Abstract
Pre-adolescence and adolescence are developmental periods associated with increased vulnerability for tobacco addiction, and exposure to tobacco during these periods may lead to long-lasting changes in behavioral and neuronal plasticity. The present study examined the short- and long-term effects of nicotine and nicotine withdrawal on fear conditioning in pre-adolescent, adolescent, and adult mice, and potential underlying substrates that may mediate the developmental effects of nicotine, such as changes in nicotinic acetylcholine receptor (nAChR) binding, CREB expression, and nicotine metabolism. Age-related differences existed in sensitivity to the effects of acute nicotine, chronic nicotine and nicotine withdrawal on contextual fear conditioning (no changes in cued fear conditioning were seen); younger mice were more sensitive to the acute effects and less sensitive to the effects of nicotine withdrawal 24 hours post treatment cessation. Developmental differences in nAChR binding were associated with the effects of nicotine withdrawal on contextual learning. Developmental differences in nicotine metabolism and CREB expression were also observed, but were not related to the effects of nicotine withdrawal on contextual learning 24 hours post treatment. Chronic nicotine exposure during pre-adolescence or adolescence, however, produced long-lasting impairments in contextual learning that were observed during adulthood, whereas adult chronic nicotine exposure did not. These developmental effects could be related to changes in CREB. Overall, there is a developmental shift in the effects of nicotine on hippocampus-dependent learning and developmental exposure to nicotine results in adult cognitive deficits; these changes in cognition may play an important role in the development and maintenance of nicotine addiction.
Keywords: Hippocampus, Adolescent, Addiction, Learning, Acetylcholine
1. Introduction
The developmental period of adolescence is associated with a greater risk for the onset of nicotine addiction (Giovino, 2002; Nelson, Mowery, Asman, Pederson, O’Malley, Malarcher, Maibach, and Pechacek, 2008), and thus the high prevalence of tobacco use by adolescents is cause for concern. The initial use of tobacco most commonly occurs during pre-adolescence or early adolescence, with the majority of smokers having tried their first cigarette before age 18 (Everett, Warren, Sharp, Kann, Husten, and Crossett, 1999; Johnston, O’Malley, Bachman, and Schulenberg, 2009; Lantz, 2003). Furthermore, an earlier onset of smoking is predictive of a more severe addiction (Everett et al., 1999) and is associated with higher rates of drug use later in life (Hanna and Grant, 1999), suggesting that the effects of nicotine during childhood or adolescence are more detrimental than if nicotine use begins during adulthood. The effects of nicotine on the developing brain may play a critical role in the development and maintenance of nicotine addiction, yet there is much that remains unknown about the behavioral, cellular, and molecular changes that occur during adolescence following exposure to nicotine.
Nicotine-induced alterations of cognitive processes during adolescence may play an important role in the continued use of nicotine and could lead to addiction. A key feature of adolescent neurodevelopment is the maturation of brain areas that underlie cognitive processes, and the effects of adolescent nicotine exposure on executive function could lead to increased risk for nicotine addiction (Casey, Tottenham, Liston, and Durston, 2005; DeBry and Tiffany, 2008). Using a mouse model to investigate the effects of nicotine on learning, we have demonstrated in adult mice that acute nicotine enhances hippocampus-dependent learning, chronic nicotine has no effect, and nicotine withdrawal impairs hippocampus-dependent learning (Davis and Gould, 2009; Davis, James, Siegel, and Gould, 2005; Davis, Kenney, and Gould, 2007; Gould and Higgins, 2003; Kenney, Adoff, Wilkinson, and Gould, 2011). Developmental differences in sensitivity to the effects of nicotine on hippocampus-dependent learning may contribute to nicotine addiction; however, the developmental effects of nicotine on learning and the neural substrates that mediate these effects remain unknown.
Exposure to nicotine during adolescence can lead to changes in neural function, but few studies have linked developmental differences in the neural response to nicotine to changes in behavior. Radioligand binding studies have shown that adolescent nicotine exposure can produce long-lasting increases in nicotinic acetylcholine receptor (nAChR) upregulation relative to adults (Abreu-Villaca, Seidler, Qiao, Tate, Cousins, Thillai, and Slotkin, 2003; Trauth, Seidler, McCook, and Slotkin, 1999); this increased nAChR upregulation could enhance sensitivity to nicotine which may lead to changes in behavior. Adolescents also metabolize nicotine more rapidly than adults (O’Dell, Bruijnzeel, Smith, Parsons, Merves, Goldberger, Richardson, Koob, and Markou, 2006; Trauth, Seidler, and Slotkin, 2000a), and this enhanced metabolism may partially explain differences in nicotine sensitivity between adolescents and adults. It is also possible that developmental differences in the effects of nicotine may depend upon changes to gene transcription factors such as cAMP response element-binding protein (CREB), which plays a critical role in long-term memory (Abel and Lattal, 2001; Bourtchuladze, Frenguelli, Blendy, Cioffi, Schutz, and Silva, 1994). Changes in CREB activity after nicotine administration have previously been reported in adult mice (Walters, Cleck, Kuo, and Blendy, 2005), but nicotine-induced changes in CREB activity have not been characterized at earlier developmental time points. Thus, the developmental effects of nicotine exposure on associative learning may depend upon changes to nAChR upregulation, nicotine metabolism and/or CREB expression; however, no studies to date have examined developmental differences in the effects of nicotine on learning and potential underlying biological substrates.
In addition to potentially producing short-term alterations in learning, adolescent nicotine exposure during development may also have long-lasting consequences on learning that manifest later during adulthood. Longitudinal studies have reported that cigarette smoking may be a risk factor for cognitive impairment later in life (Cervilla, Prince, and Mann, 2000; Nooyens, van Gelder, and Verschuren, 2008; Richards, Jarvis, Thompson, and Wadsworth, 2003), but a causal link between cigarette smoking and cognitive impairment is difficult to establish as numerous factors in a longitudinal study could contribute to cognitive impairment. However, research in rodents has demonstrated that nicotine exposure during adolescence produces long-lasting changes in reward (Adriani, Deroche-Gamonet, Le Moal, Laviola, and Piazza, 2006; Adriani, Spijker, Deroche-Gamonet, Laviola, Le Moal, Smit, and Piazza, 2003), anxiety (Slawecki, Thorsell, El Khoury, Mathe, and Ehlers, 2005), and attention (Counotte, Spijker, Van de Burgwal, Hogenboom, Schoffelmeer, De Vries, Smit, and Pattij, 2009). In contrast, it is unclear if developmental exposure to nicotine produces changes in fear conditioning during adulthood, as one study in rats reported improved fear conditioning in adulthood (Smith, McDonald, Bergstrom, Brielmaier, Eppolito, Wheeler, Falco, and Smith, 2006) but a recent study found deficits (Spaeth, Barnet, Hunt, and Burk, 2010). If developmental nicotine exposure produces long-lasting changes in cognition, the underlying neurodevelopmental changes may facilitate the maintenance of addiction during adulthood by interfering with learning adaptive strategies to remain drug-free. In support, data suggest that poorer cognitive function may be a risk factor for development of addiction (Block, Erwin, and Ghoneim, 2002).
In the present study, we investigated the effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning in mice during three developmental periods: pre-adolescence (post-natal day (PND) 23, start of treatment), adolescence (PND 38, start of treatment), and adulthood (PND 53, start of treatment). Both hippocampus-dependent contextual fear conditioning (Kim and Fanselow, 1992; Logue, Paylor, and Wehner, 1997; Phillips and LeDoux, 1992) and hippocampus-independent cued fear conditioning (Kim and Fanselow, 1992; Logue et al., 1997; Phillips and LeDoux, 1992) and potential underlying neural and biological substrates were examined. Analyses of nicotine binding and CREB and pCREB levels were conducted 24 hours after nicotine withdrawal to determine whether nicotine treatment produced developmental changes in nAChR upregulation and CREB function. In addition, changes in metabolism of acute and chronic nicotine were also examined. Finally, we investigated whether exposure to chronic nicotine during pre-adolescence, adolescence, or adulthood would alter fear conditioning that occurred later during adulthood.
2. Material and Methods
2.1. Subjects
Subjects were male C57BL/6J mice that were obtained from Jackson Laboratory (Bar Harbor, ME). Mice received ad libitum access to food and water and were maintained on a 12 hour light/dark cycle (lights on at 7:00 a.m.). Mice acclimated to the colony and laboratory for at least one week prior to the start of experiments. Pre-adolescent mice were shipped with dams and were PND 16 on the day of arrival, whereas adolescents were PND 31 and adults were PND 46 on arrival day. The Temple University Institutional Animal Care and Use Committee approved all behavioral and surgical procedures. The age span of adolescence in humans is not defined by clear boundaries (see Spear, 2000 for review); thus, it is difficult to define a similar developmental period in mice. However, research in mice suggests that events consistent with adolescence in humans may be observed by PND 28 – 30. For example, PND 30 male mice exhibit initial signs of puberty, increased impulsivity, and social behavior characteristic of adults (Johnston et al., 2009; Keene, Suescun, Bostwick, Chandrashekar, Bartke, and Kopchick, 2002; Terranova, Laviola, and Alleva, 1993; Terranova, Laviola, de Acetis, and Alleva, 1998). Therefore, at the start of all experiments, pre-adolescent mice were PND 23, adolescent mice were PND 38, and adults were PND 53.
