Abstract
Introduction
Ultrasound guided access allows for direct visualization of the access artery during percutaneous endovascular aortic aneurysm repair. We hypothesize that the use of ultrasound guidance allowed us to safely increase the utilization of percutaneous endovascular aortic aneurysm repair to almost all patients and decrease access complications.
Methods
A retrospective chart review of all elective endovascular aortic aneurysm repairs, both abdominal and descending thoracic, from 2005-2010 was performed. Patients were identified using ICD9 codes and stratified based on access type: percutaneous vs. cutdown. We examined the success rate of percutaneous access and the cause of failure. Sheath size was large (18-24 Fr) or small (12-16 Fr). Minimum access vessel diameter was also measured. Outcomes were wound complications (infections or clinically significant hematomas that delayed discharge or required transfusion), operative and incision time, length of stay, and discharge disposition. Predictors of percutaneous failure were identified.
Results
168 patients (296 arteries) had percutaneous access (P-EVAR) while 131 patients (226 arteries) had femoral cutdown access (C-EVAR). Ultrasound guided access was introduced in 2007. P-EVAR increased from zero cases in 2005 to 92.3% of all elective cases in 2010. The success rate with percutaneous access was 96%. Failures requiring open surgical repair of the artery included 7 for hemorrhage and 6 for flow limiting stenosis or occlusion of the femoral artery. P-EVAR had fewer wound complications (0.7% vs. 7.4%, P = .001) shorter operative time (153.3 vs. 201.5 minutes, P < .001) and larger minimal access vessel diameter (6.7 mm vs. 6.1 mm, P < .01). Patients with failed percutaneous access had smaller minimal access vessel diameters when compared to successful P-EVAR (4.9 mm vs. 6.8 mm, P < .001). More failures occurred in small sheaths than large ones (7.4% vs. 1.9%, P = .02). Access vessel diameter < 5 mm is predictive of percutaneous failure (16.7% of vessels < 5 mm failed vs. 2.4% of vessels ≥ 5 mm, P < .001) (OR 7.3, 95% CI [1.58-33.8], P = .01).
Conclusion
Ultrasound guided percutaneous EVAR can be performed in the vast majority of patients with a high success rate, shorter operative times, and fewer wound complications. Access vessel diameters less than 5 mm are at greater risk for percutaneous failure and should be treated selectively.
Introduction
Endovascular aortic aneurysm repair has replaced open surgical repair as the preferred method for treating anatomically suitable infrarenal abdominal aortic aneurysms (AAA) and descending thoracic aortic aneurysms (DTA) in the elective setting 1. Endovascular repair requires the placement of large diameter sheaths into the common femoral arteries (CFA) for stent graft deployment. Access to the CFA has typically been achieved by open femoral cutdown incisions. Complications related to femoral cutdown include wound infections, lymphoceles, and hematomas. With the development of suture mediated closure devices and the “preclose” technique 2 for closure of large femoral arteriotomies, some vascular surgeons are now performing percutaneous EVAR. Among prior reports including 50 or more patients, the technical success rates of percutaneous access have ranged between 76% and 96% 3-7. The utilization of ultrasound guidance has been suggested to improve accuracy and lower the complication rate associated with percutaneous access 7, 8.
Several patient factors have been stated to be relative contraindications for percutaneous arteriotomy closure. Some studies have demonstrated that morbid obesity (Body Mass Index > 35), femoral artery calcification, groin scars from prior interventions, and large sheath size are more commonly associated with percutaneous failure and wound complications 3, 9-15. Other studies have demonstrated no association between sheath size, morbid obesity, and femoral artery calcification with percutaneous failure and wound complications 7, 16, 17. Percutaneous access has been suggested to decrease operating room (OR) time as well as patient length of stay (LOS) in the hospital 9, 11.
The majority of the data are from single institution case series and small single center randomized trials where patients had percutaneous access in one limb and femoral cutdown access in the contralateral limb. Our study is the first to group patients into purely percutaneous access, purely femoral cutdown access, or both. The purpose of this study is to describe our experience with percutaneous endovascular aortic aneurysm repair and to compare our outcomes with the published literature.
Methods
Overview
We performed a retrospective study of all elective endovascular aortic aneurysm repairs (abdominal and thoracic) from January 1, 2005 to December 31, 2010 at a single tertiary care medical center. Patients were stratified based on their type of access for stent graft delivery: percutaneous (P-EVAR) vs. femoral cutdown access (C-EVAR). Only limbs accessed with a 12 French (Fr) sheath size or larger were included in the study. Sheaths were categorized as small (12-16 Fr) or large (18-24 Fr). The majority of cases were performed in an operating room endovascular suite with fixed imaging and most patients received general anesthesia. All patients were anticoagulated with heparin during the procedure and received protamine reversal at the end of the case. Patients were seen for follow up typically at 1 month where each CFA puncture site and cutdown incision was assessed via clinical examination. A pulse examination was performed as well as a history taken to identify symptoms of claudication. A computed tomography (CT) scan is obtained that goes through the femoral heads to evaluate the femoral artery access site. Demographic data were recorded as well as BMI. Intraoperative variables analyzed included sheath size and minimum access vessel diameter. Minimum access vessel diameter was measured by CT scan and is the minimum intraluminal diameter of the CFA or external iliac artery as determined by the PEMS M2S Patient Evaluation and Management System.
