Abstract
Background
Extremely obese adolescents are increasingly undergoing bariatric procedures, which restrict dietary intake. However, there are as yet no data available which describe the change in caloric density or composition of the adolescent bariatric patient’s diet pre- and post-operatively.
Objective
Assess the 1-year change in dietary composition of adolescents undergoing bariatric surgery.
Setting
Tertiary care children’s hospital
Methods
Twenty-seven subjects [67% female, 77% white, age 16.7 ± 1.4 years, baseline body mass index (BMI) 60.1 ± 14.1 kg/m2] were prospectively enrolled into an observational cohort study one month prior to laparoscopic Roux-en-Y gastric bypass (RYGB) between August 2005 and March 2008. Three-day dietary intake was recorded at baseline (n=24), at 2 weeks (n=16), 3 months (n=11), and 1 year (n=9) post-operatively. Dietary record data were verified by structured interview and compared with Dietary Reference Intake (DRI) values for ages 14–18.
Results
By 1 year post-surgery, mean caloric intake adjusted for BMI was 1015 ± 182 kcal/day, a 35% reduction from baseline. The proportion of fat, protein and carbohydrate intake did not differ from baseline. However, protein intake was lower than recommended postoperatively. Calcium and fiber intake was also persistently lower than recommended. Calcium and vitamin B12 supplementation increased the likelihood of meeting daily minimal recommendations (p≤0.02).
Conclusions
One year after RYGB, adolescents’ caloric intake remained restricted with satisfactory macronutrient composition, but with lower than desirable intake of calcium, fiber and protein.
Keywords: bariatric surgery, Roux-en-Y gastric bypass, adolescent, dietary assessment
Introduction
Obese adults and children are at risk for nutritional deficiencies.(1–6) Significant dietary restriction and bypass of the gastric body, duodenum and proximal jejunum following RYGB could potentiate suboptimal intake and absorption of important macro- and micronutrients.(7) RYGB has been associated with deficiencies in iron, folate, calcium, vitamins D and B12.(8–10) Higher rates of non-adherence to recommended vitamin supplementation correlate with higher rates of post-operative deficiencies.(11)
Little is known about the nutritional intake of adolescents undergoing bariatric surgery. This information is particularly important, as adherence to dietary and supplement regimens may differ between adults and adolescents.(12) The objective of this study was to determine dietary intake of adolescent bariatric surgery patients at baseline and during the first year following surgery.
Materials and Methods
The study was conducted at the Cincinnati Children’s Hospital Medical Center (CCHMC) and approved by the Institutional Review Board. Written informed consent was obtained from participants/parents. Patients were eligible to participate if they were previously enrolled in a study examining diabetic precursors and outcome after bariatric surgery that collected detailed dietary data. Sample size for this pilot study was a convenience sample, determined by the number of participants with completed dietary records. Exclusion criteria included: cirrhosis, total bilirubin >1 mg/dL, prothrombin time > 13.3 sec, prior myocardial infarction, serum creatinine ≥ 1.7 mg/dL, and systemic glucocorticoid therapy within the previous six weeks.
Study visits included assessments at baseline (1 month prior to surgery), 2 weeks, 3 months and 1 year post-operatively. At each visit, subjects completed a 3-day food record, and those with at least one record (n=27 participants) were included in the analysis. Height and weight were measured and BMI calculated (kg/m2). Height was measured using a wall-mounted stadiometer and weight was measured using a calibrated Scale-Tronix 5200 scale (Scale Tronix, White Plains, NY) with the subject wearing light clothing and socks. Measurements were made in duplicate by trained coordinators and averaged for analysis.
Pre-operatively, patients were counseled by a clinical dietician who did not participate in data collection. A low-fat, portion controlled diet was prescribed with the goal of 5–15% loss of baseline total weight during a 2–3 month pre-operative phase designed to promote weight loss prior to surgery. The baseline data therefore reflects participant intake during this preparation period. Post-surgically, the clinical dietician prescribed progressive stages of nutritional therapy. The final diet consisted of a recommended intake of 60 grams (g) of protein daily and 3–4 meals daily. Recommended supplements in the first six months post-surgery included a multivitamin, calcium plus vitamin D, vitamins B1 and B12, with supplementation of iron in all females, and in males if clinically indicated. The B1 supplement was discontinued at 6 months.