2.2. Apparatus
The training and testing of contextual fear conditioning occurred in four identical chambers (17.78 cm × 19.05 cm × 38.10 cm) that were housed in sound attenuating boxes (Med-Associates, St. Albans, VT), for details see Gould and Higgins (2003). Background noise (69 dB) during training and testing was provided by ventilation fans. Stimulus administration during training and testing was controlled by a computer using Med-PC software. Cued fear conditioning was tested in four identical altered chambers (20.32 × 22.86 × 17.78 cm) housed in sound attenuating boxes located in a different room from the training chambers. These chambers differed from the training chambers in size, construction, visual cues, tactile cues, and olfactory cues (for details see Gould and Higgins (2003)).
2.3. Behavioral Procedures: Contextual and Cued Fear Conditioning
Fear conditioning was measured with a time-sampling procedure that has been described in detail previously (Gould and Higgins, 2003). Mice were observed for freezing behavior (defined as the absence of all movement except for respiration (Blanchard and Blanchard, 1969) ) for one second during ten second intervals. At the start, baseline activity was scored for 120 seconds. Following baseline, a 30 second white noise conditioned stimulus (CS,85 dB) was activated that co-terminated with a 2 second 0.57 mA footshock unconditioned stimulus (US). Immediate freezing was scored during a 120 second inter-trial interval and was followed by a second CS-US pairing. Training ended with a 30 second interval during which freezing behavior was not recorded. Mice were tested for contextual fear conditioning twenty-four hours later; mice were returned to the training chambers and freezing was scored for 5 minutes. One hour later, mice were placed in an altered context to test for both generalized freezing (i.e. freezing over a 3 minute period in response to a context distinct from the training context in the absence of the auditory cue) and auditory cued freezing over 3 minutes.
2.4. Drug Administration and Experimental Design
For all acute nicotine experiments, nicotine hydrogen tartrate salt (Sigma Co., St. Louis, MO) was dissolved in physiological saline and administered via an intraperitoneal (i.p.) injection. For chronic nicotine and nicotine withdrawal experiments, nicotine was administered subcutaneously by mini-osmotic pump (model 1002; Alzet, Cupertino, CA); doses were calculated based on starting weight, changes in weight over the course of treatment could influence dose. All doses of nicotine are reported in freebase weight. For experiments in which the effects of nicotine withdrawal were examined, mini-osmotic pumps were removed 12 days after chronic nicotine treatment via an intrascapular incision. To ensure proper delivery of nicotine and saline solutions, pumps were checked for the remaining liquid following pump removal. Solutions were extracted from the pumps and the volumes were measured. The remaining volume of solution was then compared to the anticipated amount based upon the flow rate and the duration of implantation. Any pump that was outside the range of the calculations was considered ineffective and the corresponding animal was removed from data collection. However, none of the pumps used in this study fell outside of the expected values and thus no mice were excluded.
Developmental effects of acute nicotine on fear conditioning
Pre-adolescent (PND 23), adolescent (PND 38) and adult (PND 53) mice were administered saline, 0.045, 0.09, or 0.18 mg/kg nicotine i.p. five minutes before both the training and testing of contextual and cued fear conditioning (n = 9 – 14 per group). This range of acute nicotine doses was based on prior work (André, Gulick, Portugal, and Gould, 2008; Davis and Gould, 2007; Davis, Porter, and Gould, 2006; Gould and Higgins, 2003; Gould and Wehner, 1999; Portugal, Kenney, and Gould, 2008; Raybuck and Gould, 2007). All doses of acute nicotine enhanced contextual fear conditioning in pre-adolescent mice (see results); thus, a follow up experiment was conducted to identify the lowest dose of acute nicotine that would enhance contextual fear conditioning in pre-adolescent mice. The design of the experiment was identical to the acute nicotine experiments described above, except that pre-adolescent and adult mice were administered saline or 0.023 mg/kg nicotine; adolescent mice were not examined.
Developmental effects of chronic nicotine on fear conditioning
To investigate the effects of chronic nicotine on fear conditioning, mini-osmotic pumps were implanted subcutaneously that contained saline 3.0, 6.3 or 12.0 mg/kg/day nicotine (n = 9 – 12 per group; Figure 1). The pre-adolescent group was PND 23, adolescent group was PND 38, and adult group was PND 53 when pumps were implanted. Doses were based on prior work (Davis and Gould, 2009; Davis et al., 2005; Portugal and Gould, 2007; Raybuck, Portugal, Lerman, and Gould, 2008). The training of fear conditioning began 13 days after chronic nicotine treatment commenced. Thus, pre-adolescent mice were PND 36, adolescent mice were PND 51, and adults were PND 66 on training day. Chronic nicotine treatment continued on testing day (24 hours later).
Figure 1.
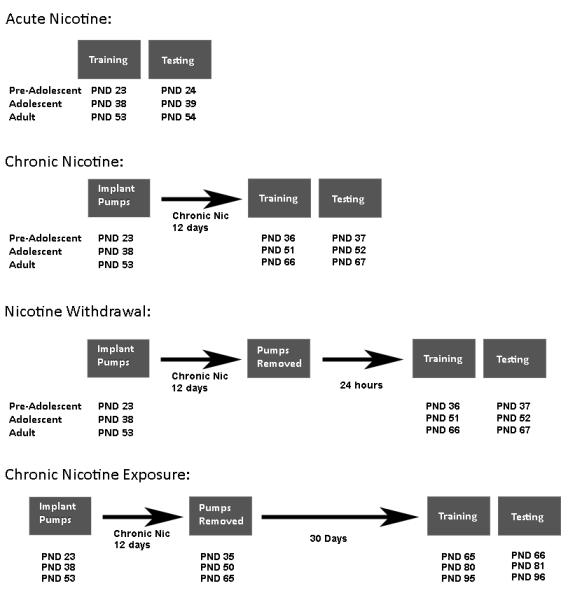
A schematic of the design for the experiments conducted in this study. Each box represents a phase of the experiment, and the text indicates the age of pre-adolescent, adolescent, and adult mice during each phase of the experiment.
Developmental effects of nicotine withdrawal on fear conditioning
At the start of nicotine withdrawal experiments, mice were implanted with mini-osmotic pumps that contained 3.0, 6.3, 12.0 mg/kg/day nicotine or saline (n = 7 – 12 per group). Mice were PND 23, PND 38, or PND 53 when pumps were implanted. Chronic nicotine was administered for 12 days, and all pumps were removed on day 12. The training of fear conditioning occurred on day 13, and mice were tested for contextual and cued fear conditioning 24 hours later. On training day, pre-adolescent, adolescent, and adult mice were PND 36, PND 51, or PND 66, respectively. Withdrawal from all doses of chronic nicotine disrupted contextual fear conditioning in PND 38 mice (see results). Therefore, an experiment was conducted to determine whether withdrawal from lower doses of chronic nicotine would impair contextual fear conditioning in adolescent mice. In this experiment, mini-osmotic pumps were implanted in PND 38 mice that contained 0.5, 1.1 mg/kg/day nicotine, or saline.
Developmental effects of prior chronic nicotine exposure on fear conditioning
To investigate the effects of prior chronic nicotine exposure on future learning, PND 23, PND 38, or PND 53 mice were implanted with mini-osmotic pumps that delivered saline, 8.8, or 12.0 mg/kg/d nicotine subcutaneously for 12 days (n = 8 – 14 per group). Pre-adolescent mice were PND 35, adolescent mice were PND 50, and adults were PND 65 at the time of pump removal. Following pump removal, mice remained in the colony room for 30 days and the training of fear conditioning began when pre-adolescent mice were PND 65, adolescent mice were PND 80, and adults were PND 95 (see Figure 1 for a schematic of the experiment). The testing of contextual and cued fear conditioning occurred 24 hours later for all groups. The 12.0 mg/kg/d dose of chronic nicotine was used because mice from all age groups withdrawn from 12.0 mg/kg/d chronic nicotine exhibited nicotine withdrawal-related deficits in contextual fear conditioning (see results). A group of pre-adolescent, adolescent, and adult mice were mistakenly implanted with pumps containing 8.8 mg/kg/d chronic nicotine; the results were informative so the groups were completed and these data were added to the experiment.
2.5. Plasma nicotine collection
Developmental differences in nicotine metabolism may play a role in the effects of acute nicotine and nicotine withdrawal on contextual fear conditioning. Therefore, plasma samples were collected from mice that were treated with the intermediate doses of acute and chronic nicotine used in this study (0.09 mg/kg and 6.3 mg/kg/d, respectively; n = 8 per group). Mice were administered 0.09 mg/kg acute nicotine and blood samples were collected 10 minutes later via cardiac puncture. For chronic nicotine, mini-osmotic pumps containing 6.3 mg/kg/d nicotine were implanted subcutaneously and blood samples were collected 12 days later via cardiac puncture. Blood samples were collected in lithium heparin tubes and centrifuged to isolate plasma. The analysis of plasma samples was conducted by Dr. J. Randy James (Virginia Commonwealth University) using a Micromass Quattro II liquid chromatography/tandem mass spectrometer (LC/MS; Blue Lion Biotech, Snoqualmie, WA). The procedure for analyzing plasma nicotine was adapted from Naidong and colleagues (2001) and was validated for selectivity, calibration model fit, sensitivity, accuracy, and precision. Mice used for plasma nicotine and cotinine experiments were not used for behavioral experiments.
