Abstract
Purpose
To evaluate the safety and possible efficacy of subconjunctival sirolimus for the treatment of chronic active anterior uveitis
Design
Prospective, non-randomized, open-label clinical trial.
Methods
This single-center pilot trial enrolled 5 patients with chronic active anterior uveitis. The study drug was administered as single subconjunctival injection of 30μL (1,320μg) sirolimus in the study eye at the baseline visit. Study visits were performed at baseline, 2 weeks, 4 weeks and monthly until 4 months, and included a complete ophthalmic exam, review of systems, adverse event assessment at each visit, physical exam and ancillary ophthalmic testing at some visits. The primary outcome measure was a 2-step reduction in the anterior chamber inflammation within 4 weeks of injection of the study drug.
Results
There were 3 females and 2 males; 4 patients had idiopathic anterior uveitis and one had psoriatic arthritis-associated anterior uveitis. Three of the five patients met the primary outcome criteria by showing at least a 2-step decrease in inflammation within 4 weeks, 2 patients showed a 1-step decrease in inflammation within the same time frame. No recurrence was encountered during a 4 month follow-up. There were no serious adverse events.
Conclusions
Subconjunctival sirolimus appears to be well tolerated in this pilot trial and shows promise as a treatment for active inflammation in patients with chronic anterior uveitis. Larger studies are needed to assess its usefulness in uveitis.
Introduction
Uveitis is an important cause of visual loss both in adults and children. Its prevalence and incidence appears to be much higher than initially thought, with anterior uveitis being the most common type.1,2,3 It is believed that over 100,000 American citizens currently require the use of systemic corticosteroids or other immunosuppressive agents as treatment for ocular inflammation. Management and prevention of iatrogenic complications of immunosuppressive therapy accounts for most of the medical resources devoted to these individuals.2
Anterior uveitis is the most common form of uveitis, and it can be chronic and recurrent in up to two-thirds of cases. The recurrent episodes of anterior uveitis can ultimately lead to vision loss secondary to cataract, band keratopathy, glaucoma and macular edema.4,5,6 When topical corticosteroids are insufficient, recurrences are often managed with periocular corticosteroid injections which can cause intraocular pressure (IOP) elevation or cataracts.7 More severe cases require systemic corticosteroids or immunosuppressive medications which can cause significant adverse effects, and many patients continue to experience recurrent inflammation despite systemic therapy.4
Both preclinical and clinical evidence indicate that uveitis is a predominantly T-cell mediated immune disease. Drugs targeting T-cells, such as cyclosporine, have been shown to be effective.4,8,9 Sirolimus, an mTOR (mammalian target of rapamycin) inhibitor, exerts its effect by a mechanism that is distinct from other immunusuppressives. It suppresses cytokine-driven T-cell proliferation and inhibits the production, signaling and activity of many growth factors relevant to uveitis.10 Sirolimus tablets and oral solution are currently approved by the Food and Drug Administration (FDA) for the prevention of transplant rejection.11 It has been used off-label in one small uncontrolled case series with the suggestion of benefit for uveitis.12
The purpose of this non-randomized, open-label, prospective pilot study was to assess the safety and possible efficacy of a new subconjunctivally-administered sirolimus formulation in the treatment of patients with chronic active anterior uveitis.
Methods
This was a Phase I, non-randomized, prospective single-center study that evaluated subconjunctival sirolimus as a treatment for chronic active anterior uveitis. All participants received a single 30 μL (1,320 μg) subconjunctival sirolimus injection in the study eye at baseline and were followed for 16 weeks following the study drug injection. The study duration was 16 weeks and included six study appointments including the baseline visit followed by visits at weeks 2, 4, 8, 12 and 16.
Inclusion and Exclusion Criteria
Inclusion criteria included age ≥18 years and a diagnosis of noninfectious chronic active anterior uveitis. The study eye was required to have ≥1+ anterior chamber (AC) cells despite treatment with topical corticosteroids. At the time of enrollment, the study eye was required to have been treated with systemic and/or topical anti-inflammatory medications (3 times daily or more frequent doses of topical corticosteroids) unless local corticosteroid treatment was contraindicated because of significant IOP elevation in the past (corticosteroid responder). Participants were required to have visual acuity (VA) of 20/400 or better in the study eye and were not anticipated to need ocular surgery during the study. If both eyes met the inclusion criteria, the eye with worse inflammation (higher grade of AC cells) was chosen as the study eye.
