Abstract
In women, pain symptoms and nociceptive thresholds vary with reproductive cycle suggesting the role of estrogen receptors (ERs) in modulating nociception. Our previous data strongly suggest interaction between ERs and ATP-induced purinergic (P2X3) as well as ERs and capsaicin-induced vanilloid (TRPV1) receptors at the level of dorsal root ganglion (DRG) neurons. In this study we investigated the expression of P2X3 and TRPV1 receptors by western blotting and immunohistochemistry in lumbosacral DRGs from wild type, estrogen receptor-α and estrogen receptor-β knockout mice. We found a significant decrease for both P2X3 and TRPV1 in ERαKO and ERβKO. This phenomenon was visualized in L1, L2, L4, and L6 levels for P2X3 receptors and in L1, L2, and S2 levels for TRPV1 receptors. This tan interaction between P2X3/TRPV1 and ERs expression in sensory neurons may represent a novel mechanism that can explain sex differences in nociception observed in clinical practice. The DRG is an important site of visceral afferent convergence and cross-sensitization and potential target for designing new anti-nociceptive therapies.
Keywords: DRG, P2X3, TRPV1, ERα, ERβ, mouse
Introduction
The incidence of episodic or persistent visceral functional pain disorders such as irritable bowel syndrome (IBS), painful bladder syndrome (PBS) and fibromyalgia are much more prevalent in women than men. Sensitization of primary afferent neurons may play a role in the enhanced perception of visceral sensation leading to pain. Several lines of evidence indicate that 17β-estradiol (E2) directly influence the functions of primary afferent neurons. Both subtypes of estrogen receptors (ERα and ERβ) are present in DRG neurons including the small-diameter putative nociceptors [1]. ATP-sensitive DRG neurons in primary culture respond to E2 [2–3]. E2 modulates cellular activity by altering ion channel opening, G-protein signaling, and activation of trophic factor-like signal transduction pathways [4]. DRG neurons can be activated or modulated by the activation of chemosensitive receptors and ion channels on peripheral terminals. Once released into the intercellular areas, the action of ATP is mediated by purinergic receptors which are expressed on primary afferent fibers and cell bodies within DRG, suggesting a significant role for ATP release in neuropathic pain [5]. Although various types of P2X receptors are expressed in the DRG, the predominant ATP receptor is the P2X3 [6].
The capsaicin-sensitive primary afferent neurons of small- and medium-diameter neurons mediate nociceptive-like behaviors suggesting that TRPV1 expressing neurons are nociceptors. Activation of P2X3 and TRPV1 receptors results in the production of a cation current with a subsequent depolarization and opening of voltage-gated Ca2+ channels (VGCC) and the sensation of pain is produced by depolarization of the peripheral nerve terminals [7]. Recently we showed that E2 rapidly attenuates ATP-induced P2X3-mediated [Ca2+]i signaling through membrane-associated ERα [3]. E2 may also play a key role in pain modulation by inhibiting transient receptor TRPV1 activation by capsaicin in nociceptor neurons [8]. The mechanism underlying the effect of E2 on the P2X3 and TRPV1-mediated nociceptive response needs further investigation and in this study we showed that the expression of both P2X3 and TRPV1 receptors was significantly reduced in estrogen receptor-α (ERαKO) and estrogen receptor-β (ERβKO) mice compare with the expression wild type (Wt) mouse.
Materials and Methods
Animals
We have used 6~8 week old female wild type (Wt, C57Bl/6J), ERαKO (B6.129P2-Esr1tm1Ksk/J), and ERβKO (B6.129P2-Esr2tm1Unc/J) mice (Jackson Laboratory, Bar Harbor, ME). Upon arrival mice were housed in microisolator caging and maintained on a 12-h light/dark cycle in a temperature-controlled environment with access to food and water ad libitum for two weeks. All studies were carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Charles R. Drew University and the NIH Guide for the Care and Use of Laboratory Animals. In some experiments we used animals from our breeding colony.
