Abstract
The mechanisms supporting temporal processing of pain remain poorly understood. To determine the involvement of opioid mechanisms in temporal processing of pain, responses to dynamic noxious thermal stimuli and offset analgesia were assessed following administration of naloxone, a μ-opioid antagonist, and on a separate day, during and following intravenous administration of remifentanil, a μ-opioid agonist, in 19 healthy human volunteers. Multiple end points were sampled from real time computerized visual analog scale ratings (VAS, 1–10) to assess thermal sensitivity, magnitude and duration of offset analgesia, and painful after sensations. It was hypothesized that the magnitude of offset analgesia would be reduced by direct opioid antagonism and during states of acute opioid-induced hypersensitivity (OIH), as well as diminished by the presence of exogenous opioids. Surprisingly, the magnitude of offset analgesia was not altered following naloxone administration, during remifentanil infusion, or following the termination of remifentanil infusion. Since thermal hyperalgesia was observed following both drugs, 8 of the original 19 subjects returned for an additional session without drug administration. Thermal hyperalgesia and increased magnitude of offset analgesia were observed across conditions of remifentanil, naloxone and no drug within this subset analysis, indicating that repeated heat testing induced thermal hyperalgesia which potentiated the magnitude of offset analgesia. Thus, it is concluded that the mechanisms subserving temporal processing of nociceptive information are largely opioid-independent, but that offset analgesia may be potentiated by heat-induced thermal hyperalgesia in a proportion of individuals.
Keywords: offset analgesia, temporal sharpening, real-time VAS, opioid-induced hyperalgesia, thermal hyperalgesia
1. Introduction
Alterations in temporal processing, such as after sensations, occur during acute central sensitization and chronic pain [32, 14, 17]. Moreover, chronic pain patients exhibit increased temporal summation of pain [35, 55, 50]. However, little is known about the mechanisms subserving temporal processing of nociceptive information. A novel putative model of temporal contrast, offset analgesia, may provide insight to how pain is temporally altered in disease states and may be useful to test for altered temporal processing of nociceptive information. Offset analgesia occurs when small incremental decreases in noxious stimulus intensity produce disproportionately large decreases in perceived pain intensity [18]. Offset analgesia is thought to be centrally mediated since regions of the brainstem implicated in descending control of pain are activated during offset analgesia in healthy subjects [62]. Since many of these regions, such as the periaqueductal gray, are associated with opioid analgesia, it was hypothesized that offset analgesia may be supported by opioid mechanisms.
The most direct method to test for opioid involvement is to administer an opioid antagonist. Diffuse noxious inhibitory control (DNIC), decreased pain to an acute stimulus when an additional heterotopic stimulus is applied simultaneously, is blocked by opioid antagonists [59]. Accordingly, it was hypothesized that naloxone (μ-opioid antagonist) administration would decrease the magnitude of offset analgesia by direct opioid antagonism.
Additionally, exogenous opioids can be administered to test for involvement of endogenous opioids in pain phenomena. Exogenous opioids may outcompete or saturate receptors and effectively block endogenously released neurotransmitters. For example, DNIC is blocked by morphine administration [29]. Thus, during opioid infusion endogenous opioid effects supporting offset analgesia might be eliminated or enhanced.
Finally, states of opioid-induced hypersensitivity (OIH) provide an alternative method to assess mechanisms subserving offset analgesia. During OIH increased sensitivity to acute stimuli often occurs following administration of opioid analgesics [37, 8, 23]. Clinically, increased post-operative pain and increased morphine requirement occur following procedures involving high doses of remifentanil [15, 19], and opioid tolerance / hyperalgesia to acute stimuli occurs in opioid-treated patients [16, 12, 21]. OIH reflects similarities to states of opioid tolerance and dependence [38]. Interestingly, OIH is distinct from effects of direct opioid antagonism and is thought to involve a more complicated sequence of physiological events [26] by several possible mechanisms including altered activity of opioid receptors (eg. dynorphin), upregulation of N-methyl D-aspartate receptors (NMDARs) and altered descending control of pain [56, 36, 2]. Thus, the final hypothesis was that following the period of opioid analgesia, subjects would exhibit increased sensitivity to noxious thermal stimuli and the magnitude of offset analgesia would decrease due to disruption of descending inhibition and local inhibitory mechanisms that may normally contribute to offset analgesia.
To test these hypotheses, healthy volunteers were administered naloxone and remifentanil on separate days, and rated pain from noxious heat stimuli. It was predicted that if opioid mechanisms support offset analgesia, 1) subjects would exhibit decreased magnitude of offset analgesia following administration of naloxone, 2) the presence of exogenous opioids would eliminate or enhance offset analgesia during remifentanil infusion, and 3) the magnitude of offset analgesia would be reduced during OIH.
2. Methods
2.1. Subjects
All study procedures were approved by the Institutional Review Board (IRB) at Wake Forest University School of Medicine. Before participating in the study, every subject gave written, informed consent acknowledging that they understood all methods and procedures used in the experiment, that they would experience painful stimuli and receive intravenous administration of naloxone and remifentanil on separate days, and that they were free to withdraw from the study at any time. Nineteen healthy, pain-free volunteers with no history of chronic pain or any neurological disorder participated in the study (8 females, 11 males; 2 black, 17 white; 25 ± 5.9 yrs, mean ± SEM). Two additional subjects were excluded due to insensitivity to the thermal stimuli. All subjects were asked not to take analgesics within 48 hours before study sessions. All female subjects reported using a reliable method of birth control and were not pregnant while participating in this study. Negative urine pregnancy test results from all female subjects and negative urine drug screen results from all subjects were required prior to administration of study drugs. Eight control subjects were recruited from the original group of volunteers (3 females, 5 males; 27 ± 7.9 yrs, mean ± SEM).
2.2. Psychophysical Training and Assessment
Psychophysical training and assessment was similar to that used previously [39]. Subjects participated in a psychophysical training session prior to the study visits when drugs were administered. Thermal stimuli were delivered using a 16 × 16 mm2 peltier device that was applied to the lower left leg (Medoc, Ramat-Yishai, Israel, TSA-II). Subjects were first presented with four series of thermal stimuli (35°C, 43–49°C, 5 s) to familiarize them with using a computerized sliding visual analog scale (VAS; end points of “no pain sensation” and “most intense pain sensation imaginable”) [49, 47, 27].
Subjects were then familiarized with the sequence of thermal stimuli that would be used at multiple time points on the days involving drug administration. At the beginning of each testing sequence, short duration stimuli (35, 45, and 49°C, 5 s) were presented in random order to assess thermal sensitivity, and subjects were asked to report pain ratings using a sliding scale non-electronic VAS after each stimulus. Next, long duration (30 s) stimuli were presented in two pseudo-randomized blocks. Each of these blocks consisted of three stimuli: a constant 49°C stimulus (30 s), a three-temperature stimulus [T1=49°C (5 s), T2= 50°C (5 s), T3=49°C (20 s)], and a control stimulus [T1=49°C (5 s), T2= 50°C (5 s), T3=35°C (20 s)] (Fig. 1). Offset analgesia is evoked by the 1°C decrease from 50°C to 49°C in the three-temperature 49-50-49°C stimulus. A block of 4 short-duration stimuli (49°C, 4–6 seconds) with varying fall rates of 0.5°C/s or 5.0°C/s was also administered to test for alterations in VAS fall rates. The order of stimuli within blocks as well as the order of block presentation was pseudo-randomized to minimize effects of expectation. Additionally, after each stimulus, the thermal probe was moved to a completely distinct, yet adjacent area of skin.
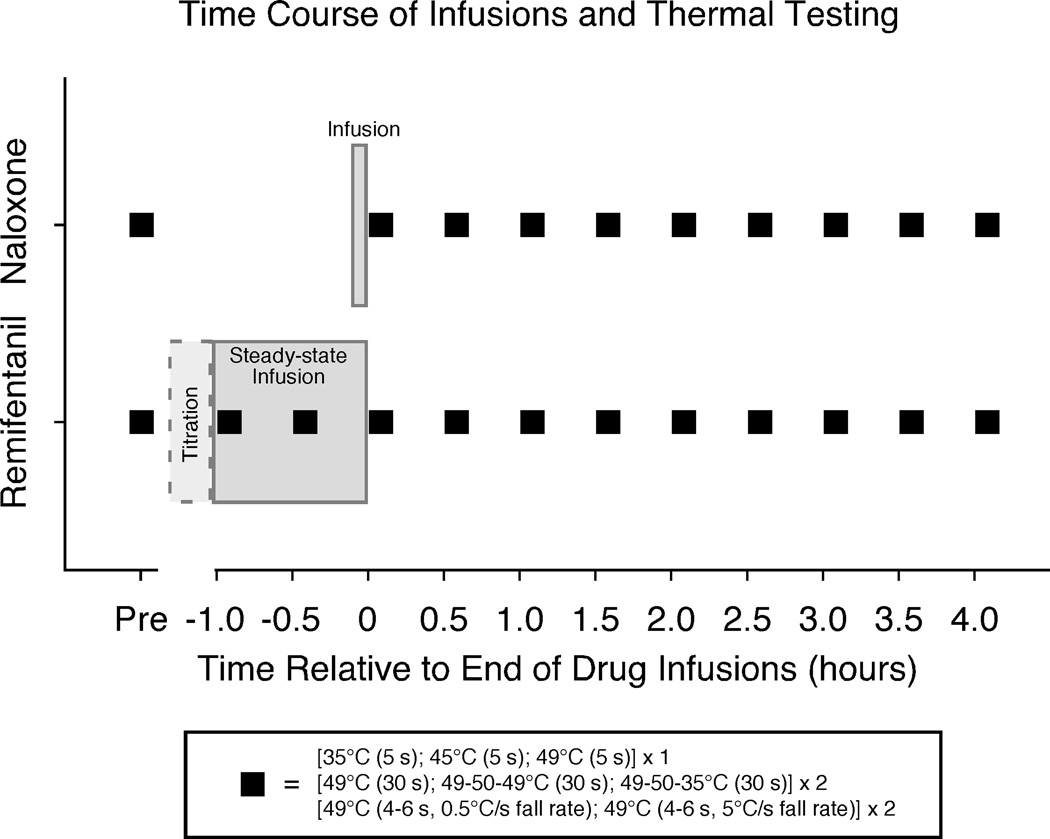
Figure 1. Time course of infusions and thermal testing.