Important exclusion criteria included active systemic or joint inflammation requiring immediate addition of or increase in systemic anti-inflammatory medications, active anterior uveitis in the fellow eye requiring more than topical treatment and significant active infection or history of cancer diagnosed within the past 5 years. Participants who used latanoprost within 2 weeks prior to study enrollment, and those with a media opacity that precluded assessment of AC inflammation were excluded. Participants who were pregnant or lactating, or those who refused to use contraception during the study, were also not eligible.
Ophthalmic and medical evaluations
At the baseline and close-out visits patients underwent an ophthalmic examination that included grading of AC cells/flare and vitreous cells/haze according to Standardization of Uveitis Nomenclature (SUN) criteria,13 manifest refraction, review of systems, physical examination, fundus photography, optical coherence tomography (OCT) and intravenous fluorescein angiography (FA). Ophthalmic exams (including visual acuity assessment using the standardized Early Treatment Diabetic Retinopathy Study (ETDRS) refraction protocol at 4 meters, inflammation grading, and dilated fundus exams), review of systems, adverse event assessment, concomitant medication assessment, complete blood count with differential (CBC/diff), chemistry panel (chem20) and serum sirolimus levels were done at each visit.
Primary and Secondary Outcome Assessment
The primary efficacy outcome was a two-step reduction in AC inflammation out of a scale of +0 to +4 (where 0.5+ is recognized as an ordinal step between 1+ and 0) according to SUN criteria within 4 weeks from the time of study drug administration in the absence of an increase in concomitant treatments (topical or systemic). AC cell grading, as the primary outcome measure, was assessed by two investigators independently. Any difference in observation was adjudicated by a third examiner. Secondary efficacy outcomes included changes in VA, FA or retinal thickening as measured by OCT.
Study Drug Administration
All patients received a single injection of subconjunctival sirolimus (30 uL, 1320 ug) on the day of study enrollment. Subconjunctival sirolimus injections were done according to the manufacturer's guidelines. Briefly, the study drug was thawed immediately prior to use, drawn into the syringe using sterile technique [0.3 cc insulin Becton Dickinson (BD) syringe] and injected within two hours. Following topical anesthesia, the needle was engaged approximately 5 mm away from the intended location in the inferior bulbar conjunctiva. Following slow advancement of the needle, the drug was injected into the subconjunctival space. Immediately following needle withdrawal, a sterile, cotton-tipped applicator was placed over the conjunctival entry site for approximately 1 minute. All patients received 0.3% ofloxacin drops immediately following the procedure and three times daily for two days.
Topical treatments and systemic immunosuppressives, including corticosteroids, were maintained at the baseline doses or reduced, but not increased, for the study duration. The non-study eye, if active, was treated with topical corticosteroids, and the participant was instructed to apply punctal pressure for two minutes in an attempt to prevent systemic absorption of topical corticosteroids.
Safety assessment and adverse event reporting
Safety assessments were made routinely during the study, with a review of the previous visit interval performed at each scheduled visit. Each participant was contacted one day after the injection for safety, and participants were encouraged to report any apparent adverse events between scheduled visits. Serum sirolimus levels were checked at each visit.
Results
Five patients with chronic active anterior uveitis were enrolled in this pilot trial. There were 3 females and 2 males, the average age was 46 (range: 36–52). Four patients had idiopathic anterior uveitis, and one had psoriatic arthritis-associated anterior uveitis. The median duration of uveitis was 27 months, and the median duration of active inflammation despite topical therapy was 8 weeks immediately prior to enrollment. The severity of inflammation ranged from 1+ to 3+ AC cells despite topical corticosteroids three times daily or more (prednisolone acetate 1% in 4 patients and difluprednate 0.005% in one patient). One patient was using systemic methotrexate in addition to topical therapy. Four of the 5 patients were known corticosteroid responders or had the diagnosis of glaucoma-suspect in the study eye which limited a higher frequency of corticosteroids (patients 1,2,4,5). Three of these patients were on topical IOP lowering medications in the study eye at the baseline visit (Table).
