Abstract
Injuries can induce adaptations in pain processing that result in amplification of signaling. One mechanism may be analogous to long-term potentiation (LTP) and involve the atypical protein kinase C, PKMζ The possible contribution of PKMζ-dependent, and independent amplification mechanisms to experimental neuropathic pain was explored in rats with spinal nerve ligation (SNL) injury. SNL increasedp-PKMζin the rostral anterior cingulate cortex (rACC) a site that mediates, in part, the unpleasant aspects of pain. Inhibition of PKMζ within the rACC by a single administration of ζ-pseudosubstrate inhibitory peptide (ZIP)reversedSNL-induced aversivenesswithin24 hrswhereasNMDA receptor blockade with MK-801 had no effects. The SNL-induced aversive state (reflecting “spontaneous” pain),was re-established in a time-dependent manner, with full recovery observed 7 days post-ZIP administration. Neither rACC ZIPnor MK-801altered evoked responses. In contrast, spinal ZIP or MK-801, but not scrambled peptide,transiently reversedevoked hypersensitivity but had no effect on nerve-injury induced spontaneous pain.PKMζ phosphorylation was not altered by SNL in the spinal dorsal horn.These data suggest thatamplification mechanisms contribute to different aspects of neuropathic pain at different levels of the neuraxis. Thus, PKMζ-dependent amplification contributes to nerve-injury induced aversivenesswithin the rACC. Moreover, unlike mechanisms maintaining memory, the consequences of PKMζ inhibition within the rACC are not permanent in neuropathic pain, possibly reflecting the re-establishment of amplification mechanisms by ongoing activity of injured nerves. In the spinal cord, however, both PKMζ-dependent and independent mechanisms contribute to amplification of evoked responses, but apparently not spontaneous pain.
Keywords: rACC, spinal cord, PKMζ, ZIP, aversiveness, spontaneous pain, neuropathic pain
Introduction
Neuropathic pain is often characterized by spontaneouspainand,in some patients, allodynia to touch and cold[3-5]. Following injury, pain resulting from previously innocuous touch stimuli may result from altered processing of low threshold inputs within the central nervous system[24; 25; 36; 42; 46; 50]. Enhanced afferent drive is thought to elicit a state of central sensitization resulting in amplification of signaling [12; 24; 59]. A proposed mechanism that may contribute to central sensitization is synaptic long-term potentiation (LTP)[42]. Amplified signaling between C-fibers and second order spinal neurons has been demonstrated following injury[42; 58] and such plasticity also likely mediates hyperalgesia [24; 42].
Mechanisms of amplification have been studied primarily in slice preparations or in the spinal cord[24; 42]. LTP hasbeen demonstrated in the spinal cord as well as within several regions of the brain including the anterior cingulate cortex (ACC) following nerve injury [25]. Whether, or how,such mechanisms of amplification contribute to specific components of pain at different levels of the neuraxis is not known. Clinical imaging studies have demonstrated that aversiveness of pain is integrated, at least in part, within therostral ACC (rACC)[53; 54]. In preclinical models,rACC lesions abolish formalin-induced conditioned place aversion without altering sensory thresholds and/or evoked nocifensive behaviors [17]. Further, rACClesionhas been shown to remove the aversive state induced by experimental neuropathic pain without altering evoked hypersensitivity to mechanical or thermal stimuli [40]. While a role for the ACC in some of the most intractable features of clinical pain disorders is clear [1], mechanisms underlying ACC plasticity in relation to affective dimensions of pain are not well understood. Notably, the unpleasant affective dimensions of pain are thought to be the most troubling component of pain in patients [14; 34].
One well-studied mechanism of LTPinitiation is via NMDA receptors [24; 42]. A potential consequence of NMDA receptor activation is formation and phosphorylation of PKMζ[43]. PKMζ is an atypical protein kinase C (aPKC) that is constitutively active following translation and believed to be necessary and sufficient for late LTP (l-LTP; [29; 43]). PKMζ phosphorylation coincides with the initiation of LTP and persists after l-LTP consolidation [47]. Blockade of PKMζ activity with ζ-inhibitory pseudosubstratepeptide (ZIP) within the hippocampus reverses l-LTP and erases established memories in vivo [37; 48]. In contrast, blockade of NMDA receptors does not reverse established LTP [42].
While multiple lines of evidence link spinal and ACC synaptic plasticity to chronic pain states [2; 11; 27; 42; 50; 52; 57; 59], the potential contributions of these mechanisms to the aversive aspects or to evoked hypersensitivity of experimental neuropathic pain at these sites is not known. Recently, we have employed negative reinforcement to reveal the presence of an aversive state induced by nerve injury,allowing the unmasking of spontaneous neuropathic pain and the investigation of underlying mechanisms[21]. We used this principle to determine the contribution of PKMζ-dependent and independent amplification within the spinal cord and rACC to components of neuropathic pain.
Materials and methods
Animals
Male, Sprague Dawley rats(Harlan) weighing 250–350 g at time of testing, were maintained in a climate-controlled room on a 12 hr light/dark cycle, and food and water were available ad libitum. All experiments were performed in accordance with the policies and recommendations of the International Association for the Study of Pain and the National Institutes of Health guidelines for the handling and use of laboratory animals and received approval from the Institutional Animal Care and Use Committee of the University of Arizona. All efforts were made to minimize animal suffering and reduce the number of animals used.All behavioral experiments were carried out by an experimenter blinded to the treatment groups.
Rostral ventromedial medulla (RVM) and rACCcannulation
Animals were anesthetized with injection of ketamine–xylazine (100 mg/kg, i.p.) and placed in a stereotaxic apparatus. Bilateral cannulation of the RVM was performed as previously described [6; 61]. Two 26 gauge guide cannulas separated by 1.2 mm were directed toward the lateral portions of the RVM (anteroposterior, −11.0 mm from bregma; lateral, ±0.6 mm; dorsoventral, −7.5 mm from the dura mater[38]).Bi-lateral cannulation of the rACC was performed as previously described [16]. Double stainless steel guides (33-gauge) were implanted 1 mm above the ACC injection site (anterior/posterior +2.6 from bregma; lateral ± 0.6 mm; dorsoventral, −1.6 [38]. Guide cannulas were cemented in place and secured to the skull by small stainless steel machine screws.For all cannulas, stainless steel dummy cannulas extending to the tip of the guide cannulas were inserted and kept the guide free of debris until drug delivery. Rats then received gentamicin and were allowed to recover 7 days before any behavioral testing and subsequent SNL or sham surgeries. No signs of weight loss or distress were observed following cannulation surgeries.
Intrathecal cannulation
Rats were anesthetized with isofluorane and placed in a stereotaxic head holder. The atlanto-occipital membrane was exposed, cleared and an incision was made in the dura mater. A length of PE-10 tubing was advanced 8 cm caudally to the lumbar spinal cord. The tubing was exteriorized, filled with saline and plugged with wire. The wound was closed, and animals were allowed to recover for 7 days before any behavioral testing and subsequent SNL or sham surgeries.