A 2 session crossover design was used with separate sessions of naloxone administration (0.01 mg/kg, bolus dose over 5 minutes) and remifentanil administration (individually titrated dose, followed by steady-state infusion for 60 minutes)(see methods for details). Subjects were not told which drug they received, and sessions were scheduled at least 1 week apart. Thermal testing included both short and long-duration stimuli and was administered prior to drug infusions (Pre) and every half-hour for 4 hours after each drug infusion. Two additional sets of thermal testing were performed during the remifentanil infusion. The time between Pre and 0 hr thermal testing sets was approximately 30 minutes during the naloxone session and is displaced in the figure to show matched post-infusion sets of thermal testing across the 2 drug sessions. An additional control session was performed in a subset of 8 subjects out of the original 19 to assess effects of repeated heat testing. The control session involved thermal testing at time points matched to the remifentanil session and did not involve an infusion.
2.3. Drug Administration and Thermal Testing
Subjects were asked to fast after midnight prior to reporting in the morning to the General Clinical Research Center (GCRC) at Wake Forest University School of Medicine for study sessions involving drug administration. A peripheral intravenous catheter was started in the subject’s right arm, and normal saline was infused for the duration of both study sessions. A two-session, crossover design was used (Fig. 1). Study days were randomized for drug, naloxone or remifentanil, and at least one week apart. Subjects were not informed which drug they received.
2.3.1. Naloxone Administration
Naloxone (naloxone hydrochloride, International Medication Systems, Ltd., Amphastar Pharmaceuticals, USA) was administered intravenously. A 0.01 mg/kg dose was infused over a period of 5 minutes according to Koppert et al. [26]. Subjects were monitored for nausea and vomiting, sweating, possible increase in heart rate, tremor, rapid breathing, agitation, irritability, restlessness or excitement. Vital signs were taken every 15 minutes including respiration rate, oxygen saturation, end-tidal CO2.
2.3.2. Remifentanil Administration
Intravenous infusions of remifentanil (Ultiva, Glaxo, Bioniche Pharma, Hospira, USA LLC) were delivered according to Hood et al. [23]. The infusions were computer-controlled using the STANPUMP algorithm to titrate to desired blood plasma levels [20]. Initial targeted plasma concentrations were 1 ng/ml. After 8–10 minutes of remifentanil infusion, analgesia was assessed by having subjects rate 35°C, 45°C, and 49°C stimuli (5 s). This titration was then increased by 0.5 ng/ml every 8–10 minutes until a sufficient rate was reached such that pain ratings to 49°C (5 s) decreased to approximately 50% of baseline, or until side effects prevented additional increases in remifentanil dose. Once targeted analgesia was achieved, the infusion was maintained for 60 minutes. Subjects received supplemental oxygen, and vital signs of peripheral oxyhemoglobin saturation, blood pressure, heart rate, respiration rate and end-tidal CO2 were monitored and recorded every 5 minutes during the infusion (and every15 minutes after drug infusion) to minimize risks associated with respiratory and / or cardiovascular suppression.
2.4. Time Course of Psychophysical Assessment
Hood et al. found effects of OIH up to 4 hours following discontinuation of remifentanil infusion, therefore we tested responses to sequences of thermal stimuli at multiple time points throughout the sessions up to 4 hours post-infusion [23]. Each testing sequence included one block of 5-second duration stimuli (35°C, 45°C, 49°C), two blocks of three long-duration stimuli (constant 49°C, 49-50-49°C, 49-50-35°C), and one block of 4 short-duration stimuli (49°C, 4–6 seconds). The 5-second duration stimuli were rated conventionally as post-stimulus ratings. All other stimuli were rated using the real-time computerized VAS. Sequences of thermal stimuli were administered at matched time points on both drug days: prior to drug administration (Pre), immediately after delivery of the drug (0 hr), and every half hour following drug infusion for 4 hours (post-drug time points of 0.5 hr, 1.0 hr … 4.0 hr). Two additional testing sequences were used on the remifentanil day during the 60 minute steady-state infusion period, one at 0 minutes (Inf1) and a second testing block at 30 minutes into the steady-state infusion period (Inf2).
Control data (noxious heat testing with no drug administered) were acquired from 8 of the original 19 subjects during a separate visit that did not involve an infusion. This served to measure effects of possible sensitization over time due to repeated application of thermal stimuli. No infusion was involved because this session was designed to specifically assess the effects of repeated heat stimulation without possible confounds arising from placebo effects. Time points of heat testing on the control day were matched to time points of the remifentanil session (including Inf1 and Inf2 time points).
2.5. End Points of Real-Time VAS Data
Subjects reported real-time pain ratings using an electronic visual analog scale (VAS, 0–10) during delivery of each heat stimulus. The real-time VAS ratings were digitally recorded (Powerlab, Chart Software, ADInstruments, USA) and analyzed using custom-made programs (IDL).
Several end points were extracted from the real-time data to assess changes in sensitivity and the magnitude and duration of offset analgesia. Long-duration stimulus end points included:
Peak VAS, the maximum VAS rating from the constant 49°C stimulus, was the primary end point to assess changes in sensitivity following drug infusions.
MaxT2 was the maximum VAS rating during the T2 time period (5 s plus an additional 3 s to account for delayed temporal summation and response time) for the 49-50-49°C stimulus.
Min Offset, was designed as the minimum VAS rating following the T2–T3 temperature decrease in the 49-50-49°C stimulus (window of stimulus time after the T2–T3 shift to just before the end of the T3 stimulus).
Magnitude Offset Analgesia was calculated by subtracting Min Offset values from MaxT2 values from the VAS response to the same applied 49-50-49°C stimulus (within-stimulus difference).
Min Offset Latency, the measure of time for offset analgesia to occur, was calculated from the 49-50-49°C stimulus as the difference in time from the T2–T3 shift until the time at which Min Offset occurred (within stimulus-calculation).
VAS End Latency values were calculated from the constant 49°C stimulus as the time between the start of the T3 decrease to the end of the real-time VAS rating (VAS=0) to assess duration of after sensations.
Short-duration stimulus end points included:
Short-duration PeakVAS (S.PeakVAS), the maximum value from real-time VAS ratings to short-duration stimuli (49°C, 4–6 seconds) which served to assess changes in sensitivity.
VAS Fall Slope was the rate of decrease in real-time VAS ratings at the end of the short-duration 49°C stimuli. These values were calculated using a linear regression of VAS values over time between the initial decrease in VAS ratings after the temperature fall start and the real-time VAS rating end (VAS=0).
2.6. Statistical Analysis
Infusions of remifentanil were titrated to individual doses to achieve 50% reduction in pain intensity ratings. Thus, post-stimulus ratings of 49°C, 5 s stimuli obtained at the end of the drug titration were assessed with a paired t-test to determine if remifentanil significantly reduced pain ratings compared to baseline. Next, a linear regression was used to assess the relationship between remifentanil induced analgesia (final % reduction from baseline) and final steady-state infusion dose remifentanil.
Separate analyses were conducted to directly compare naloxone and remifentanil (N=19) and to compare across conditions of remifentanil, naloxone and control (N=8). Post-stimulus ratings from 45°C and 49°C (5 s) stimuli were analyzed as an additional measure for changes in sensitivity. A two-way repeated measures analysis of variance (ANOVA) was used to assess effects of time, drug condition, and time by drug condition interaction (JMP statistical software, SAS Institute Inc., Cary, NC, USA). Real-time VAS end points were averaged per subject for each testing time point and then analyzed using within-subjects, two-way repeated measures ANOVA with variables of time, drug condition, and time by drug condition interaction. Missing data for each end point and post-stimulus VAS ratings were interpolated across neighboring time points to ensure that all subjects were included in the analyses. Significance level p < 0.05. Control 49-50-35°C stimuli were not analyzed and served only as a control for expectations.
3. Results
3.1. Naloxone Infusion
The dose of naloxone was calculated based on subject weight (0.01 mg/kg) and ranged from 0.46 – 1.20 mg total administered per subject (0.77 ± 0.19 mg, mean ± SD). No side effects were observed or reported for subjects following naloxone administration.