Dietary intake was assessed using the completed 3-day food records.(13) Trained research dieticians reviewed and verified all records at each visit with the participant and parent, and probed for missing details. Participants were asked to include two weekdays and one contiguous weekend day in their 3 day food record and results were averaged for the analysis. Dietary analysis was performed using the Nutrition Data System for Research (Version 8, 2008, University of Minnesota, Minneapolis, MN). Dietary intake was compared with the Dietary Recommended Intake (DRI) for adolescents aged 14–18.(14, 15)
Statistical analysis was conducted using SAS, version 9.1 (SAS Institute Inc, Cary, North Carolina). As some dietary intake variables were not normally distributed, analyses were compared between log transformed and un-transformed variables. Transformations did not alter conclusions, so data are presented in original units. One iron intake value (723.76 mg) at 1 year was determined to be an outlier and was removed from analysis. Sensitivity analyses including only individuals with complete longitudinal data at all visits (n=7) were also conducted, with no difference in conclusions; therefore, analysis included all available data. Age, sex, race and follow-up BMI were tested as covariates, and were retained if significant at p≤0.05.
Baseline comparisons between those with complete data (n=7) and those without (n=20) were evaluated using non-parametric tests for categorical and continuous variables (Fisher’s exact or Wilcoxon Signed-Rank respectively) due to small sample size. Dietary intake was analyzed by visit using repeated measures generalized estimating equations (GEE, using PROC GENMOD). GEE models account for correlations among multiple observations per person while allowing for missing data, and are relatively robust to deviations from normality. Least squares (LS) means (95% confidence intervals (CI)) or LS means ± SE were calculated from GEE models adjusted for significant covariates, as noted. Pairwise differences between visits were evaluated using a Bonferroni-corrected significance threshold of p≤0.05/6=0.008. Marginal significance is indicated for p-values between 0.05 and 0.008. Significant differences from DRI were determined when the 95% CI excluded the DRI.
Results
