Abstract
Organophosphorus (OP) nerve agents and pesticides inhibit acetylcholinesterase (AChE), and this is thought to be a primary mechanism mediating the neurotoxicity of these compounds. However, a number of observations suggest that mechanisms other than or in addition to AChE inhibition contribute to OP neurotoxicity. There is significant experimental evidence that acute OP intoxication elicits a robust inflammatory response, and emerging evidence suggests that chronic repeated low-level OP exposure also upregulates inflammatory mediators. A critical question that is just beginning to be addressed experimentally is the pathophysiologic relevance of inflammation in either acute or chronic OP intoxication. The goal of this article is to provide a brief review of the current status of our knowledge linking inflammation to OP intoxication, and to discuss the implications of these findings in the context of therapeutic and diagnostic approaches to OP neurotoxicity.
Keywords: Acute toxicity, biomarkers, chronic toxicity, cytokines, microglia, neuroinflammation, neuroprotection, neurotoxicity, occupational exposure, organophosphorus pesticides, reactive astrocytes
1.0 Introduction
Organophosphorus (OP) nerve agents and pesticides inhibit acetylcholinesterase (AChE), and this activity is widely accepted as a primary mechanism underlying the neurotoxicity of these compounds. AChE inhibition increases acetylcholine in cholinergic synapses resulting initially in overstimulation of nicotinic and muscarinic receptors followed by receptor downregulation. Acute cholinergic toxicity (OP poisoning) is thought to be mediated by overstimulation of receptors secondary to AChE inhibition, resulting in peripheral parasympathomimetic effects as well as seizures and respiratory arrest; whereas it is hypothesized that chronic OP neurotoxicity is mediated in part by receptor downregulation (Costa, 2006, Echbichon and Joy, 1995). However, a number of observations suggest that OP neurotoxicity is not due entirely to perturbations of cholinergic systems. For example, different OPs have different effects despite similar changes in AChE activity and other cholinergic markers (Bushnell and Moser, 2006, Jett and Lein, 2006, Pope et al., 2005, Pope, 1999), and AChE knockout mice exhibit symptoms of neurotoxicity comparable to those observed in wildtype mice following OP exposure (Duysen et al., 2001). There are also reports in the human and animal literature that OP neurotoxicity, particularly in response to chronic OP exposure, occurs in the absence of cholinesterase (ChE) inhibition (Abou-Donia, 2003, Costa, 2006, Kamel and Hoppin, 2004). For example, studies of humans with occupational exposures to OPs have consistently failed to find a significant association between blood cholinesterase activity and neurobehavioral deficits (Rohlman et al., 2011). A review of the animal literature presents a more complicated picture. In general, the most significant and prolonged motor effects are obtained following OP exposures that markedly inhibit brain ChE activity; however, cognitive deficits are not as clearly correlated with ChE inhibition (Bushnell and Moser, 2006). Considered together, these observations suggest that mechanisms in addition to or other than AChE inhibition mediate OP neurotoxicity. This conclusion has significant implications for the development of effective medical countermeasures for OP neurotoxicity and the use of AChE inhibition as a predictive or diagnostic biomarker of OP-induced neurotoxicity.
Of the various alternative molecular targets and mechanisms proposed to mediate OP-neurotoxicity (Casida and Quistad, 2005, Hernandez et al., 2004, Jett and Lein, 2006, Lockridge and Schopfer, 2010, Pancetti et al., 2007, Soltaninejad and Abdollahi, 2009), inflammation is of interest because of evidence suggesting that anti-inflammatory agents are neuroprotective following acute intoxication with OP nerve agents (Amitai et al., 2006) and because of the availability of experimentally validated quantitative peripheral biomarkers of inflammation that correlate well with neurobehavioral deficits observed consequent to neurodegenerative disease (Dziedzic, 2006, Mrak and Griffin, 2005). In this review, we will provide an overview of experimental evidence that links inflammation to acute and chronic OP intoxication, discuss mechanisms by which OPs may elicit inflammatory response and the potential pathophysiologic consequences of inflammation in the context of OP toxicity, and finally suggest how information regarding OP-induced inflammation may provide insight regarding novel therapeutic strategies for mitigating the neural damage consequent to OP intoxication.
2.0 Overview of inflammation
Inflammation is the natural response of the immune system to injury or infection. The inflammatory response is initiated via activation of macrophages in the periphery and microglia and/or astrocytes in the central nervous system (CNS), which leads to the release of proinflammatory mediators, such as cytokines. These compounds induce the dilation of blood vessels to promote migration of leukocytes, typically neutrophils, to the area of injury. Neutrophils and macrophages induce apoptosis of cellular targets via the release of nitric oxide and reactive oxygen species (ROS), and macrophages subsequently clear apoptotic cells via phagocytosis (Duffield, 2003).
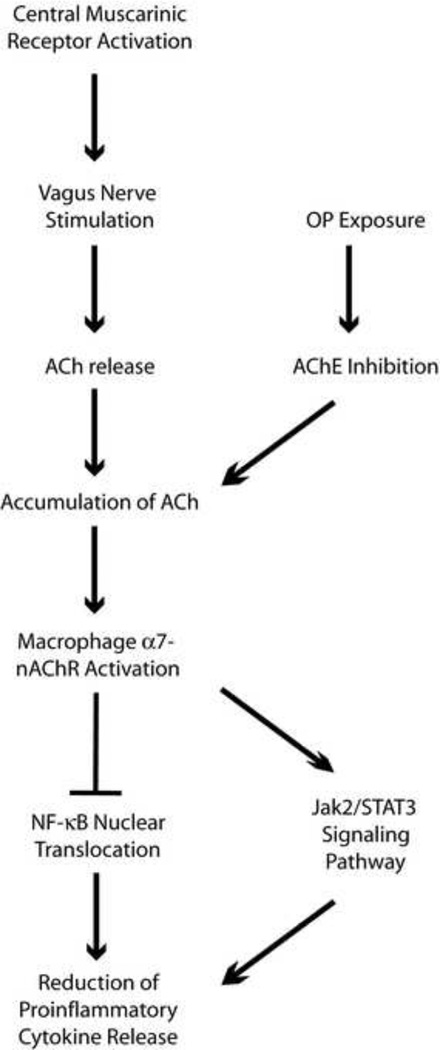
The inflammatory response is essential in maintaining homeostasis, but has the potential to cause deleterious effects if not tightly controlled (Hanisch and Kettenmann, 2007). Overproduction of proinflammatory cytokines and excessive inflammation is characteristic of many degenerative diseases (Blasko et al., 2004, Eikelenboom et al., 1998, Whitton, 2007), and can lead to systemic shock and sepsis. Anti-inflammatory mediators, such as the cytokines interleukin-10 (IL-10) and interleukin-4 (IL-4), transforming growth factor β (TGF β), and interleukin-1 (IL-1) receptor antagonists, in addition to neuroendocrine pathways, such as the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system, serve to regulate the inflammatory response (Elenkov et al., 2000, Pavlov et al., 2003, Turnbull and Rivier, 1999, Webster et al., 2002). A more recently discovered pathway, termed the cholinergic anti-inflammatory pathway (CAP) is a parasympathetic pathway designed to reduce inflammatory responses via the activity of the vagus nerve (Figure 1). This was first reported by Borovikova and colleagues (Borovikova et al., 2000) who demonstrated that acetylcholine suppresses inflammatory cytokine release from lipopolysaccharide (LPS)-treated human macrophage cultures, and that this activity is mediated by α7-nicotinic acetylcholine receptors. Vagotomy in rats increases tumor necrosis factor α (TNFα) levels in serum and liver, suggesting efferent vagus nerve signaling is crucial for reduction of inflammatory cytokine production (Borovikova et al., 2000). Stimulation of the efferent vagus nerve induces acetylcholine release, which binds to α7-nAChRs on macrophages to inhibit NF-κB nuclear translocation and proinflammatory cytokine release (Pavlov and Tracey, 2006). Additionally, activation of central muscarinic receptors (M1 and M2) in rats contributes to the reduction of serum TNFα levels during LPS-induced inflammation (Pavlov et al., 2006).
Figure 1.
Schematic diagram of the cholinergic anti-inflammatory pathway (CAP). Stimulation of the vagus nerve induces acetylcholine (ACh) release, which binds to a7-nicotinic acetylcholine receptors (nAChR) on macrophages. Activation of a7-nAChR initiates the Jak2- STAT3 signaling pathway, and inhibits NF-kB nuclear translocation, leading to the inhibition of pro-inflammatory cytokine release. OP exposure should activate CAP by inhibiting acetylcholinesterase (AChE), causing an increase in acetylcholine levels.
Evidence of the pathophysiologic relevance of the cholinergic anti-inflammatory pathway is emerging from studies of the therapeutic efficacy of anticholinergic agents as anti-inflammatory agents. For example, the AChE inhibitor galantamine has been reported to reduce levels of LPS-induced TNFα in plasma and serum (Liu et al., 2010, Pavlov et al., 2009). This anti-inflammatory response appears to involve activation of CAP, since vagotomy and non-functional α7-nicotinic AChRs abolish the anti-inflammatory effects of galantamine (Liu, Ma, 2010, Pavlov, Parrish, 2009). Additionally, treatment with atropine sulfate (which crosses the blood brain barrier) reverses the effects of galantamine on serum TNFα levels, suggesting the importance of central muscarinic AChRs in this pathway (Pavlov, Parrish, 2009). Further evidence of the role of CAP in modulating inflammatory responses is provided by a recent report that the anti-cholinergic agent donepezil decreases LPS-induced TNFα and interleukin-1β (IL-1β) in rat brains and this anti-inflammatory effect is diminished by administration of an α7-nAChR antagonist (Tyagi et al., 2010). There is also substantial evidence that direct activation of α7 receptors with selective agonists activates CAP and attenuates inflammatory processes (Bencherif et al., 2011). For example, the α7-selective agonist GTS-21 reduces LPS-induced TNFα and IL-1β levels in human whole blood and monocytes (Rosas-Ballina et al., 2009). GTS-21 also blocks TNFα release in mouse alveolar macrophages (Giebelen et al., 2007). Nicotine prevents microglial activation and reduces LPS-induced TNFα in rat brain (Park et al., 2007), and inhibits cytokine production by synovial tissue within joints (van Maanen et al., 2009).