Spinal Nerve Ligation
The surgical procedure for L5/L6 spinal nerve ligation was performed according to that described previously [19]. Sham-operated control rats were prepared in an identical manner except that the L5/L6 spinal nerves were not ligated. The behavior of the rats was monitored carefully for any visual indication of motor disorders or change in weight or general health.
Drug administration
All injections were delivered in a separate room with rats placed into the conditioned place preference (CPP) chambers within 2 min.RVM microinjections were performed by slowly expelling 0.5 l of saline or lidocaine (4% w/v) through a 33 gauge injection cannula protruding 1 mm beyond the guide cannula into fresh brain tissue as previously demonstrated to elicit CPP in rats with nerve injury[21].Microinjections into the rACC were performed by slowly expelling 0.5 μl volume of ZIP (20 μg/0.5 μl; 10 nmol), scrambled ZIP (20 μg/0.5 μl; 10 nmol)or MK-801 (67μg/0.5 μl,(200nmol)across a 1 min period through injectors protruding 1 mm beyond the guide tip.Cocaine (1mg/kg) was administered into awake rats by intravenous injection into the tail vein.Spinal clonidine (10 μg), MK-801 (3 μg) and ZIP (20 μg)were administered in a 5 μl volume followed by a 9 μl saline flush. Progress was monitored with an air bubble.Myristoylated-ZIP andmyristoylated-scrambled ZIP were purchased from Tocris (Ellisville, MO), MK-801 was purchased from Sigma Chemicals (St Louis, MO) andcocaine was obtained from NIDA drug supply program.
Tactile hypersensitivity
The withdrawal threshold of the hindpaw was measured in response to probing of the plantar surface with a series of calibrated von Frey filaments and analyzed using a Dixon nonparametric test as previously described [8; 13; 21].
Thermal hypersensitivity
The withdrawal latency of the hindpaw to an infrared radiant heat source was performed as previously described [6; 61]. Baseline latencies were established at 17-25 sec to allow a sufficient window for the detection of possible hyperalgesia. A maximal cutoff of 33 sec was used to prevent tissue damage.
Conditioned place preference
The single trial CPP protocol was performed as previously described [20; 21; 40].The CPP procedure depends on the association of a context with the effect of an experimental manipulation (i.e., administration of drug). For this reason, the time at which the context is paired with the drug is critical.Previous studies have demonstrated that MK-801 rapidly elicits blockade of NMDA receptors[51]. For this reason, possible direct effects of MK-801 on CPP in sham- and SNL-rats were assessed as described in detail below. In contrast, ZIP is believed to act through time-dependent modulation of GluR2 AMPA receptor trafficking to the membrane[33, 42]. As the time point at which ZIP might produce effects on aversiveness or evoked hypersensitivity is uncertain, we determined whether ZIP (or scrambled peptide) treatment would alter CPP to an established pain-relieving mechanism (i.e., RVM lidocaine for rACC studies or spinal clonidine for intrathecal studies)[21]. A limitation of the present studies is our inability to assess whether ZIP could directly induce CPP.
rACC ZIP or scrambled ZIP
Starting 7 days post nerve-injury/sham surgery, all rats were exposed to the environment with full access to all chambersfor 15 min and behavior recorded and analyzed to verify no pre-conditioning chamber preference. ZIP or scrambled peptide was delivered immediately following BL for the D1 time-point. The following day, rats received RVM saline paired with the appropriate chamber in the morning, and RVM lidocainepaired with the other chamber 4 hr later. Chamber pairings were counterbalanced. Following the afternoon pairing, rats were placed in the CPP box with access to all chambers and their behavior recorded for 15 min for analysis ofchamber preference. Separate groups of rats received ZIP or scrambled peptide3 or 7 days prior to conditioning day for analysis of the time-course of ZIP’s effects.We have previously used this procedure to demonstrate that lesion of the rACC prevents CPP to RVM lidocaine in SNL-injured rats [40].
rACC ZIP or scrambled peptide effects on cocaine-induced CPP was assessed in separate rats to verify that rACC ZIP treatment does not impair ability to acquire CPP. Pre-conditioning baselines were assessed 7 days following rACCcannulation.ZIP or scrambled peptide was delivered immediately following BL as the most robust effect of ZIP on CPP induced by RVM lidocaine was observed at the 24 hour time-point (see below). The following day, rats received saline (i.v.) paired with the appropriate chamber. Four hours later, rats received cocaine (1 mg/kg,i.v.) paired with the opposite chamber. On test day, 20-24 hours following the afternoon pairing, rats were placed in the CPP box with access to all chambers and their behavior recorded across 15 min for analysis of chamber preference.We have previously used this procedure to demonstrate that lesion of the rACC fails to impair cocaine-induced CPP [40].
rACC MK-801
Rats underwent preconditioning analysis to verify no preconditioning chamber preference 7 days following nerve injury/sham surgery. The following day, rats received rACC saline paired with the appropriate chamber in the morning. Four (4) hours later, rats received rACC MK-801 (67μg/0.5 μl per side) paired with the opposite chamber. This dose was chosen based on a previous study that reported that unilateral injection of this dose of MK-801 into the contralateral ACC at the time of axotomyproduced a decrease in autotomy behaviors when evaluated over the subsequent 25 days[30]. Test day occurred 20-24 hours following the afternoon pairing. MK-801 has been shown to produce a rapid effect (i.e., within 20 min) reversal of evoked responses; this time-course is similar to other pain alleviating manipulations known to induce CPP (e.g., spinal clonidine or ω-conotoxin or RVM lidocaine [21]).
Spinal ZIP or scrambled ZIP peptide
Our studies showed that ZIP-induced reversal of SNL-induced thermal hypersensitivity has a slow onset, peaking 4 hrs following administration (see below). Thus, we assessed whether spinal ZIP would block spinal clonidine-induced CPP [20]. Starting 7 days post nerve-injury/sham surgery, all rats were exposed to the environment with full access to all chambers for 15 min and behavior recorded and analyzed to verify no pre-conditioning chamber preference. The following day, rats received spinal saline paired with the appropriate chamber followed immediately by spinal administration of ZIP or scrambled peptide. The afternoon pairing occurred 4 hours later with spinal clonidine (10 μg). Test day occurred 20-24 hours following the afternoon pairing.
Spinal MK-801
Starting 7 days post nerve-injury/sham surgery, all rats were exposed to the CPP box with full access to all chambers for 15 min and behavior recorded and analyzed to verify no pre-conditioning chamber preference. The following day, rats received spinal saline paired with the appropriate chamber followed 4 hrs later with spinal MK-801 (3 μg) paired with the opposite chamber. Test day occurred 20-24 hrs following the afternoon pairing. This dose of MK-801 has been shown to reverseSNL-induced thermal, but not tactile, hypersensitivity [30]. Higher doses elicit significant motor impairment preventing assessment of possible effects on tactile responses [26].