Western Blot Analysis
The expressions of P2X3 and TRPV1 receptors in L1-S1 DRGs were studied by using Western blot analyses. Tissues from Wt (C57Bl/6J), ERαKO, and ERβKO mice were quick frozen in tubes on dry ice during collection. L1-S1 DRG were combined, homogenized by mechanical disruption on ice-cold RIPA buffer plus protease inhibitors and incubated on ice for 30 minutes. Homogenates were then spun at 5000g for 15 minutes and supernatants collected. Total protein was determined on the supernatants using the BCA microtiter method (Pierce, Rockford, IL, USA). Samples containing equal amounts of protein (40µg) were electrophoresed under denaturing conditions using Novex Mini-cell system (San Diego, CA, USA) and reagents (NuPage 4–12% Bis-Tris gel and MOPS running buffer). After electrophoretic transfer onto nitrocellulose membrane using the same system, the membrane was blocked with 5% non-fat dry milk (NFDM) in 25 mM TRIS buffered saline, pH 7.2, plus 0.1% Tween 20 (TBST) for 1 hour at room temperature, followed by incubation with polyclonal rabbit antibody against P2X3 and TRPV1 receptor (1:1000, Neuromics, Edina, MN) for overnight at 4°C. The membrane was then washed in TBST plus NFDM, and proteins were visualized using a horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, CA). Following a final wash in TBST without NFDM, the membrane was incubated with ECL+ (Amersham, Arlington Heights, Ill., USA) substrate for HRP. Membranes were probed with primary antibody and corresponding secondary antibodies, signals were scanned and quantified by Image J version 1.28U and NIH Image 1.60 scan software. Following enhanced chemiluminescence detection of proteins, the membranes were stripped with stripping buffer (Pierce, Rockford, IL, USA) and re-hybridized with β-actin antibody as a loading control. At least three independent cell preparations were used.
Immunohistochemistry
For tissue collection DRG from bilateral spinal levels L1-S1 were removed and fixed in 4% paraformaldehyde for overnight at 4°C. DRGs were rinsed in Delbecco’s Phosphate Buffered Saline (DPBS) and kept in sucrose (20%, 4°C) for cryoprotection (48 h), after which excess liquid was removed. Each DRG was mounted in Tissue-Tek® OCT embedding medium (Sakura Finetek, Torrance, CA), and sectioned at −20°C in a MICROM H505E (Viocompare, San Francisco, CA) cryostat. Sections were cut at 20µm and collected in PBS. Endogenous tissue peroxidase activity was quenched by soaking the sections for 10 min in 3% hydrogen peroxide solution in 0.01 M PBS. The specimens were washed and then treated for 60 min in blocking solution, 0.01 M PBS containing 0.5% Triton X-100 and 1% normal donkey serum (NDS) at room temperature. They were processed for wild type (n=4), ERαKO (n=4), or ERβKO (n=4) immunohistochemistry by the free floating method using polyclonal rabbit TRPV1 receptor antibody (1:50000, Neuromics) or P2X3 receptor antibody (1:15000, Neuromics) for overnight at 4°C, washed in 0.01 M phosphate-buffered saline (PBS) and 0.01M Tris Buffered Saline (TBS), followed by incubation in solutions of donkey anti-rabbit fluorophore-conjugated secondary antibodies (1:200, Invitrogen) in 0.01M Tris Buffered Saline (TBS) for 3 hours at room temperature. Cells showing no apparent or only faint membrane ⁄ intracellular labeling were considered to be negative for TRPV1 or P2X3. P2X3-positive neurons showed diffuse membrane ⁄ intracellular labeling. Mounted sections were air dried and coverslipped with Aqua Poly Mount (Polisciences, Warrington, PA). TRPV1-positive cells included those with strong plasma membrane labeling that formed a discernible clustered pattern, and those with strong intracellular labeling that formed a punctuate pattern. Some neurons showed both strong plasma membrane and intracellular labeling. Images from at least three sections in each level were taken using Leica DMLB M130X microscope. The total numbers of DRG neurons expressing TRPV1 and P2X3 were counted. TRPV1/P2X3-positive neurons were categorized according to their labeling patterns and were expressed as a percentage of the total number of TRPV1/P2X3-positive cells. Immunohistochemical signal percent was measured by computerized image analysis (Image Pro-Plus, Media Cybernetics, Silver Spring, MD, USA).
Statistical analysis
The amplitude of [Ca2+]i response represents the difference between baseline concentration and the transient peak response to drug stimulation. Significant differences in response to chemical stimulation were obtained by comparing [Ca2+]i increases during the first stimulation with the second. All of the data are expressed as the mean ± SEM. Statistical analysis was performed using Statistical Package for the Social Sciences 15.0 (SPSS, Chicago, IL, USA). To assess the significance among different groups, data were analyzed with one-way ANOVA followed by Schéffe post hoc test. A p <0.05 was considered statistically significant.
