Abstract
BACKGROUND
Hepatitis C virus (HCV) is a controversial indication for liver transplantation (LT) in HIV-infected patients due to reportedly poor outcomes.
METHODS
This prospective U.S. multicenter cohort study compared patient and graft survival in 89 HCV-HIV coinfected versus 2 different controls groups: 235 HCV monoinfected LT controls and all U.S. transplant recipients ≥65 years.
RESULTS
The 3-year patient and graft survival rates (95% CI) were 60% (47–71%) and 53% (40–64%) in HCV-HIV versus 79% (72–84%) and 74% (66–79%) in HCV recipients (both p<0.001) and HIV infection was the only factor significantly associated with reduced patient and graft survival. Among HCV-HIV patients, older donor age (HR=1.3 per decade), combined kidney-LT (HR=3.8), HCV-positive donor (HR=2.5), and body mass index (BMI) less than 21 kg/m2 (HR=3.2) were independent predictors of graft loss. In patients without these latter 3 factors, patient and graft survival were similar to those in U.S. LT recipients. The 3-year incidence of treated acute rejection was 1.6-fold higher in HCV-HIV versus HCV (log rank p=0.02) but cumulative incidence of severe HCV disease (29% versus 23% at 3 years, respectively) were not significantly different (p=0.21).
CONCLUSIONS
Patient and graft survival are lower in HCV-HIV compared to HCV alone LT patients. Importantly, rates of treated acute rejection but not HCV disease severity are significantly higher in HCV-HIV compared to HCV recipients. Our results indicate that HCV per se is not a contraindication to LT in HIV patients but recipient and donor selection as well as management of acute rejection strongly influence outcomes.
Keywords: cirrhosis, acute rejection, body mass index, donor age, recurrent hepatitis
INTRODUCTION
With dramatic improvements in the prognosis of individuals living with human immunodeficiency virus (HIV) on long-term antiretroviral therapy, end-stage liver disease has emerged as an important cause of morbidity and mortality.1, 2 Historically, HIV infection was viewed as a contraindication to LT due to concerns of immunosuppression-related opportunistic infections and reduced survival, but this is no longer the case. Several studies, including the NIH-sponsored Organ Transplantation in HIV Study (HIV-TR), demonstrate acceptable short-term survival and no increased risk of HIV-related complications.3 However, results in HIV-infected liver transplant recipients with HCV are generally worse than those without HCV4–7 making HCV a controversial indication for LT in HIV-infected patients. Natural history studies of HCV-infected patients with HIV demonstrate an accelerated rate of hepatic fibrosis8–10 and higher rate of complications of cirrhosis11 compared to HCV-infected patients without HIV. Following liver transplantation, patients with HCV also experience an accelerated rate of disease progression and recurrent HCV cirrhosis is the leading cause of graft loss among LT recipients.12 Thus, HCV-HIV coinfected transplant recipients may be at high risk for recurrent cirrhosis following transplantation. We conducted a prospective multicenter U.S. cohort study in HCV-HIV co-infected liver transplant recipients to define the natural history of disease and predictors of graft survival and HCV disease recurrence. We hypothesized that the outcomes of HCV-HIV coinfected patients would be similar to other higher risk liver transplant recipients without HIV, such as those over the age of 65 years.
METHODS
Study Population
This is a prospective cohort study of 89 HIV-infected patients with chronic HCV infection and complications of end-stage liver disease transplanted at 17 U.S. centers from October 2003 to February 2010 (ClinicalTrials.gov number NCT00074386). The HIV-specific inclusion criteria for transplantation were as previously described.3 No HCV-specific selection criteria were used. All patients with hepatocellular carcinoma (HCC) were within Milan criteria. The research protocol was approved and monitored by the institutional review boards at all participating centers and the NIAID-DSMB. Each patient provided written informed consent to participate in the trial.
Two non-HIV control groups were used for analytic purposes. The first control group was HCV mono-infected liver transplant recipients (up to 3 per HIV-HCV subject) matched by study clinical site, calendar time, and two additional clinical parameters (i) single versus combined kidney-liver transplantation, and (ii) HCC. This control group was used for comparison of survival, rejection and HCV recurrence. The second control group, used for survival analyses only, was all liver-transplant recipients and older (≥ 65 years) liver transplant recipients from the U.S. Scientific Registry of Transplant Recipients (SRTR) with data cutoff date of August 23, 2010. The latter group was selected to represent a patient population potentially similar to HIV patients in that LT is offered selectively due to reduced patient and graft survival.
Post-transplant Follow-up in the HCV-HIV Cohort
A standardized protocol for post-transplant follow-up was utilized. Liver biopsies were obtained at least annually to assess HCV disease severity and were read centrally and scored with the Ludwig-Batts method.13 Additionally, liver biopsies were performed for abnormal liver tests, suspected rejection, or suspected drug hepatotoxicity. The number of central biopsy readings performed in the 1st, 2nd and 3rd year post-transplant was 203, 33 and 15, respectively.
Antiretroviral therapy was reinitiated post-transplantation when the patient was able to take oral agents and renal function and clinical status were stable. Antiretroviral therapy was determined by the HIV provider in consultation with transplant team physicians having expertise in HIV management. Most patients continued their pre-transplant regimen in the post-transplant period.
Prophylaxis for opportunistic infections was as previously described.3 Immunosuppression was not standardized but the study protocol strongly recommended specific immunosuppressive drugs and adherence to those recommendations was high. Maintenance immunosuppression included a calcineurin inhibitor (cyclosporine or tacrolimus), mycophenolate mofetil, and prednisone. Due to potential anti-HIV effects of cyclosporine, there was higher use of cyclosporine in HCV-HIV patients than in HCV monoinfected controls (below). Sirolimus was used at the discretion of the investigator and typically used as a renal-sparing drug strategy. Target trough immunosuppressive levels were site specified. Induction with an IL-2 receptor inhibitor (anti-CD25 antibody) or thymoglobulin was not recommended and alemtuzumab use was prohibited due to risk of infectious complications and severe HCV recurrence.14, 15 Health care providers followed calcineurin inhibitor dosing guidelines for patients on protease inhibitor (PI) or combined PI-nonnucleoside reverse transcriptase inhibitor (NNRTI)-based regimens.16 Calcineurin inhibitor and sirolimus doses were adjusted to obtain therapeutic trough serum levels. Steroid induction, tapering, and maintenance were performed according to local site practice. Treatment of acute rejection was dictated by local site protocols and all treated rejection episodes were biopsy-proven. Acute rejection was treated with amplification of baseline immunosuppression (e.g. change of calcineurin inhibitor, addition of mycophenolate mofetil) or administration of corticosteroids. OKT3 and polyclonal anti-lymphocyte preparation use was restricted to those with severe rejection.
