Abstract
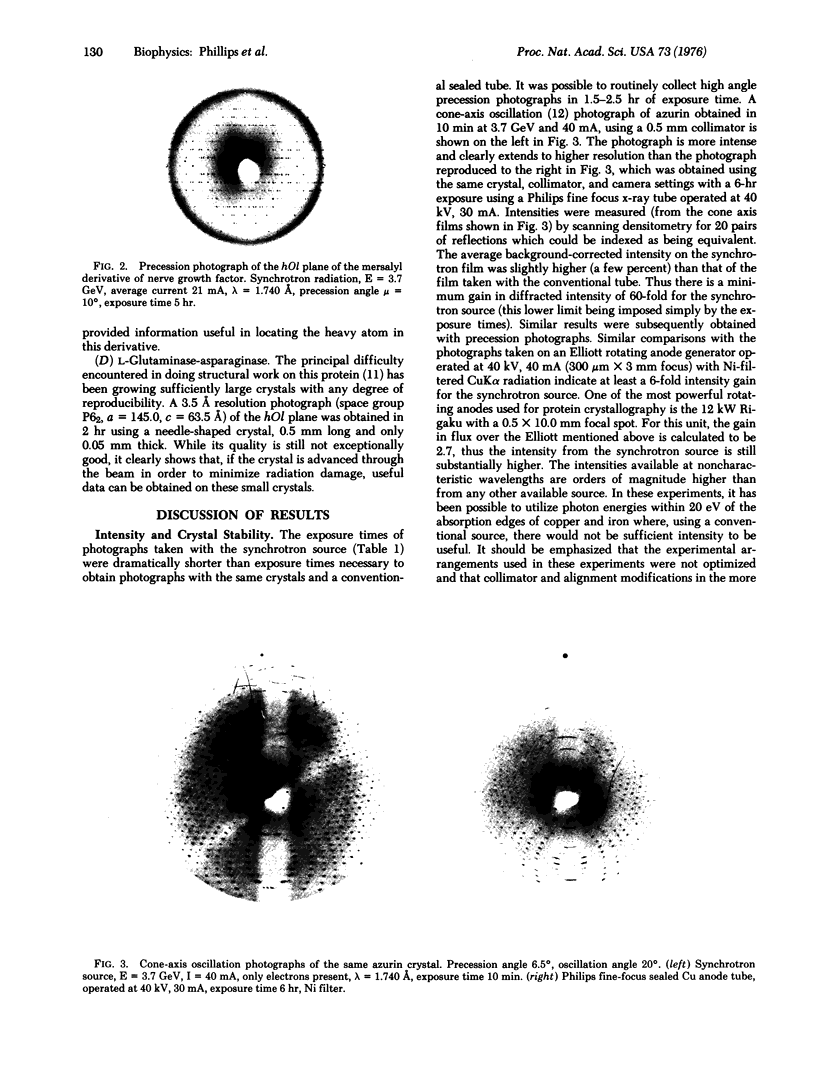
X-ray diffraction photographs of protein single crystals have been obtained using synchrotron radiation produced by an electron-positron storage ring. The diffracted intensities observed with this unconventional source are a factor of at least 60 greater than those obtained with a sealed x-ray tube using the same crystal and instrumental parameters. Diffraction data have been collected by the precession method to higher resolution and using smaller protein crystals than would have been possible with a conventional source. The crystal decay rate in the synchrotron beam for several proteins appears to be substantially less than that observed with Ni-filtered Cu radiation. The tunable nature of the source (which allows selective optimization of anomalous contributions to the scattering factors) and the low angular divergence of the beam make the source very useful for single crystal protein diffraction studies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Wlodawer A., Hodgson K. O., Shooter E. M. Crystallization of nerve growth factor from mouse submaxillary glands. Proc Natl Acad Sci U S A. 1975 Mar;72(3):777–779. doi: 10.1073/pnas.72.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]