2.6. Nicotinic acetylcholine receptor binding
To test whether changes in nAChR upregulation are associated with nicotine-induced alterations of behavior, pre-adolescent and adult mice were treated with 6.3 mg/kg/d nicotine or saline for 12 days and all pumps were removed on day 12 (n = 4 per group). This dose of chronic nicotine was selected because withdrawal from 6.3 mg/kg/d nicotine disrupted contextual fear conditioning in adults but not pre-adolescent mice (see results). Twenty-four hours later, mice were euthanized via cervical dislocation and the cortex, cerebellum, and hippocampus were collected for radioligand binding. Tissues were homogenized in 50 mM Tris HCl (Sigma-Aldrich, St. Louis, MO) buffer, pH 7.4 at 24°C, and centrifuged twice at 35,000 x g for 10 min in fresh buffer. The membrane pellets were resuspended in fresh buffer and added to tubes containing a saturating concentration (2 nM) of [3H]Epibatidine ([3H]EB) (PerkinElmer, Boston, MA), which binds with very high affinity to all heteromeric nAChR subtypes in brain. Incubations were performed in Tris buffer at pH 7.4 for 2 h at 24°C with [3H]EB. Bound receptors were separated from free ligand by vacuum filtration over GF/C glass-fiber filters (Brandel, Gaithersburg, MD) that were pretreated with 0.5% polyethyleneimine (Sigma-Aldrich, St. Louis, MO), and the filters were then counted in a liquid scintillation counter. Nonspecific binding was determined in the presence of 300μM nicotine, and specific binding was defined as the difference between total binding and nonspecific binding. Binding data was expressed as fmol/mg tissue (Turner, Castellano, and Blendy, 2011; Turner, Ortinski, Sherrard, and Kellar, 2011). Previous work has demonstrated that the effects of nicotine on contextual fear conditioning are mediated through high-affinity heteromeric nAChRs, such as the α4β2 nAChRs but not homomeric nAChRs, such as α7 nAChRs (Davis and Gould, 2006; 2009; Davis et al., 2007; Portugal et al., 2008). Thus, epibatidine binding was examined because epibatidine has higher affinity for heteromeric nAChRs versus homomeric nAChRs (Avalos, Parker, Maddox, Carroll, and Luetje, 2002; Houghtling, Davila-Garcia, and Kellar, 1995; Marks, Smith, and Collins, 1998; Xiao and Kellar, 2004).
2.7. Western blot analysis
To test whether changes in CREB activity would be seen 24 hours after withdrawal from chronic nicotine in pre-adolescent mice, osmotic mini-pumps containing 6.3 mg/kg/d nicotine or saline were implanted in pre-adolescent and adult mice (n = 4 per group). Mice were treated with chronic nicotine for 12 days and all pumps were removed on day 12. Twenty-four hours later, mice were euthanized via cervical dislocation and the cortex, cerebellum, and hippocampus were collected. Tissues were homogenized in 200 μl of ice-cold extraction buffer containing 50mM Tris, 1mM EGTA, 1mM EDTA, 1% SDS, and 1mM PMSF (pH 7.4). Protein concentrations were determined using a BCA assay, with bovine serum albumin as the standard. Prior to loading, 6x SDS Sample buffer (50mM Tris, 2.5% SDS, 36% glycerol, 0.03% bromophenol blue and 1M DTT) was added to each sample, which were then boiled for 5 min. Equivalent amounts of protein (30 μg) for each sample were resolved in 10% SDS-PAGE. After electrophoresis, proteins were transferred to nitrocellulose membranes. Membranes were incubated with LI-COR blocking buffer (LI-COR, Lincoln, Nebraska) for 1 hr at room temperature to block non-specific binding. The blots were reacted overnight at 4°C with primary antibodies (pCREB (1:1000, CREB phosphorylated at Ser-133, Cell Signaling Technology, Lake Placid, NY) and beta-tubulin (1:2000, BD biosciences, San Jose, CA)). After washing in PBS-T, the blots were incubated in fluorescent secondary antibodies (1:20,000, LI-COR, Lincoln, Nebraska) in LI-COR blocking buffer for 1 hr at RT. Membranes were then washed three times with PBS-T. Immunolabeling detection and densitometry measurements were performed using the LI-COR Odyssey System (LI-COR, Lincoln, Nebraska). The blots were stripped using LI-COR Newblot stripping buffer (5x) (LI-COR, Lincoln, Nebraska), washed, and reprobed with antibodies for both CREB (1:1000, Cell Signaling Technology, Lake Placid, NY) and the reference antibody (β-tubulin). Blots were re-scanned following stripping to ensure complete removal of signal. Ratios of pCREB or CREB to β-tubulin densities were calculated for each sample and analyzed across conditions.
2.8. Data Analysis
For each age group, data from all experiments investigating the effects of acute nicotine, chronic nicotine, and nicotine withdrawal on fear conditioning were analyzed with one-way ANOVAs. Data from an experiment investigating the effects of 0.023 mg/kg nicotine was analyzed with a 2 (age: pre-adolescent vs. adult) × 2 (drug: 0.023 mg/kg nicotine vs. saline) ANOVA. One-way ANOVAs were used for each age group to determine whether exposure to chronic nicotine during adolescence altered learning that occurred during adulthood. For plasma nicotine experiments, one-way ANOVAs were used to compare plasma nicotine, plasma cotinine, and the nicotine/cotinine ratio between pre-adolescent, adolescent, and adult mice. Nicotine/cotinine ratios are used to assess metabolic rate: lower nicotine/cotinine ratios indicates a rapid metabolism of nicotine or slower metabolism of cotinine (Rao, Hoffmann, Zia, Bodin, Zeman, Sellers, and Tyndale, 2000). Using the GraphPad Prism 5.0 software package (GraphPad Software, San Diego, CA), statistical analyses of data from nicotine binding and western blot experiments were assessed using two-way ANOVAs and when an interaction or main effect of drug treatment was detected, planned Bonferroni multiple comparisons were conducted comparing treatment conditions within age groups. A Levene statistic was used following each ANOVA to test for homogeneity of variance. Tukey post-hoc tests were used on data sets that did not violate the assumption of homogeneity of variance, whereas Games-Howell post-hoc tests were used when the assumption of homogeneity of variance was not met.
3. Results
3.1. Developmental effects of acute nicotine on fear conditioning
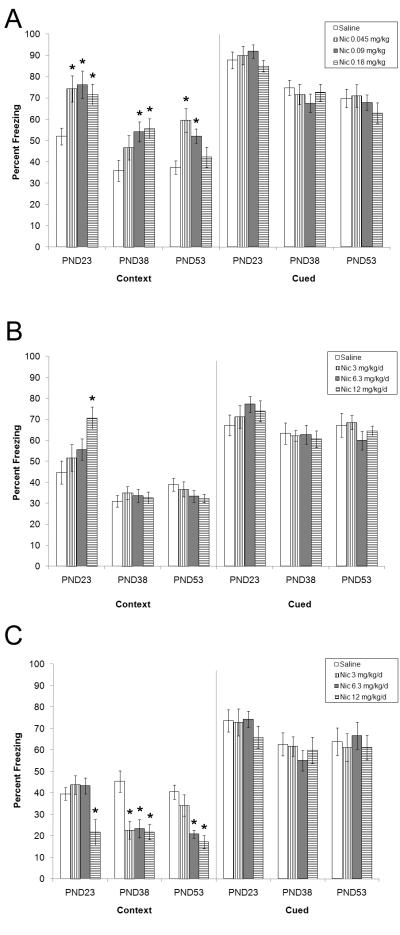
To investigate the effects of acute nicotine on fear conditioning across development, pre-adolescent, adolescent, and adult mice were administered saline, 0.045, 0.09, or 0.18 mg/kg nicotine prior to the training and testing of contextual and cued fear conditioning (Figure 2a). One-way ANOVAs revealed a significant main effect for drug treatment on contextual fear conditioning for all three age groups (pre-adolescent: [F(3, 35) = 4.88, p < 0.05]; adolescent: [F(3, 46) = 3.71, p < 0.05]; adult: [F(3, 42) = 6.80, p < 0.05]). Tukey post-hoc comparisons determined that pre-adolescent mice treated with 0.045, 0.09, or 0.18 mg/kg nicotine exhibited enhanced contextual fear conditioning relative to saline-treated pre-adolescent mice (p < 0.05), and adolescent mice that received 0.09 or 0.18 mg/kg nicotine had significantly greater contextual fear conditioning when compared to saline-treated adolescent mice (p < 0.05). Furthermore, adult mice that were administered 0.045 or 0.09 mg/kg nicotine exhibited enhanced contextual fear conditioning relative to saline-treated adults (p < 0.05). No significant effects of acute nicotine were observed for baseline freezing, immediate freezing, generalized freezing, or cued fear conditioning for all three age groups; means and standard errors for baseline freezing, immediate freezing, and generalized freezing can be found in Supplementary Table 1 (p > 0.05).
Figure 2.
The effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning in pre-adolescent, adolescent, and adult mice. A: Compared to adults, pre-adolescent mice were more sensitive to the effects of acute nicotine on contextual fear conditioning whereas adolescent mice were less sensitive. B: Pre-adolescent mice exhibited enhanced contextual fear conditioning when treated with 12 mg/kg/d chronic nicotine. C: Compared to adults, pre-adolescent mice were less sensitive to the effects of nicotine withdrawal on contextual fear conditioning whereas adolescent mice were more sensitive. No significant changes in cued fear conditioning were observed. Error bars indicate SEM, (*) indicates p < 0.05.
Given that both pre-adolescents and adults exhibited enhanced contextual fear conditioning when they were treated with 0.045 mg/kg nicotine, a separate experiment was conducted to identify the lowest dose of acute nicotine that would enhance contextual fear conditioning for both age groups. A 2 (age) × 2 (drug) ANOVA revealed a significant main effect of age on immediate freezing [F(1, 36) = 15.92, p < 0.05], contextual fear conditioning [F(1, 36) = 9.22, p < 0.05], and cued fear conditioning [F(1, 36) = 8.65, p < 0.05] (data not shown). The main effect of drug and the interaction between age and drug was not significant (p > 0.05; data not shown). Thus, these data demonstrate that 0.023 mg/kg nicotine has no effect on fear conditioning for both pre-adolescents and adults, but that pre-adolescents exhibit greater immediate freezing, contextual fear conditioning, and cued fear conditioning when compared to adults, regardless of drug treatment.
