Abstract
Trace fear conditioning, in which a brief empty “trace interval” occurs between presentation of the CS and UCS, differs from standard delay conditioning in that contributions from both the hippocampus and prelimbic medial prefrontal cortex (PL mPFC) are required to form a normal long term memory. Little is currently known about how the PL interacts with various temporal lobe structures to support learning across this temporal gap between stimuli. We temporarily inactivated PL along with either ventral hippocampus or amygdala in a disconnection design to determine if these structures functionally interact to acquire trace fear conditioning. Disconnection (contralateral injections) of the PL with either the ventral hippocampus or amygdala impaired trace fear conditioning; however, ipsilateral control rats were also impaired. Follow-up experiments examined the effects of unilateral inactivation of the PL, ventral hippocampus, or amygdala during conditioning. The results of this study demonstrate that unilateral inactivation of the ventral hippocampus or amygdala impairs memory, while bilateral inactivation of the PL is required to produce a deficit. Memory deficits after unilateral inactivation of the ventral hippocampus or amygdala prevent us from determining whether the mPFC functionally interacts with the medial temporal lobe using a disconnection approach. Nonetheless, our findings suggest that the trace fear network is more integrated than previously thought.
Keywords: trace fear conditioning, prelimbic, temporal lobe, disconnection, lidocaine, fear memory
1. Introduction
Trace fear conditioning provides a powerful model system for studying the contributions of prefrontal cortex and the medial temporal lobe to memory. Trace fear conditioning, like standard “delay” fear conditioning, requires the association of a neutral conditional stimulus (CS) and an aversive unconditional stimulus (UCS). Trace conditioning differs from delay conditioning by the addition of a stimulus-free “trace” interval of several seconds separating the CS and UCS. While delay conditioning is largely dependent on the amygdala (Phillips & LeDoux, 1992), trace conditioning requires a more distributed network of structures. Several studies have demonstrated a role for the dorsal (DH) and ventral (VH) hippocampus (Czerniawski, Yoon, & Otto, 2009; Esclassan, Coutureau, Di Scala, & Marchand, 2009b; McEchron, Bouwmeester, Tseng, Weiss, & Disterhoft, 1998; Quinn, Oommen, Morrison, & Fanselow, 2002; Yoon & Otto, 2007), and extra-hippocampal areas (Bang & Brown, 2009; Esclassan, Coutureau, Di Scala, & Marchand, 2009a; Kholodar-Smith, Boguszewski, & Brown, 2008) in the acquisition of trace fear conditioning. The amygdala has received surprisingly little attention in trace fear conditioning, but existing data support a role for this structure in trace fear acquisition (Kwapis, Jarome, Schiff, & Helmstetter, 2011; Selden, Everitt, Jarrard, & Robbins, 1991, but see Raybuck & Lattal, 2010). Recent data from our laboratory showed that disruption of protein synthesis in the amygdala immediately following trace fear conditioning impairs consolidation of CS and context memories (Kwapis et al., 2011). The medial prefrontal cortex (mPFC) has also been implicated in trace fear memory processes (Baeg et al., 2001; Blum, Hebert, & Dash, 2006; Gilmartin & McEchron, 2005b; Runyan, Moore, & Dash, 2004) and we have shown that the prelimbic (PL) area of the mPFC is necessary for trace, but not delay, fear conditioning (Gilmartin & Helmstetter, 2010). Temporary inactivation of PL with the GABAA agonist muscimol or temporary inhibition of prelimbic NMDA receptors with APV prior to training impaired the formation of CS and context memory for trace, but not delay, fear conditioning (Gilmartin & Helmstetter, 2010).
The dependence on mPFC and medial temporal lobe in trace fear conditioning suggests that memory formation may require communication between these two regions, but a functional interaction between mPFC and hippocampus or amygdala has yet to be demonstrated. Anatomical and physiological evidence support the possibility of functional communication between mPFC and VH or mPFC and amygdala. The PL mPFC receives direct projections from the ventral, but not dorsal, hippocampus (Cenquizca & Swanson, 2007; Hoover & Vertes, 2007; Jay, Glowinski, & Thierry, 1989), which are monosynaptic and glutamatergic (Jay et al., 1989). These synapses undergo NMDA receptor-dependent LTP (Jay, Burette, & Laroche, 1995; Laroche, Jay, & Thierry, 1990). The connections between the mPFC and amygdala are reciprocal (Hoover & Vertes, 2007; McDonald, Mascagni, & Guo, 1996; Vertes, 2004). Prelimbic LTP is supported in the amygdala to mPFC pathway, is dependent on NMDA receptors, and can be blocked by acute stress exposure (Maroun & Richter-Levin, 2003; Tan, Lauzon, Bishop, Bechard, & Laviolette, 2010). Using a disconnection approach, a functional interaction between the hippocampus and mPFC has been demonstrated in tasks that require integration of working memory processes in spatial learning (Churchwell, Morris, Musso, & Kesner, 2010; Floresco, Seamans, & Phillips, 1997). This approach uses asymmetrical lesions or inactivation to cut off communication between two structures. Because VH-mPFC connections are ipsilateral, unilateral inactivation of mPFC and unilateral inactivation of the contralateral VH effectively disrupts communication between these two regions. The same disconnection procedure has been used to show a functional interaction between the mPFC and amygdala in tasks that require behavioral flexibility in reward anticipation (Churchwell, Morris, Heurtelou, & Kesner, 2009) and in the effects of stress on memory formation (Maeng, Waddell, & Shors, 2010; Roozendaal, McReynolds, & McGaugh, 2004; Roozendaal et al., 2009). Using asymmetric microinjections of a cannabinoid CB1 receptor antagonist, Laviolette and colleagues showed that CB1 mediated transmission between the amygdala and mPFC is necessary for olfactory fear conditioning (Tan et al., 2010). In the present study, we used asymmetric temporary inactivation with muscimol of the PL mPFC and either the VH or amygdala to test whether a functional interaction between the mPFC and medial temporal lobe is necessary for trace fear conditioning. The PL mPFC may be important for helping to maintain the CS across the empty trace interval (Gilmartin & McEchron, 2005b), and we hypothesize that communication between the mPFC and medial temporal lobe is necessary for the association of the CS and UCS across this interval.
2. Materials and Methods
2.1 Subjects and surgery
The experiments were performed on adult male Long-Evans rats (325–400 g; Harlan, IN). All rats were housed individually and received food and water ad libitum. All procedures were in accordance with the National Institutes of Health guidelines and approved by the University of Wisconsin-Milwaukee Institutional Animal Care and Use Committee. Rats were anesthetized with isoflurane in 100% oxygen (induction, 4%; maintenance, 1–2%) and positioned in a stereotaxic frame. 4-mm diameter holes were drilled in the skull above the mPFC, VH, or amygdala. Stainless steel guide cannulae (26 ga; Plastics One, Inc., Roanoke, VA) were stereotaxically lowered to the target site: for PL mPFC, cannulae were implanted at a 15° angle to vertical, AP +3.2 mm; ML 1.6 mm; DV −3.2 mm from the skull; for VH, cannulae were implanted vertically (0°) at AP −5.6 mm; ML 5.5 mm; DV −7.0 mm from the skull; for amygdala, cannulae were implanted vertically (0°) at AP −2.8 mm; ML 5.0 mm; DV −7.3 mm from the skull. Acrylic cement was used to secure the cannulae to the skull and 33-ga dummy cannulae were screwed into the guide cannulae to prevent clogging.
2.2 Microinjection of drugs
Following recovery from surgery (4–7 days), rats received three days of acclimation to transport from their home cages to the procedure rooms. During this time, the rats were also acclimated to gentle restraint in a towel and to the sound of the infusion pump that would be used for intracerebral injections. No injections were delivered during these acclimation sessions. On the day of conditioning, rats received microinjections of the GABAA agonist muscimol (1 µg/µl; 5-aminomethyl-3-hydroxyisoxazole, MP Biomedicals, Solon, OH) or sterile saline vehicle 30 minutes prior to training. The injection was delivered at a rate of 0.5 µl/min through 33-ga injection cannulae, which extended 0.5 mm below the end of the guide cannulae. The injection volume was 0.3 µl/hemisphere for PL and 0.5 µl/hemisphere for VH and amygdala. For a group of trace conditioning rats in Experiment 4, the sodium-channel blocker lidocaine (4%; lidocaine hydrochloride monohydrate, Sigma-Aldrich, St. Louis, MO) was microinjected in the amygdala 5–10 minutes prior to training. Injectors were left in place for 90s following the completion of the injection to allow for diffusion of the drug or vehicle away from the cannulae. These injection volumes were selected based on previous work (Czerniawski et al., 2009; Esclassan et al., 2009b; Gilmartin & Helmstetter, 2010; Helmstetter & Bellgowan, 1994) and the estimated spread is ~0.5–1 mm (Allen et al., 2008; Martin, 1991). Thus, diffusion is limited to each region of interest. Diffusion of drug from the VH or amygdala into the perirhinal or entorhinal cortices is also unlikely as white matter may form a natural diffusion boundary (Allen et al., 2008). Rats were returned to their home cages immediately after the injection procedure. The time points for microinjection were based on reports that muscimol takes effect within minutes and neuronal activity recovers within 4 hours post-injection and lidocaine takes effect almost immediately and wears off within an hour (Martin, 1991; van Duuren et al., 2007). Training was complete within 1 hour of drug injection.
2.3 Conditioning
Conditioning occurred in a set of four Plexiglas and stainless steel conditioning chambers (internal dimensions: 21 × 28 × 21 cm), each housed in a sound attenuating outer chamber and illuminated with a white incandescent house lamp (7.5W). Rats were placed in the chambers and after a 6-min baseline period received 6 pairings of a 10-s white noise conditional stimulus (CS; 72dB) and a 1-s footshock unconditional stimulus (UCS; 1 mA). For trace fear conditioning, the CS offset and UCS onset were separated by an empty 20-s trace interval and the intertrial interval (ITI) for this session was 240 ± 20 s. For delay conditioning, the UCS was delivered at the offset of the CS and the ITI was 260 ± 20 s. All training sessions lasted 33 minutes. Ventilation fans in each outer chamber provided 63–65 dB background noise and the white noise CS was delivered through a speaker centered in one side wall of each conditioning chamber. Stainless steel bars (4 mm diameter, spaced 12 mm apart) on the floor of each chamber served to deliver the footshock UCS. Each chamber was cleaned with 5% ammonium hydroxide solution before training each rat.
During training, rats learned to associate both the auditory CS and the training context with the occurrence of the UCS. Twenty-four hours after training, rats were tested for memory of each association separately. Contextual fear memory was assessed by measuring conditional freezing during re-exposure to the original training chamber. Conditional freezing to the CS was tested in a novel chamber. Each session lasted 17 min and these tests were separated by 4 hr. Novel testing chambers used for the CS test (internal dimensions: 20.5 × 26.5 × 21 cm) were each housed in a sound attenuating outer chamber with 58–60 dB background noise. These chambers were in a separate room and differed from the training chambers in illumination (infrared house lamp), texture (solid, textured floor), and odor (5% acetic acid solution). The CS retention test was a variation of our standard 5-min CS test (Gilmartin & Helmstetter, 2010), modified to measure rats’ fear to both the CS and CS-offset. In trace conditioning, subjects exhibit conditional responses to CS-offset in addition to the CS itself (Buhusi & Meck, 2000). Tests using a long stimulus presentation allow for sufficient detection of conditional freezing. The modified test applies this same rationale to the CS-offset (i.e., long “trace interval” presentation). After a 6 min pre-CS baseline period, a single 10-sec CS (same duration as was used in the conditioning procedure) was delivered. This CS was followed by a long 2-min stimulus-free period (SFP) to assess freezing in response to CS-offset. After this 2-min period, we presented our standard 5-min CS to assess freezing in response to the CS. The single 10-sec CS is unlikely to cause extinction before the long CS is presented. Indeed, freezing during the 5-min CS in this hybrid test is comparable to freezing levels during the 5-min CS in our standard test, which does not have a brief CS presentation (see Gilmartin & Helmstetter, 2010). For comparison with trace conditioning, this same test procedure was delivered to delay conditioned rats in Experiment 4. While SFP freezing in trace rats reflects a conditional response to the offset itself, SFP freezing in delay rats is likely an extension of CS freezing in the absence of the expected UCS. This distinction is more evident after additional training (e.g., 10 trials), when the temporal relationship of the CS and UCS may be better learned: delay animals show persistent freezing during the CS, which decays after CS-offset, while trace animals show decaying freezing during the CS, but increased and persistent freezing after CS offset (Detert, Kampa, & Moyer, 2008). Importantly, in both cases, post-CS freezing tracks CS responding and not contextual fear (Detert et al., 2008). Thus, in our study, SFP freezing in both trace and delay animals can be considered a conditional response to the CS. In Experiment 1 trace rats were re-trained in the absence of drug. Re-training and testing procedures were identical to the original training and testing procedures for each rat.
