Abstract
Mast cells, activated by antigen via the high affinity receptor for IgE (FcεRI), release an array of pro-inflammatory mediators that contribute to allergic disorders such as asthma and anaphylaxis. The KIT ligand, stem cell factor (SCF), is critical for mast cell expansion, differentiation and survival, and, under acute conditions, enhances mast cell activation. However, extended SCF exposure in vivo conversely protects against fatal antigen-mediated anaphylaxis. In investigating this dichotomy, we identified a novel mode of regulation of the mast cell activation phenotype through SCF-mediated programming. We found that mouse bone marrow-derived mast cells chronically exposed to SCF displayed a marked attenuation of FcεRI-mediated degranulation and cytokine production. The hypo-responsive phenotype was not a consequence of altered signals regulating calcium flux or protein kinase C, but of ineffective cytoskeletal reorganization, with evidence implicating a down-regulation of expression of the Src kinase Hck. Collectively, these findings demonstrate a major role for SCF in the homeostatic control of mast cell activation with potential relevance to mast cell-driven disease and the development of novel approaches for the treatment of allergic disorders.
Keywords: Mast cells, SCF, KIT, Hck, antigen, FcεRI, degranulation, cytokines, phenotype reprogramming
Introduction
Antigen-mediated mast cell (MC) activation, via the high affinity receptor for IgE (FcεRI), results in the release of an array of inflammatory mediators that underlie allergic reactions in atopic disease (1, 2). MCs are derived from bone marrow progenitors which migrate into the circulation and peripheral tissues where they expand and mature under the influence of cytokines contained within the surrounding milieu (3). KIT activation, as a consequence of binding of its ligand stem cell factor (SCF) produced within tissues by stromal cells, is critical for the development of MCs from bone marrow progenitor cells and their subsequent accumulation, maturation, and survival in tissues (4). Unlike human MCs (5), mouse MC development and survival in culture is supported by IL-3 in the absence of SCF (6). However, the supplementary presence of SCF markedly enhances the rate of growth of these cells and is described to skew the development of the cells to that of a serosal/connective tissue phenotype. In contrast, cells grown in IL-3 alone are considered to more resemble the mucosal phenotype based on the types of proteases expressed (7-10).
Little is known about how SCF and other extrinsic factors, or the combination thereof, may dictate the MC phenotype with regards to responsiveness to antigen and other stimulants. Under acute experimental conditions, SCF is one of several endogenous agents known to potentiate antigen-mediated MC degranulation and cytokine production (11-13). Nevertheless, in contrast to its acute effects on MC activation in vivo (14), it is reported that repetitive subcutaneous injection of SCF over a period of 21 days into mice may actually protect against fatal anaphylactic reactions (15). Indeed at the sites of injection, the MCs exhibited little morphological evidence of degranulation after induction of anaphylaxis via IgE in these mice (Fig. 2 in (15)), suggesting that chronic exposure to SCF may have a profoundly different impact on MC activation than short term exposure. We thus investigated the hypothesis that prolonged exposure of MCs to SCF, as likely occurs in vivo to maintain MC homeostasis, may lead to transcriptional modifications that alter the underlying activation properties of the cells.
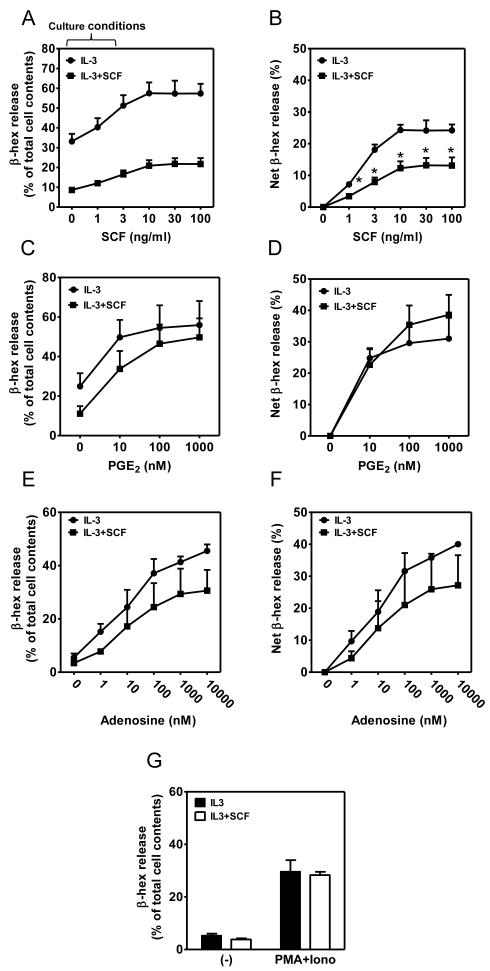
Figure 2. Differential effects of extended exposure to SCF on Kit and GPCR-enhanced MC degranulation.
(A-G) BMMCs were cultured in IL-3 in the presence or absence of SCF for 4 weeks. The cells were then starved of cytokines overnight and concurrently sensitized with IgE. The cells were challenged with the indicated concentrations of antigen Ag (10 ng/ml) concomitantly with SCF (0-100 ng/ml; A-B), PGE2 (0-100 nM; C-D), adenosine (0-10000 nM; E-F), or PMA/Ionomycin (20 nM PMA/100 nM Ionomycin; G) for 30min, degranulation (A, C, E, G) was determined, and potentiation, expressed as net β-hex release (B, D, F), evaluated. In A-G, the data represent means and SEM (A-B, n=4; C-D, n=5; and E-F, n=3) and differences between IL-3 and IL-3+SCF-cultured cells are indicated (*, p <0.05, t test).
As reported here, these studies led us to identify a novel mechanism for the regulation of the extent of MC activation through SCF-dependent induction of a hypo-responsive phenotype with respect to both cytokine production and degranulation. This phenotype was not due to down regulation of the expression of either FcεRI or KIT, but could be explained by an inability of the cells to undergo the cytoskeletal reorganization required for mediator release, potentially as a consequence of decreased expression of the Src kinase Hck. These findings reveal that the sensitivity of MCs to IgE/antigen stimulation is highly regulated by SCF and presumably other cytokines in the surrounding tissue milieu and may thus have important implications for understanding how the activation capacity of tissue MCs may be phenotypically modified in health and in disease.
Methods
Cell culture and co-culture
Experiments conducted on mice were conducted under a protocol approved by the Animal Care and Use Committee at NIH. Bone marrow-derived MCs (BMMCs) were developed from bone marrow obtained from femurs of C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) as described (16). Essentially, the cells were cultured for 4-6 weeks in media containing mouse recombinant IL-3 (30 ng/ml) (Peprotech, Rocky Hill, NJ) or a combination of mouse recombinant IL-3 (30 ng/ml) and mouse recombinant SCF (unless otherwise indicated: 100 ng/ml) (Peprotech). The cells were maintained at 37 °C in a humidified incubator gassed with 95% air and 5% CO2. The purity of the cultures, as assessed by toluidine blue staining (17) and FcεRIα and KIT expression, was >99%. The NIH 3T3 mouse fibroblast cell line (obtained from American Type Culture Collection, Manassas, VA) was grown or co-cultured (18) with BMMCs in the same media as for BMMCs but in the absence of IL-3 and SCF.
Cell sensitization, activation, degranulation, and cytokine/chemokine release
BMMCs were sensitized overnight in cytokine-containing or cytokine-free media (as indicated) with mouse anti DNP-IgE (clone SPE-7 [Sigma]; 100 ng/ml). After sensitization, the cells were processed and activated as described (16).
Degranulation after 30 min activation was monitored by the release of the granule component, β-hexosaminidase (β-hex), into the supernatants as described (19), and expressed as a percentage of β-hex released into supernatant. The amount of cytokines released from cells after 6 h activation was determined by Quantikine ELISA kits (R&D Systems, Minneapolis, MN). To measure cytokine content within the cytoplasm, the activated cells were lysed by adding distilled water followed by freezing (1 hour)/thawing, then the supernatants were collected and the amount of cytokines determined as above.
Cell fractionation, immunoblotting, intracellular calcium measurement
Sensitized BMMCs were stimulated with antigen for 2 min at 37 °C, and cell fractionation performed as described (16). The cells were lysed as described (20, 21) and proteins were separated by electrophoresis on 4-12% NuPage Bis-Tris gels (Invitrogen, Carlsbad, CA) and probed for immunoreactive proteins utilizing the following protein-specific antibodies: β-actin (Sigma), Hck (Santa Cruz Biotechnology, Santa Cruz, CA), and other phosphoprotein- and protein-specific antibodies were from Cell Signaling (Beverly, MA). The immunoreactive antibodies were visualized by probing with rabbit IgG-specific antibody (Amersham Biosciences, Piscataway, NJ), or mouse IgG Fc-specific antibody (Sigma) conjugated with horseradish peroxidase. To monitor the intracellular calcium during cell activation, the sensitized BMMCs were pretreated, activated and analyzed as described (22).
