Abstract
Social subordination in female macaques is imposed by harassment and the threat of aggression and produces reduced control over one's social and physical environment and a dysregulation of the limbic-hypothalamic-pituitary-adrenal axis resembling that observed in people suffering from psychopathologies. These effects support the contention that this particular animal model is an ethologically relevant paradigm in which to investigate the etiology of stress-induced psychological illness related to women. Here, we sought to expand this model by performing a discriminate analysis (DA) on 33 variables within three domains; behavioral, metabolic/anthropomorphic, and neuroendocrine, collected from socially housed female rhesus monkeys in order to assess whether exposure to social subordination produces a distinct phenotype. A receiver operating characteristic (ROC) curve was also calculated to determine each domain's classification accuracy. DA found significant markers within each domain that differentiated dominant and subordinate females. Subordinate females received more aggression, showed more submissive behavior, and received less of affiliation from others than did dominant females. Metabolic differences included increased leptin, and reduced adiponectin in dominant compared to subordinate females. Dominant females exhibited increased sensitivity to hormonal stimulation with higher serum LH in response to estradiol, cortisol in response to ACTH, and increased glucocorticoid negative feedback. Serum oxytocin, CSF DOPAC and serum PACAP were all significantly higher in dominant females. ROC curve analysis accurately predicted social status in all three domains. Results suggest that socially house rhesus monkeys represent a cogent animal model in which to study the physiology and behavioral consequences of chronic psychosocial stress in humans.
Keywords: social subordination, psychosocial stress, animal model, discriminate analysis
Introduction
Chronic stress is a causal and sustaining factor in a number of adverse health outcomes (Juster et al., 2010; McEwen, 2008), an observation supported by an extensive literature from both prospective animal studies and epidemiological analyses in humans. While physical stress resulting directly from infection or injury can initiate a similar cascade of biological changes, the psychogenic component of exposure to social stressors is important and involves activation of cortico-limbic circuits that modulate both sympathetic and limbic-hypothalamic pituitary-adrenal (LHPA) responses that are universally used to define “stress” (Choi et al., 2008; Herman et al., 2003; Jankord and Herman, 2008; Ulrich-Lai and Herman, 2009). In general, the specific parameters of the stress response can vary depending on whether the socio-environmental stressor is acute (short duration), chronic (prolonged duration), or acute imposed on the background of chronic stress. Whereas acute stress activates sympathetic and hormonal events which orchestrate a coordinated sequence of responses to restore homeostasis or allostasis, as described by McEwen and colleagues (McEwen and Wingfield, 2010; Schulkin et al., 1994), chronic stress can overwhelm these allostatic mechanisms and result in dysregulation or mal-adaptations of central and peripheral circuits regulating the stress response that may lead to psychiatric, immune, cardiovascular, and metabolic illnesses (McEwen, 1998).
Even though many behavioral paradigms and animal models have been developed to examine how chronic stress may produce negative health outcomes, for the most part these models elicit behavioral and hormonal responses that are unique to a particular type of stress employed in a laboratory setting. Although these studies are important from a heuristic point of view and are certainly informative, any investigation of the biobehavioral effects of stress as it relates to the development of human pathophysiology should focus on those stressors that are likely to be shared by human populations (Anisman and Matheson, 2005; Huhman, 2006; Tamashiro et al., 2005). Moreover, in many models of chronic stress animals eventually adapt to stressors and do not continue to exhibit stress hormone or behavioral responses (Armario, 2006; Bhatnagar and Dallman, 1998; Bhatnagar et al., 1998; Bhatnagar and Vining, 2003; Bhatnagar et al., 2006; Jaferi and Bhatnagar, 2006). However, when the chronic stress is uncontrollable, and/or not easily predictable, then both the sympathetic and hormonal response are continually reactivated or sustained and physiological and behavioral changes ensue.
Several rodent models of chronic stress induce sustained neurobiological and behavioral changes that resemble stress-induced disorders in people. The chronic variable stress paradigm (Herman et al., 1995) exposes rats to 6 weeks of repeated mild stressors, including physical stressors and social isolation. This paradigm produces animals that show increased corticosterone levels and a range of other phenotypes including altered fear learning, anhedonia, and dysfunction in limbic – hypothalamic circuits (Dalla et al., 2005; Flak et al., 2009; McGuire et al., 2010; Solomon et al., 2011). Two other rodent models have an ethological relevant social stressor as the central component. In the social defeat model, most typically studied in hamsters, repeated exposure to a more aggressive intruder on a single day produces sustained activation of the LHPA axis and specific changes in neurochemical circuits within mesolimbic regions in both male and female rodents (Huhman, 2006; Razzoli et al., 2009). The submissive behavior persists ~50% in males for up to a month (Huhman et al., 2003) whereas response to defeat is diminished in females. Indeed, more submissive behavior is expressed during estrous cycle days associated with elevated estradiol concentrations (Solomon et al., 2007). Finally, in the visible burrow system, groups of male rats are housed socially with several females for two weeks. Males quickly form a dominance hierarchy and subordinate males exhibit a number of changes characteristic of chronic stress, including neurobiological changes in limbic circuits as well as reproductive and metabolic deficits (Blanchard et al., 1993; Blanchard et al., 1995; Choi et al., 2006; Hardy et al., 2002; Tamashiro et al., 2004). In addition, this model has several notable features. First, animals are given intermittent recovery periods from social housing during which previously subordinate males respond differently than dominant males, most typically with excess food intake and weight gain (Tamashiro et al., 2004). However, the subordinate phenotype is maintained with re-exposure to the social housing (Lucas et al., 2004; Tamashiro et al., 2007). Secondly, a subgroup of subordinate males is classified as non-responders, showing a diminished response in corticosterone to an acute restraint during the social housing period (Lucas et al., 2004; Watanabe et al., 1995), a profile analogous to the attenuated stress hormone activity described for post traumatic stress disorder (Meewisse et al., 2007; Yehuda, 2002). Importantly, despite this reduced glucocorticoid response, these males show more altered dopaminergic tone in mesolimbic regions than other subordinate or dominant animals (Lucas et al., 2004). However, because females of the strain of rats used do not form a hierarchy when housed socially, this paradigm cannot be used to evaluate adverse consequences of chronic social stress in females (Tamashiro et al., 2004).
