Abstract
Response monitoring abnormalities have been reported in chronic schizophrenia patients, but it is unknown whether they predate the onset of psychosis, are present in early stages of illness, or are late-developing abnormalities associated with illness progression. Response-synchronized event-related potentials (ERP) recorded during a picture-word matching task yielded error-related negativity (ERN), correct-response negativity (CRN), and error positivity (Pe) from 84 schizophrenia patients (SZ), 48 clinical high risk patients (CHR), and their age-matched healthy controls (HC; n = 110 and 88, respectively). A sub-sample of 35 early illness schizophrenia patients (ESZ) was compared to 93 age-matched HC and the CHR patients (after statistically removing the effects of normal aging). Relative to HC, 1) SZ, ESZ, and CHR had smaller ERNs, and 2) SZ and ESZ had larger CRNs and smaller Pes. Within the SZ, longer illness duration was associated with larger CRNs but was unrelated to ERN or Pe. CHR and ESZ did not differ on ERN or CRN, although Pe was smaller in ESZ than CHR. These results indicate that while ERN, CRN, and Pe abnormalities are present early in the illness, only the ERN abnormality is evident prior to psychosis onset, and only the CRN abnormality appears to worsen progressively over the illness course. Brain regions subserving response monitoring may be compromised early in the illness and possibly during its clinical prodrome.
Keywords: schizophrenia, prodrome, at-risk, error-related negativity, performance monitoring
Successful performance of cognitive tasks requires the online monitoring of our own responses in order to detect errors and to make strategic adjustments to enhance accuracy. The brain’s error-monitoring system is reflected by the error-related negativity (ERN) (Falkenstein, Hohnsbein, Hoorman, & Blanke, 1990; Gehring, Goss, Coles, Meyer, & Donchin, 1993) and the error positivity (Pe; Falkenstein, Hoormann, Christ, & Hohnsbein, 2000; Mathalon et al., 2002; Nieuwenhuis, Ridderinkhof, Blom, Band, & Kok, 2001), two event-related potential (ERP) components, successively elicited by commission errors across a wide variety of choice reaction-time tasks. Patients with schizophrenia have been shown to exhibit behavioral impairments in performance monitoring during cognitive tasks (Blakemore, Smith, Steel, Johnstone, & Frith, 2000; Malenka, Angel, Hampton, & Berger, 1982; Turken, Vuilleumier, Mathalon, Swick, & Ford, 2003), likely contributing to their deficits in performance accuracy. In regard to neurophysiology, schizophrenia has been associated with abnormal reduction of ERN amplitude in response to errors (Alain, McNeely, He, Christensen, & West, 2002; Bates, Kiehl, Laurens, & Liddle, 2002; Bates, Liddle, Kiehl, & Ngan, 2004; Kim et al., 2006; Kopp & Rist, 1999; Mathalon et al., 2002; Mathalon, Jorgensen, Roach, & Ford, 2009; Morris, Heerey, Gold, & Holroyd, 2008; Morris, Yee, & Nuechterlein, 2006) as well as an abnormally enlarged ERN-like negativity following correct trials known as the correct-response negativity (CRN; Alain et al., 2002; Kim et al., 2006; Kopp & Rist, 1999; Mathalon et al., 2002; Morris et al., 2008; Morris et al., 2006). Although these electrophysiological abnormalities are evident in schizophrenia patients independent of clinical severity (Bates et al., 2004), it remains unclear whether they are present early in the illness, or indeed, whether they are present in individuals at clinical high risk for the development of psychosis.
Electrophysiological Markers of Response Monitoring
ERN
The error-related negativity (ERN) (Gehring et al., 1993), or error negativity (Ne; Falkenstein et al., 1990), component of the response-locked ERP associated with performance errors in speeded choice-response tasks is evident following the earliest electromyographic activity generated by overt error responses (Gehring, Coles, Meyer, & Donchin, 1995; Kopp & Rist, 1999; Kopp, Rist, & Mattler, 1996) and peaks 50–150 ms after the error is committed. Larger (i.e., more negative) ERNs are associated with instructions emphasizing accuracy over speed, faster errors, lower error rates, attempts to correct errors, greater post-error slowing, and greater error salience (Bernstein, Scheffers, & Coles, 1995; Falkenstein et al., 2000; Gehring et al., 1995; Scheffers & Coles, 2000). Topographic scalp maps show the ERN to have a fronto-central maximum (Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; Gehring et al., 1995). Converging evidence from dipole modeling of the ERN (Badgaiyan & Posner, 1998; Dehaene, Posner, & Tucker, 1994; Holroyd, Dien, & Coles, 1998; Luu, Flaisch, & Tucker, 2000; Milter, Braun, & Coles, 1997), functional magnetic resonance imaging (fMRI; Carter et al., 1998; Kiehl, Liddle, & Hopfinger, 2000; Mathalon et al., 2009; Mathalon, Whitfield, & Ford, 2003; van Veen & Carter, 2002), and intracranial recordings from monkeys (Brooks, 1987; Gemba, Sasaki, & Brooks, 1986; Niki & Watanabe, 1979), suggests that anterior cingulate cortex (ACC) is the principal generator of the ERN. The ERN has variously been considered by researchers to reflect simple error detection (Falkenstein et al., 1991; Gehring et al., 1993; Gehring et al., 1995; Hohnsbein, Falkenstein, Hoormann, & Blanke, 1991), high levels of response conflict (Danielmeier, Wessel, Steinhauser, & Ullsperger, 2009; Gehring & Fencsik, 2001; Hughes & Yeung, 2010; van Veen & Carter, 2002), but see (Carbonnell & Falkenstein, 2006; Masaki, Falkenstein, Sturmer, Pinkpank, & Sommer, 2007), and reward prediction errors in which outcomes are worse than expected (Holroyd & Coles, 2002). It is interesting that the ERN is evoked by errors committed outside of conscious awareness (Endrass, Reuter, & Kathmann, 2007; Nieuwenhuis et al., 2001; O’Connell, et al., 2007).
CRN
There is often a small fronto-central ERN-like negativity following correct responses known as the correct-response negativity (CRN; Falkenstein et al., 2000; Gehring & Knight, 2000; Scheffers & Coles, 2000; Scheffers, Coles, Bernstein, Gehring, & Donchin, 1996; Vidal, Hasbroucq, Grapperon, & Bonnet, 2000). As reviewed by Coles et al. (Coles, Scheffers, & Holroyd, 2001), CRNs may result when subjects: 1) detect partial errors, 2) exhaust the allotted time for a given response, 3) lack confidence that a correct response was made, or 4) experience high response conflict. Alternatively, it has been proposed that some CRN activity may be present on all responses, reflecting the response checking or monitoring process itself, with the ERN superimposed on the CRN when the outcome of this monitoring process is the detection of an error (Falkenstein et al., 2000).
Pe
The error positivity (Pe) is a positive voltage response-locked ERP component with a centro-parietal scalp maximum appearing 200–500 ms following error responses (Falkenstein et al., 1990; Falkenstein, Hohnsbein, & Hoormann, 1995; Falkenstein et al., 1991; Falkenstein et al., 2000; Scheffers & Coles, 2000; Vidal et al., 2000). The Pe has been localized to the rostral ACC and superior parietal cortex, as opposed to the more caudal ACC source of the ERN (Herrmann, Rommler, Ehlis, Heidrich, & Fallgatter, 2004; van Veen & Carter, 2002). Unlike the ERN, awareness of errors is necessary to produce a Pe (Endrass et al., 2007; Hughes & Yeung, 2011; Nieuwenhuis et al., 2001; O’Connell, et al., 2007). Indeed, the Pe may be a P300-like positivity resulting from context updating when errors are consciously recognized (Falkenstein et al., 1991; Leuthold & Sommer, 1999) and response strategies are adjusted (Falkenstein et al., 1995).
Performance Monitoring in Schizophrenia
Studies of error monitoring in schizophrenia have repeatedly demonstrated ERN amplitude reduction (Alain et al., 2002; Bates et al., 2002; Bates et al., 2004; Kim et al., 2006; Kopp & Rist, 1999; Mathalon et al., 2002; Mathalon et al., 2009; Morris et al., 2008; Morris et al., 2006). In addition, some (Alain et al., 2002; Mathalon et al., 2002; Morris et al., 2008; Morris et al., 2006), but not all (Bates et al., 2002; Bates et al., 2004; Mathalon et al., 2009) studies have reported abnormally large CRNs in schizophrenia. Many studies have shown schizophrenia patients to have normal Pe amplitudes (Alain et al., 2002; Bates et al., 2004; Kim et al., 2006; Mathalon et al., 2002; Morris et al., 2006) and normal post-error slowing (Bates et al., 2002; Bates et al., 2004; Mathalon et al., 2002) following errors, suggesting that their ERN and CRN abnormalities are not simply the result of deficient error awareness. Some (Carter, MacDonald, Ross, & Stenger, 2001; Kerns et al., 2005; Laurens, Ngan, Bates, Kiehl, & Liddle, 2003; Polli et al., 2008), but not all (Mathalon et al., 2009), fMRI studies suggest that the ACC is hypoactive in response to errors in schizophrenia, consistent with other evidence of functional (Minzenberg, Laird, Thelen, Carter, & Glahn, 2009) and structural abnormalities (Benes, 1993; Benes & Bird, 1987) in the ACC. Although error monitoring has been repeatedly studied in chronic schizophrenia patients, it has not been examined in patients early in their illness, or in putatively prodromal patients at clinical high risk for psychosis. Accordingly, it remains unclear whether the ERN/CRN abnormalities observed in schizophrenia patients predate the onset of psychosis, are present in early stages of illness, or are late-developing abnormalities associated with illness progression and/or nonspecific aspects of illness chronicity.
Prospective Definition of the Schizophrenia Prodrome
In the past decade, clinical criteria have been developed to identify individuals exhibiting a prodromal syndrome associated with increased risk for psychosis. These include the Criteria of Prodromal Syndromes (COPS; Miller et al., 2002) and the similar At-Risk Mental States criteria (ARMS; Yung & McGorry, 1996). Validation studies show these criteria to predict conversion to full-blown psychosis, most often schizophrenia (Woods et al., 2009), at a rate of 35% over a 2.5-year follow-up period (Cannon et al., 2008). Thus, about two-thirds of the individuals meeting clinical criteria for this psychosis risk syndrome do not convert to psychosis, limiting the potential for early intervention with these clinical high risk patients. This limitation has motivated recent and ongoing studies aimed at determining whether the neurobiological abnormalities associated with schizophrenia are present prior to psychosis onset (Brockhaus–Dumke et al., 2008; Karlsgodt et al., 2008; van der Stelt, Lieberman, & Belger, 2005), potentially enhancing our ability to predict future risk for psychosis and setting the stage for targeted preventive interventions.