Toluidine blue and Alcian blue/Safranin staining
Cytospins of 4 week old BMMCs were prepared, fixed, and stained with Toluidine blue or Alcian blue/Safranin as described (17, 23).
Flow cytometric analysis of FcεRI and KIT surface expression
Following blocking of Fcγ receptors with 2.4G2 (BD Pharmingen), cells were stained with FITC-anti-FcεRI (eBioscience) and PE-anti-KIT (BD Pharmingen) antibodies to respectively examine surface expression of FcεRI and KIT. Cells were incubated for 1 h at 4 °C and, after washing with PBS, the stained cells were analyzed using FACSCalibur flow cytometer and CellQuest software (BD Biosciences).
F-actin, α-tubulin
Sensitized BMMCs were stimulated with DNP-HSA (10 ng/ml) for the indicated times, fixed with 4% paraformaldehyde/5 mM EGTA and EDTA in PBS for 15 min at RT and then F-actin (polymeric, filamentous actin) was stained with FITC-labeled phalloidin (Sigma) in 2% BSA/0.1% Saponin-PBS for 1 h in the dark at RT. The stained cells were analyzed by FACSCalibur flow cytometer and CellQuest software (BD Biosciences) and the data analyzed using FlowJo software (Tree Star, Ashland, OR). Alternatively, the fixed cells were sedimented, rinsed with 2% BSA/PBS/5 mM EGTA and fixed to glass slides by cytospin. The attached cells were then incubated with an anti-α-tubulin antibody (Cell Signaling) at a dilution of 1:100 in 0.1% Saponin/2% BSA/PBS/5 mM EGTA for 1 hr at RT. After washing, FITC-labeled phalloidin (Sigma) and Alexa Fluor 568 donkey anti-rabbit IgG (Invitrogen) were added for 40 min at RT. After washing the slides, mounting solution containing DAPI (Invitrogen) was added and the stained cells were imaged by fluorescence microscopy using Zeiss LSM-510-UV Confocal Microscope, Plan-Apochromat, NA 63×/1.4 oil. The fluorescence intensity was evaluated from the acquired images using ImageJ software.
Retroviral transduction
Retroviral vector pMX-puro with/without the mouse hck cDNA (a kind gift from Dr. Toshiaki Kawakami at La Jolla Institute for Allergy and Immunology) was transfected into PLAT-E cells (Cell Biolabs Inc. San Diego CA) with FUGENE 6 transfection reagent (Roche Applied Science) in OPTI-MEM (Invitrogen). Transfected cells were grown in DMEM containing FBS (10%) and l-glutamine (4 mM) and the media was replaced 16-20 h post-transfection with fresh media containing penicillin (100 units/ml) and streptomycin (100 μg/ml). After 24 h, virus particles in the media were collected by centrifugation (8,000 rpm, overnight, 4 °C) and the pellet was resuspended in complete BMMC medium. Viral titer was determined using QuickTiter™ retrovirus quantitation kit (Cell Biolabs Inc). 0.5~1 × 107 BMMCs (2-3 weeks old) were transduced with 4.5×1010 virus particles in hexadimerthrine bromide (10 μg/ml). After 72 h, the medium was replaced with fresh BMMC medium with/without SCF, and transduced cells were selected in the presence of 1.2 μg/ml puromycin for 2 weeks.
Quantitative real-time PCR
Total RNA was isolated using RNeasy Plus mini kit (Qiagen, Valencia, CA). One μg of RNA was used for reverse transcription reaction using random hexamers and Superscript III reverse transcriptase (Invitrogen) in a 20 μl reaction. One μl of the resulting cDNA was used for Real Time PCR using HCK Taqman gene expression assay according to the manufactures instructions (Applied Biosystems, Foster City, CA).
Affymatrix gene chip analysis
Bone marrow cells were harvested from 6 three-month old male littermates in a randomized order on a single day and cells were cultured for 4 weeks in either IL-3 (30 ng/ml) alone or IL-3+SCF (100 ng/ml), cell suspensions centrifuged and each cell pellet lysed in 600 μl of RLT buffer (Qiagen, Valencia, CA). Cell lysates were then homogenized for 2 min at 21,000 × g using a QIAshredder homogenizing column (Qiagen, Valencia, CA). Each sample lysate aliquot (100 μL) was combined with 100 μL of RLT buffer, 140 μL of 100% ethanol, and β-mercaptoethanol (0.145 mM). RNAs were extracted using an RNeasy 96 kit (Qiagen, Valencia, CA) as described (24), except that each sample was treated with 27 units DNase I during the extraction process. RNA concentrations were determined by spectrophotometry measuring absorbance at 260 nm and 280 nm, and RNA quality was verified on an Agilent 2100 Bioanalyzer using the Pico analysis kit (Agilent Technologies, Palo Alto, CA). The sample RNA Integrity Number (RIN) values ranged from 8.7 to 9.8 with the average of 9.4. Affymetrix GeneChip targets were made by amplification of 50 ng RNA and using the WT-Ovation RNA Amplification System (Nugen Inc., San Carlos, CA). The resulting amplified single stranded cDNAs were purified according to the QIAquick 96-well protocol (Qiagen, Valencia, CA) with a modified centrifugation protocol (24). Each sample was quantified using A260/280 spectrophotometry (Molecular Devices, Sunnyvale, CA) and the quality was assessed using Agilent’s 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). Amplified cDNAs were fragmented and labeled following Nugen’s FL-Ovation cDNA Biotin Module V2 protocol (Nugen, San Carlos, CA).
Hybridization, fluidics and scanning were performed according to standard Affymetrix protocols (http://www.affymetrix.com). GeneChip Operating Software (GCOS v1.4) was used to convert the image files to cell intensity data (cel files). All cel files, representing individual samples, were normalized using the scaling method within expression console (EC v1.1, http://www.Affymetrix.com) and a scaled target of 1250 to produce the analyzed cel files (chp files) along with the report files. The cel files were placed into Partek Genomics Suite software (Partek, inc. St. Louis, Mo., v6.5 6091110) and quantile normalized to produce the principal components analysis (PCA) graph. An ANOVA was performed within Partek to obtain multiple test corrected p-values using the false discovery rate method at the 0.01 significance level and was combined with fold change values, signal confidence (above background), and call consistency (as a percent) as calculated using custom Excel templates for each comparison (25). The data are deposited in Gene Expression Omnibus (GEO; GSE35332; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=pngzzescaqwkwta&acc=GSE35332).
Statistical analysis
Data are presented as the mean and SEM. Statistics were analyzed using the two-tailed Student’s t-test. When p values were p<0.05, differences were considered significant. The n represents the number of preparations from individual mice.
Results
Prolonged exposure of developing BMMCs to SCF enhances IL-3-mediated growth but subsequently inhibits antigen-mediated degranulation
BMMCs were cultured in media containing IL-3, SCF, or IL-3 in combination with SCF for periods up to 8 weeks. As reported (10), the combination of IL-3 and SCF (100 ng/ml) significantly enhanced the number of MCs in culture as compared to IL-3 alone (Fig. S1A). Culturing in SCF alone also supported the growth of mouse BMMCs, however, the yields were inconsistent and significantly lower than those obtained with either IL-3 alone or IL-3 in combination with SCF (data not shown).