Increasing evidence suggests that OPs modulate these inflammatory responses. Table 1 provides a summary of the main effects of OPs on inflammation. However, the inflammatory response profile is variable, depending in part on the OP, the exposure scenario, and when after exposure inflammation is assessed. Details of the inflammatory responses identified following acute or chronic OP intoxication are discussed in the following sections.
Table 1.
Summary of inflammatory responses triggered by OPs.
| Inflammatory Response | Exposure Paradigm | Type of OP | References |
|---|---|---|---|
| Glial Activation | |||
| Microglia | Acute | Soman |
Collombet et al. 2005a (in vivo, mouse); Zimmer et al. 1997 (in vivo, rat) |
| Increased GFAP expression | Acute | Soman, Sarin |
Angoa-Perez et al. 2010 (in vivo, rat); Damodaran et al. 2006 (in vivo, rat); Baille-Le Crom et al. 2005 (in vivo, rat); Collombet et al. 2005a (in vivo, mouse); Damodaran et al. 2002 (in vivo, rat); Damodaran et al. 2000 (in vivo, hen); Zimmer et al. 1997 (in vitro, rat telencephalon); |
| Chronic | Parathion, Chlorpyrifos |
Lim et al. 2011 (in vivo, mice); Zurich et al. 2004 (in vitro, rat telencephalon); Garcia et al. 2002 (in vivo, rat) |
|
| Soluble Inflammatory Mediators | |||
| Increased cytokine levels | Acute | Soman, Sarin, Chlorpyrifos |
Johnson et al. 2011 (in vivo, rat); Dillman et al. 2009 (in vivo, rat); Stapleton et al. 2009 (in vivo, rat); Dhote et al. 2007 (in vivo, mouse); Chapman et al. 2006 (in vivo, rat); Damodaran et al. 2006 (in vivo, rat); Williams et al. 2003 (in vivo, rat); Svensson et al. 2001 (in vivo, rat); |
| Chronic | Sarin, Chlorpyrifos |
Mense et al. 2006 (in vitro, human astrocytes); Henderson et al. 2002 (in vivo, rat) |
|
| Altered chemokine levels | Acute | Soman, Acephate |
Johnson and Kan, 2010 (in vivo, rat); Dhote et al. 2007 (in vivo, mouse); Williams et al. 2003 (in vivo, rat); Singh and Jiang, 2002 (in vivo, rat) |
| Increased prostaglandin/isoprostanoid levels | Acute | Soman, Sarin, DFP |
Angoa-Perez et al. 2010 (in vivo, rat); Zaja-Milatovic et al. 2009 (in vivo, rat); Grauer et al. 2008 (in vivo, rat); Chapman et al. 2006 (in vivo, rat); Levy et al. 2004 (in vivo, guinea pig) |
| Anaphylaxis | |||
| Increased macrophage activity/mast cell degranulation | Acute | Soman, Sarin, Malathion |
Levy et al. 2004 (in vivo, guinea pigs); Xiong and Rodgers, 1997 (in vitro, rat and human mast cells); Rodgers and Xiong, 1997a, 1996 (in vivo, mouse); Rodgers and Ellefson, 1992, 1990 (in vivo, mouse); Newball et al. 1986 (in vitro, rat mast cells); Doebler et al. 1985 (in vivo, rat) |
| Chronic | Malathion | Rodgers and Xiong, 1997b, 1997c (in vivo, mouse) | |
3.0 Acute OP intoxication is associated with increased inflammation
One of the initial indicators that OPs may initiate an inflammatory response was evidence linking acute OP intoxication to the activation of microglia and astrocytes. Microglia are considered the immune cells of the brain in that they have the capability to respond to infection or injury in the CNS (Hanisch and Kettenmann, 2007, Kreutzberg, 1996). Microglial activation in response to neuronal insult is quite rapid (Stence et al., 2001), and activated microglia are characterized by cellular hypertrophy with fewer, thicker processes extending from the cell body. Activated microglia release chemokines and proinflammatory cytokines (Benveniste, 1998, Feuerstein et al., 1997) which may either protect or damage the CNS (Duffield, 2003), and have been implicated in many neurodegenerative disorders (Block and Hong, 2005, Mrak and Griffin, 2005, 2007, Sheng et al., 1996). Microglial-derived cytokines can activate astrocytes (Kaminska et al., 2009), which are similarly characterized by hypertrophy of cell bodies and processes in addition to upregulated expression of glial fibrillary acidic protein (GFAP) (Eng et al., 2000). Activated astrocytes produce proinflammatory cytokines (Benveniste, 1998), which have been implicated in the promotion of neuroinflammation. GFAP is often used as a marker of inflammation because it typically precedes neuronal damage (Dell'Anna et al., 1995), and is readily detectable in vivo and in vitro (Monnet-Tschudi et al., 2007). Acute intoxication by OP nerve agents has been linked to activation of glial cells in rodent models. Acute soman intoxication upregulates GFAP expression and activates microglia in many regions of the brain, including the hippocampus, amygdala, lateral septum, piriform cortex and entorhinal cortex (Angoa-Perez et al., 2010, Baille-Le Crom et al., 1995, Collombet et al., 2005a, Zimmer et al., 1997), and sarin upregulates GFAP mRNA in the cortex, cerebellum, midbrain, spinal cord, and brainstem of acutely intoxicated rats (Damodaran et al., 2002, Damodaran et al., 2006). Interestingly, soman induces a delayed overexpression of GFAP in the medial and lateral septum and hippocampus of mice 3 days after exposure, and levels remain elevated at 30 days (Collombet, Four, 2005a). It is probable that at least a subset of OP pesticides similarly trigger an inflammatory response based on reports that acute exposure to the OP pesticide parathion elevates GFAP immunoreactivity in both immature and differentiated cells in aggregating brain cultures (Zurich et al., 2000). However, the available evidence suggests that spatiotemporal patterns of glial activation vary between different OPs even when administered at doses that cause comparable levels of AChE inhibition. Thus, the OP diisopropylfluorophosphate (DFP) initially decreases GFAP expression in the cerebrum, cerebellum, brainstem, and spinal cord of hens, but is subsequently upregulated 5–20 days after exposure in the cerebrum (Damodaran and Abou-Donia, 2000).
3.1. Cytokines upregulated by acute OP intoxication
A major hallmark of the inflammatory response is the release of cytokines and chemokines from activated macrophages. Cytokines possess a diverse array of functions, including the ability to activate macrophages, promote leukocyte recruitment, stimulate B-cell and T-cell differentiation, increase vascular permeability and upregulate major histocompatibility complex (MHC) antigen expression (Benveniste, 1998, Feuerstein, Wang, 1997). Chemokines act as chemotactic molecules that attract leukocytes to the site of injury. Acute intoxication with OP nerve agents can directly increase secretion of proinflammatory cytokines and chemokines. Transcript and protein levels of the proinflammatory cytokines TNFα, IL-1β, and interleukin-6 (IL-6) are elevated in the hippocampus, piriform cortex and thalamus of rats and mice following acute soman exposure (Dhote et al., 2007, Dillman et al., 2009, Johnson and Kan, 2010, Svensson et al., 2001, Williams et al., 2003). Sarin acts similarly to soman, increasing levels of TNFα, IL-1β, and IL-6 in the cortex and hippocampus of rats. Cytokine levels return to control values 1–2 days after exposure, but a second increase in cytokine levels is observed in a subset of rats 30 days after exposure, indicating that inflammation can persist long after the initial exposure (Chapman et al., 2006).
OP nerve agents and pesticides also modulate neuroinflammatory responses. Soman induces the upregulation of genes important for cytokine signaling and neutrophil migration (Dhote, Peinnequin, 2007, Williams, Berti, 2003), including suppressor of cytokine stimulating-3 (SOCS3), which negatively regulates cytokine signaling (Croker et al., 2003, Fujimoto and Naka, 2003), as well as intercellular adhesion molecule-1 (ICAM1), vascular cell adhesion molecule-1 (VCAM1) and endothelial leukocyte adhesion molecule-1 (E-selectin), which mediate the binding of leukocytes to endothelial cells during extravasation of leukocytes from blood vessels into affected tissues (Frijns and Kappelle, 2002). Soman increases protein levels of the chemokines C-X-C motif ligand-1 (CXCL1) and macrophage inflammatory protein-1α (MIP-1α) in the hippocampus, piriform cortex, and thalamus (Johnson et al., 2011). CXCL1 and MIP-1α guide neutrophils to damaged tissues (Appelberg, 1992, Shaftel et al., 2007), and activate granulocytes (Rot et al., 1992) which promote the initiation of neuroinflammatory responses via the generation of proinflammatory cytokines. Interestingly, sarin also upregulates gene expression of IL-10, an anti-inflammatory cytokine (Damodaran, Greenfield, 2006). An acute dose of the OP pesticide acephate prior to injury reduces immune cell counts and blocks stimulatory effects of IL-1 on immune cells, which may lead to a decreased ability to combat infection or injury. Additionally, acephate exposure concurrent with injury increases neutrophil counts and enhances the acute-phase response, which may exacerbate cytokine toxicity (Singh and Jiang, 2002). The pesticide chlorpyrifos has been reported to modulate IL-6 and TNFα/NF-κB signaling pathways by downregulating genes that encode signaling molecules in these pathways (Stapleton and Chan, 2009).