Many of the individuals at clinical high risk for psychosis are adolescents and young adults, underscoring the need to take normal brain maturational processes into account when examining neurobiological markers such as the ERN. Segalowitz (Davies, Segalowitz, & Gavin, 2004; Mathewson, Dywan, & Segalowitz, 2005; Santesso & Segalowitz, 2008; Santesso, Segalowitz, & Schmidt, 2006) and others (Ladouceur, Dahl, & Carter, 2004) have shown ERN amplitudes to increase from childhood to young adulthood, whereas the CRN has been shown to increase from about age 10 to 16 years, leveling off thereafter (Davies et al., 2004). In contrast, the centro-parietal Pe was generally stable over this same developmental period (Davies et al., 2004; Ladouceur et al., 2004; Santesso et al., 2006). Thus, the various neurophysiological signals associated with performance monitoring show distinct neurodevelopmental trajectories from childhood to early adulthood. Segalowitz and colleagues (Segalowitz, Santesso, & Jetha, 2010) suggest that these data are indicative of late maturation of the ACC and/or late involvement of the ACC during performance monitoring.
Design of Present Study
In the current study, the ERN, CRN, and Pe elicited during a picture–word verification task were assessed in patients with schizophrenia across the illness course, including a subsample of early illness patients assessed within 2 years of their first psychiatric hospitalization for psychosis, and in patients at clinical high risk for psychosis. Each group was compared to a group of age-matched healthy control subjects, and after removing the effects of normal aging, the early illness schizophrenia and clinical high risk patients were directly compared. Based on the hypothesis that electrophysiological abnormalities associated with error monitoring are a core pathophysiological feature of schizophrenia, we predicted that patients early in their illness course would show an attenuated ERN, an increased CRN, and a normal Pe, similar to our prior observations in chronic patients (Mathalon et al., 2002). Additionally, positing that these electrophysiological abnormalities reflect either genetic vulnerability for schizophrenia or result from faulty neurodevelopment, we hypothesized that this pattern of abnormalities would be evident in patients at clinical high risk for psychosis, albeit in attenuated form, given that only a subset of these patients will ultimately convert to schizophrenia. Moreover, since the clinical high risk patients have not been exposed to antipsychotic medication, study of this group provides an opportunity to assess whether these performance-related ERP components are abnormal in the absence of the antipsychotic confound associated with studies of schizophrenia patients. Furthermore, in order to examine progression over the illness course, we asked whether the degree of abnormality present in each ERP component, relative to values expected based on normal aging, is correlated with illness duration across the full schizophrenia sample.
Method
Participants
Study participants included 50 patients at clinical high risk (CHR) for psychosis based on the Structured Interview for Prodromal Syndromes (Miller et al., 2003; Miller et al., 2002), 88 patients with schizophrenia (SZ; per the Diagnostic and Statistical Manual of Mental Disorders–IV) based on the Structured Clinical Interview for DSM–IV (SCID), and 135 healthy control (HC) subjects. CHR patients met criteria for at least one of the three subsyndromes defined by the COPS (Miller et al., 2003; Miller et al., 2002): 1) attenuated positive symptoms (APS); 2) brief intermittent psychotic states (BIPS); 3) genetic risk with deterioration in social/occupational functioning (GRD). Among the SZ, a sub-sample of 38 patients were identified as early illness (ESZ), defined as being within 2 years of initial hospitalization for psychosis or initiation of antipsychotic medication. Interviews were conducted by a trained research assistant, psychiatrist, or clinical psychologist.
Out of the 135 HC subjects, three overlapping age-matched groups were organized according to the age range of each patient group: 1) a group of 115 healthy controls (age range 16–59 years) age-matched to SZ patients; 2) a group of 96 healthy controls (age range 16–37 years) age-matched to ESZ patients; and 3) a group of 92 healthy controls (age range 12–28 years) age-matched to CHR patients.
Patients were referred by community clinicians. HC participants were recruited by advertisements and word of mouth. Exclusion criteria for the HC group included a past or current DSM–IV major Axis I disorder based on a SCID interview or having a first-degree relative with a psychotic disorder. Exclusion criteria for all groups included a history of illicit substance dependence or substance abuse within the past year, a history of significant medical or neurological illness, or a history of head injury resulting in loss of consciousness. The study was approved by the institutional review boards of the University of California, San Francisco and Yale University, and all adult participants provided written informed consent. In the case of minor participants, parents provided written informed consent and youths provided written informed assent. Clinical and demographic data for all groups are presented in Table 1.
Table 1.
Group Demographics
| Schizophrenia Patients n = 84 |
Healthy Controls age-matched to Schizophrenia Patients n = 110 |
Statistic | p-value | Early Illness Schizophrenia Patients n = 35 |
Healthy Controls age-matched to Early Illness Patients n = 93 |
Statistic | p-value | Clinical High-Risk Patients n = 48 |
Healthy Controls age-matched to Clinical High-Risk Patients n = 88 |
Statistic | p-value | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |||||||
| Age (years) | 29.58 | 11.57 | 27.93 | 9.77 | t(192) = −1.08 | ns | 22.75 | 5.0 | 24.62 | 5.9 | t(126) = 1.68 | ns | 18.93 | 4.1 | 19.99 | 4.3 | t(134) = 1.09 | ns | ||||||
| Parental SESa,b | 39.79 | 14.85 | 34.36 | 15.12 | t(190) = −2.45 | <.05 | 40.1 | 15.2 | 32.92 | 14.9 | t(126) = −2.44 | <.05 | 36.43 | 16.4 | 30.34 | 13.4 | t(134) = −2.33 | <.05 | ||||||
| Gender | χ2(1, 194) = 4.34 | <.05 | χ2(1, 128) = 2.5 | ns | χ2(1, 136) = .51 | ns | ||||||||||||||||||
| Male | 64 | 68 | 27 | 58 | 29 | 47 | ||||||||||||||||||
| Female | 20 | 42 | 8 | 35 | 19 | 41 | ||||||||||||||||||
| Handednessc | χ2(2, 194) = 2.0 | ns | χ2(2, 128) = 0.9 | ns | χ2(2, 136) = 5.15 | ns | ||||||||||||||||||
| Right | 75 | 103 | 33 | 87 | 38 | 81 | ||||||||||||||||||
| Left | 6 | 6 | 1 | 5 | 7 | 6 | ||||||||||||||||||
| Ambidextrous | 3 | 1 | 1 | 1 | 3 | 1 | ||||||||||||||||||
| Schizophrenia subtype | ||||||||||||||||||||||||
| Paranoid | 49 | 18 | ||||||||||||||||||||||
| Disorganized | 9 | 6 | ||||||||||||||||||||||
| Undifferentiated | 15 | 6 | ||||||||||||||||||||||
| Residual | 9 | 3 | ||||||||||||||||||||||
| Schizophreniform | 2 | 2 | ||||||||||||||||||||||
| CHR criteriad | ||||||||||||||||||||||||
| APS | 47 | |||||||||||||||||||||||
| BPS | 2 | |||||||||||||||||||||||
| GRD | 7 | |||||||||||||||||||||||
| Antipsychotic type | ||||||||||||||||||||||||
| Atypical | 67 | 32 | 0 | |||||||||||||||||||||
| Typical | 6 | 1 | 0 | |||||||||||||||||||||
| Both | 5 | 0 | 0 | |||||||||||||||||||||
| None | 6 | 2 | 48 | |||||||||||||||||||||
| PANSS total | 66.38 | 16.4 | 67.34 | 16.6 | ||||||||||||||||||||
| SOPS total | 32.3 | 13.7 | ||||||||||||||||||||||
Note. SES = socioeconomic status; CHR = clinical high risk; APS = attenuated positive symptoms; BPS = brief intermittent psychotic symptoms; GRD = genetic risk and functional decline; PANSS = Positive and Negative Syndrome Scale; SOPS = Scale of Prodromal Symptoms. Values are given as number of subjects for gender, handedness, diagnostic subtype, clinical high risk criteria, and antipsychotic type. Group means with the standard deviation for age, parental socioeconomic status, PANSS, and SOPS are reported. Age and parental socioeconomic status were analyzed with independent samples t-tests; gender and handedness were analyzed with chi-square analyses.
The Hollingshead (1975) four-factor index of parental SES is based on a composite of maternal education, paternal education, maternal occupational status, and paternal occupational status. Lower scores represent higher SES.
SES values are missing from two schizophrenia patients (n = 2).
The Crovitz-Zener (1962) questionnaire was used to measure handedness.
Clinical high-risk criteria APS, BIPS, and GRD are not mutually exclusive.
Procedure
Clinical ratings
Within 4 weeks of ERP assessment (mean [M] = 12.51, standard deviation [SD] = 17.7 days), a clinically trained research assistant, psychiatrist, or clinical psychologist rated schizophrenia symptoms using the Positive and Negative Syndrome Scale (PANSS; Kay & Opler, 1987). Prodromal symptoms were rated using the Scale of Prodromal Symptoms (SOPS; Miller et al., 2003; Miller et al., 2002).
Task
A variant of our earlier picture–word verification task (Mathalon et al., 2002) was used. In this task, each trial consists of consecutive presentations of a picture for 250 ms, a blank screen for 75 ms, and a word for 250 ms. Subjects are instructed to indicate whether or not the word matched the preceding picture by pressing one of two buttons. The pictures consisted of 102 line drawings, selected for nameability from a set of 120 (Snodgrass & Vanderwart, 1980) based on pilot testing in young adults. Pictures were classified into 10 natural categories (clothing, animal, bird, appliance, tool, vehicle, vegetable, fruit, toy, and musical instrument). The full set of pictures was presented in each of four blocks. Pictures were paired with different words in the different blocks, and the order of the pictures was varied across blocks. The inter-stimulus interval between successive trials was jittered between 1,625 ms and 3,225 ms.
Each picture was paired with a word that either matched (50%) or did not match (50%) the picture. Of those that did not match, half (25%) were words within the category and half (25%) were outside the category. For example, a picture of a camel was followed by the word “camel” (match), by “cow” (in category non-match), or by “candle” (out of category non-match). Subjects pressed buttons with right and left index fingers to indicate whether or not the word matched the picture. Subjects pressed one button to matches and the other button to nonmatches, with hand of press counterbalanced across subjects. Prior to starting the task, participants listened to recorded instructions that emphasized both response speed and accuracy and completed a 20-trial practice session to ensure task comprehension. In order to assess ERPs associated with performance accuracy (errors vs. correct responses) using the maximum number of trials, trials were collapsed across the various match/nonmatch conditions.
ERP data acquisition and preprocessing
Electroencephalographic (EEG) data were recorded from 64 scalp sites using a BioSemi ActiveTwo system (www.biosemi.com). EEG data were continuously digitized at 1024 Hz, referenced offline to averaged earlobe electrodes, and digitally bandpass-filtered between 0.5 and 12 Hz. Continuous data were separated into 850 ms epochs time-locked to the button-press response, encompassing 100 ms before and 750 ms after the response. Electrodes placed at the outer canthi of both eyes and above and below the right eye recorded vertical and horizontal electrooculogram data, which were used in a regression-based algorithm (Gratton, Coles, & Donchin, 1983) to correct EEG epochs for eye movements and blinks.