All subjects enrolled in this study underwent laparoscopic RYGB resulting in a mean BMI reduction from 60 to 37 kg/m2 (Table 1). Twenty-four of the 27 subjects (89%) completed food records at baseline, with fewer complete records at follow-up (n=16 at 2 weeks, n=11 at 3 months, n=9 at 1 year). The 7 subjects with complete data at all visits did not differ in any characteristics from those with data missing (all p>0.17; Table 1). Follow-up BMI and dietary intake also did not differ between groups, except at the 2 week visit post-surgery when those with complete data reported higher percent kilocalorie intake of carbohydrate (p=0.03) and lower percent intake of fat (p=0.02, data not shown).
Table 1.
Demographic, dietary and BMI characteristics of the study cohort at baseline, by availability of complete data
| Total | Complete data | Incomplete data | |
|---|---|---|---|
| N | 27 | 7 | 20 |
| Age at surgery, years (mean ± SD]) | 16.7 ± 1.4 | 16.6 ± 1.6 | 16.7 ± 1.5 |
| Sex (% male) | 33% | 25% | 57% |
| Race (% white) | 77% | 71% | 79% |
| Baseline Dietary intake (mean ± SD)a | |||
| Kilocalories | 1403 ± 786 | 1681 ± 1056 | 1289 ± 651 |
| Carbohydrate (g) | 168 ± 96 | 187 ± 127 | 160 ± 83 |
| Protein (g) | 72.8 ± 28.6 | 86.4 ± 26.7 | 67.2 ± 28.2 |
| Fat (g) | 58.1 ± 37.7 | 65.7 ± 55.7 | 55.0 ± 29.0 |
| BMI (mean ± SD)bat: | |||
| Baseline | 60.0 ± 14.1 | 62.5 ± 8.4 | 59.0 ± 15.9 |
| 2 weeks | 53.7 ± 12.0 | 54.8 ± 7.3 | 53.1 ± 14.4 |
| 3 months | 46.8 ± 8.6 | 45.6 ± 5.8 | 49.0 ± 13.0 |
| 1 year | 36.6 ± 7.5 | 35.8 ± 7.3 | 39.3 ± 10.6 |
Includes all individuals (n=24) with baseline dietary data (n=7 complete longitudinal data, n=17 incomplete longitudinal data)
Includes all individuals with data at each study visit
Caloric and Macronutrient Intake
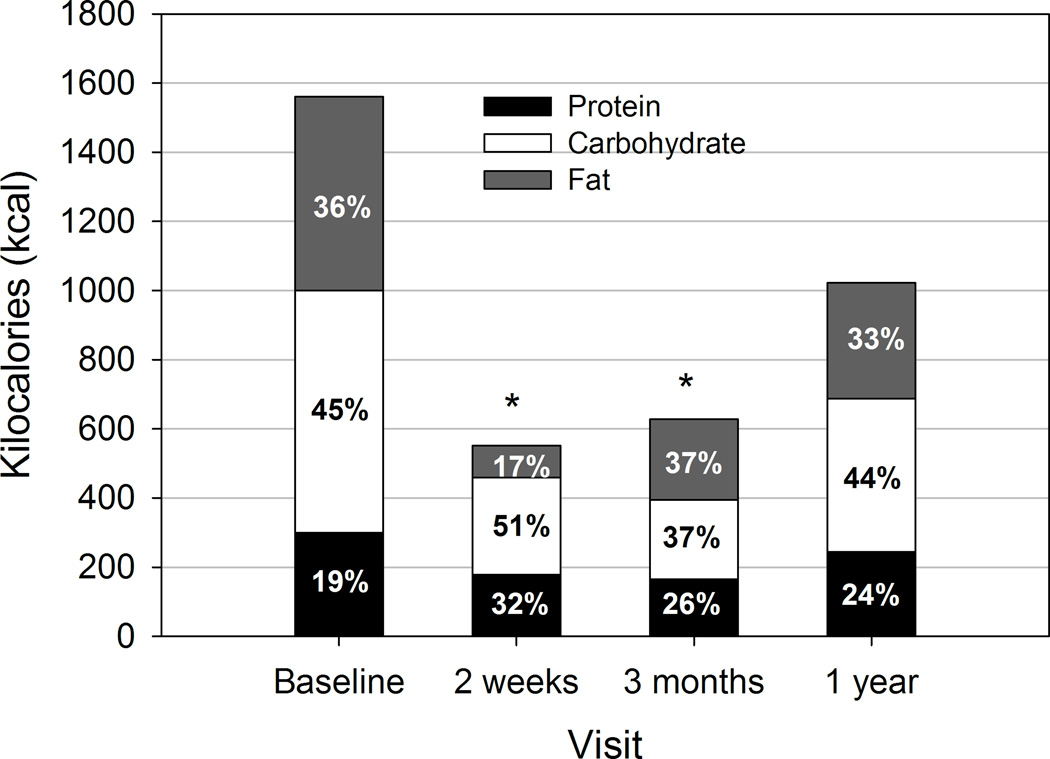
Adjusting for BMI at each visit, caloric intake at one year was 1015 ± 182 kcal/day, which did not differ significantly from baseline (1555 ± 166 kcal/day, p=0.06; Figure). At baseline, 21 of 24 (88%) patients’ intake of protein met or exceeded DRI (46–52 g protein/day), but few achieved DRI for protein intake (g/day) by 2 weeks (6 of 16, 38%) and 3 months post-surgery (2 of 11, 18%). The proportion meeting the goal of ≤30% of calories from fat was 29% at baseline, 81% at 2 weeks, 27% at 3 months and 33% at 1 year.
Figure.
Macronutrient composition of dietary intake at baseline (n=24), 2 weeks (n=16), 3 months (n=11) and 1 year (n=9) post-surgery. Values (means and percents of total intake) presented are from generalized estimating equations (GEE) models adjusted for body mass index (BMI) at each visit, with least square (LS) means and 95% confidence intervaIs (CI) of total kcal/day as follows: Baseline: 1555 (CI: 1230, 1880); 2 weeks: 552 (CI: 428, 675); 3 months: 625 (CI: 450, 801); 1 year: 1015 (CI: 658, 1373). Mean percent of total intake as protein, carbohydrate and fat are indicated within each stacked bar. * p≤0.0001 compared with baseline.
