Abstract
Functional repair of focal cartilage defects requires filling the space with neotissue that has compressive properties comparable to native tissue and integration with adjacent host cartilage. While poor integration is a common complication with current clinical treatments, reports of tissue engineering advances in the development of functional compressive properties rarely include analyses of their potential for integration. Our objective was thus to assess both the maturation and integration of mesenchymal stem cell (MSC) laden hyaluronic acid (HA) hydrogels in an in vitro cartilage defect model. Furthermore, we considered the effects of an initial period of pre-maturation as well as various material formulations to maximize both construct compressive properties and integration strength. MSCs were encapsulated in 1%, 3%, and 5% methacrylated HA (MeHA) or 2% agarose (Ag) and gelled directly (in situ) within an in vitro cartilage defect or were formed and then pre-cultured for 4 weeks before implantation. Results showed the integration strength of pre-cultured repair constructs was equal to (1% MeHA) or greater than (2% Ag) the integration of in situ repaired cartilage. Moreover, MSC chondrogenesis and maturation was restricted by the in situ repair environment with constructs maturing to a much lesser extent than pre-matured constructs. These results indicate that construct pre-maturation may be an essential element of functional cartilage repair.
Keywords: cartilage tissue engineering, hydrogel, tissue engineering, cell encapsulation, hyaluronic acid/hyaluronan, mechanical test, mechanical properties, progenitor cell
Introduction
The role of articular cartilage is to provide a low friction joint surface that resists wear while distributing stresses in a demanding joint environment [1]. Together with the limited regenerative capacity of native cartilage, these functional demands have made cartilage repair a rather intractable problem. Regenerative strategies (e.g. autologous chondrocyte implantation [ACI] or microfracture) for the repair of cartilage defects arising from disease or traumatic injury often result in fibrocartilaginous tissue that does not restore function [2–4]. Successful lateral integration is likewise a complication in both ACI and osteochondral grafting procedures [2, 5–7]. Failed graft integration results in changes in mechanical stress which can damage adjacent cartilage and result in the early onset of osteoarthritis [8–11]. The clinical demand for functional, biological cartilage replacement strategies has motivated the efforts of many researchers in the fields of biomaterials and tissue engineering to create a graft material. However, many challenges remain in the identification of biomaterials that have sufficient mechanical stiffness and the ability to integrate with host tissue to provide clinically relevant functional outcomes.
Considerable progress has been made towards the in vitro tissue engineering of neocartilage with compressive properties approaching native levels. Of note, articular chondrocytes encapsulated within various biomaterials have generated constructs with native mechanical properties when exposed to specialized media and dynamic loading conditions [12–14]. While these advances are significant, obtaining sufficient quantities of healthy chondrocytes from a patient to generate autologous tissue engineered cartilage remains a challenge. Towards this end, the autologous use of mesenchymal stem cells (MSCs) has become increasingly popular as MSCs are easily expanded in vitro while maintaining the capacity for chondrogenic differentiation [15, 16]. Despite their potential, one report suggests that in vitro repair with MSCs results in a fraction of the integration strength obtained by chondrocytes [17]. To date, no reported studies of MSC-based cartilage constructs have examined the simultaneous development of compressive and integrative properties, both of which are crucial for successful cartilage repair.
While many in vitro studies show histological data to demonstrate ‘good’ orthotopic graft to host tissue integration, relatively few provide biomechanical evidence to support these claims [18–20]. The intricacies and cost of such studies are perhaps the primary reason that more studies are not routinely conducted [21]. When mechanics have been assessed in in vitro or ectopic in vivo models, integration strength is reported as a function of tension or shear to failure of the cartilage repair interface. Results from these studies show that integration is dependent on biomaterial properties [22, 23], cell type [17], construct pre-culture [22, 24], and growth factor supplementation [25]. Cartilage age [25, 26], surface degradation [24, 27, 28], and the application of tissue adhesives [29–31] also modulate integration strength. In general, integration occurs under conditions of active biosynthesis, can be improved by increased permissiveness of the host cartilage matrix, and by the use of tissue adhesives.
Yet to be established is whether in situ formed constructs or the implantation of in vitro matured constructs is more appropriate for cartilage repair. Two important questions emerge: first, while in situ gelation is the most direct and practical clinical application [29], it is yet to be demonstrated that in situ formed constructs can completely integrate and develop sufficient compressive properties to protect adjacent host cartilage. Obradovich et al. in their study seeded chondrocytes on polyglycolic acid (PGA) scaffolds and found that immature constructs (5 day pre-culture) integrated better than mature (5 week pre-culture) constructs [24]. Conversely, an in vitro study by Hunter et al reported that construct maturation had a limited effect on the integration strength of PGA scaffolds, concluding also that in situ maturation is inhibited by adjacent cartilage [22]. Moreover, it is not clear whether the implantation of a mature construct will limit the degree to which integration occurs. The Obradovic study implanted chondrocyte-seeded PGA constructs with an equilibrium modulus of 224 kPa (confined compression) which resulted in an adhesive strength of ~250 kPa; however, this work, like the rest of the literature did not report the modulus of the repair material after in vitro culture within the defect model. It thus remains to be determined when implantation should take place and the subsequent maturation that can occur in the defect site.
To address some of these issues, we have optimized a photocrosslinkable methacrylated hyaluronic acid (MeHA) hydrogel [32] to maximize chondrogenesis of encapsulated MSCs [33, 34]. Our findings demonstrated that low MeHA macromer densities (1% w/v) provide a permissive environment for the formation and diffusion of cartilage matrix components and for the functional maturation of MSC-based tissue engineered cartilage. Still unknown, however, is the potential for MSC-laden MeHA to mature and integrate in a cartilage repair model. Further, the extent to which pre-culture (an initial period of construct maturation) modulates integration and compressive properties is unknown.
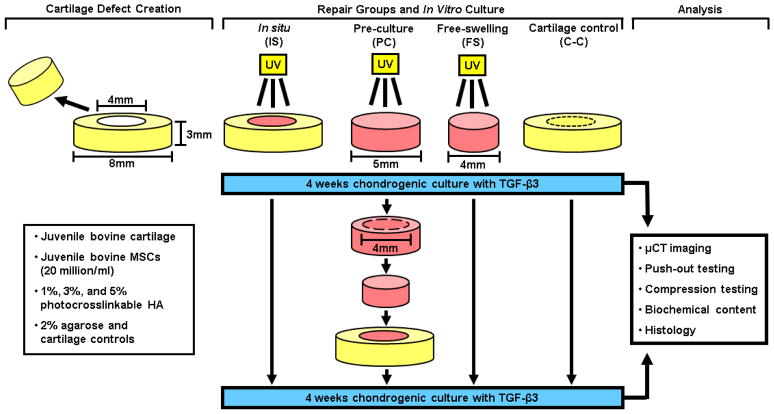
Thus, the objective of the present study was to determine the effects of tissue prematuration on the potential of MSC-laden MeHA for functional cartilage repair. Two independent factors were considered: 1) which MeHA macromer concentration (1%, 3%, or 5%) and 2) which construct maturation state (0 or 4 weeks) would result in the greatest combination of both compressive properties and integration strength. To accomplish this design, MSCs were encapsulated in MeHA and either UV polymerized within explant cartilage defects (in situ repair group: IS) or pre-cultured for 4 weeks (PC) before being press-fit into a defect (Fig. 1). The integration strength of repair and the simultaneous development of compressive properties were determined, as were the biochemical content, histological appearance, and the 3D space filling of repair constructs was assessed by contrast enhanced μCT.