EEG epochs were baseline-corrected relative to the 100 ms pre-response baseline and artifact rejected for voltages exceeding ± 50 μV in nine central electrodes (F3, Fz, F4, C3, Cz, C4, P3, Pz, P4). For both error and correct responses, epochs surviving artifact rejection were averaged to form individual participant error and correct ERP waveforms. Grand average ERP waveforms were then produced from these individual averages. Based on reports regarding stability of the ERN component (Olvet & Hajcak, 2009; Pontifex et al., 2010), individuals had to have a minimum of n = 6 error trials to be included in the analyses.
The ERN and CRN were measured as the most negative peaks between 0 ms and 125 ms in each subject’s error and correct ERP waveforms, respectively. The Pe was measured as the average voltage between 200 ms and 500 ms from the error ERP waveforms. Only subjects with a discernable ERN peak (i.e., change in slope from negative to positive) in the 0 – 125 ms post-response time window were included for analysis, resulting in exclusion of six HC, two CHR, and six SZ (four of whom were also characterized as ESZ) with noisy ERPs lacking an identifiable ERN. The final samples consisted of (1) 84 SZ age-matched with 110 HC; (2) 35 ESZ age-matched with 93 HC; and 3) 48 CHR age-matched with 88 HC.
Statistical Analyses
Statistical correction for normal aging effects
Our primary approach to controlling for the effects of normal aging when evaluating whether the patient groups showed ERP abnormalities was to compare each patient group or subgroup with an age-matched HC group. However, in order to directly compare the CHR and ESZ groups, whose ages significantly differed (p < .001), it was necessary to first remove the effects of normal brain maturation and aging from the ERP measures. To model these normal maturation/aging effects, each ERP component was regressed on age in the full sample of HC (n = 130; age range 12 to 59 years). For each ERP measure, the resulting regression equation was then used to derive age-specific predicted values for all participants (patients and HC). Differences between actual values and predicted values were then divided by the standard error (SE) of regression from the HC regression model, yielding age-corrected z-scores for each ERP component (ERN, CRN, Pe) for all subjects. This method has been used in previous brain imaging studies of patient groups to control for normal aging effects (Pfefferbaum et al., 1992) and is preferable to a standard analysis of covariance (ANCOVA) model because it only removes normal aging effects while preserving any abnormal aging effects related to the disease process. The resulting age-corrected z-scores expressed each ERP component in standard units of deviation from the value expected for a healthy individual of a given age.
Behavioral data
Error rates were compared for each patient-control group pair with t tests for independent samples. Individual subject median reaction times (RTs) were extracted for correct and error trials. The effects of group (patients vs. controls) and accuracy (error vs. correct) on RT were assessed using a two-way analysis of variance (ANOVA) for each patient–control group pair.
To measure post-error slowing within an individual subject, the median RT for correct trials following correct trials (correct–correct) was subtracted from the median RT for correct trials following error trials (error–correct). Because the different trial types in our task were associated with RT differences (i.e., in-category nonmatch > out-of-category nonmatch > match trials), correct–correct trial pairs were pseudorandomly selected such that the relative proportions of various trial type combinations matched the proportions present among the error–correct trial pairs. Although the proportions of various trial type combinations were matched, the absolute number of correct–correct trial pairs exceeded the number of error–correct trial pairs, maximizing the number trials used to derive the correct trial median RT estimates. Post-error slowing median RT difference scores were compared between patient and HC groups using t-tests for independent samples.
Electrophysiological data
For each patient–control group pair, ERN/CRN amplitudes were analyzed in a Group (patients, controls) × Accuracy (ERN, CRN) × Lead (Fz, FCz, Cz) ANOVA, and Pe amplitudes were analyzed in a Group × Lead (FCz, Cz, Pz) ANOVA. Interactions involving group were parsed with follow-up ANOVAs. Greenhouse–Geisser corrections were applied to the tests of all within-subjects factors containing more than two levels in the repeated-measures ANOVAs.
Because the CHR group was significantly younger than the ESZ group, direct comparisons of these groups were based on one-way ANOVAs of the age-corrected z-scores for each ERP component (ERN, CRN, Pe) at the lead showing the greatest patient– control differences in the raw ERP amplitude analyses. As described above, the z-scores reflected deviations in standard units from the ERP amplitudes expected for a healthy person of a given age, based on modeled normal aging effects in the full HC sample.
To assess for patient–control group differences in the relationship between behavior and ERP component amplitudes, we regressed each ERP component on the behavioral measure, group (dummy coded), and the Group × Behavioral measure interaction. The interaction term tested for slope differences between the groups. When the slope difference was not significant, the interaction term was dropped and the analysis focused on the test of the common slope estimated within both groups. The ERP components considered were the ERN, CRN, and Pe. The behavioral measures considered were error rate and post-error slowing scores.
In order to determine whether each ERP component abnormality progressively worsened over the illness course, independent of normal aging effects, duration of illness was correlated with age-corrected z-scores for ERN, CRN, and Pe, within the full SZ sample.
To assess the relationship between symptom severity and ERP component amplitudes, the amplitude of each ERP component (ERN, CRN, Pe) was correlated with positive and negative symptom subscale scores from the PANSS in the full SZ sample. Correlations of ERP component amplitudes with SOPS positive and negative symptom subscale scores were performed in the CHR group. Because of the exploratory nature of these symptom correlations, a Bonferroni correction for the number of correlations performed was applied within each patient group.
Results
Demographic and Behavioral Data
Demographics
Group demographic data and analyses are presented in Table 1. Independent sample t-tests confirmed that there were no significant differences in age between each patient group and its respective age-matched control group, although the ESZ group was significantly older than the CHR group, t(81) = 3.7, p < .001. Each patient group had significantly lower parental socioeconomic status (SES; Hollingshead, 1975) than their age-matched control group, but ESZ and CHR patients did not differ from each other (p > .3). Chi-square tests showed that the full sample of SZ patients had a significantly greater proportion of males than females relative to the age-matched HC group, although gender distributions did not significantly differ between the remaining patient and matched control groups or between ESZ and CHR groups. There were no significant differences in handedness (Crovitz & Zener, 1962) between patients and their respective control groups or between ESZ and CHR groups. To address potential confounds associated with group differences in parental SES and gender, group effects on the ERP components were assessed with ANCOVA models using each of these variable as covariates.
Behavioral data
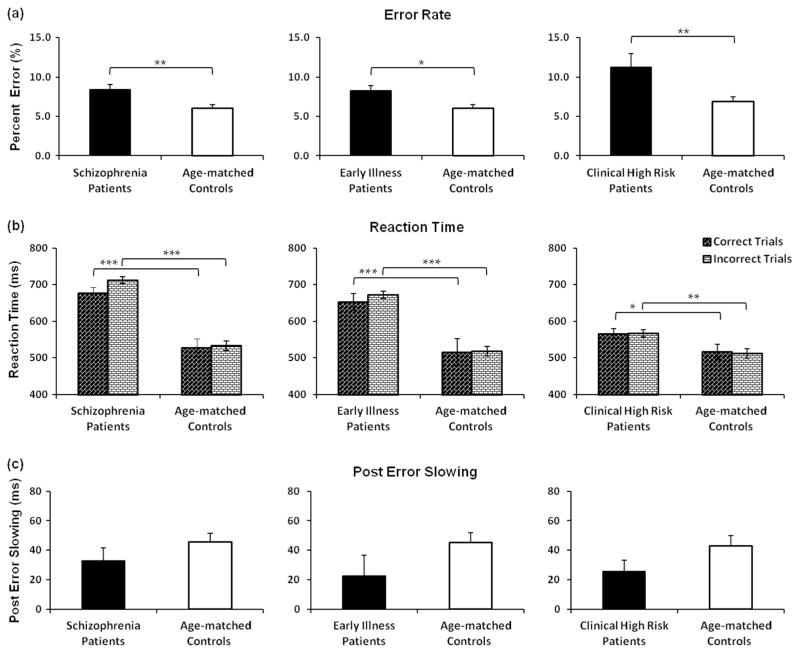
The SZ group, ESZ subgroup, and CHR group made significantly more errors than their respective age-matched HC (Figure 1a). Error rates did not differ between CHR and ESZ (p = .18). For RT (Figure 1b), the Group × Accuracy ANOVAs for each patient–control group pairing revealed significant main effects of group [for SZ vs. HC, F(1, 192) = 61.85, p < .001; for ESZ vs. HC, F(1, 126) = 32.35, p < .001; for CHR versus HC, F(1, 134) = 7.31, p < .01] indicating that all patient groups responded more slowly than their age-matched HCs. The main effect of accuracy, F(1, 192) = 7.93, p < .005) and the Group × Accuracy interaction, F(1, 192) = 4.18, p < .05 were significant in the SZ versus HC ANOVA, but not for the other group pairings. Follow-up paired t-tests indicated significantly slower errors than correct responses in SZ, t(83) = −2.5, p < .05, but not in their age-matched HC (p = .42). All groups exhibited post-error slowing, and there were no significant pair-wise patient–control differences (Figure 1c).
Figure 1.
Behavioral performance in the picture-word verification task across the illness course of schizophrenia and their age-matched control groups. In (a), error rates are shown as percent error (%), revealing increased error rates in all patient groups; (b), increased response time across all patient groups is shown in median reaction time (RT) values (ms) for correct and incorrect trials; (c), post-error slowing is displayed (ms), revealing intact strategic adjustment in all groups. Bars = standard error. Significant values are displayed as * p < 0.05, ** p < 0.01, *** p < 0.001.
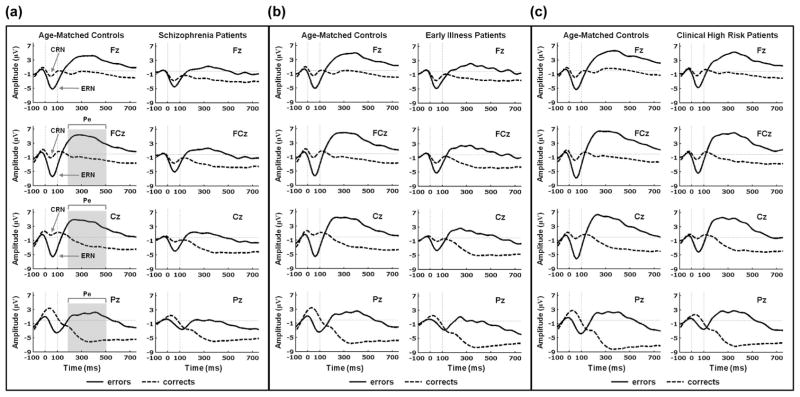
Grand Average ERP Waveforms for Error and Correct Responses
As can be seen in the grand average ERP waveforms shown in Figure 2, errors elicited a large fronto-central (Fz, FCz, Cz) ERN peaking at approximately 60 ms (SD = 21.6 ms) post-response, and correct responses elicited a smaller fronto-central (Fz, FCz, Cz) CRN peaking at about 55 ms (SD = 27.6 ms) post-response. Subsequent to the ERN, a slow positive fronto-central-parietal (FCz, Cz, Pz) Pe was evident from 200 to 500 ms. Scalp topography maps for the ERN, CRN, and Pe for all of the patient and HC groups are presented in Figure 1 of the supplementary materials.
Figure 2.