Together, these paradigms represent well-established approaches that have significantly advanced our understanding of how chronic exposure to social stressors produces lasting changes in behavior and physiology. While each of these paradigms employs a repeated uncontrollable or unpredictable type of stressor, because the stressor is discontinued after a specific duration these paradigms only partially model the continual daily exposure to stressors experienced by people and implicated in the development of stress-induced diseases. The use of socially housed non-human primates provides an opportunity to assess the socially-induced consequences on a number of health-related phenotypes (Abbott et al., 2003a; McKenzie-Quirk and Miczek, 2008; Sapolsky, 2005). Notably, socially housed macaque monkeys provide an opportunity to study the impact of continual exposure to social stress over an extended period of time on a number of health-related phenotypes (Sapolsky, 2005). Macaque social groups, regardless of size, are organized by a linear dominance hierarchy that functions to maintain group stability. While an animal's position within the hierarchy can be enforced through contact aggression, most often subordinate status is imposed by the threat of aggression or harassment from more dominant animals (Bernstein, 1976b; Bernstein and Gordon, 1974b; Bernstein et al., 1974b; Shively and Kaplan, 1984). Subordinates terminate these interactions by emitting submissive behavior, which is the defining feature of social subordination in macaque groups (Bernstein, 1976b; Bernstein and Gordon, 1974b; Bernstein et al., 1974b; Shively and Kaplan, 1984). In addition to the frequent harassment, control over an individual's social and physical environment decreases with more subordinate status (Bernstein, 1970).
Several unique features differentiate this model from more typical laboratory animal paradigms. Because the social housing mimics the organization of free-ranging populations, the stress of subordination is a part of this species' natural history (Bernstein and Gordon, 1977). Secondly, the dominance hierarchy is defined by matrilineal relations and is thus female based (Bernstein, 1970), providing an important opportunity to study stress-induced disorders in females. Finally, groups are stable for extended periods, even when experimentally established (Jarrell et al., 2008), providing the opportunity to study long-term consequences of either high or low social status (Kaplan, 2008). The social subordination model in macaques is being used to study the adverse effects of psychosocial stress on a range of health related conditions known to be stress dependent (Troisi, 2002), including cardiovascular disease (Kaplan et al., 2009), addictive behavior (Morgan et al., 2002), reproductive compromise (Kaplan and Manuck, 2004; Wilson and Kinkead, 2008; Zehr et al., 2005), immune dysfunction (Gust et al., 1991; Paiardini et al., 2009), appetite (Arce et al., 2010), and an increase in emotionality (Shively et al., 2005; Wilson et al., 2008). Despite the value of using this model for these targeted problems, the subordinate phenotype remains incompletely defined across a number of behavioral and physiological parameters (Abbott et al., 2003b). To rectify this, the present analysis used multivariate analysis of variance (MANOVA) and discriminant analysis on prospectively collected data to test the hypothesis that social subordination in female rhesus monkeys produces a distinct phenotype from that of dominant females. Moreover, we predict that differences in phenotype elicited by social subordination in this species will yield new avenues of investigation into the origins of stress-related psychopathologies, particularly as they relate to human females.
Methods
Data were collected on 39 adult female rhesus monkeys (Macaca mulatta), aged 12 – 16 yr, that were housed in 8 social groups each containing 4 or 5 females and one adult male. Females were ovariectomized four to six years prior to the present analysis. Groups were housed in indoor – outdoor runs (3.7 × 3.7 × 2.1 m). Animals had continuous access to water and commercial monkey chow, described below, supplemented with a piece of seasonal fresh fruit or vegetable. The Emory University Institutional Animal Care and Use Committee approved all procedures in accordance with the Animal Welfare Act and the NIH “Guide for the Care and Use of Laboratory Animals”.
Groups had been established for four years as described previously (Jarrell et al., 2008). Briefly, females were removed from their large natal breeding groups and formed into in eight small member groups. All females were taken from the middle portions of the social hierarchy in their natal groups. The new groups were formed by sequentially adding unfamiliar females to the new housing area. The dominance hierarchy quickly formed and after several adjustments (females primarily switching between ranks 2, 3, or 4), dominance positions have been stable for several years. Dominance rankings were determined by the outcome of dyadic agonistic interactions with subordinate status defined by an animal emitting an unequivocal submissive gesture to another animal (Bernstein, 1976b; Bernstein and Gordon, 1974b; Bernstein et al., 1974b; Shively and Kaplan, 1984). In accordance with previously established conventions (Arce et al., 2010; Collura et al., 2009; Jarrell et al., 2008; Kaplan et al., 2010; Riddick et al., 2009; Shively, 1998; Shively et al., 1997; Stavisky et al., 2001; Wilson et al., 2008), females ranked 1 and 2 were classified as dominant, while those of ranks 3, 4, and 5 were considered subordinate, yielding 16 dominant and 23 subordinate females. A single male was added to each group some three years after the groups were formed and assumed the alpha status of each group. Because no subordinate males were members of these groups, data from males was not considered for the present analysis. These females were used in a series of NIH-supported studies investigating how social status modifies a number of estradiol-dependent behavioral and physiological systems (e.g., (Michopoulos et al., 2009a; Michopoulos et al., 2011b; Michopoulos and Wilson, 2011). Consequently, since the time of ovariectomy, each subject had received periodic replacement therapy with ovarian steroids.
The intent of the study was to describe a broad range of phenotypical endpoints to determine what differentiates dominant from subordinate females. Most data were obtained prospectively as described below while selected previously published data from this same cohort of females were also included. Specifically, social status differences in serum concentrations of LH in response to low dose estradiol negative feedback inhibition (Michopoulos et al., 2009a) as well as status differences in serum oxytocin during estradiol replacement (Michopoulos et al., 2011a) were included. In addition, the samples used for the oxytocin analyses were additionally analyzed for pituitary adenylate cyclase activating peptide (PACAP), a retroactive molecule thought to be stress sensitive in females (Ressler et al., 2011).