Fig. 1.
Schematic illustrating the experimental design, creation of in vitro repair groups, and analysis techniques utilized in this study.
Materials and Methods
Methacrylated hyaluronic acid (MeHA) macromer synthesis
Methacrylated HA (MeHA) was synthesized by reacting methacrylic anhydride (Sigma, St. Louis, MO) and 74 kDa HA (Lifecore, Chaska, MN) followed by 1H NMR characterization as previously described [32]. MeHA (25% methacrylated) was lyophilized and stored at −20° C before being sterilized by exposure to a biocidal UV lamp for 15 minutes. The MeHA macromer was then dissolved to 1, 3, and 5% (mass/volume) in sterile PBS with 0.05% photoinitiator Irgacure-2959 (2-methyl-1-[4-(hydroxyethoxy)phenyl]-2-methyl-1-propanone; Ciba-Geigy, Tarrytown, NY).
Mesenchymal stem cell (MSC) isolation and cartilage repair model
MSCs from the bone marrow of juvenile bovine femurs were isolated and expanded through passage 3 in basal medium consisting of DMEM with 10% fetal bovine serum and 1% penicillin-streptomycin-fungizone (PSF) (Invitrogen, Carlsbad, CA) [35]. Cartilage was cored from the trochlear grooves of juvenile bovine femurs (3–6 months of age) using 8 mm diameter biopsy punches (Miltex, York, PA) and cultured in basal medium. Three days before beginning the experiment, cartilage was trimmed to ~3 mm in thickness and concentric 4 mm diameter cores were removed from each cartilage sample using biopsy punches with a custom device to create cartilage defects. The design of the experiment is shown in Fig. 1.
Experimental groups and culture conditions
Expanded MSCs were seeded at a density of 20 million cells per ml into 1%, 3%, and 5% MeHA and photopolymerized inside of the previously prepared cartilage rings. Photopolymerization was carried out within a custom made chamber wherein oxygen was purged with N2 gas throughout the 10 minute UV exposure to ensure complete polymerization (365 nm Blak-Ray UV lamp, Model #UVL-56, San Gabriel, CA). These constructs were referred to as in situ (IS) repaired constructs.
To investigate the effects of construct maturation on integration, MSCs were encapsulated in 1%, 3%, and 5% MeHA via UV polymerization between glass plates spaced by 2.25 mm as in [36]. Sterile 5 mm diameter biopsy punches were used to create MSC-laden hydrogel cylinders. After 4 weeks of pre-culture, biopsy punches were used to core a 4 mm construct from the 5 mm hydrogels and these cores were press fit within the additional cartilage defects. This experimental repair group was referred to as pre-culture (PC) repaired constructs.
To serve as a control for the effects of construct maturation within a cartilage defect, additional MSC-seeded MeHA hydrogels were fabricated at the same time (4 mm), but maintained in free-swelling conditions for the duration of the study. Samples from this control group were referred to as free-swelling (FS) constructs.
MSCs were also encapsulated within agarose (Ag; 2% w/v; Type VII, Sigma, St. Louis, MO) hydrogels (a well established scaffold for cartilage tissue engineering [35]) to provide a comparison and control for the unique microenvironment of the MeHA hydrogels. Molten MSC-laden Ag was gelled within cartilage defects (to produce IS repair group) and press-fit after 4 weeks of pre-culture (to form a PC group). Lastly, cartilage defects were re-fitted with 4 mm cartilage plugs that were obtained from the initial defect preparation. This cartilage-to-cartilage (C-C) control offered an in vitro analog to osteochondral transplantation, an established surgical repair approach.
All FS hydrogel constructs (1 ml/construct) and repaired defects (3 ml/construct) were cultured in TGF-β3 (10 ng/ml, R&D Systems, Minneapolis, MN) supplemented chemically defined medium consisting of high glucose DMEM with 1x PSF, 0.1 μm dexamethasone, 50 μg/mL ascorbate 2-phosphate, 40 μg/mL l-proline, 100 μg/mL sodium pyruvate, ITS+ (6.25 μg/ml insulin, 6.25 μg/ml transferrin, 6.25 ng/ml selenous acid, 1.25 mg/ml bovine serum albumin, and 5.35 μg/ml linoleic acid) in non-tissue culture treated 6-well plates with feedings thrice weekly.
Micro-computed tomography (μCT)
Contrast-enhanced micro-computed tomography (μCT) has been used to analyze 3D structure and proteoglycan content of articular cartilage [37]. 3D μCT imaging was utilized for analysis of cartilage defect filling to visualize the cartilage repair interface and proteoglycan accumulation within the repair material. Samples (n=3) at 4 and 8 weeks were first prepared by staining in Lugol’s solution (5% w/w I2 and 10% KI in dH2O) for 24 hours [37] and then scanned at an energy level of 70 kV and intensity of 114 μA (vivaCT 40, SCANCO USA, inc, Wayne, PA). 3D reconstructions provided visualization of defect filling and related to proteoglycan content for each hydrogel repair group and the C-C controls.
Mechanical testing
The integration strength of the in vitro repaired cartilage was assessed using a push-out test [27, 38]. A custom 3.8 mm indenter affixed to an Instron 5848 mechanical testing device pushed the hydrogel repair out of the cartilage annulus (0.2 mm/sec) while recording load. Failure stress (integration strength) was calculated as the quotient of the load at failure and the interface area (height x circumference).
After push out testing, the unconfined equilibrium compressive modulus was derived from a stress relaxation test (10% strain; 1000 sec relaxation) [39]. For each group and time point 4–5 samples were analyzed. After equilibration, the dynamic modulus was determined by applying 5 sinusoidal cycles of compression at 1 Hz (1% strain amplitude) [40]. Free swelling constructs were similarly assessed.
Biochemical content
Following the mechanical testing of FS and recently removed repair constructs (PC and IS), each (n = 4–5) was weighed and digested in papain before being analyzed for DNA, sulfated glycosaminoglycan (sGAG), and collagen content [35]. DNA content was analyzed using the Picogreen dsDNA assay kit (Molecular Probes, Eugene, OR), sGAG using the 1,9-dimethylmethylene blue (DMMB) dye binding assay, and the orthohydroxyproline (OHP) was measured and converted to collagen as previously described [41–43].