Grand average ERP waveforms for response-locked error (solid) and correct (dashed) trials for age-matched healthy control participants and schizophrenia, early illness schizophrenia, and clinical high risk patients. Data shown in waveforms are from Fz, FCz, Cz, and Pz channels. The x-axis presents time in milliseconds from -100 ms (pre-response) to 750 ms (post-response) relative to the button press (0 ms). The y-axis presents amplitude in microvolts (μV). ERN and CRN peaks are signified by arrows at Fz, FCz and Cz, and the Pe time window (200 to 500 ms) is depicted by the gray bar at FCz, Cz and Pz in (a). (a) age-matched healthy control participants (left) and schizophrenia patients (right); (b) age-matched healthy control participants (left) and early illness schizophrenia patients (right); (c) age-matched healthy control participants (left) and clinical high risk patients (right).
ERN/CRN Analyses
Table 2 presents the ERN/CRN ANOVA results for each patient–control group comparison. Summary plots in Figure 3a present the mean ERN and CRN amplitudes at Cz for each of the patient and HC groups. None of the ANOVA results involving group or accuracy effects were substantially changed when reanalyzed as ANCOVAs with either parental SES or gender as covariates.
Table 2.
Summary of ERP Analysis Assessing ERN/CRN and Pe Across Each Patient Group Compared to Their Respective Age-Matched Healthy Control Sample
| ERP component | Schizophrenia patients
|
Early Illness Schizophrenia patients
|
Clinical High Risk patients
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | p-value | df | F | p-value | df | F | p-value | |
| ERN/CRN | |||||||||
| Group (patients, controls) | 1,192 | 0.01 | ns | 1,126 | 0.01 | ns | 1,134 | 2.36 | ns |
| Accuracy (ERN, CRN) | 1,192 | 154.53 | <.001 | 1,126 | 83.19 | <.001 | 1,134 | 133.26 | <.001 |
| Lead (Fz, FCz, Cz) | 2,384 | 44.46 | <.001 | 2,252 | 25.43 | <.001 | 2,268 | 22.18 | <.001 |
| Accuracy × Group | 1,192 | 14.38 | <.001 | 1,126 | 5.03 | <.05 | 1,134 | 3.75 | 0.055 |
| Lead × Group | 2,384 | 0.27 | ns | 2,252 | 1.34 | ns | 2,268 | 1.88 | ns |
| Accuracy × Lead | 2,384 | 54.81 | <.001 | 2,252 | 17.52 | <.001 | 2,268 | 17.64 | <.001 |
| Accuracy × Lead × Group | 2,384 | 7.36 | <.005 | 2,252 | 7.84 | <.005 | 2,268 | 4.99 | <.05 |
| Fz-Group × Accuracy | 1,192 | 9.81 | <.005 | 1,126 | 2.14 | ns | 1,134 | 1.11 | ns |
| Effect of accuracy in each group | |||||||||
| Effect of accuracy for controls | 1,109 | 101.28 | <.001 | 1,92 | 91.58 | <.001 | 1,87 | 79.57 | <.001 |
| Effect of accuracy for patients | 1,83 | 30.87 | <.001 | 1,34 | 14.98 | <.001 | 1,47 | 49.28 | <.001 |
| Effect of group for ERN and CRN | |||||||||
| Group effects for ERN | 1,192 | 1.98 | 0.161 | 1,126 | 0.12 | 0.720 | 1,134 | 2.40 | 0.120 |
| Group effects for CRN | 1,192 | 7.58 | 0.006 | 1,126 | 4.45 | 0.037 | 1,134 | 0.12 | 0.730 |
| FCz-Group × Accuracy | 1,192 | 15.01 | <.001 | 1,126 | 4.45 | <.05 | 1,134 | 3.74 | 0.055 |
| Effect of accuracy in each group | |||||||||
| Effect of accuracy for controls | 1,109 | 135.61 | <.001 | 1,92 | 117.51 | <.001 | 1,87 | 106.71 | <.001 |
| Effect of accuracy for patients | 1,83 | 40.41 | <.001 | 1,34 | 17.72 | <.001 | 1,47 | 64.48 | <.001 |
| Effect of group for ERN and CRN | |||||||||
| Group effects for ERN | 1,192 | 4.16 | 0.043 | 1,126 | 1.04 | 0.310 | 1,134 | 5.57 | 0.020 |
| Group effects for CRN | 1,192 | 9.41 | 0.002 | 1,126 | 4.45 | 0.038 | 1,134 | 0.08 | 0.780 |
| Cz-Group × Accuracy | 1,192 | 15.76 | <.001 | 1,126 | 7.95 | <.01 | 1,134 | 5.69 | <.05 |
| Effect of accuracy in each group | |||||||||
| Effect of accuracy for controls | 1,109 | 133.33 | <.001 | 1,92 | 114.28 | <.001 | 1,87 | 103.84 | <.001 |
| Effect of accuracy for patients | 1,83 | 46.44 | <.001 | 1,34 | 16.73 | <.001 | 1,47 | 43.16 | <.001 |
| Effect of group for ERN and CRN | |||||||||
| Group effects for ERN | 1,192 | 4.17 | 0.042 | 1,126 | 4.28 | 0.042 | 1,134 | 7.84 | 0.006 |
| Group effects for CRN | 1,192 | 13.20 | <.001 | 1,126 | 5.24 | 0.025 | 1,134 | 0.01 | 0.910 |
| Pe | |||||||||
| Group (patients, controls) | 1,192 | 28.65 | <.001 | 1,126 | 15.74 | <.001 | 1,134 | 0.58 | ns |
| Lead (FCz, Cz, Pz) | 2,384 | 60.33 | <.001 | 2,252 | 48.91 | <.001 | 2,268 | 92.35 | <.001 |
| Lead × Group | 2,384 | 8.45 | <.001 | 2,252 | 4.72 | <.05 | 2,268 | 0.83 | ns |
| Effect of lead in each group | |||||||||
| Effect of lead for controls | 2,218 | 54.12 | <.001 | 2,184 | 69.09 | <.001 | |||
| Effect of lead for patients | 2,166 | 65.97 | <.001 | 2,68 | 11.46 | <.001 | |||
| Effect of group for each lead | |||||||||
| FCz-group effects for Pe | 1,192 | 34.89 | <.001 | 1,126 | 27.36 | <.001 | |||
| Cz-group effects for Pe | 1,192 | 31.69 | <.001 | 1,126 | 16.16 | <.001 | |||
| Pz-group effects for Pe | 1,192 | 12.95 | <.001 | 1,126 | 7.34 | <.005 | |||
Note. Significant effects of interest are in bold font.
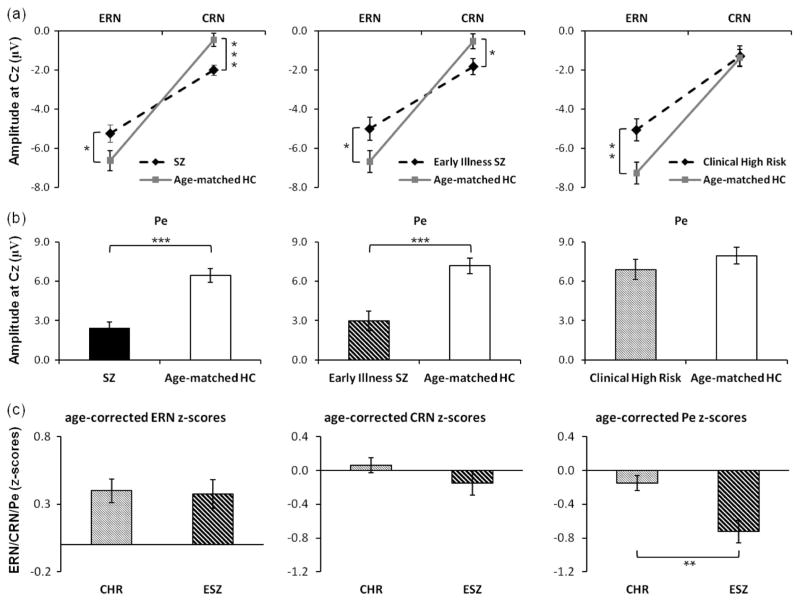
Figure 3.
(a), Group mean (± standard error) ERN and CRN amplitudes at Cz for each patient group and their age-matched healthy controls. (b), Group mean (± standard error) Pe amplitudes at Cz for each patient group and their age-matched healthy controls. (c) Mean (± standard error) age-corrected ERN, CRN, and Pe z-scores at Cz, reflecting the degree of deviation of each patient group from the healthy control (HC) group, are plotted for Clinical High Risk (CHR) and Early Illness Schizophrenia (ESZ) patients. Significant patient differences are shown for age-corrected Pe waveforms, while patient groups show statistical equivalence to each other on age-corrected ERN and CRN waveforms. Error bars indicate standard error. Significant values are displayed as * p < 0.05. ** p < 0.01. *** p < 0.001.
Schizophrenia patients versus healthy controls
For the comparison of the full sample of SZ patients with their age-matched controls, there was a significant main effect of accuracy, confirming that the amplitude of the ERN was significantly larger (more negative) than the CRN, as well as a significant main effect of lead, indicating greater overall negativity at FCz relative to Fz and Cz. In addition, there were significant Accuracy × Group and Accuracy × Group × Lead interactions, leading us to examine the Accuracy × Group interaction at each lead. The Group × Accuracy interaction was significant at each lead, although the effect was slightly less significant at Fz relative to FCz and Cz. To further parse the Accuracy × Group and Accuracy × Group × Lead interactions, the accuracy effect was examined separately for each group and each lead. For both patients and controls, the accuracy effect was significant at each lead, indicating that the ERN was significantly more negative than the CRN in both groups. Next, the group effect was examined separately for errors and corrects at each lead. Relative to the HC group, the SZ group had a significantly smaller ERN at FCz and Cz, but not at Fz, and a significantly larger (i.e., more negative) CRN at Fz, FCz, and Cz (Figure 3a).
Early illness schizophrenia patients versus healthy controls
For the comparison of the ESZ subsample to their age-matched controls, there were significant main effects of accuracy and lead, similar to those described for the SZ versus HC analysis. In addition, there were significant Accuracy × Group and Accuracy × Group × Lead interactions, which were parsed by examining the Accuracy × Group effects at each lead. The Accuracy × Group effect was significant at FCz and Cz, but not at Fz. Further examining the accuracy effect within each group and lead, both ESZ and HC groups showed significant accuracy effects at each lead, indicating that the ERN was significantly more negative than the CRN in both groups. Further follow-up analyses examined the group effect separately for ERN and CRN at each lead. Relative to HC, ESZ patients showed a significantly reduced ERN at Cz, but not at Fz or FCz, and a significantly larger CRN at Fz, FCz, and Cz (Figure 3a).