3.2. Remifentanil Infusion
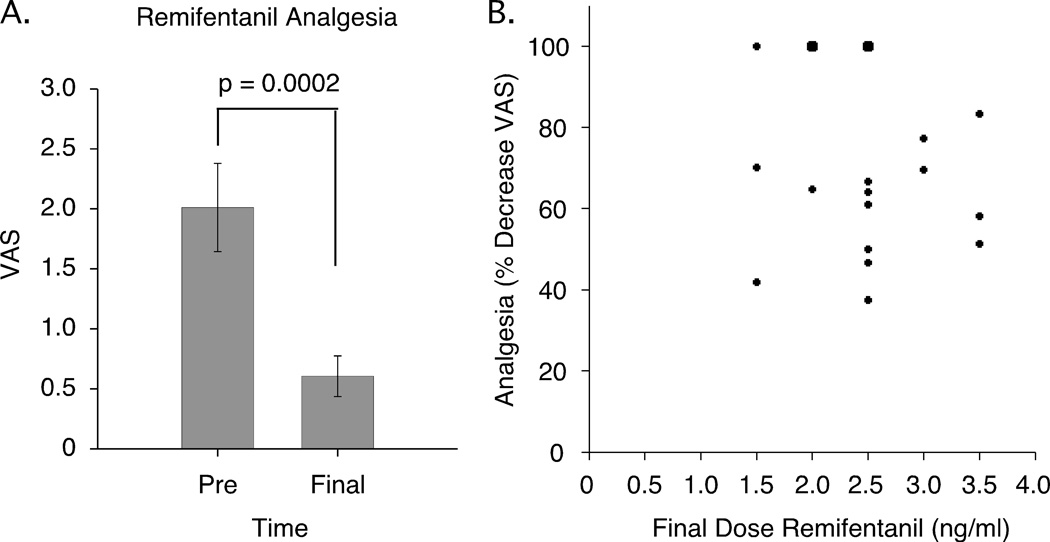
Individual differences in metabolism of and sensitivity to opioids exist, therefore an individualized titration of remifentanil was used [53]. Total dosage of remifentanil administered ranged from 0.269 mg – 1.333 mg per subject (0.675 ± 0.285 mg, mean ± SD). In all subjects, VAS ratings to 49°C stimuli (5 s) were significantly reduced from baseline (p = 0.0002) (Fig. 2). Most subjects displayed moderate sedation during the remifentanil administration but were able to attend to and adequately rate thermal stimuli and respond to the study staff. Additional side effects were observed in a majority of the subjects including nausea (7/19), vomiting (4/19), pruritus (13/19), sweating (5–19), dry mouth (1/19), light-headedness (2/19), dizziness (3/19), headache (3/19), and anxiety (2/19). These occurred during or immediately after the 60 minute infusion, and most side effects were resolved by 15 minutes post-infusion.
Figure 2. Remifentanil analgesia.
(A) Remifentanil produced a mean 56% decrease in VAS pain ratings to 49°C stimuli (5 s) at the end of the titration period (Mean ± SEM). (B) Analgesia at the end of the titration period (percent decreases in VAS ratings) was not significantly related to final steady-state infusion doses (maximum titration rate, ng/ml) per subject (N=19*). *Two subjects showed 100% reductions (VAS=0) at each dose of 2 and 2.5 ng/ml (large dots). VAS, visual analogue scale.
3.3. Thermal Assessment during Remifentanil Infusion
Thermal sensitivity and offset analgesia were assessed during remifentanil infusion at two time points: Inf1 (immediately following the titration period / at the beginning of the 60 minute steady state infusion) and Inf2 (30 minutes into the steady-state infusion).
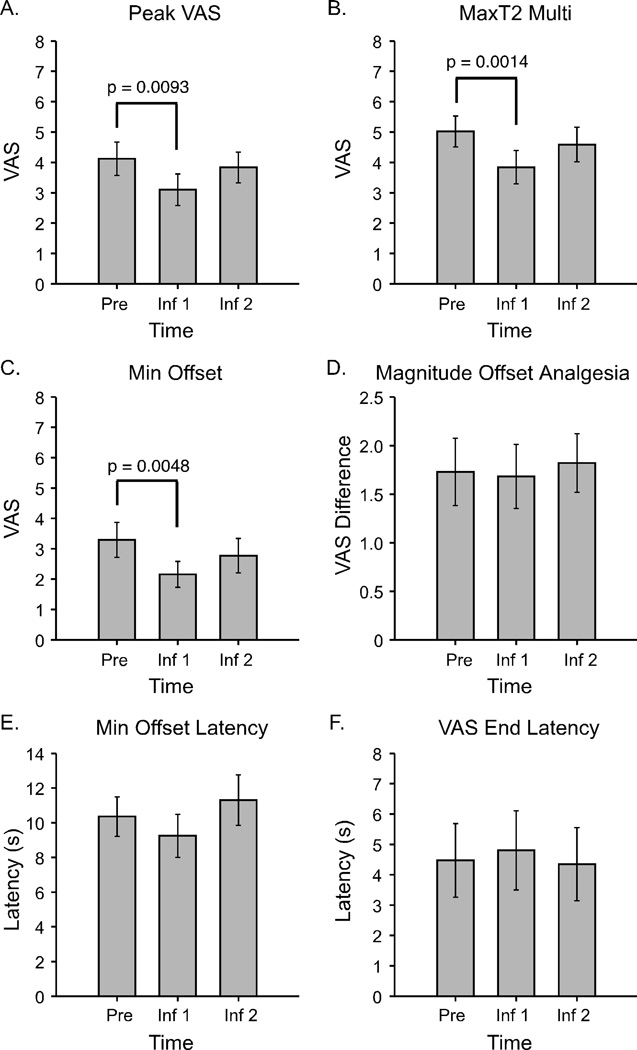
During remifentanil infusion Peak VAS values from constant 49°C stimuli decreased significantly at the Inf1 time point compared to baseline (p = 0.0093), however, the decrease was not significant at the Inf2 time point (p = 0.3318) (overall effect of time p = 0.0050, Fig. 3A).
Figure 3. Sensitivity to noxious heat stimuli and offset analgesia during remifentanil infusion (N=19).
Reductions in maximum VAS ratings to constant 49°C stimuli, 40 s (A, Peak VAS) and to 49-50-49°C stimuli (B, MaxT2) occurred at the Inf1 time point, but not at the Inf2 time point. Similarly, Min Offset values from 49-50-49°C stimuli were significantly reduced at the Inf1 time point (C). However, the difference between MaxT2 and Min Offset values was not significantly altered during infusion, resulting in no reductions in Magnitude Offset Analgesia (D). No significant temporal alterations in offset analgesia were observed during remifentanil infusion (E, Min Offset Latency) and after sensations did not occur (F, VAS End Latency). Mean ± SEM; VAS, visual analogue scale.
MaxT2 values were significantly decreased at remifentanil infusion time points compared to baseline with an overall effect of time (p = 0.0023), but post-hoc tests revealed that the decrease was only significant for the Inf1 time point (p = 0.0014) and not at the Inf2 time point (p = 0.2228) (Fig. 3B). Min Offset values also significantly decreased during remifentanil infusion compared to baseline (p = 0.0184), and post-hoc tests showed that the decrease was significant at the Inf1 time point (p = 0.0048) but not at the Inf2 time point (p = 0.1694) (Fig. 3C). Analysis of Magnitude Offset Analgesia for the infusion time points revealed no significant effect of time (p = 0.9310) (Fig. 3D).
Min Offset Latency values were not significantly altered at infusion time points (p = 0.3816) (Fig. 3E). Similarly, VAS End Latency values were not altered during the remifentanil infusion compared to baseline (p = 0.9664) (Fig. 3F).
3.4. Naloxone vs. Remifentanil
Responses to thermal stimulation were compared over time, initially across conditions of remifentanil and naloxone administration (N=19), since it was expected that drug effects would follow highly distinct time courses.