Results
Expression of P2X3 and TRPV1 receptors in whole cell lysates in DRG neurons from Wt, ERαKO, and ERβKO mice
P2X3 and TRPV1 receptors expression was examined by Western Blot analysis of lysates from Wt, ERαKO, and ERβKO DRG tissues using a P2X3 specific primary antiserum (Fig.1a). Intense bands representing a ~64 kDa protein (P2X3) and a ~130 kDa (TRPV1) were seen in DRG lysates from Wt animals. There was a dramatic decrease in intensity of these bands using lysates made from the both ERαKO and ERβKO knock-outs DRG tissues compared with Wt animals (>4 fold decrease Fig.1a). A representative result of P2X3/TRPV1 receptors and the standardization ratio statistics are shown in Fig.1 b. The average intensities of the bands in both knock-out mice decreased significantly. When the density in the control group was standardized to 1.0, the average densities were 0.172 ± 0.08 for ERαKO and 0.262 ± 0.10 for ERβKO in P2X3 receptors, and 0.59 ± 0.06 in ERαKO and 0.391 ± 0.04 in ERβKO for TRPV1 receptors (n=4, p<0.05). Our data strongly suggest that both P2X3 and TRPV1 protein decreased in DRG from ERs knock-out mice
Figure 1.

Western blot analysis of DRG lysates shows reduced expression of P2X3 and TRPV1 in both knock-out mice. Equal amounts of lysates (40 µg) generated from ERαKO and ERβKO DRG neurons, as well as from Wt mice, were electrophoresed under denaturing conditions and probed with a anti-P2X3/TRPV1 antibodies (n=4 per group). a) Expression of P2X3/TRPV1 in DRG neurons. b) Both DRG neurons of knock-out mice showed a significant reduction in band intensity, 2~3 fold changes of knock-out compared with neurons from Wt (control) DRGs. Quantification of signals from Western blots shows statistically significant difference between the intensity of the bands from both knock-out DRG neurons when compared with wild type animals. Values are expressed as mean ± SEM p<0.05, n=4. * indicate significant difference from control.
Distribution of P2X3 and TRPV1 receptors in L1-S1 levels of DRG neurons from WT, ERαKO, and ERβKO mice
Nociceptive capsaicin-sensitive ATP-sensitive P2X3 receptors and capsaicin-sensitive TRPV1 receptors are expressed in lumbosacral small DRG neurons. DRGs from each level (L1-S1) were immunostained with primary antibodies against P2X3 and TRPV1. Neuronal profiles from ERαKO, ERβKO as well as Wt mice (n=4 in each group) were quantified for different fluorescent probe. Both nociceptive mediating P2X3 and TRPV1 receptors present in DRG neurons (Fig. 2a, 3a). The distributions of labeled dorsal root ganglion neurons represent the statistically significant difference between L1, L2, L4, and L6 levels of P2X3 receptors and L1, L2, S1 levels of TRPV1 receptors in both ERαKO and ERβKO DRG neurons in comparison to Wt mice. There was also statistically significant difference L5 level of P2X3, and L3 and L4 of TRPV1 in Wt compared to ERαKO (Fig. 2b, 3b; P<0.05, n=4 in each group).
Figure 2.