Table 1.
Sirolimus for the treatment of chronic active anterior uveitis: patient and study eye characteristics
| Patient | Age/ gender/ race |
Duration of uveitis (months ) |
Etiology of uveitis |
Glaucoma status |
Medications in the study eyea | AC cells | Vision | ocular AEsc |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| baseline | 4 wks | 16 wks | baseline | 4 wks | 8 wks | 16 wks | baseline | 4 wks | 16 wks | ||||||
| 1 | 54/F/W | 258 | idiopathic | Glaucoma | Alphagan BID Cosopt BID PF QID |
Alphagan TID Cosopt TID PF QID |
Alphagan BID Cosopt BID PF TID |
+1 | +0.5 | 0 | +0.5 | 20/25 | 20/32 | 20/20 | Chemosi s, redness, ocular irritation |
| 2 | 51/M/A | 13 | Psoriatic arthritis | Steroid responder Glaucoma suspect |
PF OD QID MTX 15mg |
PF TID MTX 15mg |
Timolol BIDb MTX 15mg |
+2 | 0 | 0 | 0 | 20/50 | 20/20 | 20/16 | - |
| 3 | 43/M/W | 27 | idiopathic | None | PF TID | PF BID | PF BID | +1 | +0.5 | +0.5 | 0 | 20/16 | 20/16 | 20/16 | Mild ocular irritation |
| 4 | 36/F/AA | 182 | idiopathic | Steroid responder | Combigan BID Durezol TID |
Cosopt BID Durezol BID |
Combigan BID |
+3 | 0 | 0 | +0.5 | 20/25 | 20/25 | 20/20 | Mild ocular irritation |
| 5 | 52/F/W | 3 | idiopathic | Steroid responder | Timolol BID PF x6/day |
Timolol BID PF QID |
Timolol BID PF BID |
+2 | +0.5 | 0 | 0 | 20/25 | n/a | 20/20 | Mild foreign body sensation |
Primary outcome visit is performed at 4 weeks, study duration is 16 weeks
Medications listed are those that are used for uveitis or uveitis related complications
This participant was able to stop timolol at the end of the study
Ocular adverse events that are considered to be related to the study drug are listed
(PF = Pred Forte or prednisolone actetate 1%, MTX = methotrexate, A=Asian, W=white, AA=AfricanAmerican)
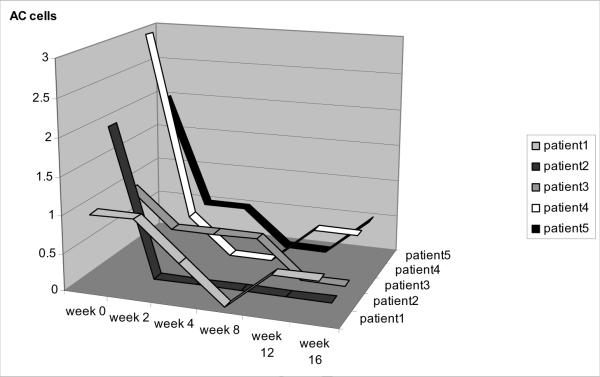
All patients completed the study and showed a decrease in the AC cell grade following injection (Figure). Three of the 5 patients met the primary efficacy outcome measure by achieving a ≥2-step reduction in inflammation within 4 weeks, two showed a one-step decrease within the same time frame. All patients were able to decrease (3 patients) or discontinue (2 patients) their topical corticosteroids. None of the study eyes showed a recurrence in inflammation which was defined as ≥2-step increase in AC cell grading compared to the lowest grading achieved. The fellow eyes (non-study eyes) in 2 patients developed mild recurrences (0.5 and 1+ AC cells) at weeks 4 (patient #3) and 12 (patient #5). At the close-out visit, visual acuity had improved by 1 to 5 lines in 4 patients and remained same in one patient (table 1). There were no patients with cystoid macular edema (CME) detected clinically or on OCT at baseline, and no change was observed during the study. One patient who had angiographic evidence of minimal perifoveal leakage at baseline showed improvement at the following visits which was likely independent of the intervention. Of the four patients with a known history of corticosteroid response or glaucoma, two had >10mmHg IOP elevations (patient #1 at 2 weeks and patient #2 at 8 weeks) while on corticosteroid drops. Elevated IOP resolved with IOP lowering drops.