It is interesting that acute OP intoxication increases both expression of cytokines that propagate the inflammatory response (TNFα, IL-1β, and IL-6), and molecules that are typically considered to be anti-inflammatory (IL-10 and SOCS3). It remains to be determined whether OPs directly induce anti-inflammatory molecules, or whether proinflammatory cytokines secreted from microglia and glial cells trigger upregulation of anti-inflammatory cytokines indirectly via negative feedback mechanisms that maintain homeostasis.
3.2. Prostaglandins and isoprostanoids associated with acute OP intoxication
Prostaglandins (PG) are lipid-derived compounds produced by fatty acid oxidation. They are synthesized by cyclooxygenase (COX) enzymes following arachidonic acid liberation from the plasma membrane by phospholipases (Ricciotti and FitzGerald, 2011). Prostaglandin E2 (PGE2) is intimately connected with inflammation, and possesses a wide variety of functions that include modulation of vascular permeability and blood flow, and induction of hyperalgesia (Ricciotti and FitzGerald, 2011). Isoprostanoids, such as F2-isoprostanes (F2-IsoPs) and neuron-specific F4-neuroprostanes (F4-NeuroPs), are prostaglandin-like compounds derived from lipid peroxidation of arachidonic acid, and these are commonly used as biomarkers of oxidative stress (Milatovic et al., 2006). F2-ISoPs are potent vasoconstrictors (Cracowski et al., 2001, Kromer and Tippins, 1996), and are generally considered to be proinflammatory products of oxidative stress, since they are linked to inflammatory conditions, such as sepsis, asthma, atherosclerosis, and rheumatic disease (Basu, 2010). Isoprostanes enhance neutrophil adhesion to endothelial cells (Zahler and Becker, 1999), and may potentiate the inflammatory response via increased neutrophil activity.
There are numerous reports of increased prostaglandin production following acute OP intoxication. Acutely intoxicating doses of sarin decrease PGE2 receptor transcripts in the brain (Damodaran, Greenfield, 2006) coincident with elevated PGE2 protein levels in the cortex and hippocampus of rats (Chapman, Kadar, 2006, Grauer et al., 2008); interestingly, the duration of increased PGE2 appears to vary with route of exposure. Additionally, a secondary increase in prostaglandins is observed 1–6 months after acute intoxication with OP nerve agents. Sarin vapor also increases prostaglandin and eosinophil levels in the bronchoalveolar lavage of guinea pigs (Levy et al., 2004). Soman increases inducible cyclooxygenase-2 (COX-2) expression in neurons of the rat hippocampus, piriform cortex and amygdala (Angoa-Perez, Kreipke, 2010), which is consistent with an increase in PG synthesis. Interestingly, pretreatment with PGE2 prior to soman exposure reduces toxicity and delays the onset of seizure activity by reducing the rate of AChE inactivation in the brain (Lundy and Frew, 1984). The authors postulated that pretreatment with PGE2 modulates the central effects of soman by reducing cerebral blood flow, which in turn reduces soman uptake in the brain.
Acute OP intoxication also stimulates isoprostanoid production. A single acute dose of DFP elevates both F2-IsoPs and F4-NeuroPs in the rat brain (Zaja-Milatovic et al., 2009). This response is not unique to OPs since increased isoprostanoids are observed following treatment with other anticholinesterase agents, such as the carbamate pesticide carbofuran (Milatovic, Gupta, 2006), suggesting that increased levels of isoprostanoids may be a consequence of acute AChE inhibition and/or OP-induced seizures. Treatment with antioxidants or the N-methyl D-aspartate (NMDA) receptor antagonist memantine attenuates isoprostanoid elevation, indicating that oxidative stress and/or excitotoxicity are also involved in OP-induced lipid peroxidation.
3.3 OP-induced anaphylaxis
OP-induced cholinergic crisis is often accompanied by toxicity induced by noncholinergic mechanisms, including anaphylactic shock (Cowan et al., 1996). OP-induced anaphylaxis is induced by autacoids, such as histamine and platelet activating factor (PAF), and serine proteases (Cowan, Shih, 1996). Activation and degranulation of mast cells and basophils release histamine, cytokines (TNFα), eicosanoids (prostaglandin D2 and leukotriene C4), and other mediators into the extracellular environment which sets the development of anaphylaxis. Histamine released from mast cells binds to H1 histamine receptors to increase capillary permeability and initiate vasodilation and inflammatory responses. Histamine also binds to H2 histamine receptors to regulate the immune response. This includes the stimulation of cytokine IL-6 and IL-10 production, and the inhibition of interferon-γ (IFNγ), interleukin-12 (IL-12) and TNFα in various cell types (Elenkov et al., 2005). PAF also contributes to the inflammatory response by promoting vasodilation, aggregation and chemotaxis of leukocytes, and endothelial cell permeability (Penna et al., 2011).
There is substantial evidence that at least some OPs induce anaphylaxis via the release of autacoids from mast cells. Soman exposure induces dose-dependent mast cell degranulation in rats (Doebler et al., 1985), and induces a calcium-dependent release of histamine from rat peritoneal mast cells (Newball et al., 1986). Sarin vapor elevates histamine levels in bronchoalveolar lavage of guinea pigs (Levy, Chapman, 2004). Oral exposure to malathion increases macrophage function, measured by respiratory burst activity of peritoneal macrophages in mice (Rodgers and Ellefson, 1992, Rodgers and Ellefson, 1990b) and malathion-induced macrophage activity is influenced by inflammatory mediators released from mast cells (Rodgers and Xiong, 1996, 1997a). Malathion metabolites also induce histamine release from basophils and peritoneal mast cells (Xiong and Rodgers, 1997). Antihistamines are anti-inflammatory agents that act by preventing histamine release from mast cells and/or stabilizing histamine receptors in an inactive conformation. Additionally, antihistamines inhibit expression of adhesion molecules (ICAM-1), prevent prostaglandin release, and regulate cytokine release from T lymphocytes and epithelial cells (Nettis et al., 2005). It is unknown whether antihistamines would attenuate OP-induced toxicity, but these observations suggest that victims may benefit from their anti-inflammatory properties.
3.4 Acute OP intoxication causes inflammation in peripheral tissues
Inflammation in response to OP exposure is not restricted to the CNS, but has also been detected in cardiac and pancreatic tissue following acute OP intoxication. An examination of hearts from 13 patients who died as a result of OP poisoning revealed myocarditis, pericarditis, and interstitial inflammation and edema, among other histopathological findings (Anand et al., 2009). Myocarditis has been documented in other cases of acute OP intoxication (Chharba et al., 1970, Dalvi et al., 1986) and appears to be a prominent symptom of OP poisoning. Pulmonary edema, which may have been caused by myocarditis, was seen in necropsy of individuals poisoned by OPs (Limaye, 1966). The OP pesticide fenthion induces inflammation, edema, vacuolization, and necrosis in myocardial tissue, and these features are alleviated by the anticholinergic/antihistaminic agent diphenhydramine (Yavuz et al., 2008). Pancreatitis (Hamaguchi et al., 2006, Harputluoglu et al., 2003, Roeyen et al., 2008) and parotitis (Gokel et al., 2002) have also been reported in acute OP poisoning case studies. Additionally, dogs exposed to low levels of the OP insecticide methidathion for 1 year were reported to experience mild chronic inflammation of the liver, as determined by lymphocyte infiltration (Chang et al., 1992).
4.0 Chronic OP exposures and inflammation
While there is clear and compelling evidence that acute OP intoxication is associated with inflammatory responses, there is still a question as to whether chronic OP exposures that have been linked to neurobehavioral deficits (Bouchard et al., 2011, Engel et al., 2011, Rohlman, Anger, 2011) are also associated with inflammation. Certainly traditional biomarkers of OP exposure (urinary OP metabolites and blood cholinesterase inhibition) have not been reliably associated with changes in cognitive ability following chronic OP exposures and there is significant interest in identifying noncholinergic biomarkers that are better biomarkers of OP neurotoxicity following low-level chronic OP exposure (Farahat et al., 2011, Rohlman, Anger, 2011). Evidence linking inflammation to neurodegeneration and cognitive defects (Dziedzic, 2006, McGeer and McGeer, 2003), suggest the possibility that induction of inflammation by chronic exposure to OPs may be mechanistically related to deficits in cognitive ability.
There is emerging evidence to suggest a link between chronic exposure to OPs and inflammation. Rats repeatedly exposed to low doses of sarin vapor have elevated levels of IL-1β, TNFα, and IL-6 in the brain (Henderson et al., 2002). Administration of low levels of the OP pesticide malathion for 14 or 90 days increases macrophage function and mast cell degranulation in mice, but does not elevate histamine levels in the blood (Rodgers and Xiong, 1997c, d). Treatment of astrocyte cultures for 1 week with the OP pesticide chlorpyrifos upregulates GFAP, IL-6, and eight genes (LFAP, NYREN18, HSPB2, PSMB10, PSMB8, PRKCA, IL6R, and PDCD5) involved in the signaling pathway of the proinflammatory cytokine IFNγ (Mense et al., 2006). Parathion and chlorpyrifos induce an increase in GFAP expression in mixed-cell aggregate cultures from fetal rat telencephalon, but only parathion induces astrogliosis (Zurich et al., 2004). Consistent with these in vitro observations, dermal exposure to subtoxic doses of chlorpyrifos for 7 days increases GFAP expression and astrocytic density in mouse brains (Lim et al., 2011). High doses of chlorpyrifos that induce systemic toxicity in dams and fetuses following administration to pregnant rat dams over gestational days 17–20, increase GFAP expression in the brain of fetal animals. Postnatal administration of chlorpyrifos causes a sex-specific initial decrease in GFAP expression followed by a rebound of GFAP to levels significantly higher than controls days after chlorpyrifos exposure is removed (Garcia et al., 2002). The authors postulate that gestational GFAP elevation is due to astrogliosis, whereas postnatal GFAP deficits are caused by a depression of cell differentiation during periods of glial proliferation. A similar decrease in GFAP expression is observed upon a single exposure of developing rat pups on postnatal day 7 to chlorpyrifos at 2 mg/kg (Ray et al., 2010). This suggests that developing glia are susceptible to the toxic effects of chlorpyrifos, but that the effect of chlorpyrifos on GFAP expression varies not only quantitatively but also qualitatively as a function of the developmental stage at the time of exposure.