Overall dietary macronutrient composition adjusting for BMI at each visit was not significantly different between baseline and 1 year post-surgery (Figure). There was a trend toward correlation between BMI change and total caloric intake at 1 year, but this finding was not statistically significant due to small sample size. At 2 weeks post-surgery, % fat intake was lower than all other visits (all p≤0.0002), while % carbohydrate was marginally higher than at 3 months (p=0.01). The % protein intake at 2 weeks was also significantly higher than baseline (p<0.0001) or 1 year (p=0.008), and marginally higher than at 3 months (p=0.04). Percent protein intake at 3 months was also significantly higher compared with baseline (p=0.001), and by 1 year, % protein intake remained marginally higher than baseline (p=0.02). Thus, while protein intake in g/day declines along with reduced caloric intake, protein represents a greater proportion of the dietary intake throughout the first year post-surgery compared with baseline.
Micronutrient Intake and Supplementation
At baseline, mean intake of vitamins B1 and B12 exceeded DRI levels, while intake of vitamin E, calcium and fiber were deficient (Table 2). At 2 weeks post-surgery, fiber intake remained deficient and declined further from baseline (p<0.0001), while all other micronutrients met or exceeded DRI. Deficiencies in the calcium and fiber intake persisted through 1 year, while iron, folate, vitamins E, D, B1, and B12 intake met or exceeded DRI levels at all time points. Only vitamin B1 intake decreased significantly between baseline and 1 year post-surgery (p≤0.008), likely corresponding to discontinuing the B1 supplement at 6 months.
Table 2.
Dietary intake and supplementation of select micronutrients pre-surgery and post-surgery, compared with Dietary Reference Intake (DRI) thresholds for 14–18 year olds
| Post-surgical Visit | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| DRI | Baselinea | 2 weeksa | 3 monthsa | 1 yeara | |||||
| Dietary Intake | |||||||||
| Vitamin E (µg)b | 15 µg | 8.3 (5.6, 11.1) ∇ | 11.3 (7.1, 15.5) | 11.5 (7.3, 15.8) | 11.9 (5.3, 18.6) | ||||
| Vitamin D (µg)b | 5 µg | 7.1 (4.4, 9.7) | 17.8 (11.3, 24.3) **∆ | 11.9 (6.1, 17.6) ∆ | 10.2 (2.5, 17.8) | ||||
| Vitamin B1 (mg)c | 1 mg (females) 1.2 mg (males) |
12.0 (3.6, 20.4) ∆ | 27.3 (12.9, 41.8) ∆ | 36.8 (18.6, 54.9) ∆ | −1.03 (−5.3, 3.2) ** | ||||
| Folate (µg)c | 400 µg | 434 (318, 549) | 780 (453, 1106) ∆ | 682 (370, 994) | 581 (116, 1045) | ||||
| Fiber (g)d | 38 g (males) 26 g (females) |
8.1 (6.4, 9.8) ∇ | 1.7 (0.2, 3.2) **∇ | 4.0 (2.5, 5.4) **∇ | 8.2 (4.9, 11.5) ∇ | ||||
| Calcium (mg)c | 1300 mg | 998 (776, 1219) ∇ | 1780 (1341, 2220)**∆ | 893 (486, 1299) ∇ | 814 (491, 1138) ∇ | ||||
| Vitamin B12 (µg)b | 2.4 µg | 189 (59.4, 320) ∆ | 243 (116, 370) ∆ | 330 (138, 521) ∆ | 168 (0.32, 335) | ||||
| Iron (mg)e | 11 mg (males) 15 mg (females) |
15.5 (6.5, 24.4) | 26.5 (16.6, 36.4) ∆ | 32.2 (13.4, 51.0) | 25.4 (13.5, 37.0) | ||||
| % meeting DRI | With Supp |
Without Supp |
With Supp |
Without Supp |
With Supp |
Without Supp |
With Supp |
Without Supp |
|
| Calciumf | 80% | 16%* | 80% | 50% | 60% | 0% | 100% | 14% | |
| Vitamin B12f | 100% | 69% | 100% | 83% | 100% | 25%* | 100% | 88% | |
| Irong | 100% | 70% | 100% | 73% | 100% | 60% | 100% | 38% | |
Least squares means and 95% confidence intervals (CI) presented, from repeated measures analysis (GENMOD), with each model adjusted as indicated. Negative estimates of mean intake and lower CI bound for vitamin B1 occur due to model estimation across all visits, and may be considered to be 0 for the purposes of actual reported intake.