Figure.
Anterior chamber (AC) inflammation in the study eyes. Change in inflammation over 16 weeks following subconjunctival sirolimus injection in the study eyes of 5 patients with chronic active anterior uveitis is shown.
Serum sirolimus levels were undetectable at all times. All adverse events were mild or moderate, and most were deemed unrelated to the study medication. There were no serious adverse events. All patients tolerated the injection well. As expected, three patients (patients #3,4,5) reported mild ocular irritation or foreign body sensation following the injection which resolved within several days. One patient (#1) developed significant chemosis, redness and ocular irritation one day after the injection which resolved within a week as the subconjunctival sirolimus depot was depleted. Other patients did not experience a similar reaction. Regularly performed labs including CBC, chemistry, and urinalysis did not show any significant changes that could be attributed to the study drug.
Discussion
Chronic anterior uveitis may require intensive topical corticosteroids, periocular injections or systemic immunosuppression, but all of these therapies carry significant side effects. In adult anterior uveitis, local treatments such as periocular injections often are used in the absence of associated systemic disease, but they can be associated with corticosteroid-induced glaucoma and cataract. In addition, patients often have difficulty complying with corticosteroid drop regimens that exceed three times a day.
The efficacy of orally administered sirolimus (Rapamune®) was previously reported in a case series of eight patients with refractory uveitis. Systemic sirolimus was moderately successful for controlling inflammation and improving macular edema, but intolerable side effects limited the use of high doses. Patients who were able to tolerate systemic sirolimus were able to decrease their systemic corticosteroid dependence and eventually taper the sirolimus dose.12 Systemic sirolimus can have anti-angiogenic properties14 and has been successfully used in a case of uveitic choroidal neovascularization (CNV).15
In this pilot trial, a single subconjunctival sirolimus injection was generally well tolerated and reduced active inflammation in all patients, with 3 of the 5 meeting the primary outcome of a two-step reduction in anterior chamber cell grade. Interestingly, patients who achieved the primary outcome were those with a higher grade of inflammation at baseline (grade 2+ to 3+), whereas those who could not achieve the primary outcome by 4 weeks had milder baseline AC inflammation (grade 1+).
The most common side effect of subconjunctival sirolimus was ocular irritation and redness which was mild in most cases with the exception of one patient who experienced significant conjunctival edema, redness and ocular irritation. The irritation is most likely attributable to the vehicle components, although it is possible that high doses of sirolimus can exert some local toxicity and/or exacerbate the effects of the vehicle.10
Clinical data indicate that the occurrence and the severity of adverse events correlate with blood levels of sirolimus.12 Therefore, local administration of sirolimus offers the advantage of sparing patients from systemic side effects while providing a local effect. In pharmacokinetic studies, the whole blood concentrations of sirolimus were shown to be very low following subconjunctival injections. Our patients had undetectable serum levels at all times probably due to the small quantities of sirolimus administered (microgram doses), as well as slow diffusion from the sirolimus depot.
Subconjunctival sirolimus is estimated to be effective for approximately two months. Therefore, it may alleviate the issue of patient compliance with frequent eye drops. Since our follow up extended beyond the estimated duration of a single injection, we anticipated that some patients would develop recurrent inflammation in the second half of the study. The observed absence of recurrence potentially could be explained by regression to the mean or other variations in natural history, longer than expected duration of the sirolimus depot, remittive effects of the treatment, or other factors. This pilot trial is not sufficient to make definitive conclusions about efficacy since it was designed to assess the safety of subconjunctival sirolimus for the treatment of active uveitis.