Histological analysis
Constructs were fixed in 4% paraformaldehyde after μCT scanning, dehydrated, infiltrated with Citrisolv (Fisher), and embedded with paraffin. Sections (8 μm) were stained for collagens (picrosirius red) and imaged at 100 X magnification [44].
Statistical analysis
All statistical analyses were performed using SYSTAT (v13, San Jose, CA). Three-way ANOVA was used for biochemical and integration data with hydrogel formulation (Ag, 1%, 3%, and 5% MeHA), culture condition (FS, IS, and PC), and time (4 and 8 weeks) as independent variables. Two-way ANOVA was used for equilibrium modulus with hydrogel formulation (Ag, 1%, 3%, and 5% MeHA) and culture condition (FS, IS, and PC) as independent variables. Fisher’s least significant difference post hoc test was used for each analysis of pair-wise comparisons and a threshold of p<0.05 was used to discern significant differences between experimental groups.
Results
Repair construct morphology and interface characteristics
Photopolymerization of MSC-laden MeHA within cartilage defects resulted in full defect filling with stable integration to the host cartilage at time 0 (i.e. constructs could be easily handled without dislodging the repair material). In some samples, a slight contraction of the IS 1% MeHA hydrogel core was observed with time in culture, but the majority of the repair interface remained intact. No hydrogel contraction was observed for the IS 3%, 5%, or Ag groups with time (data not shown). Constructs maintained in FS conditions for the first 4 weeks increased in opacity, with 1% MeHA and Ag constructs appearing more opaque and palpably stiffer. PC constructs from all groups were stable after being press fit into cartilage defects and remained in place for the final 4 weeks.
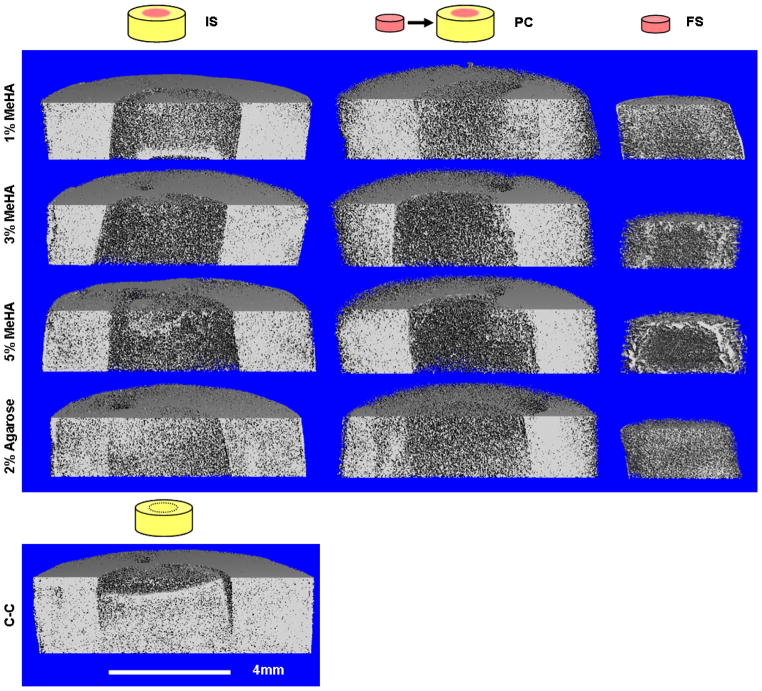
Cartilage and constructs were rapidly and effectively infiltrated by the charged contrast agent (I2KI) and, based on an inverse relationship to the contrast agent to content of proteoglycan, the repair interface between native cartilage and the experimental repair constructs was visualized in 3D. Most IS and all PC constructs completely filled the defect space (Fig. 2). Notable gaps were observed, however, at the repair interface in the cartilage to cartilage (C-C) control. No tissue engineered construct presented signal intensity levels as high as natural cartilage. However, greater signal attenuation was observed for 1% MeHA FS and PC constructs than in the IS repair hydrogels, indicating increased proteoglycan content (Fig. 2). Similarly, increased attenuation in each culture condition (IS, PC, and FS) was observed in both 1% MeHA and 2% Ag compared to either 3% or 5% MeHA.
Fig. 2.
Contrast enhanced μCT imaging of in vitro repaired cartilage defects after 8 weeks. Some contraction was observed in the 1% IS repair group (black arrows), while the PC repaired constructs showed no evidence of contraction or gapping. Proteoglycan-associated signal attenuation increased in 1% MeHA and 2% Ag indicating more accumulated proteoglycan than the remaining MeHA groups, yet still less than native cartilage (ring). Signal in FS controls was greater than in IS polymerized samples. C-C controls often contained large gaps between repair cartilage and adjacent host cartilage (black arrows).
Mechanical properties
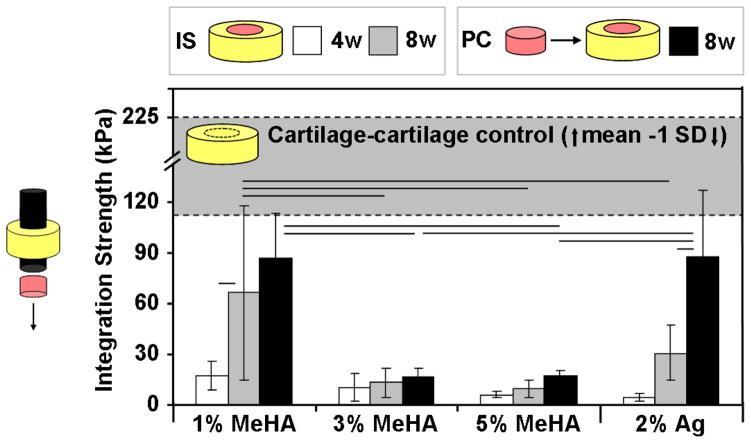
After 4 weeks of IS repair, the integration strength of each hydrogel group was less than 20 kPa (Fig. 3). The integration strength of IS 1% MeHA constructs at 4 weeks (17 kPa) increased 4 fold (67 kPa) by 8 weeks (p=0.002). Ag IS controls likewise increased from 5 to 31 kPa over this same period (p=0.075). The C-C control constructs reached 93 kPa at 4 weeks (not shown) and increased to 225 kPa by 8 weeks (shaded region, Fig. 3). The PC technique (at 8 weeks) resulted in a 30% increase (87 kPa) in integration over IS repair for 1% MeHA (p=0.168) and a near 3 fold increase for Ag controls (p<0.001; 88 kPa). The integration strength of 3% and 5% MeHA peaked at 17 kPa for PC, with no significant effects observed for either time (4 or 8 weeks) or repair condition (IS or PC).