3.2. Developmental effects of chronic nicotine on fear conditioning
The effects of 3.0, 6.3, or 12.0 mg/kg/d chronic nicotine on fear conditioning were examined in mice that began chronic nicotine treatment during pre-adolescence, adolescence, or adulthood (Figure 2b). One-way ANOVAs revealed no effects of chronic nicotine on contextual fear conditioning for adolescent and adult mice (p > 0.05), suggesting the development of tolerance to the effects of nicotine. In contrast, a main effect of drug treatment was observed in pre-adolescent mice for contextual fear conditioning [F(3, 38) = 4.33, p < 0.05] and generalized freezing [F(3, 38) = 2.86, p < 0.05]. Subsequent Tukey post-hoc tests revealed that pre-adolescent mice treated with 12.0 mg/kg/d chronic nicotine exhibited enhanced contextual fear conditioning relative to saline-treated pre-adolescent mice (p < 0.05); no significant differences between groups were found for generalized freezing (p > 0.05). Chronic nicotine had no effect on baseline freezing, immediate freezing, and cued fear conditioning for all three age groups (p > 0.05); means and standard errors for baseline freezing, immediate freezing, and generalized freezing can be found in Supplementary Table 1.
3.3. Developmental effects of nicotine withdrawal on fear conditioning
To investigate the effects of nicotine withdrawal on fear conditioning, pre-adolescent, adolescent, or adult mice were withdrawn from 3.0, 6.3, or 12.0 mg/kg/d chronic nicotine and were trained in fear conditioning (Figure 2c). Withdrawal from chronic nicotine produced deficits in contextual fear conditioning for pre-adolescent [F(3, 28) = 6.63, p < 0.05], adolescent [F(3, 38) = 7.91, p < 0.05], and adult mice [F(3, 27) = 11.16, p < 0.05]. One-way ANOVAs also revealed an effect of drug treatment on immediate freezing in pre-adolescent mice [F(3, 28) = 3.66, p < 0.05], and generalized freezing in adult mice [F(3, 27) = 4.01, p < 0.05]. Tukey post-hoc tests found that pre-adolescent mice withdrawn from 12.0 mg/kg/d chronic nicotine showed impaired contextual fear conditioning relative to pre-adolescents that were withdrawn from chronic saline (p < 0.05), whereas adolescent mice withdrawn from all 3 doses of chronic nicotine had disrupted contextual fear conditioning when compared to adolescents that were withdrawn from chronic saline (p < 0.05). In addition, adult mice withdrawn from 6.3 or 12.0 mg/kg/d chronic nicotine exhibited withdrawal-related deficits in contextual fear conditioning relative to adult saline controls (p < 0.05). Games-Howell post-hoc tests found no differences between groups for immediate freezing in pre-adolescent mice, and for generalized freezing in adult mice (p > 0.05). Withdrawal from chronic nicotine had no effect on baseline freezing, or cued fear conditioning for all three age groups (p > 0.05); means and standard errors for baseline freezing, immediate freezing, and generalized freezing can be found in Supplementary Table 1.
As adolescents exhibited disrupted contextual fear conditioning following withdrawal from all 3 doses of chronic nicotine, we sought to determine whether withdrawal from lower doses of chronic nicotine (0.5 or 1.1 mg/kg/d) would also produce deficits. A one-way ANOVA found a significant main effect of withdrawal on contextual fear conditioning [F(2, 27) = 5.43, p < 0.05; data not shown], and Tukey post-hoc comparisons revealed that mice withdrawn from 1.1 mg/kg/d chronic nicotine had impaired contextual fear conditioning when compared to saline-withdrawn mice (data not shown; p < 0.05). Nicotine withdrawal did not alter baseline freezing, immediate freezing, generalized freezing, or cued fear conditioning (data not shown; p > 0.05). These data suggest that mice that start treatment during adolescence are very sensitive to the effects of nicotine withdrawal on hippocampus-dependent learning as withdrawal from low doses of chronic nicotine disrupted contextual fear conditioning.
3.4. Plasma nicotine and cotinine in pre-adolescent, adolescent, and adult mice
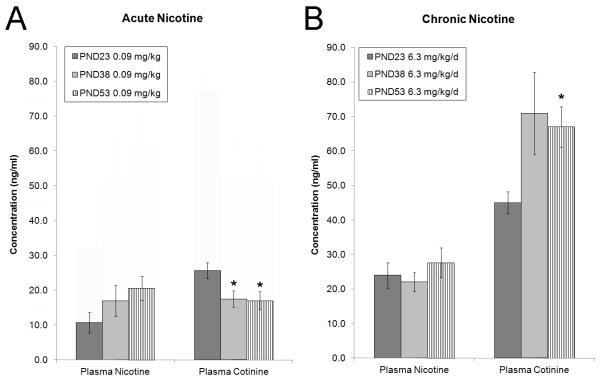
Plasma nicotine and cotinine samples were collected from pre-adolescent, adolescent, and adult mice to determine whether there were differences in nicotine and cotinine metabolism between the three age groups (Figure 3). One-way ANOVAs found no differences in plasma nicotine levels between all three age groups when mice were treated with acute nicotine or chronic nicotine (p > 0.05). However, an effect of age was observed for plasma cotinine levels when mice were treated with acute nicotine [F(2, 21) = 4.67, p < 0.05] or chronic nicotine [F(2, 21) = 3.63, p < 0.05]. A main effect of age was also observed for the nicotine/cotinine ratio in mice that received chronic nicotine [F(2, 21) = 3.55, p < 0.05]; no other significant differences were observed for nicotine/cotinine ratios and post-hoc analysis following up the main effect for chronic nicotine on nicotine/cotinine ratio did not detect any significant group differences. Tukey post-hoc tests revealed that pre-adolescent mice treated with 0.09 mg/kg nicotine had significantly higher plasma cotinine levels than adolescents and adults that were administered 0.09 mg/kg acute nicotine. Additionally, Games-Howell post hoc tests found that 6.3 mg/kg/d chronic nicotine produced significantly lower plasma cotinine levels in the pre-adolescent condition relative to adults.
Figure 3.
Developmental differences in plasma nicotine and cotinine following acute or chronic nicotine administration. A: Plasma cotinine was significantly higher in pre-adolescent mice that received 0.09 mg/kg nicotine relative to adolescents and adults. B: Plasma cotinine levels differed between pre-adolescent and adult mice treated with 6.3 mg/kg/d chronic nicotine. Error bars indicate SEM, (*) indicates p < 0.05.
3.5. Nicotine receptor binding
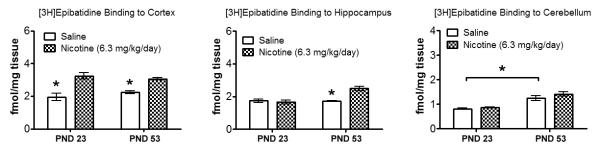
Changes in radioligand binding for [3H]Epibatidine in the cortex, hippocampus, and cerebellum were examined in pre-adolescent and adult mice that were withdrawn from 6.3 mg/kg/d chronic nicotine for 24 hours because age groups differed in withdrawal phenotype for this treatment (Figure 4). For cortical binding data, a 2 (treatment: nicotine, saline) × 2 (age: pre-adolescent, adult) ANOVA revealed a significant main effect for treatment [F(1, 12) = 45.12, p < 0.05], whereas the main effect of age and the interaction between age and treatment were not significant (p > 0.05). Analysis of hippocampal data by a 2 × 2 ANOVA revealed significant main effects of age [F(1, 12) = 16.66, p < 0.05] and treatment [F(1, 12) = 11.62, p < 0.05], and a significant interaction between age and treatment [F(1, 12) = 14.81, p < 0.05]. In the cerebellum, receptor binding differed between pre-adolescents and adults [F(1, 12) = 14.81, p < 0.05], but nicotine did not produce changes in receptor binding, nor was there an interaction between age and treatment (p > 0.05). Bonferroni post-hoc tests revealed that pre-adolescents and adults withdrawn from 6.3 mg/kg/d nicotine had a significant upregulation of cortical nAChRs relative to saline treated mice (p < 0.05). In contrast, hippocampal nAChRs were upregulated in the adults (p < 0.05), but no such effects were observed in the pre-adolescents.
Figure 4.
[3H]Epibatidine binding in the cortex, hippocampus, and cerebellum 24 hours after withdrawal from chronic nicotine. Hippocampal nAChRs were upregulated in adult mice but not in pre-adolescent mice, whereas cortical nAChRs were upregulated during nicotine withdrawal for both age groups. Adult mice had a greater number of cerebellar nAChRs, regardless of treatment. Error bars indicate SEM, (*) indicates p < 0.05.