2.4 Analyses
Freezing was defined as the cessation of all movement except that needed for respiration and was used as the measure of conditional fear during all training and testing sessions (Fanselow & Bolles, 1979). Freezing was scored automatically in real-time using the FreezeScan 1.0 detection software (Clever Sys, Inc.; Reston, VA). The scoring parameters used to detect freezing have been previously validated in our laboratory against hand-scoring methods (see Parsons, Gafford, & Helmstetter, 2010). All statistical analyses were performed with Statistica version 9 (Statsoft, Inc.; Tulsa, OK). Each training group was analyzed separately and drug differences in freezing were analyzed using one-way ANOVAs (context retention) or mixed model ANOVAs with repeated measures (acquisition; CS retention), which included the following factors: a repeated measure of Period (for acquisition: Baseline, Trials 1–3, Trials 1–4; for CS retention: Baseline, SFP, and CS) and a between factor of Group. Only the first 2 min of the CS were analyzed in order to temporally match CS-onset freezing and CS-offset freezing. Fisher LSD post-hoc tests were used to test the significance of mean differences. An α level of 0.05 was required for significance in all analyses.
2.5 Histology
At the end of the experiment, rats were deeply anesthetized with 5% isoflurane, transcardially perfused with 0.9% saline followed by 10% buffered formalin, and the brains were placed in a 10% formalin solution (in 0.9% saline). Brains were transferred to a 20% sucrose/formalin solution prior to processing for histology. Brains were then frozen, sectioned coronally, mounted on glass slides, and stained with Cresyl violet.
3. Results
3.1 Experiment 1: VH-PL disconnection
Both the ventral hippocampus (VH) and the prelimbic (PL) mPFC are necessary for the association of the CS and UCS in trace fear conditioning (Czerniawski et al., 2009; Esclassan et al., 2009b; Gilmartin & Helmstetter, 2010). This experiment used a disconnection design to test the importance of communication between these two structures in trace fear conditioning. On the day of training, each rat received a unilateral injection of muscimol into the PL and a unilateral injection of muscimol into the contralateral VH (MUS-CONTRA group) or ipsilateral VH (MUS-IPSI group) 30 minutes before trace fear conditioning. Projections from VH to PL are almost exclusively ipsilateral (Hoover & Vertes, 2007). Thus, simultaneous inactivation of one PL and the contralateral VH effectively disconnects the PL from the VH. Inactivation of each structure ipsilaterally leaves one VH-PL pathway intact, providing a control group that should acquire trace fear conditioning. Two additional groups of rats received saline vehicle injections in the same pattern as the muscimol groups (SAL-CONTRA group; SAL-IPSI group). Twenty-four hours after trace fear conditioning, all rats were tested for retention of fear to the CS and to the training context, and freezing was used as the measure of conditional fear.
3.1.1. Histology
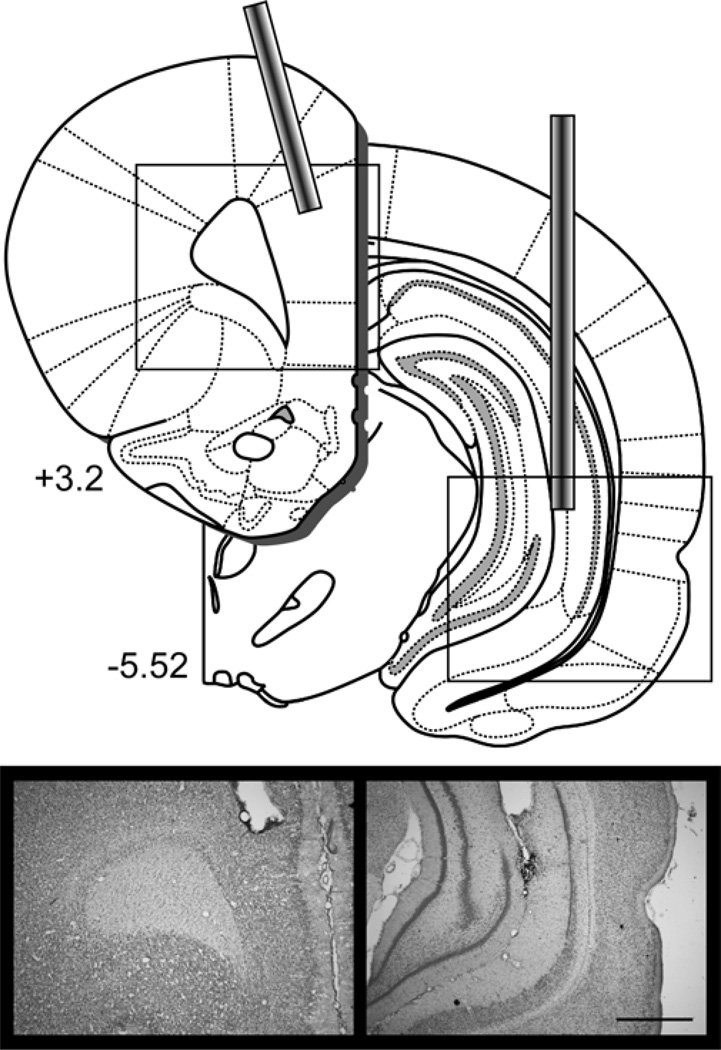
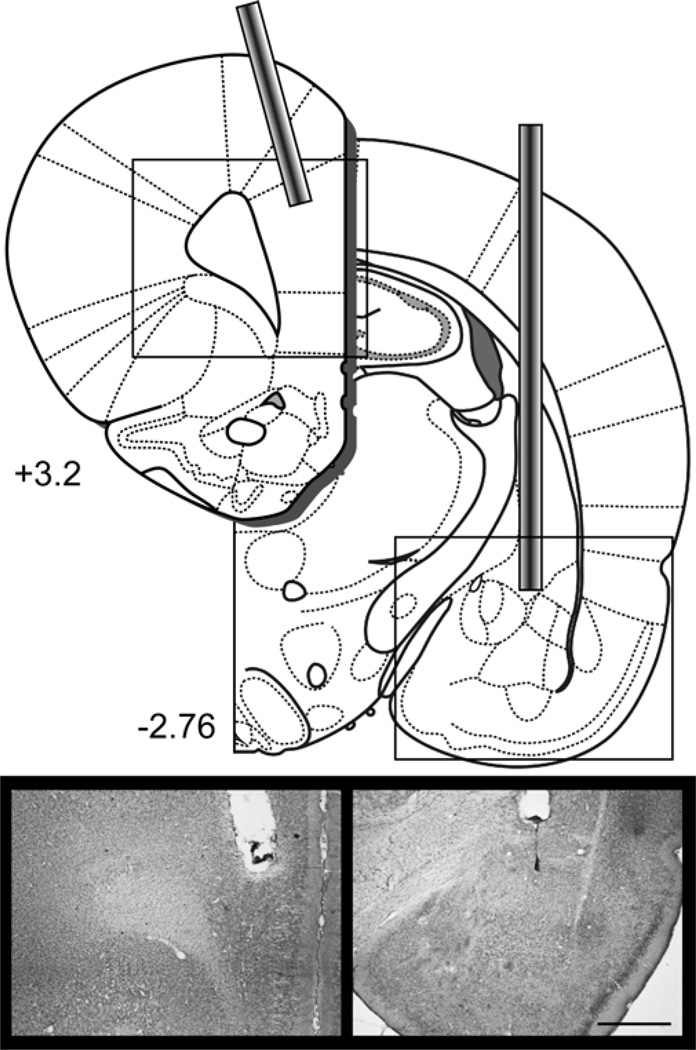
Behavioral analyses were conducted on the 22 rats with accurate cannula placement in the PL of the mPFC and VH out of 24 rats that underwent cannula implantation surgery. One rat (SAL-CONTRA) was excluded from analyses because of extensive tissue damage to the PL beyond the immediate area of the cannula site and one rat (MUS-IPSI) was excluded because the prefrontal cannula was misplaced in anterior medial orbital cortex. Table 1 shows the final group sizes included in analyses. Figure 1 shows a diagram depicting injector tip locations in the PL and contralateral VH and an example placement in each structure (see Figure S1 for individual placements).
Table 1.
Final number of rats per group for each experiment. VH (v) = ventral hippocampus; PL (p) = prelimbic mPFC; AMY (a) = amygdala; MUS = muscimol; SAL = saline; LIDO = lidocaine; IPSI = ipsilateral injections; CONTRA = contralateral injections; BI = bilateral injection; UNI = unilateral injection.
| Experiment 1 | |||
| VH-PL injection | IPSI | CONTRA | Collapsed |
| SAL(v)-SAL(p) | 6 | 5 | 11 |
| MUS(v)-MUS(p) | 5 | 6 | N/A |
| Experiment 2 | |||
| VH-PL injection | IPSI | CONTRA | Collapsed |
| SAL(v)-SAL(p) | 6 | 5 | 11 |
| SAL(v)-MUS(p) | 5 | 6 | 11 |
| MUS(v)-SAL(p) | 5 | 5 | 10 |
| MUS(v)-MUS(p) | 4 | 4 | 8 |
| Experiment 3 | |||
| AMY-PL injection | IPSI | CONTRA | Collapsed |
| SAL(a)-SAL(p) | 7 | 6 | 13 |
| MUS(a)-MUS(p) | 9 | 11 | N/A |
| Experiment 4 | |||
| AMY-AMY injection | TRACE | DELAY | Group |
| SAL(a)-SAL(a) | 5 | 6 | SAL |
| SAL(a)-MUS(a) | 6 | 5 | MUS-UNI |
| MUS(a)-MUS(a) | 6 | 5 | MUS-BI |
| Experiment 5 | |||
| Injection | AMY | VH | Group |
| SAL-SAL | 5 | 8 | SAL |
| SAL-LIDO | 6 | 7 | LIDO-UNI |
| LIDO-LIDO | 6 | 8 | LIDO-BI |
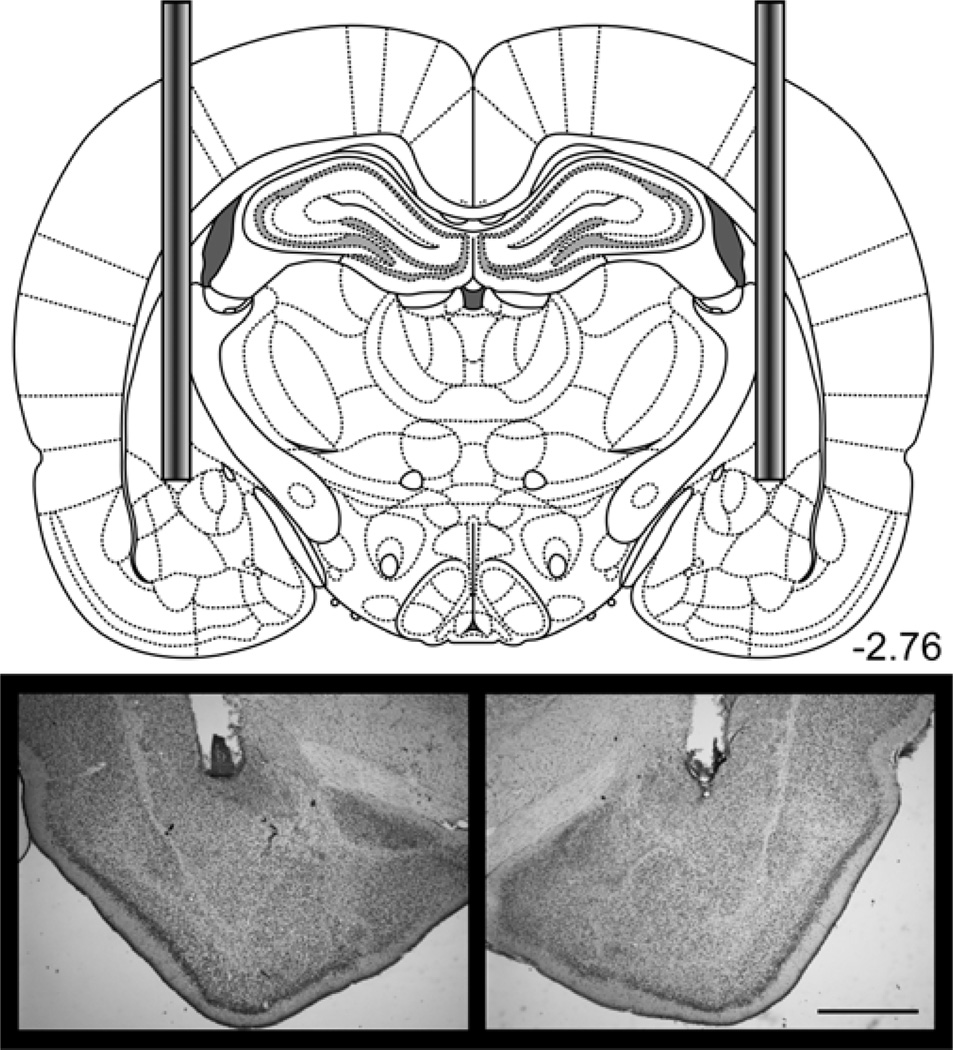
Figure 1.
Disconnection of the ventral hippocampus from the prelimbic mPFC in Experiments 1 and 2 required implanting cannula in one VH and one PL. The diagram shows the placement of cannulae in the PL and contralateral VH. Photomicrograph images show an example placement of the prefrontal cannula and the ventral hippocampal cannula. See Figure S1 and S2 for individual placements. Scale bar = 1 mm. Diagrams were adapted from Paxinos & Watson, 2007.