Fig. 3.
Integration strength of in vitro cartilage repair was dependent on both hydrogel formulation and repair technique. The integration of MSC-laden MeHA (1%) and Ag reached nearly half the C-C controls (top grey region), while higher macromer concentration MeHA gels did not support integrative repair. Pre-culture (black bars) improved integration strength in both 1% MeHA and Ag repaired constructs. (n=4–5/group/timepoint; lines indicate p < 0.05)
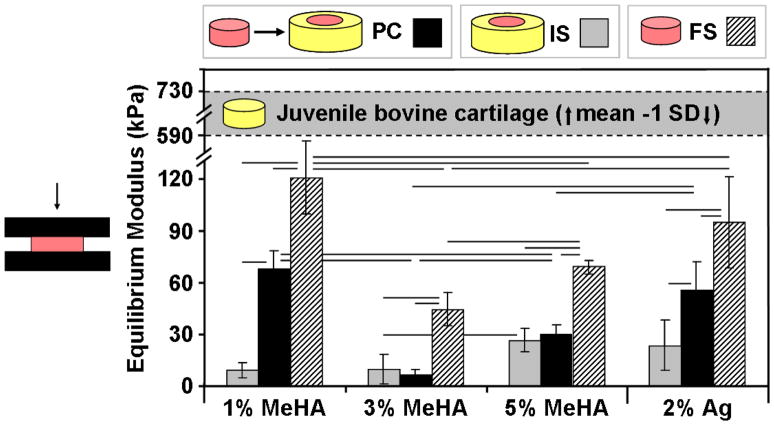
Compressive properties of the inner core from IS and PC repaired constructs were evaluated after measurement of the integration strength. Strikingly, the equilibrium modulus of FS 1% MeHA reached 120 kPa by 8 weeks, while 8 weeks of IS repair with the same MeHA concentration resulted in a modulus of only 9 kPa (Fig. 4; p<0.05). Similarly, IS culture resulted in reductions of equilibrium modulus of at least 50% for each additional hydrogel formulation (p<0.05). The modulus of 1% MeHA PC constructs reached 68 kPa, a value that was ~60% of FS controls and more than 7 times greater than IS constructs (p<0.001). Likewise, Ag constructs achieved a modulus of 56 kPa for PC compared to 24 kPa for IS at 8 weeks (p=0.001). The modulus of both 3% (7 kPa) and 5% (30 kPa) PC MeHA controls was not statistically different than the IS values.
Fig. 4.
Compressive properties of repair constructs were dependent on both hydrogel formulation and culture condition. IS repair construct properties (grey bars) were severely limited, while FS controls (hatched bars) attained the greatest equilibrium modulus. PC (black bars) improved the compressive properties of the hydrogel repair constructs for 1% MeHA and Ag, but did not match FS controls. (n=4–5/group/timepoint; lines indicate p < 0.05)
Biochemical content
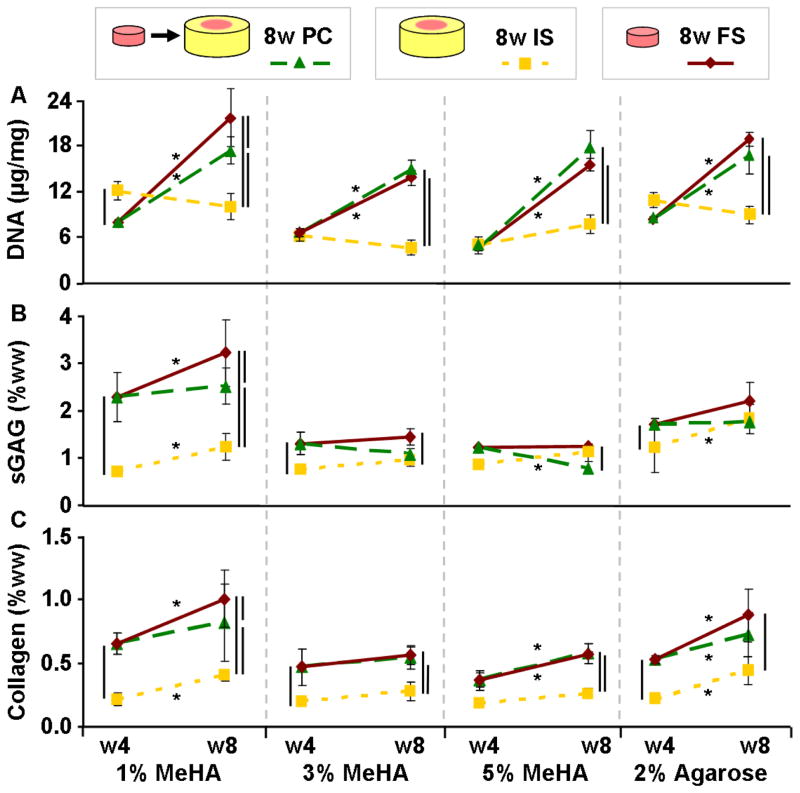
Trends in biochemical content followed the measured compressive properties of repair constructs. For example, sGAG content in FS 1% MeHA constructs after 8 weeks (3.2% w/w) was ~25% greater than in PC (2.5%) and ~3 fold greater than in IS (1.2%) constructs (p<0.05; Fig. 5A). The sGAG content in 3% and 5% MeHA hydrogels (in all conditions) reached ~1% of wet weight after 4 weeks and did not increase with an additional 4 weeks of culture. sGAG content in Ag FS, PC, and IS constructs reached ~2% of wet weight at 8 weeks, independent of repair condition. The highest level of sGAG in PC constructs was observed in 1% MeHA (2.5%), ~2–3 fold greater than sGAG in the other MeHA-based PC repair groups (p<0.001; Fig. 5A).
Fig. 5.
Biochemical content was dependent on hydrogel formulation and culture conditions. sGAG content (A) in 1% MeHA increased significantly in both FS and PC conditions compared to IS repair. IS repair similarly limited collagen accumulation (B) for every MeHA concentration and Ag. DNA content (C) generally increased from week 4 to 8 for PC and FS hydrogels, while DNA content in IS groups did not significantly change. (n=4–5/group/timepoint; lines indicate p < 0.05)
Collagen content in 1% MeHA constructs mirrored patterns observed for sGAG, with IS constructs containing less than half that of FS or PC constructs (Fig 5B). While collagen increased through 8 weeks in both 1% MeHA FS (p<0.001) and PC (p=0.062) constructs, PC constructs contained ~25% less collagen at week 8 (p=0.029). While IS collagen levels were equivalent for all MeHA macromer densities, collagen in FS and PC constructs was uniformly greater in 1% MeHA than in either 3% or 5% MeHA (p<0.05). Regardless of hydrogel formulation, IS cultured constructs contained less than 50% of the collagen in FS and PC constructs (p<0.05). Similarly, collagen in the Ag IS constructs at 8 weeks was 50% of the PC construct levels, while collagen in FS constructs was ~20% higher than in PC constructs (p=0.144; Fig. 5B).