Outcome measures evaluated were technical success rate of percutaneous access, conversion to open femoral artery repair, cause of percutaneous failure, LOS, discharge disposition, and wound complications. Technical success was defined as a successful arterial closure without the need for conversion to open femoral artery repair as well as no change in the patient’s baseline pulse examination. Percutaneous failures were due to either hemorrhage or flow limiting stenosis or occlusion of the CFA. Hemorrhage was defined as persistent bleeding or an expanding hematoma that required surgical exploration after tying the perclose sutures. Flow limitation was identified immediately in the operating room based on a change in the patient’s baseline pulse exam at the end of the procedure or at the 1 month follow-up visit if the patient had symptoms of claudication or a change in their pulse examination. OR time (time from entry into the OR until discharge to the recovery room) and incision time (time from initial groin puncture or skin incision to skin closure or completion of manual compression) were measured as well. OR and incision time were compared only in aneurysm repairs without concomitant procedures such as hypogastric coiling and renal and iliac stents. Wound complications included wound infections requiring antibiotic treatment, seromas, pseudoaneurysms, and clinically significant hematomas that delayed routine discharge from the hospital or required transfusion. This study was approved by the Institutional Review Board at Beth Israel Deaconess Medical Center and Harvard Medical School.
Statistical analysis
We calculated the total number of patients and arteries that underwent percutaneous or femoral cutdown access and how the proportion of each has changed over time. Analyses of mean sheath size, proportion of sheaths that were large (18-24 Fr), and technical success of percutaneous access were performed on a per limb basis. Analyses of wound complications and other outcomes were calculated on a per patient basis. Preoperative characteristics and outcomes are reported as proportions of the sample and median with [interquartile range] (IQR). Length of stay is reported as both mean ± standard deviation as well as median with IQR. Patient variables were compared utilizing univariate analysis. Categorical variables were analyzed using chi-square and the Fisher exact test where appropriate. Continuous variables were compared using the Wilcoxon rank sum test. A test of trend over time was used to test for significance in changes in the minimum access vessel diameter in P-EVAR patients over the study period. Multivariable logistic regression was used to identify predictors of percutaneous failure. Statistical significance was defined as P < .05. All statistical tests were performed using STATA 12 software (StataCorp, College Station, TX).
Patient Selection
Early in our experience, the decision to perform endovascular aortic aneurysm repair via percutaneous access varied by attending. Percutaneous access was initially performed on the contralateral 12 Fr side when deploying the Gore Excluder stent graft (W.L. Gore & Associates, Flagstaff, AZ). Percutaneous access was generally avoided in patients with small arteries and femoral artery calcification in our early experience. Ultrasound (US) guidance for percutaneous access was introduced and routinely used beginning in 2007. As our experience broadened with the utilization of US guidance, patient selection for percutaneous access expanded rapidly and now virtually every elective EVAR is performed with percutaneous access.
Preclose Technique
All patients receive preoperative intravenous antibiotics. After sterile drapes are placed, the US probe is used to identify the CFA and the femoral bifurcation in all patients. The best location for CFA puncture is determined by the extent of calcification on the anterior wall and plaque both anteriorly and posteriorly. A small stab incision is made in the skin inferior to the expected arterial puncture site. Blunt dissection with a hemostat is then carried down through the subcutaneous tissue to the anterior wall of the CFA under US guidance. A micropuncture needle is inserted into the CFA under direct visualization with the US probe. Fluoroscopy is used to confirm puncture over the femoral head. A microsheath and 0.035 inch wire are inserted into the CFA followed by a 7 Fr dilator. After dilation of the artery and subcutaneous tissue a 6 Fr Perclose Proglide device (Abbott Vascular, Redwood City, CA) is inserted over the wire into the CFA. Pulsatile blood flow from the marker lumen confirms proper positioning of the Proglide device within the CFA. The Proglide device is then fired and the wire is replaced before the device is withdrawn from the CFA. The sutures are not tied down as they are secured to the sterile drapes with a Kelly clamp. A second Proglide device is inserted into the same artery over the wire, confirmation of intra-arterial placement is obtained, the wire is removed and the device is fired, the wire is then replaced, and the sutures are secured with a Crile clamp to distinguish the second knot from the first. A 7 Fr 25 cm sheath is then inserted into the CFA. The above steps are repeated on the contralateral groin leaving two sets of untied Proglide sutures in each groin. Initially, we deployed the two devices at 10 and 2 o’clock and changed over time to 11 and 1 o’clock and currently aim for 11:59 and 12:01 with the goal of slightly offsetting the devices without puncturing the side wall of the CFA to avoid narrowing the vessel or failure to puncture the vessel wall. The patient is then heparinized. If there is hemorrhage around the 7 Fr sheath a 12 Fr sheath is inserted over an Amplatz or Lunderquist wire. Serial dilations are employed with Coons dilators before placement of the large sheath and device.
Standard EVAR then commences. The majority of the stent grafts placed at our institution during the study period were Gore and Zenith (Cook, Bloomington, IN) stent grafts. In general, we deliver the main body through the larger access vessel. At the completion of the operation the previously placed perclose sutures are irrigated to remove any debris that may be present. It is important to maintain wire access until hemostasis is assured. Initially we used a floppy guide wire, but now we use a Benstson wire. We prefer to avoid a stiff wire as this may hamper hemostasis with the initial knot. Gentle manual pressure is applied to the proximal CFA as the sheath is removed over a wire. After removal of the sheath, manual pressure is released to allow the knot pusher to advance properly through the subcutaneous tissue and cinch down the preformed knot of the perclose suture. If hemostasis is adequate after tying of the first perclose suture, the wire is removed and the suture is locked and cut. The second suture is then tied down, locked and cut. Manual pressure is then held for approximately 5 minutes. If hemostasis is inadequate after tying the first knot, a third perclose device is inserted over the wire and deployed and this usually results in adequate hemostasis. If hemostasis is still not achieved, a fourth perclose device may be deployed or a 7 Fr sheath may be introduced over the wire to provide hemostasis while the CFA is surgically explored (this can be upsized to a 12 Fr sheath or larger as needed). The pedal pulses are always checked at the end of the operation and compared to the preoperative pulse exam. Duplex examination of the puncture site may be performed to assess the patency of the femoral vessels. In our series, initially, a duplex examination was never performed, but now it is performed selectively. All patients with a groin hematoma received manual compression followed by a duplex examination to evaluate for ongoing hemorrhage or pseudoaneurysm. If a patient has no palpable pulses preoperatively, a duplex examination should be performed because it may affect the decision making regarding open surgical repair of the femoral artery. If pulses are lost or the duplex examination shows significant stenosis, the ipsilateral groin is then explored surgically.