Verification of cannula placement
Following the experiments, rats were euthanized and India ink was injected in the same manner as drug delivery. Brains were then removed and post-fixed in 10% formalin for a minimum of 4 hrs. Brains were frozen and sliced at 30 μm, and location of the ink recorded. Animals with incorrectly placed cannulas were not included within the data analysis.
Western blotting and quantitative PCR
Punch biopsies of rostral ACC were taken from stereotaxically identified 2 mm thick slices (bregma +3.7 to +1.7) on the contralateral side from SNL or sham surgery 10 days following surgery. Protein was extracted from biopsies in lysis buffer (50 mMTrisHCl, 1% Triton X-100, 150 mMNaCl, and 1 mM EDTA at pH 7.4) containing protease and phosphatase inhibitor mixtures (Sigma) with an ultrasonicator on ice, and cleared of cellular debris and nuclei by centrifugation at 14,000 RCF for 15 min at 4°C. Tenmicrograms of protein per well were loaded and separated by standard 7.5% SDS-PAGE. Proteins were transferred to Immobilon-P membranes (Millipore) and then blocked with 5% dry milk for 3 hrs at room temperature. The blots were incubated with primary antibody overnight at 4°C (phospho (p)-PKMζand p-PKCλantibody from Cell Signaling technologies, that recognize the Thr410 site of phosphorylation for PKMζ and PKCλ; and total-PKMζ and -PKCλ antibody from Santa Cruz Biotechnologies; βIII tubulin antibody from Millipore) and detected the following day with donkey anti-rabbit antibody (p-PKC/Mζ and PKC/Mζ) or donkey anti-mouse antibody (βIII tubulin) conjugated to horseradish peroxidase (Jackson Immunoresearch). PKCλand PKMζ were distinguished by size (PKCλ~ 70kD; PKMζ ~ 50 kD).Signal was detected by ECL on chemiluminescent films. Each phosphoprotein was normalized to the expression of the corresponding total protein on the same membrane. Total proteins were normalized to βIII tubulin on the same membrane.Densitometric analyses were performed with ImageJ software (NIH).For quantitative PCR (qPCR), punch biopsies were taken and RNA was isolated with the RNeasy mini kit (QIAGEN). Reverse transcription was performed with RT Superscript III (Invitrogen). PCR reactions were performed on an ABI 7500 Fast Real-time PCR System with SYBRGreen PCR master mix (Applied Biosystems). Each reaction was normalized to the expression of GAPDH and expressed as relative copy number. QPCR primers used were: PKCλ - TGAGCAGCCATTCACCATGA and GGGAACACGTGGATCAGGAG, PKCζ - GTCAGGGCAGGGACGAAGT and GGCGGTAGATGGACTTGTCTTCT, PKMζ - GCTCCTTAAAGGGACGGAAGAT and GGCTCCACGGCGGTAGAT and GAPDH – AGGTCGGTGTGAACGGATTTG and GGGGTCGTTGATGGCAACA.
Statistical Analysis
For analysis of evoked pain behaviors, significant changes from pre-surgery baseline control values were detected by ANOVA, followed by Bonferroni post-test. These evaluations were all performed using GraphPad Prism 5.03 for Windows (GraphPad Software, San Diego CA, www.graphpad.com). For CPP experiments, data was analyzed before conditioning (baseline) and after conditioning using two-factor ANOVA (chambers vs. treatment) followed by Bonferroni test. Difference from baseline scores were calculated for each rat using the formula: test time in chamber - preconditioning time spent in chamber. Difference scores from baseline for the drug paired chamber between nerve injured and sham operated rats were analyzed using paired t-tests. Western blot densitometry measures were compared by student’s t-test. For all analyses, significance was set at p<0.05.
Results
SNL leads to a specific increase in p-PKMζ in the rACC
Three aPKC isoforms have been described in rodents, PKCζ, PKMζ and PKCλ[49]. We first determined which aPKC isoforms are expressed in the rat rACC. QPCR analyses of isoform-specific expression, normalized to housekeeping gene GAPDH controland expressed as relative copy-number,demonstrated robust PKMζ (64,894 ± 2,685) and PKCλ (26,269 ± 2,016) mRNA expression in the rACC, whereas PKCζ mRNA expression was close to the lower detection limit (116 ±3.53), despite robust expression in kidney (not shown).The relative expression of the isoforms is shown in Fig 1A.We then determined if SNL altered PKMζ or PKCλexpression and/or phosphorylation in the rACC.Ten days following SNL or sham surgery,rACC punch biopsies were taken and PKMζ and PKCλexpression and phosphorylation status were assessed by Western blot.SNL led to a significant increase in PKMζ phosphorylation in the rACC compared to sham animals (Fig 1B; 100.0 ±12.6 vs. 144.4 ±15.5 %; n = 6) whereas PKCλ phosphorylation was not significantly altered (Fig 1C; 100.0 ±13.4 vs. 106.3 ±13.7 %; n = 6).Neither total PKMζ (Fig 1D; 100.0 ±5.16 vs. 93.0 ±5.26 %; n = 6) nor total PKCλ(Fig 1E; 100.0 ±5.13 vs. 95.8 ± 9.16 %; n = 6) were altered in the rACCwhencomparedto the loading control, βIII tubulin at post-SNL day 10.
Figure 1.

SNL leads to a specific increase in p-PKMζ in the rostral ACC. (A) qPCR analysis indicates that PKCλand PKMζ, but not PKCζ, mRNA is expressed in rACC. Tissue biopsies were taken from the contralateral ACC 10 days following SNL or sham surgery. SNL led to a significant increase in p-PKMζ (B) but not p-PKCλ (C). SNL did not change total PKMζ (D) or total PKCλ (E) compared to loading control (βIII tubulin) in the rostral ACC. * p < 0.05. Graphs represent mean ±SEM, n=6.
Inhibition of PKMζ in rACCreverses SNL-induced spontaneous, but not evoked, pain
To determine the role of rACC PKMζ in SNL-induced spontaneous pain, separate groups of rats received rACC ZIP (10 nmol per side) or scrambled peptide (10 nmol per side, control injection) 9 days following SNL or sham surgery and underwent the single trial conditioning to RVM lidocaine 1, 3, or 7 days later. Pre-conditioning time spent in the CPP chambers were equivalent in all groups, so data were pooled for graphical representation (Fig 2A). Post-conditioning time spent in the CPP chambers did not change in sham-treated rats irrespective of treatment group, therefore data were pooled for graphical representation (Fig 2A,B). RVM lidocaine induced CPP in SNL rats that received rACC injection of scrambled ZIP peptide (Fig 2A,B; *p<0.05vs BL). rACCZIP administration1 day prior to conditioning day, however,blocked the RVM lidocaine induced CPP (Fig 2A).Separate groups of animals were evaluated at 3 and 7 days after rACC ZIP in order to determine the duration of the effect of a single ZIP treatment.At 3 days post-ZIP, a blockade of the RVM lidocaine CPP effect was observed (Fig 2A)whereasa single injection of ZIP into the rACC7 days prior to conditioning failed to block RVM lidocaine induced CPP (Fig 2A, *p<0.05 vs BL). Difference scores confirmed that SNL rats treated with scrambled ZIP showed increased time spent in the RVM lidocaine paired chamber, while ZIP treatment elicited a time-dependent reversal of RVM lidocaine induced CPP in SNL rats (Fig 2B, *p<0.05vs Sham).