Follow-up in the HCV Mono-infected Control Group
Controls were identified retrospectively within strata as the next HCV-infected LT recipient in calendar time after the transplanted HCV-HIV coinfected patient. Donor information was obtained from SRTR and recipient and post-transplant complications including HCV disease severity by liver biopsy, HCV treatment, treatment of acute cellular rejection, and cause of graft loss and patient death were obtained by medical chart review. Biopsies were not read centrally but scored based on histopathologic review by the local pathologist and scored for presence or absence of severe HCV disease, defined as bridging fibrosis or cirrhosis (Ludwig-Batts F3-4) or histological features of cholestatic hepatitis.17 The number of biopsy readings performed in the 1st, 2nd and 3rd year post-transplant was 382, 136 and 60, respectively.
Statistical Methods
The primary study endpoints were patient and graft survival. Secondary endpoints included the rate of severe HCV disease (defined by presence of cholestatic hepatitis or bridging fibrosis/cirrhosis, or graft loss due to HCV) and rate of treated acute rejection. Other outcomes, limited to the HCV-HIV group, included the incidence of HIV-associated opportunistic complications, and changes in CD4+ T-cell counts.
Descriptive statistics included median, interquartile range (IQR) and range as appropriate. Comparison of baseline characteristics was conducted using the Fisher’s exact test (categorical variables) or Wilcoxon rank-sum test (continuous variables). Changes from baseline in CD4+ T-cell counts were analyzed using the Wilcoxon signed-rank test. Estimated rates of patient survival, graft survival, graft rejection and severe HCV recurrence were calculated with the Kaplan–Meier method, and 95% confidence intervals were estimated with the Greenwood’s formula. Survival distributions were compared by the log-rank test. Predictors of outcomes were analyzed in Cox proportional hazards models; all variables from the univariate analysis with P<0.10 were included in an initial multivariate model. Subsequently, variables with P≥0.1 were excluded, the model was re-fit, and all interactions examined. Post-transplant characteristics were analyzed as time-dependent covariates. Select antiretrovirals previously linked with liver related outcomes in non-transplant patients (protease inhibitors, nevirapine, didanosine, stavudive) were evaluated as predictors of graft loss in HCV-HIV patients. Models were developed for the overall cohort, and the HCV-HIV group alone. Models for the overall cohort were stratified by the case-matched group and the predictor variable of interest was HIV-HCV coinfection. A two-sided P value of less than 0.05 was considered to indicate statistical significance. Statistical analyses were performed with SAS software, version 9.2 (SAS Institute, Cary, NC).
RESULTS
Baseline Characteristics
A total of 89 HCV-HIV coinfected patients and 235 HCV monoinfected controls were included. Survivors were followed for a median [IQR] of 2.7 years [1.4–3.7] in the HCV-HIV coinfected group and 2.4 years [1.3–3.5] in the HCV controls (p=0.45). When all subjects are included, the median [IQR] follow-up was 2.0 years [1.1–3.4] in HIV negative compared to 1.8 years [0.7–3.4] in HIV positive HCV subjects (p=0.51). Recipient, donor, and transplant-related characteristics are shown in Table 1. Compared to HCV controls, HCV-HIV transplant recipients were younger, had a lower BMI at listing, more HBV coinfection, more donor allografts from non heart beating donors, longer median warm ischemia time and less use of tacrolimus-based (versus cyclosporine) initial immunosuppression. In the HIV-HCV group, the median [IQR] CD4+ T-cell count at LT was 283 [187–408] cells per cubic mmand 88% had undetectable plasma HIV RNA at the time of transplantation. The majority (97%) of coinfected patients were on antiretrovirals up to the time of LT and 80% resumed antiretrovirals within the first post-operative week. Among the 8 HCV-HIV recipients of combined kidney-liver transplants, the causes of renal dysfunction were diabetic nephropathy (n=3), hypertensive nephrosclerosis (n=2), HIV nephropathy (n=1), acute renal failure (n=1) and hepatorenal syndrome (n=1). Two patients were on long-term dialysis with compensated cirrhosis; the remainder had decompensated cirrhosis with acute or acute on chronic kidney disease.
Table 1.
Characteristics of HCV-HIV Coinfected and HCV Monoinfected Transplant Recipients
| HCV-HIV (N=89) | HCV (N=235) | P Value | |
|---|---|---|---|
| Recipient Characteristics | |||
| Age – yr (median [IQR]) | 49 [44, 53] | 54 [51, 60] | <.0001 |
| Male Gender – no. (%) | 67 (75) | 166 (71) | 0.49 |
| Caucasian Race – no. (%) | 58 (65) | 126 (54) | 0.08 |
| BMI at Listing (median [IQR]) | 25 [23–28] | 28 [25–32] | <.0001 |
| MELD at LT (median [IQR]) | 20 [15–27] | 20 [15–28] | 0.80 |
| Hepatocellular Carcinoma – no. (%) | 30 (34) | 73 (31) | 0.69 |
| HCV Genotype 1/4/other – no. (%) | 71 (80) | 192 (82) | 0.85 |
| HBV Coinfection – no. (%) | 5 (6) | 2 (1) | 0.02 |
| Donor/Transplant Characteristics | |||
| Donor Age – yr (median [IQR]) | 37 [24, 49] | 42 [30, 53] | 0.07 |
| Black Donor Race – no. (%) | 10 (11) | 36 (15) | 0.38 |
| Living Donor – no. (%) | 1 (1) | 15 (6) | 0.08 |
| Donor Risk Index (DRI) - median [IQR] | 1.31 [1.15–1.62] | 1.42 [1.21–1.77] | 0.08 |
| Cold Ischemia Time – hours (median [IQR]) | 7.6 [5.8, 9.9] | 8.0 [6.2, 10.1] | 0.23 |
| Non-heart Beating Donor – no. (%) | 15 (17) | 8 (4) | 0.0002 |
| Warm Ischemia Time – min. (median [IQR]) | 41 [36, 55] | 21 [13, 26] | 0.001 |
| Anti-HCV Positive Donor – no. (%) | 12 (13) | 26 (11) | 0.14 |
| HBV Core Antibody + Donor – no. (%) | 12 (13) | 24 (10) | 0.16 |
| Combined Kidney-Liver Transplant – no. (%) | 8 (9) | 17 (7) | 0.64 |
| Split/Partial Transplant – no. (%) | 1 (1) | 2 (1) | 1.00 |
| Post-Transplant Characteristics | |||
| Initial calcineurin inhibitor Use – no. (%) | <.0001 | ||
| Cyclosporine | 31 (35) | 23 (10) | |
| Tacrolimus | 52 (58) | 188 (80) | |
| None | 6 (7) | 9 (4) | |
| Not reported | 15 (6) | ||
| HCV Treatment – no. (%) | 37 (42) | 57 (24) | 0.002 |
| Follow-up Post-LT – yr (median [IQR]) | 1.8 [0.7, 3.4] | 2.0 [1.1, 3.4] | 0.51 |
| HIV-Specific Characteristics | |||
| CD4+ T-Cell (cells/mm3) a – median [IQR] | 283 [187–408] | ||
| HIV RNA Undetectable a – no. (%) | 78 (88) | ||
| On Antiretrovirals within 1st Week Post-LT (%) | 71 (80) | ||
| Antiretroviral Therapyb – no. (%) | |||
| Protease Inhibitor (PI) | 45 (51) | ||
| Non-Nucleoside (NNRTI) | 22 (25) | ||
| PI & NNRTI | 4 (4) | ||
| Nucleosides only | 15 (17) | ||
| None | 3 (3) | ||
Most recent pre-transplant value, within 16 weeks of transplant.