Prospective data were obtained on females in the absence of hormone replacement with an average washout period 4.85 ± 0.13 weeks with a range of 4–6 weeks since their last estradiol replacement. Data were collected in two different phases, separated by a minimum of 3 weeks of no assessments. The first phase was six weeks in duration. During this period, animals had continual access to the typical low fat, high fiber nonhuman primate diet (Test Diets, #5038, Richmond IN) comprised of 3.50 kcal/gram with 18% of calories derived from protein, 12% from fat, 60% from fiber, and 10% from sugar. The purpose of this phase was to obtain a range of phenotypes, including neurochemical, LHPA responsivity, metabolic, and anthropometric data, while females consumed a standard laboratory diet. During the first week, animals received a dexamethasone (Dex) suppression test to determine glucocorticoid negative feedback (Wilson et al., 2008). On day 1, a baseline serum sample was obtained at 1100 hr. At 1730 hr, each female received an injection of Dex (0.25 mg/kg, IM) and samples were obtained at 1100 hr the following morning for the assay of cortisol. Absolute values of cortisol following Dex as well as the change in cortisol from the post Dex to the baseline sample were used in the analysis. During week 3, females received an ACTH stimulation test (Shively, 1998). At 0800 hr, a serum sample was obtained followed by an injection of Dex (0.5 mg/kg, IM) to suppress endogenous ACTH. Four hours later, another serum sample was obtained followed by an injection of Cortrosyn (10 ng/kg, IV). Subsequent samples were obtained at +15 and +30 min. The area under the curve in serum cortisol was used for the analysis.
In addition to the assessment of LHPA regulation, a single blood sample was obtained at the beginning of week 4 and week 5 for the analysis of metabolic hormones, including plasma ghrelin and serum fructosamine (as an index of circulating glucose over a 2 to 3 week period), insulin, leptin, and adiponectin. Following the sample collection at week 5, females were anesthetized with ketamine HCl (10 mg/kg, IM). Whole body scans using a dual x-ray absorptiometry (DEXA; Norland Eclipse) were performed to obtain total lean, fat, and bone mass. Collars were then placed on each animal (Primate Products) and an Actical Accelerometer (MiniMiter, Bend OR) was attached to record activity bouts. The devises were programed to sum activity every 30 sec. Collars were removed 7 days later and hourly activity outs were calculated throughout a 24-hr day averaged across the 7-day period. Animals were anesthetized for the removal of the collars. At this time, a sample of cerebrospinal fluid (CSF) was obtained from the cisterna magna for the measurement of the serotonin (5HT) metabolite 5 hydroxyindoleamine (5HIAA) as well as dopamine (DA) and its metabolites homovanillic acid (HVA) and 3, 4-dihydroxyphenylacetic acid (DOPAC). Finally, body weights were obtained on each animal every week.
In addition to these metabolic and physiological measures, the behavioral data using an established ethogram (Jarrell et al., 2008) were collected for 30 min twice weekly during the 6 weeks of the initial phase as described previously (Michopoulos et al., 2011c). Affiliative behavior was comprised of proximity and grooming; aggression was defined by threats, slaps, grabs, and bites; and submissive behavior was characterized by withdrawals, grimaces, and screams. Anxiety-like behavior consisted of body shakes, yawns, self-scratching, and self-grooming (Troisi, 2002). Data was recorded using a netbook computer using a data acquisition program that records actor – behavior - recipient (Graves and Wallen, 2006). Inter-observer reliability was greater than 92%.
Finally, the intent of the second phase of the study was to characterize total caloric intake when females were presented with a choice between the typical low fat, high fiber monkey diet described above (3.54 kcal/gram) and a diet with additional fat and sugar (3.73 kcal/gram), having 18% of calories derived from protein, 36% from fat, 31% from fiber, and 15% from sugar (Test Diets, “Typical American Diet”). The rationale for including these data is that based on previous reports from our laboratory that subordinate females consume significantly more calories when a calorically dense diet is made available (Arce et al., 2010). Diets were presented to the animals through previously validated automated feeders that quantifies food intake continually for individual animals housed in a social setting (Arce et al., 2010; Wilson et al., 2008). Validation of this feeding system indicates that a pellet of food obtained by the monkey was eaten by that monkey and, while dominant females can eat at any time, they do not block access to feeders nor take food from subordinate females (Wilson et al., 2008). Each group had access two feeders one containing the low caloric diet and one containing the high caloric diet for two weeks. A complete description of feeding behavior in different dietary environments is contained in another manuscript (Michopoulos et al., 2011d).
Subjects had been previously habituated to removal from their groups for conscious venipuncture, with a particular group typically being sampled 10 minutes following entrance into the housing area thereby minimizing the arousal associated the sampling procedure (Blank et al., 1983; Walker et al., 1982). Descriptions of assays are summarized in Table 1. Except for the fructosamine and PACAP analyses, all assays were performed in the Yerkes Biomarkers Core Lab. Antech Diagnostics (Atlanta GA) performed the fructosamine assay. Dr. Victor May (University of Vermont) performed the assay of serum PACAP.
Table 1.