Adjusted for body mass index (BMI) at each visit
No adjustments significant
Adjusted for race
Adjusted for BMI at each visit and sex
Supplementation recommended at all time points
Supplementation recommended only as clinically indicated
p≤0.05 by χ2 analysis comparing proportion meeting DRI with versus without supplementation at each visit
p≤0.008 compared with baseline
Significantly lower than DRI (p<0.05)
Significantly higher than DRI (p<0.05)
Supplementation of three key micronutrients was explored in relation to achieving DRI levels (Table 2). Only 21% to 63% of individuals were taking recommended calcium supplements at each visit. The proportion of subjects meeting calcium DRI levels was higher among those taking supplements, which achieved significance at baseline (p<0.02) and for all visits combined (p<0.0002, data not shown). Eleven to 64% of patients reported B12 supplementation at each visit. Importantly, all of those taking vitamin B12 supplementation met DRI at each visit, compared with 25% to 88% at follow up visits who were not taking supplements (p<0.0002 for all visits combined, data not shown). Iron supplementation did not affect meeting DRI, perhaps due to limited iron supplementation (4–11% of participants at each visit, 7% overall).
Discussion
This is the first study to prospectively examine the changes in dietary intake in adolescents undergoing bariatric surgery and general results are reassuring. The data confirm that RYGB severely restricts energy intake within the first 3 months post-operatively in adolescents, and a reduced mean caloric intake is maintained at one year postoperatively, similar to adults.(16–21) This indicates that these adolescents were able to maintain a similar level of reduced caloric intake at one year follow-up, as they did when they were striving to meet a pre-operative calorie restrictive diet.
Reported ranges of percent energy intake in adult patients at 12 months was highest from carbohydrates (41 – 47%), followed by fat (30 to 42%) and protein (16 – 23%)(16–21), consistent with the findings in this cohort of adolescents. Although percent intake of carbohydrate, fat, and protein remained within or very near recommended DRI ranges, absolute intake of all macronutrients fell significantly within the first 3 months. Therefore absolute protein intake (g/day) was lower than recommended for adolescent patients at both 3 and 12 months post-operatively. At least 45% of the adolescents in the current study failed to meet DRI thresholds for protein intake at any point post-operatively, consistent with adults after RYGB.(22) Protein intake is a primary concern due to its role in maintaining lean body mass during rapid weight loss. Based on these early findings, it is important to monitor for adverse changes in lean mass. Given the differing conclusions drawn from evaluating absolute intake of protein (g/day) versus % protein intake, the relative importance of these metrics on adolescent bariatric outcomes warrants further investigation.
Encouragingly, the DRI of many critical micronutrients including folate, iron, and vitamins B1, B12, E and D, was maintained in this cohort of adolescents after bariatric surgery. Nonetheless, the low intake of calcium and fiber is notable. Suboptimal adherence to calcium supplementation is likely to play a role, as supplementation with calcium significantly improved the likelihood of meeting the DRI threshold. Conspicuously, fiber intake was very low both pre-and post-operatively, far below the DRI and mean levels in U.S. adolescents aged 12–19 years (13–15 gm/day).(23) Fiber may be an important co-factor to help promote satiety and weight loss and therefore of even greater importance to these patients.
Limitations of this study include the small sample size, incomplete food records in some subjects over time, and lack of serum measurements of micronutrients levels. Three day diet records are an accepted and validated tool in outpatient energy intake research, despite inherent imprecision in all self-report methods used to estimate caloric intake.(13, 24) Although completion of food records declined over time, the subjects with complete dietary data did not differ from those with incomplete data on demographics, BMI or dietary intake. While this study evaluated total intake of micronutrients, including both food and oral supplements, analysis of supplement use was limited to individual micronutrients (calcium, B12 and iron), and did not include consideration of multivitamin supplementation. This was a limitation of the nutritional analysis software which did not adequately separate out micronutrients obtained from multivitamins from those obtained from food sources. Assessment of clinical serum levels of micronutrients was attempted, but as serum micronutrient labs are not part of the usual clinical regimen within the first post-operative year and return for follow-up declined at 1 year time point, paired data were too limited to be analyzed meaningfully. Future studies should include both dietary intake and serum levels to better quantify absorption.