Most recent pre-transplant: The PI, NNRTI, PI and NNRTI, and Nucleosides only regimens included at least two nucleoside analogues (except for seven that included a single nucleoside analogue). Of the 22 NNRTI-based regimens, 18 included efavirenz, 3 nevirapine and 1 etravirine. Two PI-based regimens included raltegravir and one PI-based regimen included enfuvirtide as well.
Patient and Graft Survival
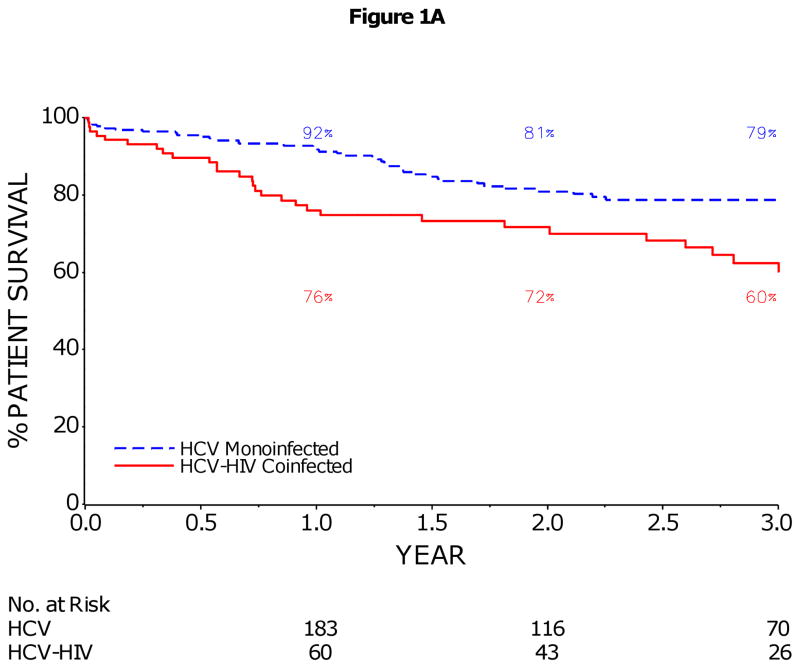
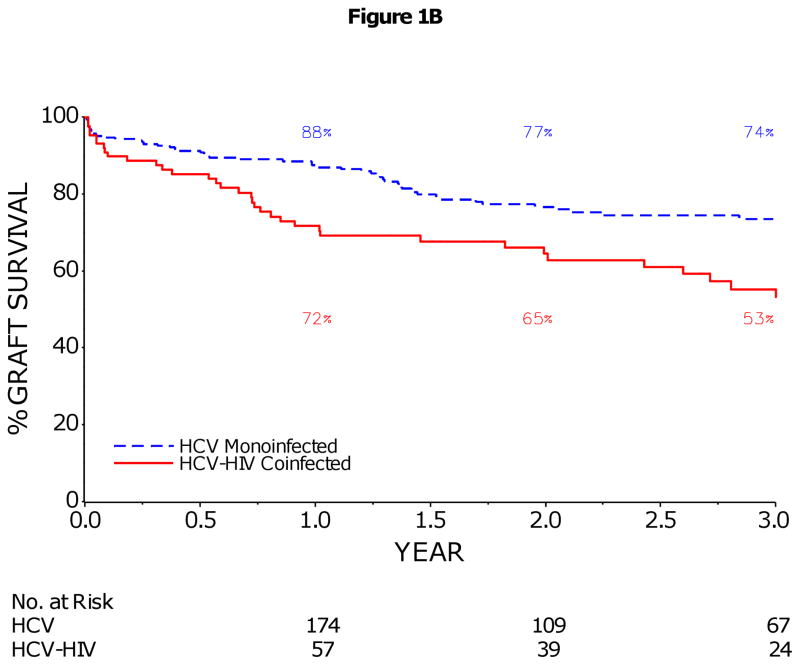
The 1- and 3-year patient survival rates (95% CI) were 76% (66–84%) and 60% (47–71%) in HCV-HIV, and 92% (87–95%) and 79% (72–84%) in HCV, p<0.001 (Figure 1A). Similarly, graft loss was significantly higher in HCV-HIV compared to HCV patients (p<0.001, Figure 1B). Patient/graft loss due to sepsis or multiorgan failure was more frequent in HCV-HIV than HCV recipients and patient/graft loss related to malignancy less frequent (Table 2). There were no deaths due to HIV-associated infections or malignancies in the HCV-HIV group. Pre-transplant history of AIDS-related opportunistic infections or neoplasms did not significantly impact post-transplant survival (HR (95% CI):1.0 (0.4, 3.0); p=0.96). In multivariate analysis, HIV infection was the only baseline factor significantly associated with increased risk of death (HR=2.3, p=0.002) and graft loss (HR=1.9, p=0.01) (Table 3). Among HCV-HIV coinfected patients, receipt of a combined kidney-liver transplant (HR=3.8, p=0.003), BMI<21 kg/m2 at enrollment (HR=3.2, p=0.01), receipt of an anti-HCV positive donor (HR=2.5, p=0.03), and older donor age (HR=1.3 per decade, p=0.04) were significant predictors of reduced graft survival (Table 4). Treated cytomegalovirus infection post-transplant (HR=9.5, p<0.001) was also significantly associated with increased risk of graft loss in univariate analysis, but was not included in multivariate model since there were only 5 cases in this group. Seven of 8 (88%) combined kidney-LT recipients died; 6 of these 8 had a MELD score greater than 25 at LT. Seven of 10 (70%) and 8 of 12 (67%) liver recipients with BMI<21 kg/m2 at enrollment and with anti-HCV positive donor had graft loss, respectively.
Figure 1.
1A: Kaplan–Meier Estimates of Patient Survival in HCV-HIV and HCV Liver Transplant Recipients
The 1 and 3 year patient survival rates (95% CI) were 76% (66–84%) and 60% (47–71%) in HCV-HIV, and 92% (87–95%) and 79% (72–84%) in HCV (p<0.001).
1B: Kaplan–Meier Estimates of Graft Survival in HCV-HIV and HCV Liver Transplant Recipients
The 1 and 3 year graft survival rates (95% CI) were 72% (61–80%) and 53% (40–64%) in HCV-HIV, and 88% (83–91%) and 74% (66–79%) in HCV (p<0.001). The rate of graft survival was calculated on the basis of graft failure from any cause.