Description of assays for ligands in serum, plasma, or CSF samples. For each ligand, all samples were analyzed in one assay.
| Ligand | Method and Source | Inter-assay CV | Intra-assay CV | Reference |
|---|---|---|---|---|
| Plasma ghrelin (acetylated) | ELISA Siemens | 4.55% | 5.51% | (44) |
| Serum fructosamine | Colorimetric Roche | 2.90% | 0.90% | - |
| Serum insulin | RIA Siemens | 9.05% | 6.75% | (44) |
| Serum leptin | RIA Millipore | 6.22% | 8.51% | (44) |
| Serum adiponectin | RIA Millipore | N/A | 3.2% | (114) |
| Serum LH | RIA – in house | 11.98% | 6.85% | (53) |
| Serum cortisol | RIA Beckman Coulter | 8.75% | 4.91% | (44) |
| Serum oxytocin | ELISA | 7.50% | 10.2% | (67) |
| Serum PACAP | RIA | 9.99% | 4.00% | (115) |
| CSF 5HIAA | HPLC | 9.50 | 4.99 | (99) |
| CSF DA | HPLC | 8.30 | 3.28 | (99) |
| CSF HVA | HPLC | 12.54 | 4.78 | (99) |
| CSF DOPAC | HPLC | 4.78 | 6.56 | (99) |
Statistical Analyses
MANOVA and Discriminant Analysis (DA) procedures were chosen to analyze these data for which variables maximize the differences between dominant and subordinate subjects, because these procedures (a) protect against Type I error inflation problems caused by running multiple separate ANOVAs for each separate variable and (b) help reveal potential combinations of variables that optimally differentiate the groups dominant and subordinate females (Field, 2009; Tabachnik and Fidell, 2007). All data were complete and no significant outliers were noted. Right skewed distributions were noted for some of the variables; however, the MANOVA and DA procedures used are robust to deviations from normality (Tabachnik and Fidell, 2007). The 33 variables were included into either a Behavioral, Metabolic, or Neuroendocrine domain as listed in Table 2. Prior to analysis, Z-scores were created for each variable to eliminate any variable comparison biases due to scale differences or offset from zero. The first step in analysis was to review the pairwise correlations among all variables within each domain. Any variables that had high correlations (r > 0.65) were considered for possible removal because highly co-linear variables will cause instabilities within the matrix inversion procedures required by MANOVA and DA. Multicollinearity problems were also handled within MANOVA and DA procedures by removing any variables that failed the tolerance test from further analysis. An initial DA procedure was performed to see which variables had discriminant function “loadings” ≥ 0.3, as variables with higher loadings contribute more to group differentiation (Field, 2009; Tabachnik and Fidell, 2007). Only one discriminant function was possible for the data set because only two groups were considered (dominant versus subordinate). Any variables with loadings < 0.3 were removed from analysis for the final DA. This final variable list was additionally run through the MANOVA procedure to calculate the extent to which each separate variable significantly differentiated between the 2 groups. The power for each discriminant analysis/MANOVA was calculated using PASS 2008 (NCSS, LLC; Kaysville, Utah) inputting the 2 group means for each of the variables retained in the final model as well as the pooled within groups covariance matrix. Additionally, t-tests, adjusting degrees of freedom for unequal variances, were performed on the resulting discriminant scores for each model comparing dominant and subordinate groups with accompanying effect sizes (Rosnow, 2003). Finally, for each discriminant function, receiver operating characteristic (ROC) curves and their associated areas under the curve (AUC) were calculated for each domain as a final comparison for each domain's classification accuracy.
Table 2.
List of Variables in Each Measurement Domain: Metabolic, Behavioral and Neuroendocrine
| Phenotype | Dominant (n = 16) | Subordinate (n = 23) | ||
|---|---|---|---|---|
| Behavioral | Mean | sem | Mean | sem |
| Activity | 6458.41 | 768.79 | 6253.27 | 485.00 |
| Frequency of affiliation initiated (per hr) | 3.28 | 0.55 | 2.04 | 0.34 |
| Duration of time in affiliation (per hr) | 14.10 | 2.02 | 14.95 | 2.96 |
| Frequency of affiliation received from others (per hr) | 1.76 | 0.32 | 2.71 | 0.35 |
| Frequency aggression directed towards others (per hr) | 2.10 | 0.56 | 1.32 | 0.56 |
| Frequency of aggression received from others (per hr) | 0.13 | 0.06 | 2.46 | 0.53 |
| Frequency of submission directed towards others (per hr) | 0.39 | 0.20 | 2.29 | 0.46 |
| Frequency of anxiety-like behavior (per hr) | 7.00 | 1.12 | 6.14 | 0.58 |
| Metabolic | ||||
| Adiponectin (ng/ml serum) | 15423.82 | 1928.09 | 20857.48 | 1522.13 |
| Average kcals from a choice diet | 130.77 | 13.65 | 184.55 | 14.90 |
| Bone mass (g) | 309.51 | 11.71 | 274.27 | 8.55 |
| Fat mass (g) | 2206.45 | 374.26 | 876.83 | 268.96 |
| Fructosamine (mg/dl serum) | 190.56 | 3.68 | 183.87 | 2.25 |
| Ghrelin (pg/ml plasma) | 11.75 | 2.64 | 13.64 | 2.12 |
| Insulin (μU/ml serum) | 60.42 | 19.55 | 45.58 | 15.96 |
| Lean mass (g) | 6363.50 | 146.49 | 6245.87 | 88.24 |
| Leptin (ng/ml serum) | 30.73 | 5.70 | 16.39 | 2.91 |
| Weight (kg) | 8.93 | 0.41 | 7.34 | 0.29 |
| Neuroendocrine | ||||
| PACAP (pM serum) | 126.73 | 7.15 | 112.89 | 5.22 |
| DOPAC (ng/ml CSF) | 5.71 | 0.63 | 4.11 | 0.56 |
| DA (ng/ml CSF) | 3.67 | 0.55 | 3.36 | 0.36 |
| HIAA (ng/ml CSF) | 90.14 | 10.34 | 80.56 | 7.80 |
| HVA (ng/ml CSF) | 501.11 | 40.07 | 461.34 | 26.48 |
| Oxytocin(pg/ml serum) | 216.52 | 24.07 | 160.09 | 12.65 |
| Cortisol response to ACTH (plasma AUC) | 79.00 | 16.59 | 37.01 | 9.29 |
| Degree of glucocorticoid negative feedback (change in cortisol (μg/dl serum by Dex compared to control) | −23.91 | 2.73 | −19.43 | 1.07 |
| Post Dex serum cortisol (μg/dl serum) | 3.95 | 0.87 | 4.10 | 0.65 |
| LH (ng/ml serum) response to estradiol negative feedback | 6.49 | 0.63 | 4.49 | 0.32 |
Results
Social status categorizations
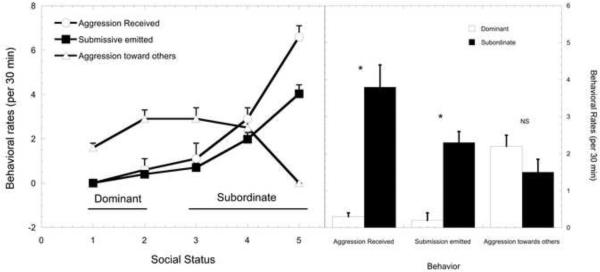
Data are shown in Figure 1 to illustrate the well-accepted social rank differences in the mean frequency of aggression received and submissive behavior that supports our categorization of dominant and subordinate females. Females ranked 3 – 5 received significantly more aggression from higher-ranking group mates (F4, 27 = 11.4, p < 0.001). This harassment was associated with rank-dependent, higher rates of submissive behavior (F4, 27 = 6.56, p < 0.001). Importantly, rates of aggression directed towards others do not differ significantly between dominant and subordinate females (p > 0.05).