3.1.2. Behavior
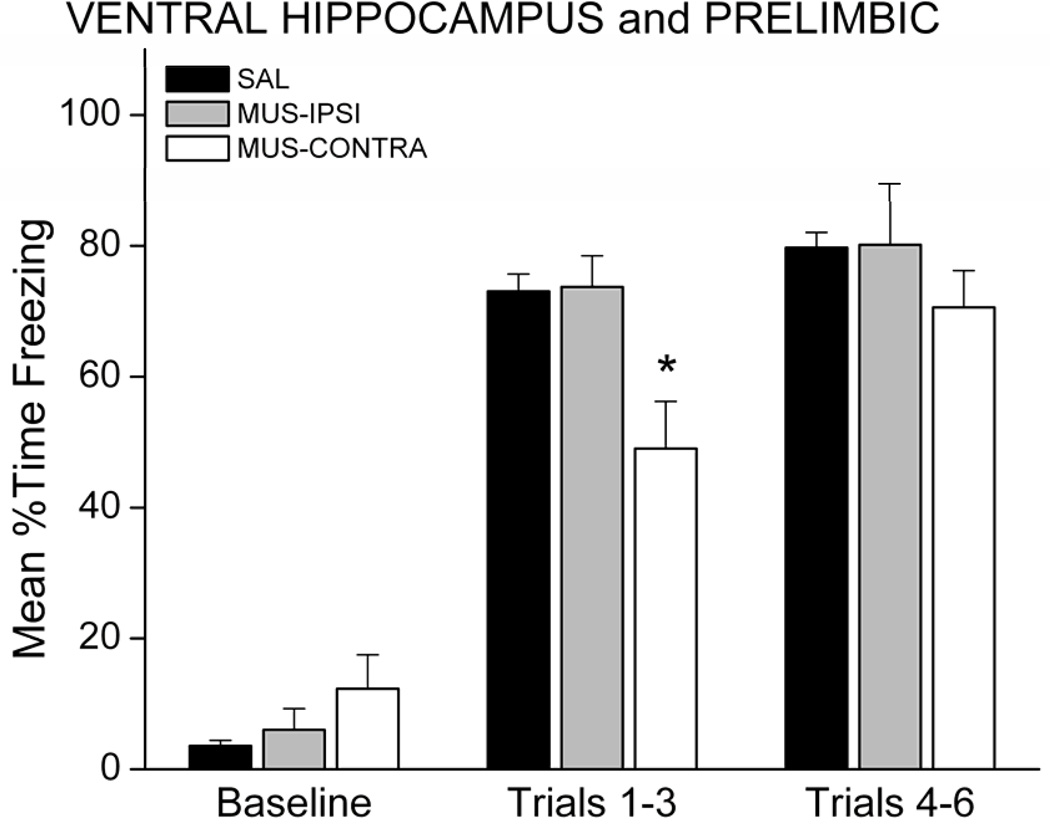
Freezing behavior during conditioning and retention testing did not differ between the contralateral and ipsilateral SAL groups (p>0.05), so rats in these conditions were collapsed into one SAL group (n=11). During the training session, MUS-IPSI rats acquired similar levels of freezing during the six CS-UCS pairings, compared with SAL rats. MUS-CONTRA rats showed a delayed acquisition curve, but reached similar levels of freezing by mid-session (Figure 2). This observation was confirmed by analysis of freezing during the conditioning session. A 3 × 3 mixed factor ANOVA revealed a Group X Period interaction, F(4,38)=4.75; p=0.0033. Follow-up post-hoc analysis showed that MUS-CONTRA rats froze less than SAL or MUS-IPSI rats during the first 3 trials (p<0.05), but showed similar freezing during the last 3 trials (p>0.05).
Figure 2.
Disconnection of the VH and PL slowed, but did not prevent freezing during the training session. Graph shows the mean percent time spent freezing during the baseline period and during the first and second halves of the conditioning session. * p<0.05 relative to SAL.
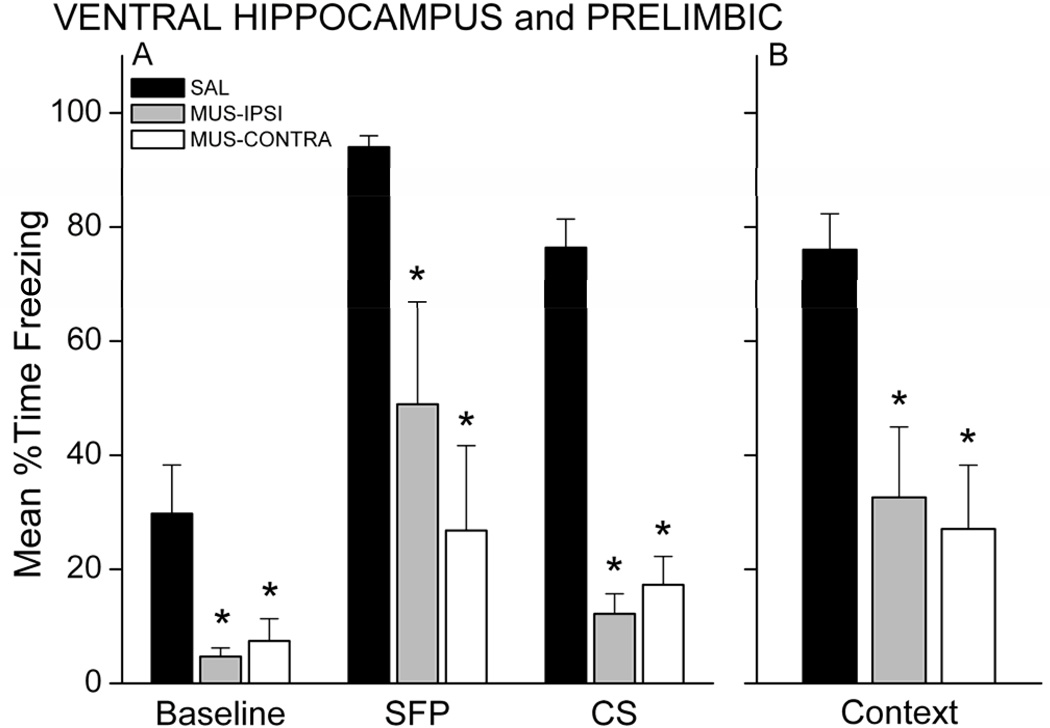
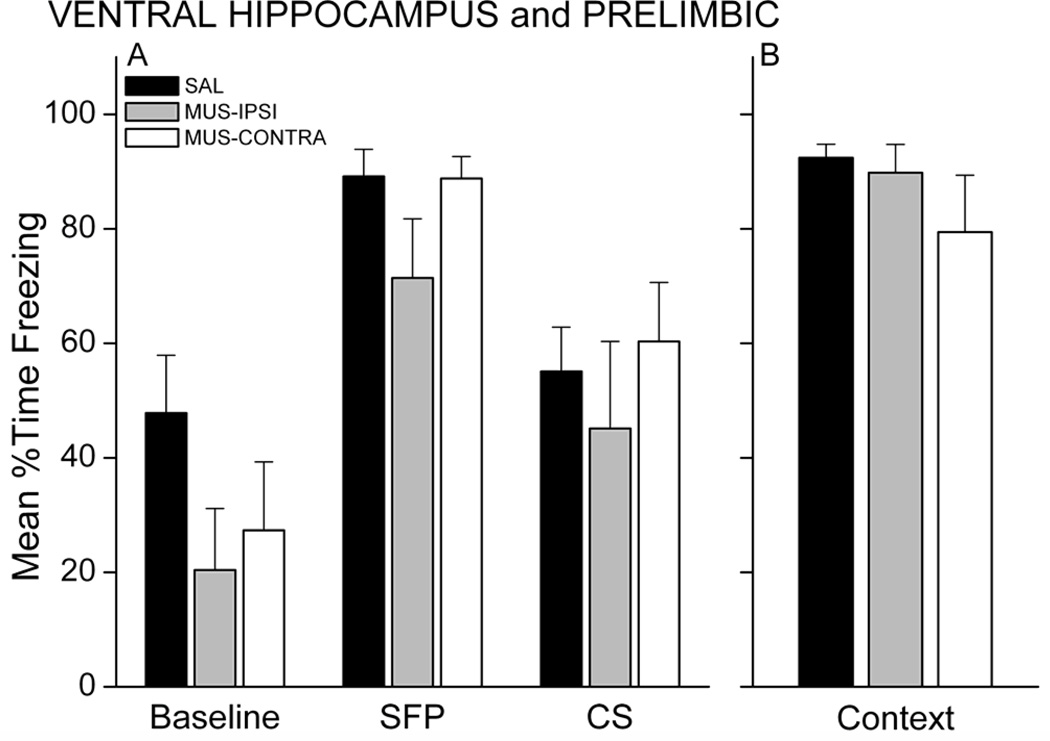
Twenty-four hours after training, rats were tested for retention of fear to the CS and context. The CS test included both a brief 10-sec CS and a long 5-min CS separated by a 2-min stimulus-free period (SFP) to assess freezing in response to CS-offset (SFP freezing) and the CS itself (CS freezing). MUS rats, regardless of injection sites, showed less freezing during the SFP and CS periods of the test, compared with SAL control rats (Figure 3A). A 3 × 3 mixed model ANOVA revealed a Group X Period interaction, F(4,38)=4.25, p=0.00607. MUS-IPSI and MUS-CONTRA rats froze significantly less than SAL rats during each period (p<0.05 for each period and group). SAL rats showed significantly more freezing during the SFP and CS relative to baseline (p<0.001), demonstrating intact conditional fear to the auditory CS. Neither MUS group showed increased freezing during the CS relative to baseline, but MUS-IPSI rats showed more freezing during the SFP relative to baseline (p<0.05). Muscimol injection also impaired contextual fear responses acquired during trace fear conditioning (Figure 3B; main effect of Group, F(2,19)=10.15, p=0.001). Follow-up analysis showed that MUS-IPSI and MUS-CONTRA rats froze significantly less than SAL rats (p<0.05 for each group). These results suggest that functional disconnection of the PL from VH prevents trace and contextual fear learning. One intact VH-PL pathway in the MUS-IPSI condition may support some learning; nonetheless, even with one VH-PL pathway intact, retention of the trace association was severely impaired in the MUS-IPSI rats.
Figure 3.
Simultaneous inactivation of unilateral PL and unilateral VH impairs trace and contextual fear memory. Graphs show the mean percent time each group spent freezing during the CS retention test (A) and context retention test (B). MUS rats showed significantly less conditional freezing during the 2-min stimulus-free period (SFP) following a brief 10s CS (not shown) and during the first 2 min of the long CS presentation (A). MUS rats also exhibited significantly impaired freezing during the 17-min context test (B). * p<0.05 relative to SAL.
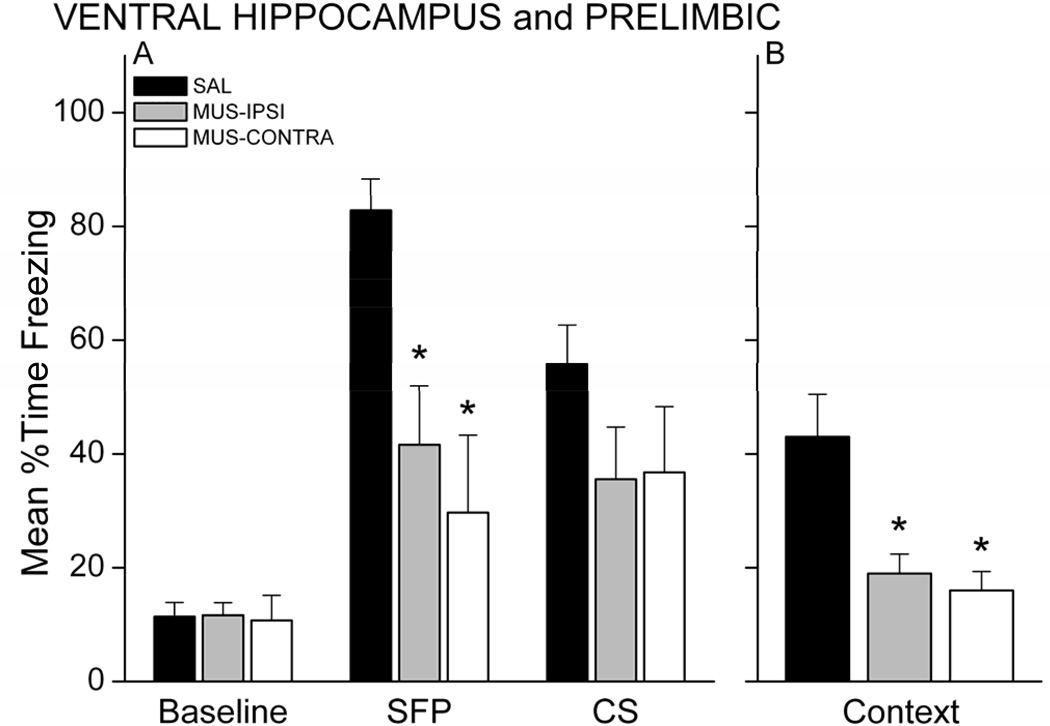
The persistence of memory deficits was examined with a test session 48 hours after the first retention test. The procedure for this second test was identical to that of the first. Figure 4 shows behavior during the CS and context retention tests for each group. As in the first test, MUS-IPSI and MUS-CONTRA rats exhibited impaired memory during the CS and context tests. A 3 × 3 mixed model ANOVA revealed a Group X Period interaction, F(4,38)=6.52, p=0.00042. MUS-IPSI and MUS-CONTRA rats froze significantly less than SAL rats during the SFP (respective p’s<0.05). CS freezing in the MUS groups was still reduced relative to SAL, but was not significantly less than CS freezing in the SAL group (p<0.08). Overall, freezing levels were lower in the SAL group relative to the initial test, which may reflect some extinction of conditional freezing to the CS after the first test 48 hours earlier. Unlike the first test, MUS-IPSI rats did not exhibit significantly greater freezing during the SFP relative to baseline. One intact VH-PL ipsilateral pathway may support some learning, but the memory is not robust across multiple testing sessions. Ipsilateral VH-PL communication severely impairs contextual fear memory at either test, suggesting that in general, one intact VH-PL pathway is not sufficient for rats to acquire trace and contextual fear conditioning. Thus, the association of the CS and UCS across a temporal gap may require bilateral communication between VH and PL. Alternatively, these results could be explained if unilateral disruption of VH or PL is sufficient to impair learning. Experiment 2 tested this possibility.