Figure 2.
Inhibition of PKMζ in rACC reversibly blocks SNL-induced spontaneous pain, but not evoked hypersensitivity. (A) Scrambled ZIP peptide into the rACC did not block RVM lidocaine-induced CPP in SNL rats. ZIP administration into the rACC blocked RVM lidocaine induced CPP when given 24 hr prior to conditioning, with a time-dependent return of RVM lidocaine induced CPP by 7 days post ZIP injection. *p<0.05 vs BL. (B) Difference scores confirmed significant increase in time spent in the RVM lidocaine paired chamber in SNL rats treated with scrambled ZIP peptide (SNL-SCR). Administration of ZIP completely blocked the RVM lidocaine induced CPP on D1 post injection, which reversed by D7 post-injection. *p<0.05 vs sham. Administration of ZIP into the rACC failed to alter tactile (C) or thermal (D) hypersensitivity. *p<0.05 vs Sham-Scr. Graphs represent mean ± SEM, n=10-12.
The role of rACC PKMζ in SNL-induced evoked behaviors was determined in separate groups of rats using the same treatment schedules.Rats received rACC ZIP or scrambled ZIP into the rACC 9 days following surgery followed by testing for tactile and thermal sensory thresholds 4, 24 and 72 hrs later. Rats with SNL showed a reduction in tactile as well as thermal paw withdrawal thresholds (Fig 2C,D, *p<0.05 vs sham). Thermal and mechanical thresholds were unchanged in SNL rats treated with rACCZIP, with paw withdrawal values equivalent to rats receiving scrambled ZIP at all test points (Fig 2C,D, *p<0.05 vs sham); no effects of ZIP or scrambled ZIP peptidewere seen in sham-operated rats.
Inhibition of PKMζ in rACC does not alter cocaine-induced CPP
To verify that inhibition of PKMζ does not alter the ability of rats to acquire reinforcement to a rewarding stimulus, rats were treated with rACC scrambled ZIP peptide or ZIP (10 nmol/0.5 μl per side) immediately following BL. The following day, rats underwent single trial conditioning with systemic administration of cocaine (1 mg/kg, i.v.). Cocaine elicited CPP irrespective of ZIP or scrambled ZIP peptide treatment (Fig 3A, *p<0.05 vs BL). Difference scores indicate that both groups show equivalent increases in time spent in the cocaine-paired chamber (Fig 3B).
Figure 3.
Inhibition of PKMζ in rACC fails to block cocaine-induced CPP. (A) Scrambled ZIP into the rACC did not block cocaine (1 mg/kg, i.v.) induced CPP when given 24 hr prior to conditioning. Administration of ZIP into the rACC also did not block cocaine induced CPP when given 24 hr prior to conditioning. (B) Difference scores confirmed significant increase in time spent in the cocaine paired chamber irrespective of rACC ZIP treatment. Graphs represent mean ± SEM, *p<0.05, n=10-12.
Blockade of NMDA receptors in rACC does not block spontaneous pain or evoked hypersensitivity
To determine whether blockade of NMDA receptors within the rACC blocks SNL-induced spontaneous pain or evoked hypersensitivity, rats underwent the single trial conditioning in which they received bilateral rACC saline injections paired with a chamber in the morning, and bilateral rACC MK-801 (67 μg in 0.5 μl per side) four hrs later. Administration of MK-801 into the rACCfailed to induce CPP in SNL or sham treated rats, suggesting that this dose did not block SNL-induced spontaneous pain (Fig 4A). Administration of this same dose of MK-801 into the rACC also failed to alter SNL-induced tactile or thermal hypersensitivity (Fig 4B,C, respectively).
Figure 4.
Blockade of rACC NMDA receptors fails to block SNL-induced spontaneous or evoked pain. (A) Administration of the NMDA receptor antagonist, MK-801 failed to induce CPP in sham or SNL treated rats. (B) Administration of MK-801 into the rACC failed to block SNL-induced tactile hypersensitivity. (C) Administration of MK-801 into the rACC failed to block SNL-induced thermal hypersensitivity. Graphs represent mean ± SEM, *p<0.05 compared to pre-surgery BL, n=10-12.
SNL does not change p-PKMζ in the dorsal spinal cord
We first determined which aPKC isoforms are expressed in the rat dorsal horn. QPCR analyses of isoform-specific expression, normalized to housekeeping gene GAPDH control and expressed as relative copy-number, demonstrated PKMζ (24,525 ± 3,056) and PKCλ (24,471 ± 1,781) mRNA expression in the dorsal horn, whereas PKCζ mRNA expression was again close to the lower detection limit (370±78.0, Fig 5A).We cannot rule out that PKCζ mRNA expression might be increased by nerve injury.We then determined if SNL altered PKMζ or PKCλexpression and/or phosphorylation in the dorsal horn. Ten days following SNL or sham surgery, dorsal horns were isolated and PKMζ and PKCλexpression and phosphorylation status were assessed by Western blot. In the spinal dorsal horn, SNL did not change PKMζ phosphorylation compared to sham animals (Fig 5B; 100.0 ±17.9vs. 115.0±16.13 %; n = 6) or PKCλphosphorylation (Fig 5C; 100.0 ±25.3 vs. 108.3±23.9 %; n = 6). Neither total PKMζ (Fig 5D; 100.0 ±3.50 vs. 98.5±6.92 %; n = 6) nor total PKCλ(Fig 5E; 100.0 ±11.0 vs. 126.3±17.4 %; n = 6) were altered in the dorsal horn when compared to the loading control, βIII tubulin at post-SNL day 10.
Figure 5.

SNL does not change p-PKMζ in the dorsal horn. (A) qPCR analysis indicates that PKCλand PKMζ, but not PKCζ, mRNA is expressed in dorsal horn. SNL did not change p-PKMζ (B) or p-PKCλ (C). SNL also did not change total PKMζ (D) or total PKCλ (E) compared to loading control (βIII tubulin) in the dorsal horn. Graphs represent mean ±SEM, n=6.
