Abstract
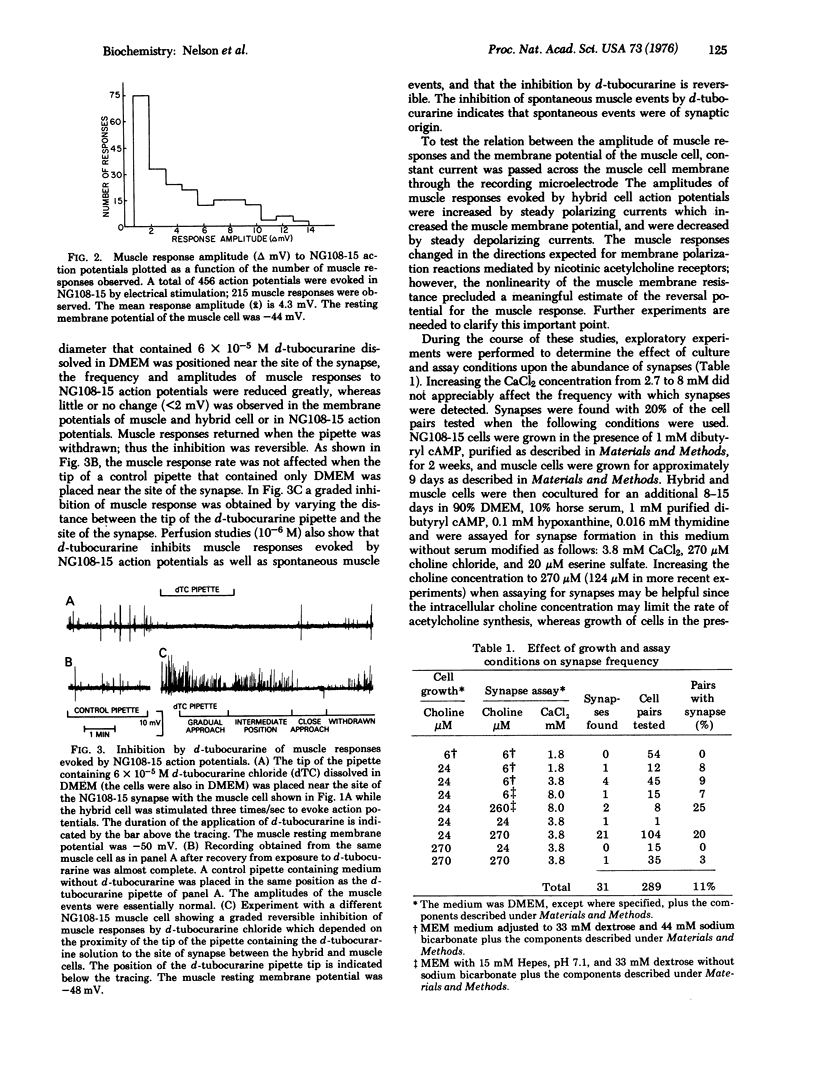
Clonal neuroblastoma X glioma hybrid cells were shown to form synapses with cultured, striated muscle cells. The properties of the synapses between hybrid and muscle cells were similar to those of the normal, neuromuscular synapse at an early stage of development. The number of synapses formed and the efficiency of transmission across synapses were found to be regulated, apparently independently, by components in the culture medium. Under appropriate conditions synapses were found with 20% of the hybrid-muscle cell pairs examined; thus, the hybrid cells form synapses with relatively high frequency.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano T., Hamprecht B., Kemper W. High activity of choline acetyltransferase induced in neuroblastoma x glia hybrid cells. Exp Cell Res. 1974 Apr;85(2):399–408. doi: 10.1016/0014-4827(74)90142-6. [DOI] [PubMed] [Google Scholar]
- Augusti-Tocco G., Sato G. Establishment of functional clonal lines of neurons from mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):311–315. doi: 10.1073/pnas.64.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in striated muscle during development. J Physiol. 1974 Sep;241(2):515–545. doi: 10.1113/jphysiol.1974.sp010670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J., MILEDI R. A study of foetal and new-born rat muscle fibres. J Physiol. 1962 Aug;162:393–408. doi: 10.1113/jphysiol.1962.sp006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels M. P., Hamprecht B. The ultrastructure of neuroblastoma glioma somatic cell hybrids. Expression of neuronal characteristics stimulated by dibutyryl adenosine 3',5' cyclic monophosphate. J Cell Biol. 1974 Nov;63(2 Pt 1):691–699. doi: 10.1083/jcb.63.2.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D., Cohen S. A. The distribution of acetylcholine sensitivity over uninnervated and innervated muscle fibers grown in cell culture. Dev Biol. 1973 Mar;31(1):147–162. doi: 10.1016/0012-1606(73)90326-6. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D. Synapse formation between dissociated nerve and muscle cells in low density cell cultures. Dev Biol. 1972 Jun;28(2):407–429. doi: 10.1016/0012-1606(72)90023-1. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y., Heinemann S. Synapse formation between clonal muscle cells and rat spinal cord explants. Nature. 1974 Dec 13;252(5484):593–594. doi: 10.1038/252593a0. [DOI] [PubMed] [Google Scholar]
- Landmesser L. Contractile and electrical responses of vagus-innervated frog sartorius muscles. J Physiol. 1971 Mar;213(3):707–725. doi: 10.1113/jphysiol.1971.sp009410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minna J., Glazer D., Nirenberg M. Genetic dissection of neural properties using somatic cell hybrids. Nat New Biol. 1972 Feb 23;235(60):225–231. doi: 10.1038/newbio235225a0. [DOI] [PubMed] [Google Scholar]
- Minna J., Nelson P., Peacock J., Glazer D., Nirenberg M. Genes for neuronal properties expressed in neuroblastoma x L cell hybrids. Proc Natl Acad Sci U S A. 1971 Jan;68(1):234–239. doi: 10.1073/pnas.68.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P. G., Peacock J. H., Amano T., Minna J. Electrogenesis in mouse neuroblastoma cells in vitro. J Cell Physiol. 1971 Jun;77(3):337–352. doi: 10.1002/jcp.1040770308. [DOI] [PubMed] [Google Scholar]
- Nurse C. A., O'Lague P. H. Formation of cholinergic synapses between dissociated sympathetic neurons and skeletal myotubes of the rat in cell culture. Proc Natl Acad Sci U S A. 1975 May;72(5):1955–1959. doi: 10.1073/pnas.72.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins N., Yonezawa T. Physiological studies during formation and development of rat neuromuscular junctions in tissue culture. J Gen Physiol. 1971 Oct;58(4):467–481. doi: 10.1085/jgp.58.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Humphreys S., Baroni C., Cohn M. In vitro differentiation of a mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):316–323. doi: 10.1073/pnas.64.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach J. H., Harris A. J., Patrick J., Schubert D., Heinemann S. Nerve-muscle interaction in vitro. Role of acetylcholine. J Gen Physiol. 1973 Sep;62(3):255–270. doi: 10.1085/jgp.62.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytkowski A. J., Vogel Z., Nirenberg M. W. Development of acetylcholine receptor clusters on cultured muscle cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):270–274. doi: 10.1073/pnas.70.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]