DNA content in IS repaired constructs was stable from 4 to 8 weeks of culture (p>0.05; Fig. 5C). Conversely, DNA in FS and PC constructs increased ~2 fold (p<0.005) during the final 4 week period.
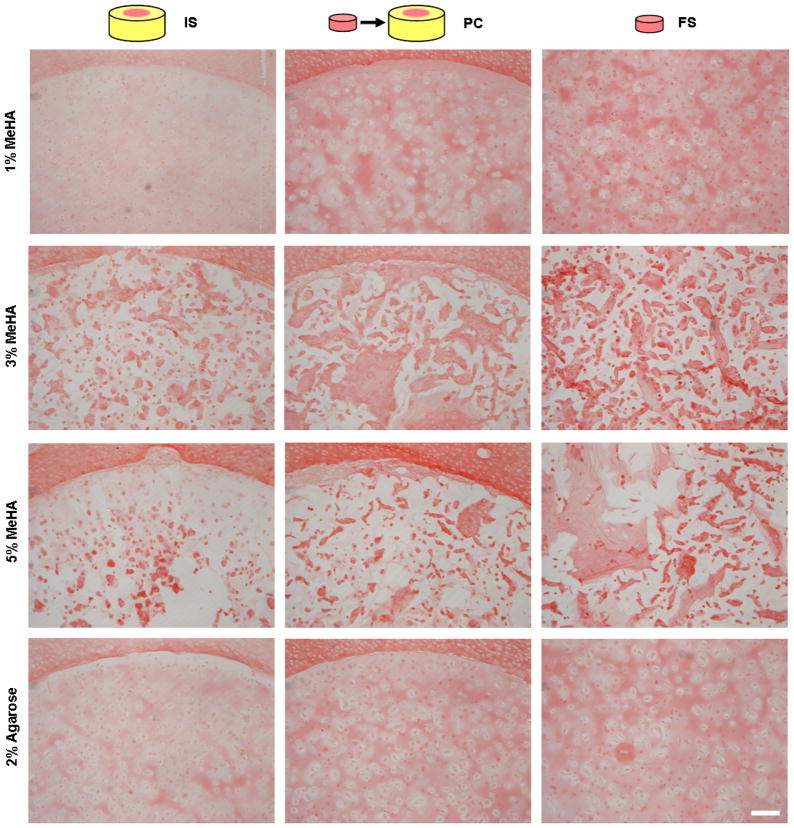
Histological analysis
Histological staining confirmed the quantitative biochemical findings for collagen content (Fig. 6). Picrosirius red staining of collagen was most intense in FS constructs, with slightly less staining in PC constructs, and much less staining in the IS repaired groups. The interface between the hydrogel and cartilage showed close apposition in all cases with visible collagen deposition at the interface. Collagen in 3% and 5% MeHA constructs was present in isolated accumulations, consistent with the limited matrix distribution previously observed in these higher macromer density gels [33].
Fig. 6.
Picrosirius red staining of collagen shows that IS repair limits construct maturation, while increased collagen density was observed in PC and FS constructs. Isolated aggregates of collagen were observed within 3% and 5% MeHA constructs from all experimental groups. (100 X original magnification; 250 μm scale bar)
Discussion
The functional repair of focal cartilage defects requires the restoration of mechanical function coupled with complete integration between the tissue engineered cartilage and adjacent host cartilage. While tissue engineered cartilage constructs with native compressive properties have been generated [12–14], quantitative analysis of the integrative potential of these constructs remains to be demonstrated. We have shown the potential for in vitro MSC-based cartilage development with photocrosslinkable HA hydrogels [33, 34], but had yet to analyze its capacity for integrative repair. One general concern with constructs that are matured in vitro is whether they are capable of integrating to host cartilage. Therefore, the two objectives of the present study were to 1) evaluate the integrative capacity of methacrylated HA hydrogels and 2) to determine the effect of in vitro pre-culture (PC) on the integration of tissue engineered cartilage to native cartilage.
The initial selection of HA hydrogels was based on its natural presence in cartilage, capacity for IS polymerization to fill irregularly shaped defects, overall biocompatibility, and in vivo degradation. Our earlier findings demonstrated that lower macromer density formulations led to increased compressive properties, however, it was also observed that higher macromer density HA increased dimensional stability [33, 45], an important factor for the IS formation of hydrogel constructs for cartilage repair. The results from the present study indicate that IS polymerized 1% MeHA remained stable (Fig. 2) and reached integration strengths greater than either of the higher macromer density formulations (Fig. 3). Quantification of matrix accumulation showed that the improved integration in IS 1% MeHA may be a result of the simultaneous increase in both sGAG and collagen with time, where matrix content in IS 3% and 5% MeHA did not increase (Fig. 5B–C). While integration strength paralleled accumulation of matrix constituents, compressive properties did not develop in either MeHA or Ag hydrogels when used for IS repair.
Given the poor maturation of IS constructs (compared to FS controls), constructs were next allowed a period of PC to mature before being press fit in the cartilage explant repair model. μCT imaging showed a good fit without gapping between tissue engineered and adjacent cartilage (Fig. 2). The integration strength of PC repaired constructs was equal to or better than that of the IS repair groups and the equilibrium modulus of the tissue engineered cartilage from the PC repairs was significantly greater for 1% MeHA and Ag (Fig. 3–4). An initial period of construct maturation resulted in repairs reaching levels that were closer to C-C control integration strength while also attaining mechanical properties that were closer to native tissue levels. Despite this progress, additional work must focus on further improving these levels to meet target benchmarks established by native cartilage properties and integration capacity (Fig. 7). Moreover, it will be important to implement additional mechanical tests of integration strength (for example the lap test employed by Dimicco and co-workers [47]), as the push-out test cannot fully decouple the contribution of mechanical integration, repair construct compressive properties, and friction between repair and native tissue due to swelling of the repair material.
Fig. 7.