Figure 1.
Mean ± SEM rates of agonistic behavior for animals categorized as dominant (ranked 1 and 2) and subordinate (ranks 3 – 5). Dominant females received less aggressive behavior (closed circle) than those categorized as subordinate while subordinate animals emitted more submissive behaviors (open square) than dominant animals.
Behavioral Results
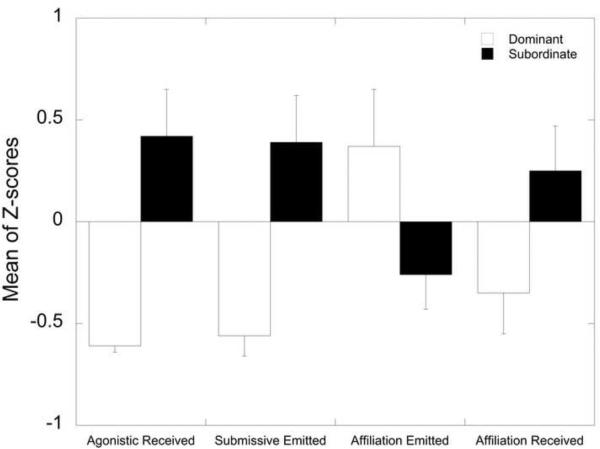
Table 3 presents the DA and MANOVA results for the variables considered in the Behavioral domain. No high correlations were noted and no variables failed the tolerance test. However, only 4 of the 8 variables in this domain yielded loadings of 0.3 or higher: aggression received from others, submission directed towards others, frequency of affiliation directed towards others, and affiliation received from others which were retained in the final model. Activity levels, duration of time in affiliation, aggression directed towards others and anxiety-like behavior all had loadings < 0.3 and were excluded from the analysis. In the final DA model, aggression received and submission emitted loaded the highest onto the discriminant function followed by affiliative behaviors initiated and received. Only aggression received and submission showed significant between group effects within the MANOVA procedure. As can be seen in Figure 2, the dominant females had lower rates of aggression received from others, submission directed towards others, and affiliation received from others yet higher rates of affiliation directed towards others.
Table 3.
Behavioral domain: DA and MANOVA Results
| Behavioral Predictor Variables (Z-scores used in analysis) | Discriminant Function Loadings | MANOVA: Between-Groups Effects F-statistic (df1=1,df2=37); (p-value) |
|---|---|---|
| Aggression received from others | 0.654 | 13.163 (0.001) |
| Submission directed towards others | 0.594 | 10.859 (0.002) |
| Affiliation directed towards others | − 0.365 | 4.099 (0.050) |
| Affiliation received from others | 0.343 | 3.622 (0.065) |
| Discriminant Analysis Results | ||
| Canonical R2 | 0.454 | |
| Eigenvalue | 0.833 | |
| Wilks' Lambda | 0.546 | |
| Chi-square,df (p-value) | 21.209,4 (<0.001) | |
| Cross-Validation % | 87.2% cases correctly classified (cross-validation using leave-one-out “jackknife” method) | |
| Area Under ROC Curve | AUC = 0.946, SE = 0.034, p < 0.001, 95% CI [0.878, 1.000] | |
| Variables Removed From Analysis | ||
| Activity levels | Loading < 0.3 | |
| Duration of time in affiliation | Loading < 0.3 | |
| Aggression directed towards others | Loading < 0.3 | |
| Anxiety-like behaviors | Loading < 0.3 | |
Figure 2.
Behavioral Domain – Variables Retained in Final DA Model Sorted from Highest to Lowest Discriminant Function Loading (absolute values). Significance p < 0.05 for all variables.
The final discriminant function explained approximately 45% (Canonical R2) of the variability between predictors and Status groups. The final discriminant analysis model correctly classified 87.2% of the cases when using the leave-one-out cross-validation procedure. This MANOVA design achieved 99% power to test the Status factor given a Wilks' Lambda of 0.546 at a 5% significance level.
Metabolic Results
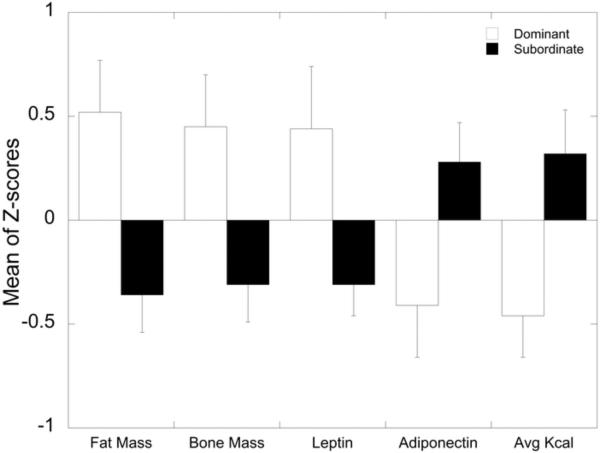
Table 4 presents the DA and MANOVA results for the variables considered in the Metabolic Domain. Weight was removed from analysis because it was highly correlated (r > 0.65) with several other measures including insulin, fat mass and bone mass. The next DA yielded 5 measures with loadings of 0.3 or greater: fat mass, average kcal consumed, bone mass, leptin, and adiponectin. Ghrelin, fructosamine, insulin, and lean mass all had loadings < 0.3 and were excluded from the analysis. In the final DA model, fat mass, average kcal consumed during the choice diet phase and bone mass loaded highest onto the discriminant function, followed by leptin and adiponectin. All of these showed significant (p<0.05) between group effects within the MANOVA procedure. As can be seen in Figure 3, the dominant females exhibited significantly higher fat mass, bone mass, and leptin, and reduced adiponectin compared to subordinate females. Again, these data were obtained while animals consumed the standard low fat monkey diet. In contrast, when presented with a choice between this diet and a high caloric diet, total average kcal consumed was significantly higher for subordinate compared to dominant females (Figure 3).