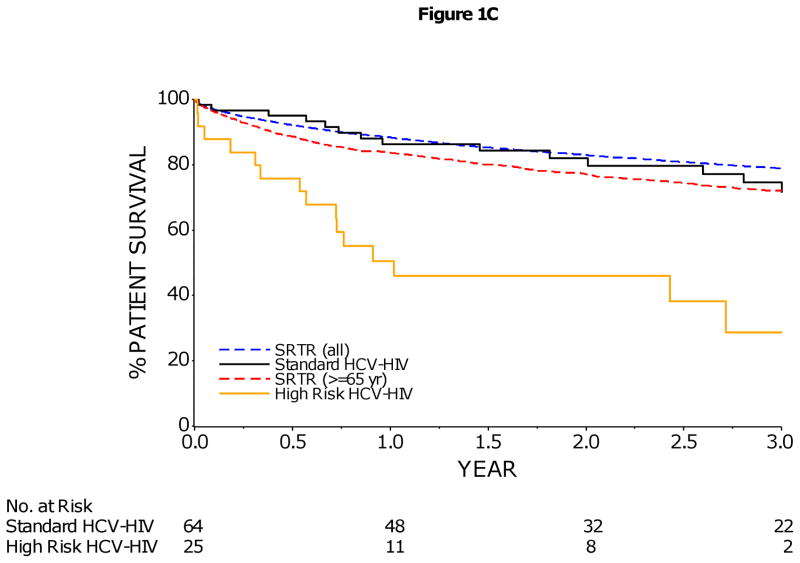
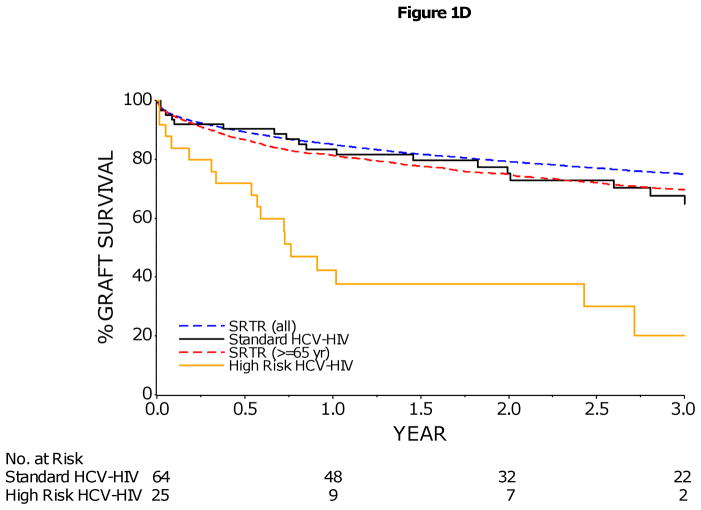
1C/D: Kaplan–Meier Estimates of Patient and Graft Survival in HCV-HIV Coinfected Liver Transplant Recipients by Risk Group
HCV-HIV coinfected liver transplant recipients were divided into two risk groups: 25 subjects who were identified to have an increased risk of graft loss (enrollment BMI < 21, or combined kidney-liver transplant, or anti-HCV positive donor were included in the High Risk group; remaining 64 subjects were included in the non-High Risk group. The 1- and 3-year patient survival rates (95% CI) were 86% (75–93%) and 72% (56–83%) in non-High Risk HCV-HIV, and 51% (30–68%) and 29% (9–52%) in High Risk HCV-HIV patients (p<0.001). The 1- and 3-year graft survival rates (95% CI) were 84% (72–91%) and 65% (49–77%) in non-High Risk HCV-HIV, and 42% (23–61%) and 20% (5–43%) in High Risk HCV-HIV patients (p<0.001). However, rates of patient survival (1C) and graft survival (1D) in non-High Risk HCV-HIV group were similar to those rates reported in the national Scientific Registry of Transplant Recipients (SRTR) for older liver-transplant recipients (≥65 years) and for all liver-transplant recipients in the United States during a similar time frame. The rate of graft survival was calculated on the basis of graft failure from any cause.
Table 2.
Causes of Graft Loss in HCV-HIV Coinfected and HCV Monoinfected Transplant Recipients*
| HCV-HIV N=38/89 |
HCV N=55/235 |
|
|---|---|---|
| Recurrent HCV – no. (%) | 9 (24) | 12 (22) |
| Multi-Organ Failure/Sepsis – no. (%) | 12 (32) | 8 (15) |
| Post-Surgical Complications – no. (%) | 6 (16) | 11 (20) |
| Rejection – no. (%) | 3 (8) | 4 (7) |
| Malignancy – no. (%) | 1 (3) | 8 (15) |
| Other/unknown – no. (%) | 7 (18) | 12 (22) |
There was no significant difference in causes of graft loss between HCV-HIV coinfected and HCV monoinfected transplant recipients (p=0.25).
Table 3.
Predictors of Death and Graft Loss in HIV-HCV Coinfected and HCV Monoinfected Liver Recipientsa
| Univariate Predictor | Death | Graft Loss | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| HIV Coinfection | 2.3 (1.3, 3.8) | 0.002 | 2.1 (1.3, 3.4) | 0.002 |
| Recipient Age | 1.0 (0.9, 1.0) | 0.14 | 1.0 (0.9, 1.0) | 0.08 |
| Recipient Female Gender | 1.0 (0.5, 1.9) | 0.98 | 0.8 (0.5, 1.5) | 0.52 |
| Recipient Caucasian Race | 1.2 (0.6, 2.2) | 0.62 | 1.1 (0.6, 2.0) | 0.65 |
| HCV Genotype 1/4/other | 0.6 (0.3, 1.5) | 0.29 | 0.6 (0.3, 1.3) | 0.16 |
| BMI at Enrollment < 21 | 2.4 (0.8, 6.9) | 0.11 | 2.0 (0.8, 5.3) | 0.16 |
| MELD Score at LT > 25 | 1.0 (0.5, 2.0) | 0.98 | 1.0 (0.5, 1.9) | 0.98 |
| TAC as Initial IMS (vs. CSA) | 0.8 (0.3, 2.1) | 0.60 | 1.1 (0.4, 2.7) | 0.88 |
| Deceased Donor | 3.1 (0.7, 14.8) | 0.15 | 1.8 (0.5, 6.8) | 0.39 |
| Donor Risk Index | 1.2 (0.6, 2.4) | 0.66 | 1.1 (0.6, 2.2) | 0.75 |
| Donor Age (by decade) | 1.0 (0.9, 1.2) | 0.59 | 1.1 (0.9, 1.2) | 0.46 |
| Non-heart Beating Donor | 1.4 (0.4, 4.3) | 0.61 | 2.1 (0.7, 5.7) | 0.16 |
| Cold Ischemia Time (hours) | 1.1 (0.9, 1.2) | 0.23 | 1.1 (1.0, 1.2) | 0.23 |
| Donor Black Race | 0.7 (0.3, 1.6) | 0.35 | 0.7 (0.3, 1.6) | 0.37 |
| HCV+ Donor | 1.6 (0.6, 4.3) | 0.32 | 1.1 (0.5, 2.7) | 0.79 |
| Treated Acute Rejectionb | 1.6 (0.8, 3.3) | 0.23 | 2.4 (1.2, 4.6) | 0.01 |
| Multivariate Predictors | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value |
| HIV Coinfection | 2.3 (1.3, 3.8) | 0.002 | 1.9 (1.2, 3.1) | 0.01 |
| Treated Acute Rejectionb | 2.0 (1.0, 4.0) | 0.06 | ||
IMS = immunosuppression, LT= liver transplant
Adjusted for clustering
As time-varying covariate
Table 4.