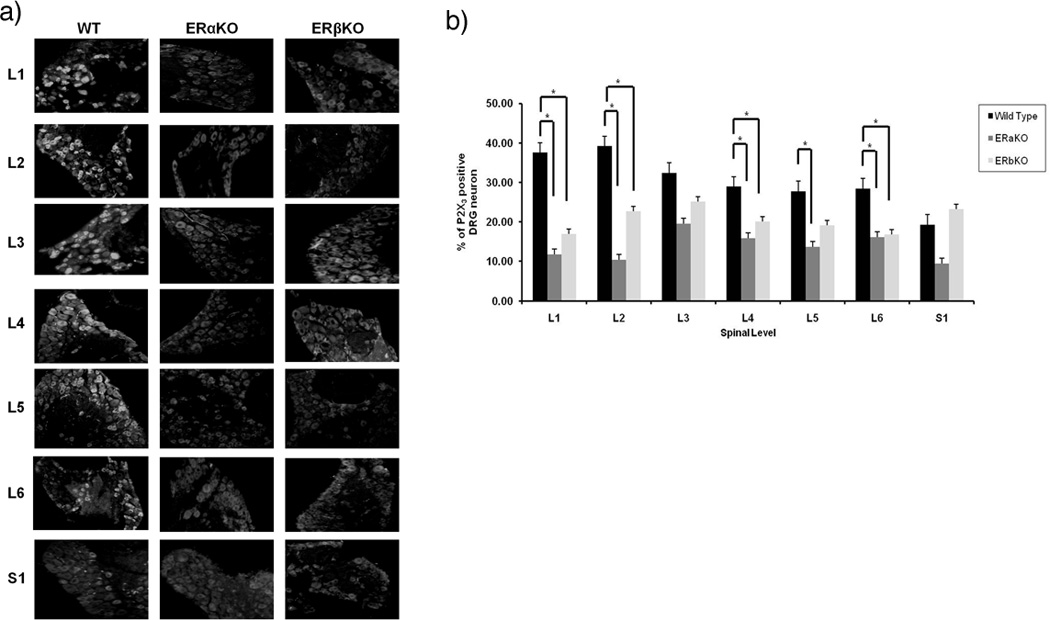
(a) Expression of P2X3 receptors in DRG neurons from Wt, ERαKO, and ERβKO mice using immunocytochemistry (b) Percentage distribution of P2X3-labeled DRG neurons in Wt, ERαKO and ERβKO mice in L1-S1 levels. * indicate statistically significant difference from control, p<0.05.
Figure 3.
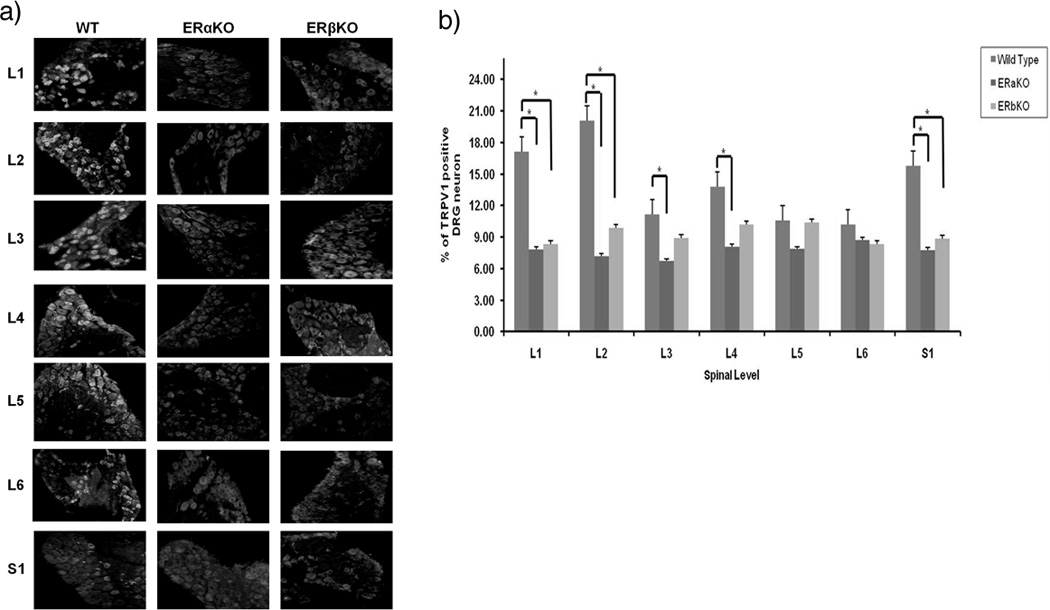
(a) Distribution of TRPV1 receptors in DRG neurons from Wt, ERαKO, and ERβKO by immunocytochemistry. DRG sections (L1 through S1 levels) were incubated in TRPV1 primary antibodies. (b) Distribution percentage of TRPV1-labeled DRG neurons in ERαKO, ERβKO and Wt mice in L1-S1 levels. * indicate statistically significant difference, p<0.05.
Discussion
The incidence of persistent, episodic, or chronic visceral pain is more prevalent in females thus defining the site(s) and mechanism through which female steroid hormones modulate pain is an important step in understanding gender difference in pain perception.