Results
We identified 294 patients who underwent elective endovascular repair of their aortic aneurysm between 2005 and 2010 at our institution; 267 had AAA and 27 had DTA. 163 patients underwent percutaneous access (P-EVAR) while 126 underwent femoral cutdown access (C-EVAR). Five patients had combined access with one groin accessed via percutaneous technique and the other accessed via cutdown. Only limbs accessed with 12 Fr sheaths or larger were evaluated: 168 patients (296 arteries) underwent P-EVAR and 131 patients (226 arteries) underwent C-EVAR. Sheath size was similar between P-EVAR and C-EVAR patients (17.0 ± 2.0 Fr vs. 17.2 ± 1.5 Fr, P = .58). Similarly, the proportion of sheaths that were considered large (18-24 Fr) was similar between P-EVAR and C-EVAR patients (54.1% vs. 60.6%, P = .13). A third perclose device was needed in 59 of 296 arteries (19.9%). Six of the 59 additional perclose devices failed to achieve hemostasis (10.2%). A fourth perclose device was placed in 2 of these arteries (0.7%) and both of these were successful in achieving hemostasis. The remaining 4 arteries underwent conversion to open surgical repair. We experienced no problems passing the 3rd and 4th perclose devices through the arteriotomy.
Percutaneous vs. Cutdown
We performed zero percutaneous repairs in 2005 and two in 2006 (6.7% of all endovascular repairs in 2006). After the introduction of ultrasound in 2007, the proportion of cases performed via percutaneous access increased from 39.3% in 2007 to 92.3% in 2010 (Figure 1). Four of the 5 femoral cutdowns performed in 2010 were for planned femoral-femoral bypasses in patients with severe occlusive disease of one of their iliac arteries while the remaining femoral cutdown was performed in a patient with distal embolization of debris from the aneurysm sac. Femoral cutdown was performed in this patient to isolate the femoral vessels and prevent further lower extremity embolization during wire manipulation and stent graft delivery.
Figure 1.
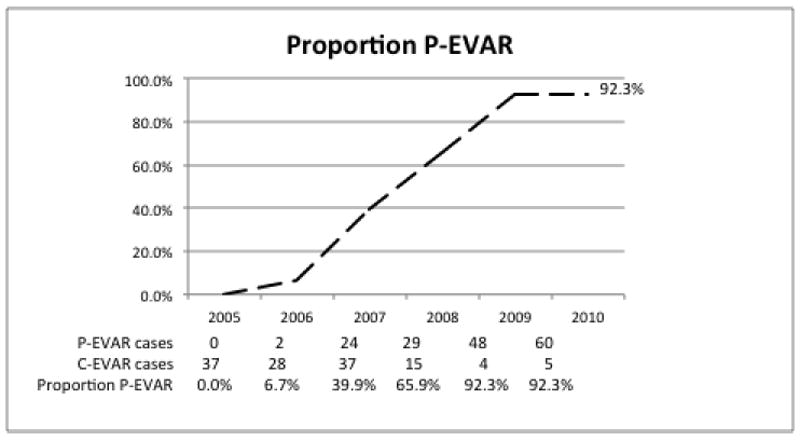
Proportion of P-EVAR over time 2005-2010
Demographic and Comorbidities
The majority of patients were white males with a similar mean age in the P-EVAR and C-EVAR patients (75.0 years vs. 75.7 years) [Table 1]. Comorbidities were similar between the P-EVAR and C-EVAR patients. The only significant differences were a higher rate of hypertension and hyperlipidemia in the P-EVAR group. Mean BMI was similar (27.0 vs. 26.7) as well as the proportion of obese patients with BMI > 30 (28.2% vs. 19.4%). The proportion of patients with prior groin operations was 6.8% in the P-EVAR group vs. 11.1% in the C-EVAR group.
Table 1.
Demographics and comorbidities of patients undergoing P-EVAR or C-EVAR, 2005-2010.
| P-EVAR | C-EVAR | |
|---|---|---|
| Male | 81.0% | 77.0% |
| Age (mean) | 75.0 ± 8.8 | 75.7 ± 8.9 |
| White race | 85.9% | 89.7% |
| Coronary artery disease | 45.4% | 43.7% |
| Myocardial Infarction | 25.2% | 27.0% |
| Percutaneous coronary intervention | 17.2% | 13.5% |
| Coronary artery bypass graft | 19.0% | 21.4% |
| Hypertension | 82.8% | 69.1% |
| Atrial fibrillation | 15.3% | 19.8% |
| Valvular heart disease | 8.0% | 12.7% |
| Congestive heart failure | 7.4% | 11.9% |
| Hyperlipidemia | 74.2% | 57.9% |
| Diabetes | 19.6% | 22.2% |
| Renal Insufficiency | 19.0% | 23.8% |
| COPD | 25.2% | 31.8% |
| Stroke | 11.7% | 10.3% |
| Peripheral Vascular disease | 19.6% | 19.1% |
| Body Mass Index (mean) | 27.0 ± 5.3 | 26.7 ± 5.7 |
| % BMI > 30 (obese) | 28.2% | 19.4% |
| Prior groin operation | 6.8% | 11.1% |
| Minimum Access Vessel Diameter | 6.7 ± 1.6mm | 6.1 ± 1.4mm |
COPD = chronic obstructive pulmonary disease
Minimum Access Vessel Diameter
The minimum access vessel diameter was significantly larger in the P-EVAR group (6.7 mm vs. 6.1 mm, P < .01). The change in minimum vessel diameter over time in arteries undergoing P-EVAR is shown in Figure 2. The mean diameter was 7.3 mm in 2006 and this decreased by over 1 mm to 6.2 mm in 2010 (P = .01, test of trend).
Figure 2.