Conclusions
Mean energy intake at the end of the first post-operative year in adolescents remained comparable to the reduced calorie preoperative diet recommended prior to bariatric surgery. Percent intake of macronutrients remained unchanged at 1 year post-surgery, but absolute intake of protein was low in many adolescents post-operatively. While the DRI of many critical micronutrients including folate, iron, and vitamins B1, B12, E and D, was maintained in this cohort of adolescents after bariatric surgery, calcium and fiber intake was persistently lower than recommended. Adherence to calcium supplementation increased the likelihood of meeting the daily minimal intake recommendation. Larger and longer prospective studies will be needed to determine how well postoperative caloric intake influences long-term weight trajectory in adolescents, and to what degree dietary intake of both macro and micro-nutrients influences biochemical markers of nutritional adequacy.
Acknowledgments
Funding sources: This publication was supported by NIH grant 5R03DK68228 to THI, NIH grant K23DK080888 to SAX and an Institutional Clinical and Translational Science Award, NIH/NCRR Grant Number 5UL1RR026314-02. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aills L, Blankenship J, Buffington C, Furtado M, Parrott J. ASMBS Allied Health Nutritional Guidelines for the Surgical Weight Loss Patient. Surg Obes Relat Dis. 2008;4(Suppl):S73–S108. doi: 10.1016/j.soard.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Alemzadeh R, Kichler J, Babar G, Calhoun M. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism. 2008;57:183–191. doi: 10.1016/j.metabol.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 3.Botella-Carretero JI, Alvarez-Blasco F, Villafruela JJ, Balsa JA, Vazquez C, Escobar-Morreale HF. Vitamin D deficiency is associated with the metabolic syndrome in morbid obesity. Clin Nutr. 2007;26:573–580. doi: 10.1016/j.clnu.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Pereira S, Saboya C, Chaves G, Ramalho A. Class III obesity and its relationship with the nutritional status of vitamin A in pre- and postoperative gastric bypass. Obes Surg. 2009;19:738–744. doi: 10.1007/s11695-008-9478-y. [DOI] [PubMed] [Google Scholar]
- 5.Pinhas-Hamiel O, Doron-Panush N, Reichman B, Nitzan-Kaluski D, Shalitin S, Geva-Lerner L. Obese children and adolescents: a risk group for low vitamin B12 concentration. Arch Pediatr Adolesc Med. 2006;160:933–936. doi: 10.1001/archpedi.160.9.933. [DOI] [PubMed] [Google Scholar]
- 6.Strauss RS. Comparison of serum concentrations of alpha-tocopherol and beta-carotene in a cross-sectional sample of obese and nonobese children (NHANES III). National Health and Nutrition Examination Survey. J Pediatr. 1999;134:160–165. doi: 10.1016/s0022-3476(99)70409-9. [DOI] [PubMed] [Google Scholar]
- 7.Xanthakos SA, Inge TH. Nutritional consequences of bariatric surgery. Curr Opin Clin Nutr Metab Care. 2006;9:489–496. doi: 10.1097/01.mco.0000232913.07355.cf. [DOI] [PubMed] [Google Scholar]
- 8.Boylan LM, Sugerman HJ, Driskell JA. Vitamin E, vitamin B-6, vitamin B-12, and folate status of gastric bypass surgery patients. J Am Diet Assoc. 1988;88:579–585. [PubMed] [Google Scholar]
- 9.Madan AK, Orth WS, Tichansky DS, Ternovits CA. Vitamin and trace mineral levels after laparoscopic gastric bypass. Obes Surg. 2006;16:603–606. doi: 10.1381/096089206776945057. [DOI] [PubMed] [Google Scholar]
- 10.Rhode BM, Shustik C, Christou NV, MacLean LD. Iron absorption and therapy after gastric bypass. Obes Surg. 1999;9:17–21. doi: 10.1381/096089299765553656. [DOI] [PubMed] [Google Scholar]
- 11.Brolin RE, Gorman RC, Milgrim LM, Kenler HA. Multivitamin prophylaxis in prevention of post-gastric bypass vitamin and mineral deficiencies. Int J Obes. 1991;15:661–667. [PubMed] [Google Scholar]
- 12.Rand CS, Macgregor AM. Adolescents having obesity surgery: a 6-year follow-up. South Med J. 1994;87:1208–1213. doi: 10.1097/00007611-199412000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Crawford PB, Obarzanek E, Morrison J, Sabry ZI. Comparative advantage of 3-day food records over 24-hour recall and 5-day food frequency validated by observation of 9- and 10-year-old girls. J Am Diet Assoc. 1994;94:626–630. doi: 10.1016/0002-8223(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 14.Institute of Medicine. Dietary Reference Intakes Table--The Complete Set. [Last accessed 6/28/2011];2004 http://www.iom.edu/Activities/Nutrition/SummaryDRIs/~/media/Files/Activity%20Files/Nutrition/DRIs/New%20Material/5DRI%20Values%20SummaryTables%2014.pdf.