Spinal PKMζmediates SNL-induced thermal hypersensitivity
To determine whether inhibition of spinal PKMζ blocks SNL-induced evoked hypersensitivity, rats had post-SNL thermal sensory thresholds measured followed by spinal administration of ZIP or scrambled ZIP peptide. Rats were tested again 1, 4, 24, and 48 hours later. Spinal ZIP produced a time-dependent reversal of SNL-induced thermal hyperalgesia, with full reversal at the 4 hr time-point (Fig 6A, *p<0.05 vs post-surgery). Thermal hyperalgesia returned in a time-dependent manner, with pre-ZIP values observed at the 48 hr time-point (Fig 6A). Scrambled ZIP had no effect on evoked behaviors. SNL induced a decrease in response thresholds to tactile stimulation with von Frey filaments from a pre-surgery baseline of 15± 0.64 to 3.9± 0.42. Spinal ZIP produced a transient, non-significantattenuation of responses to von Frey stimulus in SNL rats. At 4 hr following administration of scrambled peptide or ZIP, response thresholds to probing the hindpaw were 4.35±0.64 and 7.00± 0.86 g respectively; these treatments had no effects in animals with sham surgeries. No effects of scrambled peptide or ZIP were seen on tactile thresholds at other time points (data not shown).
Figure 6.
Inhibition of PKMζ in spinal cord transiently blocks SNL-induced evoked hypersensitivity, but not spontaneous pain. (A) Spinal ZIP fully reversed SNL-induced thermal hypersensitivity within 4 hrs. This reversal diminished within 24 hrs and sensory thresholds returned to pre-ZIP values at the 48 hr time-point.*p<0.05 vs post-surgery. (B) Spinal administration of scrambled ZIP peptide or ZIP did not block spinal clonidine-induced CPP in SNL rats. *p<0.05 vs BL. (C) Difference scores confirmed significant increase in time spent in the spinal clonidine paired chamber in SNL rats irrespective of ZIP treatment. *p<0.05 vs sham. Graphs represent mean ± SEM, *p<0.05 vs post-surgery, n=10-12.
Spinal PKMζdoes not mediate SNL-induced spontaneous pain
To determine whether spinal PKMζmediatesSNL-induced spontaneous pain, rats underwent a single trial conditioning to spinal administration of clonidine (10 μg) administered 4 hrs post-ZIP administration (peak effect, Fig 6A). On conditioning day, rats received spinal saline paired with the morning chamber. Immediately following removal from the saline-paired chamber, rats received spinal administration of ZIP (10 nmol) or scrambled peptide (10 nmol). Spinal clonidine was paired with the opposite chamber 4 hrs following spinal administration of ZIP or scrambled ZIP peptide. Pre-conditioning baselines were equivalent across all treatment groups, so data were pooled for graphical representation.Post-conditioning time spent in the conditioning chambers were equivalent in sham-operated animals irrespective of ZIP treatment, so data were pooled for graphical representation. Spinal clonidine produced CPP in SNL rats irrespective of whether they received spinal administration of scrambled ZIPpeptide or ZIP (Fig 6B, *p<0.05 vs BL). Difference from baseline scores verified that SNL rats showed CPP, with equivalent increases in the time spent in the clonidine paired chamber observed in scrambled ZIP peptide and ZIP treated rats (Fig 6C, *p<0.05 vs sham).
Spinal NMDA receptors mediate SNL-induced thermal hypersensitivity
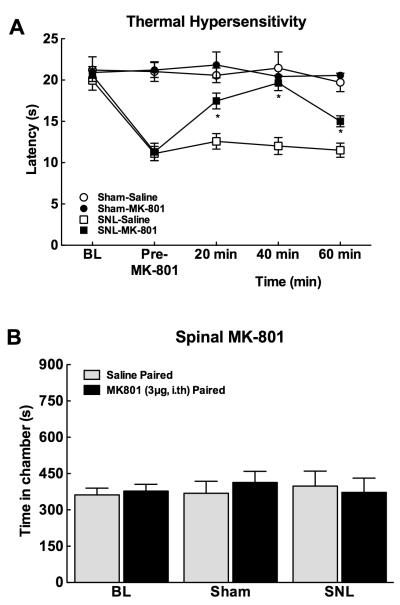
Consistent with many previous reports[31; 56], spinal administration of the NMDA receptor antagonist, MK-801, reversed SNL-induced thermal hyperalgesia. SNL produced significantly decreased response latencies to thermal stimulation (Fig 7A, p<0.05 vsBL). In accordance with our previous studies[31], spinal administration of MK-801 (3 μg) fully reversed SNL-induced thermal hypersensitivity within 20 min, with the hypersensitivity re-established at the 60 min time-point (Fig 7A, *p<0.05 vs post-surgery). As previously demonstrated[31], spinal NMDA receptor blockade with MK-801 did not alter tactile hypersensitivity (data not shown).
Figure 7.
Spinal administration of the NMDA receptor antagonist, MK-801 (3 μg) blocks evoked hypersensitivity, but not spontaneous pain. (A) Spinal administration of MK-801 reversed SNL-induced thermal hypersensitivity within 20 min, with the hypersensitivity re-established at the 60 min time-point. *p<0.05 vs post-surgery, n=6-12. (B) Spinal administration of MK-801 failed to induce conditioned place preference in sham or SNL treated rats. Graphs represent mean ± SEM, n=10-12.
Spinal NMDA receptorsdo not mediate SNL-induced spontaneous pain
To determine whether spinal administration of the NMDA receptor antagonist blocks the tonic aversive component of SNL-induced pain, rats underwent a single trial conditioning protocol in which they received spinal saline paired with a chamber in the morning followed 4 hrs later by spinal MK-801 (3 μg) paired with the opposite chamber. Pre-conditioning time spent in the conditioning chambers did not differ between sham and SNL rats, therefore values were pooled for graphical representation (Fig 7B). Spinal MK-801 failed to induce conditioned place preference in sham or the SNL treated rats.
Discussion
We have explored whether manipulations thought to inhibit amplification can modulate either evoked hypersensitivity or SNL-induced aversiveness. Our data show that PKMζwithin the rACCmaintains nerve injury-induced aversiveness, but not evoked hypersensitivity.In contrast, spinal PKMζcontributes to evoked hypersensitivity, but not spontaneous pain.These data suggest differential roles of amplification mechanisms at different sites within the neuraxis.
PKMζ is critical to the maintenance of long-term potentiation (LTP) [43; 44]via persistent enhancement ofsynaptic AMPA receptor trafficking [27-29; 47]. Phosphorylation of PKMζ maintains persistentkinase activity and is proposed to maintain LTP [18]. Here, increasedp-PKMζ, but not PKCλor p-PKCλ, was observed within the rACCat 10 days post-injury. These data are consistent with a previous report of increased p-PKMζand a decrease in synaptic efficacy induced by ZIP in the ACC in nerve-injured mice[27]. PKMζmay promote synaptic strength through maintaining AMPA receptors within the membrane through trafficking of the GluR2 subunit[35; 43]. Consistent with this, the PKMζ inhibitor, ZIP, has been shown to block the amplitude of EPSCs in ACC neurons of nerve injured mice through reduction of active AMPA receptors[27].