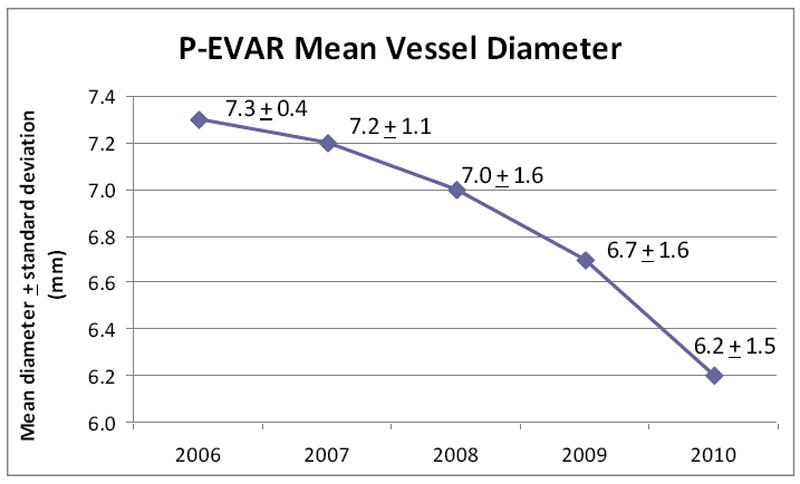
Change in P-EVAR minimum access vessel diameter over time 2006-2010
Percutaneous Failure
There were 13 percutaneous failures (12 patients) in 296 arteries requiring conversion to open surgical exploration and repair of the artery, giving a technical success rate of 96% (93% success rate per patient). Eleven failures were noted immediately in the operating room at the conclusion of the case, one failure occurred 12 hours post-operatively when the patient was on the vascular ward, and one failure was discovered on post-operative day 20 at the patient’s follow-up clinic visit [Table 2]. Figure 3 is a preoperative CT angiogram with 3-dimensional reconstruction of a patient with extensive plaque burden in the right common femoral/external iliac artery who experienced percutaneous failure. This patient was noted to have no pedal pulses at the end of the operation and after open surgical exploration the patient was noted to have a plaque disruption from the perclose device causing significant flow limiting stenosis.
Table 2.
Causes of percutaneous failure requiring open surgical repair.
|
Hemostasis |
| Tear in anterior arterial wall |
| Disrupted plaque |
5 failed perclose deployments
|
|
Flow limiting stenosis |
| 5 disrupted plaques |
| Thrombosis due to posterior intimal damage |
Figure 3.
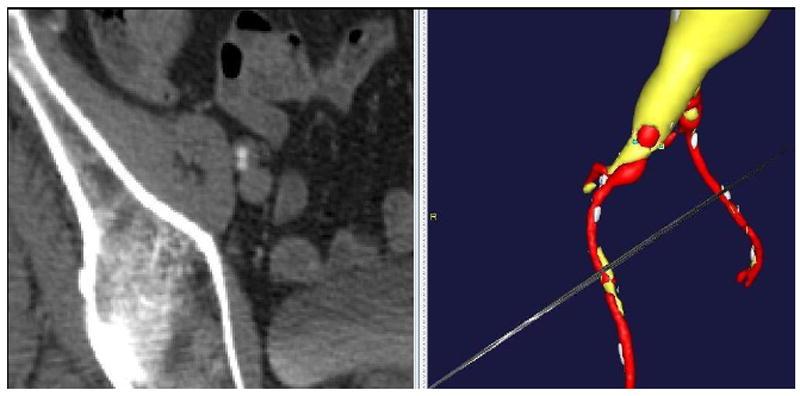
Preoperative CT angiogram with 3-dimensional reconstruction showing a patient with heavy plaque burden in the right common femoral and external iliac arteries who experienced percutaneous failure.
A greater proportion of women experienced failure compared to men, but this was not significant (12.5% vs. 5.9%, P = .18). One failure occurred in an obese patient and one occurred in a patient with groin scar tissue. Figure 4 shows the absolute number of arteries per millimeter of size along the left vertical axis (48 diameters are missing) and the proportion of arteries that failed percutaneous access per millimeter of size along the right vertical axis (1 diameter is missing). The majority of the access vessels in our study had minimum diameters between 5 and 8.9 mm (78.2%), while a small amount (4.8%) were larger vessels (≥ 9mm) and the remaining 16.9% were small vessels between 3 and 4.9 mm. The percutaneous failure rate was 16.7% for all access vessels < 5 mm, 6.3% for vessels 5-5.9 mm, 2.1% for vessels 6-8.9 mm, and 0% in vessels ≥ 9 mm. The minimum access vessel diameter was significantly smaller in patients who failed percutaneous access (4.9 mm vs. 6.8 mm, P < .001). Most failures (58.3%) in our study occurred in arteries smaller than 5 mm. Figure 5 shows the absolute number of sheaths per Fr size along the left vertical axis and the proportion of failures per sheath size along the right vertical axis. More failures occurred in small sheaths than large ones (7.4% vs. 1.9%, P = .02). Percutaneous failures have decreased over time: 9.1% in 2007, 6.8% in 2009, and 2.7% in 2010 (there were zero failures in 2008). The decrease in percutaneous failures occurred despite a greater proportion of smaller arteries (< 5mm) being treated over time: 0% of arteries were < 5mm in 2007, 8.0% in 2008, 15.8% in 2009, and 21.7% in 2010.
Figure 4.