3.6. Western blot analysis
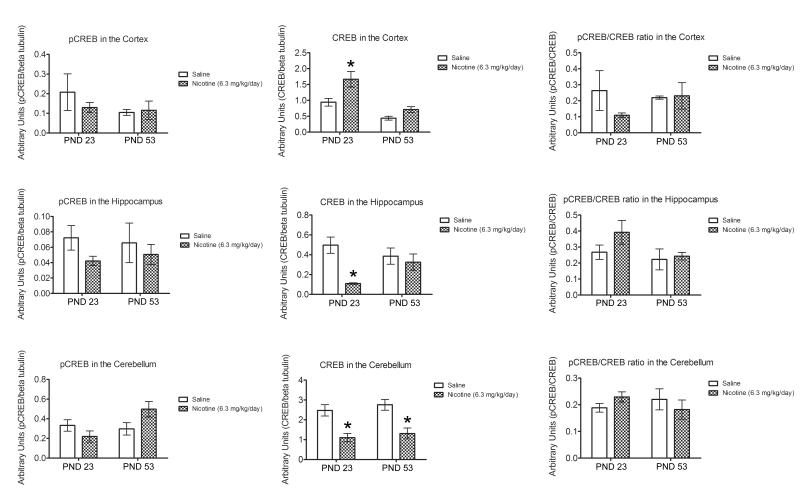
Phosphorylated CREB (pCREB), total CREB activity, and pCREB/CREB ratio were measured in the cortex, hippocampus, and cerebellum 24 hours after withdrawal from 6.3 mg/kg/d nicotine in pre-adolescent and adult groups because the age groups differed in withdrawal phenotype for this treatment (Figure 5). In the cortex, a 2 (treatment: nicotine, saline) × 2 (age: pre-adolescent, adult) ANOVA for total CREB revealed a significant main effects for treatment [F(1, 10) = 9.01, p < 0.05] and age [F(1, 10) = 19.22, p < 0.05], but the interaction between age and treatment was not significant (p > 0.05). Planned Bonferroni comparisons revealed that total CREB in the cortex was increased in pre-adolescents withdrawn from 6.3 mg/kg/d nicotine relative to saline treated mice (p < 0.05), whereas no effect was observed in adults. No significant differences were found for cortical pCREB, or for the pCREB/CREB ratio (p > 0.05).
Figure 5.
Changes in pCREB, total CREB, and a pCREB/CREB ratio in the cortex, hippocampus, and cerebellum 24 hours after withdrawal from chronic nicotine. Developmental effects in total CREB were observed in the cortex and hippocampus, whereas no effects were observed in pCREB and the pCREB/CREB ratio. Nicotine withdrawal decreased total CREB in the cerebellum for both pre-adolescents and adults. Error bars indicate SEM, (*) indicates p < 0.05.
A 2 × 2 ANOVA for total CREB in the hippocampus revealed a significant main effect of treatment [F(1, 10) = 10.61, p < 0.05] and a significant interaction between age and treatment [F(1, 10) = 5.57, p < 0.05], whereas the main effect of age was not significant (p > 0.05). Planned Bonferroni comparisons revealed that total CREB was decreased in pre-adolescents withdrawn from chronic nicotine compared to the saline group (p < 0.05), but no treatment effects were observed in adults. No significant differences were found for hippocampal pCREB, or for the pCREB/CREB ratio (p > 0.05).
In the cerebellum, a main effect of treatment on total CREB [F(1, 10) = 28.40, p < 0.05], and a significant interaction between treatment and age for pCREB [F(1, 10) = 6.00, p < 0.05] were found. Planned Bonferroni comparisons indicated that total CREB in the cerebellum was decreased in both pre-adolescents and adults withdrawn from chronic nicotine when compared to saline treated mice (p > 0.05) but found no significant group differences for pCREB. No other main effects or interactions for cerebellar data were statistically significant (p > 0.05).
3.7. Developmental effects of prior chronic nicotine exposure on fear conditioning
This experiment investigated whether exposure to chronic nicotine during development altered fear conditioning acquired during adulthood. Specifically, pre-adolescent, adolescent, and adult mice were treated with 8.8, 12.0 mg/kg/d chronic nicotine or saline, and pumps were removed after 12 days of treatment. Thirty days later, mice were trained and tested in fear conditioning (Figure 6). Mice that were treated with chronic nicotine during pre-adolescence and adolescence exhibited impaired contextual fear conditioning during adulthood (pre-adolescent: [F(2, 34) = 19.11, p < 0.05]; adolescent: [F(2, 35) = 9.81, p < 0.05]). In contrast, mice that were administered chronic nicotine during adulthood showed no changes in contextual fear conditioning when they were trained and tested later during adulthood (p > 0.05). Tukey post-hoc tests revealed that mice treated with both doses of chronic nicotine during pre-adolescence exhibited impaired contextual fear conditioning during adulthood, whereas mice that received 12.0 mg/kg/d chronic nicotine during adolescence showed disrupted contextual fear conditioning during adulthood (p < 0.05). Baseline freezing, immediate freezing, generalized freezing, and cued fear conditioning were not altered by chronic nicotine exposure for all three age groups (p > 0.05).
Figure 6.
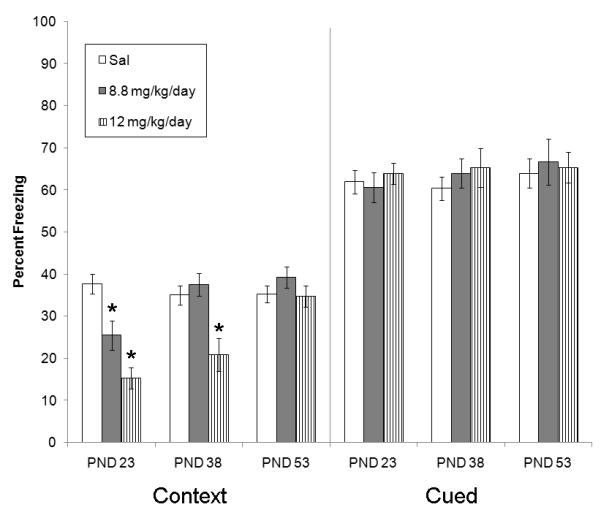
The effects of prior chronic nicotine exposure on contextual and cued fear conditioning. Pre-adolescent and adolescent chronic nicotine exposure disrupted contextual fear conditioning that occurred during adulthood, whereas adult chronic nicotine exposure did not alter fear conditioning. No significant changes in cued fear conditioning were observed. Error bars indicate SEM, (*) indicates p < 0.05.
4. Discussion
The present study is the first to characterize the developmental effects of acute, chronic, and withdrawal from chronic nicotine on contextual learning and to examine possible neural changes that may mediate these effects. Differences in sensitivity to the effects of acute nicotine, chronic nicotine, and nicotine withdrawal on contextual learning were observed in pre-adolescent, adolescent, and adult mice. Furthermore, age-dependent differences in the effects of nicotine withdrawal on contextual learning assessed 24 hours after cessation of treatment were associated with changes in hippocampal high-affinity nAChR binding but did not appear to be related to differences in nicotine metabolism or CREB levels. In addition, exposure to chronic nicotine during pre-adolescence or adolescence also disrupted contextual learning that occurred during adulthood, suggesting that chronic nicotine exposure during these developmental periods can lead to long-lasting alterations of contextual learning; it is possible that the observed nicotine-related developmental increase in cortical CREB and the decrease in hippocampal CREB contribute to this effect. Although exposure to nicotine altered contextual fear conditioning, no changes in cued fear conditioning were found in any experiments. Therefore, these data demonstrate that the developmental effects of nicotine were specific to contextual learning and that nicotine did not alter processes that could impact both types of learning.
Because adolescence is associated with increased drug use and increased vulnerability for development of addiction (Breslau and Peterson, 1996; Crews, He, and Hodge, 2007), an important issue is how developmental differences in the behavioral and neural effects of nicotine could contribute to addiction. A dramatic finding from the present study is that a relatively brief exposure to nicotine during preadolescence and adolescence lead to adult deficits in hippocampus-dependent learning. This has important implications for understanding nicotine addiction because lower levels of cognitive function may be a risk factor for addiction (Block et al., 2002) and clinical studies have reported that cigarette smoking is associated with cognitive deficits in middle-aged and elderly adults (Cervilla et al., 2000; Nooyens et al., 2008; Richards et al., 2003). In the present study, mice treated with chronic nicotine during pre-adolescence and adolescence had impaired contextual fear conditioning during adulthood, 30 days after chronic nicotine treatment ended, whereas adult chronic nicotine exposure had no effect on fear conditioning. Furthermore, earlier onset of chronic nicotine exposure was associated with greater sensitivity as treatment with a lower dose of chronic nicotine during pre-adolescence, but not adolescence, also disrupted contextual fear conditioning during adulthood. These deficits most likely do not reflect a global change but may be selective for hippocampus-dependent learning as contextual fear conditioning was disrupted but cued fear conditioning, which in contrast does not require the hippocampus (Phillips and LeDoux, 1992), was spared. Overall, these data suggest that pre-adolescent and adolescent nicotine exposure can lead to cognitive impairment during adulthood, which in turn may lead to poor or impulsive decision-making that increases the risk for drug addiction. In support, it has been shown that an earlier age of smoking initiation is associated with a more severe addiction to tobacco and an increased likelihood of drug use (Everett et al., 1999; Hanna and Grant, 1999).
Few studies to date have investigated whether adolescent nicotine exposure produces changes in neural functioning that persist during adulthood, but some evidence suggests that adolescent nicotine exposure alters cholinergic function and adenylyl cyclase activity, which may lead to changes in associative learning. Slotkin and colleagues (2007) reported that markers of acetylcholine synthesis and function were reduced in the cortex and elevated in the hippocampus in adults that received chronic nicotine during adolescence. Furthermore, adolescent nicotine treatment altered the response to chronic nicotine treatment that occurred during adulthood (Slotkin, Bodwell, Ryde, and Seidler, 2008), suggesting a change in cholinergic function. Adenylyl cyclase (AC) activity in the cortex was also decreased in adults that received adolescent nicotine exposure, whereas this effect was less robust in adults that received chronic nicotine earlier during adulthood (Slotkin, Ryde, Mackillop, Bodwell, and Seidler, 2008). Changes in AC activity can lead to alterations of gene transcription factors such as CREB (Shaywitz and Greenberg, 1999), and CREB is critically involved in long-term memory associated with contextual fear conditioning (Bourtchuladze et al., 1994). Thus, changes in CREB could contribute to the nicotine-associated cognitive deficits.