Predictors of Graft Loss in HIV-HCV Coinfected Liver Recipients
| Univariate Predictor | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Recipient Age | 1.0 (1.0, 1.1) | 0.63 |
| Recipient Female Gender | 0.8 (0.3, 1.7) | 0.52 |
| Recipient Caucasian Race | 0.9 (0.4, 1.7) | 0.68 |
| Combined Kidney-Liver Transplant | 3.7 (1.6, 8.7) | 0.002 |
| BMI at Enrollment < 21 | 3.2 (1.4, 7.5) | 0.01 |
| Transplant MELD Score > 25 | 1.6 (0.8, 3.4) | 0.17 |
| Detectable HIV RNA at Transplant | 2.6 (1.2, 5.7) | 0.02 |
| History of Splenectomy | 2.7 (0.8, 8.8) | 0.10 |
| Hepatocellular Cancer | 0.7 (0.3, 1.4) | 0.31 |
| Tacrolimus as Initial IS Med (vs. Cyclosporine) | 2.8 (1.3, 6.2) | 0.01 |
| Donor Risk Index (DRI) | 1.9 (0.9, 4.1) | 0.08 |
| Donor Age (by decade) | 1.3 (1.0, 1.5) | 0.04 |
| Anti-HCV Positive Donor | 2.7 (1.2, 6.1) | 0.01 |
| Treated Acute Rejectiona | 1.4 (0.7, 2.9) | 0.36 |
| Treated CMV Infectiona | 9.5 (3.1, 29.4) | <.0001 |
| Protease Inhibitor Use in Prior 3 Monthsa | 0.9 (0.5, 1.7) | 0.70 |
| D4T/DDI Use in Prior 3 Monthsa | 0.3 (0.0, 2.0) | 00.19 |
| Nevirapine Use in Prior 3 Monthsa | 0.6 (0.1, 4.2) | 0.58 |
| Pre-transplant history AIDS-related opportunistic infections or neoplasms | 1.0 (0.4, 3.0) | 0.96 |
| Multivariate Predictors | Hazard Ratio (95% CI) | P Value |
| BMI at Enrollment < 21 | 3.2 (1.3, 7.7) | 0.01 |
| Combined Kidney-Liver Transplant | 3.8 (1.6, 9.1) | 0.003 |
| Anti-HCV Positive Donor | 2.5 (1.1, 5.6) | 0.03 |
| Donor Age (by decade) | 1.3 (1.0, 1.6) | 0.04 |
As time-varying covariate
Patient and graft survival in High Risk HCV-HIV patients, defined by combined kidney-liver transplantation, low enrollment BMI (<21), or anti-HCV positive donor, were significantly lower than non-High Risk HCV-HIV patients without these three risk factors (p<0.001) (Figures 1C/1D). In contrast, patient and graft survival in non-High Risk HCV-HIV patients were not significantly different from those rates reported in the national Scientific Registry of Transplant Recipients (SRTR) for older (≥65 years) liver-transplant recipients during a similar time frame, (p=0.67 and 0.79, respectively) (Figures 1C/1D).
There was no significant change in survival among the HIV-HCV transplant recipients over time. When the first half of coinfected cohort was compared with second half, no significant difference was observed in the patient and graft survival curves (log-rank test; p=1.00 and 0.91, respectively). For the first half of the coinfected cohort, 1-year patient and graft survival rates (95% confidence intervals) were 75% (60–85) and 71% (55–82), respectively, while for the second half of the coinfected cohort, rates were 77% (61–88) and 73% (57–84), respectively.
HCV Disease Severity
Among recipients with functioning grafts, the median [IQR] histologic follow-up was 0.9 [0.2–1.8] years for the HCV-HIV group and 1.1 [0.3–2.3] years for the HCV group (p=0.20). Sixty-two percent of HCV-HIV and 64% of HCV recipients had 2 or more biopsies for review. The 1, 2 and 3-year cumulative incidences (95% CI) of severe HCV disease were 17% (10–28%), 27% (17–40%) and 29% (19–43%) in HCV-HIV and 6% (3–9%), 14% (9–20%) and 23% (16–31%) in HCV (p=0.21). HIV was not significantly associated with severe HCV disease (HR=1.4, p=0.41) (eTable 1). Recipient female gender (HR=3.5, p=0.01) and treated acute rejection (HR=3.3, p=0.01) were significantly associated with severe HCV disease and older recipient age was protective (HR=0.9, p=0.046). The proportion of graft losses attributed to recurrent HCV disease was similar in the HCV-HIV and HCV recipients (Table 2).
Significantly more HCV/HIV subjects (42%) received HCV therapy compared to HCV/non-HIV subjects (24%) post-transplant (p=0.002), and about 85% of the HCV/HIV subjects had fibrosis stage of 0 or 1 prior to HCV therapy initiation. A lack of detailed biopsy grading info pre treatment in the HCV monoinfected patients precluded comparison of stage of disease at treatment initiation. Receipt of HCV treatment was not included in the multivariate model of severe HCV disease (eTable 1) as it is a surrogate for severity of disease. However, when included to adjust for the impact of differing treatment rates between the two groups, HIV coinfection was still not significantly associated with severe HCV disease in the multivariate model (HR (95%CI): 1.2 (0.6, 2.7); p=0.61). The rate of sustained virologic response was similar between the two groups: 5/37 (13.5%) for coinfected and 9/57 (15.8%) for HCV monoinfected group.
Among HCV-HIV transplant recipients, the only factor independently associated with severe HCV disease was treated acute rejection (HR=2.7, p=0.04). Treated cytomegalovirus infection (HR=15.6, p<0.001), another univariate predictor, was not included in the multivariate model due to small number of cytomegalovirus infection cases. Three (3%) HCV-HIV recipients spontaneously cleared HCV infection post-LT.
Acute Rejection
The cumulative incidence of acute rejection requiring treatment was significantly higher in HCV-HIV than HCV patients (39% versus 24% at year 3, HR=2.1, p=0.01). Over half (54%) of initial rejection episodes in the HCV-HIV recipients occurred within the first 21 days post-LT. Of the 28 first treated acute rejections in coinfected, 23 were centrally read, and 16 of those were able to be graded according to Banff criteria: 2 were indeterminate, 3 were mild (Grade I), 10 were moderate (Grade II), and 1 was severe (Grade III). 25 of the 28 coinfected subjects who experienced rejection were on ART at the time of first treated acute rejection. The majority of HCV-HIV patients (82%) with acute rejection were treated with corticosteroids; only 1 patient received OKT3 for severe acute rejection. In multivariate analysis, HIV infection was the only factor significantly associated with treated acute rejection (HR=2.0, p=0.01) (eTable 2). Similar results were obtained when the multivariate analysis was restricted to the first 30 days post-transplantation (data not shown). Among HCV-HIV recipients, older recipient age (HR=0.95, p=0.03) and prednisone use post-LT (HR=0.4, p=0.04) were predictive of treated acute rejection in the multivariate model (Table 5).