5.0 Mechanisms contributing to OP-induced inflammation
A key question is whether inflammatory responses to OP intoxication are mechanistically related to AChE inhibition. As indicated earlier, AChE inhibition is a hallmark characteristic of acute OP intoxication, that can result in cholinergic crisis and seizure onset. Experimental evidence indicates that OP-induced seizures are associated with neuronal damage and the induction of inflammation. OP-induced seizures are coincident with cytokine and chemokine elevation, glial activation, increases in PG production, and neuronal damage in the hippocampus, piriform cortex and amygdala (Angoa-Perez, Kreipke, 2010, Chapman, Kadar, 2006, Collombet, Four, 2005a, Dhote, Peinnequin, 2007, Grauer, Chapman, 2008, Johnson and Kan, 2010, Svensson, Waara, 2001, Zimmer, Ennis, 1997). Administration of oxime compounds that inhibit sarin-induced seizures also inhibit sarin-induced PG synthesis, suggesting that elevated PG is linked to AChE inhibition and/or seizure activity (Levy, Chapman, 2004). This conclusion is strengthened by evidence of a direct correlation between the duration of seizure activity and the level of expression of inflammatory markers (Chapman, Kadar, 2006).
However, chronic OP exposure induces inflammation even in the absence of seizures or significant AChE inhibition. Repeated low-level doses of sarin elevate cytokine levels in rat brain in the absence of central AChE inhibition, although blood ChE activity is reduced (Henderson, Barr, 2002). Chronic chlorpyrifos administration increases GFAP expression in the hippocampus of rats without inhibiting serum cholinesterase (Lim, Tay, 2011). This indicates that prolonged exposure to low-level OPs can trigger inflammatory responses, even in the absence of AChE inhibition and overt clinical symptoms of OP poisoning. The mechanism(s) by which chronic OP exposure induces inflammation have yet to be defined, but one possibility is that OPs interact directly with inflammatory cells to trigger the release of inflammatory mediators. There is experimental evidence that OPs can activate inflammatory cells and trigger inflammatory responses. For example, in mice, the OP malathion stimulates macrophages to generate ROS and cathepsin D (Rodgers and Xiong, 1997c, Rodgers and Ellefson, 1990a, b), potentiates macrophage phagocytosis and antigen presentation (Flipo et al., 1992), increases mast cell degranulation in the intestine and skin (Rodgers and Xiong, 1997b) and causes histamine release from mast cells (Rodgers and Ellefson, 1992) and basophils (Xiong and Rodgers, 1997). In guinea pigs, inhalation of the OP sarin increases inflammatory mediators (including histamine, prostaglandins, eosinophils and macrophages) in the lungs, although the response is complex and varies with dose and time post-exposure (Levy, Chapman, 2004). Similarly, in rats exposed to a single or repeated subclinical doses of sarin, mRNA expression of the pro-inflammatory cytokines IL-1β, IL-6 and TNFα is upregulated in the lungs (Pena-Philippides et al., 2007). Exposure of human whole blood cultures to the OP chlorpyrifos potentiated LPS-induced release of IFNγ (Duramad et al., 2006), and gene and protein expression profiling of primary cultures of human fetal astrocytes demonstrated that chlorpyrifos upregulated key inflammatory mediators, including IFNγ and IL-6, as well as GFAP, a marker of inflammatory astrocytes (Mense, Sengupta, 2006). The latter observation is consistent with a preliminary report that chlorpyrifos induces expression of the pro-inflammatory cytokines TNFα and IL-6 in addition to the chemokine MCP-1 in the mouse brain in a time- and dose-dependent manner (Hirani et al., 2007).
6.0. OP-induced inflammation: Neurotoxic or neuroprotective?
The general assumption has been that OP-induced inflammation contributes to the pathology associated with OP neurotoxicity, and in particular, the delayed neuronal cell death and persistent neurobehavioral deficits observed following acute OP intoxication (Collombet, 2011). This assumption is based on the observation that many of the therapeutic agents currently used to treat OP poisoning possess anti-inflammatory properties. For example, benzodiazepines attenuate seizures and convulsions by acting on the GABA-A receptor, but also antagonize PAF receptors (Bidri et al., 1999, Tanniere-Zeller et al., 1989). Other agents used to treat OP poisoning, including atropine, oximes, and carbamates, influence inflammatory responses (Cowan et al., 2003, Cowan, Shih, 1996). Additionally, novel nonsteroidal anti-inflammatory drugs (NSAID) coupled to a pyridostigmine moiety increase survival rates and attenuate brain edema in mice challenged with soman (Amitai, Adani, 2006). These data suggest an important role for inflammation in OP-induced toxicity.
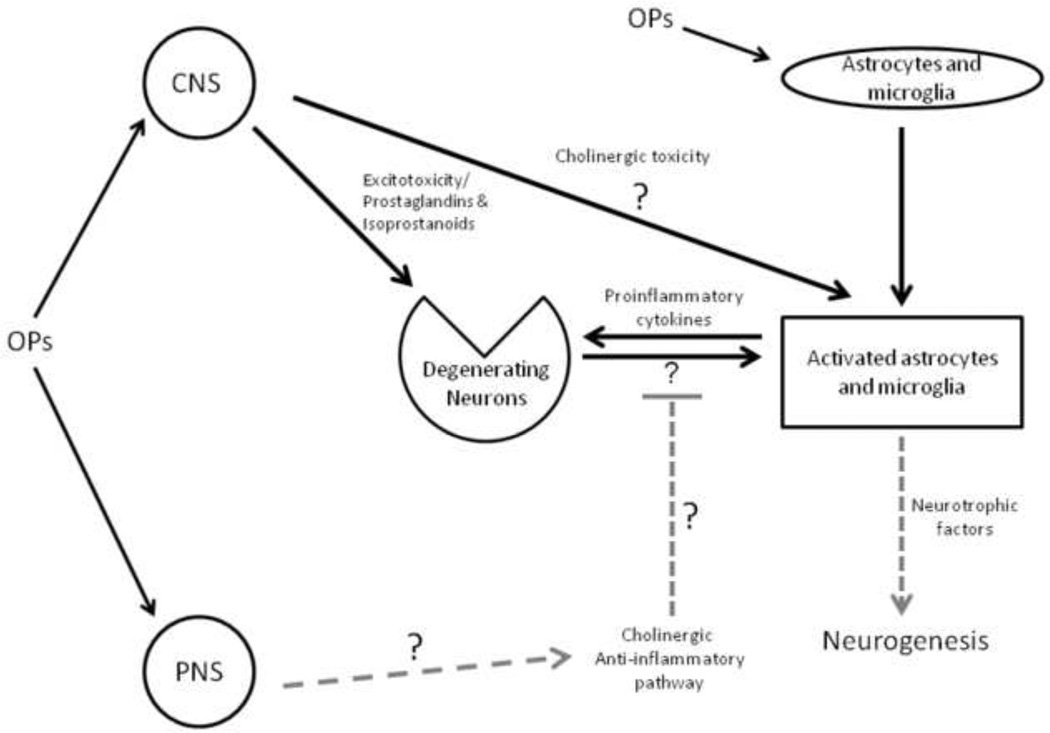
There is also evidence that neuroinflammation exacerbates neuronal damage due to excitotoxicity (Morimoto et al., 2002) in part via interactions between proinflammatory cytokines and glutamatergic pathways (Fogal and Hewett, 2008). These observations have important implications regarding the pathology associated with acute OP intoxication in that OP-induced seizures are initially cholinergic, but appear to shift towards noncholinergic pathways, predominantly glutamatergic pathways, during later stages of intoxication (Harrison et al., 2004, Lallement et al., 1998, McDonough and Shih, 1997, Shih et al., 1999). As illustrated in Figure 2, excessive activation of NMDA receptors leads to elevated calcium influx into cells, which perturbs mitochondria and increases generation of ROS. This ultimately leads to neurodegeneration, as affected cells undergo apoptosis. Whether anti-inflammatory agents decrease OP-induced excitotoxicity has yet to be systematically investigated.
Figure 2.
Schematic of the inflammatory response following acute exposure to organophosphate (OP) agents. Inhibition of acetylcholinesterase (AChE) induces cholinergic toxicity, leading to neuronal damage via the release of pro-inflammatory cytokines from activated microglia, astrocytes. Prostaglandin/isoprostanoid release and neuronal damage due to enhanced glutamatergic activity (excitotoxicity) are additional consequences of acute OP intoxication. Astrocytes secrete neurotrophic factors which lead to neurogenesis. The role of the cholinergic anti-inflammatory pathway in organophosphate toxicity is not currently known. Novel or unknown pathways are indicated with grey dashed arrows/bars.