The developmental change that results in adult deficits in learning after juvenile exposure to nicotine is unknown but it could involve nicotine-induced changes in patterns of gene expression during development. In the present study, pre-adolescent mice withdrawn from 6.3 mg/kg/d nicotine for 24 hours had increased total CREB in the cortex and decreased total CREB in the hippocampus, whereas these effects were not observed in adults. While these changes in total CREB activity were not associated with immediate nicotine withdrawal deficits in contextual fear conditioning, pre-adolescent changes in CREB due to chronic nicotine exposure could alter gene expression and brain development leading to functional changes that emerge during adulthood. This may be particularly problematic as the human brain continues to develop into the 20’s (Casey et al., 2005) and the period of adolescence to early adulthood is associated with greater experimentation with tobacco (Giovino, 1999; Lantz, 2003), and increased vulnerability to develop nicotine addiction (Breslau and Peterson, 1996).
In addition to the long-term effects of developmental nicotine exposure, altered sensitivity to the immediate acute and chronic effects of nicotine during development may also contribute to nicotine addiction. In the present study, pre-adolescent mice exhibited enhanced contextual learning over a broader range of acute nicotine doses than adolescents and adults, while adolescent mice had a rightward shift in the dose response compared to adult mice. These results suggest that the maturation of the nicotinic acetylcholine system goes through multiple phases of development that effect sensitivity to agonists. The enhanced sensitivity to acute nicotine in pre-adolescent mice is consistent with previous studies demonstrating that younger rodents exhibit greater sensitivity to the reinforcing and rewarding properties of nicotine (Chen, Matta, and Sharp, 2007; Kota, Martin, and Damaj, 2008; Kota, Martin, Robinson, and Damaj, 2007; Levin, Lawrence, Petro, Horton, Rezvani, Seidler, and Slotkin, 2007; Levin, Rezvani, Montoya, Rose, and Swartzwelder, 2003; Shram, Funk, Li, and Le, 2006; Shram and Le, 2010; Torres, Natividad, Tejeda, Van Weelden, and O’Dell, 2009; Torres, Tejeda, Natividad, and O’Dell, 2008; Vastola, Douglas, Varlinskaya, and Spear, 2002). Thus, it is possible that in younger smokers enhanced effects of acute nicotine on learning along with increased sensitivity to the rewarding properties of nicotine could promote the formation of strong drug-stimulus associations that could later trigger stimulus-induced cravings that contribute to continued nicotine use and increased risk for addiction.
Continued use of nicotine can lead to tolerance to its initial effects (Collins, Romm, Selvaag, Turner, and Marks, 1993; Grabus, Martin, Batman, Tyndale, Sellers, and Damaj, 2005) and we have demonstrated in adult mice that chronic nicotine treatment does not alter contextual fear conditioning, even though the dose selected produced similar plasma nicotine levels as an effective dose of acute nicotine (Davis et al., 2005; Portugal, Wilkinson, Kenney, Sullivan, and Gould, 2012). In the present study, pre-adolescents showed enhanced contextual fear conditioning during treatment with the highest dose of chronic nicotine, whereas chronic nicotine did not alter fear conditioning in adolescents or adults. Previous studies have reported that tolerance to the behavioral effects of nicotine is related to nAChR desensitization (Robinson, James, Lapp, Vann, Gross, Philibin, and Rosecrans, 2006; Robinson, Vann, Britton, O’Connell, James, and Rosecrans, 2007). Therefore, it is possible that nAChR desensitization does not occur or is altered in pre-adolescent mice, which in turn may explain why treatment with higher doses of chronic nicotine enhanced contextual learning. These results suggest that the younger brain reacts differently to chronic nicotine than the older brain.
It has been proposed that reduced sensitivity to the aversive effects of nicotine withdrawal during adolescence paired with increased pleasurable effects may facilitate continued use of nicotine (O’Dell, 2009). However, it is also likely that increased sensitivity to nicotine withdrawal-related deficits in cognition may lead to continued nicotine use as smokers may relapse in order to ameliorate withdrawal symptoms. In the present study, pre-adolescent mice exhibited low sensitivity to the effects of nicotine withdrawal on contextual learning whereas adolescent mice had greater sensitivity. Compared to pre-adolescents and adolescents, adults displayed an intermediate level of sensitivity to the effects and nicotine withdrawal on contextual learning. This suggests that there are multiple distinct developmental periods for the effects of nicotine withdrawal on learning. Perhaps young smokers continue to smoke assuming that they are not becoming addicted because of a lack of withdrawal effects but this continued smoking contributes to brain changes underlying addiction and the later emergence of withdrawal symptoms.
Previous studies have demonstrated that the effects of nicotine withdrawal on anxiety (Kota et al., 2007; Wilmouth and Spear, 2006), conditioned place avoidance (O’Dell, Torres, Natividad, and Tejeda, 2007), and somatic signs (Kota et al., 2007; O’Dell, Bruijnzeel, Ghozland, Markou, and Koob, 2004; O’Dell et al., 2006; Shram, Siu, Li, Tyndale, and Le, 2008) are reduced in adolescent rodents. Our finding that adolescent mice were more sensitive to the effects of nicotine withdrawal, which seems contrary to the aforementioned results, could be related to strain/species difference, a difference in the age tested, and/or differences in behavioral measures. For instance, background genotype influences the effects of acute nicotine and nicotine withdrawal on hippocampus-dependent learning (Portugal et al., 2012), and thus it is possible that a genotype by developmental stage interaction could influence the withdrawal phenotype. If such an interaction existed, indentifying genetic risk factors could inform on optimal therapeutics for treating nicotine addiction based on genotype and developmental stage.
Another factor that could contribute to the differences in results between studies of adolescent nicotine exposure is the age at which nicotine is withdrawn. Rodents in prior studies were withdrawn from chronic nicotine during adolescence, whereas in the present study mice were treated with chronic nicotine during pre-adolescence or adolescence, but withdrawn during a later developmental period (adolescence or adulthood, respectively). Therefore, it is possible that sensitivity to the effects of nicotine withdrawal on contextual learning is reduced when withdrawal occurs during adolescence, whereas sensitivity to this effect increases if nicotine is withdrawn during adulthood. It is also possible that adolescents are less sensitive to physical, anxiety, and affective withdrawal symptoms but more sensitive to symptoms related to cognitive processes. In support, Wilmouth and Spear (2006) found that adolescent rats were less sensitive than adults to the effects of nicotine withdrawal on anxiety but more sensitive to the effects of nicotine withdrawal on prepulse inhibition of the acoustic startle reflex, a measure of sensorimotor gating.
The effects of nicotine withdrawal on contextual learning require hippocampal β2-containing nAChRs (i.e, high-affinity nAChRs) but not α7 nAChRs (i.e,. low-affinity nAChRs) (Davis and Gould, 2009; Portugal et al., 2008). Therefore, developmental differences in the effect of chronic nicotine and nicotine withdrawal on high-affinity nAChR function or number may alter sensitivity to the effects of nicotine withdrawal on contextual learning. In the present study, adult mice had disrupted contextual fear conditioning and upregulated high-affinity hippocampal nAChR binding following withdrawal from 6.3 mg/kg/d chronic nicotine, whereas both effects were absent in pre-adolescent mice. Furthermore, this age-related difference in nAChR upregulation by nicotine was specific to the hippocampus, as cortical nAChR upregulation was observed during nicotine withdrawal for both age groups. Together, these data suggest that high-affinity nAChR upregulation in the hippocampus may play a critical role in nicotine withdrawal-induced deficits of contextual fear conditioning.
In adults, treatment with chronic nicotine produces both desensitization and an upregulation of β2-containing nAChRs (Buisson and Bertrand, 2001; Flores, Rogers, Pabreza, Wolfe, and Kellar, 1992; Mansvelder, van Aerde, Couey, and Brussaard, 2006; Marks, Burch, and Collins, 1983; Pidoplichko, DeBiasi, Williams, and Dani, 1997; Schwartz and Kellar, 1985). During withdrawal, nAChR upregulation may persist while nAChR shift from a desensitized state to an active state (Dani and Heinemann, 1996); this inferred increase in nAChR function during nicotine withdrawal could lead to impairments in contextual learning as seen in the present study. In support, recent evidence demonstrated that the duration of hippocampal high-affinity nAChR upregulation after nicotine withdrawal paralleled the duration of deficits in hippocampus-dependent learning (Gould, Portugal, Andre, Tadman, Marks, Kenney, Yildirim, and Adoff, 2012) and that nicotine withdrawal was associated with a persistent increase in hippocampal CA1 pyramidal cell activity (Penton, Quick, and Lester, 2011). In contrast, the absence of nAChR upregulation in pre-adolescent mice during nicotine withdrawal may prevent a withdrawal-induced change of nAChR function, such as increased sensitivity, that could contribute to deficits in contextual learning.