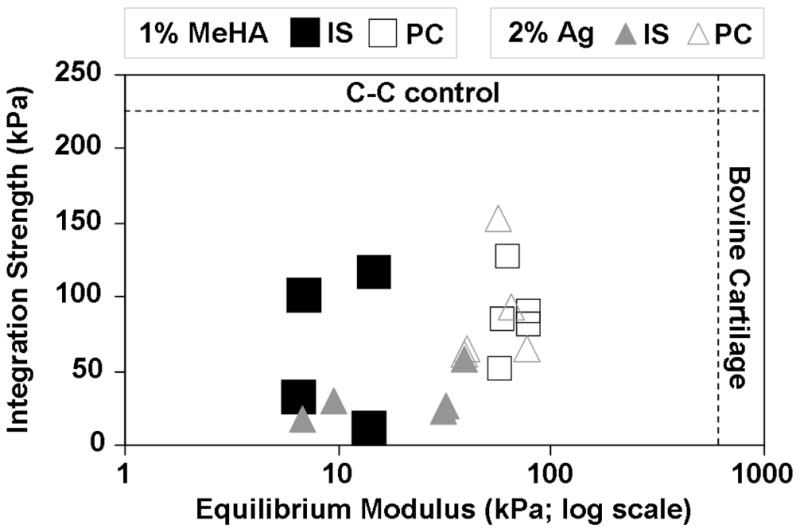
Integration strength vs. equilibrium modulus (log scale) for 1% MeHA and Ag (IS and PC) constructs compared to C-C integration control and equilibrium modulus of juvenile bovine cartilage [54]. Functional cartilage repair requires both stable lateral integration and restoration of compressive properties in the defect. The PC repairs for both hydrogels achieved levels that were closer to native tissue benchmarks compared to IS repair, though significant progress remains in matching native tissue benchmarks.
The reduction in equilibrium modulus that was observed in the IS repaired defects may be due to limited nutrient and chondrogenic factor diffusion as compared to those constructs pre-cultured in free-swelling conditions. This is supported by the intense μCT signal seen in the periphery of HA constructs grown in free swelling conditions, compared to the relative uniformity of staining in the IS and PC groups in the repair constructs (Fig. 2). However, the in vitro culture environment of the IS groups more closely mimics the in vivo scenario in that the route for diffusion of nutrients and waste is limited to the top of the repair hydrogel. Despite tripling the medium volume with thrice-weekly changes, nutrients may also have been limited by competitive consumption from the adjacent explant cartilage itself. Hunter et al suggested that soluble factors from live cartilage explants may limit the IS maturation of cartilage constructs, concluding that the implantation of mature constructs would result in the best outcomes [22]. Conversely, Bian et al recently showed that a small fraction of chondrocytes co-encapsulated with MSCs could increase differentiation substantially, but had no positive effect when cultured in adjacent gels [46]. These challenges to the undifferentiated MSCs in various hydrogel formulations may help explain lower equilibrium modulus, DNA, sGAG, and collagen content in IS repaired defects compared to PC groups. The constructs used for the PC repairs were maintained for 4 weeks in optimal growth conditions without any conflicting factors released from the adjacent cartilage. This favorable PC period allowed for chondrogenic differentiation and the accumulation of cartilage matrix constituents that persisted (or increased) with 4 additional weeks of culture within the cartilage defect.
In contrast to our findings, Obradovic et al reported that repair with immature (5 day PC) chondrocyte seeded polyglycolic acid (PGA) constructs led to greater integration strength when compared to repair with more mature (5 week PC) constructs [24]. In addition to using a different scaffold (fibrous PGA mesh) that may promote cell migration into or out of the material, they also utilized a rotating wall bioreactor, which could limit the effects of nutrient diffusion, competitive consumption, and even soluble factors that may have influenced the outcome of the present study. Also, unlike their use of chondrocytes, we used MSCs that undergo differentiation during the PC period. While this study presented a different conclusion on the effects of PC, the increased integration strength of the immature constructs paralleled higher rates of sGAG and collagen accumulation, which was a key finding of the current work. Our observations are supported by Dimicco et al who demonstrated that integration is correlated with new collagen deposition [47]. Recent work by Vinardell et al encapsulated MSCs and chondrocytes in agarose to evaluate their potential for in vitro integration [17]. In that study, sGAG and collagen accumulation was similarly limited with IS maturation, and the reported integration strength for their Agarose IS repair group (22.7 kPa) was similar to the Agarose IS results in this work (31 kPa).
In order to address our limited understanding of the effects of the IS repair environment on cartilage repair with MSC-laden MeHA, future work will consider repair and co-culture within live and devitalized cartilage as well as co-culture models where chondrocytes and MSCs are in the same gel [46]. Further, while the observed integration demonstrates the potential for successful repair, the in vitro model used here lacks the physical demands of the joint; loading may influence the formation of a stable integrated repair or disrupt a repair interface with insufficient durability. Cyclic deformation of repaired defects in vitro could offer new insights into integrative repair durability and the necessary restrictions on post-operative joint motion before the resumption of normal activities. Long-term dynamic compressive loading could also be used to overcome diffusional limitations in IS (and PC) repair by enhancing the diffusion of nutrients and soluble factors [48, 49]. Continuous loading could further improve the chondrogenic differentiation of MSCs and subsequent maturation of the repair construct [50–52]. If certain factors are identified that modulate this repair, they could likewise be delivered from the engineered construct itself with IS repair. For instance, we have recently shown that co-encapsulated alginate spheres can be used to deliver factors to direct the chondrogenic differentiation of MSCs in HA gels with subcutaneous implantation [53]; these methods may likewise improve construct maturation and integration in the cartilage defect space.
While not addressed here, the use of tissue adhesives and enzymatic degradation of interface surfaces can directly increase integration strength between tissue engineered and adjacent host cartilage [29–31]. The integration strength of the best case in this study (PC 1% MeHA) neared C-C controls and the equilibrium modulus approached native properties (~25%). Future work to improve integration strength may utilize degrading enzymes or tissue adhesives. Additionally, while we have recently demonstrated that increasing the MSC cell density by 3-fold in 1% MeHA resulted in constructs with an equilibrium modulus of ~300–400 kPa [54], the effect of these higher cell densities on integration has not yet been explored. The increased synthetic activity that markedly increased mechanical properties in this preliminary work may also prove to enhance the integration strength. Additionally, there may be a temporal component when it comes to pre-culture that is cell density related.
Conclusions
The IS polymerization of MSC-laden hydrogels resulted in a stable integrated repair interface, but addressed only one aspect of functional cartilage repair. Allowing a PC period for the pre-maturation of constructs improved both integration and the compressive properties of the tissue engineered cartilage used for in vitro cartilage repair. Future investigations to confirm the mechanism by which the IS environment inhibits construct maturation and the effects of joint motion and growth factor delivery on integration will lead to further advancements in functional cartilage repair.
Acknowledgments
This work was supported by the National Institutes of Health (R01 EB008722) and a Graduate Research Fellowship from the National Science Foundation (IEE).