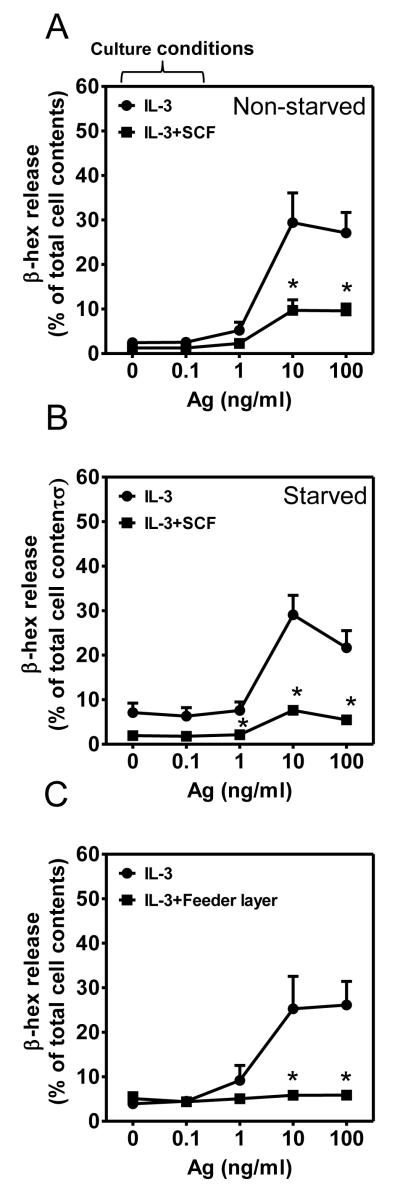
In contrast to the ability of SCF to enhance antigen-mediated degranulation on acute exposure (12, 13), cells cultured in IL-3 in the presence of SCF throughout the culture period, hereafter termed chronic SCF-cultured (cSCF)-BMMCs, displayed a marked reduction in the capacity of antigen to induce degranulation compared to the cells grown in IL-3 alone, regardless of whether the cells were non-starved (Fig. 1A) or starved overnight (Fig. 1B) of cytokines prior to antigen challenge. The attenuated degranulation observed in the cSCF-BMMCs was not a consequence of reduced surface expression of FcεRI or KIT (Fig. S1B, S1C) and there were no noticeable differences in the purity, staining characteristics or morphology of the cells cultured in IL-3 or IL-3 plus SCF (Fig. S1D, S1E), other than that cells exposed to SCF were more granulated than cells cultured in IL-3 alone. Further, the histological examination of cells revealed that, whereas the cells cultured in IL-3 alone appeared depleted of granules following antigen challenge, the cSCF-BMMCs did not (Fig. S2). These latter data are consistent with the conclusion that the reduced antigen-mediated degranulation in the cSCF-BMMCs was due to a defect in the process of degranulation rather than a defect in granule formation.
Figure 1. Extended exposure to SCF down-regulates FcεRI-mediated MC degranulation.
(A-B) BMMCs were cultured in IL-3 in the presence or absence of SCF for 4 weeks. The cells were then non-starved (A) or starved (B) of cytokines overnight and concurrently sensitized with IgE. The cells were challenged with the indicated concentrations of antigen (Ag, DNP-HSA) for 30 min and degranulation (β-hex) determined. (C) 2 week old BMMCs, incubated in IL-3 alone, were co-cultured with or without NIH 3T3 fibroblasts for 2 additional weeks and degranulation of the cytokine-free medium sensitized cells determined as in A-B. In A-C, the data represent means and SEM (A, n=4; B, n=6; C, n=5) and differences between IL-3 and IL-3+SCF-cultured cells are indicated (*, p <0.05, t test).
Both soluble and membrane-associated SCF down-regulate MC activation
In addition to its soluble form, SCF also exists as a membrane-associated moiety. Thus, fibroblast feeder layers, expressing membrane-bound SCF on the cell surface, support MC culture (26, 27). We therefore examined whether membrane-bound SCF, presented in this manner, has the same effect as soluble SCF. Two week-old BMMCs, cultured in IL-3 alone, were incubated for an additional 2 weeks on a monolayer of NIH 3T3 fibroblasts and antigen-mediated activation of BMMCs examined. The cells grown on the feeder layer, although smaller, were densely granulated and had a similar morphological appearance to the cells exposed to soluble SCF (Fig. S3). As for BMMCs cultured in the presence of soluble SCF, antigen-induced degranulation in the fibroblast-cultured BMMCs was significantly reduced (Fig. 1C). Together, these data support the conclusion that prolonged exposure of developing BMMCs to either soluble or membrane-bound SCF profoundly attenuates FcεRI-mediated degranulation.
Prolonged SCF exposure blocks enhanced degranulation mediated by acute KIT activation but not by the EP3 PGE2 and adenosine G protein coupled receptors
When added concurrently with antigen, SCF (12) via the tyrosine kinase based receptor KIT, and PGE2 or adenosine via G protein-coupled receptors (16), synergistically enhance antigen-mediated degranulation, albeit by different mechanisms (16, 28). To examine whether the hypoactive phenotype observed in the cSCF-BMMCs was restricted to FcεRI-regulated MC activation, cells starved of cytokines overnight were acutely challenged with SCF, PGE2, or adenosine in the presence of antigen. To furthermore examine whether the hypoactive phenotype was restricted to receptor-operated pathways, we examined degranulation in response to PMA/ionomycin, which circumvents receptor requirements, in BMMCs cultured in the absence or presence of SCF. As shown in Fig. 2A and 2B, the ability of acutely added SCF to enhance antigen-mediated degranulation in the cSCF-BMMCs was also attenuated when compared to that observed in the cells grown in IL-3 alone. However, the synergistic actions of PGE2 (Fig. 2C, 2D).and adenosine (Fig. 2E, 2F) were minimally affected. In addition, there was no difference in the capacity of PMA/ionomycin to elicit a degranulation response in the BMMCs regardless of whether or not they were cultured in SCF (Fig. 2G). Taken together, these data suggest that the ability of cSCF to alter degranulation may be restricted to receptors that signal through tyrosine kinases and not those that signal via GPCRs.
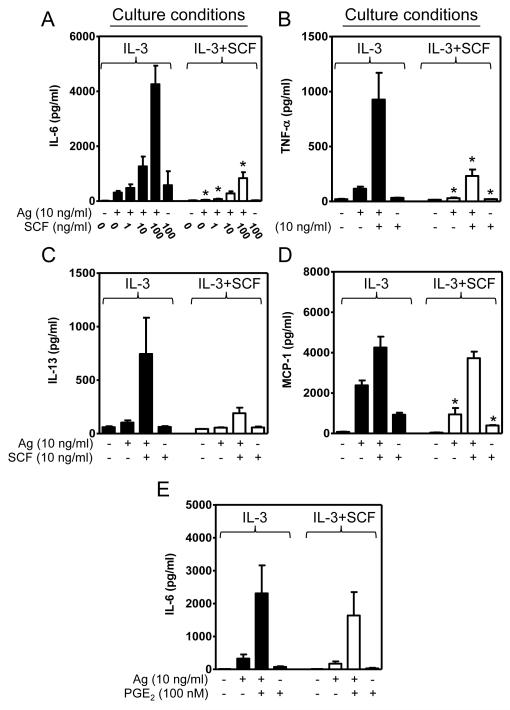
Down-regulation of cytokine production by prolonged exposure to SCF
In addition to degranulation, MCs generate cytokines (29). We therefore examined whether sustained exposure to SCF down-regulated the production of specific cytokines in the same manner as degranulation. As shown in Fig. 3, the production of IL-6 (Fig. 3A), TNF-α (Fig. 3B), and, though not statistically significantly, IL-13 (Fig. 3C) in response to antigen, alone or in combination with acute SCF, was reduced in the cSCF-BMMCs as compared to BMMCs cultured in IL-3 alone. The production of the chemokine MCP-1 (CCL2) was also reduced in the cSCF-BMMCs when cells were stimulated with antigen alone, but there was little reduction in MCP-1 production when cells were challenged with antigen and acute SCF concurrently (Fig. 3D), potentially reflecting differential transcriptional regulation. In contrast to acute SCF, PGE2 was still capable of interacting synergistically with antigen to stimulate the production of IL-6 in the cells cultured in the presence of SCF (Fig. 3E) again suggesting that this GPCR agonist can overcome the defect induced by extended SCF exposure.
Figure 3. Extended exposure to SCF down-regulates MC cytokine production.
(A-E) BMMCs were cultured in IL-3 in the absence (solid bars) or presence (white bars) of SCF for 4 weeks as indicated. The IgE-sensitized cells in cytokine-free media were then challenged for 6 h with antigen (Ag, DNP-HSA) concomitantly with SCF (A-D) or PGE2 (E) and the amount of cytokines released into the media determined. In A-E, the data represent means and SEM (A and E, n=5; B-D, n=3) and differences between IL-3 and IL-3+SCF-cultured cells are indicated (*, p <0.05, t test).
To verify that the diminution in cytokine release accurately reflected a reduction in cytokine production rather than retention within SCF-treated cells (30), intracellular and extracellular IL-6 levels were determined respectively in cell lysates and media. The data showed that relatively little IL-6 was retained within cells after their activation (Fig. S3C and S3D; note different scales). The defective cytokine production observed in the cSCF-BMMCs was thus largely a consequence of a decrease in cytokine generation.