Figure 4.
Memory impairments persist at a second retention test administered 48 hrs after the first test. MUS rats showed significantly less conditional freezing during the SFP (A) and during the context (B). * p<0.05 relative to SAL.
The observed impairments in trace and contextual fear conditioning following pre-training muscimol were not a result of permanent damage to tissue in VH or PL. This was confirmed by retraining the rats in the absence of any drug. These rats were tested for CS and context retention 24 hours later in the same manner as the original tests (Figure 5). Analysis revealed a main effect of Period F(2,38)=32.93, p=0.00000, with significantly greater freezing during SFP and CS relative to baseline (p<0.05). There was no effect of drug history on freezing as neither the main effect of Group (F(2,19) = 1.50) nor the Group × Period (F(4,38)=0.99) interaction was significant. The same analysis on just the previously impaired groups (MUS-IPSI, MUS-CONTRA) revealed a significant main effect of Period, F(2,18)=26.52, p=0.00000, with significantly greater freezing during both the SFP and CS compared with baseline freezing (p<0.05). There were no differences in context freezing, F(2,19)=1.56, p>0.05. Together, these findings demonstrate that the effects of muscimol in VH and PL were indeed temporary and that previously impaired rats were able to acquire conditional fear responses to the CS and context when re-trained in the absence of drug.
Figure 5.
Muscimol treatment does not permanently damage the PL or VH. Rats were re-trained and retested as before. Rats previously injected with MUS show intact conditional freezing to the SFP, CS, and context. * p<0.05 relative to SAL.
3.2. Experiment 2: VH-PL disconnection with unilateral control injections
Experiment 1 tested the hypothesis that communication between VH and PL is necessary for the acquisition of trace fear conditioning. The results revealed that trace conditioning is sensitive to disruption of VH and PL, and one intact VH-PL pathway (MUS-IPSI) is not sufficient to support long-term memory of the CS-trace-UCS association. A possible explanation for this finding is that unilateral disruption of VH alone or PL alone is sufficient to impair trace fear conditioning. In Experiment 2, we used the disconnection design of Experiment 1 and included two additional control groups to determine whether unilateral inactivation of VH or PL alone is sufficient to impair trace and contextual fear conditioning. On the day of training, each rat received a unilateral infusion of muscimol or saline into the VH and a unilateral infusion of muscimol or saline into the contralateral or ipsilateral PL 30 minutes before trace fear conditioning. Twenty-four hours after trace fear conditioning, all rats were tested for CS and context fear retention.
3.2.1. Histology
Behavioral analyses were conducted on the 40 rats with accurate cannula placement in the PL and VH out of 48 rats that underwent surgery. Eight rats were excluded because of cannula misplacement (n=3), incorrect infusion volume (n=2), or extensive tissue damage to the PL or VH beyond the immediate area of the cannula site (n=3). Table 1 shows the number of rats in each infusion condition that were included in analyses. See Figure S2 for individual placements of cannulae in the PL and VH.
3.2.2. Behavior
Rats for this experiment were trained and tested in two equal sized sets. Each of the three control groups (VH infusion listed first, PL, second: SAL-SAL; SAL-MUS; MUS-SAL) was collapsed across cannula placement configuration (e.g. ipsilateral and contralateral). Experiment 1 showed that MUS-IPSI and MUS-CONTRA rats showed similarly low levels of freezing during the retention tests and did not differ from each other. To confirm that the same held for this experiment, freezing during the CS test was analyzed for the MUS-MUS group independently of the other groups using a mixed model ANOVA with within-subjects Period (Baseline, SFP, CS) and between-subjects Group (MUS-IPSI, MUS-CONTRA) and Replication (Rep1, Rep2) factors. Neither Group nor Replication contributed significantly to any interaction. An ANOVA on freezing during the context with factors Group and Replication also revealed no differences. Therefore, the pattern of infusion (IPSI vs. CONTRA) did not differentially affect freezing levels in these rats and MUS-MUS IPSI and CONTRA rats were collapsed into one MUS-MUS group. All analyses were thus conducted on the four infusion groups collapsed across cannula configuration (see Table 1).
During the training session, all rats showed increased freezing during the paired trials relative to baseline. Only rats injected with MUS in both the VH and PL exhibited lower levels of freezing during the six CS-UCS pairings, compared with control groups, similar to Experiment 1 (data not shown). A 4 × 3 mixed factor ANOVA revealed a significant Group X Period interaction, F(6,72)=5.31; p=0.00014). Follow-up analysis showed that MUS-MUS rats exhibited less freezing during the paired trials, compared with each control group (p<0.05). This result, together with data from Experiment 1, suggests that simultaneous inactivation of VH and PL unilaterally can impair acquisition of trace fear conditioning. Inactivation of one VH or PL by itself did not impair freezing during the acquisition session.
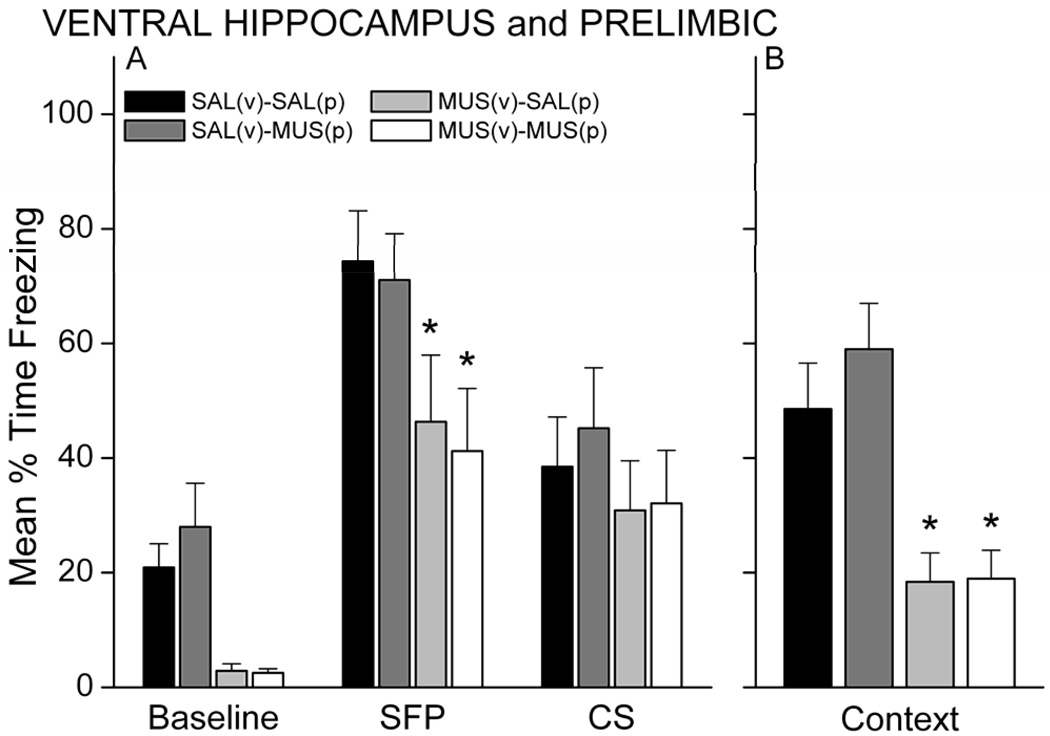
Analysis of freezing during the CS and context retention tests the next day revealed that unilateral inactivation of the VH, but not PL, did in fact impair memory. Rats injected with muscimol in the VH, regardless of injection into the PL, showed less freezing during all periods of the CS test (Figure 6A) and during the context test (Figure 6B) relative to rats injected with SAL or with MUS in the PL and SAL in the VH. A main effect of Group was found for both the CS test (F(3,36)=2.92, p=0.047) and context test (F(3,36)=8.63, p=0.00019). For the CS test, MUS-MUS and MUS-SAL rats (VH-MUS groups) froze significantly less than SAL-MUS (p<0.05, each group). These VH-MUS groups also showed a trend for decreased freezing relative to the SAL-SAL group (p<0.07). For the context test, MUS-SAL and MUS-MUS rats froze significantly less than both the SAL-MUS and SAL-SAL rats (p<0.05, respectively). No significant interaction between Group and Period was revealed for the CS test. A one-way ANOVA applied to each period of the CS test revealed an effect of Group on Baseline freezing (F(3,36)=7.06, p=0.00075) and SFP freezing (F(3,36)=2.88, p=0.049), but not CS freezing (p>0.05). MUS-SAL and MUS-MUS rats froze significantly less during the SFP period compared with SAL-SAL rats (p<0.05, each). In contrast to Experiment 1, SAL rats in Experiment 2 exhibited low (<50%) freezing during the long-CS presentation. Whether this reflects stronger conditioning to the CS-offset rather than to the CS itself in this set of rats is unclear. Despite this reduced freezing in the control rats, the pattern of impairments in this experiment is similar to that in Experiment 1. Impaired freezing during the SFP was still evident during a second CS retention test 2 days later (data not shown). At this test, all groups showed low (<15%) freezing during the baseline and there were no differences in baseline freezing during the second test (p>0.05). Analysis of freezing during the SFP revealed a main effect of Group, F(3,36)=3.04, p=0.041. Rats infused with muscimol in the VH, regardless of PL infusion, showed significantly less freezing during the SFP compared with SAL-MUS control rats (p<0.05). These data suggest that differences in CS-offset induced freezing are not simply a reflection of increased generalized fear to a novel context in control rats. The results of Experiments 1 and 2 suggest that temporary inactivation of VH unilaterally is sufficient to impair trace and contextual fear memory.
Figure 6.
Unilateral inactivation of VH but not PL impairs trace and contextual memory. Rats with MUS in the VH, regardless of whether MUS or SAL was inected into the PL, showed significantly less conditional freezing during the CS retention test (A) and context retention test (B) compared with saline control rats. In contrast, MUS in PL and SAL in VH had no effect on learning. * p<0.05 relative to SAL-SAL and MUS-SAL.
3.3. Experiment 3: Amygdala-PL disconnection
The amygdala is also important for forming a trace fear memory (Kwapis et al., 2011; Selden et al., 1991) but it is unclear whether communication between the PL and the amygdala is necessary for learning. This experiment used a disconnection design identical to Experiment 1, except that the PL was disconnected from the amygdala instead of the VH. On the day of training, each rat received a unilateral injection of muscimol into the PL and a unilateral injection of muscimol into the contralateral amygdala (MUS-CONTRA) or ipsilateral amygdala (MUS-IPSI) 30 minutes before trace fear conditioning. Two additional groups of rats received saline injections in the same pattern as the muscimol groups (SAL-CONTRA; SAL-IPSI). Twenty-four hours after trace fear conditioning, all rats were tested for CS and context fear retention.
3.3.1. Histology
Thirty-one out of 40 cannulated rats had accurate cannula placement in the PL mPFC and amygdala. Seven rats were excluded because the amygdala cannula was misplaced dorsally in the caudate. Two rats in the SAL control group had accurate placement in the amygdala, but inaccurate placement of the prefrontal cannula. The behavior of these rats did not differ from other saline-injected rats and they were included in all analyses. Exclusion of these 2 rats from the analyses did not affect the results. Table 1 shows the final group sizes included in analyses. Figure 7 shows an example placement of cannulae in the PL and contralateral amygdala (see Figure S3 for individual placements).
Figure 7.
Disconnection of the amygdala from the prelimbic mPFC in Experiments 1 and 2 required implanting cannula in one amygdala and one PL. The diagram shows the placement of cannulae in the PL and contralateral amygdala. Photomicrograph images show an example placement of the prefrontal cannula and the amygdala cannula. See Figure S3 for individual placements. Scale bar = 1 mm. Diagrams were adapted from Paxinos & Watson, 2007.