Clinical high-risk patients versus healthy controls
For the comparison of the CHR group with their age-matched controls, there were significant main effects of accuracy and lead, similar to those described for the SZ versus HC analysis. In addition, there was a marginally significant Accuracy × Group effect (p = .055) and a significant Accuracy × Group × Lead interaction, which were parsed by examining the Accuracy × Group effect separately for each lead. The Group × Accuracy interaction was significant at Cz, marginally significant at FCz (p = .055), and not significant at Fz. The Group × Accuracy interactions were further parsed at each lead by examining the accuracy effect separately for each group. For both CHR and HC, there were significant effects of accuracy at each lead indicating that the ERN was significantly more negative than the CRN in both groups. Further follow-up analyses examined the group effect separately for ERN and CRN at each lead. Relative to HC, CHR patients showed reduced ERN amplitudes at Cz and FCz but not at Fz, whereas the CRN did not differ between the groups (Figure 3a).
Pe Analyses
Table 2 presents the Pe ANOVA results for each patient–control group comparison. Summary plots in Figure 3b present the mean Pe amplitude at Cz for each of the patient and HC groups. None of the ANOVA results involving group or accuracy effects were substantially changed when reanalyzed as ANCOVAs with either parental SES or gender as covariates.
Schizophrenia patients versus healthy controls
For the comparison of the full SZ sample with their age-matched controls, there was a significant group effect indicating that SZ had a reduced (less positive) Pe relative to HC, as well as a significant lead effect indicating that the Pe was larger at FCz and Cz relative to Pz. There was also a significant Group × Lead interaction, indicating that the SZ – HC difference was somewhat larger at fronto-central sites (FCz, Cz) than at posterior sites (Pz), although SZ exhibited a significantly smaller Pe at all leads relative to HC (Figure 3b).
Early illness schizophrenia patients versus healthy controls
For the comparison of the ESZ subsample with their age-matched controls, there were significant main effects of group and lead. In addition, there was a significant Group × Lead interaction, indicating that the Pe reduction in ESZ relative to HC, which was significant at each lead, was somewhat more pronounced at fronto-central relative to parietal leads (Figure 3b).
Clinical high-risk patients versus healthy controls
For the comparison of CHR patients to their age-matched controls, there was a significant main effect of lead showing the Pe to be greater at fronto-central relative to parietal sites. However, neither the group effect nor the Group × Lead interaction was significant, indicating normal Pe amplitude in CHR patients (Figure 3b).
Effects of Normal Aging and Illness Progression on ERP Measures
Effects of normal aging on ERP measures
There were no significant associations between age and ERN at any electrode in the full HC sample, although CRN amplitude significantly decreased (i.e., became less negative) with increasing age at Cz (r = .21, p = .02) and showed a similar trend at FCz (r = .15, p = .09). Pe amplitude significantly decreased (i.e., became less positive) with increasing age at FCz (r = −0.27, p < .005) and Cz (r =−0.27, p < .005), but not at Pz. ERN, CRN, and Pe amplitudes were each regressed on age in the full HC sample (n = 130) spanning the age range from 12 to 59 years. Linear normal aging effects, regardless of significance, were removed from the ERP data for all groups based on these HC regression models, as described in the Methods. Resulting age-corrected z-scores reflected the deviation (in SD units) of each subject’s ERP component amplitude from the value expected for a healthy individual of a given age.
Comparisons of age-corrected ERP Z-scores in CHR and ESZ groups
To test the hypothesis that ERP measures of error processing are more abnormal in ESZ patients than in CHR patients while taking into account the age difference between the groups, CHR and ESZ patients were directly compared on their age-corrected z-scores for each ERP component at Cz, the lead where group abnormalities tended to be strongest (Figure 3c). Neither ERN nor CRN z-scores significantly differed between CHR and ESZ patients (p > .5). However, Pe z-scores were significantly reduced in the ESZ relative to the CHR patients (p < .01).
Behavioral and Clinical Correlations With ERPs
Correlations with behavioral data in SZ and HC groups
The ERN, CRN, and Pe components from lead Cz were regressed on the error rate, group (SZ vs. HC), and the Error Rate × Group interaction. Because error rate distributions were positively skewed in all groups, a log10 transform was applied to normalize the distributions prior to conducting the regression analyses. For the ERN regression on error rate, the Error Rate × Group interaction was not significant (p = .75), indicating similar slopes in both groups. Dropping the interaction term, the test of the common slope was highly significant (p < .001), indicating that larger (i.e., more negative) ERN amplitudes were associated with lower error rates. Covarying for error rate, the SZ versus HC group effect was not significant (p = .31). For the CRN regression on error rate, the slopes in the SZ and HC groups were not significantly different (p < .30). However, the common slope was significant (p = .014), indicating that more negative CRN amplitudes were associated with lower error rates, and the group effect was significant (p < .001), indicating that CRNs remained more negative in SZ than in HC even when controlling for error rate. For the Pe regression on error rate, the slopes significantly differed between the groups (p = .024). The slope was positive in HC, indicating that a higher error rate tended to be associated with a larger Pe (r = .18, p = .058), whereas the slope was slightly negative in the SZ (r = −.14, p = .21).
Similar regression models examined the relationships between the ERP component amplitudes and post-error slowing. For the ERN, the Group × Post-error slowing interaction showed a trend for the group slopes to differ (p = .059). In HC, greater post-error slowing was associated with a larger ERN (r = −.22, p = .02), whereas no such relationship was evident in SZ patients (r = .01, p = .97). For the CRN, the slopes significantly differed between the groups (p = .002). In HC (r = −.32, p = .001), but not in SZ (r = .03, p = .80), greater post-error slowing was associated with significantly larger (i.e., more negative) CRN amplitudes. For the Pe regression on post-error slowing, there was no significant slope difference between the groups (p = .22), nor was the common slope significant (p = .29).
Correlations with behavioral data in CHR and HC groups
Regression analyses in the CHR and HC groups examined the relationships between the ERP component amplitudes at Cz and error rate (log10 transformed). For the ERN, the Group × Error rate interaction was not significant (p = .91), indicating similar slopes in the two groups. Dropping the interaction term from the model, a significant common slope (p = .001) indicated that lower error rates were associated with more negative ERN amplitudes for both groups. Covarying for error rate, the group effect showed a trend (p = .085) for the ERN to be smaller in CHR patients than in HC. For the CRN and the Pe, the slopes did not differ between the groups (p > .24) and the common slopes were not significant (p > .27), indicating that CRN and Pe amplitudes were unrelated to error rates in the CHR and HC groups.
The ERP component amplitudes were similarly regressed on post-error slowing in the CHR and HC groups. For the ERN, there was a trend for the slopes to differ between the groups (p = .059). Greater post-error slowing was associated with more negative ERNs in the HC (r = −.26, p = .015), but not in the CHR group (r = .12, p = .42). For the CRN, the slopes did not differ between the groups (p = .57). After dropping the interaction term from the model, the common slope was significant (p = .008), indicating that greater post-error slowing was associated with more negative CRNs in both groups. Covarying for post-error slowing, the group effect on CRN amplitude was not significant (p = .74). For the Pe, the slopes did not differ between the groups (p = .40) and the common slope was not significant (p = .49), indicating no relationship with post-error slowing.
Correlational analyses with duration of illness
Given that duration of illness was significantly related to age in the full SZ sample (r = .93, p < .001), the relationship between illness duration and each ERP component was assessed using age-corrected ERP component z-scores from electrode Cz (where group effects tended to be largest). Illness duration was not significantly correlated with ERN (r = −.06, p = .50) or Pe (r = .11, p = .27) z-scores. However, illness duration showed a significant negative correlation with CRN z-scores (r = −.31, p = .001), indicating that abnormal increases in CRN amplitude (i.e., increased negativity) worsened with longer illness duration.
Correlational analyses with clinical ratings
PANSS positive and negative symptom subscales were not significantly correlated with ERN, CRN, or Pe amplitudes in the full SZ sample. Based on prior studies suggesting that ERN reductions in schizophrenia were particularly evident in patients with the paranoid subtype (Kopp & Rist, 1999; Mathalon et al., 2002), we directly compared paranoid and nonparanoid subtype schizophrenia patients. No significant subtype differences were found for ERN, CRN, or Pe. In the CHR patients, SOPS positive and negative symptom subscales were not significantly correlated with ERN, CRN, or Pe amplitudes.
Discussion
Consistent with prior reports, the present study showed that patients with schizophrenia, relative to healthy controls, have 1) a reduced ERN amplitude following commission errors in a choice–response task (Alain et al., 2002; Bates et al., 2002; Bates et al., 2004; Kim et al., 2006; Kopp & Rist, 1999; Mathalon et al., 2002; Mathalon et al., 2009; Morris et al., 2008; Morris et al., 2006), and 2) an increased CRN amplitude following correct responses (Alain et al., 2002; Kim et al., 2006; Kopp & Rist, 1999; Mathalon et al., 2002; Morris et al., 2008; Morris et al., 2006). In addition, our results extend previous findings by showing that this ERN reduction and CRN increase is evident in the early phases of schizophrenia (within 2 years of initial hospitalization or initiation of antipsychotic treatment), prior to the onset of chronicity-related sequelae. Furthermore, we found that reduced ERN amplitude, but not increased CRN amplitude, is present in patients at clinical high risk for psychosis. In contrast to previous studies (Alain et al., 2002; Bates et al., 2004; Kim et al., 2006; Mathalon et al., 2002; Morris et al., 2006), our results indicate that the Pe following the ERN on error trials is significantly reduced in schizophrenia patients, including the subset of early illness patients, but not in clinical high risk patients. All of these performance-related ERP abnormalities were present despite normal post-error slowing in all the patient groups, suggesting that patients were aware of their errors at some level. Normal post-error slowing in schizophrenia patients is consistent with some prior studies (Bates et al., 2002; Bates et al., 2004; Mathalon et al., 2002), but not others (Alain et al., 2002; Kerns et al., 2005).
None of the previously published ERP error-processing studies of patients with schizophrenia (Alain et al., 2002; Bates et al., 2002; Bates et al., 2004; Kim et al., 2006; Kopp & Rist, 1999; Mathalon et al., 2002; Mathalon et al., 2009; Morris et al., 2008; Morris et al., 2006) examined at what stage of the illness the performance-related ERP abnormalities emerged or whether they progressively worsened over the illness course. Accordingly, our study of schizophrenia patients with a wide range of illness durations, including an early illness subsample, represents a significant extension of prior studies. Our finding of reduced ERN early in the illness course, coupled with the absence of a correlation between duration of illness and ERN amplitude, suggests that reduced ERN is a non-progressive abnormality that is evident well before the sequelae of chronic illness emerge (e.g., chronic disability and medication exposure, longstanding social and occupational dysfunction). In contrast, while abnormal enhancement of the CRN was evident in the full schizophrenia sample and the early illness subsample, the magnitude of the CRN abnormality increased with illness duration, consistent with a progressive pathophysiological process that begins early in the illness. Although the nature of this progressive process is not clear, these results suggest that CRN-related response uncertainty (Coles et al., 2001) or response checking (Falkenstein et al., 2000) during cognitive tasks intensifies as the illness progresses, either as a function of neurocognitive decline or as a compensatory response to such decline. While abnormal reduction of the Pe was evident in both the full schizophrenia sample and the early illness subsample, suggesting that it is a nonprogressive marker of compromised error awareness (Endrass et al., 2007; Falkenstein et al., 1991; Hughes & Yeung, 2011; Leuthold & Sommer, 1999; Nieuwenhuis et al., 2001; O’Connell, et al., 2007), this conclusion is difficult to reconcile with the preponderance of prior studies showing normal Pe in schizophrenia (Alain et al., 2002; Bates et al., 2004; Kim et al., 2006; Mathalon et al., 2002; Morris et al., 2006).