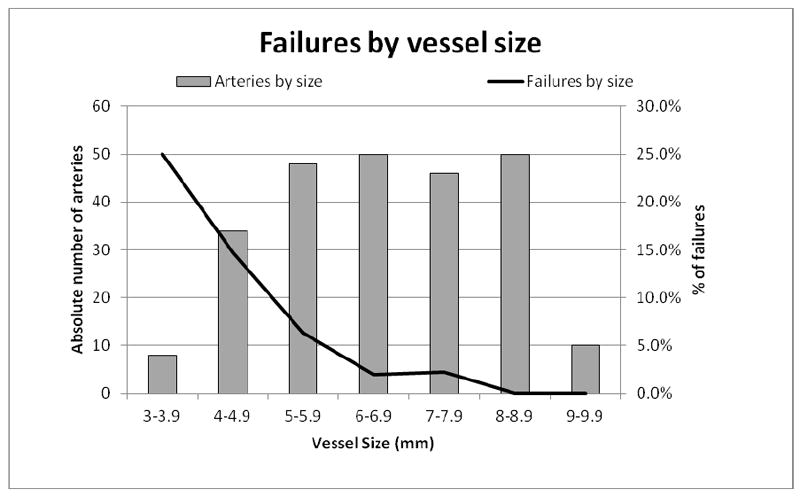
Minimum access vessel diameter in millimeters (horizontal axis) with the absolute number of treated arteries within each access vessel diameter (left axis) and the proportion of arteries with percutaneous failure within each access vessel diameter (right axis)
Figure 5.
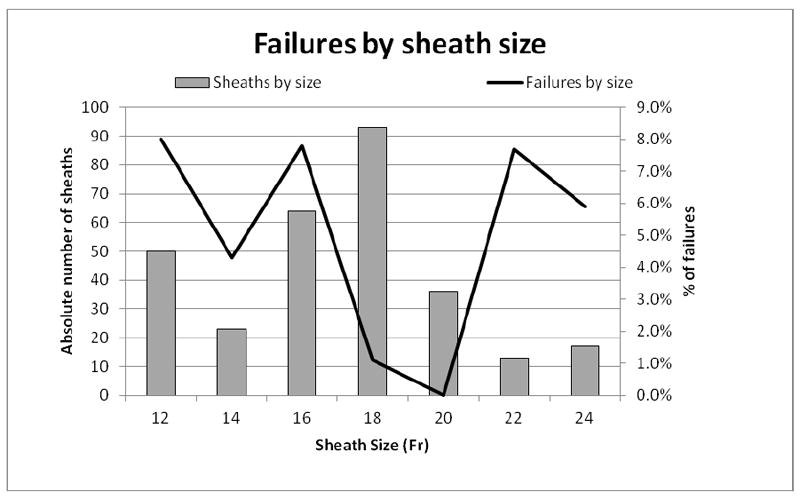
Sheath size (horizontal axis) with the absolute number of sheaths per Fr size (left axis) and the proportion of percutaneous failures per sheath size (right axis)
Postoperative Outcomes
There were no deaths. More P-EVAR patients were discharged home (91.2% vs. 84.4%, P = .11). Mean LOS was 1 day shorter in P-EVAR patients, which exhibited a trend towards significance (3.1 days vs. 4.1 days, P = .08). Median LOS was similar (2 days [1 – 3] vs. 2 days [2 – 4]). Sixteen P-EVAR patients and 31 C-EVAR patients had no 30-day follow up and were excluded from the analysis of wound complications. There was one wound complication (0.7%) in the P-EVAR group (hematoma that delayed discharge) and 7 wound complications (7.4%) in the C-EVAR group (3 wound infections, 2 hematomas that delayed discharge, 1 pseudoaneurysm, and 1 seroma), P < .01. Sixteen patients had longitudinal cutdown incisions and 52 had oblique incisions. The remaining patients did not have sufficient documentation to determine incision orientation. The wound complication rate was 6.3% with longitudinal incisions and 10.9% with oblique incisions. There were no wound complications in the 12 patients with failed percutaneous access.
OR and incision time
When examining straight forward EVAR cases only (excluding patients with concomitant procedures such as hypogastric embolization (n=11) and renal (n=6) or iliac stents (n=11) for stenosis), percutaneous access significantly reduced total OR time and incision time when compared to femoral cutdown access: OR time (143 [126 – 173] minutes vs. 196 [178 – 214] minutes, P < .001) and incision time (90 [72 – 109] minutes vs. 133 [116 – 153] minutes, P < .001). Failed percutaneous access negated the reduction in OR and incision times when compared to patients with successful percutaneous access: OR time (197 [169 – 269] minutes vs. 141 [125 – 170] minutes, P < .001) and incision time (147 [115 – 212] minutes vs. 89 [72 – 107] minutes, P < .001). Failed P-EVAR OR and incision times were longer than C-EVAR but this was not significant.
Predictors of percutaneous failure
Multivariable logistic regression was performed to identify predictors of percutaneous failure. Due to the low rate of percutaneous failures (n=13), a limited multivariable model was run. After controlling for age, female gender, and the presence of peripheral vascular disease (any patient with a diagnosis of claudication or a history of lower extremity angioplasty, stenting, or bypass), small vessel size (< 5mm, dichotomous variable) is predictive of percutaneous failure (OR 7.3, 95% CI [1.58-33.8], P = .01).
Discussion
We observed a high technical success rate with US guided percutaneous access and found that minimum access vessel diameter rather than sheath size predicts failure. In our experience, we started simple, and as we gained experience and familiarity with percutaneous access we expanded this technique to virtually all patients. In addition to experience, we feel that US guidance has allowed us to improve our technical success rate by allowing us to more accurately puncture the CFA, rather than the superficial femoral or profunda femoris arteries, and to find the best puncture site on the CFA avoiding areas with anterior calcification or plaque if present. Our study is the first to incorporate the minimum access vessel diameter as a potential predictor of percutaneous failure and found this to be the only predictor of failure even with large 24 Fr sheaths.