Table 5.
Predictors of First Treated Acute Rejection in HIV-HCV Coinfected Liver Recipients
| Univariate Predictor | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Recipient Age | 0.94 (0.90, 0.98) | 0.002 |
| Recipient Caucasian Race | 1.3 (0.6, 2.9) | 0.56 |
| Tacrolimus as Initial IS Med (vs. Cyclosporine) | 0.7 (0.3, 1.5) | 0.34 |
| Donor Risk Index (DRI) | 1.3 (0.5, 3.4) | 0.57 |
| CD4 count (per 50 cells/μL)a | 1.0 (0.9, 1.1) | 0.60 |
| PI Use in Prior 3 Months (any)a | 1.2 (0.6, 2.7) | 0.58 |
| EFV without PI in prior 3 monthsa | 1.1 (0.4, 3.0) | 0.78 |
| MMF Usea | 0.8 (0.4, 1.7) | 0.53 |
| Prednisone Usea | 0.3 (0.1, 0.7) | 0.01 |
| CsA Trough Level (ng/mL)a | 1.00 (0.99, 1.00) | 0.31 |
| Tac Trough Level (ng/mL)a | 0.86 (0.74, 0.99) | 0.04 |
| Multivariate Predictors | Hazard Ratio (95% CI) | P Value |
| Recipient Age | 0.95 (0.91, 1.00) | 0.03 |
| Prednisone Usea | 0.4 (0.2, 1.0) | 0.04 |
As time-varying covariate
HIV Disease Post-Liver Transplantation
The median [IQR] change in CD4 cell counts at 6 months, 1- and 3-years post-LT compared to baseline was −83 [−164, 1] (p<0.001), −11 [−106, 86] (p=0.82), and −2 [−74, 102] (p=0.61), respectively. HIV RNA was well-controlled post-LT. Post-transplantation, there have been three cases of esophageal candidiasis, one case of candidiasis of bronchi, and one case of pneumocystosis. All these complications responded to antimicrobial therapy. One case of cutaneous Kaposi’s sarcoma was treated successfully with a change to sirolimus-based immunosuppression.
Of the eleven subjects with detectable HIV RNA at baseline, ten had undetectable levels within 3 months and one at 9 months post transplant. Although the latter case subsequently had an episode of HIV viremia (544–63409 copies/mL over 6 months), plasma HIV-1 RNA was undetectable again at the most recent visit. Of the 78 non-viremic individuals at baseline, 14 (18%) had 2 or more consecutive detectable measures post-transplant. Among these 14 cases, there were 30 episodes of HIV viremia (median [IQR] peak HIV RNA level: 7259 [1450, 42570] copies per milliliter). HIV RNA was detectable at the most recent follow-up visit in 9 of the cases (5 of them at the time of graft loss)
DISCUSSION
In this study, HCV-infected liver transplant recipients with HIV were found to have significantly lower graft and patient survival than HCV recipients without HIV. This is consistent with previously published studies.5,6 Importantly, however, our study uniquely evaluated predictors of poor outcomes, and provides, for the first time, insights into critical patient selection and management strategies to maximize good outcomes. The association of graft survival in HCV-HIV recipients with low BMI and need for combined kidney-liver transplantation suggests wait-listed patients who are more debilitated do less well, and the findings of higher graft losses in HCV-HIV recipients with older or anti-HCV positive donors highlights the importance of both careful recipient and donor selection to achieve optimal outcomes.
In HCV transplant recipients, donor age is strongly associated with an increased risk of recurrent HCV cirrhosis, reduced responsiveness to HCV treatment and higher rates of graft loss.18–20 Among the HCV-HIV patients, older donor age was also associated with higher rates of graft loss, as was use of anti-HCV positive donors. The latter finding is important, as use of anti-HCV positive organs in HCV-infected transplant recipients without HIV has not been associated with an increased risk of severe HCV disease progression or graft loss.21 Donor biopsy data are not available so it unknown whether greater severity of disease in anti-HCV positive donors in HCV-HIV versus HCV recipients accounts for this difference.
Graft and patient losses due to hepatitis C did not differ between HCV and HIV-HCV coinfected recipients. This contrasts with a prior study from France indicating more severe HCV disease in coinfected patients 5. The rate of advanced fibrosis (F3-4) in the HCV-HIV coinfected French cohort was 25% (4/20) and 57% (8/14) at 1 and 2 years post-transplant compared with 17% and 27 % in our cohort. In addition to the recognized limitations of sample size in providing precise estimates of recurrence rates, donor, recipient and post-transplant factors including using of anti-HCV therapy likely vary from center to center and may account for differences between studies. Additionally, in our study, HCV-HIV coinfected patients had a higher rate of graft loss due to sepsis and multiorgan failure (albeit not significantly) and this may have contributed to a survivor bias in assessment of fibrosis severity. The higher frequency of sepsis/MOF in the coinfected recipients may be related to their greater level of debilitation pre-transplant, a higher rate of treated acute rejection, or the presence of underlying recurrent HCV disease.
Acute rejection treatment in HCV-infected liver transplant recipients is a well-recognized risk factor for recurrent cirrhosis and graft loss.12, 22 The finding of a significantly higher rate of acute rejection in HCV-HIV compared to HCV recipients is a critically important finding of our study, as prevention of acute rejection may offer a means of optimizing outcomes in HIV-HCV recipients. This higher rate of acute rejection may be due to a higher rate of misinterpretation of acute rejection (versus recurrent HCV or drug toxicity effects) in HCV-HIV recipients, an overly cautious use of immunosuppression due to concerns for exacerbating HIV or HCV related diseases, or a reflection of the difficulties in achieving adequate immunosuppression due to interactions between antiretroviral drugs and calcineurin inhibitors16, 23 and mirrors the experience in kidney transplant recipients.24 Our data suggest that HCV-HIV patients with acute rejection were “under-immunosuppressed”, as lower tacrolimus trough level was associated with higher rates of treated acute rejection in univariate model and prednisone use post-LT was protective in the multivariate model. Alternatively, it is also possible that higher rates of acute rejection in patients with HIV reflect an inherently enhanced immune response and dysregulation of the immune response.25 Prior infections with other viruses (e.g. CMV) lead to the generation of memory alloreactive T cells as a result of crossreactivity 26. Other studies have shown that the homeostatic expansion of T cells in HIV infection is often coupled with the acquisition of memory phenotype, which in turn, is associated with increased responsiveness of the T cell and nonspecific enhancement of alloimmunity 27. Studies investigating T cell responses in coinfected patients are ongoing and are expected to shed light on contributing mechanisms.
Similar to the kidney transplant experience24, there was no evidence of accelerated HIV disease progression, as indicated by stable or improved CD4+ T-cell counts and control of HIV viremia. HIV-specific infections/neoplasms did not contribute to morbidity or mortality in coinfected transplant recipients, with follow-up periods up to ~3.5 years.
