keep pace (Oliveras et al., 2001; Rilling and Insel, 1999a). Thus, humans have a relatively small CC for a species of our brain size followed by great apes, and then the more distantly related Old and New World monkeys. The suggestion is that as primate brains got larger, each hemisphere became increasingly disconnected which resulted in increasingly intra- rather than interhemispheric connectivity (Aboitiz et al., 2003; Hopkins and Cantalupo, 2008; Ringo et al., 1994). This, in turn, resulted in increasingly functional and anatomical specializations within each hemisphere. Because chimpanzees have a smaller ratio in CC size to brain volume compared to macaque monkeys, this theory predicts that chimpanzees would show larger asymmetries than macaques. We tested this hypothesis by comparing the absolute degree of sulci asymmetry in macaque monkeys and chimpanzees in five sulci that were common to both genera.
Sulci were quantified using a software program called BrainVISA (BV). BV focuses on cortical folding patterns of the brain and uses sulcus-based morphometry (Mangin et al., 2004). This method differs from historical approaches, because it quantifies both the surface area and mean depth of the sulci rather than relying solely on the linear length of the outer contour of the sulcus, the primary measure employed in many previous studies of brain asymmetry in human and nonhuman primates measured from cadaver specimens (Gannon et al., 2008; Gilissen, 1992; Heilbronner and Holloway, 1988; Heilbronner and Holloway, 1989; Imai et al., 2011; LeMay, 1985; Witelson and Kigar, 1992; Yeni-Komshian and Benson, 1976), cranial endocasts (Cheverud et al., 1990; Falk et al., 1986; Falk et al., 1990), and MRI (Cantalupo et al., 2003; Hopkins et al., 2000; Ide et al., 1996; Liu and Phillips, 2009; Zilles et al., 1996). This is an important distinction because measures of length, by themselves, may not capture all size dimensions of the sulci. By contrast, BrainVISA captures all dimensions of variability in organization (i.e., length and depth) and therefore offers a new and potentially more thorough means of assessing asymmetries in cortical sulci.
1.2 Methods
1.2.1 Subjects
In vivo magnetic resonance images (MRI) were obtained from 127 captive chimpanzees (Pan troglodytes), including 76 females and 51 males, ranging in age from 6 to 53 years. The chimpanzees were housed at the Yerkes National Primate Research Center (YNPRC) and the University of Texas M. D. Anderson Cancer Center (UTMDACC).
Magnetic resonance images (MRI) were obtained from 28 bonnet monkeys (Macaca radiata) and 21 rhesus monkeys (Macaca mulatta) housed at the Wake Forest University Primate Center (WFUPC). Within the bonnet monkey sample, there were 11 females and 17 males, ranging in age from 8 months to 10 years of age (M=4.24, SD=2.65). The rhesus monkey sample was comprised of 16 males and 5 females ranging in age from 6 to 11 years of age (M=9.1, SD=2.3). This study was conducted in accordance with the Guidelines of the Committee on the Care and Use of Laboratory Animal Resources (NRC, 1996) and approved by each institution’s animal care and use committee.
1.2.2 MRI Image Collection
Scanning Procedures
All chimpanzee magnetic resonance image (MRI) scans followed standard procedures at the YNPRC and UTMDACC. Subjects were scanned during their scheduled physical examination surveys and anaesthetized with propofol (40–60 mg/(kg/h)). The chimpanzee was placed in a supine position in the scanner with its head in a human-head coil. The scanning process ranged between 35–50 minutes depending on brain size. Upon completion of the MRI, chimpanzees were temporarily singly housed for 2–6 hours to allow the anesthesia to wear off before being returned to their home group.
Monkeys were given initial ketamine anesthesia (15 mg/kg, i.m.) and atropine (0.07 mg/kg, i.m.), then transported to the mobile MRI scanner, intubated and maintained under isofluorane (1.25%) throughout the scan. The subjects remained anesthetized for the duration of the scans as well as the time needed to transport them between their home cage and the imaging facility (total time ~ 1 hour). Subjects were placed in the scanner chamber in a supine position with their head fitted inside the human-knee coil. Scan duration ranged between 24 and 28 minutes as a function of brain size. After completing MRI procedures, the subjects were temporarily housed in a single cage for 1–2 h to allow the effects of the anesthesia to wear off, after which they were returned to their home cage. The archived MRI data were transferred to a PC running BrainVISA software for post-image processing.
Imaging Parameters
For the chimpanzees, 68 individuals were scanned using a 3.0 T scanner (Siemens Trio, Siemens Medical Solutions USA, Inc., Malvern, Pennsylvania, USA). T1-weighted images were collected using a three-dimensional gradient echo sequence (pulse repetition = 2300 ms, echo time = 4.4 ms, number of signals averaged = 3, matrix size = 320 × 320, with 0.6 × 0.6 × 0.6 resolution). The remaining 59 chimpanzees as well as all the macaques were scanned using a 1.5T G.E. echo-speed Horizon LX MR scanner (GE Medical Systems, Milwaukee, WI). T1-weighted images were collected in the transverse plane using a gradient echo protocol (pulse repetition = 19.0 ms, echo time = 8.5 ms, number of signals averaged 8, matrix size = 256 × 256, with 0.7 × 0.7 × 1.2 resolution). Examples of T1-images from both species are illustrated in Figure 1.
Figure 1.

Slice of T1-weighted images of a a) rhesus macaque (left) and b) chimpanzee (right) brain in the transverse plane with overlay of the grey/white segmentation processed from BrainVISA. Images are not to scale.
1.2.3 Post-Image Processing
BrainVISA 4.0.1 is freely distributed software (http://brainvisa.info) that examines the cortical folding of the brain (Mangin et al., 2004). To account for the differences in chimpanzee and monkey anatomy compared to humans, a number of adjustments were preformed before the scans were processed using the pipeline procedure within BrainVISA. Specifically, all monkey and chimpanzee MRI scans were skull-stripped, cropped, and reformatted at 0.7 cubic isotropic resolution using ANALYZE 8.1 software and subsequently imported into BrainVISA. The pipeline process of extracting the sulci from the cortex involved a number of steps (Mangin et al., 2004) (see Figure 2). To align the template brain, the anterior and posterior commissures were manually specified on the MRI at the point where they intersect with the mid-sagittal slice. The first step was to correct for spatial inhomogeneities in the signal intensity providing a spatially smooth bias field with a stable distribution of tissue intensities. Next, the analysis of the signal histogram and mathematical morphology was computed to obtain a binary mask of the brain. Adjustments were sometimes needed in the histogram process to better distinguish grey and white matter for both chimpanzee and macaque brain scans. The mask was then split into the left and right hemispheres and the cerebellum. For the monkey scans, adjustments were made to this process regarding the minimum brain size in cubic centimeters and the human-based template was turned off. Manual editing of the spit brain mask was sometimes needed during this process to properly label the hemispheres and cerebellum. A negative mold of the white matter was computed from the split brain mask. The outside boundary of this mould results from a 5 mm morphological closing of the masked hemisphere, filling up the folds. The grey/white interface is the inside boundary that preserves deformations and assures the spherical topology of the mould. Finally the mould was skeletonised to detect cortical folding, while topological constraints guaranteed the resulting surfaces would have no holes.