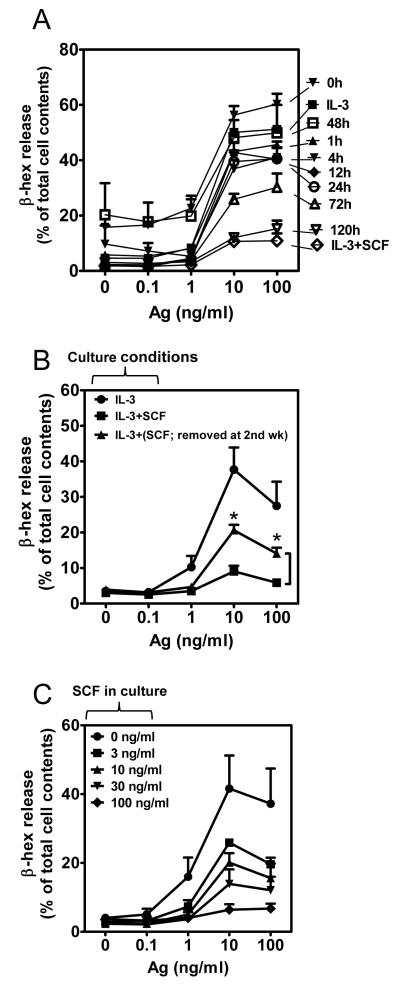
Transformation to a hypo-responsive phenotype occurs in mature BMMCs after 72h exposure to SCF
To determine whether the switch to a hypo-responsive phenotype could be induced with reduced exposure times to SCF, newly seeded bone marrow cells were, after initial culturing in media with IL-3 alone, exposed to SCF at weekly intervals after initiation of culture. Their capacity to respond to antigen was then examined as they approached maturity (4 weeks). These experiments revealed that exposure to SCF for as little as 1 week prior to reaching maturity (4 weeks) diminished the ability of antigen to elicit degranulation (data not shown). To further define the kinetics of this switch in phenotype and to determine whether this switch could be elicited in the mature cultures, SCF was added to 4 week-old IL-3-generated cultures of BMMCs for various periods up to 5 days and antigen-mediated degranulation examined. We observed that 48-72 hour exposure to SCF was required before there was substantial evidence of down-regulation of antigen-mediated degranulation and that maximal attenuation was required >120 h of exposure (Fig. 4A). These data demonstrate that the switch in cell phenotype following introduction of SCF is also observed in mature BMMCs. This switch, therefore, was not a consequence of cryptic alterations during cell development.
Figure 4. Kinetics of onset, reversibility of SCF-induced hypo-responsive phenotype and effect of different SCF concentration in culture.
(A) BMMCs were cultured in IL-3 alone for 4 weeks then SCF was added to the cultures for periods indicated and after sensitization, the cells were sensitized overnight with IgE in IL-3-containing culture medium prior to, or during, culture with SCF, depending on the time point examined. The cells were then stimulated with the indicated concentrations of antigen (Ag, DNP-HSA) for 30 min and degranulation (β-hex) determined. These data were compared to the release from BMMCs grown for the entire 6 weeks in IL-3 alone (IL-3) or in IL-3 and SCF (IL-3+SCF). (B) 2 week old BMMCs cultured in IL-3 and SCF were transferred into IL-3 only-containing media, cultured for additional 2 weeks and degranulation of these cells [IL-3+(SCF; removed at 2nd wk)] alongside with cells grown in IL-3 or IL-3+SCF-containing media for 4 weeks was determined. (C) BMMCs were grown in IL-3 in the presence or absence of SCF (0-100 ng/ml) for 4 weeks and degranulation (β-hex) was determined. In A-C, the data represent means and SEM (A and C, n=3; B, n=6) and in B, the differences between IL-3+SCF and IL-3+(SCF; removed at 2nd wk)-cultured cells are indicated (*, p <0.05, t test).
The hypo-responsive phenotype is partly reversible
We next determined whether the hypo-responsive phenotype was reversible upon removal of SCF. Since removal of SCF from mature BMMCs developed in the SCF-containing cultures leads to apoptosis (31), we attempted to analyze reversibility of the phenotype in BMMCs cultured in the presence of SCF for the initial 2 weeks then culturing in IL-3 alone for the additional 2 weeks. Under such conditions, the majority of cells were still viable as determined by annexin V staining (data not shown). As shown in Fig. 4B, removal of SCF from cSCF-BMMCs allows at least a partial reversion of these cells from the SCF-generated hypoactive phenotype.
SCF-mediated downregulation of mediator release is concentration dependent
To examine whether the SCF-induced downregulation was “tunable”, bone marrow cells were cultured in the presence of IL-3 with increasing concentrations of SCF. As shown in Fig. 4C, the responsiveness to antigen was inversely proportional to increasing concentration of SCF, demonstrating that SCF can produce a graded MC hypo-responsiveness phenotype dictated by its concentration.
SCF-mediated downregulation of mediator release minimally affects signaling processes leading to calcium mobilization
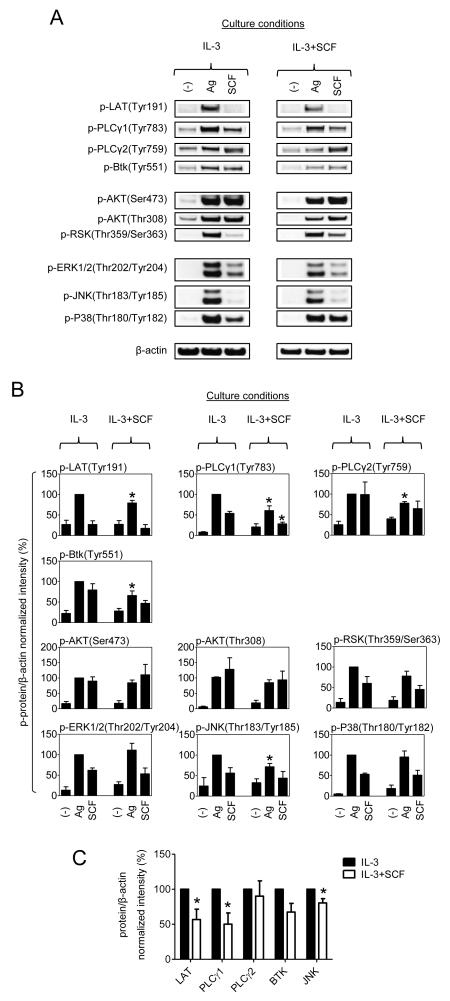
FcεRI-mediated activation of MCs requires a complex signaling cascade that leads to the activation of phospholipase (PL)Cγ and phosphoinositide 3-kinase (PI3K) ultimately resulting in an increase in cytosolic calcium concentrations, and protein kinase C (PKC) activation; obligatory signals for MC activation. In addition, such pathways, in association with those regulated by MAP kinases, lead to cytokine production (1, 29, 32, 33). We therefore examined critical signaling events initiated by FcεRI and SCF in the cSCF-BMMCs, following overnight cytokine deprivation, in an effort to identify the defect that would explain the hypoactive phenotype. We initially examined the phosphorylation of key molecules in the PLCγ activation pathway (LAT, PLCγ1, PLCγ2, and Btk), PI3K-regulated signaling molecules (AKT and RSK), and MAP kinases (ERK1/2, JNK, and p38. As can be seen, we observed very minor (albeit statistically significant) reductions in the phosphorylation of Btk, PLCγ1, PLCγ2, LAT and JNK, but no changes in other molecules examined (including AKT, as a surrogate marker for PI3K activation, RSK, ERK and p38) (Fig. 5A and 5B). With the exception of Btk and PLCγ2, this reflected a similar minor reduction in protein level (Fig. 5C). To examine whether these changes were of sufficient magnitude to influence downstream signaling, we next examined whether the consequential calcium signal or PKC translocation/activation was attenuated in the cells cultured in SCF. As shown in Fig. 6A, culturing of BMMCs in SCF had no impact on the calcium signal in response to antigen in the absence or presence of acute SCF. Furthermore, there was no evidence of defects in antigen-mediated PKC translocation in the cSCF-BMMCs (Fig. 6B). Taken together, these data demonstrate that the hypo-responsive phenotype observed in the cSCF-BMMCs is not explained by underlying defects in major regulatory pathways responsible for the calcium signal following FcεRI and, by inference, upstream signals regulating these processes. In addition, the lack of modifications of other major regulatory processes, including MAPKs and PKC, did not appear to be the contributory factors to the profound hypo-responsiveness of the cSCF-BMMCs.
Figure 5. Impact of extended SCF exposure on BMMC signaling in response to acute challenge with antigen or SCF.