Contributor Information
Isaac E. Erickson, McKay Orthopaedic Research Laboratory, Department of Orthopaedic Surgery, University of Pennsylvania, 424 Stemmler Hall, 36th Street and Hamilton Walk, Philadelphia, PA 19104, USA
Sydney R. Kestle, McKay Orthopaedic Research Laboratory, Department of Orthopaedic Surgery, University of Pennsylvania, 424 Stemmler Hall, 36th Street and Hamilton Walk, Philadelphia, PA 19104, USA
Kilief H. Zellars, McKay Orthopaedic Research Laboratory, Department of Orthopaedic Surgery, University of Pennsylvania, 424 Stemmler Hall, 36th Street and Hamilton Walk, Philadelphia, PA 19104, USA
George R. Dodge, McKay Orthopaedic Research Laboratory, Department of Orthopaedic Surgery, University of Pennsylvania, 424 Stemmler Hall, 36th Street and Hamilton Walk, Philadelphia, PA 19104, USA
Jason A. Burdick, Department of Bioengineering, University of Pennsylvania, 240 Skirkanich Hall, 210 S. 33rd Street, Philadelphia, PA 19104, USA
Robert L. Mauck, Email: lemauck@mail.med.upenn.edu, Associate Professor of Orthopaedic Surgery and Bioengineering, McKay Orthopaedic Research Laboratory, Department of Orthopaedic Surgery, University of Pennsylvania, 424 Stemmler Hall, 36th Street and Hamilton Walk, Philadelphia, PA 19104, USA, Phone: 215-898-3294, Fax: 215-573-2133
References
- 1.Ateshian GA, Soltz MA, Mauck RL, Basalo IM, Hung CT, Lai WM. The role of osmotic pressure and tension-compression nonlinearity in the frictional response of articular cartilage. Transport Porous Med. 2003;50:5–33. [Google Scholar]
- 2.Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85-A(2):185–92. doi: 10.2106/00004623-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Harris JD, Brophy RH, Siston RA, Flanigan DC. Treatment of Chondral Defects in the Athlete’s Knee. Arthroscopy. 2010;26(6):841–52. doi: 10.1016/j.arthro.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 4.Meachim G, Roberts C. Repair of the joint surface from subarticular tissue in the rabbit knee. J Anat. 1971;109(2):317–27. [PMC free article] [PubMed] [Google Scholar]
- 5.Niemeyer P, Pestka JM, Kreuz PC, Erggelet C, Schmal H, Suedkamp NP, et al. Characteristic Complications After Autologous Chondrocyte Implantation for Cartilage Defects of the Knee Joint. Am J Sports Med. 2008;36(11):2091–9. doi: 10.1177/0363546508322131. [DOI] [PubMed] [Google Scholar]
- 6.Domayer SE, Welsch GtH, Dorotka R, Mamisch TC, Marlovits S, Szomolanyi P, et al. MRI Monitoring of Cartilage Repair in the Knee: A Review. Semin Musculoskelet Radiol. 2008;12(04):302,17. doi: 10.1055/s-0028-1100638. [DOI] [PubMed] [Google Scholar]
- 7.Erggelet C, Kreuz P, Mrosek E, Schagemann J, Lahm A, Ducommun P, et al. Autologous chondrocyte implantation versus ACI using 3D-bioresorbable graft for the treatment of large full-thickness cartilage lesions of the knee. Arch Orthop Trauma Surg. 2010;130(8):957–64. doi: 10.1007/s00402-009-0957-y. [DOI] [PubMed] [Google Scholar]
- 8.Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg. 1982;64-A:460–66. [PubMed] [Google Scholar]
- 9.Guettler JH, Demetropoulos CK, Yang KH, Jurist KA. Osteochondral Defects in the Human Knee. Am J Sports Med. 2004;32(6):1451–8. doi: 10.1177/0363546504263234. [DOI] [PubMed] [Google Scholar]
- 10.Bullough PG. The role of joint architecture in the etiology of arthritis. Osteoarthritis Cartilage. 2004;12(Supplement 1):2–9. doi: 10.1016/j.joca.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Volpin G, Dowd G, Stein H, Bentley G. Degenerative arthritis after intra-articular fractures of the knee. Long-term results. J Bone Joint Surg Br. 1990;72-B(4):634–8. doi: 10.1302/0301-620X.72B4.2380219. [DOI] [PubMed] [Google Scholar]
- 12.Byers BA, Mauck RL, Chiang IE, Tuan RS. Transient Exposure to Transforming Growth Factor Beta 3 Under Serum-Free Conditions Enhances the Biomechanical and Biochemical Maturation of Tissue-Engineered Cartilage. Tissue Eng Part A. 2008;14(11):1821–34. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, et al. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15(9):1025–33. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bian L, Fong JV, Lima EG, Stoker AM, Ateshian GA, Cook JL, et al. Dynamic Mechanical Loading Enhances Functional Properties of Tissue-Engineered Cartilage Using Mature Canine Chondrocytes. Tissue Eng Part A. 2010;16(5):1781–90. doi: 10.1089/ten.tea.2009.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 16.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–72. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 17.Vinardell T, Thorpe S, Buckley C, Kelly D. Chondrogenesis and Integration of Mesenchymal Stem Cells Within an In Vitro Cartilage Defect Repair Model. Ann Biomed Eng. 2009;37(12):2556–65. doi: 10.1007/s10439-009-9791-1. [DOI] [PubMed] [Google Scholar]
- 18.Emans PJ, van Rhijn LW, Welting TJM, Cremers A, Wijnands N, Spaapen F, et al. Autologous engineering of cartilage. P Natl Acad Sci USA. 2010;107(8):3418–23. doi: 10.1073/pnas.0907774107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toh WS, Lee EH, Guo X-M, Chan JKY, Yeow CH, Choo AB, et al. Cartilage repair using hyaluronan hydrogel-encapsulated human embryonic stem cell-derived chondrogenic cells. Biomaterials. 2010;31(27):6968–80. doi: 10.1016/j.biomaterials.2010.05.064. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira JT, Gardel LS, Rada T, Martins L, Gomes ME, Reis RL. Injectable gellan gum hydrogels with autologous cells for the treatment of rabbit articular cartilage defects. J Orthop Res. 2010;28(9):1193–9. doi: 10.1002/jor.21114. [DOI] [PubMed] [Google Scholar]
- 21.Gratz KR, Wong VW, Chen AC, Fortier LA, Nixon AJ, Sah RL. Biomechanical assessment of tissue retrieved after in vivo cartilage defect repair: tensile modulus of repair tissue and integration with host cartilage. J Biomech. 2006;39(1):138–46. doi: 10.1016/j.jbiomech.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Hunter CJ, Levenston ME. Maturation and integration of tissue-engineered cartilages within an in vitro defect repair model. Tissue Eng Part A. 2004;10(5–6):736–46. doi: 10.1089/1076327041348310. [DOI] [PubMed] [Google Scholar]
- 23.Rice MA, Homier PM, Waters KR, Anseth KS. Effects of directed gel degradation and collagenase digestion on the integration of neocartilage produced by chondrocytes encapsulated in hydrogel carriers. J Tissue Eng Regen Med. 2008;2(7):418–29. doi: 10.1002/term.113. [DOI] [PubMed] [Google Scholar]
- 24.Obradovic B, Martin I, Padera RF, Treppo S, Freed LE, Vunjak-Novakovic G. Integration of engineered cartilage. J Orthop Res. 2001;19(6):1089–97. doi: 10.1016/S0736-0266(01)00030-4. [DOI] [PubMed] [Google Scholar]