Table 4.
Metabolic Domain – DA and MANOVA Results
| Metabolic Predictor Variables (Z-scores used in analysis) | Discriminant Function Loadings | MANOVA: Between-Groups Effects F-statistic (df1=1,df2=37); (p-value) |
|---|---|---|
| Fat Mass | − 0.601 | 8.790 (0.005) |
| Average kcal consumed | 0.514 | 6.432 (0.016) |
| Bone Mass | − 0.505 | 6.201 (0.017) |
| Leptin | − 0.494 | 5.939 (0.020) |
| Adiponectin | 0.453 | 4.993 (0.032) |
| Discriminant Analysis Results | ||
| Canonical R2 | 0.397 | |
| Eigenvalue | 0.657 | |
| Wilks' Lambda | 0.604 | |
| Chi-square,df (p-value) | 17.420,5 (0.004) | |
| Cross-Validation % | 84.6% cases correctly classified (cross-validation using leave-one-out/“jackknife” method) | |
| Area Under ROC Curve | AUC = 0.878, SE = 0.076, p < 0.001, 95% CI [0.729, 1.000] | |
| Variables Removed From Analysis | ||
| Ghrelin | Loading < 0.3 | |
| Fructosamine | Loading < 0.3 | |
| Insulin | Loading < 0.3 | |
| Lean Mass | Loading < 0.3 | |
| Weight | Highly correlated with Insulin (r=0.655), Fat Mass (r=0.951) and Bone Mass (r=0.698) | |
Figure 3.
Metabolic Domain – Variables Retained in Final DA Model Sorted from Highest to Lowest Discriminant Function Loading (absolute values). Significance p < 0.05 for all variables.
The final discriminant function explained 40% (Canonical R2) of the variance between the predictors and Status groups. The final DA model correctly classified 84.6% of the cases when using the leave-one-out cross-validation procedure. This MANOVA design achieved 96% power to test the Status factor given a Wilks' Lambda of 0.604 at a 5% significance level.
Neuroendocrine Results
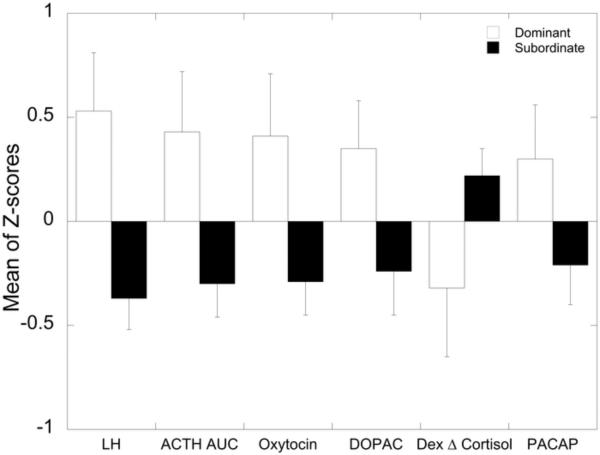
Table 5 presents the DA and MANOVA results for the variables considered in the Neuroendocrine domain. No high correlations were noted and no variables failed the tolerance test. However, only 6 of the 12 variables in this domain yielded loadings of 0.3 or higher: serum LH in response to estradiol negative feedback, cortisol in response to an ACTH challenge, serum oxytocin, CSF concentrations of DOPAC, the degree of glucocorticoid negative feedback, and serum PACAP which were retained in the final model. CSF levels of DA, HIAA, and HVA as well as post Dex cortisol concentrations had loadings < 0.3 and were removed from further analysis. Serum LH in response to estradiol negative feedback, cortisol in response to an ACTH challenge, serum oxytocin loaded the highest onto the discriminant function, followed by DOPAC, degree of glucocorticoid negative feedback, and PACAP. None of these last three had significant between group effects within the MANOVA procedure. As can be seen in Figure 4, the dominant females exhibited higher serum LH in response to estradiol negative feedback, cortisol in response to an ACTH challenge, serum oxytocin, CSF DOPAC and serum PACAP and a greater degree of glucocorticoid negative feedback than did the subordinate females.
Table 5.
Neuroendocrine domain: DA and MANOVA Results
| Neuroendocrine Predictor Variables (Z-scores used in analysis) | Discriminant Function Loadings | MANOVA: Between-Groups Effects F-statistic(df1=1,df2=37); (p-value) |
|---|---|---|
| Serum LH in response to estradiol negative feedback | 0.592 | 9.459 (0.004) |
| Serum cortisol response to ACTH challenge | 0.456 | 5.607 (0.023) |
| Serum oxytocin | 0.433 | 5.051 (0.031) |
| CSF DOPAC | 0.361 | 3.518 (0.069) |
| Degree of glucocorticoid negative feedback | − 0.331 | 2.956 (0.094) |
| Serum PACAP | 0.309 | 2.566 (0.118) |
| Discriminant Analysis Results | ||
| Canonical R2 | 0.421 | |
| Eigenvalue | 0.729 | |
| Wilks' Lambda | 0.578 | |
| Chi-square,df (p-value) | 18.612,6 (0.005) | |
| Cross-Validation % | 74.4% cases correctly classified (cross-validation using leave-one-out “jackknife” method) | |
| Area Under ROC Curve | AUC = 0.894, SE = 0.050, p < 0.001, 95% CI [0.797, 0.991] | |
| Variables Removed From Analysis | ||
| CSF DA | Loading < 0.3 | |
| CSF HIAA | Loading < 0.3 | |
| CSF HVA | Loading < 0.3 | |
| Post DEX serum cortisol | Loading < 0.3 | |
Figure 4.