(A) BMMCs were cultured for 4 weeks in IL-3 in the absence or presence of SCF and sensitized overnight in cytokine-free media. The cells were then challenged with antigen (Ag, DNP-HSA; 10 ng/ml) or SCF (100 ng/ml) for 2 minutes, the cells were lysed and analyzed by immunoblotting. (B) The proteins in A were evaluated. (C) Protein expression of the significantly regulated signaling molecules analyzed in B was evaluated. In A, the blots are representative from 3 mice, and in B and C, the data represent means and SEM (B, n=5, and C, n=3).
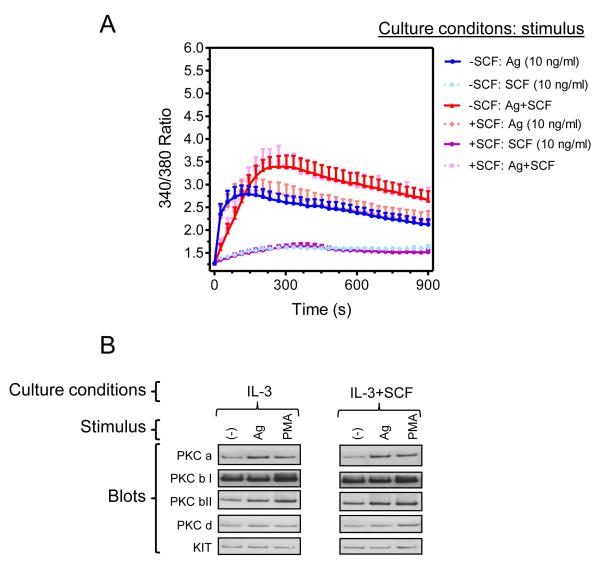
Figure 6. Extended SCF exposure does not affect the calcium signal nor PKC translocation in response to acute challenge with antigen and/or SCF.
(A) BMMCs were cultured for 4 weeks in IL-3 in the absence or presence of SCF and sensitized overnight in cytokine-free media. Fura 2-loaded cells were then challenged with antigen (Ag, DNP-HSA; 10 ng/ml) and/or SCF (10 ng/ml) as indicated and calcium response analyzed. (B) BMMCs were prepared and sensitized as in A then cells were stimulated with antigen (10 ng/ml) or the positive control, phorbol myristoyl acetate (PMA) (500 nM) for 2 min. The membrane fractions were isolated and analyzed by immunoblotting. In A, the data represent means and SEM (n=10). In B, the blots are representative from 3 mice.
Hck is downregulated following prolonged exposure of MCs to SCF
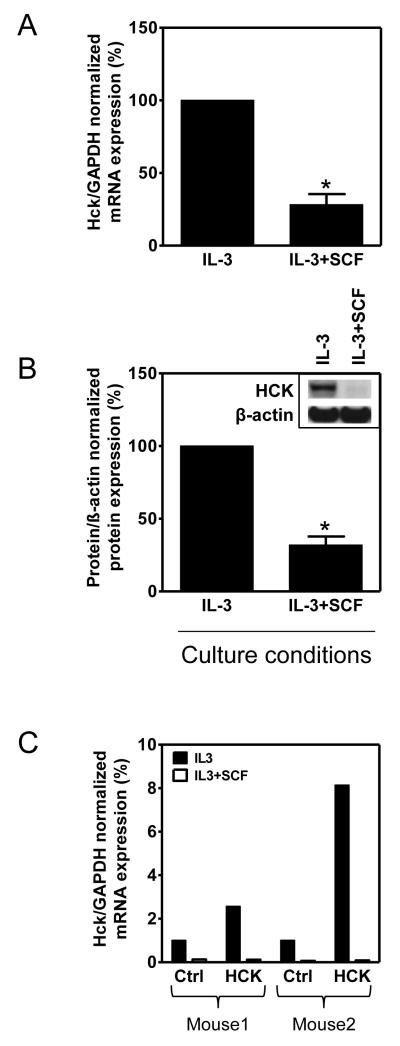
In seeking to identify an alternative explanation for the reduced FcεRI-mediated responses in the cSCF-BMMCs, we explored more global changes in expression of signaling proteins by gene expression microarray. BMMCs were developed from 6 littermate male mice and cultured in IL-3 in the absence or presence of SCF for 4 weeks, mRNA was extracted and then relative gene expression examined. As expected, a wide array of genes was either upregulated or downregulated in the cells grown in IL-3+SCF compared to the cells grown in IL-3 alone (Table S1). Some of the more notable, but predictable, differences were the marked increases in mRNA for the MC proteases, tryptase alpha/beta 1 and MC protease 4, which would reflect the increased granularity observed in the cSCF-BMMCs (Table S1). Few, if any, of the genes down-regulated by SCF would be predicted to influence FcεRI-mediated degranulation with the exception of the Src linase hck (Table S1, highlighted) which was one of the most highly down regulated genes in the cSCF-BMMCs.
This downregulation of hck was of particular interest as Hck-deficient BMMCs exhibit defective antigen-mediated degranulation and cytokine generation without impairment in the calcium signal (34) exactly as noted here for the cSCF-BMMCs. To confirm that indeed prolonged exposure to SCF downregulated Hck expression in BMMCs, we assayed for Hck mRNA and protein in the cSCF-treated cells. As shown in Fig. 7A, as observed in the gene expression array, there was a marked reduction in hck mRNA in the cSCF-BMMCs when examined by real time PCR. Furthermore, there was a pronounced reduction of Hck protein in the cSCF-BMMCs (Fig. 7B). Down-regulation of Hck protein was also observed at earlier times of SCF exposure (7 days) (data not shown).
Figure 7. Extended SCF exposure downregulates mRNA and protein expression of Hck in BMMCs.
(A) BMMCs were cultured for 4 weeks in IL-3 in the absence or presence of SCF and expression of Hck mRNA determined by quantitative real-time PCR. (B) The cells in A were lysed and Hck expression analyzed by immunoblotting and evaluated. (C) BMMCs from 2 mice were cultured in IL-3 in the absence or presence of SCF, transfected with retroviral vector pMX-puro with (HCK) or without (Ctrl) the mouse hck cDNA and Hck mRNA expression was then determined as in A. In A-B, the data represent means and SEM (n=3) and differences between IL-3 and IL-3+SCF-cultured cells are indicated (*, p <0.05, t test).
To determine whether we could reverse the hypoactive phenotype by ectopic expression of Hck, we attempted to overexpress Hck in BMMCs cultured in the absence and presence of SCF, as described (34). As shown in Figure 7C although overexpression of Hck was achieved in the BMMCs cultured in the absence of SCF, no such overexpression was observed in the cells cultured in SCF. Thus we were unable to determine whether ectopic expression of Hck would reverse the hypo-responsive phenotype in the cSCF-BMMCs.
Nevertheless, these results do support our conclusion that SCF reduces Hck expression at the mRNA level.
Thus, although down-regulation of hck correlates with, and may help to explain, the observed change to a hyporesponsive phenotype, it is also possible that modification of other genes, or reduction in the levels critical signaling proteins following proteolytic digestion, may contribute to the cSCF-BMMC hypo-responsiveness.
The hypoactive phenotype is associated with defective cytoskeletal reorganization
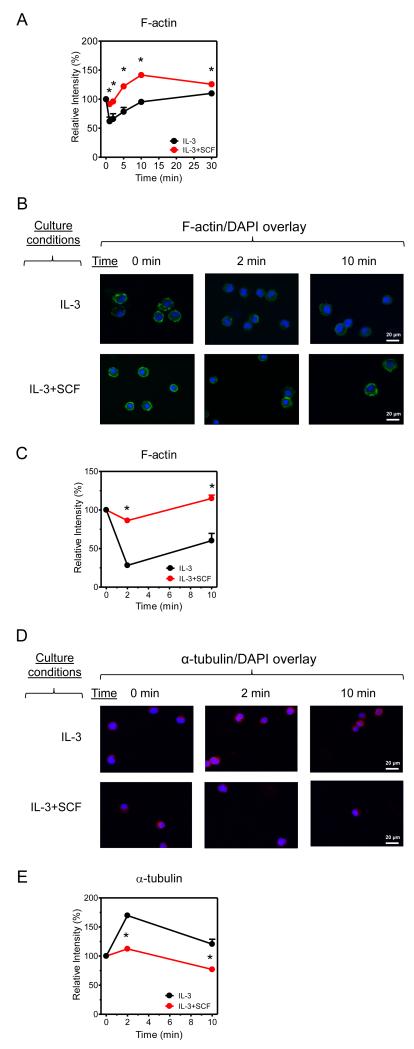
Both the calcium signal and Hck regulate cytoskeletal reorganization through processes that regulate actin polymerization/depolymerization, and formation of microtubules through tubulin polymerization. Both of these events are essential for MC mediator release (35-40). We thus examined whether SCF-dependent reduction in cytoskeletal reorganization would provide a mechanistic explanation for the attenuated mediator release observed in the cSCF-BMMCs. As revealed by flow cytometry (Fig. 8A), and confirmed by immunofluorescence microscopy (Fig. 8B-C), FcεRI-mediated actin depolymerization, which is required for degranulation, was substantially attenuated in the cSCF-BMMCs. Furthermore, immunofluorescence microscopy revealed that there was also a marked decrease in α-tubulin polymerization in the cSCF-BMMCs (Fig. 8D-E). Taken together, these data are consistent with the conclusion that the SCF-induced hypo-responsive phenotype is a consequence of ineffective cytoskeletal reorganization required for mediator release potentially as a consequence of down-regulation of the Hck expression or potentially other signaling proteins downstream of calcium mobilization.