3.4.1. Naloxone vs. Remifentanil: Real-Time VAS Ratings
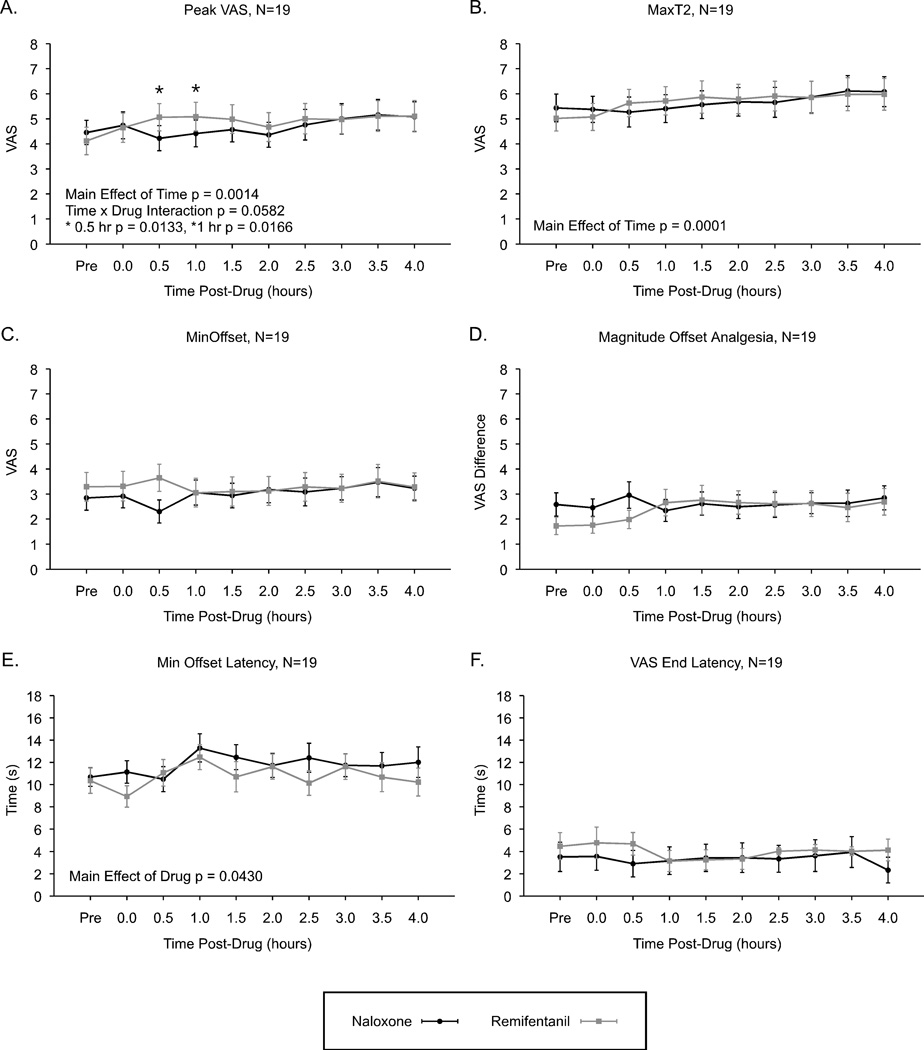
In the analysis of real-time VAS rating end points, increased sensitivity was observed at several time points with a similar time course after administration of both drugs. Peak VAS ratings increased significantly over time (p = 0.0014), but were not significantly altered by drug effects (p = 0.1220). However, Peak VAS values showed a trend for an overall time by drug interaction (p = 0.0582) with post-hoc tests indicating significantly lower Peak VAS values post-naloxone compared to post-remifentanil at 0.5 hr (p = 0.0133) and 1.0 hr (p = 0.0166) post-administration (Fig. 4A).
Figure 4. Sensitivity to noxious heat stimuli and offset analgesia at time points after termination of remifentanil infusion and after administration of naloxone (N=19).
Subjects displayed increased sensitivity over time to constant 49°C, 40 s stimuli (A, Peak VAS) and to 49-50-49°C stimuli (B, MaxT2). However, minimum values during offset analgesia were not significantly altered following naloxone or remifentanil administration (C, Min Offset). Likewise, Magnitude Offset Analgesia (MaxT2 – Min Offset) was also not significantly altered (D). Time to the minimum ratings of offset analgesia was shorter following remifentanil compared to naloxone (E, Min Offset Latency), however, no after sensations were observed (F, VAS End Latency). Mean ± SEM; VAS, visual analogue scale.
MaxT2 values similarly increased over time (p = 0.0001), but showed no effect of drug (p = 0.6936), and no time by drug interaction (p = 0.1743) (Fig. 4B). Min Offset values were not significantly altered over time (p = 0.8726), by drug (p = 0.1881), or time by drug interaction effects (p = 0.2457) (Fig. 4C).
The Magnitude Offset Analgesia (calculated as MaxT2 – Min Offset) was not significantly altered over time (p = 0.4148), or by drug (p = 0.3211), and no interaction was observed (p = 0.1088) (Fig. 4D).
Min Offset Latency values were significantly longer post-naloxone compared to post-remifentanil (p = 0.0430), however, there was no significant effect over time (p = 0.3209), and no time by drug interaction (p = 0.8951) (Fig. 4E). VAS End Latency values were not significantly altered over time (p = 0.5636), by drug (p = 0.3040), or by time / drug interaction effects (p = 0.5752) (Fig. 4F).
3.4.2. Naloxone vs. Remifentanil: Post-Stimulus VAS Ratings
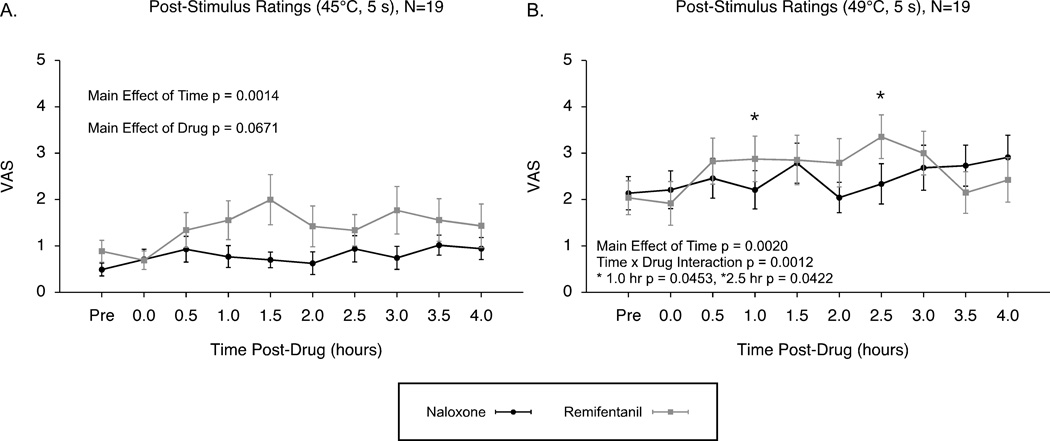
Post-stimulus ratings to 5-second duration 45°C and 49°C stimuli were assessed to compare changes in sensitivity under conditions of naloxone and remifentanil. Post-stimulus ratings to 45°C stimuli significantly increased over time during both drug conditions and showed a trend for increased hyperalgesia over time following the remifentanil administration (effect of time p = 0.0014, effect of drug p = 0.0671, time by drug interaction effects p = 0.1134; Fig. 5A). Post-stimulus ratings to the 49°C stimuli also significantly increased over time under both conditions of naloxone and remifentanil administration, and this increase was enhanced after remifentanil administration compared to naloxone (effect of time p = 0.0020, effect of condition p = 0.5731, time by condition interaction p = 0.0012; Fig. 5B).
Figure 5. Post-stimulus VAS ratings: Evidence for opioid-induced hyperalgesia by remifentanil (N=19).
Post-stimulus VAS ratings to 45°C showed significant increases over time after both infusions of remifentanil and naloxone, with a trend for greater hyperalgesia following remifentanil (A). Post-stimulus VAS ratings to 49°C stimuli also revealed significantly increased sensitivity over time under both conditions, and these effects were enhanced after remifentanil indicating the presence of opioid-induced hyperalgesia (B). Mean ± SEM; VAS, visual analogue scale.
3.5. Naloxone vs. Remifentanil vs. Control
Thermal hypersensitivity was evident after both naloxone and remifentanil administration (described above). Although it was expected that changes in thermal sensitivity would follow highly distinct time courses for both conditions, the time course of hyperalgesia was similar under both conditions. Therefore 8 of the original 19 subjects were recruited for a follow-up visit that consisted of identical thermal testing procedures without drug administration. The following results reflect analyses conducted across conditions of naloxone, remifentanil and control.