While this study represents the largest U.S. experience with transplantation of HIV-HCV coinfected patients, limitations include the lack of a uniform immunosuppressive regimen and standard antiretroviral therapy followed by all centers. Additional limitations include lack of prospective data collection and central biopsy reading in the HCV-mono-infected controls. To avoid potential biases in selection, the HCV mono-infected patients were carefully matched by center, year of transplant and key recipient factors. However, this may not completely mitigate potentially important differences in the HCV-HIV and HCV cohorts. Finally, to minimize error related to assessment of liver disease severity, we focused on advanced fibrosis (bridging fibrosis and cirrhosis) as the critical endpoint. However, liver biopsy adequacy was not assessed and thus underestimation of fibrosis in both HCV-HIV and HCV mono-infected patients with small biopsies may have occurred.28 However, any measurement bias related to assessment of fibrosis severity would be non-differential.
In summary, this study highlights that patient and graft survival are lower in HCV-infected patients with HIV compared to those without HIV, but that acceptable results are achievable in most coinfected patients. Importantly, our results indicate how recipient and donor selection and management of post-transplantation complications may be used potentially improved upon in order to maximize patient and graft survival. Firstly, recipient severity of illness, as reflected by low BMI and need for kidney transplant influences outcomes post-LT. Thus, early referral for consideration of transplantation and utilization of donor options (e.g. living donor) that shorten wait-list time are the best means of overcoming this potential barrier to transplantation. Second, donor selection is important. Older donors are already recognized as a risk for HCV-infected transplant recipients and coinfected patients have higher rates of graft loss with older donors also. Anti-HCV positive donors should be used cautiously given the significant association with graft loss in our study. Thirdly, cytomegalovirus infection was strongly associated with graft loss and we recommend use of universal prophylaxis to minimize any risk for this complication. Finally, reducing rates of early acute rejection are highly desirable, as treatment of rejection is associated with graft loss and more severe HCV disease. Vigilance for rejection and a low threshold to obtain a biopsy to evaluate abnormal liver tests is recommended. Although protease inhibitor and efavirenz use has not been shown to be associated with rejection, graft loss or death, we hypothesize that newer antiretroviral regimens that avoid the use of protease inhibitors and efavirenz may facilitate conventional immunosuppressive drug dosing with better drug exposure and potentially reduce rejection rates. Thus, while there remain many challenges, our results support the continued select use of this life-saving treatment in HCV-HIV coinfected patients.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by the Solid Organ Transplantation in HIV: Multi-Site Study (AI052748) funded by the National Institute of Allergy and infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy And Infectious Diseases or the National Institutes of Health.
Abbreviations
- Anti-HCV
antibody to hepatitis
- CALT
alanine aminotransferase
- CMV
cytomegalovirus infection
- PI
protease inhibitor
- NNRTI
Non-nucleoside reverse transcriptase inhibitor
- NRTI
Nucleoside reverse transcriptase inhibitor
- HIV
human immunodeficiency virus
- LT
liver transplantation
- HIV-TR Study
Solid Organ Transplantation in HIV Multi-Site Study
Footnotes
Author Contributions
Data integrity: Mr. Barin and Dr. Stablein have full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Terrault, Roland, Ragni, Fung, Stablein, Fox, Odim, Stock.
Acquisition of data: Terrault, Roland, Schiano, Dove, Wong, Poordad, Ragni, Simon, Olthoff, Johnson, Stosor, Jayaweera, Fung, Sherman, Subramanian, Millis, Slakey, Berg, Carlson, Ferrell, Stock.
Interpretation of data: Terrault, Roland, Schiano, Dove, Wong, Poordad, Ragni, Barin, Simon, Olthoff, Johnson, Stosor, Jayaweera, Fung, Sherman, Subramanian, Millis, Slakey, Berg, Carlson, Ferrell, Stablein, Fox, Odim, Stock.
Drafting of the manuscript: Terrault, Roland, Barin, Stablein, Stock.
Critical revision of the manuscript for important intellectual content: Terrault, Roland, Schiano, Dove, Wong, Poordad, Ragni, Barin, Simon, Olthoff, Johnson, Stosor, Jayaweera, Fung, Sherman, Subramanian, Millis, Slakey, Berg, Carlson, Ferrell, Stablein, Fox, Odim, Stock.
Statistical analysis: Terrault, Roland, Barin, Stablein, Stock.
Obtained funding: Roland, Stock.
Study supervision: Terrault, Roland, Carlson, Odim, Fox, Stock.
Conflict of Interest Disclosures: All authors have completed and submitted the Copyright Assignment, Authorship Responsibility, NIH Funding, Financial Disclosure, Institutional Review Board/Animal Care Committee Approval, and Sponsorship Form and none were reported.
Institutional Review Board Approval: The Committee on Human Research at the University of California, San Francisco approved the study protocol, as did the Internal Review Board from each participating center. Each participant provided written informed consent.
Additional Contributions:
We want to thank Rodney Rogers for this expert coordination of the study consortium. We want to thank all of the Solid Organ Transplantation in HIV: Multi-Site Study investigators and coordinators for their hard work and dedication to the study and subjects. A complete list can be found in the online materials for this manuscript.