In previous studies we showed that E2 acts through an ERα in modulating the ATP/capsaicin-mediated [Ca2+]i response in DRG, since its effect was eliminated in ERαKO mouse and retained in ERβKO ([3] and unpublished observations). In this study we found a correlation between the expression of P2X3/TRPV1 and ERα/ERβ. Expression of P2X3 receptors was significantly decreased in L1, L2, L4, and L6 levels and the expression of TRPV1 receptors in L1, L2, S1 levels of in both ERαKO and ERβKO mice.
Elucidating the mechanisms through which E2 modulates nociception is an important step in designing appropriate therapies for treating pain. This study supports the potential of the P2X3 and TRPV1 receptor as a pain therapy target in females. ATP released by noxious stimulation and tissue damage has been found near the peripheral terminal of primary sensory afferent neurons [9]. Evidence for TRPV1’s role in there pathogenesis come from studies showing that mice lacking TRP1 receptor gene have deficits in thermal- or inflammatory-induced hyperalgesia [10]. Although large numbers of clinical and animal studies have indicated that there are sex and estrous cycle differences in pain perception of various pain models [11] it remains unclear whether that occurs in capsaicin-induced pain response.
Activation of both capsaicin-sensitive TRPV1 and ATP-sensitive P2X3 receptors caused desensitization correlating to the anti-nociception. Therefore, we hypothesized that changes of capsaicin-sensitive TRPV1 receptor may show a level of DRG neuron activation by noxious cutaneous stimuli, but changes of ATP-sensitive P2X3 receptor in [Ca2+]i may affect the level of DRG neuron sensitization by noxious visceral stimulation [12]. E2 effects on visceral nociceptive signaling have been observed in clinical studies, but most of the research has been focused on the central nervous system. This study, on the other hand, was focused on the nociceptive signaling mechanisms in the peripheral nervous system, at the level of DRG and how ERs modulate encoding of nociceptive stimuli that may contribute to the development of cure for many functional pain-associated syndromes.
Acknowledgement
Supported by NIH Grant NS 063939-04 from the National Institute of Neurological Disorders and Stroke
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Papka RE, Storey-Workley M. Estrogen receptor-alpha and -beta coexist in a subpopulation of sensory neurons of female rat dorsal root ganglia. Neurosci Lett. 2002;319(2):71–74. doi: 10.1016/s0304-3940(01)02562-9. [DOI] [PubMed] [Google Scholar]
- 2.Chaban VV, et al. Estradiol inhibits ATP-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003;118(4):941–948. doi: 10.1016/s0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- 3.Cho T, Chaban VV. Interaction between P2X3 and ERalpha/ERbeta in ATP-mediated calcium signaling in mice sensory neurons. J Neuroendocrinol. 2011 doi: 10.1111/j.1365-2826.2011.02272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srivastava DP, et al. Rapid estrogen signaling in the brain: implications for the fine-tuning of neuronal circuitry. J Neurosci. 2011;31(45):16056–16063. doi: 10.1523/JNEUROSCI.4097-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnstock G. Purinergic Signalling special issue on "Purinergic Signalling in the Inner Ear"; a commentary by Professor Geoffrey Burnstock, Editor-in-Chief. Purinergic Signal. 2010;6(2):149. doi: 10.1007/s11302-010-9197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn PM, Zhong Y, Burnstock G. P2X receptors in peripheral neurons. Prog Neurobiol. 2001;65(2):107–134. doi: 10.1016/s0301-0082(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 7.Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010;16(11):1248–1257. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu S, et al. 17beta-estradiol activates estrogen receptor beta-signalling and inhibits transient receptor potential vanilloid receptor 1 activation by capsaicin in adult rat nociceptor neurons. Endocrinology. 2008;149(11):5540–5548. doi: 10.1210/en.2008-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22(4):182–188. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- 10.Davis JB, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405(6783):183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 11.Bradshaw HB, Berkley KJ. Estrogen replacement reverses ovariectomy-induced vaginal hyperalgesia in the rat. Maturitas. 2002;41(2):157–165. doi: 10.1016/s0378-5122(01)00261-4. [DOI] [PubMed] [Google Scholar]
- 12.Chaban V. Estrogen and Visceral Nociception at the Level of Primary Sensory Neurons. Pain Res Treat. 2012;2012(2012) doi: 10.1155/2012/960780. [DOI] [PMC free article] [PubMed] [Google Scholar]