Although adolescent nicotine exposure alters nAChR upregulation, it is possible that developmental differences in the metabolism of nicotine or cotinine (the primary metabolite of nicotine) may also play a role in the age-dependent effects of nicotine on contextual learning. For acute and chronic nicotine, no significant age-related differences in the nicotine levels were found. In addition, post-hoc analysis found no significant differences in nicotine/cotinine ratios, a measure of metabolism (Rao et al., 2000). Developmental differences were seen in cotinine levels for both the acute treatment and the chronic treatment. However, for the acute treatment, 0.09 mg/kg produced higher cotinine levels in only the youngest group but all three age groups showed enhanced learning with this dose of nicotine suggesting that differences in cotinine levels were not driving behavioral effects. For chronic nicotine treatment, 6.3 mg/kg/day produced the lowest cotinine levels in the youngest group and this group was the only group that did not show withdrawal-associated deficits in learning at this dose. Overall, these results suggest that changes in plasma nicotine levels and metabolism are not a primary factor in the behavioral differences and while there are differences in cotinine levels, those differences may not be a primary factor either. Studies investigating nicotine metabolism in rats have demonstrated that adolescents metabolize nicotine more rapidly than adults (O’Dell et al., 2006; Trauth, Seidler, and Slotkin, 2000b) but display fewer somatic signs during nicotine withdrawal even when plasma nicotine levels are matched (O’Dell et al., 2006). While we did not see an age-related difference in nicotine metabolism, which may be a species specific difference, the conclusion of O’Dell and colleagues (2006) that metabolism is not the primary factor underlying age-related withdrawal differences is consistent with the present study.
Taken together, a model based on the current results can be used to explain how the developmental effects of nicotine on learning could facilitate the acquisition and maintenance of nicotine addiction. The enhancement of contextual learning by acute nicotine could lead to the development of drug-context associations that may later evoke cravings that contribute to nicotine addiction; a greater sensitivity to the effects of acute nicotine in younger smokers may result in even stronger drug-context memories. During nicotine withdrawal, hippocampus-dependent learning is disrupted and this impairment of cognition may promote relapse; however, if younger smokers are less sensitive to nicotine withdrawal they may falsely believe that they are not becoming addicted and continue to smoke. Finally, pre-adolescent and adolescent nicotine exposure could alter brain development producing impairments in learning and other cognitive processes that emerge in adulthood. Altered cognitive processes could facilitate poor or impulsive decision making that might contribute to continued use of tobacco. Thus, the developmental effects of nicotine on cognition may play an important role in determining risk for nicotine addiction, and an improved understanding of how developmental nicotine exposure impacts cognition and the underlying neural substrates may lead to more effective treatments for nicotine addiction.
Supplementary Material
We examined developmental effects of nicotine on contextual learning.
We examined age-related changes in nAChR binding and CREB expression.
Age-related differences existed in the effects of nicotine on contextual learning.
Age-related differences in nAChR binding were associated with withdrawal deficits.
Chronic nicotine exposure during adolescence impaired learning during adulthood.
Acknowledgements
The authors would like to acknowledge grant support from the National Institute on Drug Abuse (DA017949 TG; DA024787 TG), and the National Cancer Institute (CA143187 PI: Caryn Lerman PhD), and thank Dr. Randy James of Virginia Commonwealth University for his analysis of plasma samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11:180–187. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- Abreu-Villaca Y, Seidler FJ, Qiao D, Tate CA, Cousins MM, Thillai I, Slotkin TA. Short-term adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology. 2003;28:1935–1949. doi: 10.1038/sj.npp.1300221. [DOI] [PubMed] [Google Scholar]
- Adriani W, Deroche-Gamonet V, Le Moal M, Laviola G, Piazza PV. Preexposure during or following adolescence differently affects nicotine-rewarding properties in adult rats. Psychopharmacology (Berl) 2006;184:382–390. doi: 10.1007/s00213-005-0125-1. [DOI] [PubMed] [Google Scholar]
- Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci. 2003;23:4712–4716. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André JM, Gulick D, Portugal GS, Gould TJ. Nicotine withdrawal disrupts both foreground and background contextual fear conditioning but not pre-pulse inhibition of the acoustic startle response in C57BL/6 mice. Behav Brain Res. 2008;190:174–181. doi: 10.1016/j.bbr.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avalos M, Parker MJ, Maddox FN, Carroll FI, Luetje CW. Effects of pyridine ring substitutions on affinity, efficacy, and subtype selectivity of neuronal nicotinic receptor agonist epibatidine. J Pharmacol Exp Ther. 2002;302:1246–1252. doi: 10.1124/jpet.102.035899. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Block RI, Erwin WJ, Ghoneim MM. Chronic drug use and cognitive impairments. Pharmacology Biochemistry and Behavior. 2002;73:491–504. doi: 10.1016/s0091-3057(02)00816-x. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL. Smoking cessation in young adults: age at initiation of cigarette smoking and other suspected influences. Am J Public Health. 1996;86:214–220. doi: 10.2105/ajph.86.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Chronic exposure to nicotine upregulates the human (alpha)4((beta)2 nicotinic acetylcholine receptor function. J Neurosci. 2001;21:1819–1829. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Cervilla JA, Prince M, Mann A. Smoking, drinking, and incident cognitive impairment: a cohort community based study included in the Gospel Oak project. J Neurol Neurosurg Psychiatry. 2000;68:622–626. doi: 10.1136/jnnp.68.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2007;32:700–709. doi: 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- Collins AC, Romm E, Selvaag S, Turner S, Marks MJ. A comparison of the effects of chronic nicotine infusion on tolerance to nicotine and cross-tolerance to ethanol in long- and short-sleep mice. J Pharmacol Exp Ther. 1993;266:1390–1397. [PubMed] [Google Scholar]
- Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer AN, De Vries TJ, Smit AB, Pattij T. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology. 2009;34:299–306. doi: 10.1038/npp.2008.96. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Dani JA, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16:905–908. doi: 10.1016/s0896-6273(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. The effects of DHBE and MLA on nicotine-induced enhancement of contextual fear conditioning in C57BL/6 mice. Psychopharmacology (Berl) 2006;184:345–352. doi: 10.1007/s00213-005-0047-y. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. beta2 subunit-containing nicotinic receptors mediate the enhancing effect of nicotine on trace cued fear conditioning in C57BL/6 mice. Psychopharmacology (Berl) 2007;190:343–352. doi: 10.1007/s00213-006-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Hippocampal nAChRs mediate nicotine withdrawal-related learning deficits. Eur Neuropsychopharmacol. 2009;19:551–561. doi: 10.1016/j.euroneuro.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. J Neurosci. 2007;27:10870–10877. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Porter J, Gould TJ. Nicotine enhances both foreground and background contextual fear conditioning. Neurosci Lett. 2006;394:202–205. doi: 10.1016/j.neulet.2005.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBry SC, Tiffany ST. Tobacco-induced neurotoxicity of adolescent cognitive development (TINACD): a proposed model for the development of impulsivity in nicotine dependence. Nicotine Tob Res. 2008;10:11–25. doi: 10.1080/14622200701767811. [DOI] [PubMed] [Google Scholar]
- Everett SA, Warren CW, Sharp D, Kann L, Husten CG, Crossett LS. Initiation of cigarette smoking and subsequent smoking behavior among U.S. high school students. Prev Med. 1999;29:327–333. doi: 10.1006/pmed.1999.0560. [DOI] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- Giovino GA. Epidemiology of tobacco use among US adolescents. Nicotine Tob Res. 1999;1(Suppl 1):S31–40. doi: 10.1080/14622299050011571. [DOI] [PubMed] [Google Scholar]
- Giovino GA. Epidemiology of tobacco use in the United States. Oncogene. 2002;21:7326–7340. doi: 10.1038/sj.onc.1205808. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Higgins SJ. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem. 2003;80:147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Portugal GS, Andre JM, Tadman MP, Marks MJ, Kenney JW, Yildirim E, Adoff M. The duration of nicotine withdrawal-associated deficits in contextual fear conditioning parallels changes in hippocampal high affinity nicotinic acetylcholine receptor upregulation. Neuropharmacology. 2012;62:2118–2125. doi: 10.1016/j.neuropharm.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behav Brain Res. 1999;102:31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Batman AM, Tyndale RF, Sellers E, Damaj MI. Nicotine physical dependence and tolerance in the mouse following chronic oral administration. Psychopharmacology (Berl) 2005;178:183–192. doi: 10.1007/s00213-004-2007-3. [DOI] [PubMed] [Google Scholar]
- Hanna EZ, Grant BF. Parallels to early onset alcohol use in the relationship of early onset smoking with drug use and DSM-IV drug and depressive disorders: findings from the National Longitudinal Epidemiologic Survey. Alcohol Clin Exp Res. 1999;23:513–522. [PubMed] [Google Scholar]
- Houghtling RA. Characterization of (+/−)(−)[3H]epibatidine binding to nicotinic cholinergic receptors in rat and human brain. Mol Pharmacol. 1995;48:280–287. [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975-2008. Volume I: Secondary school students. National Institute on Drug Abuse; Bethesda, MD: 2009. [Google Scholar]
- Keene DE, Suescun MO, Bostwick MG, Chandrashekar V, Bartke A, Kopchick JJ. Puberty is delayed in male growth hormone receptor gene-disrupted mice. J Androl. 2002;23:661–668. [PubMed] [Google Scholar]