Figure 8. The effect of extended SCF exposure on actin and α-tubulin polymerization.
(A) BMMCs cultured in IL-3 in the absence or presence of SCF were sensitized and then activated with antigen (10 ng/ml) for 0, 2, 5, 10 and 30 min. The cells were fixed/permeabilized and the amount of F-actin determined by phalloidin-FITC staining and flow cytometry. (B) The cells in A were activated with antigen (10 ng/ml) for 0, 2 and 10 min, fixed/permeabilized, and F-actin was labeled with phalloidin-FITC (green) and the nuclei were labeled with DAPI (blue). (C) The fluorescence intensity of the visualized cells in B was evaluated. (D) The cells in A were activated with antigen (10 ng/ml) for 0, 2 and 10 min, fixed/permeabilized, and α-tubulin was labeled with anti-α-tubulin/secondary-Alexa 568 Ab (red) and the nuclei were labeled with DAPI (blue). (E) The fluorescence intensity of the visualized cells in D was evaluated. In B and D, the images are representative from 4 mice. In A, C and E, the data represent means and SEM (n=4) and differences between IL-3 and IL-3+SCF-cultured cells are indicated (*, p <0.05, t test).
Discussion
The results of this study demonstrate that MCs chronically exposed to SCF have a significantly reduced capacity to degranulate and to generate cytokines in response to antigen. This phenotype was linked to ineffective cytoskeletal reorganization potentially associated with down-regulation of Hck. These findings thus provide evidence for a novel mechanism by which the extent of MC activation may be programmed through the influence of cytokines present within the immediate microenvironment.
Based on the relative lack of ability of long-term SCF exposure to reduce the capacity of PMA/ionomycin, adenosine, and PGE2 to enhance antigen-mediated degranulation and cytokine production, the hypo-responsive phenotype induced by SCF appears to be restricted to MC responses mediated by receptors that signal through tyrosine kinases rather than G proteins. Together with the extended time period (>72 h) required for the onset of inhibition by SCF, and the lack of effects on FcεRI and KIT expression, led us to conclude that SCF induced the hypo-responsive phenotype through changes in the expression of specific critical signaling protein(s) rather than as a consequence of short term post-translational modification, for example, through protein phosphorylation. The lack of marked defects in inducible critical early and intermediary signaling events including regulators of calcium mobilization, PI3K, PKC, and MAP kinases, in the hypo-responsive phenotype, further demonstrated that such down-regulation was a consequence of a restricted targeting and not merely a generalized phenomenon.
The lack of any marked defects in these early signaling events additionally indicated that the activation phenotype observed in the cSCF-BMMCs likely reflected down-regulation of gene products that control critical downstream processes. Although the expression array revealed that a multitude of genes are up-regulated or down-regulated in the cells grown in SCF and IL-3 compared to the cells grown in IL-3 alone, based on the current state of knowledge of MC signaling processes (2, 29), the vast majority of the identified genes appeared to have little relevance to the regulation of MC degranulation and/or cytokine production and release. Nevertheless, a notable exception was the substantial reduction in the expression of the Src kinase family member hck. Indeed, real time PCR and western blot analysis confirmed a marked attenuation of the expression of Hck mRNA and protein in the cSCF-BMMCs.
By employing hck−/− mice and MCs derived from the bone marrow of these mice, it has been shown that Hck deficiency results in a MC phenotype characterized by defective antigen-mediated degranulation and reduced expression of TNF-α and IL-6 (34). The corollary is that upregulation of Hck activity has been shown to produce a hyper-responsive MC phenotype (41). Of particular note was the observation that defective degranulation and cytokine production in the hck−/− BMMCs was not associated with a concomitant decrease in calcium flux (34), exactly as was observed in the cSCF-BMMCs. It should be noted, however, that not all observations in the cSCF-BMMS recapitulated those observed in the hck−/− BMMCs. In the hck−/− BMMCs, for example, there was an increase in the phosphorylation of Lyn substrates, such as Dok2, as a consequence of increase Lyn activaity. However, if anything we observed that the phosphorylation of Dok2 was slightly reduced in the cSCF-BMMCs (data not shown), suggesting that Lyn activity was not unregulated in these cells.
Hck binds to and/or tyrosine phosphorylates a number of ligands/substrates including Wiskott-Aldrcih syndrome protein (WASP), WASP-interacting protein (WIP), ELMO1, and α-tubulin (42) and, as for Hck, both WASP (43) and WIP (35) have been demonstrated to be essential for MC function. Hck, WASP and WIP in MCs and other cell types (44, 45) are known to regulate cytoskeletal and/or microtubule reorganization (34, 46, 47) through the respective control of actin and tubulin polymerization/depolymerization. Certainly, both of these processes are recognized to be essential for MC degranulation (35-40) and, indeed, it is believed that Hck, WASP, and WIP may exert their influence on MC activation through the regulation of such processes. Thus, our findings that antigen-induced actin polymerization was attenuated in the cSCF-BMMCs support the conclusion that the down regulation of Hck and subsequent defective cytoskeletal reorganization and tubulin polymerization may account for the attenuated degranulation in the cSCF-BMMCs. Nevertheless, it is possible that downregulation of other signaling molecules may also contribute to this phenotype.
The mode by which cytoskeletal reorganization would prevent FcεRI/KIT-mediated cytokine production is uncertain but, due to the reduced total cytokine protein levels observed, this is unlikely solely due to defective trafficking to the cell surface. A potential explanation may be inadequate access of signaling molecules to the nucleus in the cSCF-BMMCs. The process by which SCF leads to down-regulation of Hck expression in MCs is also currently unknown. Both of these issues thus warrant further investigation.
The identification of the MC phenotypic change induced by chronic exposure to SCF has led us to conclude that the cytokine milieu to which MCs are exposed in vivo may exert not only homeostatic control of growth and survival but also of activation. The importance of such a mechanism for regulating MC function in a physiological setting may have evolved to temper inappropriate MC activation during SCF-mediated cell division, expansion and development where the influence of SCF on MCs would be expected to be greatest. In addition, the plasticity of the phenotype in developing cells would allow reversal of the hyporesponsive phenotype to a more normal responsive phenotype when MCs are mature and resident in tissues. Of potential relevance to this argument was our observation that the degree of the phenotypic hypo-responsiveness correlated to the concentration of SCF the cells were exposed to. Local concentrations of SCF also dictates the described phenotypic differences associated with serosal (connective-tissue) compared to mucosal MCs. It is thus also possible that SCF may similarly dictate the activation capacity of MCs, as a function of their compartmentalization within their resident tissues. Of interest is the report that connective tissue and mucosal MCs display differential gene expression that may impact antigen-mediated degranulation (48). Nevertheless, the gene profiles associated with these phenotypes appear to be different to those observed in our study. For example, whereas there was markedly reduced expression of FcγRIIB (Fcgr2b), Rab-GEF1 (Rabgef1) and CD81 (Cd81) reported in connective tissue MCs associated with reduced antigen-mediated degranulation (48), we observed little change in the expression of these genes in the cSCF-BMMCS (Table S1).
Whether SCF is unique in its ability to downregulate FcεRI-mediated MC activation in the manner reported in this study is thus of interest. It has been reported for example that IL-10 (49) and TGFβ (50) downregulate MC activation in response to antigen. Although, the mechanism for the downregulation by TGF-β is unknown (50) or may be due to reduction in cell survival (51), the IL-10-mediated downregulation could be explained by downregulation of FcεRI expression (49). However, this is not the case with the hyporesponsive phenotype induced by SCF reported here. Given the role of MCs in allergic and autoimmune disorders (52), if these agents, or the pathways they regulate, could be utilized to downregulate MC activation in a clinical setting, these processes would have considerable potential for the treatment of these conditions.