While pharmacological studies with anti-inflammatory agents suggest that inflammation contributes to the pathogenesis following acute OP intoxication, emerging evidence suggests that inflammatory responses may also serve a neuroprotective role. It has recently been reported that OP-induced glial activation triggers release of not only IL-6, TNFα, and IL-1β, cytokines thought to promote inflammatory damage, but also secretion from astrocytes of neurotrophic and other growth factors that promote angiogenesis and neurogenesis, and thereby serve to repair OP-induced damage (Collombet et al., 2011). These astrocyte-derived growth factors include ciliary neurotrophic factor (CNTF), brain-derived neurotrophic factor (BDNF), fibroblast growth factor (FGF-2), nerve growth factor (NGF), and vascular endothelial growth factor (VEGF) (Collombet, 2011). Following acute exposure to soman, a decrease in neural progenitor proliferation is initially observed. However, this transient depression is followed by an increase above normal levels in progenitor proliferation in the subventricular zone and subgranular zone of the dentate gyrus a month after exposure. The duration of neurogenesis coincides with the duration of astroglial cell activation, and delayed neuronal cell death occurs after growth factor levels decrease, about 2–3 months after the initial OP exposure (Collombet, Four, 2005a, Collombet et al., 2007).
Collectively, these studies suggest the intriguing possibility that astrocyte activation plays an integral role in neural repair via secretion of neurotrophic and angiogenic growth factors and that increasing and/or prolonging the elevated expression of these factors may prove of therapeutic benefit in treating acute OP intoxication. In support of this hypothesis, administration of FGF-2 and epidermal growth factor (EGF) to mice acutely intoxicated with soman has been reported to significantly increase progenitor cells in the CA1 region of the hippocampus and amygdala 1 month after soman exposure (Collombet et al., 2005b). More recently, FGF-2 and EGF co-administration has been demonstrated to accelerate the rate of neuronal regeneration in the hippocampus, and improved anxiety profiles in soman-exposed mice as measured by elevated plus maze and fear conditioning (Collombet, Beracochea, 2011). This treatment did not, however, alter neuronal regeneration in the amygdala nor did it enhance the restoration of hippocampus-dependent memory-related tasks (Collombet, Beracochea, 2011). Interestingly, administration of these growth factors did not alter the severity or duration of the soman-induced seizures, nor did it decrease neuronal cell loss during the first 9 days after soman exposure (Collombet, Beracochea, 2011). What has yet to be established is whether endogenous growth factors released by activated astrocytes in vivo influence neuroregeneration and behavioral recovery following acute OP intoxication. Nonetheless, these data suggest that inflammation may be functionally important in the regenerative processes that occur following acute OP intoxication. However, we are still far from a clear understanding the spatiotemporal profile of inflammatory mediators that contribute to promoting damage versus repair.
Another interaction that has yet to be examined is the effect of OP intoxication on the cholinergic anti-inflammatory pathway or CAP (see Figure 1). As discussed above, the available experimental evidence suggests that enhanced binding to α7-nAChRs reduces proinflammatory cytokine levels without affecting the release of anti-inflammatory mediators, and this has been suggested as an effective therapeutic strategy for treating a broad range of inflammatory diseases (Bencherif, Lippiello, 2011, Brenner et al., 2008, Rosas-Ballina and Tracey, 2009). Theoretically, then, it would seem that OP inhibition of AChE would activate CAP as a consequence of increased acetylcholine levels, suggesting a homeostatic mechanism for limiting inflammation following OP exposure. Whether this is true and whether it is of physiological relevance following either acute or chronic OP exposure remains to be determined.
7.0 Conclusions
It is quite clear that acute OP intoxication leads to an inflammatory response that appears to be both neurotoxic and neuroprotective. Based on studies of the therapeutic efficacy of anti-inflammatory compounds and growth factors known to be secreted by activated astrocytes following acute OP intoxication, a model emerges in which inflammation is initially detrimental but may then serve to promote repair at later stages in at least some brain regions. An intriguing possibility that warrants investigation is that the extent of inflammatory damage may be determined in part by the level of activation of CAP, and changes in CAP reflective of changes in AChE levels that can be depressed for significant periods of time then rebound to levels significantly higher than normal before returning to pre-exposure levels over a period of months (Duysen and Lockridge, 2011), may contribute to the complex spatiotemporal profiles of glial activation following acute OP intoxication (Collombet, 2011). With respect to chronic OP exposures, emerging evidence supports the hypothesis that these repeated low-level exposures also trigger an inflammatory response consisting of elevated markers of glial activation and increased levels of proinflammatory cytokines in the CNS and periphery. The more significant question, however, of whether these inflammatory responses contribute to the neurotoxic effects associated with chronic OP exposure, remains to be answered.
The standard antidote for acute OP intoxication (atropine, oxime, and benzodiazepines) enhances survival following acute OP intoxication, but does not effectively prevent long-term neurological damage (McDonough and Shih, 1997). Ideally, novel medical countermeasures would focus on developing therapeutic approaches that selectively interfere with those aspects of the inflammatory response that promote damage while protecting or promoting those aspects that function in repair processes. Therapeutic candidates that may warrant investigation in this context include the selective TNFα inhibitor etanercept (Wong et al., 2008) and the IL-6 receptor inhibitor tocilizumab (Murakami and Nishimoto, 2011). Also intriguing to consider are the alpha7-nAChR agonists, which may alleviate some of the inflammatory damage during the initial stages of OP poisoning by suppressing cytokine release from macrophages (Figure 1). These types of novel therapeutics, in conjunction with standard antidotes, may protect the brain from neuronal insult following acute OP intoxication. Last but not least, these studies raise the possibility of using inflammation as a biomarker of OP effect to not only identify individuals at risk for OP-induced neurotoxicity, but also to monitor the therapeutic efficacy of medical countermeasures for OP intoxication.
Acknowledgements
This work is supported by research grants from the National Institute of Neurological Disease and Stroke (NINDS CounterACT Program, R21 NS072094, Lein and Rogawski, MPI) and the National Institute of Environmental Health Sciences (NIEHS R01 ES016308, Anger and Lein, MPI), and an NIEHS postdoctoral fellowship to Banks (T32 ES007058-33). The content is solely the authors’ responsibility and does not necessarily represent official views of the NINDS or NIEHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest to declare.
Contributor Information
Christopher N. Banks, Email: cnbanks@ucdavis.edu.
Pamela J. Lein, Email: pjlein@ucdavis.edu.
References
- Abou-Donia MB. Organophosphorus ester-induced chronic neurotoxicity. Arch Environ Health. 2003;58:484–497. doi: 10.3200/AEOH.58.8.484-497. [DOI] [PubMed] [Google Scholar]
- Amitai G, Adani R, Fishbein E, Meshulam H, Laish I, Dachir S. Bifunctional compounds eliciting anti-inflammatory and anti-cholinesterase activity as potential treatment of nerve and blister chemical agents poisoning. J Appl Toxicol. 2006;26:81–87. doi: 10.1002/jat.1111. [DOI] [PubMed] [Google Scholar]
- Anand S, Singh S, Nahar Saikia U, Bhalla A, Paul Sharma Y, Singh D. Cardiac abnormalities in acute organophosphate poisoning. Clin Toxicol (Phila) 2009;47:230–235. doi: 10.1080/15563650902724813. [DOI] [PubMed] [Google Scholar]
- Angoa-Perez M, Kreipke CW, Thomas DM, Van Shura KE, Lyman M, McDonough JH, et al. Soman increases neuronal COX-2 levels: possible link between seizures and protracted neuronal damage. Neurotoxicology. 2010;31:738–746. doi: 10.1016/j.neuro.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg R. Macrophage inflammatory proteins MIP-1 and MIP-2 are involved in T cell-mediated neutrophil recruitment. J Leukoc Biol. 1992;52:303–306. doi: 10.1002/jlb.52.3.303. [DOI] [PubMed] [Google Scholar]
- Baille-Le Crom V, Collombet JM, Carpentier P, Brochier G, Burckhart MF, Foquin A, et al. Early regional changes of GFAP mRNA in rat hippocampus and dentate gyrus during soman-induced seizures. Neuroreport. 1995;7:365–369. [PubMed] [Google Scholar]
- Basu S. Bioactive eicosanoids: role of prostaglandin F(2alpha) and F-isoprostanes in inflammation and oxidative stress related pathology. Mol Cells. 2010;30:383–391. doi: 10.1007/s10059-010-0157-1. [DOI] [PubMed] [Google Scholar]