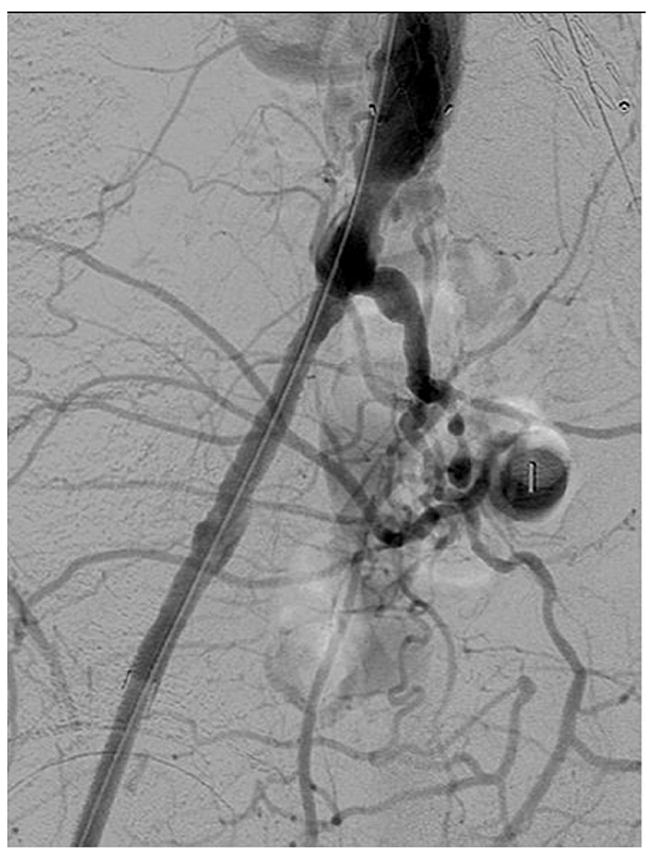
The majority of the access vessels in our study had minimum diameters between 5 and 8.9 mm (78.3%), while a small amount (4.8%) were larger vessels (≥ 9mm) and the remaining 16.9% were small vessels between 3 and 4.9 mm. As we have expanded P-EVAR to nearly all patients who present for elective AAA repair, it is of no surprise that the minimum access vessel diameter of patients undergoing P-EVAR has decreased over time from a mean size of 7.3 mm in 2006 to a mean size of 6.2 mm in 2010 (P = .01). Of the 12 percutaneous failures with documented minimum vessel diameters, 7 (58.3%) were in vessels smaller than 5 mm (16.7% of all vessels < 5 mm failed compared to 2.4% of all vessels ≥ 5 mm). The minimum access vessel diameter was significantly smaller in patients who failed percutaneous access (4.9 mm vs. 6.8 mm, P < .001). Women typically have smaller arteries than men, and a greater proportion of women in our study experienced percutaneous failure when compared to men (12.5% vs. 5.9%, P = .19), but this was not significant. When performing multivariable logistic regression and controlling for age and gender, access vessel size < 5 mm was the only predictor of percutaneous failure. This does not mean that patients with small access vessels should not be offered percutaneous repair, but rather that care must be exercised when deciding which small vessels upon which to attempt percutaneous repair. More data is needed regarding small access vessels and percutaneous repair as none of the existing grafts are designed for use in access vessels < 5 mm in diameter. However, we have found that most arteries will tolerate this with a “Dotter” technique. Figure 6a shows a patient with small common femoral and external iliac arteries where the 7 Fr sheaths are occlusive when introduced into the arteries. Figures 6b and 6c show widely patent common femoral and external iliac arteries at the completion of the operation and the “Dotter” technique. We feel that small vessel size predicts failure because small diseased CFAs and EIAs may not allow adequate room for proper function of the perclose ProGlide device as the foot plate may not have adequate clearance for proper function without disrupting plaque or even suturing the back wall of the artery to the anterior wall. The Instructions For Use of the perclose ProGlide device state that “its’ effectiveness has not been evaluated in small femoral arteries < 5 mm in diameter”18. We feel that future improvements in percutaneous EVAR will be realized due to improvements in percutaenous closure devices rather than solely based on lower profile devices.
Figure 6.
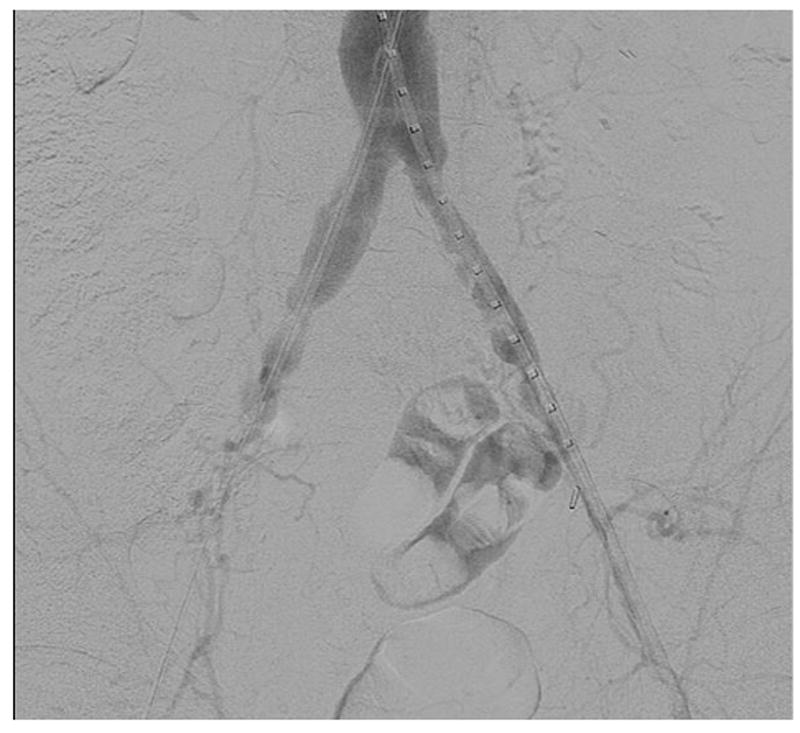
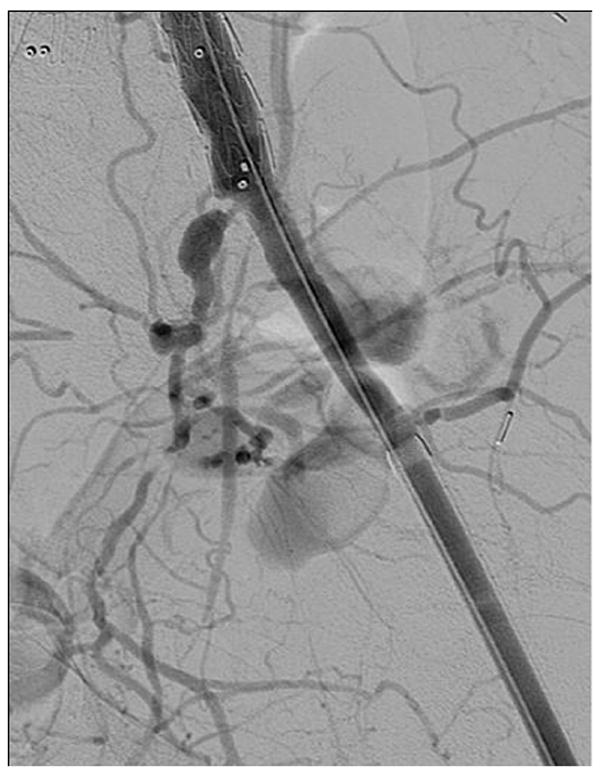
a. Angiogram showing a patient with small diameter common femoral and external iliac arteries bilaterally where the 7 Fr sheaths are occlusive
b and c. Post EVAR angiogram showing improved blood flow through the common femoral and external iliac arteries bilaterally after utilizing the “Dotter” technique during AAA repair.