In summary, subconjunctival sirolimus appears safe in this small trial and may represent a novel therapeutic option in patients with chronic active anterior uveitis. It shows promise in reducing inflammation in patients with chronic anterior uveitis and if validated by larger studies, can offer an alternative for patients who are known corticosteroid responders for whom topical or local corticosteroids may no longer be a safe option. Larger studies are currently ongoing and should provide more evidence regarding both safety and efficacy in uveitis.
Acknowledgements
a. Funding/Support: The study is supported by the National Eye Institute (NEI) Intramural Research Program.
e. Other Acknowledgments: none
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
b. Financial Disclosure: None of the authors have financial interest. The study drug was provided in-kind by Santen, Inc
c. Contributions of Authors: design and conduct of the study (HNS); collection (HNS, TAL, DM, WS), management, analysis, and interpretation of the data (HNS, RBN); and preparation, review, or approval of the manuscript (HNS, TAL, DM, WS, RBN).
d. Statement about Conformity with Author Information: The study protocol was reviewed and approved by the Institutional Review Board of the National Institutes of Health, a HIPAA compliant institution, and all procedures conformed to the tenets of the Declaration of Helsinki (Clinical Trials registration: NCT00876434, NEI protocol ID: 09-EI-0116).
References
- 1.Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14(5–6):303–8. doi: 10.1007/BF00163549. [DOI] [PubMed] [Google Scholar]
- 2.Annual Report. Vol. 9. Research to Prevent Blindness, Inc.; New York: 2000. [Google Scholar]
- 3.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111(3):491–500. doi: 10.1016/j.ophtha.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Whitcup SM. Anterior Uveitis. In: Nussenblatt RB, Whitcup SM, editors. Uveitis: Fundamentals and clinical practice. Elsevier Health Sciences; St Louis: 2003. pp. 273–85. [Google Scholar]
- 5.Wakefield D, Dunlop I, McClusky PJ, Penny R. Uveitis: aetiology and disease associations in an Australian population. Aust NZ J Ophthalmol. 1986;14:181–7. doi: 10.1111/j.1442-9071.1986.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 6.Loh AR, Acharya NR. Incidence rates and risk factors for ocular complications and vision loss in HLA-B27-associated uveitis. Am J Ophthalmol. 2010;150(4):534–42. doi: 10.1016/j.ajo.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helm CJ, Holland GN. The effects of posterior subtenon injection of triamcinolone acetonide in patients with intermediate uveitis. Am J Ophthalmol. 1995;120(1):55–64. doi: 10.1016/s0002-9394(14)73759-6. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda E, Hikita N, Eto K, Mochizuki M. Tacrolimus-rapamycin combination therapy for experimental autoimmune uveoretinitis. Jpn J Ophthalmol. 1997;41(6):396–402. doi: 10.1016/s0021-5155(97)00083-x. [DOI] [PubMed] [Google Scholar]
- 9.Martin DF, DeBarge LR, Nussenblatt RB, Chan CC, Roberge FC. Synergistic effect of rapamycin and cyclosporin A in the treatment of experimental autoimmune uveoretinitis. J Immunol. 1995;154(2):922–7. [PubMed] [Google Scholar]
- 10.Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31(5):335–40. doi: 10.1016/s0009-9120(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 11.Rapamune [package insert] Wyeth Pharmaceuticals Inc; Philadelphia (PA): Jul, 2008. [Google Scholar]
- 12.Shanmuganathan VA, Casely EM, Raj D, et al. The efficacy of sirolimus in the treatment of patients with refractory uveitis. Br J Ophthalmol. 2005;89(6):666–9. doi: 10.1136/bjo.2004.048199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dejneka NS, Kuroki AM, Fosnot J, Tang W, Tolentino MJ, Bennett J. Systemic rapamycin inhibits retinal and choroidal neovascularization in mice. Mol Vis. 2004;22(10):964–72. [PubMed] [Google Scholar]
- 15.Nussenblatt RB, Coleman H, Jirawuthiworavong G, et al. The treatment of multifocal choroiditis associated choroidal neovascularization with sirolimus (rapamycin) Acta Ophthalmol Scand. 2007;85(2):230–1. doi: 10.1111/j.1600-0420.2006.00858.x. [DOI] [PubMed] [Google Scholar]