- 25.Ionescu LC, Lee GC, Garcia GH, Zachry TL, Shah RP, Sennett BJ, et al. Maturation State-Dependent Alterations in Meniscus Integration: Implications for Scaffold Design and Tissue Engineering. Tissue Eng Part A. 2011;17(1–2):193–204. doi: 10.1089/ten.tea.2010.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiMicco MA, Waters SN, Akeson WH, Sah RL. Integrative articular cartilage repair: dependence on developmental stage and collagen metabolism. Osteoarthritis Cartilage. 2002;10(3):218–25. doi: 10.1053/joca.2001.0502. [DOI] [PubMed] [Google Scholar]
- 27.van de Breevaart Bravenboer J, In der Maur CD, Koen Bos PK, Feenstra L, Verhaar JAN, Weinans H, et al. Improved cartilage integration and interfacial strength after enzymatic treatment in a cartilage transplantation model. Arthritis Res Ther. 2004;6(5):469–76. doi: 10.1186/ar1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tam HK, Srivastava A, Colwell CW, D’Lima DD. In vitro model of full-thickness cartilage defect healing. J Orthop Res. 2007;25(9):1136–44. doi: 10.1002/jor.20428. [DOI] [PubMed] [Google Scholar]
- 29.Wang D-A, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, et al. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat Mater. 2007;6(5):385–92. doi: 10.1038/nmat1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverman RP, Passaretti D, Huang W, Randolph MA, Yaremchuk MJ. Injectable Tissue-Engineered Cartilage Using a Fibrin Glue Polymer. Plast Reconstr Surg. 1999;103(7):1809–18. doi: 10.1097/00006534-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Peretti GM, Zaporojan V, Spangenberg KM, Randolph MA, Fellers J, Bonassar LJ. Cell-based bonding of articular cartilage: An extended study. J Biomed Mater Res A. 2003;64(3):517–24. doi: 10.1002/jbm.a.10367. [DOI] [PubMed] [Google Scholar]
- 32.Burdick JA, Chung C, Jia X, Randolph MA, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6(1):386–91. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erickson IE, Huang AH, Sengupta S, Kestle S, Burdick JA, Mauck RL. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthritis Cartilage. 2009;17(12):1639–48. doi: 10.1016/j.joca.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung C, Burdick JA. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2009;15(2):243–54. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mauck RL, Yuan X, Tuan RS. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage. 2006;14(2):179–89. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Erickson IE, Huang AH, Chung C, Li RT, Burdick JA, Mauck RL. Differential maturation and structure-function relationships in mesenchymal stem cell- and chondrocyte-seeded hydrogels. Tissue Eng Part A. 2009;15(5):1041–52. doi: 10.1089/ten.tea.2008.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer AW, Guldberg RE, Levenston ME. Analysis of cartilage matrix fixed charge density and three-dimensional morphology via contrast-enhanced microcomputed tomography. P Natl Acad Sci USA. 2006;103(51):19255–60. doi: 10.1073/pnas.0606406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moretti M, Wendt D, Schaefer D, Jakob M, Hunziker EB, Heberer M, et al. Structural characterization and reliable biomechanical assessment of integrative cartilage repair. J Biomech. 2005;38(9):1846–54. doi: 10.1016/j.jbiomech.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 39.Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122(3):252–60. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 40.Park S, Nicoll S, Mauck R, Ateshian G. Cartilage mechanical response under dynamic compression at physiological stress levels following collagenase digestion. Ann Biomed Eng. 2008;36(3):425–34. doi: 10.1007/s10439-007-9431-6. [DOI] [PubMed] [Google Scholar]
- 41.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18(2):267–73. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 42.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883(2):173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 43.Neuman RE, Logan MA. The determination of hydroxypoline. J Biol Chem. 1949:299–306. [PubMed] [Google Scholar]
- 44.Mauck RL, Wang CC, Oswald ES, Ateshian GA, Hung CT. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis Cartilage. 2003;11(12):879–90. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Chung C, Beecham M, Mauck RL, Burdick JA. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells. Biomaterials. 2009;30(26):4287–96. doi: 10.1016/j.biomaterials.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bian L, Zhai DY, Mauck RL, Burdick JA. Coculture of Human Mesenchymal Stem Cells and Articular Chondrocytes Reduces Hypertrophy and Enhances Functional Properties of Engineered Cartilage. Tissue Eng Part A. 2011;17(7–8):1137–45. doi: 10.1089/ten.tea.2010.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DiMicco MA, Sah RL. Integrative cartilage repair: adhesive strength is correlated with collagen deposition. J Orthop Res. 2001;19(6):1105–12. doi: 10.1016/S0736-0266(01)00037-7. [DOI] [PubMed] [Google Scholar]
- 48.Mauck RL, Hung CT, Ateshian GA. Modeling of neutral solute transport in a dynamically loaded porous permeable gel: implications for articular cartilage biosynthesis and tissue engineering. J Biomech Eng. 2003;125(5):602–14. doi: 10.1115/1.1611512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Albro MB, Chahine NO, Li R, Yeager K, Hung CT, Ateshian GA. Dynamic loading of deformable porous media can induce active solute transport. J Biomech. 2008;41(15):3152–7. doi: 10.1016/j.jbiomech.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hunter CJ, Levenston ME. The influence of repair tissue maturation on the response to oscillatory compression in a cartilage defect repair model. Biorheology. 2002;39(1–2):79–88. [PubMed] [Google Scholar]
- 51.Mauck RL, Byers BA, Yuan X, Tuan RS. Regulation of Cartilaginous ECM Gene Transcription by Chondrocytes and MSCs in 3D Culture in Response to Dynamic Loading. Biomech Model Mechanobiol. 2006 doi: 10.1007/s10237-006-0042-1. [DOI] [PubMed] [Google Scholar]
- 52.Huang AH, Farrell MJ, Kim M, Mauck RL. Long-term dynamic loading improves the mechanical properties of chondrogenic mesenchymal stem cell-laden hydrogel. Eur Cell Mater. 2010;19:72–85. doi: 10.22203/ecm.v019a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bian L, Zhai DY, Tous E, Rai R, Mauck RL, Burdick JA. Enhanced MSC chondrogenesis following delivery of TGF-beta3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011;32(27):6425–34. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erickson IE, Kestle SR, Zellars KH, Burdick JA, Mauck RL, editors. High Density MSC-Seeded Hyaluronic Acid Constructs Produce Engineered Cartilage with Near-Native Properties. The 57th Annual Meeting of the Orthopaedic Research Society; January 13–16, 2011; Long Beach, CA. [Google Scholar]