- Kenney JW, Adoff MD, Wilkinson DS, Gould TJ. The effects of acute, chronic, and withdrawal from chronic nicotine on novel and spatial object recognition in male C57BL/6J mice. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Damaj MI. Age-dependent differences in nicotine reward and withdrawal in female mice. Psychopharmacology (Berl) 2008;198:201–210. doi: 10.1007/s00213-008-1117-8. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Lantz PM. Smoking on the rise among young adults: implications for research and policy. Tob Control. 2003;12(Suppl 1):i60–70. doi: 10.1136/tc.12.suppl_1.i60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol. 2007;29:458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology (Berl) 2003;169:141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci. 1997;111:104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, van Aerde KI, Couey JJ, Brussaard AB. Nicotinic modulation of neuronal networks: from receptors to cognition. Psychopharmacology (Berl) 2006;184:292–305. doi: 10.1007/s00213-005-0070-z. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther. 1983;226:817–825. [PubMed] [Google Scholar]
- Marks MJ, Smith KW, Collins AC. Differential agonist inhibition identifies multiple epibatidine binding sites in mouse brain. J Pharmacol Exp Ther. 1998;285:377–386. [PubMed] [Google Scholar]
- Naidong W, Shou W, Chen YL, Jiang X. Novel liquid chromatographic-tandem mass spectrometric methods using silica columns and aqueous-organic mobile phases for quantitative analysis of polar ionic analytes in biological fluids. J Chromatogr B Biomed Sci Appl. 2001;754:387–399. doi: 10.1016/s0378-4347(01)00021-4. [DOI] [PubMed] [Google Scholar]
- Nelson DE, Mowery P, Asman K, Pederson LL, O’Malley PM, Malarcher A, Maibach EW, Pechacek TF. Long-term trends in adolescent and young adult smoking in the United States: metapatterns and implications. Am J Public Health. 2008;98:905–915. doi: 10.2105/AJPH.2007.115931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooyens AC, van Gelder BM, Verschuren WM. Smoking and cognitive decline among middle-aged men and women: the Doetinchem Cohort Study. Am J Public Health. 2008;98:2244–2250. doi: 10.2105/AJPH.2007.130294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE. A psychobiological framework of the substrates that mediate nicotine use during adolescence. Neuropharmacology. 2009;56(Suppl 1):263–278. doi: 10.1016/j.neuropharm.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Ghozland S, Markou A, Koob GF. Nicotine withdrawal in adolescent and adult rats. Ann N Y Acad Sci. 2004;1021:167–174. doi: 10.1196/annals.1308.022. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Richardson HN, Koob GF, Markou A. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology (Berl) 2006;186:612–619. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Torres OV, Natividad LA, Tejeda HA. Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicol Teratol. 2007;29:17–22. doi: 10.1016/j.ntt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penton RE, Quick MW, Lester RA. Short- and long-lasting consequences of in vivo nicotine treatment on hippocampal excitability. J Neurosci. 2011;31:2584–2594. doi: 10.1523/JNEUROSCI.4362-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Bupropion dose-dependently reverses nicotine withdrawal deficits in contextual fear conditioning. Pharmacol Biochem Behav. 2007;88:179–187. doi: 10.1016/j.pbb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Kenney JW, Gould TJ. beta2 subunit containing acetylcholine receptors mediate nicotine withdrawal deficits in the acquisition of contextual fear conditioning. Neurobiol Learn Mem. 2008;89:106–113. doi: 10.1016/j.nlm.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Wilkinson DS, Kenney JW, Sullivan C, Gould TJ. Strain-dependent Effects of Acute, Chronic, and Withdrawal from Chronic Nicotine on Fear Conditioning. Behav Genet. 2012;42:133–150. doi: 10.1007/s10519-011-9489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Y, Hoffmann E, Zia M, Bodin L, Zeman M, Sellers EM, Tyndale RF. Duplications and defects in the CYP2A6 gene: identification, genotyping, and in vivo effects on smoking. Mol Pharmacol. 2000;58:747–755. doi: 10.1124/mol.58.4.747. [DOI] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. Extracellular signal-regulated kinase 1/2 involvement in the enhancement of contextual fear conditioning by nicotine. Behav Neurosci. 2007;121:1119–1124. doi: 10.1037/0735-7044.121.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Portugal GS, Lerman C, Gould TJ. Varenicline ameliorates nicotine withdrawal-induced learning deficits in C57BL/6 mice. Behav Neurosci. 2008;122:1166–1171. doi: 10.1037/a0012601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M, Jarvis MJ, Thompson N, Wadsworth ME. Cigarette smoking and cognitive decline in midlife: evidence from a prospective birth cohort study. Am J Public Health. 2003;93:994–998. doi: 10.2105/ajph.93.6.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SE, James JR, Lapp LN, Vann RE, Gross DF, Philibin SD, Rosecrans JA. Evidence of cellular nicotinic receptor desensitization in rats exhibiting nicotine-induced acute tolerance. Psychopharmacology (Berl) 2006;184:306–313. doi: 10.1007/s00213-005-0049-9. [DOI] [PubMed] [Google Scholar]
- Robinson SE, Vann RE, Britton AF, O’Connell MM, James JR, Rosecrans JA. Cellular nicotinic receptor desensitization correlates with nicotine-induced acute behavioral tolerance in rats. Psychopharmacology (Berl) 2007;192:71–78. doi: 10.1007/s00213-006-0687-6. [DOI] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. In vivo regulation of [3H]acetylcholine recognition sites in brain by nicotinic cholinergic drugs. J Neurochem. 1985;45:427–433. doi: 10.1111/j.1471-4159.1985.tb04005.x. [DOI] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl) 2006;186:201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Le AD. Adolescent male Wistar rats are more responsive than adult rats to the conditioned rewarding effects of intravenously administered nicotine in the place conditioning procedure. Behav Brain Res. 2010;206:240–244. doi: 10.1016/j.bbr.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Siu EC, Li Z, Tyndale RF, Le AD. Interactions between age and the aversive effects of nicotine withdrawal under mecamylamine-precipitated and spontaneous conditions in male Wistar rats. Psychopharmacology (Berl) 2008;198:181–190. doi: 10.1007/s00213-008-1115-x. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Thorsell AK, El Khoury A, Mathe AA, Ehlers CL. Increased CRF-like and NPY-like immunoreactivity in adult rats exposed to nicotine during adolescence: relation to anxiety-like and depressive-like behavior. Neuropeptides. 2005;39:369–377. doi: 10.1016/j.npep.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Bodwell BE, Ryde IT, Seidler FJ. Adolescent nicotine treatment changes the response of acetylcholine systems to subsequent nicotine administration in adulthood. Brain Res Bull. 2008;76:152–165. doi: 10.1016/j.brainresbull.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Rudder CL, Ryde IT, Tate CA, Seidler FJ. Permanent, sex-selective effects of prenatal or adolescent nicotine exposure, separately or sequentially, in rat brain regions: indices of cholinergic and serotonergic synaptic function, cell signaling, and neural cell number and size at 6 months of age. Neuropsychopharmacology. 2007;32:1082–1097. doi: 10.1038/sj.npp.1301231. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Ryde IT, Mackillop EA, Bodwell BE, Seidler FJ. Adolescent nicotine administration changes the responses to nicotine given subsequently in adulthood: adenylyl cyclase cell signaling in brain regions during nicotine administration and withdrawal, and lasting effects. Brain Res Bull. 2008;76:522–530. doi: 10.1016/j.brainresbull.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Smith LN, McDonald CG, Bergstrom HC, Brielmaier JM, Eppolito AK, Wheeler TL, Falco AM, Smith RF. Long-term changes in fear conditioning and anxiety-like behavior following nicotine exposure in adult versus adolescent rats. Pharmacol Biochem Behav. 2006;85:91–97. doi: 10.1016/j.pbb.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Spaeth AM, Barnet RC, Hunt PS, Burk JA. Adolescent nicotine exposure disrupts context conditioning in adulthood in rats. Pharmacol Biochem Behav. 2010 doi: 10.1016/j.pbb.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Terranova ML, Laviola G, Alleva E. Ontogeny of amicable social behavior in the mouse: gender differences and ongoing isolation outcomes. Dev Psychobiol. 1993;26:467–481. doi: 10.1002/dev.420260805. [DOI] [PubMed] [Google Scholar]
- Terranova ML, Laviola G, de Acetis L, Alleva E. A description of the ontogeny of mouse agonistic behavior. J Comp Psychol. 1998;112:3–12. doi: 10.1037/0735-7036.112.1.3. [DOI] [PubMed] [Google Scholar]
- Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O’Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology (Berl) 2009;206:303–312. doi: 10.1007/s00213-009-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Tejeda HA, Natividad LA, O’Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol Biochem Behav. 2008 doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851:9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA. An animal model of adolescent nicotine exposure: effects on gene expression and macromolecular constituents in rat brain regions. Brain Res. 2000a;867:29–39. doi: 10.1016/s0006-8993(00)02208-3. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA. Persistent and delayed behavioral changes after nicotine treatment in adolescent rats. Brain Res. 2000b;880:167–172. doi: 10.1016/s0006-8993(00)02823-7. [DOI] [PubMed] [Google Scholar]
- Turner JR, Castellano LM, Blendy JA. Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res. 2011;13:41–46. doi: 10.1093/ntr/ntq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR, Ortinski PI, Sherrard RM, Kellar KJ. Cerebellar nicotinic cholinergic receptors are intrinsic to the cerebellum: implications for diverse functional roles. Cerebellum. 2011;10:748–757. doi: 10.1007/s12311-011-0285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Walters CL, Cleck JN, Kuo YC, Blendy JA. Mu-opioid receptor and CREB activation are required for nicotine reward. Neuron. 2005;46:933–943. doi: 10.1016/j.neuron.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Withdrawal from chronic nicotine in adolescent and adult rats. Pharmacol Biochem Behav. 2006;85:648–657. doi: 10.1016/j.pbb.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Kellar KJ. The comparative pharmacology and up-regulation of rat neuronal nicotinic receptor subtype binding sites stably expressed in transfected mammalian cells. J Pharmacol Exp Ther. 2004;310:98–107. doi: 10.1124/jpet.104.066787. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.