3.5.1. Naloxone vs. Remifentanil vs. Control: Real-Time VAS Ratings
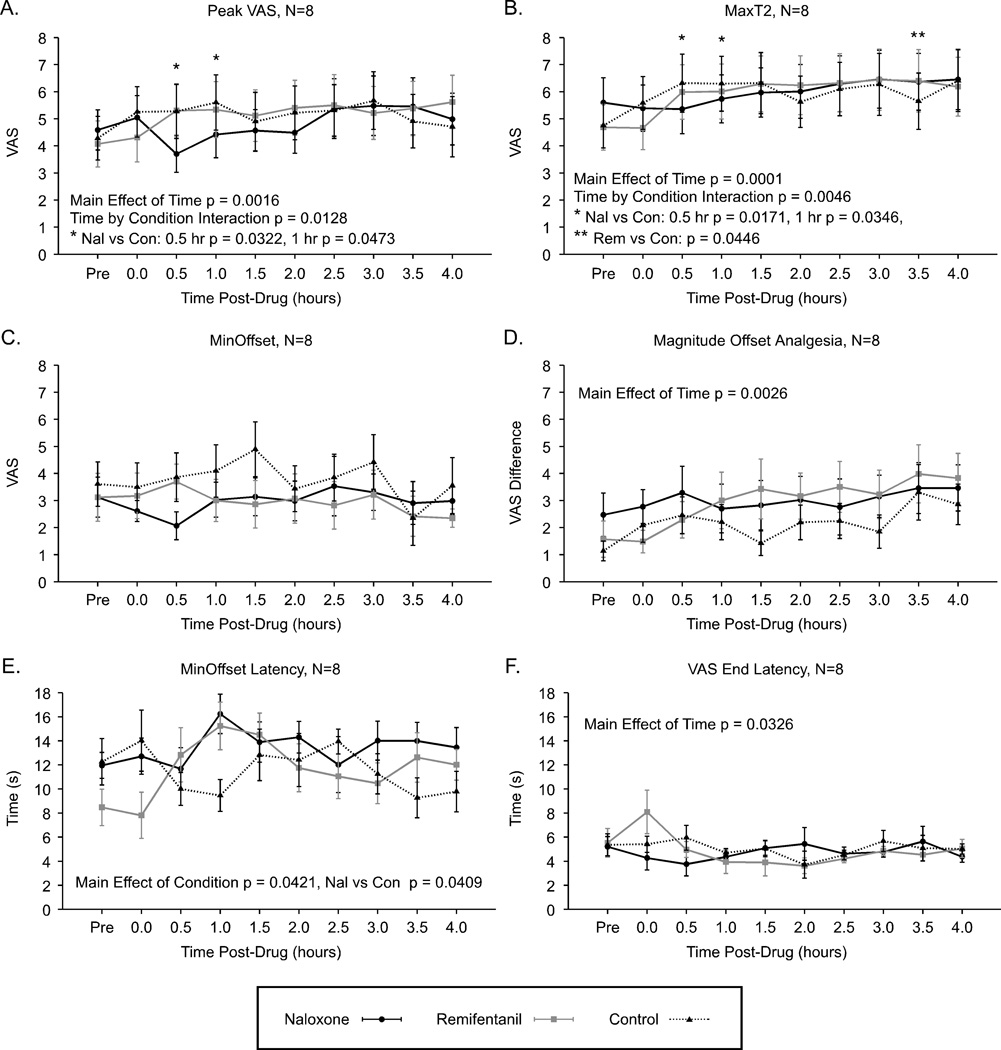
Peak VAS values increased over time for all three conditions (N=8, ANOVA, time p = 0.0016). The magnitude of hyperalgesia observed after remifentanil analgesia and naloxone administration was not significantly different from the hyperalgesia for the control condition (condition effect p = 0.1918). However, hyperalgesia occurred differently over time for remifentanil and naloxone compared to control (interaction p = 0.0128), with post-hoc tests indicating significantly lower Peak VAS values at 0.5 hr (p = 0.0322) and 1.0 hr (p = 0.0473) time points following naloxone compared to control (Fig. 6A).
Figure 6. Sensitivity to noxious heat stimuli and offset analgesia after naloxone and remifentanil compared to control condition with no drug infusion (N=8).
Increased sensitivity occurred over time under all conditions for constant 49°C stimuli, 40 s (A, Peak VAS), and for 49-50-49°C stimuli (B, MaxT2). Conversely, Min Offset values remained unaltered under all conditions (C). The Magnitude Offset Analgesia increased over time under all conditions reflecting heat-induced hyperalgesia in this subset analysis (D). The time to minimum values during offset analgesia were significantly longer following naloxone compared to control condition (E, Min Offset Latency) and VAS End Latency values significantly decreased over time, but this was influenced by after sensations at the 0 hr time point in the remifentanil condition (F). Mean ± SEM; VAS, visual analogue scale.
MaxT2 ratings significantly increased over time for all conditions (overall effect of time p = 0.0001). No significant overall effect of condition was observed for remifentanil and naloxone compared to control (overall effect of condition p = 0.9862). However, there was a significant time by condition interaction (p = 0.0046) indicating that at later time points during the remifentanil condition MaxT2 ratings increased more than control [rem vs. con, post-hoc test 3.5 hr (p = 0.0446)], while at earlier time points MaxT2 ratings were slightly lower post-naloxone compared to control [nal vs. con, post-hoc test 0.5 hr (p = 0.0171), 1 hr (p = 0.0346)] (Fig. 6B). Min Offset values were not significantly altered over time (p = 0.2195), by conditions of remifentanil or naloxone compared to control (condition p = 0.1082), or by time / condition interaction (p = 0.5567) (Fig. 6C).
In contrast to the N=19 remifentanil vs. naloxone data, the N=8 analysis including control data showed that Magnitude Offset Analgesia significantly increased over time under all conditions (p = 0.0026). No significant overall effect of condition was observed (p = 0.0956). No significant time by drug interaction effect was observed (p = 0.2371) (Fig. 6D).
Min Offset Latency values were not significantly altered over time (p = 0.3643), but were significantly increased following naloxone compared to control (overall effect of condition p = 0.0421, post-hoc naloxone vs. control p = 0.0409), however, only a trend for an interaction effect was observed (p = 0.0790) (Fig. 6E). VAS End Latency values were significantly altered over time (p = 0.0326), but this was likely due to outlier effects at the 0 hr time point. These values were not significantly altered by condition (p = 0.8196) or interaction (p = 0.0612) (Fig. 6F).
3.5.2. Naloxone vs. Remifentanil vs. Control: Post-Stimulus VAS Ratings
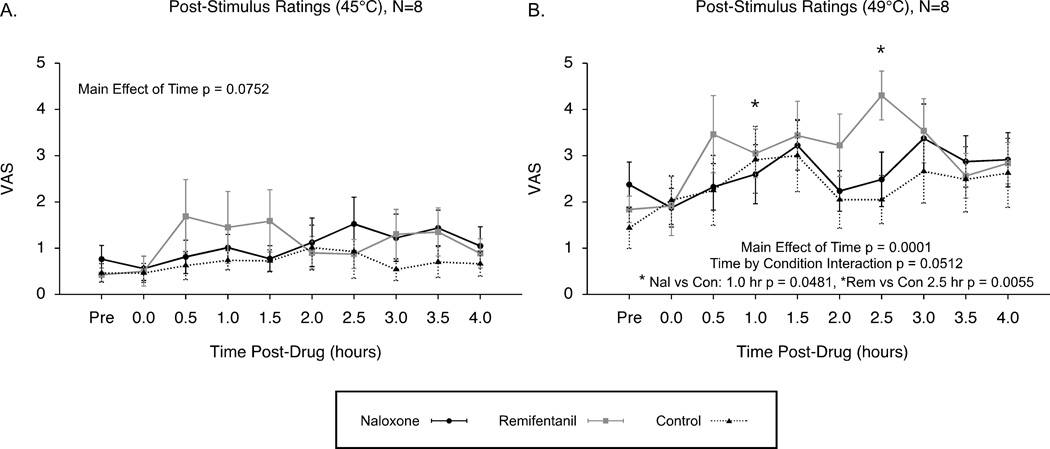
Post-stimulus ratings to 45°C and 49°C stimuli (5 s duration) were assessed to compare changes in sensitivity under conditions of naloxone, remifentanil and control. Post-stimulus ratings to 45°C stimuli were not significantly altered in the N=8 analysis across conditions of remifentanil, naloxone and control, but showed a trend for hyperalgesia over time (effect of time p = 0.0752, effect of condition p = 0.4231, time by condition interaction p = 0.1586; Fig. 7A). Post-stimulus ratings to the 49°C stimuli significantly increased over time under conditions of remifentanil, naloxone and control, and showed a trend for enhanced hyperalgesia following remifentanil (effect of time p = 0.0001, effect of condition p = 0.5193, time by condition interaction p = 0.0512; Fig. 7B).
Figure 7. Post-stimulus VAS ratings: Opioid-induced hyperalgesia by remifentanil compared to naloxone and control conditions (N=8).
Post-stimulus VAS ratings to 45°C showed only a trend for increased sensitivity over time across the naloxone, remifentanil and control conditions (A). Post-stimulus VAS ratings to 49°C stimuli revealed significantly increased sensitivity over time for all conditions with a trend for opioid-induced hyperalgesia following remifentanil compared to the control condition (B). Mean ± SEM; VAS, visual analogue scale.