Figure 2.
BrainVISA’s pipline processing steps a) MR image of a skull-stripped chimpanzee brain, b) stable tissue intensities creating bias field, c) binary mask of the brain, d) split mask of left and right hemispheres and cerebellum, e) grey and white interface, f) A negative mould of the white matter, g) skeletonised mould of cortical folding, h) cortical fold graph of chimpanzee sulci.
1.2.4 Sulci Labeling and Quantification
Eleven sulci in the chimpanzee brain and seven for the macaques were manually labeled following the definitions of Bailey et al (1950) and Connolly (1936) (see Figure 3a & b). For the chimpanzees, the sulci selected in the frontal lobe were the central (CS), superior precentral (SPC), precentral inferior (PCI), inferior frontal (IFS), and fronto-orbital (FO) sulci (Keller et al., 2009b). The superior precentral sulcus in the chimpanzees is triradiate in formation, where one branch extends anteriorly toward the frontal pole and is considered a part of the superior frontal sulcus (Bailey et al., 1950) while the posterior end runs medial to lateral, with the lateral branch sometimes considered the superior precentral sulcus. In this study, we include this anterior limb as a portion of SPC rather than distinct. PCI often includes the superior limb running parallel to the central sulcus, however, for the purpose of this study, we were interested in including only that portion of PCI that is used to define the IFG in chimpanzees (Keller et al., 2009b). Thus, we obtained measures on the inferior limb of PCI, which is considered the posterior border of the IFG in the chimpanzee brain. Furthermore, PCI can be bifurcated (Keller et al., 2009b; Sherwood et al., 2003), and we included all inferior branches of the PCI in our measurement of this sulcus. FO in the chimpanzee constitutes the anterior border of IFG and is analogous to the human ascending ramus (Keller et al., 2009b). The temporal lobe consisted of the sylvian fissure (SF) and superior temporal sulcus (STS), while the parietal and occipital lobe included the superior postcentral (POCS), inferior postcentral (POCI), intraparietal (IP) and lunate (LU) sulci. For the macaques, seven sulci were labeled including the principal/rectus (PR), arcuate (ARC), central (CS), sylvian (SF), superior temporal (STS), intraparietal (IP) and lunate (LU).
Figure 3.
Cortical brain sulci for the a) Macaque brain, including seven sulci: red = central, yellow = principal/rectus, light purple = arcuate, dark blue = sylvian fissure, dark pink = superior temporal, dark green = intraparietal, and black = lunate. b) chimpanzee brain, including eleven sulci: red = central, light green = superior precentral, orange = fronto-orbital, yellow = precentral inferior, light purple = inferior frontal, dark blue = sylvian fissure, dark pink = superior temporal, light blue = inferior postcentral, dark purple = superior postcentral, dark green = intraparietal, and black = lunate.
Measures from each sulci included surface area (mm2) and mean depth (mm). The sulcus surface area was computed as the sum of the areas of all the triangles required to mesh the sulcus medial surface. The sulcus mean depth is the average depth computed across all the bottom points of the sulcus along its principal axis of projection (i.e., dorsal-ventral or anterior-posterior). The bottom points are defined from topological properties and correspond to the sulcus edge that is not connected to the cortex hull. Hence, this definition can be used even with interrupted sulci. The depth of a bottom point is the length of the shortest path from this point to the cortex hull embedded in the sulcus medial surface (mathematically speaking, the shortest geodesic path). Asymmetry quotients (AQ) for all measures were calculated following the formula: [AQ = (R−L)/(R+L)], where R and L represent right and left hemisphere values, respectively. Positive AQ values reflected a right hemisphere bias whereas negative values reflected a left hemisphere bias. Absolute AQ values were calculated for each subject by taking the absolute value of the AQ.
Using individual AQ scores, we also classified each individual as having either a left (AQ ≤ −0.0125), right (AQ ≥ 0.0125), or no bias (AQ > −0.0125 and AQ < 0.0125) for each sulcus and measure. We adopted these cut-off points because they have been used in previous studies of brain asymmetry in human and nonhuman primates and we sought to use this approach as an additional means of characterizing asymmetry within species (Cantalupo et al., 2003; Knaus et al., 2006). Inferential statistics were used for all analyses with alpha set to p < 0.05 unless normality or homogeneity of variance assumptions for the tests could not be met. Post-hoc comparisons on inferential statistics, when used, were conducted using Tukey’s Honestly Significant Difference test.
1.3 Results
1.3.1 Chimpanzees
Sex and Scanner Effects
In the initial analysis, we tested for sex and scanner effects on the AQ scores for both surface area and mean depth. For this analysis, we performed two MANOVA tests with all AQ scores for each sulcus serving as a dependent measure while sex (male = 51, female = 76) and scanner (3T = 68 versus 1.5T = 59) served as the between-group factors. No overall significant main effects or interactions were found for either surface area or mean depth. Chi-square tests of independence confirmed the MANOVA results. For these analyses, we performed chi-square tests of independence comparing the distribution of left, right and no biased subjects for surface area and mean depth as a function of sex and scanner. None of these associations were significant, thus confirming that neither sex nor scanner explained a significant portion of variability in sulci asymmetries. In short, the patterns of asymmetry were consistent between males and females and between chimpanzees scanned at 3T compared to 1.5T.
Test of Population Asymmetry
In the next set of analyses, we tested whether population-level asymmetries were evident for each sulcus and measure. Because neither sex nor colony influenced the AQ scores, we used the entire dataset for these analyses. The mean AQ values for each sulcus and measure are shown in Figure 4. For these analyses, one sample t-tests were performed on the AQ scores for each sulcus and measure. For surface area, significant leftward asymmetries were found for SPC t(126) = −3.10, p < 0.003, FO t(126) = −2.14, p < 0.04 and SF t(126) = −3.62, p < 0.001, while a rightward bias was found for POCI t(126) = 2.04, p < 0.05 (Figure 4a). For mean sulcus depth, the chimpanzees showed significant leftward biases for the SPC t(126)=−2.31, p < 0.03, FO t(126) = −3.56, p < 0.002, SF t(126) = −4.17, p < 0.001, , IP t(126) = −4.23, p < 0.001 and the LU t(126) = −2.36, p < 0.03 sulci (Figure 4b).
Figure 4.