The rostral ACC (rACC) has been implicated in integrating, in part, the aversive component of pain [16; 17; 23; 27; 40].We found that rACCZIP fully blocked the CPP induced by RVM lidocaine treatment in SNLrats when tested 24 hrs after a single administration.This result suggests that the aversive state reflecting nerve injury-induced spontaneous pain requires rACCPKMζ activity.The present studies cannot address the precise mechanisms by which PKMζ might contribute to nerve injury induced aversiveness. Based on previous understanding of the role of PKMζ in sustaining LTP, it may be reasonable to speculate that this mechanism may also apply to some of the complex circuits that could underlie the aversive state. rACC MK801, however, failed to elicit place preference in SNL rats.Aprevious study demonstrated that this dose of MK-801 blocked axotomy-induced autotomy behaviors [30]. How this finding relates to our present results is not clear in light of differences in the time-frame of evaluation and outcome measures. If LTP-like processes in the rACC are important in maintaining aversiveness, then it is notable that blockade of NMDA receptors does not reverse established LTP[42]. In contrast, ZIP has been demonstrated to reverse established LTP [40, 42].Notably, MK-801 has been repeatedly demonstrated to modulate states of central sensitization[24].
Importantly, the effect of ZIP on nerve injury-induced aversiveness ofpain was not permanent.The CPP produced by RVM lidocaine was partially re-established 3 days after ZIP and fully recovered after 7 days. Previous studies on mechanisms driving long-term memory maintenance have demonstrated that blockade of PKMζeffectively reversed several forms of long-term memory, an effect that lasts weeks to months [43]. Notably, after ZIP washout, new memories can be made and maintained with retraining [43].The transient reversal of aversiveness elicited by rACC ZIP raises the possibility that peripheral nerve injury-induced afferent drive [9; 15; 33; 60]mightre-establish LTP in the rACC.
AsPKMζ is implicated in memory maintenance, it is possible that administration of ZIP prevented acquisition of reinforcement to reward in a manner not specific to the relief of pain.The rACC is known to mediate the emotional-affective component of pain whereas the caudal ACC has been proposed to mediate cognitive tasks including attention[7].The present studies are dependent on reward induced by pain relief, so we determined whether inhibition of PKMζ within the rACCprevents the acquisition of CPP to systemic cocaine. Neither rACCZIP,nor scrambled ZIP, given 24 hour prior to conditioning blocked cocaine-induced CPP. Thus, inhibition of PKMζ in the rACC did not interfere with reinforcement. Our previous findings show that rACC lesions do not reduce acquisition of CPP to positive reinforcement[40; 55] or CPA to aversive, nonpainful reinforcement[17].Thus, PKMζwithin the rACC is associated with aversive aspects of pain.
Consistent with clinical and preclinical reports, we found that disruption of rACC function fails to alter evoked sensory thresholds. Previous studies demonstrated that lesion of the rACC, or pharmacological treatments within this region, failed to alternocifensive behaviors to formalin [16; 17] and did not alter nerve-injury induced thermal and mechanical hypersensitivity [23; 40]. Some studies, however, have reported disruption of evoked behaviors by rACCmanipulations[62].Recently, bilateral ACC ZIP reduced allodynia within 2 hrs of administration in nerve injured mice with thresholds returning to pre-ZIP levels within 24 hours [27]. Consistent with our previous findings and other reports where rACC lesion did not alter evoked thresholds in nerve injured animals [23; 40] and with human studies showing a change in affect but not sensory features of pain [10; 41], our findings show that neither ZIP nor MK-801 altered SNL-induced thermal or mechanical hypersensitivity when evaluated at 4 or 24 hr or 3 days following administration. In contrast, aversiveness reflecting spontaneous pain was fully blocked at 24 hr following rACCPKMζ inhibition and this effect remained partially present at 3 days post ZIP.
The role of amplified signaling within the spinal dorsal horn has been well established in nerve injury-induced hypersensitivity to externally applied stimuli, such as noxious heat[24; 42; 45] or tactile stimuli [22; 32]. Spinal LTP is expressed at synapses between nociceptive primary afferents and NK-1 receptor expressing projection neurons [42] that relay nociceptive information to the brain and are necessary for development of both evoked and spontaneous pain [20]. Moreover, pharmacological studies have demonstrated that drugs that interfere with amplification mechanisms such as NMDA receptor antagonists, also effectively block injury-induced hyperalgesia [42]. Our recent studies have suggested that PKMζmaintains a state of spinal sensitization for long periods of time following an initial insult[2]possibly reflecting spinal late-LTP as observed in preclinical studies [45; 46] and suggested in humans [39].Recent studies have demonstrated differential effects of PKMζ in inflammation-induced hypersensitivity as opposed to nerve injury induced by chronic constriction injury (CCI)[22; 32]. Whereas spinal administration of the PKMζ inhibitor, ZIP, reversed tactile hypersensitivity following injection of formalin, capsaicin, or CFA, ZIP failed to reverse CCI-induced tactile hypersensitivity [22; 32]. Consistent with these observations, we found that spinal ZIP did not fully reverse SNL-induced tactile hypersensitivity, producing a slight, transient attenuation of the tactile hypersensitivity. We further expanded analysis of the role of spinal PKMζ by determining its role in nerve-injury induced ongoing (spontaneous) pain as well as thermal hypersensitivity. Spinal blockade of either NMDA receptors or PKMζ activity fully reversed thermal hyperalgesia in a transient manner. However, there were no detectable changes in levels of spinal PKMζ phosphorylation and expression, consistent with previous reports in mice[27]following nerve injury.It should be noted that neither spinal ZIP nor MK-801 raised thermal thresholds above pre-surgery baselines indicating that these treatments did not induce antinociception.
The reversal of enhanced responses to evoked thermal stimuli by either spinal ZIP or MK-801 suggests likely normalization of central processing in injured animals. It is not known how normalization might result from these mechanistically divergent agents. However, spinal blockade of NMDA receptors or inhibition of PKMζdid not affect nerve-injury induced spontaneous pain.These data suggest that although spinal PKMζor NMDA signaling may play a role in maintaining a sensitized state within the spinal cord, these amplification mechanisms do not appear to contribute to spontaneous pain elicited by experimental peripheral nerve-injury.Whether mechanisms of spinal amplification may contribute to modulation of injury induced afferent drive to higher centers that may underlie the aversive aspects of other types of injury, or under different conditions,is not known.
In summary, our data indicate that nerve-injury induced evoked hypersensitivity and spontaneous pain can be differentiated by PKMζdependent and independent mechanisms of amplification at multiple levels of the neuraxis.Thus, the aversive aspects reflecting nerve injury induced spontaneous pain, but not evoked hypersensitivity, are dependent upon PKMζwithin the rACC but not at the spinal level. In contrast, nerve injury induced evoked hypersensitivity but not spontaneous pain is dependent upon amplification mechanisms that may include PKMζand LTP-like mechanisms in the spinal cord as well as signaling via NMDA receptors.It should be noted that a relatively brief period ofinjury-induced afferent drive can establish a state of central sensitization within the spinal cord that can includePKMζ-dependent persistent sensitization[2].In animals with nerve injury, spinal inhibition of NMDA receptors or PKMζresults, however, in only a transient reversal of evoked hypersensitivity, likely reflecting re-establishment of PKMζ-dependent and/or independent plasticity.Similarly, inhibition of PKMζ activity within the rACC does not lead to permanent reversal of nerve injury induced aversivenessimplicating continuous afferent drive in possibly resettingrACC PKMζ-dependent processes. Finally, our results suggest that although spinal sensitization may be an important feature of enhanced evoked responses (i.e., hyperalgesia), whether the presence or absence of this state influences other dimensions of pain that may be of clinical significance is not clear and requires further study.