3.3.2. Behavior
Freezing behavior during conditioning and retention testing did not differ between the contralateral and ipsilateral SAL groups (p>0.05), so rats in these conditions were collapsed into one SAL group (n=13). During the training session, MUS-IPSI and MUS-CONTRA rats acquired similar levels of freezing during the six CS-UCS pairings, compared with SAL rats (data not shown). Prior to CS-UCS pairings, MUS rats exhibited less exploratory behavior during the baseline period compared with SAL rats. A one-way ANOVA on activity during the 6-min baseline period revealed a main effect of Group (F(2,30)=11.41; p=0.00021). Follow-up post-hoc analysis showed that MUS rats exhibited less movement than SAL rats during the baseline (p<0.05). A general reduction in locomotion and grooming behavior after muscimol injection in the amygdala has been reported elsewhere (e.g., Mahler & Berridge, 2009) and may reflect reduced alertness via central amygdala connections with cholinergic brainstem structures (Gallagher & Holland, 1994). Nonetheless, all rats showed similar freezing during the CS-UCS pairings (p>0.05). This suggests that muscimol injected into the amygdala may reduce exploration of the training context, but does not affect the expression of freezing during training.
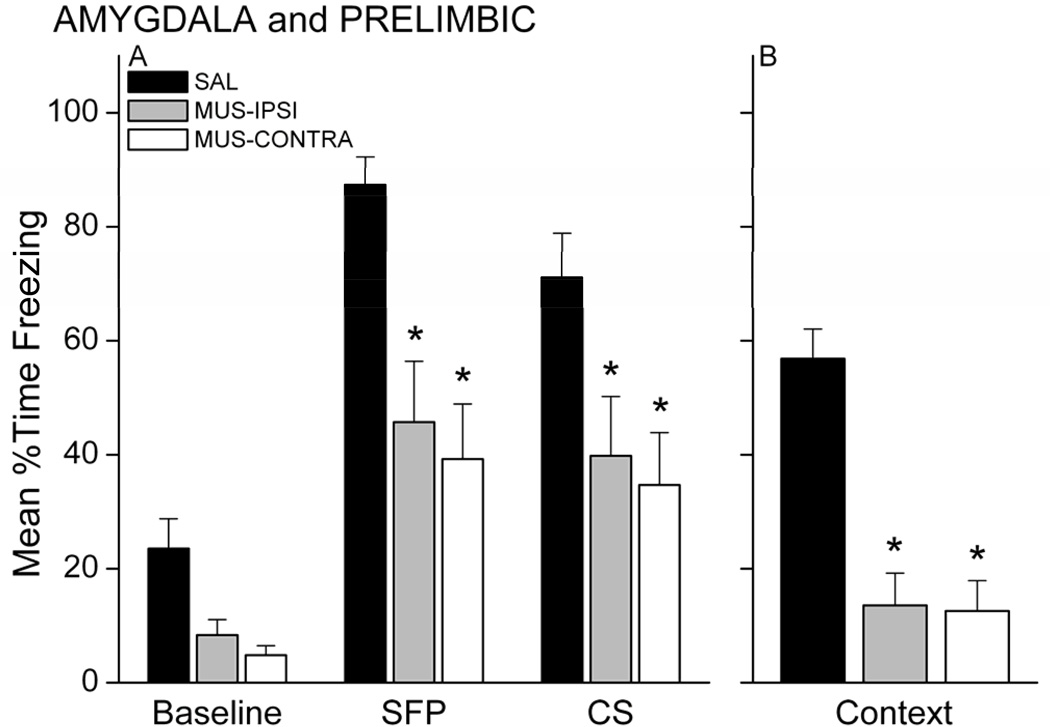
Twenty-four hours after training, rats were tested for retention of conditional fear to the CS in a novel chamber and for fear of the context in the original training chamber. Figure 8A shows the freezing data during the CS retention test. MUS rats, regardless of injection sites, showed less freezing during the SFP and CS periods of the test compared with SAL rats. A 3 × 3 mixed model ANOVA revealed a main effect of Group, F(2,30)=10.87, p=0.00028 and Period, F(2,60)=56.41, p=0.00000. Follow-up post-hoc analysis on the group effect showed that both MUS-IPSI and MUS-CONTRA groups exhibited significantly less freezing compared with the SAL control group (p<0.05, each). The ANOVA also revealed a near significant Group X Period interaction, F(4,60)=2.42, p=0.05835. Follow-up analysis of this interaction showed that MUS-IPSI and MUS-CONTRA rats froze significantly less than SAL rats during the SFP and CS periods of the test (p<0.05 for each period and group). Each group of rats showed significantly more freezing during the SFP and CS relative to its baseline (p<0.05), suggesting that rats with one PL and one amygdala intact are able to acquire some fear to the auditory CS. Nonetheless, retention of the trace association was severely impaired in the MUS-injected rats. Muscimol injection into the amygdala also impaired contextual fear responses. Figure 8B shows the average freezing during the context test for each group. Trace conditioned rats that had received muscimol injections, regardless of ipsilateral or contralateral cannula configuration, exhibited less freezing than saline-infused controls. Analysis of this freezing data revealed a significant effect of Group, F(2,30)=24.88, p<0.000001. MUS-IPSI and MUS-CONTRA rats froze significantly less than SAL rats (p<0.05 for each group). Together, these findings suggest that simultaneous inactivation of one PL and one amygdala impairs trace and contextual fear conditioning. Like Experiment 1, this could suggest that communication between these two structures in both hemispheres is necessary for trace conditioning or that unilateral inactivation of either structure is sufficient to impair learning. Unilateral pre-training lesions of the amygdala do not significantly impair conditional fear to the CS and context in delay fear conditioning (Goosens & Maren, 2001), but it is possible that trace conditioning is more sensitive to amygdala disruption than delay conditioning. Experiment 4 compared unilateral and bilateral inactivation of the amygdala in both trace and delay conditioning.
Figure 8.
Simultaneous inactivation of the PL mPFC and amygdala impairs trace fear conditioning. Both amygdala-PL disconnected rats and rats with one ipsilateral amygdala-PL pathway intact showed significantly less conditional freezing during the CS retention test (A) and context retention test (B) compared with saline control rats. * p<0.05 relative to SAL.
3.4. Experiment 4: Unilateral inactivation of amygdala in fear memory formation
This experiment compared unilateral and bilateral inactivation of the amygdala in trace fear conditioning. For comparison, we also included a delay conditioning group. Previous work has shown that bilateral lesions or temporary inactivation of the amygdala significantly impair standard delay fear conditioning, while unilateral lesions produce an intermediate or mild impairment (Goosens & Maren, 2001; LaBar & LeDoux, 1996; LeDoux, Cicchetti, Xagoraris, & Romanski, 1990; J. Muller, Corodimas, Fridel, & LeDoux, 1997). Bilateral lesions of the amygdala impair trace fear conditioning (Selden et al., 1991), but the effect of unilateral inactivation of the amygdala on trace fear conditioning has not been examined.
3.4.1 Histology
Thirty-three out of 36 rats had accurate cannula placement in the amygdala. The tips of the infusion cannulae were positioned at the border of the central and lateral subregions of the amygdala, both of which are necessary for delay fear conditioning (Goosens & Maren, 2001; Wilensky, Schafe, Kristensen, & LeDoux, 2006). This area also receives dense projections from the PL mPFC (McDonald et al., 1996; Vertes, 2004). Two rats were excluded from analysis because of cannula misplacement, and one rat was excluded because of extensive tissue damage to the amygdala beyond the immediate area of the cannula site. Figure 9 shows an example placement of bilateral amygdala cannulae in Experiment 4 and 5 (see below; see Figure S4 for individual placements).
Figure 9.
Bilateral cannulae were implanted in the amygdala for Experiments 4 and 5. The diagram shows the placement of cannulae in the amygdala. Photomicrograph images show an example placement of the bilateral cannulae. See Figure S4 for individual placements. Scale bar = 1 mm. Diagrams were adapted from Paxinos & Watson, 2007.
3.4.2. Behavior
Rats for this experiment were trained and tested in two equal sized sets; one set received trace conditioning, the other, delay conditioning. In each set, rats were infused with muscimol into the amygdala of one hemisphere and muscimol (MUS-BI) or saline (MUS-UNI) into the contralateral amygdala 30 minutes before training. A separate group of rats in each set was infused with saline in both hemispheres (SAL). Similar to Experiment 3, MUS-injected rats exhibited reduced exploration during the baseline period (p<0.05), but acquired similar levels of freezing during the six CS-UCS pairings, compared with SAL rats (p>0.05; data not shown).
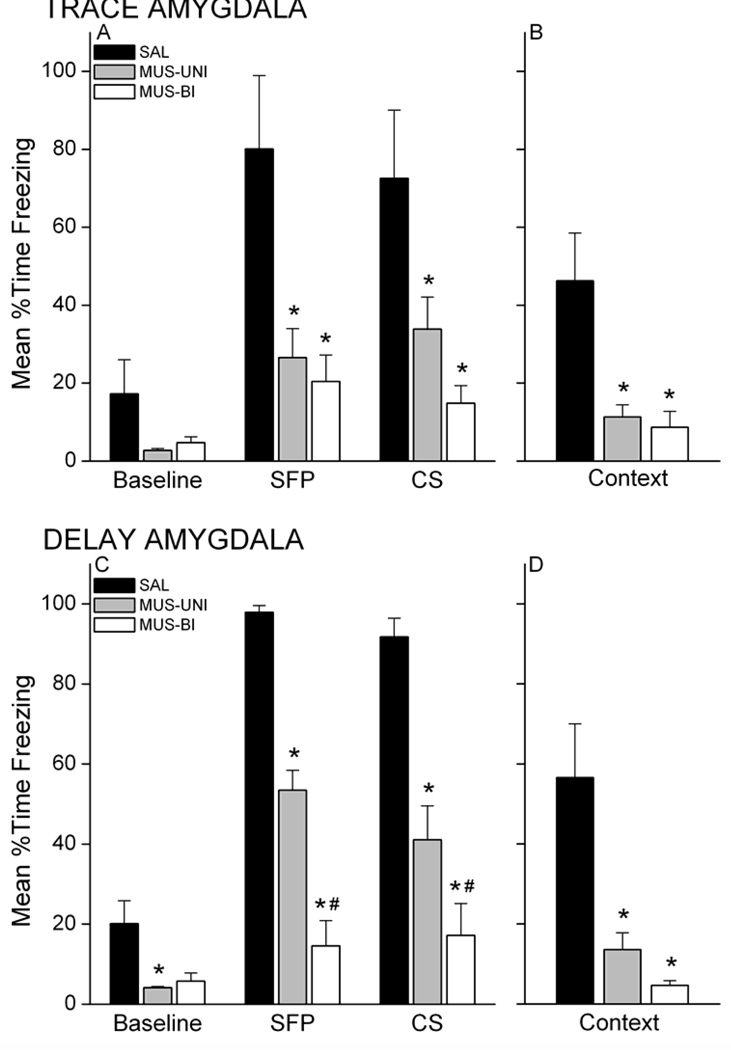
Rats were tested for retention of conditional fear to the CS and context 24 hours after training. Rats injected with muscimol in the amygdala unilaterally or bilaterally showed less freezing at test compared with saline rats for both training groups (Figure 10). An analysis of freezing during the CS retention test for trace conditioned rats revealed a Group X Period interaction, F(4,26)=5.38, p=0.0027. This analysis omitted one rat in the SAL group that exhibited extremely high (>75%) freezing during the 6-min baseline period (>3 s.d. from the mean of the remaining rats). Follow-up post-hoc analyses showed that MUS-UNI and MUS-BI rats exhibited significantly less freezing during the SFP and CS periods compared with SAL rats (p<0.05). Furthermore, only the SAL group exhibited significantly greater freezing during the SFP and CS periods relative to its own baseline freezing (p<0.05). These data demonstrate that unilateral or bilateral inactivation of the amygdala prevents memory formation during trace conditioning. Inactivation of the amygdala also impaired conditional fear to the training context acquired simultaneously with the auditory CS during trace conditioning (Figure 10B). A one-way ANOVA on context freezing data in the trace group revealed a main effect of Group, F(2,14)=8.57, p=0.0037. Post-hoc analysis showed that MUS-UNI and MUS-BI rats froze significantly less than SAL rats (p<0.05, each).
Figure 10.
Unilateral and bilateral inactivation of the amygdala with muscimol impairs both trace and delay conditional freezing. Trace conditioned rats injected with muscimol prior to training exhibit impaired conditional freezing during the CS retention test (A) and during the context retention test (B) compared with saline controls. Similarly, muscimol-injected delay conditioned rats show impaired conditional freezing to the CS (C) and context (D). Unilateral inactivation of the amygdala produces an intermediate deficit in memory formation for delay conditioned rats. * p<0.05 relative to SAL; # p<0.05 relative to MUS-UNI.
Muscimol injection into the amygdala also impaired delay fear conditioning, consistent with published findings. Analysis of freezing during the CS retention test for the delay conditioned rats (Figure 10C) revealed a Group X Period interaction, F(4,26)=17.01, p<0.000001. Follow-up analysis showed that MUS-UNI and MUS-BI rats exhibited significantly less freezing during the SFP and CS periods compared with SAL rats (p<0.05). MUS-BI rats froze significantly less than MUS-UNI rats during both the SFP and CS periods (p<0.05). In addition, SAL and MUS-UNI rats exhibited significantly greater freezing during the SFP and CS periods relative to its own baseline freezing (p<0.05), but MUS-BI rats showed no difference from baseline during these periods. These findings show that while bilateral inactivation of the amygdala prevents conditional responses to the CS, unilateral inactivation produces an intermediate impairment. Delay rats with one intact amygdala are able to exhibit some learning. One intact amygdala, however, does not support contextual fear learning. Both unilateral and bilateral injection of muscimol produce a similar impairment of contextual fear memory (Figure 10D). A one-way ANOVA applied to the delay group revealed a main effect of Group, F(2,13)=9.73, p=0.0026. Follow-up analysis showed that both MUS-UNI and MUS-BI rats froze less than SAL rats. Together, these results suggest that trace and contextual fear conditioning are equally sensitive to unilateral and bilateral amygdala disruption, while delay conditioning is only partially impaired by unilateral amygdala inactivation.