Mean chimpanzee population AQ of all11 sulci for a) surface area and b) mean depth. Significant population-level asymmetry is indicated by a red asterisk (*). Sulci: CS = central, FO = fronto-orbital, PCI = precentral inferior, IFS = inferior frontal, SPC = superior precentral, SF = sylvian fissure, STS = superior temporal, IP = intraparietal, POCI = inferior postcentral, POCS = superior postcentral, and LU = lunate.
We next assessed the distribution of asymmetries using the classification data. For both surface area and mean depth, the overall distribution of asymmetry differed significantly from a hypothetical random model (see Table 1). Of specific interest to these analyses were the differences in the number of individuals classified as either left or right biased. For surface area, there were significantly more left than right biased chimpanzees for the SF χ2(1, 110) = 9.31, p < 0.004 and SPC χ2 (1, 117) = 9.31, p < 0.004. For mean depth, there were significantly more left than right biased chimpanzees for FO χ2 (1, 104) = 9.85, p < 0.004, SPC χ2 (1, 104) = 4.65, p < 0.05, SF χ2 (1, 115) = 10.65, p < 0.004, IP χ2 (1, 111) = 12.33, p < 0.004 and LU sulci χ2 (1, 111) = 5.63, p < 0.03.
Table 1.
Distribution of cortical asymmetries for surface area and mean depth in chimpanzees across 11 sulci.
| Sulci | Surface area | Mean Depth | ||||||
|---|---|---|---|---|---|---|---|---|
| #L | #NB | #R | Chi-Square | #L | #NB | #R | Chi-Square | |
| Frontal | ||||||||
| CS | 53 | 37 | 37 | 4.03 | 40 | 48 | 39 | 1.15 |
| FO | 63 | 17 | 47 | 25.76 ** | 68 | 23 | 36 | 25.34 ** |
| IFS | 62 | 6 | 59 | 46.88 ** | 53 | 25 | 49 | 10.84 ** |
| PCI | 62 | 3 | 62 | 54.82 ** | 63 | 7 | 57 | 44.66 ** |
| SPC | 75 | 10 | 42 | 49.91 ** | 63 | 23 | 41 | 18.96 ** |
| Temporal | ||||||||
| SF | 71 | 17 | 39 | 34.83** | 75 | 12 | 40 | 47.07 ** |
| STS | 55 | 26 | 46 | 10.41** | 50 | 27 | 50 | 8.33 * |
| Parietal | ||||||||
| IP | 64 | 7 | 56 | 44.99** | 74 | 16 | 37 | 40.74 ** |
| POCI | 50 | 13 | 64 | 32.80** | 47 | 18 | 62 | 23.64 ** |
| POCS | 60 | 10 | 57 | 37.15** | 54 | 16 | 57 | 24.68 ** |
| Occipital | ||||||||
| LU | 63 | 11 | 53 | 35.97 ** | 68 | 16 | 43 | 31.95 ** |
indicates p < 0.01 and
indicates p < 0.05 for chi-square goodness-of-fit tests χ2 (2, N=127).
L = left, NB = no bias, R = right. Sulci: CS = central, FO = fronto-orbital, IFS = inferior frontal, PCI = precentral inferior, SPC = superior precentral, SF = sylvian fissure, STS = superior temporal, IP = intraparietal, POCI = inferior postcentral, POCS = superior postcentral, and LU = lunate.
1.3.2 Macaques
Species and Sex Effects
In the initial analysis, we tested for differences in the AQ scores between the two macaque species as well as potential sex differences using a multiple analysis of variance. The AQ scores for the surface area and mean depth of each sulcus served as the dependent measures while species and sex served as the between-group factors. No significant main effects or interactions were found. Thus, for the analyses examining population-level asymmetries, we combined the data from rhesus and bonnets to form a single representing population for the genus Macaca.
Test of Population-Level Asymmetries
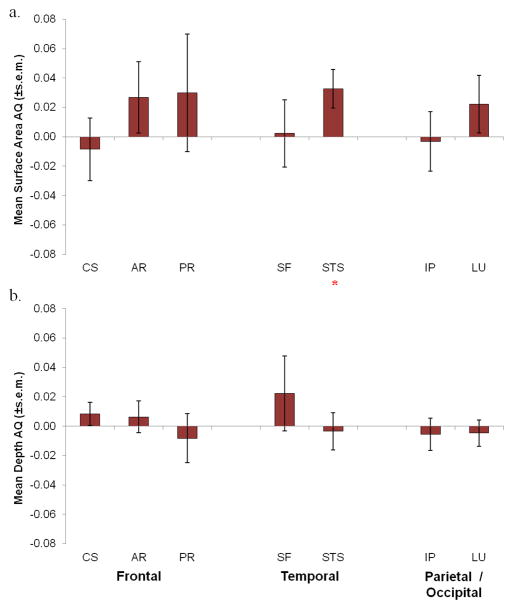
As was done with the chimpanzee data, one sample t-tests were performed on the AQ scores for each measure and sulcus. The mean AQ scores for each sulcus and measure are shown in Figure 5. For surface area, a significant rightward asymmetry was found for the STS t(48) = 2.47, p < 0.02 (Figure 5a). For mean depth, no significant population-level asymmetries were found for any of the seven sulci (Figure 5b). When considering the classification data, the results largely confirmed to the t-test findings (see Table 2). For mean depth and surface area, significant individual asymmetries were found for every measure and sulcus except mean depth of the arcuate, STS, and lunate and this was attributed to the fact that approximately equal amount of monkeys showed no bias and either the left or right classification (Table 2). However, when considering the distributions of only the left and right-lateralized subjects, a significant rightward bias was found for surface area STS χ2 (1, 47) = 6.15, p < 0.02 and mean depth AR χ2 (1, 34) = 4.24, p < 0.05.
Figure 5.
Mean macaque population AQ of all seven sulci for a) surface area and b) mean depth. Significant population-level asymmetry is indicated by a red asterisk (*). Sulci: CS = central, AR = arcuate, PR = principal/rectus, SF = sylvian fissure, STS = superior temporal, IP = intraparietal, and LU = lunate.
Table 2.