In summary, in this paper we have described that antigen-mediated MC degranulation and cytokine release is dependent on phenotypic programming in response to extended culture of the cells with SCF, conditions likely to exist in vivo. As such, these observations would imply that the capacity of MCs to release inflammatory mediators in a physiological setting may be as much dictated by the cytokine microenvironment present during expansion and maturation of the MCs as that of the extent of antigen-mediated FcεRI-aggregation. It is therefore tempting to speculate that polymorphisms or mutations resulting in disruption to such a regulatory process could result in exaggerated mediator release with the potential of leading to MC-driven pathology. Such observations may aid in the design of novel approaches to downregulate MC activation providing potential therapeutic approaches for the treatment of allergic disorders.
Supplementary Material
Acknowledgments
The authors would like to thank Kishore Kanakabandi, Dan Sturdevant, Kimmo Virtaneva, and Steve Porcella, of Rocky Mountain Laboratories, NIAID, NIH, Montana, for performing the gene arrays and for providing the methods section related to these studies. We would also like to thank Dr. Toshiaki Kawakami (La Jolla Institute for Allergy and Immunology) for providing the Hck construct.
Financial support for this work was provided by the Division of Intramural Research of NIAID and NHLBI within the National Institutes of Health.
Abbreviations
- MC
mast cell
- FcεRI
high affinity receptor for IgE
- SCF
stem cell factor
- BMMCs
bone marrow-derived MCs
Footnotes
Conflict-of-interest disclosure
The authors declare no financial or commercial conflict of interest.
References
- 1.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 2.Kalesnikoff J, Galli SJ. Anaphylaxis: mechanisms of mast cell activation. Chem Immunol Allergy. 2010;95:45–66. doi: 10.1159/000315937. [DOI] [PubMed] [Google Scholar]
- 3.Liu C, Liu Z, Li Z, Wu Y. Molecular regulation of mast cell development and maturation. Mol Biol Rep. 2010;37:1993–2001. doi: 10.1007/s11033-009-9650-z. [DOI] [PubMed] [Google Scholar]
- 4.Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunol Res. 2006;34:97–115. doi: 10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirshenbaum AS, Goff JP, Semere T, Foster B, Scott LM, Metcalfe DD. Demonstration that human mast cells arise from a progenitor cell population that is CD34(+), c-kit(+), and expresses aminopeptidase N (CD13) Blood. 1999;94:2333–2342. [PubMed] [Google Scholar]
- 6.Razin E, Ihle JN, Seldin D, Mencia-Huerta JM, Katz HR, LeBlanc PA, Hein A, Caulfield JP, Austen KF, Stevens RL. Interleukin 3: A differentiation and growth factor for the mouse mast cell that contains chondroitin sulfate E proteoglycan. J Immunol. 1984;132:1479–1486. [PubMed] [Google Scholar]
- 7.Nakano T, Sonoda T, Hayashi C, Yamatodani A, Kanayama Y, Yamamura T, Asai H, Yonezawa T, Kitamura Y, Galli SJ. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985;162:1025–1043. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karimi K, Redegeld FA, Blom R, Nijkamp FP. Stem cell factor and interleukin-4 increase responsiveness of mast cells to substance P. Exp Hematol. 2000;28:626–634. doi: 10.1016/s0301-472x(00)00161-2. [DOI] [PubMed] [Google Scholar]
- 9.Lukacs NW, Kunkel SL, Strieter RM, Evanoff HL, Kunkel RG, Key ML, Taub DD. The role of stem cell factor (c-kit ligand) and inflammatory cytokines in pulmonary mast cell activation. Blood. 1996;87:2262–2268. [PubMed] [Google Scholar]
- 10.Tsai M, Takeishi T, Thompson H, Langley KE, Zsebo KM, Metcalfe DD, Geissler EN, Galli SJ. Induction of mast cell proliferation, maturation, and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc Natl Acad Sci U S A. 1991;88:6382–6386. doi: 10.1073/pnas.88.14.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill PB, MacDonald AJ, Thornton EM, Newlands GF, Galli SJ, Miller HR. Stem cell factor enhances immunoglobulin E-dependent mediator release from cultured rat bone marrow-derived mast cells: activation of previously unresponsive cells demonstrated by a novel ELISPOT assay. Immunology. 1996;87:326–333. doi: 10.1046/j.1365-2567.1996.455545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hundley TR, Gilfillan AM, Tkaczyk C, Andrade MV, Metcalfe DD, Beaven MA. Kit and FcεRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood. 2004;104:2410–2417. doi: 10.1182/blood-2004-02-0631. [DOI] [PubMed] [Google Scholar]
- 13.Tkaczyk C, Horejsi V, Iwaki S, Draber P, Samelson LE, Satterthwaite AB, Nahm DH, Metcalfe DD, Gilfillan AM. NTAL phosphorylation is a pivotal link between the signaling cascades leading to human mast cell degranulation following Kit activation and FcεRI aggregation. Blood. 2004;104:207–214. doi: 10.1182/blood-2003-08-2769. [DOI] [PubMed] [Google Scholar]
- 14.Wershil BK, Tsai M, Geissler EN, Zsebo KM, Galli SJ. The rat c-kit ligand, stem cell factor, induces c-kit receptor-dependent mouse mast cell activation in vivo. Evidence that signaling through the c-kit receptor can induce expression of cellular function. J Exp Med. 1992;175:245–255. doi: 10.1084/jem.175.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ando A, Martin TR, Galli SJ. Effects of chronic treatment with the c-kit ligand, stem cell factor, on immunoglobulin E-dependent anaphylaxis in mice. Genetically mast cell-deficient Sl/Sld mice acquire anaphylactic responsiveness, but the congenic normal mice do not exhibit augmented responses. J Clin Invest. 1993;92:1639–1649. doi: 10.1172/JCI116749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuehn HS, Beaven MA, Ma HT, Kim MS, Metcalfe DD, Gilfillan AM. Synergistic activation of phospholipases Cγ and Cβ: a novel mechanism for PI3K-independent enhancement of FcεRI-induced mast cell mediator release. Cell Signal. 2008;20:625–636. doi: 10.1016/j.cellsig.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirshenbaum AS, Metcalfe DD. Growth of human mast cells from bone marrow and peripheral blood-derived CD34+ pluripotent progenitor cells. Methods Mol Biol. 2006;315:105–112. doi: 10.1385/1-59259-967-2:105. [DOI] [PubMed] [Google Scholar]
- 18.Levi-Schaffer F, Austen KF, Caulfield JP, Hein A, Bloes WF, Stevens RL. Fibroblasts maintain the phenotype and viability of the rat heparin-containing mast cell in vitro. J Immunol. 1985;135:3454–3462. [PubMed] [Google Scholar]
- 19.Choi OH, Lee JH, Kassessinoff T, Cunha-Melo JR, Jones SVP, Beaven MA. Antigen and carbachol mobilize calcium by similar mechanisms in a transfected mast cell line (RBL-2H3 cells) that expresses ml muscarinic receptors. J Immunol. 1993;151:5586–5595. [PubMed] [Google Scholar]
- 20.Tkaczyk C, Metcalfe DD, Gilfillan AM. Determination of protein phosphorylation in FcεRI-activated human mast cells by immunoblot analysis requires protein extraction under denaturing conditions. J Immunol Methods. 2002;268:239–243. doi: 10.1016/s0022-1759(02)00210-7. [DOI] [PubMed] [Google Scholar]
- 21.Smrž D, Iwaki S, McVicar DW, Metcalfe DD, Gilfillan AM. TLR-mediated signaling pathways circumvent the requirement for DAP12 in mast cells for the induction of inflammatory mediator release. Eur J Immunol. 2010;40:3557–3569. doi: 10.1002/eji.201040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tkaczyk C, Beaven MA, Brachman SM, Metcalfe DD, Gilfillan AM. The phospholipase Cγ1-dependent pathway of FcεRI-mediated mast cell activation is regulated independently of phosphatidylinositol 3-kinase. J Biol Chem. 2003;278:48474–48484. doi: 10.1074/jbc.M301350200. [DOI] [PubMed] [Google Scholar]