The technical success rate in our study reached 96%. This compares favorably to the 76% to 96% success rates of other studies with > 50 patients3-7. Since 2007, we have used US guidance when performing all percutaneous EVARs and the percutaneous failure rate per artery has decreased despite expanding P-EVAR to more patients and smaller diameter arteries. Starnes et al7 demonstrated, in a study of 59 and 93 arteries accessed with and without US respectively, that US guidance significantly decreased their conversion to open repair in sheaths > 20 Fr (0% vs. 14%, P < .05) as well as significantly increased their technical success rate in sheaths > 20 Fr (100% vs. 82%, P < .05). In a study of 52 patients and 85 arteries by Oquzkurt et al8, technical success was achieved in 49 patients (94%) and there were no wound complications at the end of the case and at their 30 day follow-up visit.
Femoral cutdown access for EVAR has complications that include wound infections, lymphoceles, and hematomas. These complications can occur after percutaneous access as well. Morasch et al11reported a wound complication rate of 22.8% in 35 patients who received femoral cutdown (3 femoral neuropathies, 3 lymphoceles, 1 patient with scrotal edema, and 1 wound infection) vs. zero complications in 47 patients who received percutaneous access. Dalainas et al19, in a study of 186 patients undergoing femoral cutdown for EVAR, reported an 8% wound infection rate and a 6.5% rate of wound necrosis and dehiscence. There was no percutaneous comparison group. Our results demonstrate that patients undergoing percutaneous access have significantly fewer wound complications than those undergoing femoral cutdown as well. One patient (0.6%) in the P-EVAR group (hematoma) and 7 patients (6.5%) in the C-EVAR group (3 wound infections, 2 hematomas, 1 pseudoaneurysm, and 1 seroma) experienced wound complications, P < .01. Although we did not see an increase in wound complications with failed access, it is intuitive that this may be the case. The OR time is longer with failed access and it would be best to avoid this if failure could be accurately predicted. At this time we do not have any rigid criteria for cutdown, however, we would likely perform a planned femoral cutdown for CFA occlusion or in patients with an indication for femoral endarterectomy independent of their aneurysm.
Several studies have demonstrated that morbid obesity6, 7, 11-15, large sheath size11-13, 15, and femoral artery calcification10, 15 are associated with failed percutaneous access. Our study revealed no such findings. We looked at obesity (BMI > 30) rather than morbid obesity (BMI > 35) in our study and found that 1 obese patient experienced failure (2.7%) versus 11 non-obese patients (8.7%), P = .22. Interestingly, large sheath size (18-24 Fr) was not associated with percutaneous failure in our study. In fact, we found the exact opposite. There was a significant increased risk of percutaneous failure with small sheaths in our study (7.4% vs. 1.9%, P = .02). It is hard to draw any concrete conclusions from this other than to reiterate that even small sheaths can cause serious complications when placed into diseased arteries and care needs to be taken when performing percutaneous access. In fact, we use the smaller artery as the contralateral side and deliver the main body through the larger artery. We made no effort to quantify femoral artery calcification in this study and we do not exclude patients with calcification from receiving percutaneous access. With ultrasound guidance we avoid heavily calcified areas of the CFA when locating the best puncture site. Since 2005 we have only had 2 failures (out of 13 total failures) attributed to femoral artery calcification.
Limitations to this study include its retrospective design. Certainly, earlier in the study period there was a selection bias as patients with small vessels and calcification were not offered percutaneous access. As virtually all patients who now undergo elective AAA repair are doing so via percutaneous access, the earlier selection bias has been eliminated. The low number of events can lend itself to misinterpretation of the data as well as limit the amount of multivariable modeling one can perform.
Conclusions
Percutaneous EVAR can be performed in the vast majority of patients using US guidance with a high success rate, shorter operative times, and fewer wound complications. Access vessel diameters less than 5 mm are at greater risk for percutaneous failure and should be treated selectively.
Acknowledgments
This work was supported by the NIH T32 Harvard-Longwood Research Training in Vascular Surgery grant HL007734.
Footnotes
Presented at the 38th New England Society for Vascular Surgery Annual Meeting, Providence, RI, September 16, 2011; plenary presentation.