3.6. Short-Duration Stimuli: S.PeakVAS and VAS Fall Slopes
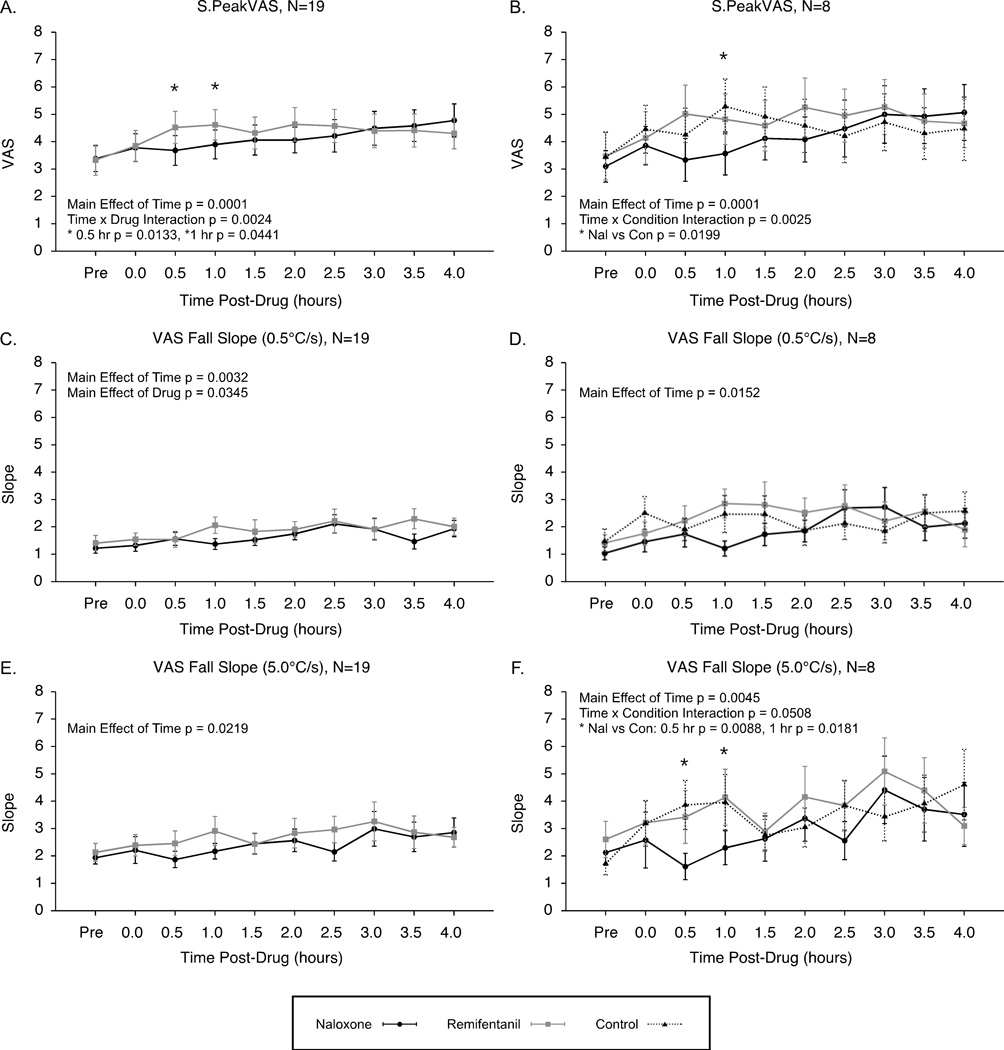
S.PeakVAS values were significantly increased over time after both remifentanil and naloxone infusions, and these increases were enhanced following remifentanil compared to naloxone (N=19, effect of time p = 0.0001, effect of drug p = 0.1986, time by drug interaction p = 0.0024)(Fig. 8A). In the subset analysis with the control condition, S.PeakVAS values showed significant increases over time because hyperalgesia was also produced during the control session. Additionally, the control condition increases were greater compared to the naloxone condition, but not remifentanil (N=8 analysis, effect of time p = 0.0001, effect of condition p = 0.2017, time by condition interaction p = 0.0025)(Fig. 8B).
Figure 8. Real-time VAS ratings to short-duration stimuli: Hyperalgesia and increased VAS fall slopes.
Maximum ratings to short-duration (4–6 s) stimuli, S.PeakVAS, were increased over time after both naloxone and remifentanil administrations (A, N=19) and under conditions of naloxone, remifentanil and control (B, N=8). VAS fall slopes also became steeper over time under all conditions for both slow stimulus fall rates of 0.5°C/s (C, N=19)(D, N=8) and fast stimulus fall rates of 5.0°C/s (E, N=19)(F, N=8) indicating that temporal sharpening mechanisms were conserved under conditions inducing thermal hyperalgesia.
VAS Fall Slopes also became significantly steeper over time after both remifentanil and naloxone infusions (N=19) for both stimuli with the slow fall rate (0.5°C/s, effect of time p = 0.0032, effect of drug p = 0.0345, time by drug interaction p = 0.4624)(Fig. 8C) and stimuli with the fast fall rate (5°C/s, effect of time p = 0.0219, effect of condition p = 0.1462, time by condition interaction p = 0.6815)(Fig. 8E). Steeper slopes were also observed during the control condition (N=8) for both slow (0.5°C/s, effect of time p = 0.0152, effect of condition p = 0.0956, time by condition interaction p = 0.2262)(Fig. 8D) and fast stimulus fall rates (0.5°C/s, effect of time p = 0.0045, effect of condition p = 0.0999, time by condition interaction p = 0.0508)(Fig. 8F).
4. Discussion
The main finding of this study was that the magnitude of offset analgesia was not reduced following naloxone infusion or at any time point during or following remifentanil infusion. These multiple converging lines of evidence indicate that the inhibitory mechanisms supporting offset analgesia are largely opioid-independent.
4.1. Offset Analgesia under Altered Opioid States
4.1.1. Naloxone
At the same dose used in the present study, naloxone (0.01 mg/kg) effectively produces hyperalgesia to electrical and mechanical stimuli in humans, presumably through blockade of endogenous opioids [26]. These effects have a rapid onset, and maximum effects occur after 30 minutes [26]. It was therefore expected that thermal hyperalgesia would occur between the 0 and 1 hr time points, however, hyperalgesia was not observed after naloxone infusion in the present study. Previous investigations show that hyperalgesia is not always produced by naloxone infusions and that paradoxical analgesia may occur at small doses [31, 30]. Nonetheless, naloxone can reliably reverse / block effects of endogenous opioids, even when acute changes in sensitivity by naloxone are not directly observed [59].
It was hypothesized that offset analgesia would be disrupted by administration of naloxone due to blockade of endogenous opioid effects. Naloxone (0.02 mg/kg) can completely reverse the analgesia produced by small doses of morphine in humans (0.1–0.3 mg/kg) [58]. Additionally, diffuse noxious inhibitory control (DNIC), an opioid-mediated inhibitory phenomenon, is blocked from 5 minutes until almost an hour after administration of naloxone (0.4 mg total dose) [59]. In the present study, similar doses of naloxone (0.01 mg/kg) failed to block offset analgesia. Thus, offset analgesia is not disrupted by opioid antagonism, and these findings provide evidence for distinct mechanisms subserving DNIC and offset analgesia.
4.1.2. Remifentanil: Infusion
Significant analgesia was produced among all subjects during the remifentanil infusion, however, no changes in the magnitude of offset analgesia were observed during the remifentanil infusion. The stability of offset analgesia is surprising since other pain inhibitory phenomena are typically altered by opioid administration. Temporal summation of pain is decreased by opioid administration in both humans and animals [51, 33]. Additionally, DNIC is blocked 15–20 minutes after low dose administration of morphine (0.05 mg/kg) [29]. Additionally, Min Offset Latency and VAS End Latency values were not significantly altered by sedation during remifentanil infusion. Thus, changes in reaction times and motor coordination were not responsible for any variations in latencies between conditions.
4.1.3. Remifentanil: Opioid-Induced Hyperalgesia
In humans, OIH produces increased pain to acute electrical, mechanical and noxious heat stimuli [26, 57]. Additional studies demonstrate increased post-operative pain and enlarged regions of secondary mechanical hyperalgesia following opioid analgesia [6, 23]. Conversely, some studies show a lack of OIH to thermal stimuli [23] and do not detect a change in thermal thresholds [34]. The present study used the same procedure as a previous investigation that showed enlarged regions of secondary hyperalgesia following remifentanil administration [23]. Findings from the analysis of post-stimulus ratings indicate that remifentanil may have induced thermal hyperalgesia to a greater extent compared to the naloxone and control conditions. In contrast, real-time VAS ratings showed less consistent hyperalgesia after remifentanil compared to naloxone and control conditions. OIH may have been more clearly detected in the post-stimulus ratings due to lower variability.
Nonetheless, contrary to the hypothesis, offset analgesia was not disrupted during the period of OIH. Recently, the NMDA antagonist ketamine has been shown to eliminate DNIC but not alter offset analgesia [44]. These new findings support the present observations that offset analgesia is not disrupted during OIH, because OIH is subserved, in part, by NMDA mechanisms [37, 8, 10].
4.2. Offset Analgesia in the Presence of Heat-Induced Hyperalgesia
The magnitude of offset analgesia remained consistent after both naloxone and remifentanil administrations. Unexpectedly, however, in the N=8 analyses, the magnitude of offset analgesia increased over time across all conditions, and this was apparently due to effects of heat-induced thermal hyperalgesia. In contrast, minimum VAS ratings during offset analgesia (Min Offset) remained notably constant under all conditions (N=19 and N=8 analyses) indicating that the lowest limit of offset analgesia is not altered by heat-induced thermal hyperalgesia. Additionally, the Min Offset Latency was not altered despite increased Magnitude Offset Analgesia in the N=8 analyses. This further indicates that temporal sharpening mechanisms were not disrupted because the duration of offset analgesia was conserved. Similarly, VAS fall slopes became significantly steeper (increased) in conjunction with hyperalgesia to short-duration stimuli (increased S.PeakVAS values). Together, these findings support the conclusion that temporal sharpening mechanisms are not disrupted, but rather, enhanced during thermal hyperalgesia.
4.3. Alternative Mechanisms Supporting Offset Analgesia
Activation of brainstem regions consistent with the periaqueductal gray, rostral ventral medulla and locus coeruleus as well as thalamic and cortical regions occurs during offset analgesia [62]. These regions are modulated by the presence of opioid antagonists [5] and exogenous opioids [7, 60]. Therefore, activation of these regions may represent supraspinal opioid mechanisms of offset analgesia. However, since offset analgesia was not altered by the presence of opioids or during opioid antagonism, several alternative hypotheses are proposed. One possibility is that because offset analgesia is dynamic in nature, it may involve both facilitatory and inhibitory mechanisms. Both descending facilitation and inhibition are thought to increase during OIH, thus overall changes in thermal sensitivity, and possibly offset analgesia, at later time points may be cancelled out [9].