- Bencherif M, Lippiello PM, Lucas R, Marrero MB. Alpha7 nicotinic receptors as novel therapeutic targets for inflammation-based diseases. Cell Mol Life Sci. 2011;68:931–949. doi: 10.1007/s00018-010-0525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste EN. Cytokine actions in the central nervous system. Cytokine Growth Factor Rev. 1998;9:259–275. doi: 10.1016/s1359-6101(98)00015-x. [DOI] [PubMed] [Google Scholar]
- Bidri M, Royer B, Averlant G, Bismuth G, Guillosson JJ, Arock M. Inhibition of mouse mast cell proliferation and proinflammatory mediator release by benzodiazepines. Immunopharmacology. 1999;43:75–86. doi: 10.1016/s0162-3109(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Blasko I, Stampfer-Kountchev M, Robatscher P, Veerhuis R, Eikelenboom P, Grubeck-Loebenstein B. How chronic inflammation can affect the brain and support the development of Alzheimer's disease in old age: the role of microglia and astrocytes. Aging Cell. 2004;3:169–176. doi: 10.1111/j.1474-9728.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, et al. Prenatal Exposure to Organophosphate Pesticides and IQ in 7-Year-Old Children. Environ Health Perspect. 2011;119:1189–1195. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner T, Nizri E, Irony-Tur-Sinai M, Hamra-Amitay Y, Wirguin I. Acetylcholinesterase inhibitors and cholinergic modulation in Myasthenia Gravis and neuroinflammation. J Neuroimmunol. 2008;201–202:121–127. doi: 10.1016/j.jneuroim.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Moser VC. Behavioral toxicity of cholinesterase inhibitors. In: Gupta RC, editor. Toxicology of Organophosphate and Carbamate Compounds. San Diego, CA: Elsevier; 2006. pp. 347–360. [Google Scholar]
- Casida JE, Quistad GB. Serine hydrolase targets of organophosphorus toxicants. Chemicobiological interactions. 2005;157–158:277–283. doi: 10.1016/j.cbi.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Chang JC, Walberg JA, Campbell WR. One-year dietary toxicity study with methidathion in beagle dogs. Fundam Appl Toxicol. 1992;19:307–314. doi: 10.1016/0272-0590(92)90165-e. [DOI] [PubMed] [Google Scholar]
- Chapman S, Kadar T, Gilat E. Seizure duration following sarin exposure affects neuro-inflammatory markers in the rat brain. Neurotoxicology. 2006;27:277–283. doi: 10.1016/j.neuro.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Chharba ML, Sepaha GC, Jain SR, Bhagwat RR, Khandekar JD. E.C.G. and necrosy changes in organophosphorus compound (malathion) poisoning. Indian J Med Sci. 1970;24:424–429. [PubMed] [Google Scholar]
- Collombet JM. Nerve agent intoxication: recent neuropathophysiological findings and subsequent impact on medical management prospects. Toxicol Appl Pharmacol. 2011;255:229–241. doi: 10.1016/j.taap.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Collombet JM, Beracochea D, Liscia P, Pierard C, Lallement G, Filliat P. Long-term effects of cytokine treatment on cognitive behavioral recovery and neuronal regeneration in soman-poisoned mice. Behav Brain Res. 2011;221:261–270. doi: 10.1016/j.bbr.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Collombet JM, Four E, Bernabe D, Masqueliez C, Burckhart MF, Baille V, et al. Soman poisoning increases neural progenitor proliferation and induces long-term glial activation in mouse brain. Toxicology. 2005a;208:319–334. doi: 10.1016/j.tox.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Collombet JM, Four E, Burckhart MF, Masqueliez C, Bernabe D, Baubichon D, et al. Effect of cytokine treatment on the neurogenesis process in the brain of soman-poisoned mice. Toxicology. 2005b;210:9–23. doi: 10.1016/j.tox.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Collombet JM, Four E, Fauquette W, Burckhart MF, Masqueliez C, Bernabe D, et al. Soman poisoning induces delayed astrogliotic scar and angiogenesis in damaged mouse brain areas. Neurotoxicology. 2007;28:38–48. doi: 10.1016/j.neuro.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta. 2006;366:1–13. doi: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Cowan FM, Broomfield CA, Lenz DE, Smith WJ. Putative role of proteolysis and inflammatory response in the toxicity of nerve and blister chemical warfare agents: implications for multi-threat medical countermeasures. J Appl Toxicol. 2003;23:177–186. doi: 10.1002/jat.901. [DOI] [PubMed] [Google Scholar]
- Cowan FM, Shih TM, Lenz DE, Madsen JM, Broomfield CA. Hypothesis for synergistic toxicity of organophosphorus poisoning-induced cholinergic crisis and anaphylactoid reactions. J Appl Toxicol. 1996;16:25–33. doi: 10.1002/(SICI)1099-1263(199601)16:1<25::AID-JAT303>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Cracowski JL, Devillier P, Durand T, Stanke-Labesque F, Bessard G. Vascular biology of the isoprostanes. J Vasc Res. 2001;38:93–103. doi: 10.1159/000051036. [DOI] [PubMed] [Google Scholar]
- Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- Dalvi CP, Abraham P, Iyer SS. Correlation of electrocardiographic changes with prognosis in organophosphorus poisoning. J Postgrad Med. 1986;32:115–119. [PubMed] [Google Scholar]
- Damodaran TV, Abou-Donia MB. Alterations in levels of mRNAs coding for glial fibrillary acidic protein (GFAP) and vimentin genes in the central nervous system of hens treated with diisopropyl phosphorofluoridate (DFP) Neurochem Res. 2000;25:809–816. doi: 10.1023/a:1007565407341. [DOI] [PubMed] [Google Scholar]
- Damodaran TV, Bilska MA, Rahman AA, Abou-Doni MB. Sarin causes early differential alteration and persistent overexpression in mRNAs coding for glial fibrillary acidic protein (GFAP) and vimentin genes in the central nervous system of rats. Neurochem Res. 2002;27:407–415. doi: 10.1023/a:1015508132137. [DOI] [PubMed] [Google Scholar]
- Damodaran TV, Greenfield ST, Patel AG, Dressman HK, Lin SK, Abou-Donia MB. Toxicogenomic studies of the rat brain at an early time point following acute sarin exposure. Neurochem Res. 2006;31:367–381. doi: 10.1007/s11064-005-9023-5. [DOI] [PubMed] [Google Scholar]
- Dell'Anna ME, Geloso MC, Draisci G, Luthman J. Transient changes in Fos and GFAP immunoreactivity precede neuronal loss in the rat hippocampus following neonatal anoxia. Exp Neurol. 1995;131:144–156. doi: 10.1016/0014-4886(95)90016-0. [DOI] [PubMed] [Google Scholar]
- Dhote F, Peinnequin A, Carpentier P, Baille V, Delacour C, Foquin A, et al. Prolonged inflammatory gene response following soman-induced seizures in mice. Toxicology. 2007;238:166–176. doi: 10.1016/j.tox.2007.05.032. [DOI] [PubMed] [Google Scholar]
- Dillman JF, 3rd, Phillips CS, Kniffin DM, Tompkins CP, Hamilton TA, Kan RK. Gene expression profiling of rat hippocampus following exposure to the acetylcholinesterase inhibitor soman. Chem Res Toxicol. 2009;22:633–638. doi: 10.1021/tx800466v. [DOI] [PubMed] [Google Scholar]
- Doebler JA, Shih TM, Anthony A. Quantitative cytophotometric analyses of mesenteric mast cell granulation in acute soman intoxicated rats. Experientia. 1985;41:1457–1458. doi: 10.1007/BF01950034. [DOI] [PubMed] [Google Scholar]
- Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin Sci (Lond) 2003;104:27–38. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Duramad P, Tager IB, Leikauf J, Eskenazi B, Holland NT. Expression of Th1/Th2 cytokines in human blood after in vitro treatment with chlorpyrifos, and its metabolites, in combination with endotoxin LPS and allergen Der p1. J Appl Toxicol. 2006;26:458–465. doi: 10.1002/jat.1162. [DOI] [PubMed] [Google Scholar]
- Duysen EG, Li B, Xie W, Schopfer LM, Anderson RS, Broomfield CA, et al. Evidence for nonacetylcholinesterase targets of organophosphorus nerve agent: supersensitivity of acetylcholinesterase knockout mouse to VX lethality. J Pharmacol Exp Ther. 2001;299:528–535. [PubMed] [Google Scholar]
- Duysen EG, Lockridge O. Induction of plasma acetylcholinesterase activity in mice challenged with organophosphorus poisons. Toxicol Appl Pharmacol. 2011;255:214–220. doi: 10.1016/j.taap.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Dziedzic T. Systemic inflammatory markers and risk of dementia. Am J Alzheimers Dis Other Demen. 2006;21:258–262. doi: 10.1177/1533317506289260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echbichon DJ, Joy RM. Pesticides and Neurological Diseases. 2nd ed. Boston and London: CRC Press; 1995. [Google Scholar]
- Eikelenboom P, Rozemuller JM, van Muiswinkel FL. Inflammation and Alzheimer's disease: relationships between pathogenic mechanisms and clinical expression. Exp Neurol. 1998;154:89–98. doi: 10.1006/exnr.1998.6920. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12:255–269. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, et al. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect. 2011;119:1182–1188. doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahat FM, Ellison CA, Bonner MR, McGarrigle BP, Crane AL, Fenske RA, et al. Biomarkers of chlorpyrifos exposure and effect in Egyptian cotton field workers. Environ Health Perspect. 2011;119:801–806. doi: 10.1289/ehp.1002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein GZ, Wang X, Barone FC. Inflammatory gene expression in cerebral ischemia and trauma. Potential new therapeutic targets. Ann N Y Acad Sci. 1997;825:179–193. doi: 10.1111/j.1749-6632.1997.tb48428.x. [DOI] [PubMed] [Google Scholar]
- Flipo D, Bernier J, Girard D, Krzystyniak K, Fournier M. Combined effects of selected insecticides on humoral immune response in mice. Int J Immunopharmacol. 1992;14:747–752. doi: 10.1016/0192-0561(92)90071-r. [DOI] [PubMed] [Google Scholar]