In addition to showing that ERN, CRN, and Pe abnormalities are present in schizophrenia patients within the first 2 years of illness onset, the present study is the first to examine which of these electrophysiological error monitoring signals are abnormal prior to the onset of psychosis in clinical high risk patients. Although the CRN and Pe were normal in at-risk patients, suggesting that they may become abnormal during the transition to, or after the onset of, schizophrenia, the ERN showed a significant reduction in clinical high risk patients that was not significantly different from the reduction evident in early illness patients. These results are consistent with those from a study of pre-prodromal 9–12-year-old children exhibiting putative antecedents of schizophrenia (Laurens et al., 2010). The presence of an ERN abnormality in clinical high risk patients suggests that this neurophysiological dysfunction predates schizophrenia onset and may be a trait marker of the underlying risk for the disorder. However, whether reduced ERN amplitude predicts conversion to psychosis, and more specifically, schizophrenia, in clinical high risk patients awaits longitudinal follow-up data.
One methodological challenge associated with comparing young clinically at-risk patients and older early illness schizophrenia patients is that these groups typically differ in age. In order to detect differences between these groups that may reflect progressive pathophysiological processes, the effects of normal development and aging on the measure of interest must be taken into account. The problem with ANCOVA models is that the effect of age is estimated by pooling across the different groups, potentially removing pathological aging effects along with normal aging effects. Our approach to this challenge involves modeling the effects of normal development and aging in healthy controls, and using the resulting model to remove these effects from both the healthy control and patient groups. In the current study, we found significant age-relationships with the CRN and Pe in the healthy controls, but failed to find an age relationship with the ERN. Those studies that have documented increases in ERN amplitude during normal development have focused on age ranges from childhood to early adulthood (Davies, Segalowitz, Dywan, & Pailing, 2001; Mathewson et al., 2005; Santesso et al., 2006). The absence of such relationships in our sample may reflect the fact that our age range began in early adolescence and extended to middle age. Nonetheless, removal of normal aging effects, even nonsignificant ones, enabled a direct comparison of clinical high risk and early schizophrenia patients despite their age differences.
Several relationships were observed between behavioral performance measures and the ERP measures, providing some behavioral validation of the ERP components as reflections of performance monitoring. Greater post-error slowing was significantly related to larger ERN amplitudes in healthy controls, but not in the SZ or CHR patient groups. This correlation in healthy subjects is consistent with a number of prior reports (Debener et al., 2005; Gehring et al., 1993; Kerns et al., 2004; West & Travers, 2008) although some studies failed to find this relationship (Gehring & Fencsik, 2001; van Meel, Heslenfeldb, Oosterlaana, & Seargeanta, 2007) or showed the opposite relationship (Dudschig & Jentzsch, 2009). Post-error slowing was also related to larger CRN amplitudes in healthy subjects and clinical high risk patients, consistent with the view of post-error slowing as a strategic adjustment (Rabbitt & Rodgers, 1977) that is more likely during heightened response monitoring (reflected by larger CRNs, as discussed below). However, it should be noted that other interpretations of post-error slowing have been proposed and supported by empirical data, including the view that it reflects ongoing error processing that interferes with processing the subsequent trial (Dudschig & Jentzsch, 2009; Gehring & Fencsik, 2001; Welford & Levin, 1979) or orienting to an infrequent event (Notebaert et al., 2009). In any case, the absence of relationships between post-error slowing and the ERN or CRN in patients with schizophrenia suggests that illness-related processes uncouple these normal brain–behavior relationships.
Schizophrenia patients, clinical high risk patients, and healthy controls showed significant associations between larger ERN amplitudes and lower error rates, consistent with prior studies (e.g., (Falkenstein et al., 1991; Falkenstein et al., 2000; Gehring et al., 1995; Scheffers et al., 1996). Larger CRN amplitudes were also associated with lower error rates in schizophrenia patients and their age-matched controls, although this relationship was not evident in the clinical high risk patients and their controls. In light of the younger age distribution in clinical high risk patients and their controls, it is possible that the CRN’s correlation with error rate emerges sometime after adolescence. This correlation suggests that larger CRNs may reflect greater efforts to be vigilant during task performance or intensified response monitoring (Falkenstein et al., 2000), an interpretation that has also been invoked to account for enhanced CRN amplitudes in patients with obsessive–compulsive disorder (Endrass et al., 2007). Although the relationship between Pe amplitude and error rate significantly differed between schizophrenia patients and healthy controls, the correlations within each group were not significant. Moreover, the Pe was unrelated to error rate in the clinical high risk patients and their controls.
Among the factors that may contribute to ERN reductions in schizophrenia (and by extension, in clinical high risk patients), ACC dysfunction has been the most consistently considered (Alain et al., 2002; Bates et al., 2002; Bates et al., 2004; Kim et al., 2006; Kopp & Rist, 1999; Mathalon et al., 2002; Mathalon et al., 2009; Morris et al., 2008; Morris et al., 2006). This interpretation is based on 1) dipole modeling studies implicating the ACC as the generator of the ERN (Badgaiyan & Posner, 1998; Dehaene et al., 1994; Holroyd et al., 1998; Luu et al., 2000; Miltner et al., 2003), and 2) fMRI studies of error processing showing deficient ACC activation in schizophrenia (Becerril, Repovs, & Barch, 2011; Carter et al., 2001; Kerns et al., 2005; Laurens et al., 2003; Polli et al., 2008). Of note, in a previous multimodal ERP/fMRI study of error processing, we found schizophrenia patients to have reduced ERN amplitudes but normal fMRI ACC activation to errors (Mathalon et al., 2009), underscoring the dissociability of ERP and fMRI measures of specific cognitive processes. More generally, the ACC dysfunction implicated by these ERN abnormalities is consistent with other research demonstrating functional and structural ACC abnormalities in schizophrenia (Benes, 1993; Benes & Bird, 1987; Fornito, Yucel, Dean, Wood, & Pantelis, 2009; Minzenberg et al., 2009). Related to these ACC interpretations of abnormal ERN, the prominent Holroyd and Coles (2002) model relating the ACC’s ERN signal to transient reductions in dopamine release following outcomes that are worse than expected suggests that the reduced ERN in schizophrenia may reflect disruption of the dopamine pathways that subserve reinforcement learning and reward.
While these pathophysiological interpretations of ERN abnormalities in schizophrenia are compelling because of their links to the larger theoretical and empirical schizophrenia literature, our results also implicate the increased error rate in schizophrenia patients and clinical high risk patients, relative to healthy controls, as a possible contributor to their reduced ERN amplitudes. Not only did our results corroborate the well-established relationship between a higher error rate and a smaller ERN, they showed that once the error rate difference between the groups was taken into account, the abnormal reduction of ERN amplitude in the patients was no longer significant (although a trend persisted in the clinical high risk group). These results suggest the possibility that the ERN reduction in schizophrenia is simply attributable to their increased error rate and the tendency for higher rates to be associated with smaller ERNs. In consulting the prior literature to evaluate this possibility, it was striking that none of the prior studies showing reduced ERNs in schizophrenia (Alain et al., 2002; Bates et al., 2002; Bates et al., 2004; Kim et al., 2006; Kopp & Rist, 1999; Laurens et al., 2010; Mathalon et al., 2002; Mathalon et al., 2009; Morris et al., 2008; Morris et al., 2006) had explicitly conducted an analysis controlling for error rate, despite the fact that many of the studies reported significantly greater error rates in patients than controls (Bates et al., 2002; Kim et al., 2006; Mathalon et al., 2009; Morris et al., 2008; Morris et al., 2006). Given the caveats about conducting ANCOVA models when groups differ on the covariate (Miller & Chapman, 2001), we repeated our analyses on performance-matched subgroups of patients and healthy controls and again observed that the ERN difference between the groups was not significant. We believe it would be premature to conclude that the ERN reduction in schizophrenia is spurious based on our results, in part because the prior literature neither corroborates nor refutes our results, and in part because it is possible that the pathophysiological mechanisms that give rise to increased error rates on cognitive tasks in schizophrenia patients may be inextricably linked to the mechanisms that produce deficient error detection signals. This complex issue needs to be examined and disentangled in future schizophrenia ERN studies.
While the CRN was also correlated with error rate in our study, this relationship did not account for the CRN enlargement observed in the schizophrenia patients. This suggests that abnormal CRN enhancement in schizophrenia, a finding that has been replicated numerous times (Alain et al., 2002; Kim et al., 2006; Kopp & Rist, 1999; Mathalon et al., 2002; Morris et al., 2008; Morris et al., 2006) may reflect pathophysiological processes that are unrelated to deficits in performance accuracy per se. Given the prior report showing enhancement of the CRN in patients with lesions of the dorsolateral prefrontal cortex (DLPFC; Gehring & Knight, 2000), it seems likely that DLPFC dysfunction may contribute to the pathological enhancement of CRN in schizophrenia. This hypothesis is consistent with a large body of evidence implicating DLPFC dysfunction in schizophrenia (see Bunney & Bunney, 2000; Minzenberg et al., 2009). However, it is also possible that the CRN enhancement reflects an intensification of response monitoring or checking (Falkenstein et al., 2000) in schizophrenia patients in an effort to compensate for the cognitive impairments (e.g., in attention and cognitive control) that compromise their task performance. Indeed, a similar compensatory strategy may have contributed to the CRN enhancement in the patients with DLPFC lesions described by Gehring and Knight (2000).
Schizophrenia patients also showed a reduced Pe, contrary to our previous finding (Mathalon et al., 2002) and other findings in the literature (Alain et al., 2002; Bates et al., 2004; Kim et al., 2006; Morris et al., 2006). Although Pe has been suggested to reflect error awareness (Falkenstein et al., 1991), it was not related to post-error slowing in any group. To the extent that both Pe and post-error slowing might reflect error awareness, it is important to note that schizophrenia patients had smaller Pe but normal post-error slowing. That is, although Pe reductions suggest patients have a weakened sense of error awareness, normal post-error slowing suggests that the effects of error commission on subsequent behavior was intact. It is possible that the reduced Pe in schizophrenia patients reflects deficient neural processes associated with detection of infrequent events more generally (Arbel & Donchin, 2009), consistent with the widely replicated P300 amplitude deficits observed in schizophrenia (Ford, 1999; Jeon & Polich, 2003). A number of researchers have suggested that the Pe is a P300-like response to the detection of an error (Leuthold & Sommer, 1999; Nieuwenhuis et al., 2001; O’Connell et al., 2007), supporting this interpretation. Moreover, P300 reduction in schizophrenia is much less consistent in visual paradigms than auditory paradigms (Jeon & Polich, 2003), perhaps explaining the inconsistency of our Pe finding relative to other error monitoring studies using visual tasks. In any case, despite abnormal ERN, CRN, and Pe amplitudes, patients are still able to make use of the neural circuitry underlying these compromised components, or are able to recruit other compensatory neural mechanisms, to slow down after making an error.