Distribution of cortical asymmetries for surface area and mean depth in macaques across 7 sulci.
| Sulci | Surface area | Mean Depth | ||||||
|---|---|---|---|---|---|---|---|---|
| #L | #NB | #R | Chi-Square | #L | #NB | #R | Chi-Square | |
| Frontal | ||||||||
| CS | 24 | 5 | 20 | 12.29 ** | 20 | 8 | 21 | 6.41 * |
| AR | 18 | 4 | 27 | 16.45 ** | 11 | 15 | 23 | 4.57 |
| PR | 22 | 4 | 23 | 14.00 ** | 18 | 8 | 23 | 7.14 * |
| Temporal | ||||||||
| SF | 22 | 4 | 23 | 14.00 ** | 20 | 6 | 23 | 10.08 ** |
| STS | 15 | 2 | 32 | 27.71 ** | 21 | 9 | 19 | 5.06 |
| Parietal | ||||||||
| IP | 25 | 4 | 20 | 14.73 ** | 21 | 10 | 18 | 3.96 |
| Occipital | ||||||||
| LU | 24 | 1 | 24 | 21.59 ** | 22 | 11 | 16 | 3.71 |
indicates p < 0.01 and
indicates p < 0.05 for chi-square goodness-of-fit tests χ2 (2, N=49).
L = left, NB = no bias, R = right. Sulci: CS = central, AR = arcuate, PR = principal/rectus, SF = sylvian fissure, STS = superior temporal, IP = intraparietal, and LU = lunate.
1.3.3 Between Species Comparisons
In this final set of analyses, we compared the direction and strength of asymmetry scores for the five sulci that were common to both chimpanzees and macaques including the CS, SF, STS, IP and LU sulci. Because the distribution of asymmetries differed between the species (see Tables 1 and 2), directly comparing the species on their AQ violated the assumptions of normality in the analysis of variance. Therefore, we compared the chimpanzees and macaques using the non-parametric Mann-Whitney U-test. For the AQ values, we found significant species differences for the STS in surface area (U = 2,328.00, N = 176, p < 0.02), with macaques showing greater rightward asymmetries than chimpanzees. For the mean depth measures, a significant species difference was found for the SF (U = 2,409.00, N = 176, p < 0.03), with chimpanzees showing a greater leftward asymmetry than macaques.
For the ABS-AQ values, in terms of surface area, significant species differences were found for the CS (U = 1,335.00, N = 176, p < 0.001), SF (U = 2,280.00, N = 176, p < 0.01) and STS (U = 2,373.00, N = 176, p < 0.02). For all three sulci, macaques showed higher ABS-AQ scores than chimpanzees, indicating stronger directional asymmetries. For the ABS-AQ mean depth values, significant species differences were found for the CS (U = 2,094.00, N = 176, p < 0.002) sulci. Macaques showed higher ABS-AQ scores than chimpanzees in the central sulcus, suggesting greater individual lateralization in macaques.
1.4 Discussion
Several significant findings emerged from this study. First, contrary to the saltational perspective, this study demonstrates that chimpanzees exhibit population-level asymmetries for a number of cortical sulci in terms of both their surface area and mean depth. In contrast, macaques showed population-level asymmetries for only one sulcus and one measure. Thus, chimpanzees as a population appear to show much more robust directional asymmetries in cortical organization as it relates to surface area and depth of sulci compared to macaques. As noted above, cortical asymmetries have long been considered unique in hominin evolution (Corballis, 1992; Ettlinger, 1988), however, many recent studies in nonhuman primates, specifically great apes, demonstrate opposing results (Cantalupo and Hopkins, 2001; Cantalupo et al., 2009; Hopkins, 2007; Hopkins et al., 2008). The findings reported here are consistent with the evidence that population-level asymmetries are not unique to humans. Further, this study’s results do not support the saltational view of the evolution of brain (and presumably behavioral) asymmetries. Indeed, these results suggest that population-level cortical asymmetries were likely present in the common ancestor of humans and chimpanzees. With specific reference to asymmetries in cortical sulci, one mechanism by which this could have evolved is via short- and long-range development of cortical connections between different cortical regions (Herculano-Houzel et al., 2010; Van Essen, 1997). Studies have shown that there has been a disproportional increase in white matter expansion during primate evolution (Rilling and Insel, 1999a; Rilling and Insel, 1999b; Schoenemann et al., 2005; Semendeferi et al., 2001) and this appears to be accompanied by increased gyrification (Rogers et al., 2010; Zilles et al., 1989). Further to this point, it has been hypothesized that increased intra- rather than interhemipsheric white matter connectivity is a driving force toward the emergence of individual and phylogenetic variation in behavioral and brain asymmetries (Aboitiz et al., 2003; Rilling and Insel, 1999a). Therefore, the differences in population-level asymmetries in sulci surface area and depth between chimpanzees and macaque monkeys reported here may be attributable to lateralization in connectivity between cortical regions that expressed more strongly in one hemisphere compared to another. Finally, contrary to our prediction, we found that macaques showed larger individual asymmetries, independent of direction, compared to chimpanzees. Comparison of the ABS-AQ scores revealed significant species differences for both sulcus surface area and mean depth. However, caution in the interpretation of these results is warranted due to possible residual effects of image processing, generating greater individual variation among macaque brains as an outcome of the difficulties associated with processing these through this human-based program, as compared to the chimpanzee.
With respect to the chimpanzees, it is of particular note that significant leftward asymmetries were found for the fronto-orbital sulcus. FO is the sulcus used to define the anterior border of the pars opercularis in the chimpanzee brain, a region considered the anatomical homolog to a portion of Broca’s area. The evidence of leftward asymmetry in surface area and mean depth is consistent with at least one other report from 65 chimpanzees (Hopkins & Cantalpuo, 2004) and indicates that this bias is consistent using different methodologies across populations of apes. Chimpanzees failed to show leftward asymmetries in PCI, the posterior sulcus used to define the IFG of chimpanzees. Others have noted that PCI is highly variable across subjects (Keller et al., in press; Sherwood et al., 2003). Therefore, even though we had a relatively large sample of subjects in the current study, the variability in the bifurcation pattern and branching of this sulcus may be too inconsistent across subjects and hemispheres to yield reliable data on asymmetries.
We also found significant leftward asymmetries in the surface area and depth of the sylvian fissure in chimpanzees but not in macaques. These results are consistent with those of some previous comparative studies on asymmetries in SF length in chimpanzees and, to a lesser extent, Old and New World monkeys (Gannon et al., 1998; Gilissen, 1992; Hopkins and Nir, 2010). Thus, leftward asymmetries in SF appear to be quite robust in chimpanzees and appear to be manifested in its length, surface area and depth, and presumably overall cortical folding. The evidence of population-level asymmetries in SF length in macaque monkeys are less clear and the findings reported here do not necessarily resolve this issue. When considering the length of the SF, Heilbronner and Holloway (1988) reported significant leftward asymmetries in Macaca mulatta and Macaca fasicularis when measured from cadaver specimens. In contrast, others have not found population-level asymmetries for the SF length in macaques (Falk et al., 1990; Gannon et al., 2008; Imai et al., 2011; Yeni-Komshian and Benson, 1976). When viewed together, the available evidence suggests that macaques do not show population-level asymmetries in SF length, depth or surface area, with some exceptions.