- 15.Yates AA, Schlicker SA, Suitor CW. Dietary Reference Intakes: the new basis for recommendations for calcium and related nutrients, B vitamins, and choline. J Am Diet Assoc. 1998;98:699–706. doi: 10.1016/S0002-8223(98)00160-6. [DOI] [PubMed] [Google Scholar]
- 16.Kruseman M, Leimgruber A, Zumbach F, Golay A. Dietary, weight, and psychological changes among patients with obesity, 8 years after gastric bypass. J Am Diet Assoc. 2010;110:527–534. doi: 10.1016/j.jada.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 17.Bobbioni-Harsch E, Huber O, Morel P, et al. Factors influencing energy intake and body weight loss after gastric bypass. Eur J Clin Nutr. 2002;56:551–556. doi: 10.1038/sj.ejcn.1601357. [DOI] [PubMed] [Google Scholar]
- 18.Coughlin K, Bell RM, Bivins BA, Wrobel S, Griffen WO., Jr Preoperative and postoperative assessment of nutrient intakes in patients who have undergone gastric bypass surgery. Arch Surg. 1983;118:813–816. doi: 10.1001/archsurg.1983.01390070025006. [DOI] [PubMed] [Google Scholar]
- 19.Dias MC, Ribeiro AG, Scabim VM, Faintuch J, Zilberstein B, Gama-Rodrigues JJ. Dietary intake of female bariatric patients after anti-obesity gastroplasty. Clinics (Sao Paulo) 2006;61:93–98. doi: 10.1590/s1807-59322006000200002. [DOI] [PubMed] [Google Scholar]
- 20.Moize V, Geliebter A, Gluck ME, et al. Obese patients have inadequate protein intake related to protein intolerance up to 1 year following Roux-en-Y gastric bypass. Obes Surg. 2003;13:23–28. doi: 10.1381/096089203321136548. [DOI] [PubMed] [Google Scholar]
- 21.Olbers T, Bjorkman S, Lindroos A, et al. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg. 2006;244:715–722. doi: 10.1097/01.sla.0000218085.25902.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bavaresco M, Paganini S, Lima TP, et al. Nutritional course of patients submitted to bariatric surgery. Obes Surg. 2010;20:716–721. doi: 10.1007/s11695-008-9721-6. [DOI] [PubMed] [Google Scholar]
- 23.Alaimo K, McDowell MA, Briefel RR, et al. Advance Data From Vital and Health Statistics. Hyattsville, MD: National Center for Health Statistics; 1994. Dietary Intake of Vitamins, Minerals, and Fiber of Persons Ages 2 Months and Over in the United States: Third National Health and Nutrition Examination Survey, Phase 1, 1998–91. No. 258. [PubMed] [Google Scholar]
- 24.Howat PM, Mohan R, Champagne C, Monlezun C, Wozniak P, Bray GA. Validity and reliability of reported dietary intake data. J Am Diet Assoc. 1994;94:169–173. doi: 10.1016/0002-8223(94)90242-9. [DOI] [PubMed] [Google Scholar]