- Fogal B, Hewett SJ. Interleukin-1beta: a bridge between inflammation and excitotoxicity? J Neurochem. 2008;106:1–23. doi: 10.1111/j.1471-4159.2008.05315.x. [DOI] [PubMed] [Google Scholar]
- Frijns CJ, Kappelle LJ. Inflammatory cell adhesion molecules in ischemic cerebrovascular disease. Stroke. 2002;33:2115–2122. doi: 10.1161/01.str.0000021902.33129.69. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Naka T. Regulation of cytokine signaling by SOCS family molecules. Trends Immunol. 2003;24:659–666. doi: 10.1016/j.it.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Seidler FJ, Qiao D, Slotkin TA. Chlorpyrifos targets developing glia: effects on glial fibrillary acidic protein. Brain Res Dev Brain Res. 2002;133:151–161. doi: 10.1016/s0165-3806(02)00283-3. [DOI] [PubMed] [Google Scholar]
- Giebelen IA, van Westerloo DJ, LaRosa GJ, de Vos AF, van der Poll T. Local stimulation of alpha7 cholinergic receptors inhibits LPS-induced TNF-alpha release in the mouse lung. Shock. 2007;28:700–703. doi: 10.1097/shk.0b013e318054dd89. [DOI] [PubMed] [Google Scholar]
- Gokel Y, Gulalp B, Acikalin A. Parotitis due to organophosphate intoxication. J Toxicol Clin Toxicol. 2002;40:563–565. doi: 10.1081/clt-120014648. [DOI] [PubMed] [Google Scholar]
- Grauer E, Chapman S, Rabinovitz I, Raveh L, Weissman BA, Kadar T, et al. Single whole-body exposure to sarin vapor in rats: long-term neuronal and behavioral deficits. Toxicol Appl Pharmacol. 2008;227:265–274. doi: 10.1016/j.taap.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Hamaguchi M, Namera A, Tsuda N, Uejima T, Maruyama K, Kanai T, et al. Severe acute pancreatitis caused by organophosphate poisoning. Chudoku Kenkyu. 2006;19:395–399. [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Harputluoglu MM, Kantarceken B, Karincaoglu M, Aladag M, Yildiz R, Ates M, et al. Acute pancreatitis: an obscure complication of organophosphate intoxication. Hum Exp Toxicol. 2003;22:341–343. doi: 10.1191/0960327103ht347cr. [DOI] [PubMed] [Google Scholar]
- Harrison PK, Sheridan RD, Green AC, Scott IR, Tattersall JE. A guinea pig hippocampal slice model of organophosphate-induced seizure activity. J Pharmacol Exp Ther. 2004;310:678–686. doi: 10.1124/jpet.104.065433. [DOI] [PubMed] [Google Scholar]
- Henderson RF, Barr EB, Blackwell WB, Clark CR, Conn CA, Kalra R, et al. Response of rats to low levels of sarin. Toxicol Appl Pharmacol. 2002;184:67–76. [PubMed] [Google Scholar]
- Hernandez A, Gomez MA, Pena G, Gil F, Rodrigo L, Villanueva E, et al. Effect of long-term exposure to pesticides on plasma esterases from plastic greenhouse workers. Journal of Toxicology & Environmental Health Part A. 2004;67:1095–1108. doi: 10.1080/15287390490452371. [DOI] [PubMed] [Google Scholar]
- Hirani A, Lee WH, Kang S, Ehrich M, Lee YW. Chlorpyrifos induces pro-inflammatory environment in discrete regions of mouse brain. Faseb J. 2007;21:785.4. [Google Scholar]
- Jett DA, Lein PJ. Non-cholinesterase mechanisms of central and peripheral neurotoxicity: Muscarinic receptors and other targets. In: Gupta RC, editor. Toxicology of Organophosphate and Carbamate Compounds. San Diego, CA: Elsevier; 2006. pp. 233–246. [Google Scholar]
- Johnson EA, Dao TL, Guignet MA, Geddes CE, Koemeter-Cox AI, Kan RK. Increased expression of the chemokines CXCL1 and MIP-1alpha by resident brain cells precedes neutrophil infiltration in the brain following prolonged soman-induced status epilepticus in rats. J Neuroinflammation. 2011;8:41. doi: 10.1186/1742-2094-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EA, Kan RK. The acute phase response and soman-induced status epilepticus: temporal, regional and cellular changes in rat brain cytokine concentrations. J Neuroinflammation. 2010;7:40. doi: 10.1186/1742-2094-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel F, Hoppin JA. Association of pesticide exposure with neurologic dysfunction and disease. Environ Health Perspect. 2004;112:950–958. doi: 10.1289/ehp.7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminska B, Gozdz A, Zawadzka M, Ellert-Miklaszewska A, Lipko M. MAPK signal transduction underlying brain inflammation and gliosis as therapeutic target. Anat Rec (Hoboken) 2009;292:1902–1913. doi: 10.1002/ar.21047. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kromer BM, Tippins JR. Coronary artery constriction by the isoprostane 8-epi prostaglandin F2 alpha. Br J Pharmacol. 1996;119:1276–1280. doi: 10.1111/j.1476-5381.1996.tb16033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallement G, Dorandeu F, Filliat P, Carpentier P, Baille V, Blanchet G. Medical management of organophosphate-induced seizures. J Physiol Paris. 1998;92:369–373. doi: 10.1016/S0928-4257(99)80007-2. [DOI] [PubMed] [Google Scholar]
- Levy A, Chapman S, Cohen G, Raveh L, Rabinovitz I, Manistersky E, et al. Protection and inflammatory markers following exposure of guinea pigs to sarin vapour: comparative efficacy of three oximes. J Appl Toxicol. 2004;24:501–504. doi: 10.1002/jat.1008. [DOI] [PubMed] [Google Scholar]
- Lim KL, Tay A, Nadarajah VD, Mitra NK. The effect of consequent exposure of stress and dermal application of low doses of chlorpyrifos on the expression of glial fibrillary acidic protein in the hippocampus of adult mice. J Occup Med Toxicol. 2011;6:4. doi: 10.1186/1745-6673-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limaye MR. Acute organophosphorous compound poisoning. A study of 76 necropsies. J Indian Med Assoc. 1966;47:492–498. [PubMed] [Google Scholar]
- Liu ZH, Ma YF, Wu JS, Gan JX, Xu SW, Jiang GY. Effect of cholinesterase inhibitor galanthamine on circulating tumor necrosis factor alpha in rats with lipopolysaccharide-induced peritonitis. Chin Med J (Engl) 2010;123:1727–1730. [PubMed] [Google Scholar]
- Lockridge O, Schopfer LM. Review of tyrosine and lysine as new motifs for organophosphate binding to proteins that have no active site serine. Chemico-biological interactions. 2010;187:344–348. doi: 10.1016/j.cbi.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy PM, Frew R. Evidence of reduced uptake of convulsant in brain following prostaglandin E2. Prostaglandins. 1984;27:725–735. doi: 10.1016/0090-6980(84)90010-8. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr, Shih TM. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci Biobehav Rev. 1997;21:559–579. doi: 10.1016/s0149-7634(96)00050-4. [DOI] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. Inflammatory processes in Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:741–749. doi: 10.1016/S0278-5846(03)00124-6. [DOI] [PubMed] [Google Scholar]
- Mense SM, Sengupta A, Lan C, Zhou M, Bentsman G, Volsky DJ, et al. The common insecticides cyfluthrin and chlorpyrifos alter the expression of a subset of genes with diverse functions in primary human astrocytes. Toxicol Sci. 2006;93:125–135. doi: 10.1093/toxsci/kfl046. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Gupta RC, Aschner M. Anticholinesterase toxicity and oxidative stress. ScientificWorldJournal. 2006;6:295–310. doi: 10.1100/tsw.2006.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnet-Tschudi F, Zurich MG, Honegger P. Neurotoxicant-induced inflammatory response in three-dimensional brain cell cultures. Hum Exp Toxicol. 2007;26:339–346. doi: 10.1177/0960327107074589. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Murasugi T, Oda T. Acute neuroinflammation exacerbates excitotoxicity in rat hippocampus in vivo. Exp Neurol. 2002;177:95–104. doi: 10.1006/exnr.2002.7991. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Griffin WS. Potential inflammatory biomarkers in Alzheimer's disease. J Alzheimers Dis. 2005;8:369–375. doi: 10.3233/jad-2005-8406. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Griffin WS. Common inflammatory mechanisms in Lewy body disease and Alzheimer disease. J Neuropathol Exp Neurol. 2007;66:683–686. doi: 10.1097/nen.0b013e31812503e1. [DOI] [PubMed] [Google Scholar]
- Murakami M, Nishimoto N. The value of blocking IL-6 outside of rheumatoid arthritis: current perspective. Current opinion in rheumatology. 2011;23:273–277. doi: 10.1097/BOR.0b013e3283456797. [DOI] [PubMed] [Google Scholar]
- Nettis E, Colanardi MC, Ferrannini A, Tursi A. Antihistamines as important tools for regulating inflammation. Curr Med Chem. 2005;4:81–89. [Google Scholar]
- Newball HH, Donlon MA, Procell LR, Helgeson EA, Franz DR. Organophosphate-induced histamine release from mast cells. J Pharmacol Exp Ther. 1986;238:839–845. [PubMed] [Google Scholar]
- Pancetti F, Olmos C, Dagnino-Subiabre A, Rozas C, Morales B. Noncholinesterase effects induced by organophosphate pesticides and their relationship to cognitive processes: implication for the action of acylpeptide hydrolase. J Toxicol Environ Health B Crit Rev. 2007;10:623–630. doi: 10.1080/10937400701436445. [DOI] [PubMed] [Google Scholar]
- Park HJ, Lee PH, Ahn YW, Choi YJ, Lee G, Lee DY, et al. Neuroprotective effect of nicotine on dopaminergic neurons by anti-inflammatory action. Eur J Neurosci. 2007;26:79–89. doi: 10.1111/j.1460-9568.2007.05636.x. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Ochani M, Gallowitsch-Puerta M, Ochani K, Huston JM, Czura CJ, et al. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Natl Acad Sci U S A. 2006;103:5219–5223. doi: 10.1073/pnas.0600506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K, et al. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun. 2009;23:41–45. doi: 10.1016/j.bbi.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. Controlling inflammation: the cholinergic anti-inflammatory pathway. Biochem Soc Trans. 2006;34:1037–1040. doi: 10.1042/BST0341037. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med. 2003;9:125–134. [PMC free article] [PubMed] [Google Scholar]