Population-level asymmetries found for several other sulci in chimpanzees, but not macaque monkeys, warrant some discussion. Specifically, we found significant leftward asymmetries in the mean depth, but not the surface area, of the intraparietal and lunate sulci. The results indicate that these sulci show greater depth in the left compared to right hemisphere. To our knowledge, there are no other published reports of asymmetries of these sulci in chimpanzees and only a few studies in macaques (Heilbronner and Holloway, 1989; Imai et al., 2011). The leftward asymmetries in IP are of note because many have suggested that there have been significant changes in the size and connectivity of the parietal lobe in humans as compared to apes and to more distantly related monkeys (Wilkens, 2005). Furthermore, involvement of the parietal lobe in some linguistic function, as well as planned motor actions, particularly those associated with tool use, is well-documented (Johnson-Frey, 2004; Lewis, 2006). For example, Peeters and colleagues (2009) used fMRI in humans and rhesus monkeys and found activation of the left inferior parietal lobule was unique to humans, thus suggesting that this region may be part of a cortical network that recently evolved. Chimpanzees are proficient tool users; however, they differ from humans in their understanding of properties of tool use (Beck, 1980; Boesch and Boesch, 1990; McGrew, 1992). Further investigation into the parietal region and sulci in chimpanzees and monkeys might provide useful insight into the evolution of the neural basis of tool use in primates.
Regarding the lunate sulcus, anatomically, much of the comparative and paleological debate concerns the migration of this sulcus in humans relative to nonhuman primates, which some believe reflects expansion of the parietal-temporal cortex (Allen et al., 2006; Armstrong et al., 1991; Holloway, 1992; Holloway et al., 2003). Basically, the suggestion is that the expansion of parietal and temporal regions during primate evolution effectively displaced the lunate into a more posterior position in human brains as compared to those of great apes and monkeys. In humans, there is little evidence of asymmetries in the lunate. It should be noted however, that due to substantial individual differences in the folding pattern, the lunate is difficult to quantify reliably across subjects. Allen et al. (2006) did not report data on asymmetries in the lunate but indicated that it could only be reliably estimated in approximately 30% of their sample. Laria and Petrides (2007) reported that they could reliably measure the lunate in about 50% of their sample of humans, with 14 of 22 demonstrating a left bias. In 20 cynomologous monkeys, Imai et al. (2011) failed to find left-right differences in the length of the lunate sulcus, as did LeGros Clark, Cooper and Zuckerman (1936) from five endocasts of chimpanzee brains. Thus, the functional and evolutionary significance of the leftward asymmetry in the depth of the lunate sulcus observed in this study is unclear and warrants further investigation. Notwithstanding, the position of the lunate, may be linked to variability within the temporo-parietal cortex, as suggested by some (Allen et al., 2006; de Sousa et al., 2010).
Macaques showed a rightward asymmetry in the surface area of the STS whereas the chimpanzees did not. This finding is consistent with a previous study in great apes and Old World monkeys that also reported a rightward asymmetry in the length of the STS in monkeys but not apes (Hopkins et al., 2000). Interestingly, a recent study in human infants using the same software as employed in this study reported a significant rightward asymmetry in one section of the STS (Glasel et al., 2011). The functional significance of the species difference in STS asymmetry is unclear. Furthermore, some caution is warranted because the topography and organization of the STS is not the same between monkeys, apes and humans. Notably, the posterior STS typically merges with the SF in New and Old World monkeys. In contrast, the STS and SF do not merge but remain distinct sulci separated by a gyrus in humans and apes. These differences likely reflect variation between human and nonhuman primate primates in parieto-temporal cortex organization and connectivity.
The hypothesis that chimpanzees would show larger absolute asymmetries compared to the macaque monkeys were not supported by the findings. Indeed, for three sulci, macaque monkeys were found to have larger ABS-AQ values than chimpanzees. Thus, at face value, the results are not consistent with the notion that smaller ratios in the size of the corpus callosum relative to brain size results in increased brain asymmetries in primates. One limitation or problem with this interpretation stems from the challenges in comparing the distributions in AQ scores between the species. For example, for the surface area of SF, macaque monkeys were found to show larger individual asymmetries than chimpanzees; however, chimpanzees showed a leftward skewed population bias whereas the macaque monkeys showed a bimodal distribution. The differences in distributions of asymmetries are clear from the data reported in Table 1 and 2. In terms of strength of asymmetry, one can see that there are a smaller proportion of subjects categorized as having no bias in the macaques compared to the chimpanzees. Thus, in absolute terms, the monkeys showed greater asymmetries but chimpanzees show a species-level bias in one direction while the monkeys did not. This leads to the challenge of defining which species is more lateralized? How asymmetry is defined (directionally or individually) is certainly relevant to the theory of the role of interhemispheric connectivity on the evolution and development of brain asymmetries, but this distinction is not explicitly defined within this theoretical framework and therefore may need further refinement.
1.5 Conclusions
The results reported here are the first systematic study of asymmetries in cortical sulci in monkeys and chimpanzees with a focus on differences in the surface areas and depth of select sulci within each species. The collective findings suggest that macaque monkeys show larger individual asymmetries in sulci surface area and depth than chimpanzees for sulci common to the two species. Chimpanzees, however, show more consistent and robust population-level asymmetries than the monkeys. In particular, they have significant population leftward asymmetry in the fronto-orbital sulcus, superior precentral sulcus, and sylvian fissure in both surface area and mean depth, while the inferior postcentral is significantly right biased for surface area in the chimpanzee population. Further, the intraparietal and lunate sulci are significantly left bias for mean depth. In contrast, the macaque population only indicates a significant hemispheric bias (rightwards) for one measure, surface area, for the sulcus of STS.
The mechanisms that underline these asymmetries are not clear, but increasing selection for motor and cognitive functions associated with tool use and tool manufacture, as well as language, may have resulted in changes in the cortical organization, including both anatomical and functional asymmetries in the chimpanzee and monkey brain. These differences in population-level sulcal asymmetries in surface area and depth may have developed as a consequence of stronger lateralization in connectivity between cortical regions in one hemisphere compared to another.
We believe that the collective results reinforce the view that hemispheric specialization may be a fundamental feature of primate brains (and indeed vertebrates) that provides for certain evolutionary advantages, as has been proposed by Vallortigara and Rogers (2005). Importantly though, and contrary to historical and contemporary saltational views, the results reported here suggest that various species may have developed different specializations as they relate to species-specific ecological, social and cognitive adaptations. Further research on behavioral and brain asymmetries should provide important data on what selection factors guided the emergence of asymmetries in primates, including humans.