References
- 1.Lesens O, Deschenes M, Steben M, Belanger G, Tsoukas CM. Hepatitis C virus is related to progressive liver disease in human immunodeficiency virus-positive hemophiliacs and should be treated as an opportunistic infection. J Infect Dis. 1999;179:1254–8. doi: 10.1086/314720. [DOI] [PubMed] [Google Scholar]
- 2.Weber R, Sabin CA, Friis-Moller N, Reiss P, El-Sadr WM, Kirk O, Dabis F, Law MG, Pradier C, De Wit S, Akerlund B, Calvo G, Monforte A, Rickenbach M, Ledergerber B, Phillips AN, Lundgren JD. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 3.Roland ME, Barin B, Carlson L, Frassetto LA, Terrault NA, Hirose R, Freise CE, Benet LZ, Ascher NL, Roberts JP, Murphy B, Keller MJ, Olthoff KM, Blumberg EA, Brayman KL, Bartlett ST, Davis CE, McCune JM, Bredt BM, Stablein DM, Stock PG. HIV-infected liver and kidney transplant recipients: 1- and 3-year outcomes. Am J Transplant. 2008;8:355–65. doi: 10.1111/j.1600-6143.2007.02061.x. [DOI] [PubMed] [Google Scholar]
- 4.de Vera ME, Dvorchik I, Tom K, Eghtesad B, Thai N, Shakil O, Marcos A, Demetris A, Jain A, Fung JJ, Ragni MV. Survival of Liver Transplant Patients Coinfected with HIV and HCV Is Adversely Impacted by Recurrent Hepatitis C. Am J Transplant. 2006;6:2983–2993. doi: 10.1111/j.1600-6143.2006.01546.x. [DOI] [PubMed] [Google Scholar]
- 5.Duclos-Vallee JC, Feray C, Sebagh M, Teicher E, Roque-Afonso AM, Roche B, Azoulay D, Adam R, Bismuth H, Castaing D, Vittecoq D, Samuel D. Survival and recurrence of hepatitis C after liver transplantation in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2008;47:407–17. doi: 10.1002/hep.21990. [DOI] [PubMed] [Google Scholar]
- 6.Mindikoglu AL, Regev A, Magder LS. Impact of human immunodeficiency virus on survival after liver transplantation: analysis of United Network for Organ Sharing database. Transplantation. 2008;85:359–68. doi: 10.1097/TP.0b013e3181605fda. [DOI] [PubMed] [Google Scholar]
- 7.Vennarecci G, Ettorre GM, Antonini M, Santoro R, Perracchio L, Visco G, Santoro E. Liver transplantation in HIV-positive patients. Transplant Proc. 2007;39:1936–8. doi: 10.1016/j.transproceed.2007.05.076. [DOI] [PubMed] [Google Scholar]
- 8.Benhamou Y, Di Martino V, Bochet M, Colombet G, Thibault V, Liou A, Katlama C, Poynard T. Factors affecting liver fibrosis in human immunodeficiency virus-and hepatitis C virus-coinfected patients: impact of protease inhibitor therapy. Hepatology. 2001;34:283–7. doi: 10.1053/jhep.2001.26517. [DOI] [PubMed] [Google Scholar]
- 9.Rai R, Wilson LE, Astemborski J, Anania F, Torbenson M, Spoler C, Vlahov D, Strathdee SA, Boitnott J, Nelson KE, Thomas DL. Severity and correlates of liver disease in hepatitis C virus-infected injection drug users. Hepatology. 2002;35:1247–55. doi: 10.1053/jhep.2002.33151. [DOI] [PubMed] [Google Scholar]
- 10.Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS. 2008;22:1979–91. doi: 10.1097/QAD.0b013e32830e6d51. [DOI] [PubMed] [Google Scholar]
- 11.Pineda JA, Romero-Gomez M, Diaz-Garcia F, Giron-Gonzalez JA, Montero JL, Torre-Cisneros J, Andrade RJ, Gonzalez-Serrano M, Aguilar J, Aguilar-Guisado M, Navarro JM, Salmeron J, Caballero-Granado FJ, Garcia-Garcia JA. HIV coinfection shortens the survival of patients with hepatitis C virus-related decompensated cirrhosis. Hepatology. 2005;41:779–89. doi: 10.1002/hep.20626. [DOI] [PubMed] [Google Scholar]
- 12.Aytaman A, Kaufman M, Terrault NA. Management of posttransplant hepatitis C infection. Curr Opin Organ Transplant. 2010;15:301–9. doi: 10.1097/MOT.0b013e3283398237. [DOI] [PubMed] [Google Scholar]
- 13.Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–17. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Marcos A, Eghtesad B, Fung JJ, Fontes P, Patel K, Devera M, Marsh W, Gayowski T, Demetris AJ, Gray EA, Flynn B, Zeevi A, Murase N, Starzl TE. Use of alemtuzumab and tacrolimus monotherapy for cadaveric liver transplantation: with particular reference to hepatitis C virus. Transplantation. 2004;78:966–71. doi: 10.1097/01.tp.0000142674.78268.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen HR, Shackleton CR, Higa L, Gralnek IM, Farmer DA, McDiarmid SV, Holt C, Lewin KJ, Busuttil RW, Martin P. Use of OKT3 is associated with early and severe recurrence of hepatitis C after liver transplantation. Am J Gastroenterol. 1997;92:1453–7. [PubMed] [Google Scholar]
- 16.Frassetto LA, Browne M, Cheng A, Wolfe AR, Roland ME, Stock PG, Carlson L, Benet LZ. Immunosuppressant pharmacokinetics and dosing modifications in HIV-1 infected liver and kidney transplant recipients. Am J Transplant. 2007;7:2816–20. doi: 10.1111/j.1600-6143.2007.02007.x. [DOI] [PubMed] [Google Scholar]
- 17.Taga SA, Washington MK, Terrault N, Wright TL, Somberg KA, Ferrell LD. Cholestatic hepatitis C in liver allografts. Liver Transpl Surg. 1998;4:304–10. doi: 10.1002/lt.500040401. [DOI] [PubMed] [Google Scholar]
- 18.Berenguer M, Aguilera V, Prieto M, Ortiz C, Rodriguez M, Gentili F, Risalde B, Rubin A, Canada R, Palau A, Rayon JM. Worse recent efficacy of antiviral therapy in liver transplant recipients with recurrent hepatitis C: impact of donor age and baseline cirrhosis. Liver Transpl. 2009;15:738–46. doi: 10.1002/lt.21707. [DOI] [PubMed] [Google Scholar]
- 19.Lake JR, Shorr JS, Steffen BJ, Chu AH, Gordon RD, Wiesner RH. Differential effects of donor age in liver transplant recipients infected with hepatitis B, hepatitis C and without viral hepatitis. Am J Transplant. 2005;5:549–57. doi: 10.1111/j.1600-6143.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- 20.Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999–2008. Am J Transplant. 2010;10:1003–19. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 21.Northup PG, Argo CK, Nguyen DT, McBride MA, Kumer SC, Schmitt TM, Pruett TL. Liver allografts from hepatitis C positive donors can offer good outcomes in hepatitis C positive recipients: a US National Transplant Registry analysis. Transpl Int. 2010;23:1038–44. doi: 10.1111/j.1432-2277.2010.01092.x. [DOI] [PubMed] [Google Scholar]
- 22.Prieto M, Berenguer M, Rayon JM, Cordoba J, Arguello L, Carrasco D, Garcia-Herola A, Olaso V, De Juan M, Gobernado M, Mir J, Berenguer J. High incidence of allograft cirrhosis in hepatitis C virus genotype 1b infection following transplantation: relationship with rejection episodes. Hepatology. 1999;29:250–6. doi: 10.1002/hep.510290122. [DOI] [PubMed] [Google Scholar]
- 23.Jain A, Venkataramanan R, Shapiro R, Scantlebury V, Potdar S, Bonham C, Ragni M, Fung J. The interaction between anti-retroviral agents and tacrolimus in liver and kidney transplant patients. American Transplant Congress; Washington DC. 2002. [DOI] [PubMed] [Google Scholar]
- 24.Stock PG, Barin B, Murphy B, Hanto D, Diego JM, Light J, Davis C, Blumberg E, Simon D, Subramanian A, Millis JM, Lyon GM, Brayman K, Slakey D, Shapiro R, Melancon J, Jacobson JM, Stosor V, Olson JL, Stablein DM, Roland ME. Outcomes of kidney transplantation in HIV-infected recipients. N Engl J Med. 2010;363:2004–14. doi: 10.1056/NEJMoa1001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, Markmann JF, Kassaee A, Rosengard BR, Hancock WW, Sayegh MH, Turka LA. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selin L, Brehm M, Naumov Y, Cornberg M, Kim S, Clute S, Welsh R. Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity. Immunol Rev. 2006;211:164–81. doi: 10.1111/j.0105-2896.2006.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Z, Bensinger S, Zhang J, Chen C, Yuan X, Huang X, Markmann J, Kassaee A, Rosengard B, Hancock W, Sayegh M, Turka L. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2003;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39:239–44. doi: 10.1016/s0168-8278(03)00191-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.