- Pena-Philippides JC, Razani-Boroujerdi S, Singh SP, Langley RJ, Mishra NC, Henderson RF, et al. Long- and short-term changes in the neuroimmune-endocrine parameters following inhalation exposures of F344 rats to low-dose sarin. Toxicol Sci. 2007;97:181–188. doi: 10.1093/toxsci/kfm017. [DOI] [PubMed] [Google Scholar]
- Penna C, Bassino E, Alloatti G. Platelet activating factor: the good and the bad in the ischemic/reperfused heart. Exp Biol Med (Maywood) 2011;236:390–401. doi: 10.1258/ebm.2011.010316. [DOI] [PubMed] [Google Scholar]
- Pope C, Karanth S, Liu J. Pharmacology and toxicology of cholinesterase inhibitors: uses and misuses of a common mechanism of action. Environ Toxicol Pharmacol. 2005;19:433–446. doi: 10.1016/j.etap.2004.12.048. [DOI] [PubMed] [Google Scholar]
- Pope CN. Organophosphorus pesticides: do they all have the same mechanism of toxicity? J Toxicol Environ Health B Crit Rev. 1999;2:161–181. doi: 10.1080/109374099281205. [DOI] [PubMed] [Google Scholar]
- Ray A, Liu J, Ayoubi P, Pope C. Dose-related gene expression changes in forebrain following acute, low-level chlorpyrifos exposure in neonatal rats. Toxicol Appl Pharmacol. 2010;248:144–155. doi: 10.1016/j.taap.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers K, Ellefson D. Mechanism of the modulation of murine peritoneal cell function and mast cell degranulation by low doses of malathion. Agents Actions. 1992;35:57–63. doi: 10.1007/BF01990952. [DOI] [PubMed] [Google Scholar]
- Rodgers K, Xiong S. Contribution of mast cell mediators to alterations in macrophage function after malathion administration. Fundam Appl Toxicol. 1996;33:100–108. doi: 10.1006/faat.1996.0147. [DOI] [PubMed] [Google Scholar]
- Rodgers K, Xiong S. Contributions of inflammatory mast cell mediators to alterations in macrophage function after malathion administration. Int J Immunopharmacol. 1997a;19:149–156. doi: 10.1016/s0192-0561(96)00073-2. [DOI] [PubMed] [Google Scholar]
- Rodgers K, Xiong S. Effect of acute administration of malathion by oral and dermal routes on serum histamine levels. Int J Immunopharmacol. 1997b;19:437–441. doi: 10.1016/s0192-0561(97)00098-2. [DOI] [PubMed] [Google Scholar]
- Rodgers K, Xiong S. Effect of administration of malathion for 14 days on macrophage function and mast cell degranulation. Fundam Appl Toxicol. 1997c;37:95–99. doi: 10.1006/faat.1997.2302. [DOI] [PubMed] [Google Scholar]
- Rodgers K, Xiong S. Effect of administration of malathion for 90 days on macrophage function and mast cell degranulation. Toxicol Lett. 1997d;93:73–82. doi: 10.1016/s0378-4274(97)00069-6. [DOI] [PubMed] [Google Scholar]
- Rodgers KE, Ellefson DD. Modulation of macrophage protease activity by acute administration of O,O,S trimethyl phosphorothioate. Agents Actions. 1990a;29:277–285. doi: 10.1007/BF01966458. [DOI] [PubMed] [Google Scholar]
- Rodgers KE, Ellefson DD. Modulation of respiratory burst activity and mitogenic response of human peripheral blood mononuclear cells and murine splenocytes and peritoneal cells by malathion. Fundam Appl Toxicol. 1990b;14:309–317. doi: 10.1016/0272-0590(90)90210-b. [DOI] [PubMed] [Google Scholar]
- Roeyen G, Chapelle T, Jorens P, de Beeck BO, Ysebaert D. Necrotizing pancreatitis due to poisoning with organophosphate pesticides. Acta Gastroenterol Belg. 2008;71:27–29. [PubMed] [Google Scholar]
- Rohlman DS, Anger WK, Lein PJ. Correlating neurobehavioral performance with biomarkers of organophosphorous pesticide exposure. Neurotoxicology. 2011;32:268–276. doi: 10.1016/j.neuro.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, Yang L, Valdes-Ferrer SI, Patel NB, et al. The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med. 2009;15:195–202. doi: 10.2119/molmed.2009.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med. 2009;265:663–679. doi: 10.1111/j.1365-2796.2009.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rot A, Krieger M, Brunner T, Bischoff SC, Schall TJ, Dahinden CA. RANTES and macrophage inflammatory protein 1 alpha induce the migration and activation of normal human eosinophil granulocytes. J Exp Med. 1992;176:1489–1495. doi: 10.1084/jem.176.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaftel SS, Carlson TJ, Olschowka JA, Kyrkanides S, Matousek SB, O'Banion MK. Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J Neurosci. 2007;27:9301–9309. doi: 10.1523/JNEUROSCI.1418-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JG, Ito K, Skinner RD, Mrak RE, Rovnaghi CR, Van Eldik LJ, et al. In vivo and in vitro evidence supporting a role for the inflammatory cytokine interleukin-1 as a driving force in Alzheimer pathogenesis. Neurobiol Aging. 1996;17:761–766. doi: 10.1016/0197-4580(96)00104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T, McDonough JH, Jr, Koplovitz I. Anticonvulsants for soman-induced seizure activity. J Biomed Sci. 1999;6:86–96. doi: 10.1007/BF02256439. [DOI] [PubMed] [Google Scholar]
- Singh AK, Jiang Y. Immunotoxicity of acute acephate exposure in control or IL-1-challenged rats: correlation between the immune cell composition and corticosteroid concentration in blood. J Appl Toxicol. 2002;22:279–291. doi: 10.1002/jat.852. [DOI] [PubMed] [Google Scholar]
- Soltaninejad K, Abdollahi M. Current opinion on the science of organophosphate pesticides and toxic stress: a systematic review. Med Sci Monit. 2009;15:RA75–RA90. [PubMed] [Google Scholar]
- Stapleton AR, Chan VT. Subtoxic chlorpyrifos treatment resulted in differential expression of genes implicated in neurological functions and development. Arch Toxicol. 2009;83:319–333. doi: 10.1007/s00204-008-0346-2. [DOI] [PubMed] [Google Scholar]
- Stence N, Waite M, Dailey ME. Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia. 2001;33:256–266. [PubMed] [Google Scholar]
- Svensson I, Waara L, Johansson L, Bucht A, Cassel G. Soman-induced interleukin-1 beta mRNA and protein in rat brain. Neurotoxicology. 2001;22:355–362. doi: 10.1016/s0161-813x(01)00022-5. [DOI] [PubMed] [Google Scholar]
- Tanniere-Zeller M, Rochette L, Bralet J. PAF-acether-induced mortality in mice: protection by benzodiazepines. Drugs Exp Clin Res. 1989;15:553–558. [PubMed] [Google Scholar]
- Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- Tyagi E, Agrawal R, Nath C, Shukla R. Cholinergic protection via alpha7 nicotinic acetylcholine receptors and PI3K-Akt pathway in LPS-induced neuroinflammation. Neurochem Int. 2010;56:135–142. doi: 10.1016/j.neuint.2009.09.011. [DOI] [PubMed] [Google Scholar]
- van Maanen MA, Stoof SP, van der Zanden EP, de Jonge WJ, Janssen RA, Fischer DF, et al. The alpha7 nicotinic acetylcholine receptor on fibroblast-like synoviocytes and in synovial tissue from rheumatoid arthritis patients: a possible role for a key neurotransmitter in synovial inflammation. Arthritis Rheum. 2009;60:1272–1281. doi: 10.1002/art.24470. [DOI] [PubMed] [Google Scholar]
- Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson's disease. Br J Pharmacol. 2007;150:963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AJ, Berti R, Yao C, Price RA, Velarde LC, Koplovitz I, et al. Central neuro-inflammatory gene response following soman exposure in the rat. Neurosci Lett. 2003;349:147–150. doi: 10.1016/s0304-3940(03)00818-8. [DOI] [PubMed] [Google Scholar]
- Wong M, Ziring D, Korin Y, Desai S, Kim S, Lin J, et al. TNFalpha blockade in human diseases: mechanisms and future directions. Clin Immunol. 2008;126:121–136. doi: 10.1016/j.clim.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong S, Rodgers K. Effects of malathion metabolites on degranulation of and mediator release by human and rat basophilic cells. J Toxicol Environ Health. 1997;51:159–175. doi: 10.1080/00984109708984019. [DOI] [PubMed] [Google Scholar]
- Yavuz Y, Yurumez Y, Ciftci IH, Sahin O, Saglam H, Buyukokuroglu M. Effect of diphenhydramine on myocardial injury caused by organophosphate poisoning. Clin Toxicol (Phila) 2008;46:67–70. doi: 10.1080/15563650701261470. [DOI] [PubMed] [Google Scholar]
- Zahler S, Becker BF. Indirect enhancement of neutrophil activity and adhesion to cultured human umbilical vein endothelial cells by isoprostanes (iPF2alpha-III and iPE2-III) Prostaglandins Other Lipid Mediat. 1999;57:319–331. doi: 10.1016/s0090-6980(98)00079-3. [DOI] [PubMed] [Google Scholar]
- Zaja-Milatovic S, Gupta RC, Aschner M, Milatovic D. Protection of DFP-induced oxidative damage and neurodegeneration by antioxidants and NMDA receptor antagonist. Toxicol Appl Pharmacol. 2009;240:124–131. doi: 10.1016/j.taap.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer LA, Ennis M, Shipley MT. Soman-induced seizures rapidly activate astrocytes and microglia in discrete brain regions. J Comp Neurol. 1997;378:482–492. doi: 10.1002/(sici)1096-9861(19970224)378:4<482::aid-cne4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Zurich MG, Honegger P, Schilter B, Costa LG, Monnet-Tschudi F. Use of aggregating brain cell cultures to study developmental effects of organophosphorus insecticides. Neurotoxicology. 2000;21:599–605. [PubMed] [Google Scholar]
- Zurich MG, Honegger P, Schilter B, Costa LG, Monnet-Tschudi F. Involvement of glial cells in the neurotoxicity of parathion and chlorpyrifos. Toxicol Appl Pharmacol. 2004;201:97–104. doi: 10.1016/j.taap.2004.05.003. [DOI] [PubMed] [Google Scholar]