Acknowledgments
This research was supported by NIH grants NS-42867, NS-73134, HD-56232 and HD-60563 and Cooperative Agreement RR-15090. Additional research was provided by NIH grants MH084980 (AJB, PJP), AA013973 (MLL) and the Translational Center for Neurobehavioral Alcohol Research AA017056 (AJB, PJP). We would like to thank Yerkes National Primate Research Center, the University of Texas M. D. Anderson Cancer Center and the Wake Forest Primate Center and their respective veterinary staff for assistance in MR imaging. Further assistance was appreciated from Jamie Russell, Jennifer Schaeffer, Jared Taglialatela, Joseph McIntyre, Christopher Corcoran, Jeremy Bailoo, and Cynthia Lees. Two chimpanzee scans were provided by the Language Research Center, Georgia State University. Michelle Haddad, Anna J. Hall, and Ruth Reveal of Agnes Scott College assisted in MRI processing of chimpanzee brains. American Psychological Association guidelines for the treatment of animals were followed during all aspects of this study. Assistance in graphic editing by K. McKee is appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboitiz F, Lopez J, Monitel J. Long distance communication in the human brain: timing constraints for inter-hemispheric synchrony and the origin of brain lateralization. Biological Research. 2003;36:89–99. doi: 10.4067/s0716-97602003000100007. [DOI] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Damasio H. Looking for the lunate sulcus: A magnetic resonance imaging study in modern humans. The Anatomical Record Part A. 2006;288A:867–876. doi: 10.1002/ar.a.20362. [DOI] [PubMed] [Google Scholar]
- Annett M. Left, right, hand, and brain: The right-shift theory. Lawrence Erlbaum Associates; London: 1985. [Google Scholar]
- Annett M. Handedness and brain asymmetry: The right shift theory. Psychology Press; Hove: 2002. [Google Scholar]
- Armstrong E, Zilles K, Schleicher A. Cortical folding, the lunate sulcus and the evolution of the human brain. Journal of Human Evolution. 1991;20:341–348. [Google Scholar]
- Bailey P, von Bonin G, McCulloch WS. The isocortex of the chimpanzee. University of Illinois Press; Urbana-Champaign: 1950. [Google Scholar]
- Balzeau A, Gilissen E. Endocranial shape asymmetries in Pan panuscus, Pan troglodytes, and Gorilla gorilla assessed via skull based landmark analysis. Journal of Human Evolution. 2010;59:54–69. doi: 10.1016/j.jhevol.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Beaton AA. The relation of planum temporale asymmetry and morphology of the corpus callosum to handedness, gender and dyslexia: A review of the evidence. Brain and Language. 1997;60:255–322. doi: 10.1006/brln.1997.1825. [DOI] [PubMed] [Google Scholar]
- Beck BB. Animal tool behavior: The use and manufacture of tools by animals. Garland; New York: 1980. [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. The Journal of Neuroscience. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesch C, Boesch H. Tool use and tool making in wild chimpanzees. Folia Primatologica. 1990;54:86–99. doi: 10.1159/000156428. [DOI] [PubMed] [Google Scholar]
- Bruner E, Holloway RL. A bivariate approach to the widening of the frontal lobes in the genus Homo. Journal of Human Evolution. 2010;58:138–146. doi: 10.1016/j.jhevol.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Cantalupo C, Hopkins WD. Asymmetric Broca’s area in great apes. Nature. 2001;414:505. doi: 10.1038/35107134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalupo C, Oliver J, Smith J, Nir T, Taglialatela JP, Hopkins WD. The chimpanzee show human-like perisylvian asymmetries in white matter. European Journal of Neuroscience. 2009;30:431–438. doi: 10.1111/j.1460-9568.2009.06830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalupo C, Pilcher D, Hopkins WD. Are planum temporale and sylvian fissure asymmetries directly related? A MRI study in great apes. Neuropsychologia. 2003;41:1975–1981. doi: 10.1016/s0028-3932(02)00288-9. [DOI] [PubMed] [Google Scholar]
- Cheverud JM, Falk D, Hildebolt C, Moore AJ, Helmkamp RC, Vannier J. Heritability and association of cortical petalia in rhesus monkeys (Macaca mulatta) Brain, Behavior, and Evolution. 1990;35:368–372. doi: 10.1159/000115881. [DOI] [PubMed] [Google Scholar]
- Connolly CJ. The fissural pattern of the primate brain. American Journal of Physical Anthropology. 1936;11:301–421. [Google Scholar]
- Corballis MC. The lopsided brain: Evolution of the generative mind. Oxford University Press; New York: 1992. [Google Scholar]
- Corballis MC. From hand to mouth: The origins of language. Princeton University Press; Princeton, NJ: 2002. [Google Scholar]
- Corballis MC. From mouth to hand: gesture, speech, and the evolution of right-handedness. Behavioral and Brain Sciences. 2003;26:199–260. doi: 10.1017/s0140525x03000062. [DOI] [PubMed] [Google Scholar]
- Crow T. Directional asymmetry is the key to the origin of modern Homo sapiens (the Broca-Annett axiom): A reply to Rogers’ review of The Speciation of Modern Homo Sapiens. Laterality: Asymmetries of Body, Brain and Cognition. 2004;9:233–242. [Google Scholar]
- Crow TJ. A theory of the origin of cerebral asymmetry: Epigenetic variation superimposed on a fixed right-shift. Laterality. 2009;15:289–303. doi: 10.1080/13576500902734900. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Cerebral Asymmetry, Emotion and Affective Style. In: Davidson RJ, Hugdahl K, editors. Brain Asymmetry. MIT Press; Cambridge, MA: 1995. pp. 361–387. [Google Scholar]
- de Sousa AA, Sherwood CC, Mohlberg A, Amunts K, Schleicher A, MacLeod CE, Hof PR, Frahm H, Zilles K. Hominoid visual brain structure volumes and the position of the lunate sulcus. Journal of Human Evolution. 2010;58:281–292. doi: 10.1016/j.jhevol.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Ettlinger GF. Hand preference, ability and hemispheric specialization. How far are these factors related in the monkey? Cortex. 1988;24:389–398. doi: 10.1016/s0010-9452(88)80002-9. [DOI] [PubMed] [Google Scholar]
- Falk D, Cheverud J, Vannier MW, Conroy GC. Advanced computer-graphics technology reveals cortical asymmetry in endocasts of rhesus-monkeys. Folia Primatologica. 1986;46:98–103. doi: 10.1159/000156242. [DOI] [PubMed] [Google Scholar]