3.5 Experiment 5: Inactivation of VH or amygdala with lidocaine
Temporary inactivation of neuronal firing using muscimol is achieved through activation of GABAA receptors by the drug, which increases the local inhibitory tone in the target structure. We next sought to determine if impaired trace and contextual fear conditioning is specific to GABAergic mechanisms or is more general to disrupted activity. We used another method of temporary inactivation of the VH or amygdala: blocking sodium channels with lidocaine. This experiment tests the effects of bilateral or unilateral lidocaine injected into the VH or amygdala on trace fear conditioning.
3.5.1 Histology
The contribution of each structure to trace conditioning was examined independently. For the amygdala set, all 17 rats had accurate cannula placement in the amygdala (see Figure 9 for a typical placement). For the VH set, 23 of 24 rats had accurate cannula placement in VH. One rat in the unilateral lidocaine group was excluded for misplacement of a cannula. Figure 11 shows an example bilateral placement in the VH (see Figure S5 for individual placements).
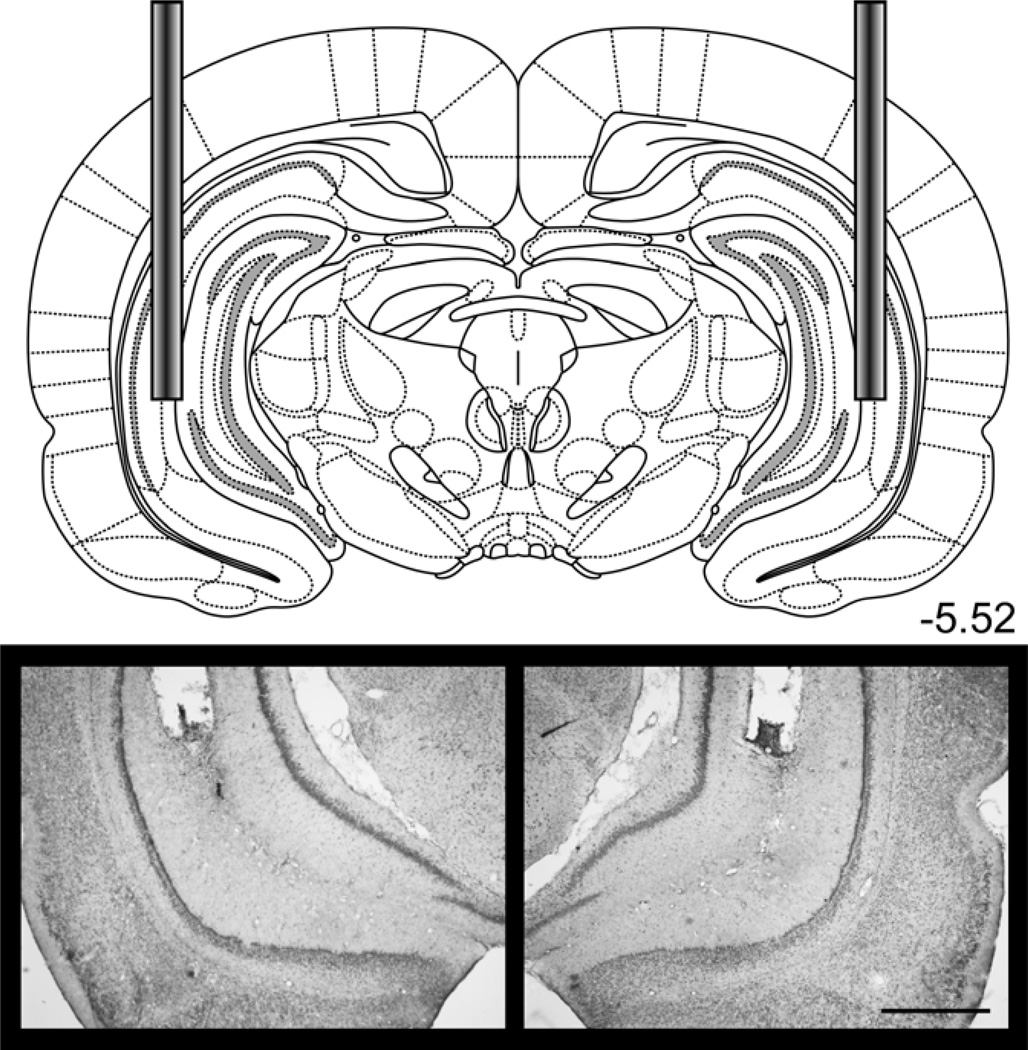
Figure 11.
Bilateral cannulae were implanted in the VH for Experiment 5. The diagram shows the placement of cannulae in the VH. Photomicrograph images show an example placement of the bilateral cannulae. See Figure S5 for individual placements. Scale bar = 1 mm. Diagrams were adapted from Paxinos & Watson, 2007.
3.5.2. Behavior
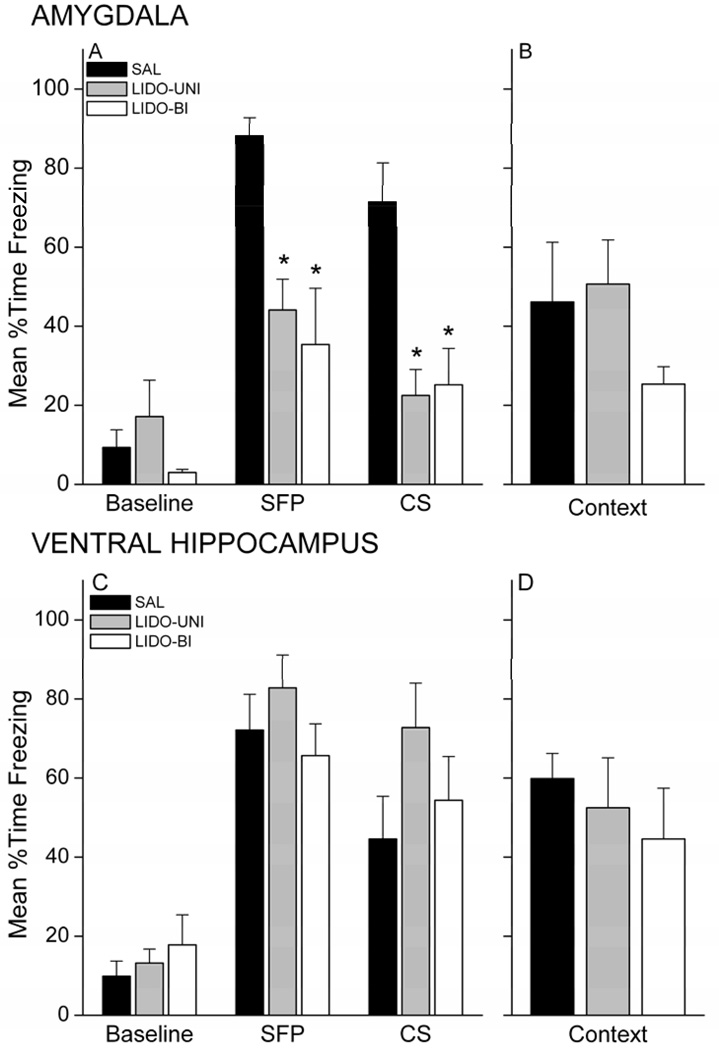
All amygdala rats exhibited comparable levels of freezing by the end of the training session, regardless of infusion (data not shown). A 3 × 3 mixed factor ANOVA on freezing in amygdala rats revealed a nearly significant effect of Group X Period, F(4,28)=2.71; p=0.0502. Freezing during the CS-UCS pairings was significantly greater than baseline levels for each group (p<0.05), but LIDO-injected rats showed less freezing during the first 3 pairings compared with SAL (p<0.05). All rats showed similar freezing during the last 3 pairings. This suggests that lidocaine injection into the amygdala, either bilaterally or unilaterally, slows the acquisition of freezing during training. The same analysis applied to the VH rats revealed a main effect of Period, F(2,40)=265.54; p=0.0000, but no effect of Group, F<2.0. Freezing during the CS-UCS pairings was significantly greater than baseline levels for each group (p<0.05).
Lidocaine injection into the amygdala, but not into the VH, prior to trace conditioning impaired retention of the CS memory 24 hours later. Figure 12 shows the freezing levels during each retention test for amygdala rats (A,B) and VH rats (C,D). Rats infused with lidocaine in the amygdala, unilaterally or bilaterally, showed less freezing during the SFP and CS of the CS retention test compared with saline rats (Figure 12A). Analysis of freezing revealed an Group X Period interaction, F(4,28)=4.34, p=0.00743. Follow-up analyses showed that LIDO-UNI and LIDO-BI rats froze significantly less than SAL rats during the SFP and CS periods (p<0.05). In contrast, lidocaine in the VH did not impair memory for the CS (Figure 12C). Analysis of freezing revealed a main effect for Period, F(2,40)=81.58, p<0.000001, but no effect of Group, F<1.0. Follow-up LSD post-hoc analyses showed that rats froze significantly more during the SFP and CS periods relative to baseline (p<0.05).
Figure 12.
Unilateral and bilateral inactivation with lidocaine of the amygdala, but not the ventral hippocampus, impairs trace conditional freezing. Trace conditioned rats injected with lidocaine into the amygdala prior to training exhibit impaired conditional freezing during the CS retention test (A). Context freezing (B) was diminished in bilaterally injected rats, but did not reach significance. In contrast, lidocaine infused into the ventral hippocampus did not impair conditional freezing during either the CS (C) or context (D) tests. * p<0.05 relative to SAL.
Lidocaine injection did not impair contextual fear when injected into either the amygdala or the VH (Figure 12B,D). LIDO-Bi rats in general showed somewhat less freezing during the context re-exposure, but this difference was not statistically reliable for VH or amygdala, Fs<2.0. At best, amygdala LIDO-Bi rats showed a trend towards impaired freezing during the first 8 minutes of the context retention test (p=0.0959). This finding with lidocaine corroborates previous work from our lab: bilateral inactivation of the amygdala with lidocaine prior to a single contextual fear conditioning session failed to significantly impair conditional freezing to the context at test (Helmstetter, 1992). Taken together, our findings suggest that the degree of inactivation of ventral hippocampal neurons produced by lidocaine infusion is insufficient to impair trace and contextual fear memory, while the same delivery to the amygdala impairs trace, but not contextual fear conditioning. In contrast, both trace and contextual fear conditioning are sensitive to GABAergic disruption of activity unilaterally in VH and amygdala.
4. Discussion
Trace fear conditioning differs from standard delay fear conditioning by the presence of a stimulus-free trace interval separating the CS and UCS. The additional demands of trace conditioning render it dependent on additional brain circuitry: the hippocampus, prelimbic area of the mPFC, amygdala, and rhinal cortices (Esclassan et al., 2009a, 2009b; Gilmartin & Helmstetter, 2010; Guimarais, Gregorio, Cruz, Guyon, & Moita, 2012; Kholodar-Smith et al., 2008; Kwapis et al., 2011; McEchron et al., 1998). Each structure in this distributed network likely cooperates to support the association of the CS and UCS across the trace interval (Navaroli, Zhao, Boguszewski, & Brown, 2011), but at this stage we know little about how these structures interact during the acquisition process. This study was designed to determine whether the mPFC functionally interacts with the VH or amygdala. Although we were unable to address this hypothesis with a disconnection approach, the present study provides important new information about the contribution of the mPFC, VH, and amygdala to trace fear conditioning. While previous work has demonstrated that bilateral inactivation or lesion of the mPFC or VH impairs trace conditioning, we provide evidence that trace fear conditioning is sensitive to unilateral disruption of the VH, but not the PL. In addition, we show that trace fear conditioning requires bilateral activity within the amygdala. This not only provides further support for a role for the amygdala in this paradigm but also provides new information about the relative sensitivity of trace and delay conditioning to unilateral disruption of the amygdala. Together, these findings demonstrate that the association of the CS and UCS across a temporal gap requires the bilateral participation of temporal lobe structures in the distributed network supporting trace fear conditioning.