Some prior studies have found ERN reductions in schizophrenia to be associated with more severe positive symptoms or to the paranoid illness subtype (Kopp & Rist, 1999; Mathalon et al., 2002; Morris et al., 2008). At least one study found reduced ERN to be associated with greater disorganization and psychomotor poverty (Bates et al., 2002). In the present study, we found no significant clinical correlations, similar to the report by Kim et al. (2006). Several other studies did not report clinical correlations (Alain et al., 2002; Mathalon et al., 2009; Morris et al., 2006). Thus, whether the ERN abnormalities are a general characteristic of schizophrenia or are more specifically associated with specific symptom domains remains unclear.
In all of the previous reports of ERN reduction in schizophrenia, the patients were treated with dopamine D2-receptor blocking antipsychotic medication. The confounding effect of medication in these studies is particularly concerning in light of the Holroyd and Coles (2002) model implicating dopamine pathways in ERN generation, as well as evidence that acute administration of a dopamine D2 antagonist reduces the ERN in healthy volunteers (Zirnheld et al., 2004). Accordingly, the fact that we observed an ERN reduction in antipsychotic-free clinical high risk patients suggests that the ERN reduction in schizophrenia is unlikely to be caused by antipsychotic medication.
In conclusion, this study indicates that the neurophysiological substrates of early error monitoring during cognitive tasks are compromised in schizophrenia across the illness course, and in patients with attenuated psychotic symptoms who are at risk for developing the full-blown disorder. To the extent that these error monitoring processes subserve learning and successful task performance, their compromise in schizophrenia may significantly contribute to the behavioral impairments patients experience when faced with cognitive tasks, and may further interfere with their ability to learn new skills. Whether the ERN abnormality in schizophrenia patients is simply a function of increased error rate is a critical question that must be pursued in future studies. In addition, it remains to be determined whether reduced ERN in clinical high risk patients predicts conversion to psychosis. This is important because improvement in our ability to detect which clinical high risk patients will convert to psychosis sets the stage for preventive intervention, allowing early treatment to be targeted to those patients who need it most.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health–supported grants R01 MH076989, R01 MH058262, and K02 MH067967. It was also supported by Department of Veterans Affairs Merit Review and the National Alliance for Research on Schizophrenia and Depression. Drs. Perez, Ford, Woods, McGlashan, Srihari, Loewy, Vinogradov, and Mathalon and Mr. Roach report no competing interests.
Contributor Information
Veronica B. Perez, Department of Psychiatry, University of California, San Francisco (UCSF) and San Francisco Veterans Administration Medical Center
Judith M. Ford, Department of Psychiatry, UCSF, San Francisco Veterans Administration Medical Center, and Yale University
Brian J. Roach, Department of Psychiatry, University of California, San Francisco (UCSF) and San Francisco Veterans Administration Medical Center
Scott W. Woods, Department of Psychiatry, Yale University
Thomas H. McGlashan, Department of Psychiatry, Yale University
Vinod H. Srihari, Department of Psychiatry, Yale University
Rachel L. Loewy, Department of Psychiatry, UCSF
Sophia Vinogradov, Department of Psychiatry, University of California, San Francisco (UCSF) and San Francisco Veterans Administration Medical Center.
Daniel H. Mathalon, Department of Psychiatry, UCSF, San Francisco Veterans Administration Medical Center, and Yale University
References
- Alain C, McNeely HE, He Y, Christensen BK, West R. Neurophysiological evidence of error-monitoring deficits in patients with schizophrenia. Cerebral Cortex. 2002;12:840–846. doi: 10.1093/cercor/12.8.840. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental health disorders. 4. Washington, DC: 1994. [Google Scholar]
- Arbel Y, Donchin E. Parsing the componential structure of post-error ERPs: A principal component analysis of ERPs following errors. Psychophysiology. 2009;46:1179–1189. doi: 10.1111/j.1469-8986.2009.00857.x. [DOI] [PubMed] [Google Scholar]
- Badgaiyan RD, Posner MI. Mapping the cingulate cortex in response selection and monitoring. Neuroimage. 1998;7:255–260. doi: 10.1006/nimg.1998.0326. [DOI] [PubMed] [Google Scholar]
- Bates AT, Kiehl KA, Laurens KR, Liddle PF. Error-related negativity and correct response negativity in schizophrenia. Clinical Neurophysiology. 2002;113:1454–1463. doi: 10.1016/s1388-2457(02)00154-2. [DOI] [PubMed] [Google Scholar]
- Bates AT, Liddle PF, Kiehl KA, Ngan ET. State dependent changes in error monitoring in schizophrenia. Journal of Psychiatric Research. 2004;38:347–356. doi: 10.1016/j.jpsychires.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Becerril KE, Repovs G, Barch DM. Error processing network dynamics in schizophrenia. NeuroImage. 2011;54:1495–1505. doi: 10.1016/j.neuroimage.2010.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM. Neurobiological investigations in cingulate cortex of schizophrenic brain. Schizophrenia Bulletin. 1993;19:537–549. doi: 10.1093/schbul/19.3.537. [DOI] [PubMed] [Google Scholar]
- Benes FM, Bird ED. An analysis of the arrangement of neurons in the cingulate cortex of schizophrenic patients. Archives of General Psychiatry. 1987;44:608–616. doi: 10.1001/archpsyc.1987.01800190024004. [DOI] [PubMed] [Google Scholar]
- Bernstein PS, Scheffers MK, Coles MG. “Where did I go wrong?” A psychophysiological analysis of error detection. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:1312–1322. doi: 10.1037//0096-1523.21.6.1312. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Smith J, Steel R, Johnstone CE, Frith CD. The perception of self-produced sensory stimuli in patients with auditory hallucinations and passivity experiences: Evidence for a breakdown in self-monitoring. Psychological Medicine: A Journal of Research in Psychiatry and the Allied Sciences. 2000;30:1131–1139. doi: 10.1017/s0033291799002676. [DOI] [PubMed] [Google Scholar]
- Brockhaus–Dumke A, Schultze–Lutter F, Mueller R, Tendolkar I, Bechdolf A, Pukrop R, Ruhrmann S. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biological Psychiatry. 2008;64:376–384. doi: 10.1016/j.biopsych.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Brooks VB. How does the limbic system assist motor learning? A limbic comparator hypothesis. Brain, Behavior and Evolution. 1987;29:29–53. doi: 10.1159/000118670. [DOI] [PubMed] [Google Scholar]
- Bunney WE, Bunney BG. Evidence for a compromised dorsolateral prefrontal cortical parallel circuit in schizophrenia. Brain Research Reviews. 2000;31:138–146. doi: 10.1016/s0165-0173(99)00031-4. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Heinssen R. Prediction of psychosis in youth at high clinical risk: A multisite longitudinal study in North America. Archives of General Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonnell L, Falkenstein M. Does the error negativity reflect the degree of response conflict? Brain Research. 2006;1095:124–130. doi: 10.1016/j.brainres.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, MacDonald AW, 3rd, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: An event-related fMRI study. American Journal of Psychiatry. 2001;158:1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- Coles MG, Scheffers MK, Holroyd CB. Why is there an ERN/Ne on correct trials? Response representations, stimulus-related components, and the theory of error-processing. Biological Psychology. 2001;56:173–189. doi: 10.1016/s0301-0511(01)00076-x. [DOI] [PubMed] [Google Scholar]
- Crovitz HF, Zener K. A group-test for assessing hand- and eye-dominance. American Journal of Psychology. 1962;75:271–276. [PubMed] [Google Scholar]
- Danielmeier C, Wessel JR, Steinhauser M, Ullsperger M. Modulation of the error-related negativity by response conflict. Psychophysiology. 2009;46:1288–1298. doi: 10.1111/j.1469-8986.2009.00860.x. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Dywan J, Pailing PE. Error-negativity and positivity as they relate to other ERP indices of attentional control and stimulus processing. Biological Psychology. 2001;56:191–206. doi: 10.1016/s0301-0511(01)00080-1. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7- to 25-year-olds. Developmental Neuropsychology. 2004;25:355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. The Journal of Neuroscience. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–305. [Google Scholar]
- Dudschig C, Jentzsch I. Speeding before and slowing after errors: Is it all just strategy? Brain Research. 2009;1296:56–62. doi: 10.1016/j.brainres.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Endrass T, Reuter B, Kathmann N. ERP correlates of conscious error recognition: Aware and unaware errors in an antisaccade task. European Journal of Neuroscience. 2007;26:1714–1720. doi: 10.1111/j.1460-9568.2007.05785.x. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoorman J, Blanke L, editors. Effects of errors in choice reaction tasks on the ERP under focused and divided attention. Tilburg, The Netherlands: Tilburg University Press; 1990. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J. Event-related potential correlates of errors in reaction tasks. Electroencephalography and Clinical Neurophysiology, Supplement. 1995;44:287–296. [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: A tutorial. Biological Psychology. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Ford JM. Schizophrenia: The broken P300 and beyond. Psychophysiology. 1999;36:667–682. [PubMed] [Google Scholar]
- Fornito A, Yucel M, Dean B, Wood SJ, Pantelis C. Anatomical abnormalities of the anterior cingulate cortex in schizophrenia: Bridging the gap between neuroimaging and neuropathology. Schizophrenia Bulletin. 2009;35:973–993. doi: 10.1093/schbul/sbn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Coles MG, Meyer DE, Donchin E. A brain potential manifestation of error-related processing. Electroencephalography and Clinical Neurophysiology, Supplement. 1995;44:261–272. [PubMed] [Google Scholar]
- Gehring WJ, Fencsik DE. Functions of the medial frontal cortex in the processing of conflict and errors. The Journal of Neuroscience. 2001;21:9430–9437. doi: 10.1523/JNEUROSCI.21-23-09430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nature Neuroscience. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Gemba H, Sasaki K, Brooks VB. ‘Error’ potentials in limbic cortex (anterior cingulate area 24) of monkeys during motor learning. Neuroscience Letters. 1986;70:223–227. doi: 10.1016/0304-3940(86)90467-2. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography & Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Herrmann MJ, Rommler J, Ehlis AC, Heidrich A, Fallgatter AJ. Source localization (LORETA) of the error-related-negativity (ERN/Ne) and positivity (Pe) Cognitive Brain Research. 2004;20:294–299. doi: 10.1016/j.cogbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Hohnsbein J, Falkenstein M, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. I. Simple and choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78:438–446. doi: 10.1016/0013-4694(91)90061-8. [DOI] [PubMed] [Google Scholar]
- Hollingshead AA. Four-factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Dien J, Coles MG. Error-related scalp potentials elicited by hand and foot movements: Evidence for an output-independent error-processing system in humans. Neuroscience Letters. 1998;242:65–68. doi: 10.1016/s0304-3940(98)00035-4. [DOI] [PubMed] [Google Scholar]