Another possibility is that offset analgesia may be supported by non-opioid supraspinal mechanisms since noradrenergic, dopaminergic, serotonergic and cannabinergic processes are also involved in pain modulation [61, 24, 42, 43, 22, 46]. For example, the nucleus accumbens is implicated in reward-like activity at the offset of noxious heat stimuli suggesting activation of supraspinal glycinergic and dopaminergic pathways under these conditions [4, 3]. In contrast to placebo effects by expectation or conditioning with opioids, placebo effects produced by conditioning with non-opioid analgesics (eg. ketorolac) are naloxone-insensitive and therefore involve non-opioid mechanisms [1]. Recent evidence further shows that naloxone fails to block analgesia produced by the presentation of highly pleasant emotional picture stimuli [28]. Similar non-opioidergic mechanisms that occur under these conditions may therefore also be supporting pain relief during offset analgesia.
Additionally, supraspinal activation during offset analgesia could be in response to perceived decreases in pain, with the analgesia being mediated primarily by spinal GABA-ergic / glycinergic or serotonergic mechanisms such as those involved in analgesia by spinal cord stimulation [13, 54]. Spinal neurons that follow a time course of activation similar to offset analgesia have been observed; these neurons are inhibited during presentation of a noxious stimulus and display strong after discharges following the end of the noxious stimulus [40, 41]. Administration of the glycine antagonist strychnine blocks the inhibition phase and enhances the after discharge phase of the neural responses. However, the enhancement of after discharges by strychnine does not significantly exceed baseline levels in spinalized rats, suggesting a supraspinal component. Thus, spinal neurons such as these may be involved in offset analgesia with a similar combination of spinal and supraspinal mechanisms across phases of the response.
4.4. Clinical Relevance of Offset Analgesia
Altered temporal summation of pain, after sensations and altered DNIC are observed in chronic pain states suggesting dysfunctional inhibition of nociceptive information [48, 55, 17, 25]. Similarly, patients with various types of neuropathic pain exhibit decreased offset analgesia compared to matched controls [45].
Inhibitory phenomena also are differentially altered in patients depending on whether their therapy includes opioid or non-opioid medications. Temporal summation of pain is increased in opioid-treated patients compared to non-opioid treated patients [11]. DNIC is reduced in opioid-treated chronic pain patients compared to non-opioid treated patients [52]. The present findings indicate that, unlike temporal summation and DNIC, offset analgesia is not an opioid-dependent phenomenon. Thus, offset analgesia may be altered in conditions of chronic pain, regardless of opioid responsiveness.
4.5. Limitations
Thermal hyperalgesia occurred at later time points after administration of both naloxone and remifentanil with similar time courses. Consequently, we employed an additional control condition without drug administration, which also revealed thermal hyperalgesia at later testing time points. Therefore, repeated administration of heat stimuli was a putative contributor to thermal hyperalgesia and presents a potential confound for testing thermal sensitivity over long periods of time.
Additionally, the control analysis was conducted in only a subset of subjects and may be biased to reflect a unique set of individual sensitivities to thermal stimuli. Further, the control sessions were conducted after collection of the original data set and may have been influenced by order effects.
4.6. Conclusions
The magnitude of offset analgesia was not reduced or significantly altered following naloxone administration, during remifentanil analgesia, or following remifentanil infusion. Therefore, endogenous opioids do not constitute the primary mechanism subserving offset analgesia. Additionally, these observations support the conclusion that offset analgesia is distinct from other pain processing phenomena such as temporal summation of pain and DNIC. While the precise mechanisms responsible for offset analgesia remain unknown, a combination of peripheral and central mechanisms may contribute to offset analgesia and may involve opioid-independent spinal and/or supraspinal antinociceptive activity.
Acknowledgements
Funded by the U.S. National Institutes of Health grant DA20168 (RCC) and DA026278 (KTM). Thanks to the Wake Forest University Healthy Sciences General Clinical Research Center staff and Sharon Warren, R.N.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors have no conflicts of interest to disclose.
Reference List
- 1.Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci. 1999;19(1):484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104(3):570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66(1):149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becerra L, Borsook D. Signal valence in the nucleus accumbens to pain onset and offset. Eur J Pain. 2008;12(7):866–869. doi: 10.1016/j.ejpain.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borras MC, Becerra L, Ploghaus A, Gostic JM, DaSilva A, Gonzalez RG, Borsook D. fMRI measurement of CNS responses to naloxone infusion and subsequent mild noxious thermal stimuli in healthy volunteers. J Neurophysiol. 2004;91(6):2723–2733. doi: 10.1152/jn.00249.2003. [DOI] [PubMed] [Google Scholar]
- 6.Bowdle TA, Camporesi EM, Maysick L, Hogue CW, Jr, Miguel RV, Pitts M, Streisand JB. A multicenter evaluation of remifentanil for early postoperative analgesia. Anesth Analg. 1996;83(6):1292–1297. doi: 10.1097/00000539-199612000-00028. [DOI] [PubMed] [Google Scholar]
- 7.Casey KL, Svensson P, Morrow TJ, Raz J, Jone C, Minoshima S. Selective opiate modulation of nociceptive processing in the human brain. J Neurophysiol. 2000;84(1):525–533. doi: 10.1152/jn.2000.84.1.525. [DOI] [PubMed] [Google Scholar]
- 8.Celerier E, Laulin J, Larcher A, Le Moal M, Simonnet G. Evidence for opiate-activated NMDA processes masking opiate analgesia in rats. Brain Res. 1999;847(1):18–25. doi: 10.1016/s0006-8993(99)01998-8. [DOI] [PubMed] [Google Scholar]
- 9.Celerier E, Laulin JP, Corcuff JB, Le Moal M, Simonnet G. Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process. J Neurosci. 2001;21(11):4074–4080. doi: 10.1523/JNEUROSCI.21-11-04074.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celerier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P, Simonnet G. Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology. 2000;92(2):465–472. doi: 10.1097/00000542-200002000-00029. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Malarick C, Seefeld L, Wang S, Houghton M, Mao J. Altered quantitative sensory testing outcome in subjects with opioid therapy. Pain. 2009;143(1–2):65–70. doi: 10.1016/j.pain.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu LF, Clark DJ, Angst MS. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: a preliminary prospective study. J Pain. 2006;7(1):43–48. doi: 10.1016/j.jpain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Cui JG, O'Connor WT, Ungerstedt U, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain. 1997;73(1):87–95. doi: 10.1016/s0304-3959(97)00077-8. [DOI] [PubMed] [Google Scholar]
- 14.Eide PK, Rabben T. Trigeminal neuropathic pain: pathophysiological mechanisms examined by quantitative assessment of abnormal pain and sensory perception. Neurosurgery. 1998;43(5):1103–1110. doi: 10.1097/00006123-199811000-00055. [DOI] [PubMed] [Google Scholar]
- 15.Eisenach JC. Preemptive hyperalgesia, not analgesia? Anesthesiology. 2000;92(2):308–309. doi: 10.1097/00000542-200002000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Fishbain DA, Lewis JE, Gao J. Are Psychoactive Substance (Opioid)-Dependent Chronic Pain Patients Hyperalgesic? Pain Pract. doi: 10.1111/j.1533-2500.2010.00437.x. [DOI] [PubMed] [Google Scholar]
- 17.Gottrup H, Kristensen AD, Bach FW, Jensen TS. After sensations in experimental and clinical hypersensitivity. Pain. 2003;103(1–2):57–64. doi: 10.1016/s0304-3959(02)00415-3. [DOI] [PubMed] [Google Scholar]
- 18.Grill JD, Coghill RC. Transient analgesia evoked by noxious stimulus offset. J Neurophysiol. 2002;87(4):2205–2208. doi: 10.1152/jn.00730.2001. [DOI] [PubMed] [Google Scholar]
- 19.Guignard B, Bossard AE, Coste C, Sessler DI, Lebrault C, Alfonsi P, Fletcher D, Chauvin M. Acute opioid tolerance: intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000;93(2):409–417. doi: 10.1097/00000542-200008000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Gustafsson LL, Ebling WF, Osaki E, Harapat S, Stanski DR, Shafer SL. Plasma concentration clamping in the rat using a computer-controlled infusion pump. Pharm Res. 1992;9(6):800–807. doi: 10.1023/a:1015863824277. [DOI] [PubMed] [Google Scholar]