- Falk D, Hildebolt C, Cheverud J, Vannier M, Helmkamp RC, Konigsberg L. Cortical asymmetries in the frontal lobe of rhesus monkeys (Macaca mulatta) Brain Research. 1990;512:40–45. doi: 10.1016/0006-8993(90)91167-f. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Eure KF, Luevano LF, Weinberger DR. MRI Asymmetries of Broca’s Area: The Pars Triangularis and Pars Opercularis. Brain and Language. 1998;64:282. doi: 10.1006/brln.1998.1974. [DOI] [PubMed] [Google Scholar]
- Gannon PJ, Holloway RL, Broadfield DC, Braun AR. Asymmetry of chimpanzee Planum Temporale: Humanlike pattern of Wernicke’s language area homolog. Science. 1998;279:220–222. doi: 10.1126/science.279.5348.220. [DOI] [PubMed] [Google Scholar]
- Gannon PJ, Kheck N, Hof PR. Leftward interhemispheric asymmetry of macaque monkey temporal lobe language area homolog is evident at the cytoarchitectural, but not gross anatomic level. Brain Research. 2008;1199:62–73. doi: 10.1016/j.brainres.2007.12.041. [DOI] [PubMed] [Google Scholar]
- Gilissen E. The neocortical sulci of the capuchin monkey (Cebus): evidence for asymmetry in the sylvian sulcus and comparison with other primates. Comptes Rendus de l’Academie de Sciences Paris, Series III. 1992;314:165–170. [Google Scholar]
- Gilissen E. Structural symmetries and asymmetries in human and chimpanzee brains. In: Falk D, Gibson KR, editors. Evolutionary anatomy of the primate cerebral cortex. Cambridge University; Cambridge: 2001. pp. 187–215. [Google Scholar]
- Glasel H, Leroy F, Dubois J, Hertz-Pannier L, Mangin JF, Dehaene-Lambertz G. A robust cerebral asymmetry in the infant rbain: A rightward superior temporal sulcus. NeuroImage. 2011;58:716–723. doi: 10.1016/j.neuroimage.2011.06.016. [DOI] [PubMed] [Google Scholar]
- Heilbronner PL, Holloway RL. Anatomical brain asymmetries in New World and Old World monkeys. Stages of temporal lobe development in primate evolution. American Journal of Physical Anthropology. 1988;76:39–48. doi: 10.1002/ajpa.1330760105. [DOI] [PubMed] [Google Scholar]
- Heilbronner PL, Holloway RL. Anatomical brain asymmetry in monkeys: Frontal, Temporoparietal, and Limbic cortex in Macaca. American Journal of Physical Anthropology. 1989;80:203–211. doi: 10.1002/ajpa.1330800208. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S, Mota B, Wong P, Kaas JH. Connectivity-driven white matter scaling and folding in primate cerebral cortex. Proceedings of the National Academy of Sciences. 2010;107:19008–19013. doi: 10.1073/pnas.1012590107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway RL. The failure of the gyrification index (GI) to account for volumetric reorganization in the evolution of the human brain. Journal of Human Evolution. 1992;22:163–170. [Google Scholar]
- Holloway RL, Broadfield DC, Yuan MS. Morphology and histology of the chimpanzee primary visual striate cortex indicate that brain reorganization predated brain expansion in early hominid evolution. The Anatomical Record Part A. 2003;273A:594–602. doi: 10.1002/ar.a.10071. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, editor. Evolution of hemispheric specialization in primates. Elsevier; Oxford: 2007. [Google Scholar]
- Hopkins WD, Cantalupo C. Theoretical speculations on the evolutionary origins of hemispheric specialization. Current Direction in Psychological Science. 2008;17:233–237. [Google Scholar]
- Hopkins WD, Nir T. Planum temporale surface area and grey matter asymmetries in chimpanzees (Pan troglodytes): The effect of handedness and comparison within findings in humans. Behavioural Brain Research. 2010;208:436–443. doi: 10.1016/j.bbr.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Pilcher DL, MacGregor L. Sylvian fissure length asymmetries in primates revisited: A comparative MRI study. Brain, Behavior and Evolution. 2000;56:293–299. doi: 10.1159/000047213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Taglialatela JP, Meguerditchian A, Nir T, Schenker NM, Sherwood CC. Gray matter asymmetries in chimpanzees as revealed by voxel-based morphometry. NeuroImage. 2008;42:491–497. doi: 10.1016/j.neuroimage.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaria G, Petrides M. Occipital sulci of the human brain: Variability and probability maps. The Journal of Comparative Neurology. 2007;501:243–259. doi: 10.1002/cne.21254. [DOI] [PubMed] [Google Scholar]
- Ide A, Rodriguez E, Zaidel E, Aboitiz F. Bifurcation patterns in the human sylvian fissure: hemispheric and sex differences. Cerebral Cortex. 1996;6:717–725. doi: 10.1093/cercor/6.5.717. [DOI] [PubMed] [Google Scholar]
- Imai N, Sawada K, Fukunishi K, Sakata-Haga H, Fukui Y. Sexual dimorphism of sulcal length asymmetry in the cerebrum of adult cynomolgus monkeys (Macaca fascicularis) Congenital Anomalies. 2011;51:161–166. doi: 10.1111/j.1741-4520.2011.00330.x. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH. The neural basis of complex tool use in humans. Trends in Cognitive Sciences. 2004;8:71–78. doi: 10.1016/j.tics.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Keller SS, Crow T, Foundas A, Amunts K, Roberts N. Broca’s area: Nomenclature, anatomy, typology and asymmetry. Brain and Language. 2009a;109:29–48. doi: 10.1016/j.bandl.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Keller SS, Deppe M, Herbin M, Gilissen E. Variabilty and asymmetry of the suclal contours defining Broca’s area homologue in the chimpanzee brain. Journal of Comparative Neurology. doi: 10.1002/cne.22747. in press. [DOI] [PubMed] [Google Scholar]
- Keller SS, Highley JR, Garcia-Finana M, Sluming V, Rezaie R, Roberts N. Sulcal variability, stereological measurement and asymmetry of Broca’s area on MR images. Journal of Anatomy. 2007;211:534–555. doi: 10.1111/j.1469-7580.2007.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SS, Roberts N, Hopkins W. A Comparative Magnetic Resonance Imaging Study of the Anatomy, Variability, and Asymmetry of Broca’s Area in the Human and Chimpanzee Brain. Journal of Neuroscience. 2009b;29:14607–14616. doi: 10.1523/JNEUROSCI.2892-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus TA, Bollich AM, Corey DM, Lemen LC, Foundas AL. Variability in perisylvian brain anatomy in healthy adults. Brain and Language. 2006;97:219–232. doi: 10.1016/j.bandl.2005.10.008. [DOI] [PubMed] [Google Scholar]
- LeGros Clark WE, Cooper DM, Zuckerman S. The endocranial cast of the chimpanzee. The Journal fo the Royal Anthropological Institute of Great Britain and Ireland. 1936;66:249–268. [Google Scholar]