Neuroendocrine Domain – Variables Retained in Final DA Model Sorted from Highest to Lowest Discriminant Function Loading (absolute values). Significance p < 0.05 for all variables.
The final discriminant function explained approximately 42% (Canonical R2) of the variability between predictors and Status groups. The final discriminant analysis correctly classified 74.4% of the cases using the leave-one-out cross-validation procedure. This MANOVA design achieved 96% power to test the Status factor given a Wilks' Lambda of 0.578 at a 5% significance level.
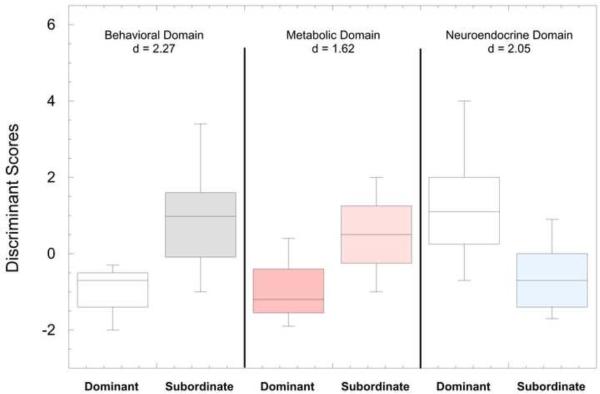
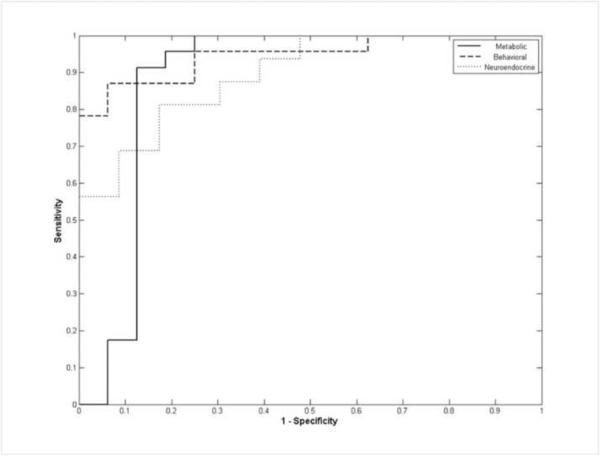
Shown in Figure 5 are boxplots of the discriminant scores for dominant and subordinate females. As a comparison of the models fit for each of the 3 domains, t-tests and effect sizes were performed on the scores for each model comparing dominant and subordinate groups. The Behavioral domain yielded the largest effect size (ES) of d=−2.27 (t31.16 = 6.34, p < 0.01), followed by the Neuroendocrine domain model (ES d=2.05; t37 = 4.68, p < 0.01) and finally the Metabolic domain model (ES d=−1.62, t20.87 = 4.93, p < 0.01). Similar comparison of the 3 domains was also made using receiver operating characteristic curves (ROC; Figure 6) generated from the discriminant analysis scores (Figure 5) and associated areas under the curve (AUC) calculated where the positive state was the subordinate rank (Table 4, 5, 6). The behavioral domain had the highest AUC (0.946) indicating that the discriminant function based on the behavioral domain achieved the best classification accuracy, followed by the neuroendocrine domain (AUC = 0.894) and the metabolic domain (AUC = 0.878).
Figure 5.
Box plots of the discriminant scores for dominant and subordinate groups in each domain (Behavioral, Metabolic, Neuroendocrine). Also listed are the effect sizes (d) comparing the scores within each domain between dominant and subordinate females.
Figure 6.
Receiver Operating Characteristic (ROC) curves of discriminant analysis scores for all 3 domains (Positive State = Subordinate Rank) (Metabolic Solid Line, Behavioral Dashed Line, Neuroendocrine Dotted Line).
Discussion
The current discriminate analysis of a range of behavioral, metabolic/anthropometric, and neuroendocrine variables demonstrates that social status in female rhesus monkeys produces two distinct phenotypes and is consistent with the hypothesis that social subordination in female macaques is a potent and chronic psychosocial stressor (Sapolsky, 2005). This analysis of phenotypic social status differences in female rhesus monkeys compliments the well-established social status differences observed in behavior and physiology of cynomolgus macaques (Kaplan and Manuck, 2008; Shively and Willard, 2011) and marmoset monkeys (Saltzman et al., 2008).
As expected, the behavioral domain best discriminated social status in socially housed female rhesus monkeys based on ROC curve analysis. It is not surprising that rates of aggression received and submission emitted were the best predictors of social status, as these behaviors are used to determine social ranking in macaques (Bernstein, 1976a; Bernstein and Gordon, 1974a; Bernstein et al., 1974a; Bernstein, 1970; Shively and Kaplan, 1984). The frequency of engaging in and receiving affiliative behavior from cage-mates also contributed to the classification of dominant and subordinate females, as dominant females most often initiate affiliation with subordinates being the most typical target. The observation that dominant animals are most often the target of affiliative behaviors in groups devoid of males (Michopoulos et al., 2011a) underscores the importance of the social context for determining the pattern of prosocial behaviors (Jacobs and Petit, 2011; Lehmann and Ross, 2011). In addition, subordination in female macaques has been associated with higher rates of anxiety (Wilson et al., 2008) or depressive behaviors (Shively et al., 1997). However, in the present analysis measures of emotionality were excluded because of low loading scores. The emergence of anxiety-like behaviors in macaques may depend on a number of contextual situations such as competition for mates or other resources and because subordination delays access to those resources the situation may produce increases in these emotional behaviors. Furthermore, analyses of female cynomolgus monkeys indicate only a subset of subordinate females, with even fewer dominant females, exhibit depressive-like behavior (Shively and Willard, 2011). Nonetheless, the present analysis shows that within the context of the present social configuration the incidence of prosocial behaviors is influenced by social status.