- 21.Hay JL, White JM, Bochner F, Somogyi AA, Semple TJ, Rounsefell B. Hyperalgesia in opioid-managed chronic pain and opioid-dependent patients. J Pain. 2009;10(3):316–322. doi: 10.1016/j.jpain.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Hayashida K, DeGoes S, Curry R, Eisenach JC. Gabapentin activates spinal noradrenergic activity in rats and humans and reduces hypersensitivity after surgery. Anesthesiology. 2007;106(3):557–562. doi: 10.1097/00000542-200703000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Hood DD, Curry R, Eisenach JC. Intravenous remifentanil produces withdrawal hyperalgesia in volunteers with capsaicin-induced hyperalgesia. Anesth Analg. 2003;97(3):810–815. doi: 10.1213/01.ANE.0000078811.80093.88. [DOI] [PubMed] [Google Scholar]
- 24.Jones SL. Descending noradrenergic influences on pain. Prog Brain Res. 1991;88:381–394. doi: 10.1016/s0079-6123(08)63824-8. [DOI] [PubMed] [Google Scholar]
- 25.Julien N, Goffaux P, Arsenault P, Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain. 2005;114(1–2):295–302. doi: 10.1016/j.pain.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 26.Koppert W, Angst M, Alsheimer M, Sittl R, Albrecht S, Schuttler J, Schmelz M. Naloxone provokes similar pain facilitation as observed after short-term infusion of remifentanil in humans. Pain. 2003;106(1–2):91–99. doi: 10.1016/s0304-3959(03)00294-x. [DOI] [PubMed] [Google Scholar]
- 27.Koyama Y, Koyama T, Kroncke AP, Coghill RC. Effects of stimulus duration on heat induced pain: the relationship between real-time and post-stimulus pain ratings. Pain. 2004;107(3):256–266. doi: 10.1016/j.pain.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Kut E, Candia V, von Overbeck J, Pok J, Fink D, Folkers G. Pleasure-related analgesia activates opioid-insensitive circuits. J Neurosci. 2011;31(11):4148–4153. doi: 10.1523/JNEUROSCI.3736-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Bars D, Willer JC, De Broucker T. Morphine blocks descending pain inhibitory controls in humans. Pain. 1992;48(1):13–20. doi: 10.1016/0304-3959(92)90126-V. [DOI] [PubMed] [Google Scholar]
- 30.Levine JD, Gordon NC. Method of administration determines the effect of naloxone on pain. Brain Res. 1986;365(2):377–378. doi: 10.1016/0006-8993(86)91653-7. [DOI] [PubMed] [Google Scholar]
- 31.Levine JD, Gordon NC, Fields HL. Naloxone dose dependently produces analgesia and hyperalgesia in postoperative pain. Nature. 1979;278(5706):740–741. doi: 10.1038/278740a0. [DOI] [PubMed] [Google Scholar]
- 32.Lindblom U. Assessment of Abnormal Evoked Pain in Neurological Pain Patients and Its Relation to Spontaneous Pain - a Descriptive and Conceptual-Model with Some Analytical Results. Advances in Pain Research and Therapy. 1985;9:409–423. [Google Scholar]
- 33.Lomas LM, Picker MJ. Behavioral assessment of temporal summation in the rat: sensitivity to sex, opioids and modulation by NMDA receptor antagonists. Psychopharmacology (Berl) 2005;180(1):84–94. doi: 10.1007/s00213-005-2153-2. [DOI] [PubMed] [Google Scholar]
- 34.Luginbuhl M, Gerber A, Schnider TW, Petersen-Felix S, Arendt-Nielsen L, Curatolo M. Modulation of remifentanil-induced analgesia, hyperalgesia, and tolerance by small-dose ketamine in humans. Anesth Analg. 2003;96(3):726–732. doi: 10.1213/01.ANE.0000048086.58161.18. table of contents. [DOI] [PubMed] [Google Scholar]
- 35.Maixner W, Fillingim R, Sigurdsson A, Kincaid S, Silva S. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain. 1998;76(1–2):71–81. doi: 10.1016/s0304-3959(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 36.Mao J. Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. Pain. 2002;100(3):213–217. doi: 10.1016/S0304-3959(02)00422-0. [DOI] [PubMed] [Google Scholar]
- 37.Mao J, Price DD, Mayer DJ. Thermal hyperalgesia in association with the development of morphine tolerance in rats: roles of excitatory amino acid receptors and protein kinase C. J Neurosci. 1994;14(4):2301–2312. doi: 10.1523/JNEUROSCI.14-04-02301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62(3):259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- 39.Martucci KT, Yelle MD, Coghill RC. Differential Effects of Experimental Central Sensitization on the Time-course and Magnitude of Offset Analgesia. Pain. 2012;153(2):463–472. doi: 10.1016/j.pain.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGaraughty S, Henry JL. Relationship between mechano-receptive fields of dorsal horn convergent neurons and the response to noxious immersion of the ipsilateral hindpaw in rats. Pain. 1997;70(2–3):133–140. doi: 10.1016/s0304-3959(97)03328-9. [DOI] [PubMed] [Google Scholar]
- 41.McGaraughty S, Henry JL. The effects of strychnine, bicuculline, and ketamine on 'immersion-inhibited' dorsal horn convergent neurons in intact and spinalized rats. Brain Res. 1998;784(1–2):63–70. doi: 10.1016/s0006-8993(97)01153-0. [DOI] [PubMed] [Google Scholar]
- 42.Meng ID, Manning BH, Martin WJ, Fields HL. An analgesia circuit activated by cannabinoids. Nature. 1998;395(6700):381–383. doi: 10.1038/26481. [DOI] [PubMed] [Google Scholar]
- 43.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66(6):355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 44.Niesters M, Dahan A, Swartjes M, Noppers I, Fillingim RB, Aarts L, Sarton EY. Effect of ketamine on endogenous pain modulation in healthy volunteers. Pain. 2011;152(3):656–663. doi: 10.1016/j.pain.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 45.Niesters M, Hoitsma E, Sarton E, Aarts L, Dahan A. Offset analgesia in neuropathic pain patients and effect of treatment with morphine and ketamine. Anesthesiology. 2011;115(5):1063–1071. doi: 10.1097/ALN.0b013e31822fd03a. [DOI] [PubMed] [Google Scholar]
- 46.Potvin S, Grignon S, Marchand S. Human evidence of a supra-spinal modulating role of dopamine on pain perception. Synapse. 2009;63(5):390–402. doi: 10.1002/syn.20616. [DOI] [PubMed] [Google Scholar]
- 47.Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994;56(2):217–226. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 48.Price DD, Long S, Huitt C. Sensory testing of pathophysiological mechanisms of pain in patients with reflex sympathetic dystrophy. Pain. 1992;49(2):163–173. doi: 10.1016/0304-3959(92)90139-3. [DOI] [PubMed] [Google Scholar]
- 49.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 50.Price DD, Staud R, Robinson ME, Mauderli AP, Cannon R, Vierck CJ. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99(1–2):49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 51.Price DD, Von der Gruen A, Miller J, Rafii A, Price C. A psychophysical analysis of morphine analgesia. Pain. 1985;22(3):261–269. doi: 10.1016/0304-3959(85)90026-0. [DOI] [PubMed] [Google Scholar]
- 52.Ram KC, Eisenberg E, Haddad M, Pud D. Oral opioid use alters DNIC but not cold pain perception in patients with chronic pain - new perspective of opioid-induced hyperalgesia. Pain. 2008;139(2):431–438. doi: 10.1016/j.pain.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 53.Smith HS. Opioid metabolism. Mayo Clin Proc. 2009;84(7):613–624. doi: 10.1016/S0025-6196(11)60750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song Z, Meyerson BA, Linderoth B. Spinal 5-HT receptors that contribute to the pain-relieving effects of spinal cord stimulation in a rat model of neuropathy. Pain. 2011 doi: 10.1016/j.pain.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 55.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91(1–2):165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 56.Vanderah TW, Ossipov MH, Lai J, Malan TP, Jr, Porreca F. Mechanisms of opioid-induced pain and antinociceptive tolerance: descending facilitation and spinal dynorphin. Pain. 2001;92(1–2):5–9. doi: 10.1016/s0304-3959(01)00311-6. [DOI] [PubMed] [Google Scholar]
- 57.Wanigasekera V, Lee MC, Rogers R, Hu P, Tracey I. Neural correlates of an injury-free model of central sensitization induced by opioid withdrawal in humans. J Neurosci. 2011;31(8):2835–2842. doi: 10.1523/JNEUROSCI.5412-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Willer JC. Studies on pain. Effects of morphine on a spinal nociceptive flexion reflex and related pain sensation in man. Brain Res. 1985;331(1):105–114. doi: 10.1016/0006-8993(85)90719-x. [DOI] [PubMed] [Google Scholar]
- 59.Willer JC, Le Bars D, De Broucker T. Diffuse noxious inhibitory controls in man: involvement of an opioidergic link. Eur J Pharmacol. 1990;182(2):347–355. doi: 10.1016/0014-2999(90)90293-f. [DOI] [PubMed] [Google Scholar]
- 60.Wise RG, Rogers R, Painter D, Bantick S, Ploghaus A, Williams P, Rapeport G, Tracey I. Combining fMRI with a pharmacokinetic model to determine which brain areas activated by painful stimulation are specifically modulated by remifentanil. Neuroimage. 2002;16(4):999–1014. doi: 10.1006/nimg.2002.1146. [DOI] [PubMed] [Google Scholar]
- 61.Yaksh TL, Wilson PR. Spinal serotonin terminal system mediates antinociception. J Pharmacol Exp Ther. 1979;208(3):446–453. [PubMed] [Google Scholar]
- 62.Yelle MD, Oshiro Y, Kraft RA, Coghill RC. Temporal filtering of nociceptive information by dynamic activation of endogenous pain modulatory systems. J Neurosci. 2009;29(33):10264–10271. doi: 10.1523/JNEUROSCI.4648-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]