- LeMay M. Asymmetries of the brains and skulls of nonhuman primates. In: Glick SD, editor. Cerebral lateralization in nonhuman species. Academic Press; New York: 1985. pp. 223–245. [Google Scholar]
- Lewis JW. Cortical networks related to human use of tools. The Neuroscientist. 2006;12:211–231. doi: 10.1177/1073858406288327. [DOI] [PubMed] [Google Scholar]
- Liu ST, Phillips KA. Sylvian fissure asymmetries in capuchin monkeys (Cebus apella) Laterality. 2009;14:217–227. doi: 10.1080/13576500802344404. [DOI] [PubMed] [Google Scholar]
- Lyn HL, Pierre P, Bennett AJ, Fears SC, Woods RP, Hopkins WD. Planum temporale grey matter asymmetries in chimpanzees (Pan troglodytes), vervet (Chlorocebus aethiops sabaeus), rhesus (Macaca mulatta) and bonnet (Macaca radiata) monkeys. Neuropsychologia. 2011;49:2004–2012. doi: 10.1016/j.neuropsychologia.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin JF, Riviere D, Cachia A, Duchesnay E, Cointepas Y, Papadopoulos-Orfanos D, Collins DL, Evans AC, Regis J. Object-based morphometry of the cerebral cortex. Medical Imaging. 2004;23:968–982. doi: 10.1109/TMI.2004.831204. [DOI] [PubMed] [Google Scholar]
- Marchant LF, McGrew WC. Laterality of function in apes: A meta-analysis of methods. Journal of Human Evolution. 1991;21:425–438. [Google Scholar]
- McGrew WC. Tool-use by free-ranging chimpanzees: The extent of diversity. Journal of Zoology (London) 1992;228:689–694. [Google Scholar]
- Oliveras R, Montiel J, Aboitiz F. Species differences and similarities in the fine structure of the mammalian corpus callosum. Brain, Behavior and Evolution. 2001;57:98–105. doi: 10.1159/000047229. [DOI] [PubMed] [Google Scholar]
- Peeters R, Simone L, Nelissen K, Fabbri-Destro M, Vanduffel W, Rizzolatti G, Orban GA. The Representation of Tool Use in Humans and Monkeys: Common and Uniquely Human Features. Journal of Neuroscience. 2009;29:11523–11539. doi: 10.1523/JNEUROSCI.2040-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Insel TR. Differential expansion of neural projection systems in primate brain evolution. Neuroreport. 1999a;10:1453–1459. doi: 10.1097/00001756-199905140-00012. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Insel TR. The primate neocortex in comparative perspective using magnetic resonance imaging. Journal of Human Evolution. 1999b;37:191–223. doi: 10.1006/jhev.1999.0313. [DOI] [PubMed] [Google Scholar]
- Ringo J, Doty R, Demeter S, Simard P. Timing is of essence: A conjecture that hemispheric specialization arises from inter-hemispheric conduction delay. Cerebral Cortex. 1994;4:331–343. doi: 10.1093/cercor/4.4.331. [DOI] [PubMed] [Google Scholar]
- Rogers J, Kochunov PV, Zilles K, Shelledy W, Lancaster JL, Thompson P, Duggirala R, Blangero J, Fox PT, Glahn DC. On the genetic architecture of cortical folding and brain volume in primates. NeuroImage. 2010;53:1103–1108. doi: 10.1016/j.neuroimage.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenker NM, Hopkins WD, Spocter MA, Garrison A, Stimpson CD, Erwin JM, Hof PR, Sherwood CC. Broca’s area homologue in chimpanzees (Pan troglodytes): probabilistic mapping, asymmetry and comparison to humans. Cerebral Cortex. 2010;20:730–742. doi: 10.1093/cercor/bhp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenemann PT, Sheehan MJ, Glotzer LD. Prefrontal white matter volume is disproportionately larger in humans than in other primates. Nature Neuroscience. 2005;8:242–252. doi: 10.1038/nn1394. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW. Prefrontal cortex of humans and apes: A comparative study of area 10. American Journal of Physical Anthropology. 2001;114:224–241. doi: 10.1002/1096-8644(200103)114:3<224::AID-AJPA1022>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Shapleske J, Rossell SL, Woodruff PW, David AS. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Research Reviews. 1999;29:26–49. doi: 10.1016/s0165-0173(98)00047-2. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Broadfield DC, Holloway RL, Gannon PJ, Hof PR. Variability of Broca’s area homologue in great apes: Implication for language evolution. The Anatomical Record. 2003;217A:276–285. doi: 10.1002/ar.a.10046. [DOI] [PubMed] [Google Scholar]
- Spocter MA, Hopkins WD, Garrison AR, Stimpson CD, Erwin JM, Hof PR, Sherwood CS. Wernicke’s area homolog in chimpanzees (Pan troglodytes): Probabilstic mapping, asymmetry and comparison with humans. Proceedings of the Royal Society B, Biological Sciences. 2010;277:2165–2174. [Google Scholar]
- Uylings H, Jacobsen A, Zilles K, Amunts K. Left-right asymmetry in volume and number of neurons in adult Broca’s area. Cortex. 2006;42:652–658. doi: 10.1016/s0010-9452(08)70401-5. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Rogers LJ. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behavioral and Brain Sciences. 2005;28:574. doi: 10.1017/S0140525X05000105. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- Warren JM. Handedness and laterality in humans and other animals. Physiological Psychology. 1980;8:351–359. [Google Scholar]
- Wilkens W. Anatomy matters. The Linguistic Review. 2005;22:271–288. [Google Scholar]
- Williams NA, Close JP, Giouzeli M, Crow TJ. Accelerated evolution of Protocadherin 11X/Y: A candidate gene-pair for cerebral asymmetry and language. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2006;141B:623.633. doi: 10.1002/ajmg.b.30357. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Kigar DL. Sylvian fissure morphology and asymmetry in men and women: bilateral differences in relation to handedness in men. Journal of Comparative Neurology. 1992;323:326–340. doi: 10.1002/cne.903230303. [DOI] [PubMed] [Google Scholar]
- Yeni-Komshian G, Benson D. Anatomical study of cerebral asymmetry in the temporal lobe of humans, chimpanzees and monkeys. Science. 1976;192:387–389. doi: 10.1126/science.816005. [DOI] [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Moser KH, Schleicher A, Stephan H. Gyrification in the cerebral cortex of primates. Brain, Behavior and Evolution. 1989;34:143–150. doi: 10.1159/000116500. [DOI] [PubMed] [Google Scholar]
- Zilles K, Dabringhaus A, Geyer S, Amunts K, Qu M, Schleicher A, Gilissen E, Schlaug G, Steinmetz H. Structural asymmetries in the human forebrain and the forebrain of non-human primates and rats. Neuroscience and Biobehavioral Reviews. 1996;20:593–605. doi: 10.1016/0149-7634(95)00072-0. [DOI] [PubMed] [Google Scholar]