The hippocampus has received extensive attention for its role in trace fear conditioning. While the precise role of this structure is still unknown, we have a better understanding about hippocampal molecular signaling and electrophysiology supporting trace fear conditioning (Czerniawski, Ree, Chia, & Otto, 2011; Czerniawski, Ree, Chia, Ramamoorthi et al., 2011; Gao et al., 2010; Gilmartin & McEchron, 2005a; Quinn, Loya, Ma, & Fanselow, 2005; Runyan & Dash, 2005). Much of this work has focused on the dorsal hippocampus (DH), but in recent years, there has been a growing appreciation for a functional segregation of the hippocampus along its septo-temporal axis (Fanselow & Dong, 2010), with the DH having a greater role in spatial and contextual learning and the VH having a greater role in fear and anxiety processes, although clear overlap exists (Bast, Zhang, & Feldon, 2001; Czerniawski et al., 2009). The VH has recently been shown to be as important as the DH for the acquisition and expression of trace fear conditioning (Czerniawski, Ree, Chia, & Otto, 2011; Czerniawski, Ree, Chia, Ramamoorthi et al., 2011; Czerniawski et al., 2009; Esclassan et al., 2009b; Rogers, Hunsaker, & Kesner, 2006; Yoon & Otto, 2007). Given that the VH, but not the DH, is directly connected to the mPFC and amygdala, it is necessary to gain a better understanding of the contribution of this structure and its interaction with cortical and subcortical structures to the formation of memory. The studies that have investigated the role of the VH in trace conditioning have used bilateral excitotoxic lesions or bilateral infusion of muscimol into hippocampal subregions prior to training or testing. Our study, using the same dose and volume of muscimol as Czerniawski et al. (2009), corroborates their finding that the VH is necessary for trace fear conditioning, but further demonstrates that inactivation of VH in just one hemisphere is sufficient to disrupt learning. This suggests that the acquisition of trace and contextual fear conditioning requires hippocampal activity in both hemispheres. Unilateral manipulations are not routinely included in lesion and inactivation studies; yet when they are, unilateral manipulations often produce an intermediate impairment or none at all. An intermediate impairment suggests that optimal learning requires both hemispheres, but one intact structure may compensate for damage in the opposite hemisphere. In our study the contralateral VH could not compensate for unilateral disruption. This requirement for bilateral activity could reflect an active communication within the VH between hemispheres to acquire trace conditioning. Alternatively, it could reflect a multi-stage involvement of one then the other VH across the course of training. The challenge to understanding the mechanisms supporting learning in the VH will be to determine which of these possibilities is at work. Furthermore, given that the VH also has a role in the acquisition and expression of delay fear conditioning (Bast et al., 2001; Esclassan et al., 2009b; Hunsaker & Kesner, 2008; Maren & Holt, 2004; Sierra-Mercado, Padilla-Coreano, & Quirk, 2010), it would be of interest to determine whether trace, delay, and contextual fear learning depend on similar or distinct mechanisms in the VH.
An important finding from this study is that inactivation of the amygdala, unilateral or bilateral, severely impairs trace fear conditioning. Unlike the hippocampus, the amygdala has received far less attention for its role in trace fear conditioning. With the exception of one study (Selden et al., 1991), no direct investigations of the role of the amygdala in trace fear conditioning have been conducted until very recently (Guimarais et al., 2012; Kwapis et al., 2011; Raybuck & Lattal, 2010), with conflicting results. The Selden et al. study found that quninolinic acid or 6-OHDA lesions of the amygdala in rats impaired conditioned lick suppression after trace fear conditioning (Selden et al., 1991). Our lab has recently shown that blocking protein synthesis in the amygdala after trace fear conditioning in rats disrupts the consolidation of trace fear memory (Kwapis et al., 2011). In contrast, Raybuck and Lattal (2010) did not find an impairment in conditional freezing to the CS following pre-training muscimol injections in mice. However, a very recent study by Moita and colleagues supports our findings and shows that bilateral muscimol injections into the amygdala of rats impair one-trial trace fear conditioning (Guimarais et al., 2012). Evidence for a role for the amygdala in trace conditioning continues to grow and we sought to determine whether this structure interacts with the mPFC during the acquisition of trace fear conditioning. In parallel to the results of our VH-PL disconnection experiment, simultaneous inactivation of the PL and amygdala impaired learning, regardless of whether the injections were ipsilateral or contralateral to each other. Experiment 2 had already demonstrated that unilateral inactivation of the PL does not impair learning, so we followed up the amygdala-PL experiment by directly comparing unilateral with bilateral inactivation of the amygdala on trace fear conditioning. For comparison with published work, we included a delay conditioning group. We found that bilateral inactivation of the amygdala prevented learning in delay conditioning, consistent with the literature (J. Muller et al., 1997). Unilateral inactivation produced an intermediate impairment, also in keeping with previous reports (Baker & Kim, 2004; Goosens & Maren, 2001; LaBar & LeDoux, 1996). For trace conditioning, we found that both unilateral and bilateral inactivation of the amygdala severely impaired learning. Furthermore, we found impaired memory for trace conditioning after inactivating the amygdala with either muscimol or lidocaine. Thus, our findings demonstrate that trace fear conditioning does indeed depend on the amygdala, but unlike delay conditioning, one intact amygdala cannot compensate for unilateral disruption. Interestingly, for both trace and delay conditioning, unilateral inactivation of the amygdala with muscimol impaired conditional responses to the context to a similar degree as bilateral inactivation. The hippocampus has been proposed to be critical for the association of discontiguous stimuli, as in trace conditioning or contextual learning (Wallenstein, Eichenbaum, & Hasselmo, 1998). It is possible that such associations with an emotional component as in fear conditioning place a greater demand on the hippocampus and amygdala alike.
Our study demonstrates that both the amygdala and VH are necessary for trace and contextual fear conditioning, but the specific mechanisms may differ. GABAergic signaling in VH is necessary for trace fear conditioning, but reducing neuronal firing with the sodium channel blocker lidocaine in VH did not impair learning. In contrast, trace fear conditioning is sensitive to both methods of inactivation in the amygdala. There are a number of possible explanations for these results. For one, the amygdala, but not the VH, may be sensitive to weak suppression of firing. Activation of GABAA receptors with muscimol is a robust method of inactivating a large population of neurons in a target structure (van Duuren et al., 2007). Extracellular unit recording in combination with reverse microdialysis delivery of muscimol in the rat frontal cortex showed that muscimol produces a reduction of firing rate around 95% in over 80% of the neuronal population (van Duuren et al., 2007). In contrast, the sodium channel blocker lidocaine produces this level of firing rate reduction in only 30% of the population (van Duuren et al., 2007). Lidocaine produces a much weaker suppression of firing and the effects wear off within an hour, while the effects of muscimol start to subside around 3 hours (Boehnke & Rasmusson, 2001; van Duuren et al., 2007). In our study, the first trial was delivered approximately 10–15 min after lidocaine infusion and the session lasted 30 minutes. Clearly, the duration of action and degree of firing suppression was sufficient to impair learning in the amygdala, but the VH may require a more extensive inactivation, as is obtained using muscimol. An alternative explanation is that GABAergic signaling specifically is important in the VH for trace conditioning and in both the VH and amygdala for contextual fear conditioning. Previous work from our lab has shown that muscimol, but not lidocaine, injections into the amygdala impair contextual fear conditioning (Helmstetter, 1992; Helmstetter & Bellgowan, 1994), similar to this study. The majority of GABAergic neurons are local inhibitory neurons, but long-range GABAergic projection neurons have been identified in several systems, including in the hippocampus. Currently identified targets of hippocampal GABAergic projections include cortical areas such as the subiculum and retrosplenial cortex, as well as subcortical areas including the medial septum (Jinno & Kosaka, 2009). Furthermore, the dentate gyrus sends GABAergic projections to the contralateral dentate gyrus (Jinno et al., 2007; Ribak et al., 1986). More recently, GABAergic projections cells from the CA1 hippocampus to the amygdala have been identified (M. Muller et al., 2012). Such projections are thought to rapidly coordinate activity in distant regions and may be a means by which the hippocampus interacts with the mPFC and amygdala during the acquisition of trace and contextual fear conditioning. If GABAergic projection cells are important for trace or contextual fear conditioning, unilateral injection of muscimol would affect signaling in the contralateral VH as well as the amygdala. Our pattern of results is consistent with this possibility, but additional work will need to be done to identify the mechanisms underlying these results.
An interesting finding from this study is that unlike the VH and amygdala, unilateral inactivation of the PL does not impair trace or contextual fear conditioning. We have previously shown that bilateral disruption of PL with muscimol or an NMDA receptor antagonist does impair both trace and simultaneously acquired contextual fear conditioning (Gilmartin & Helmstetter, 2010). Our present data suggests that trace fear conditioning is less sensitive to unilateral disruption of the mPFC relative to the hippocampus and amygdala. If the role of the PL is to bridge the gap and provide a neural representation of the CS to the hippocampus, parahippocampal regions, or amygdala, it is possible that either hemisphere could complete this task. The amygdala and hippocampus may serve to bind this CS representation with the UCS into a complete episodic memory, a process that may require interhemispheric communication. Further work is needed to test this speculation.
In conclusion, the neural circuit underlying memory formation in trace fear conditioning may be more integrated than previously thought, requiring bilateral activity in some temporal lobe structures. We initially sought to determine whether the mPFC functionally interacts with the VH or amygdala using a disconnection procedure. However, our finding that unilateral inactivation of the VH or amygdala impairs memory suggests that disconnection procedures with lesions or temporary inactivation are not suitable for testing questions about functional interaction in trace and contextual fear conditioning. With the development of projection-specific optogenetic inactivation methods, the contribution of PL-projecting hippocampal neurons to trace fear conditioning can be directly tested, including a distinction of GABAergic vs. glutamatergic projections. Furthermore, selective inhibition of the amygdala to PL pathway separately from the PL to amygdala may provide critical insight into how CS information is processed in the trace fear circuit and whether the amygdala serves a similar role in trace and delay fear conditioning.
Highlights.
Trace fear conditioning requires bilateral participation of the ventral hippocampus
The amygdala is necessary for both trace and delay fear learning
Trace conditioning is more sensitive than delay to unilateral amygdala disruption
Supplementary Material
Disconnection of the ventral hippocampus from the prelimbic mPFC in Experiment 1 required implanting cannula in one VH and one PL. Each symbol depicts the placement of a cannula in the PL or VH for each group (SAL = black; MUS-IPSI = grey; MUS-CONTRA = white). Only one hemisphere of each structure is shown but both ipsilateral and contralateral placements are included. Diagrams were adapted from Paxinos & Watson, 2007.
Disconnection of the ventral hippocampus from the prelimbic mPFC in Experiment 2 included unilateral control animals. Cannulae placements are depicted for each group: SAL(v)-SAL(p) = black; SAL(v)-MUS(p) = dark grey; MUS(v)-SAL(p) = light grey; MUS(v)-MUS(p) = white. Diagrams were adapted from Paxinos & Watson, 2007.
Disconnection of the amygdala from the prelimbic mPFC in Experiments 3 required implanting cannula in one amygdala and one PL. Each symbol depicts the placement of a cannula in the PL or amygdala (SAL = black; MUS-IPSI = grey; MUS-CONTRA = white). Only one hemisphere of each structure is shown but both ipsilateral and contralateral placements are included. Diagrams were adapted from Paxinos & Watson, 2007.
Bilateral cannulae were implanted in the amygdala for Experiments 4 and 5. Each symbol depicts the placement of a cannula in the amygdala for each group (SAL = black; DRUG-UNI = grey; DRUG-BI = white. For Experiment 4 (muscimol), the trace conditioning group is represented by circles and the delay group by squares. For experiment 5 (lidocaine), placements are represented by triangles. Diagrams were adapted from Paxinos & Watson, 2007.
Bilateral cannulae were implanted in the VH for Experiment 5. Each symbol depicts the placement of a cannula in the VH (SAL = black; LIDO-UNI = grey; LIDO-BI = white). Diagrams were adapted from Paxinos & Watson, 2007.