- 23.Malbec O, Roget K, Schiffer C, Iannascoli B, Dumas AR, Arock M, Daëron M. Peritoneal cell-derived mast cells: an in vitro model of mature serosal-type mouse mast cells. J Immunol. 2007;178:6465–6475. doi: 10.4049/jimmunol.178.10.6465. [DOI] [PubMed] [Google Scholar]
- 24.Virtaneva K, Porcella SF, Graham MR, Ireland RM, Johnson CA, Ricklefs SM, Babar I, Parkins LD, Romero RA, Corn GJ, Gardner DJ, Bailey JR, Parnell MJ, Musser JM. Longitudinal analysis of the group A Streptococcus transcriptome in experimental pharyngitis in cynomolgus macaques. Proc Natl Acad Sci U S A. 2005;102:9014–9019. doi: 10.1073/pnas.0503671102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M, Lai Y, Villaruz AE, Cha DJ, Sturdevant DE, Otto M. Gram-positive three-component antimicrobial peptide-sensing system. Proc Natl Acad Sci U S A. 2007;104:9469–9474. doi: 10.1073/pnas.0702159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flanagan JG, Leder P. The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell. 1990;63:185–194. doi: 10.1016/0092-8674(90)90299-t. [DOI] [PubMed] [Google Scholar]
- 27.Levi-Schaffer F, Austen KF, Gravallese PM, Stevens RL. Coculture of interleukin 3-dependent mouse mast cells with fibroblasts results in a phenotypic change of the mast cells. Proc Natl Acad Sci U S A. 1986;83:6485–6488. doi: 10.1073/pnas.83.17.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwaki S, Tkaczyk C, Satterthwaite AB, Halcomb K, Beaven MA, Metcalfe DD, Gilfillan AM. Btk plays a crucial role in the amplification of FcεRI-mediated mast cell activation by kit. J Biol Chem. 2005;280:40261–40270. doi: 10.1074/jbc.M506063200. [DOI] [PubMed] [Google Scholar]
- 29.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 30.Baumgartner RA, Yamada K, Deramo VA, Beaven MA. Secretion of TNF from a rat mast cell line is a brefeldin A-sensitive and a calcium/protein kinase C-regulated process. J Immunol. 1994;153:2609–2617. [PubMed] [Google Scholar]
- 31.Alfredsson J, Puthalakath H, Martin H, Strasser A, Nilsson G. Proapoptotic Bcl-2 family member Bim is involved in the control of mast cell survival and is induced together with Bcl-XL upon IgE-receptor activation. Cell Death Differ. 2005;12:136–144. doi: 10.1038/sj.cdd.4401537. [DOI] [PubMed] [Google Scholar]
- 32.Kim MS, Rådinger M, Gilfillan AM. The multiple roles of phosphoinositide 3-kinase in mast cell biology. Trends Immunol. 2008;29:493–501. doi: 10.1016/j.it.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nechushtan H, Razin E. Studies of different aspects of the role of protein kinase C in mast cells. Int Arch Allergy Immunol. 2001;124:130–132. doi: 10.1159/000053690. [DOI] [PubMed] [Google Scholar]
- 34.Hong H, Kitaura J, Xiao W, Horejsi V, Ra C, Lowell CA, Kawakami Y, Kawakami T. The Src family kinase Hck regulates mast cell activation by suppressing an inhibitory Src family kinase Lyn. Blood. 2007;110:2511–2519. doi: 10.1182/blood-2007-01-066092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kettner A, Kumar L, Anton IM, Sasahara Y, de la Fuente M, Pivniouk VI, Falet H, Hartwig JH, Geha RS. WIP regulates signaling via the high affinity receptor for immunoglobulin E in mast cells. J Exp Med. 2004;199:357–368. doi: 10.1084/jem.20030652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tolarová H, Dráberová L, Heneberg P, Dráber P. Involvement of filamentous actin in setting the threshold for degranulation in mast cells. Eur J Immunol. 2004;34:1627–1636. doi: 10.1002/eji.200424991. [DOI] [PubMed] [Google Scholar]
- 37.Pendleton A, Koffer A. Effects of latrunculin reveal requirements for the actin cytoskeleton during secretion from mast cells. Cell Motil Cytoskeleton. 2001;48:37–51. doi: 10.1002/1097-0169(200101)48:1<37::AID-CM4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 38.Smith AJ, Pfeiffer JR, Zhang J, Martinez AM, Griffiths GM, Wilson BS. Microtubule-dependent transport of secretory vesicles in RBL-2H3 cells. Traffic. 2003;4:302–312. doi: 10.1034/j.1600-0854.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- 39.Martin-Verdeaux S, Pombo I, Iannascoli B, Roa M, Varin-Blank N, Rivera J, Blank U. Evidence of a role for Munc18-2 and microtubules in mast cell granule exocytosis. J Cell Sci. 2003;116:325–334. doi: 10.1242/jcs.00216. [DOI] [PubMed] [Google Scholar]
- 40.Nishida K, Yamasaki S, Ito Y, Kabu K, Hattori K, Tezuka T, Nishizumi H, Kitamura D, Goitsuka R, Geha RS, Yamamoto T, Yagi T, Hirano T. FcεRI-mediated mast cell degranulation requires calcium-independent microtubule-dependent translocation of granules to the plasma membrane. J Cell Biol. 2005;170:115–126. doi: 10.1083/jcb.200501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samayawardhena LA, Pallen CJ. PTPα activates Lyn and Fyn and suppresses Hck to negatively regulate FcεRI-dependent mast cell activation and allergic responses. J Immunol. 2010;185:5993–6002. doi: 10.4049/jimmunol.1001261. [DOI] [PubMed] [Google Scholar]
- 42.Scott MP, Zappacosta F, Kim EY, Annan RS, Miller WT. Identification of novel SH3 domain ligands for the Src family kinase Hck. Wiskott-Aldrich syndrome protein (WASP), WASP-interacting protein (WIP), and ELMO1. J Biol Chem. 2002;277:28238–28246. doi: 10.1074/jbc.M202783200. [DOI] [PubMed] [Google Scholar]
- 43.Mani M, Venkatasubrahmanyam S, Sanyal M, Levy S, Butte A, Weinberg K, Jahn T. Wiskott-Aldrich syndrome protein is an effector of Kit signaling. Blood. 2009;114:2900–2908. doi: 10.1182/blood-2009-01-200733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ziegler SF, Marth JD, Lewis DB, Perlmutter RM. Novel protein-tyrosine kinase gene (hck) preferentially expressed in cells of hematopoietic origin. Mol Cell Biol. 1987;7:2276–2285. doi: 10.1128/mcb.7.6.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramesh N, Geha R. Recent advances in the biology of WASP and WIP. Immunol Res. 2009;44:99–111. doi: 10.1007/s12026-008-8086-1. [DOI] [PubMed] [Google Scholar]
- 46.Blundell MP, Worth A, Bouma G, Thrasher AJ. The Wiskott-Aldrich syndrome: The actin cytoskeleton and immune cell function. Dis Markers. 2010;29:157–175. doi: 10.3233/DMA-2010-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antón IM, Jones GE. WIP: a multifunctional protein involved in actin cytoskeleton regulation. Eur J Cell Biol. 2006;85:295–304. doi: 10.1016/j.ejcb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Takano H, Nakazawa S, Okuno Y, Shirata N, Tsuchiya S, Kainoh T, Takamatsu S, Furuta K, Taketomi Y, Naito Y, Takematsu H, Kozutsumi Y, Tsujimoto G, Murakami M, Kudo I, Ichikawa A, Nakayama K, Sugimoto Y, Tanaka S. Establishment of the culture model system that reflects the process of terminal differentiation of connective tissue-type mast cells. FEBS Lett. 2008;582:1444–1450. doi: 10.1016/j.febslet.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 49.Kennedy Norton S, Barnstein B, Brenzovich J, Bailey DP, Kashyap M, Speiran K, Ford J, Conrad D, Watowich S, Moralle MR, Kepley CL, Murray PJ, Ryan JJ. IL-10 suppresses mast cell IgE receptor expression and signaling in vitro and in vivo. J Immunol. 2008;180:2848–2854. doi: 10.4049/jimmunol.180.5.2848. [DOI] [PubMed] [Google Scholar]
- 50.Zhao W, Gomez G, Yu SH, Ryan JJ, Schwartz LB. TGF-β1 attenuates mediator release and de novo Kit expression by human skin mast cells through a Smad-dependent pathway. J Immunol. 2008;181:7263–7272. doi: 10.4049/jimmunol.181.10.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Norozian F, Kashyap M, Ramirez CD, Patel N, Kepley CL, Barnstein BO, Ryan JJ. TGFβ1 induces mast cell apoptosis. Exp Hematol. 2006;34:579–587. doi: 10.1016/j.exphem.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Weller CL, Collington SJ, Williams T, Lamb JR. Mast cells in health and disease. Clin Sci (Lond) 2011;120:473–484. doi: 10.1042/CS20100459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.