Summary.
PKMζ-dependent amplification contributes to nerve-injury induced aversiveness within the rACC whereas both PKMζ-dependent and independent mechanisms contribute to amplification of evoked responses but not spontaneous pain in the spinal cord.
Acknowledgments
Supported by NS066958 (FP),NS065926 (TJP) and CA149258 (SG). EKM was supported by CA009213.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest to declare.
References
- [1].Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87(2):81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Asiedu M, Tillu D, Melemedjian O, Shy A, Bodell B, Sanoja R, Porreca F, Price T. Spinal PKM underlies the maintenance mechanism of persistent nociceptive sensitization. Journal of Neuroscience. 2011;31(18):6646–6653. doi: 10.1523/JNEUROSCI.6286-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Backonja MM, Stacey B. Neuropathic pain symptoms relative to overall pain rating. J Pain. 2004;5(9):491–497. doi: 10.1016/j.jpain.2004.09.001. [DOI] [PubMed] [Google Scholar]
- [4].Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9(8):807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- [5].Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, Cunin G, Fermanian J, Ginies P, Grun-Overdyking A, Jafari-Schluep H, Lantéri-Minet M, Laurent B, Mick G, Serrie A, Valade D, Vicaut E. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4) Pain. 2005;114(1-2):29–36. doi: 10.1016/j.pain.2004.12.010. [DOI] [PubMed] [Google Scholar]
- [6].Burgess SE, Gardell LR, Ossipov MH, Malan TP, Jr., Vanderah TW, Lai J, Porreca F. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci. 2002;22(12):5129–5136. doi: 10.1523/JNEUROSCI.22-12-05129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- [8].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- [9].Chen Y, Devor M. Ectopic mechanosensitivity in injured sensory axons arises from the site of spontaneous electrogenesis. Eur J Pain. 1998;2(2):165–178. doi: 10.1016/s1090-3801(98)90009-x. [DOI] [PubMed] [Google Scholar]
- [10].Craig AD, Reiman EM, Evans A, Bushnell MC. Functional imaging of an illusion of pain. Nature. 1996;384(6606):258–260. doi: 10.1038/384258a0. [DOI] [PubMed] [Google Scholar]
- [11].Descalzi G, Kim S, Zhuo M. Presynaptic and postsynaptic cortical mechanisms of chronic pain. Mol Neurobiol. 2009;40(3):253–259. doi: 10.1007/s12035-009-8085-9. [DOI] [PubMed] [Google Scholar]
- [12].Devor M. Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp Brain Res. 2009;196(1):115–128. doi: 10.1007/s00221-009-1724-6. [DOI] [PubMed] [Google Scholar]
- [13].Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- [14].Fields HL. Pain: an unpleasant topic. Pain. 1999;(Suppl 6):S61–69. doi: 10.1016/S0304-3959(99)00139-6. [DOI] [PubMed] [Google Scholar]
- [15].Govrin-Lippmann R, Devor M. Ongoing activity in severed nerves: source and variation with time. Brain Res. 1978;159(2):406–410. doi: 10.1016/0006-8993(78)90548-6. [DOI] [PubMed] [Google Scholar]
- [16].Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat Neurosci. 2004;7(4):398–403. doi: 10.1038/nn1207. [DOI] [PubMed] [Google Scholar]
- [17].Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2001;98(14):8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kelly MT, Crary JF, Sacktor TC. Regulation of Protein Kinase M Synthesis by Multiple Kinases in Long-Term Potentiation. The Journal of Neuroscience. 2007;27(13):3439–3444. doi: 10.1523/JNEUROSCI.5612-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50(3):355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- [20].King T, Qu C, Okun A, Mercado R, Ren J, Brion T, Lai J, Porreca F. Contribution of afferent pathways to nerve injury-induced spontaneous pain and evoked hypersensitivity. Pain. 2011;152(9):1997–2005. doi: 10.1016/j.pain.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12(11):1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Laferriere A, Pitcher MH, Haldane A, Huang Y, Cornea V, Kumar N, Sacktor TC, Cervero F, Coderre TJ. PKMzeta is essential for spinal plasticity underlying the maintenance of persistent pain. Mol Pain. 2011;7(1):99. doi: 10.1186/1744-8069-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].LaGraize SC, Labuda CJ, Rutledge MA, Jackson RL, Fuchs PN. Differential effect of anterior cingulate cortex lesion on mechanical hypersensitivity and escape/avoidance behavior in an animal model of neuropathic pain. Exp Neurol. 2004;188(1):139–148. doi: 10.1016/j.expneurol.2004.04.003. [DOI] [PubMed] [Google Scholar]
- [24].Latremoliere A, Woolf CJ. Central Sensitization: A Generator of Pain Hypersensitivity by Central Neural Plasticity. The Journal of Pain. 2009;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Latremoliere A, Woolf CJ. Synaptic Plasticity and Central Sensitization: Author Reply. The Journal of Pain. 2010;11(8):801–803. doi: 10.1016/j.jpain.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lee Y-W, Chaplan SR, Yaksh TL. Systemic and supraspinal, but not spinal, opiates suppress allodynia in a rat neuropathic pain model. Neuroscience Letters. 1995;199(2):111–114. doi: 10.1016/0304-3940(95)12034-2. [DOI] [PubMed] [Google Scholar]
- [27].Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park SW, Shim J, Lee K, Collingridge GL, Kaang BK, Zhuo M. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science. 2010;330(6009):1400–1404. doi: 10.1126/science.1191792. [DOI] [PubMed] [Google Scholar]
- [28].Ling DS, Benardo LS, Sacktor TC. Protein kinase Mzeta enhances excitatory synaptic transmission by increasing the number of active postsynaptic AMPA receptors. Hippocampus. 2006;16(5):443–452. doi: 10.1002/hipo.20171. [DOI] [PubMed] [Google Scholar]
- [29].Ling DS, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC. Protein kinase Mzeta is necessary and sufficient for LTP maintenance. Nat Neurosci. 2002;5(4):295–296. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- [30].Lopez-Avila A, Coffeen U, Ortega-Legaspi JM, del Angel R, Pellicer F. Dopamine and NMDA systems modulate long-term nociception in the rat anterior cingulate cortex. Pain. 2004;111(1-2):136–143. doi: 10.1016/j.pain.2004.06.010. [DOI] [PubMed] [Google Scholar]
- [31].Malan TP, Ossipov MH, Gardell LR, Ibrahim M, Bian D, Lai J, Porreca F. Extraterritorial neuropathic pain correlates with multisegmental elevation of spinal dynorphin in nerve-injured rats. Pain. 2000;86(1-2):185–194. doi: 10.1016/s0304-3959(00)00243-8. [DOI] [PubMed] [Google Scholar]