Acknowledgments
This research was supported by the National Institute of Mental Health (NIMH) grants R01MH069558 and R03MH090426 to Fred J. Helmstetter and NIMH grant F32MH083422 to Marieke R. Gilmartin. We would like to thank Mary Lonergan for assistance with the drug microinjections.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen TA, Narayanan NS, Kholodar-Smith DB, Zhao Y, Laubach M, Brown TH. Imaging the spread of reversible brain inactivations using fluorescent muscimol. J Neurosci Methods. 2008;171(1):30–38. doi: 10.1016/j.jneumeth.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cereb Cortex. 2001;11(5):441–451. doi: 10.1093/cercor/11.5.441. [DOI] [PubMed] [Google Scholar]
- Baker KB, Kim JJ. Amygdalar lateralization in fear conditioning: evidence for greater involvement of the right amygdala. Behav Neurosci. 2004;118(1):15–23. doi: 10.1037/0735-7044.118.1.15. [DOI] [PubMed] [Google Scholar]
- Bang SJ, Brown TH. Muscarinic receptors in perirhinal cortex control trace conditioning. J Neurosci. 2009;29(14):4346–4350. doi: 10.1523/JNEUROSCI.0069-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. The ventral hippocampus and fear conditioning in rats. Different anterograde amnesias of fear after tetrodotoxin inactivation and infusion of the GABA(A) agonist muscimol. Exp Brain Res. 2001;139(1):39–52. doi: 10.1007/s002210100746. [DOI] [PubMed] [Google Scholar]
- Blum S, Hebert AE, Dash PK. A role for the prefrontal cortex in recall of recent and remote memories. Neuroreport. 2006;17(3):341–344. doi: 10.1097/01.wnr.0000201509.53750.bc. [DOI] [PubMed] [Google Scholar]
- Boehnke SE, Rasmusson DD. Time course and effective spread of lidocaine and tetrodotoxin delivered via microdialysis: an electrophysiological study in cerebral cortex. J Neurosci Methods. 2001;105(2):133–141. doi: 10.1016/s0165-0270(00)00348-4. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Timing for the absence of a stimulus: the gap paradigm reversed. J Exp Psychol Anim Behav Process. 2000;26(3):305–322. doi: 10.1037//0097-7403.26.3.305. [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev. 2007;56(1):1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Heurtelou NM, Kesner RP. Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behav Neurosci. 2009;123(6):1185–1196. doi: 10.1037/a0017734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Musso ND, Kesner RP. Prefrontal and hippocampal contributions to encoding and retrieval of spatial memory. Neurobiol Learn Mem. 2010;93(3):415–421. doi: 10.1016/j.nlm.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Czerniawski J, Ree F, Chia C, Otto T. Dorsal versus ventral hippocampal contributions to trace and contextual conditioning: Differential effects of regionally selective nmda receptor antagonism on acquisition and expression. Hippocampus. 2011 doi: 10.1002/hipo.20992. [DOI] [PubMed] [Google Scholar]
- Czerniawski J, Ree F, Chia C, Ramamoorthi K, Kumata Y, Otto TA. The importance of having Arc: expression of the immediate-early gene Arc is required for hippocampus-dependent fear conditioning and blocked by NMDA receptor antagonism. J Neurosci. 2011;31(31):11200–11207. doi: 10.1523/JNEUROSCI.2211-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniawski J, Yoon T, Otto T. Dissociating space and trace in dorsal and ventral hippocampus. Hippocampus. 2009;19(1):20–32. doi: 10.1002/hipo.20469. [DOI] [PubMed] [Google Scholar]
- Detert JA, Kampa ND, Moyer JR., Jr Differential effects of training intertrial interval on acquisition of trace and long-delay fear conditioning in rats. Behav Neurosci. 2008;122(6):1318–1327. doi: 10.1037/a0013512. [DOI] [PubMed] [Google Scholar]
- Esclassan F, Coutureau E, Di Scala G, Marchand AR. A cholinergic-dependent role for the entorhinal cortex in trace fear conditioning. J Neurosci. 2009a;29(25):8087–8093. doi: 10.1523/JNEUROSCI.0543-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esclassan F, Coutureau E, Di Scala G, Marchand AR. Differential contribution of dorsal and ventral hippocampus to trace and delay fear conditioning. Hippocampus. 2009b;19(1):33–44. doi: 10.1002/hipo.20473. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Bolles RC. Naloxone and shock-elicited freezing in the rat. J Comp Physiol Psychol. 1979;93(4):736–744. doi: 10.1037/h0077609. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17(5):1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Holland PC. The amygdala complex: multiple roles in associative learning and attention. Proc Natl Acad Sci U S A. 1994;91(25):11771–11776. doi: 10.1073/pnas.91.25.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Gill MB, Tronson NC, Guedea AL, Guzman YF, Huh KH, et al. Hippocampal NMDA receptor subunits differentially regulate fear memory formation and neuronal signal propagation. Hippocampus. 2010;20(9):1072–1082. doi: 10.1002/hipo.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Helmstetter FJ. Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learn Mem. 2010;17(6):289–296. doi: 10.1101/lm.1597410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the dentate gyrus and CA1 of the hippocampus exhibit inverse patterns of encoding during trace fear conditioning. Behav Neurosci. 2005a;119(1):164–179. doi: 10.1037/0735-7044.119.1.164. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behav Neurosci. 2005b;119(6):1496–1510. doi: 10.1037/0735-7044.119.6.1496. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem. 2001;8(3):148–155. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarais M, Gregorio A, Cruz A, Guyon N, Moita MA. Time determines the neural circuit underlying associative fear learning. Front Behav Neurosci. 2012;5:89. doi: 10.3389/fnbeh.2011.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ. Contribution of the amygdala to learning and performance of conditional fear. Physiol Behav. 1992;51(6):1271–1276. doi: 10.1016/0031-9384(92)90320-2. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Bellgowan PS. Effects of muscimol applied to the basolateral amygdala on acquisition and expression of contextual fear conditioning in rats. Behav Neurosci. 1994;108(5):1005–1009. doi: 10.1037//0735-7044.108.5.1005. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212(2):149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. Dissociations across the dorsal-ventral axis of CA3 and CA1 for encoding and retrieval of contextual and auditory-cued fear. Neurobiol Learn Mem. 2008;89(1):61–69. doi: 10.1016/j.nlm.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM, Burette F, Laroche S. NMDA receptor-dependent long-term potentiation in the hippocampal afferent fibre system to the prefrontal cortex in the rat. Eur J Neurosci. 1995;7(2):247–250. doi: 10.1111/j.1460-9568.1995.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Jay TM, Glowinski J, Thierry AM. Selectivity of the hippocampal projection to the prelimbic area of the prefrontal cortex in the rat. Brain Res. 1989;505(2):337–340. doi: 10.1016/0006-8993(89)91464-9. [DOI] [PubMed] [Google Scholar]
- Jinno S, Klausberger T, Marton LF, Dalezios Y, Roberts JD, Fuentealba P, et al. Neuronal diversity in GABAergic long-range projections from the hippocampus. J Neurosci. 2007;27(33):8790–8804. doi: 10.1523/JNEUROSCI.1847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno S, Kosaka T. Neuronal circuit-dependent alterations in expression of two isoforms of glutamic acid decarboxylase in the hippocampus following electroconvulsive shock: A stereology-based study. Hippocampus. 2009;19(11):1130–1141. doi: 10.1002/hipo.20576. [DOI] [PubMed] [Google Scholar]
- Kholodar-Smith DB, Boguszewski P, Brown TH. Auditory trace fear conditioning requires perirhinal cortex. Neurobiol Learn Mem. 2008;90(3):537–543. doi: 10.1016/j.nlm.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Schiff JC, Helmstetter FJ. Memory consolidation in both trace and delay fear conditioning is disrupted by intra-amygdala infusion of the protein synthesis inhibitor anisomycin. Learn Mem. 2011;18(11):728–732. doi: 10.1101/lm.023945.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE. Partial disruption of fear conditioning in rats with unilateral amygdala damage: correspondence with unilateral temporal lobectomy in humans. Behav Neurosci. 1996;110(5):991–997. doi: 10.1037//0735-7044.110.5.991. [DOI] [PubMed] [Google Scholar]
- Laroche S, Jay TM, Thierry AM. Long-term potentiation in the prefrontal cortex following stimulation of the hippocampal CA1/subicular region. Neurosci Lett. 1990;114(2):184–190. doi: 10.1016/0304-3940(90)90069-l. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10(4):1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng LY, Waddell J, Shors TJ. The prefrontal cortex communicates with the amygdala to impair learning after acute stress in females but not in males. J Neurosci. 2010;30(48):16188–16196. doi: 10.1523/JNEUROSCI.2265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Berridge KC. Which cue to "want?" Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci. 2009;29(20):6500–6513. doi: 10.1523/JNEUROSCI.3875-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Holt WG. Hippocampus and Pavlovian fear conditioning in rats: muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behav Neurosci. 2004;118(1):97–110. doi: 10.1037/0735-7044.118.1.97. [DOI] [PubMed] [Google Scholar]
- Maroun M, Richter-Levin G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. J Neurosci. 2003;23(11):4406–4409. doi: 10.1523/JNEUROSCI.23-11-04406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JH. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett. 1991;127(2):160–164. doi: 10.1016/0304-3940(91)90784-q. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71(1):55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8(6):638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111(4):683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- Muller M, Faber-Zuschratter H, Yanagawa Y, Stork O, Schwegler H, Linke R. Synaptology of ventral CA1 and subiculum projections to the basomedial nucleus of the amygdala in the mouse: relation to GABAergic interneurons. Brain Struct Funct. 2012;217(1):5–17. doi: 10.1007/s00429-011-0326-9. [DOI] [PubMed] [Google Scholar]
- Navaroli VL, Zhao Y, Boguszewski P, Brown TH. Muscarinic receptor activation enables persistent firing in pyramidal neurons from superficial layers of dorsal perirhinal cortex. Hippocampus. 2011 doi: 10.1002/hipo.20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Helmstetter FJ. Regulation of extinction-related plasticity by opioid receptors in the ventrolateral periaqueductal gray matter. Front Behav Neurosci. 2010;4 doi: 10.3389/fnbeh.2010.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106(2):274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Loya F, Ma QD, Fanselow MS. Dorsal hippocampus NMDA receptors differentially mediate trace and contextual fear conditioning. Hippocampus. 2005;15(5):665–674. doi: 10.1002/hipo.20088. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Oommen SS, Morrison GE, Fanselow MS. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus. 2002;12(4):495–504. doi: 10.1002/hipo.10029. [DOI] [PubMed] [Google Scholar]
- Raybuck JD, Lattal KM. Double dissociation of amygdala and hippocampal contributions to trace and delay fear conditioning. PLoS One. 2010;6(1):e15982. doi: 10.1371/journal.pone.0015982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak CE, Seress L, Peterson GM, Seroogy KB, Fallon JH, Schmued LC. A GABAergic inhibitory component within the hippocampal commissural pathway. J Neurosci. 1986;6(12):3492–3498. doi: 10.1523/JNEUROSCI.06-12-03492.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, Hunsaker MR, Kesner RP. Effects of ventral and dorsal CA1 subregional lesions on trace fear conditioning. Neurobiol Learn Mem. 2006;86(1):72–81. doi: 10.1016/j.nlm.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, McGaugh JL. The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. J Neurosci. 2004;24(6):1385–1392. doi: 10.1523/JNEUROSCI.4664-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, Van der Zee EA, Lee S, McGaugh JL, McIntyre CK. Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala. J Neurosci. 2009;29(45):14299–14308. doi: 10.1523/JNEUROSCI.3626-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyan JD, Dash PK. Inhibition of hippocampal protein synthesis following recall disrupts expression of episodic-like memory in trace conditioning. Hippocampus. 2005;15(3):333–339. doi: 10.1002/hipo.20055. [DOI] [PubMed] [Google Scholar]
- Runyan JD, Moore AN, Dash PK. A role for prefrontal cortex in memory storage for trace fear conditioning. J Neurosci. 2004;24(6):1288–1295. doi: 10.1523/JNEUROSCI.4880-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden NR, Everitt BJ, Jarrard LE, Robbins TW. Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1991;42(2):335–350. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2010;36(2):529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Lauzon NM, Bishop SF, Bechard MA, Laviolette SR. Integrated cannabinoid CB1 receptor transmission within the amygdala-prefrontal cortical pathway modulates neuronal plasticity and emotional memory encoding. Cereb Cortex. 2010;20(6):1486–1496. doi: 10.1093/cercor/bhp210. [DOI] [PubMed] [Google Scholar]
- van Duuren E, van der Plasse G, van der Blom R, Joosten RN, Mulder AB, Pennartz CM, et al. Pharmacological manipulation of neuronal ensemble activity by reverse microdialysis in freely moving rats: a comparative study of the effects of tetrodotoxin, lidocaine, and muscimol. J Pharmacol Exp Ther. 2007;323(1):61–69. doi: 10.1124/jpet.107.124784. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Wallenstein GV, Eichenbaum H, Hasselmo ME. The hippocampus as an associator of discontiguous events. Trends Neurosci. 1998;21(8):317–323. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26(48):12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T, Otto T. Differential contributions of dorsal vs. ventral hippocampus to auditory trace fear conditioning. Neurobiol Learn Mem. 2007;87(4):464–475. doi: 10.1016/j.nlm.2006.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disconnection of the ventral hippocampus from the prelimbic mPFC in Experiment 1 required implanting cannula in one VH and one PL. Each symbol depicts the placement of a cannula in the PL or VH for each group (SAL = black; MUS-IPSI = grey; MUS-CONTRA = white). Only one hemisphere of each structure is shown but both ipsilateral and contralateral placements are included. Diagrams were adapted from Paxinos & Watson, 2007.
Disconnection of the ventral hippocampus from the prelimbic mPFC in Experiment 2 included unilateral control animals. Cannulae placements are depicted for each group: SAL(v)-SAL(p) = black; SAL(v)-MUS(p) = dark grey; MUS(v)-SAL(p) = light grey; MUS(v)-MUS(p) = white. Diagrams were adapted from Paxinos & Watson, 2007.
Disconnection of the amygdala from the prelimbic mPFC in Experiments 3 required implanting cannula in one amygdala and one PL. Each symbol depicts the placement of a cannula in the PL or amygdala (SAL = black; MUS-IPSI = grey; MUS-CONTRA = white). Only one hemisphere of each structure is shown but both ipsilateral and contralateral placements are included. Diagrams were adapted from Paxinos & Watson, 2007.
Bilateral cannulae were implanted in the amygdala for Experiments 4 and 5. Each symbol depicts the placement of a cannula in the amygdala for each group (SAL = black; DRUG-UNI = grey; DRUG-BI = white. For Experiment 4 (muscimol), the trace conditioning group is represented by circles and the delay group by squares. For experiment 5 (lidocaine), placements are represented by triangles. Diagrams were adapted from Paxinos & Watson, 2007.
Bilateral cannulae were implanted in the VH for Experiment 5. Each symbol depicts the placement of a cannula in the VH (SAL = black; LIDO-UNI = grey; LIDO-BI = white). Diagrams were adapted from Paxinos & Watson, 2007.