Author Disclosures: R.P. Bensley, None; R. Hurks, None; Z. Huang, None; F. Pomposelli, None; A. Hamdan, None; M. Wyers, None; E. Chaikof, None; M.L. Schermerhorn, Endologix Data Safety and Monitoring Board, Medtronic Consultant.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Giles KA, Pomposelli F, Hamdan A, Wyers M, Jhaveri A, Schermerhorn ML. Decrease in total aneurysm-related deaths in the era of endovascular aneurysm repair. J Vasc Surg. 2009;49(3):543–550. doi: 10.1016/j.jvs.2008.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haas PC, Krajcer Z, Diethrich EB. Closure of large percutaneous access sites using the Prostar XL Percutaneous Vascular Surgery device. J Endovasc Surg. 1999;6(2):168–170. doi: 10.1177/152660289900600209. [DOI] [PubMed] [Google Scholar]
- 3.Howell M, Villareal R, Krajcer Z. Percutaneous access and closure of femoral artery access sites associated with endoluminal repair of abdominal aortic aneurysms. J Endovasc Ther. 2001;8(1):68–74. doi: 10.1177/152660280100800112. [DOI] [PubMed] [Google Scholar]
- 4.Lee WA, Brown MP, Nelson PR, Huber TS. Total percutaneous access for endovascular aortic aneurysm repair (“Preclose” technique) J Vasc Surg. 2007;45(6):1095–1101. doi: 10.1016/j.jvs.2007.01.050. [DOI] [PubMed] [Google Scholar]
- 5.Quinn SF, Kim J. Percutaneous femoral closure following stent-graft placement: use of the Perclose device. Cardiovasc Intervent Radiol. 2004;27(3):231–236. doi: 10.1007/s00270-003-2713-y. [DOI] [PubMed] [Google Scholar]
- 6.Rachel ES, Bergamini TM, Kinney EV, Jung MT, Kaebnick HW, Mitchell RA. Percutaneous endovascular abdominal aortic aneurysm repair. Ann Vasc Surg. 2002;16(1):43–49. doi: 10.1007/s10016-001-0127-3. [DOI] [PubMed] [Google Scholar]
- 7.Arthurs ZM, Starnes BW, Sohn VY, Singh N, Andersen CA. Ultrasound-guided access improves rate of access-related complications for totally percutaneous aortic aneurysm repair. Ann Vasc Surg. 2008;22(6):736–741. doi: 10.1016/j.avsg.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Oguzkurt L, Gurel K, Eker E, Gur S, Ozkan U, Gulcan O. Ultrasound-guided puncture of the femoral artery for total percutaneous aortic aneurysm repair. Diagn Interv Radiol. doi: 10.4261/1305-3825.DIR.4061-10.1. [DOI] [PubMed] [Google Scholar]
- 9.Borner G, Ivancev K, Sonesson B, Lindblad B, Griffin D, Malina M. Percutaneous AAA repair: is it safe? J Endovasc Ther. 2004;11(6):621–626. doi: 10.1583/04-1291MR.1. [DOI] [PubMed] [Google Scholar]
- 10.Eisenack M, Umscheid T, Tessarek J, Torsello GF, Torsello GB. Percutaneous endovascular aortic aneurysm repair: a prospective evaluation of safety, efficiency, and risk factors. J Endovasc Ther. 2009;16(6):708–713. doi: 10.1583/08-2622.1. [DOI] [PubMed] [Google Scholar]
- 11.Morasch MD, Kibbe MR, Evans ME, Meadows WS, Eskandari MK, Matsumura JS, et al. Percutaneous repair of abdominal aortic aneurysm. J Vasc Surg. 2004;40(1):12–16. doi: 10.1016/j.jvs.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Starnes BW, Andersen CA, Ronsivalle JA, Stockmaster NR, Mullenix PS, Statler JD. Totally percutaneous aortic aneurysm repair: experience and prudence. J Vasc Surg. 2006;43(2):270–276. doi: 10.1016/j.jvs.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Teh LG, Sieunarine K, van Schie G, Goodman MA, Lawrence-Brown M, Prendergast FJ, et al. Use of the percutaneous vascular surgery device for closure of femoral access sites during endovascular aneurysm repair: lessons from our experience. Eur J Vasc Endovasc Surg. 2001;22(5):418–423. doi: 10.1053/ejvs.2001.1495. [DOI] [PubMed] [Google Scholar]
- 14.Torsello GB, Kasprzak B, Klenk E, Tessarek J, Osada N, Torsello GF. Endovascular suture versus cutdown for endovascular aneurysm repair: a prospective randomized pilot study. J Vasc Surg. 2003;38(1):78–82. doi: 10.1016/s0741-5214(02)75454-2. [DOI] [PubMed] [Google Scholar]
- 15.Traul DK, Clair DG, Gray B, O’Hara PJ, Ouriel K. Percutaneous endovascular repair of infrarenal abdominal aortic aneurysms: a feasibility study. J Vasc Surg. 2000;32(4):770–776. doi: 10.1067/mva.2000.107987. [DOI] [PubMed] [Google Scholar]
- 16.Singh N, Adams E, Neville R, Deaton D. Percutaneous endovascular AAA repair. Endovascular Today. 2005:39–44. [Google Scholar]
- 17.Starnes BW, O’Donnell SD, Gillespie DL, Goff JM, Rosa P, Parker MV, et al. Percutaneous arterial closure in peripheral vascular disease: a prospective randomized evaluation of the Perclose device. J Vasc Surg. 2003;38(2):263–271. doi: 10.1016/s0741-5214(03)00291-x. [DOI] [PubMed] [Google Scholar]
- 18. [September 28, 2011]; ProGlide Instructions For Use http://www.abbottvascular.com/static/cms_workspace/pdf/ifu/vessel_closure/Perclose_ProGlide.pdf.
- 19.Dalainas I, Nano G, Casana R, Tealdi Dg D. Mid-term results after endovascular repair of abdominal aortic aneurysms: a four-year experience. Eur J Vasc Endovasc Surg. 2004;27(3):319–323. doi: 10.1016/j.ejvs.2003.12.009. [DOI] [PubMed] [Google Scholar]