- [32].Marchand F, D’Mello R, Yip P, Calvo M, Muller E, Pezet S, Dickenson A, McMahon S. Specific involvement of atypical PKCzeta/PKMzeta in spinal persistent nociceptive processing following peripheral inflammation in rat. Mol Pain. 2011;7(1):86. doi: 10.1186/1744-8069-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Matzner O, Devor M. Hyperexcitability at sites of nerve injury depends on voltage-sensitive Na+ channels. J Neurophysiol. 1994;72(1):349–359. doi: 10.1152/jn.1994.72.1.349. [DOI] [PubMed] [Google Scholar]
- [34].Melzack R, Casey KL. Sensory, motivational and central control determinants of chronic pain: A new conceptual model. In: Kenshalo DR, editor. The Skin Senses. Springfield. Thomas; Illinois: C.C: 1968. pp. 423–439. [Google Scholar]
- [35].Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K. PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci. 2010;13(5):630–634. doi: 10.1038/nn.2531. [DOI] [PubMed] [Google Scholar]
- [36].Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest. 2010;120(11):3779–3787. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313(5790):1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- [38].Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1986. [Google Scholar]
- [39].Pfau DB, Klein T, Putzer D, Pogatzki-Zahn EM, Treede RD, Magerl W. Analysis of hyperalgesia time courses in humans after painful electrical high-frequency stimulation identifies a possible transition from early to late LTP-like pain plasticity. Pain. 2011 doi: 10.1016/j.pain.2011.02.037. [DOI] [PubMed] [Google Scholar]
- [40].Qu C, King T, Okun A, Lai J, Fields HL, Porreca F. Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain. 2011;152(7):1641–1648. doi: 10.1016/j.pain.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277(5328):968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- [42].Ruscheweyh R, Wilder-Smith O, Drdla R, Liu XG, Sandkuhler J. Long-term potentiation in spinal nociceptive pathways as a novel target for pain therapy. Mol Pain. 2011;7:20. doi: 10.1186/1744-8069-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sacktor TC. How does PKMzeta maintain long-term memory? Nat Rev Neurosci. 2011;12(1):9–15. doi: 10.1038/nrn2949. [DOI] [PubMed] [Google Scholar]
- [44].Sacktor TC, Osten P, Valsamis H, Jiang X, Naik MU, Sublette E. Persistent activation of the zeta isoform of protein kinase C in the maintenance of long-term potentiation. Proc Natl Acad Sci U S A. 1993;90(18):8342–8346. doi: 10.1073/pnas.90.18.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sandkuhler J. Understanding LTP in pain pathways. Mol Pain. 2007;3:9. doi: 10.1186/1744-8069-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sandkuhler J. Central sensitization versus synaptic long-term potentiation (LTP): a critical comment. The journal of pain : official journal of the American Pain Society. 2010;11(8):798–800. doi: 10.1016/j.jpain.2010.05.002. [DOI] [PubMed] [Google Scholar]
- [47].Serrano P, Yao Y, Sacktor TC. Persistent phosphorylation by protein kinase Mzeta maintains late-phase long-term potentiation. J Neurosci. 2005;25(8):1979–1984. doi: 10.1523/JNEUROSCI.5132-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science. 2007;317(5840):951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- [49].Suzuki A, Akimoto K, Ohno S. Protein kinase C lambda/iota (PKClambda/iota): a PKC isotype essential for the development of multicellular organisms. J Biochem. 2003;133(1):9–16. doi: 10.1093/jb/mvg018. [DOI] [PubMed] [Google Scholar]
- [50].Suzuki R, Dickenson A. Spinal and supraspinal contributions to central sensitization in peripheral neuropathy. Neurosignals. 2005;14(4):175–181. doi: 10.1159/000087656. [DOI] [PubMed] [Google Scholar]
- [51].Suzuki R, Matthews EA, Dickenson AH. Comparison of the effects of MK-801, ketamine and memantine on responses of spinal dorsal horn neurones in a rat model of mononeuropathy. Pain. 2001;91(1-2):101–109. doi: 10.1016/s0304-3959(00)00423-1. [DOI] [PubMed] [Google Scholar]
- [52].Toyoda H, Zhao MG, Zhuo M. Enhanced quantal release of excitatory transmitter in anterior cingulate cortex of adult mice with chronic pain. Mol Pain. 2009;5:4. doi: 10.1186/1744-8069-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tracey I, Bushnell MC. How neuroimaging studies have challenged us to rethink: is chronic pain a disease? J Pain. 2009;10(11):1113–1120. doi: 10.1016/j.jpain.2009.09.001. [DOI] [PubMed] [Google Scholar]
- [54].Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55(3):377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- [55].Tzschentke TM, Schmidt WJ. Functional heterogeneity of the rat medial prefrontal cortex: effects of discrete subarea-specific lesions on drug-induced conditioned place preference and behavioural sensitization. Eur J Neurosci. 1999;11(11):4099–4109. doi: 10.1046/j.1460-9568.1999.00834.x. [DOI] [PubMed] [Google Scholar]
- [56].Wegert S, Ossipov MH, Nichols ML, Bian D, Vanderah TW, Malan TP, Jr., Porreca F. Differential activities of intrathecal MK-801 or morphine to alter responses to thermal and mechanical stimuli in normal or nerve-injured rats. Pain. 1997;71(1):57–64. doi: 10.1016/s0304-3959(97)03337-x. [DOI] [PubMed] [Google Scholar]
- [57].Wei F, Zhuo M. Potentiation of sensory responses in the anterior cingulate cortex following digit amputation in the anaesthetised rat. J Physiol. 2001;532(Pt 3):823–833. doi: 10.1111/j.1469-7793.2001.0823e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 2011;152(3, Supplement 1):S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Woolf CJ, Salter MW. Neuronal Plasticity: Increasing the Gain in Pain. Science. 2000;288(5472):1765–1768. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- [60].Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, Meyer RA. Early Onset of Spontaneous Activity in Uninjured C-Fiber Nociceptors after Injury to Neighboring Nerve Fibers. J Neurosci. 2001;21(8; RC140:):1–5. doi: 10.1523/JNEUROSCI.21-08-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Xie JY, Herman DS, Stiller CO, Gardell LR, Ossipov MH, Lai J, Porreca F, Vanderah TW. Cholecystokinin in the rostral ventromedial medulla mediates opioid-induced hyperalgesia and antinociceptive tolerance. J Neurosci. 2005;25(2):409–416. doi: 10.1523/JNEUROSCI.4054-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Xu H, Wu LJ, Wang H, Zhang X, Vadakkan KI, Kim SS, Steenland HW, Zhuo M. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci. 2008;28(29):7445–7453. doi: 10.1523/JNEUROSCI.1812-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]