- Hughes G, Yeung N. Dissociable correlates of response conflict and error awareness in error-related brain activity. Neuropsychologia. 2011;49:405–415. doi: 10.1016/j.neuropsychologia.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: Patients, paradigms, and practical implications. Psychophysiology. 2003;40:684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Sun D, Jimenez AM, Lutkenhoff ES, Willhite R, van Erp TG, Cannon TD. Developmental disruptions in neural connectivity in the pathophysiology of schizophrenia. Development and Psychopathology. 2008;20:1297–1327. doi: 10.1017/S095457940800062X. [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler L. The positive-negative dimension in schizophrenia: Its validity and significance. Psychiatric Development. 1987;5:79–103. [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Johnson MK, Stenger VA, Aizenstein H, Carter CS. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. American Journal of Psychiatry. 2005;162:1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: An event-related fMRI study. Psychophysiology. 2000;37:216–223. [PubMed] [Google Scholar]
- Kim MS, Kang SS, Shin KS, Yoo SY, Kim YY, Kwon JS. Neuropsychological correlates of error negativity and positivity in schizophrenia patients. Psychiatry and Clinical Neurosciences. 2006;60:303–311. doi: 10.1111/j.1440-1819.2006.01506.x. [DOI] [PubMed] [Google Scholar]
- Kopp B, Rist F. An event-related brain potential substrate of disturbed response monitoring in paranoid schizophrenic patients. Journal of Abnormal Psychology. 1999;108:337–346. doi: 10.1037//0021-843x.108.2.337. [DOI] [PubMed] [Google Scholar]
- Kopp B, Rist F, Mattler U. N200 in the flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology. 1996;33:282–294. doi: 10.1111/j.1469-8986.1996.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Carter CS. ERP correlates of action monitoring in adolescence. Annals of the New York Academy of Sciences. 2004;1021:329–336. doi: 10.1196/annals.1308.040. [DOI] [PubMed] [Google Scholar]
- Laurens KR, Hodgins S, Mould GL, West SA, Schoenberg PL, Murray RM, Taylor EA. Error-related processing dysfunction in children aged 9 to 12 years presenting putative antecedents of schizophrenia. Biological Psychiatry. 2010;67:238–245. doi: 10.1016/j.biopsych.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Laurens KR, Ngan ET, Bates AT, Kiehl KA, Liddle PF. Rostral anterior cingulate cortex dysfunction during error processing in schizophrenia. Brain: A Journal of Neurology. 2003;126:610–622. doi: 10.1093/brain/awg056. [DOI] [PubMed] [Google Scholar]
- Leuthold H, Sommer W. ERP correlates of error processing in spatial S-R compatibility tasks. Clinical Neurophysiology. 1999;110:342–357. doi: 10.1016/s1388-2457(98)00058-3. [DOI] [PubMed] [Google Scholar]
- Luu P, Flaisch T, Tucker DM. Medial frontal cortex in action monitoring. Journal of Neuroscience. 2000;20:464–469. doi: 10.1523/JNEUROSCI.20-01-00464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Angel RW, Hampton B, Berger PA. Impaired central error-correcting behavior in schizophrenia. Archives of General Psychiatry. 1982;39:101–107. doi: 10.1001/archpsyc.1982.04290010073013. [DOI] [PubMed] [Google Scholar]
- Masaki H, Falkenstein M, Sturmer B, Pinkpank T, Sommer W. Does the error negativity reflect response conflict strength? Evidence from a Simon task. Psychophysiology. 2007;44:579–585. doi: 10.1111/j.1469-8986.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Fedor M, Faustman WO, Gray M, Askari N, Ford JM. Response-monitoring dysfunction in schizophrenia: An event-related brain potential study. Journal of Abnormal Psychology. 2002;111:22–41. [PubMed] [Google Scholar]
- Mathalon DH, Jorgensen KW, Roach BJ, Ford JM. Error detection failures in schizophrenia: ERPs and FMRI. International Journal of Psychophysiology. 2009;73:109–117. doi: 10.1016/j.ijpsycho.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Whitfield SL, Ford JM. Anatomy of an error: ERP and fMRI. Biological Psychology. 2003;64:119–141. doi: 10.1016/s0301-0511(03)00105-4. [DOI] [PubMed] [Google Scholar]
- Mathewson KJ, Dywan J, Segalowitz SJ. Brain bases of error-related ERPs as influenced by age and task. Biological Psychology. 2005;70:88–104. doi: 10.1016/j.biopsycho.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, Woods SW. Prodromal assessment with the Structured Interview for Prodromal Syndromes and the Scale of Prodromal Symptoms: Predictive validity, interrater reliability, and training to reliability. Schizophrenia Bulletin. 2003;29:703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, Woods SW. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: Preliminary evidence of interrater reliability and predictive validity. American Journal of Psychiatry. 2002;159:863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- Milter WH, Braun CH, Coles MGH. Event-related potentials following incorrect feedback in a time-estimation task: Evidence for a ‘generic’ neural system for error detection. Journal of Cognitive Neuroscience. 1997;9:787–797. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Miltner WH, Lemke U, Weiss T, Holroyd C, Scheffers MK, Coles MG. Implementation of error-processing in the human anterior cingulate cortex: A source analysis of the magnetic equivalent of the error-related negativity. Biological Psychology. 2003;64:157–166. doi: 10.1016/s0301-0511(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Archives of General Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SE, Heerey EA, Gold JM, Holroyd CB. Learning-related changes in brain activity following errors and performance feedback in schizophrenia. Schizophrenia Research. 2008;99:274–285. doi: 10.1016/j.schres.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SE, Yee CM, Nuechterlein KH. Electrophysiological analysis of error monitoring in schizophrenia. Journal of Abnormal Psychology. 2006;115:239–250. doi: 10.1037/0021-843X.115.2.239. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: Evidence from an antisaccade task. Psychophysiology. 2001;38:752–760. [PubMed] [Google Scholar]
- Niki H, Watanabe M. Prefrontal and cingulate unit activity during timing behavior in the monkey. Brain Research. 1979;171:213–224. doi: 10.1016/0006-8993(79)90328-7. [DOI] [PubMed] [Google Scholar]
- Notebaert W, Houtman F, Opstal FV, Gevers W, Fias W, Verguts T. Post-error slowing: An orienting account. Cognition. 2009;111:275–279. doi: 10.1016/j.cognition.2009.02.002. [DOI] [PubMed] [Google Scholar]
- O’Connell RG, Dockree PM, Bellgrove MA, Kelly SP, Hester R, Garavan H, Foxe JJ. The role of cingulate cortex in the detection of errors with and without awareness: A high-density electrical mapping study. European Journal of Neuroscience. 2007;25:2571–2579. doi: 10.1111/j.1460-9568.2007.05477.x. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The stability of error-related brain activity with increasing trials. Psychophysiology. 2009;46:957–961. doi: 10.1111/j.1469-8986.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosen-bloom MJ, Lane B, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: A quantitative MRI study. Alcoholism: Clinical and Experimental Research. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Polli FE, Barton JJ, Thakkar KN, Greve DN, Goff DC, Rauch SL, Manoach DS. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain: A Journal of Neurology. 2008;131:971–986. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- Pontifex MB, Scudder MR, Brown ML, O’Leary KC, Wu CT, Themanson JR, Hillman CH. On the number of trials necessary for stabilization of error-related brain activity across the life span. Psychophysiology. 2010;47:767–773. doi: 10.1111/j.1469-8986.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Rodgers B. What does a man do after he makes an error? an analysis of response programming. The Quarterly Journal of Experimental Psychology. 1977;29:727–743. [Google Scholar]
- Santesso DL, Segalowitz SJ. Developmental differences in error-related ERPs in middle- to late-adolescent males. Developmental Psychology. 2008;44:205–217. doi: 10.1037/0012-1649.44.1.205. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ, Schmidt LA. Error-related electrocortical responses in 10-year-old children and young adults. Developmental Science. 2006;9:473–481. doi: 10.1111/j.1467-7687.2006.00514.x. [DOI] [PubMed] [Google Scholar]
- Scheffers MK, Coles MG. Performance monitoring in a confusing world: Error-related brain activity, judgments of response accuracy, and types of errors. Journal of Experimental Psychology: Human Perception and Performance. 2000;26:141–151. doi: 10.1037//0096-1523.26.1.141. [DOI] [PubMed] [Google Scholar]
- Scheffers MK, Coles MG, Bernstein P, Gehring WJ, Donchin E. Event-related brain potentials and error-related processing: An analysis of incorrect responses to go and no-go stimuli. Psychophysiology. 1996;33:42–53. doi: 10.1111/j.1469-8986.1996.tb02107.x. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Santesso DL, Jetha MK. Electrophysiological changes during adolescence: A review. Brain and Cognition. 2010;72:86–100. doi: 10.1016/j.bandc.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart MA. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Turken AU, Vuilleumier P, Mathalon DH, Swick D, Ford JM. Are impairments of action monitoring and executive control true dissociative dysfunctions in patients with schizophrenia? American Journal of Psychiatry. 2003;160:1881–1883. doi: 10.1176/appi.ajp.160.10.1881. [DOI] [PubMed] [Google Scholar]
- van der Stelt O, Lieberman JA, Belger A. Auditory P300 in high-risk, recent-onset and chronic schizophrenia. Schizophrenia Research. 2005;77:309–320. doi: 10.1016/j.schres.2005.04.024. [DOI] [PubMed] [Google Scholar]
- van Meel CS, Heslenfeldb DJ, Oosterlaana J, Sergeanta JA. Adaptive control deficits in attention-deficit/hyperactivity disorder (ADHD): The role of error processing. Psychiatry Research. 2007;151:211–220. doi: 10.1016/j.psychres.2006.05.011. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: FMRI and ERP studies. Physiology and Behavior. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Vidal F, Hasbroucq T, Grapperon J, Bonnet M. Is the ‘error negativity’ specific to errors? Biological Psychology. 2000;51:109–128. doi: 10.1016/s0301-0511(99)00032-0. [DOI] [PubMed] [Google Scholar]
- Welford NT, Levin HS. Time on target meter. Cortex. 1979;15:157–160. doi: 10.1016/s0010-9452(79)80018-0. [DOI] [PubMed] [Google Scholar]
- West R, Travers S. Tracking the temporal dynamics of updating cognitive control: An examination of error processing. Cerebral Cortex. 2007;18:1112–1124. doi: 10.1093/cercor/bhm142. [DOI] [PubMed] [Google Scholar]
- Woods SW, Addington J, Cadenhead KS, Cannon TD, Cornblatt BA, Heinssen R, McGlashan TH. Validity of the prodromal risk syndrome for first psychosis: Findings from the North American Prodrome Longitudinal Study. Schizophrenia Bulletin. 2009;35:894–908. doi: 10.1093/schbul/sbp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung AR, McGorry PD. The initial prodrome in psychosis: Descriptive and qualitative aspects. Australian and New Zealand Journal of Psychiatry. 1996;30:587–599. doi: 10.3109/00048679609062654. [DOI] [PubMed] [Google Scholar]
- Zirnheld PJ, Carroll CA, Kieffaber PD, O’Donnell BF, Shekhar A, Hetrick WP. Haloperidol impairs learning and error-related negativity in humans. Journal of Cognitive Neuroscience. 2004;16:1098–1112. doi: 10.1162/0898929041502779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.