In addition to distinct differences in social behavior, metabolic and anthropometric variables differentiated subordinate from dominant females. When animals had access to a standard laboratory monkey diet, body composition, such as fat and bone mass, as well as the peripheral metabolic markers leptin and adiponectin, best discriminate social status categories. The pattern of these markers in subordinate animals compared to dominant animals is consistent with the notion that exposure to chronic stress and resulting LHPA dysregulation produces a subclinical state of negative energy balance (Arce et al., 2010; Belzung and Anderson, 1986; Gamaro et al., 2003; Tamashiro et al., 2004). Peripheral adiponectin levels are inversely related to adiposity (Spranger et al., 2003) and act to increase insulin sensitivity (Galic et al., 2010). However, despite adiponectin clearly differentiating dominant and subordinate females, the status differences in serum insulin did not load to contribute significantly to the DA. Because activity levels did not vary by social status, it is likely these differences are attributable to differences in ingestion of this low caloric diet (Michopoulos et al., 2009b), a notion consistent with a number of studies in laboratory rodents of stress-induced anorexia (Gamaro et al., 2003; Jochman et al., 2005; Marti et al., 1994; Smagin et al., 1999). However, when the dietary environment of the monkeys changed to include a choice of a high caloric diet more closely modeling what people experience, food intake clearly differentiated social status categorizations with subordinate females consuming more overall calories than dominant animals. This result is consistent with the idea that exposure to psychosocial stressors increases preference for and intake of highly palatable food in laboratory animals (Berridge, 1996; Dallman et al., 2005; Hagan et al., 2003; Tamashiro et al., 2006) and women (Adam and Epel, 2007; Epel et al., 2001). Because the monkeys only had access to the diets for two weeks, no significant change in adiposity markers were observed, despite the significantly higher caloric intake by subordinates (Michopoulos et al., 2011d). Longer exposure to this rich dietary environment will determine how indices of adiposity increase in subordinates. Indeed, studies of female cynomolgus females fed a high fat, atherogenic diet for 32 mo indicates more often subordinates develop more abdominal fat than do dominant monkeys (Shively et al., 2009). However, because food intake was not quantified, it is not known whether this increased visceral fat is due to increased consumption of the diet and/or stress-induced redistribution of fat stores.
A number of neuroendocrine endpoints contributed significantly to the DA. The hypersensitivity to estradiol negative feedback inhibition of LH secretion in subordinates (Michopoulos et al., 2009a) is consistent with subordination-induced increase in the incidence of anovulation in macaque (Kaplan et al., 2010; Pope et al., 1986; Shively et al., 1997) and marmoset monkeys (Abbott and Hearn, 1978; Saltzman et al., 2008) as well as psychosocial stress-induced infertility in ewes (Pierce et al., 2008) and women (Homan et al., 2007; Nakamura et al., 2008; Wilson and Kopitzke, 2002). DOPAC reflects dopamine turnover and elevations in cortico-limbic regions are associated with enhanced executive control over emotion in both animals and people (Robbins and Arnsten, 2009). Thus, elevations in central DOPAC in dominant females may suggest enhanced emotional control. In addition, studies in macaques indicate the social subordination is associated with a hypodopaminergic condition (Grant et al., 1998; Kaplan et al., 2002; Morgan et al., 2002; Shively, 1998) and increased vulnerability to psychostimulant self-administration (Morgan et al., 2002). Although CSF 5HIAA levels were lower in subordinates, this factor did not differentiate social status ranks, a finding consistent with previous studies (Kaplan et al., 2002). However, subordinate females do show reduced 5HT1A receptor binding in cortico-limbic regions (Shively, 1998) and reduced expression of the 5HT precursor, tryptophan hydroxylase in the raphe (Shively et al., 2003). Our previous report of higher serum oxytocin levels in dominant females (Michopoulos et al., 2011c) contributed significantly to the DA as well. Oxytocin is implicated in increasing a vast array of social behaviors in both animals and human beings (Goodson and Thompson, 2010) and has distinct anxiolytic effects in animals (Steckler, 2010). Furthermore, increased oxytocin in dominant females is likely associated increased motivation to initiate affiliative behaviors described here. Finally, dysregulation of PACAP has recently been implicated in increased PTSD symptomology in women (Ressler et al., 2011), and reduced levels of this neurotropic peptide may render subordinate females more vulnerable to aversive events.
In the past, measures of LHPA axis function, including morning cortisol (Czoty et al., 2009; Gust et al., 1993; Sassenrath, 1970; Stavisky et al., 2001) as well as overall diurnal cortisol levels (Arce et al., 2010; Collura et al., 2009), have been inconsistent in differentiating dominant from subordinate female macaques. Rather, socially subordinate female rhesus and cynomolgus macaques most often have increased adrenal size (Shively and Kaplan, 1984; Shively, 1998) and decreased glucocorticoid negative feedback (Collura et al., 2009; Jarrell et al., 2008; Kaplan et al., 2010; Shively, 1998; Shively et al., 1997; Wilson et al., 2008) as clear indicators of LHPA dysregulation. The current analysis showed that the suppression of cortisol due to glucocorticoid negative feedback as assessed by a dexamethasone suppression test and the cortisol response to ACTH administration (Riddick et al., 2009; Shively, 1998; Shively et al., 1997) are the most potent and reliable LHPA axis predictors of social status in female rhesus macaques. Interestingly, dominant monkeys show significantly more cortisol release after ACTH administration than do subordinate monkeys. This has not been shown in previous studies in monkeys (Riddick et al., 2009; Shively, 1998; Shively et al., 1997) and may be due to increased body fat in the dominant monkeys. It has been shown that increases in body fat can lead to a more robust glucocorticoid response in rats (Tannenbaum et al., 1997). In contrast to this pattern observed in macaques, subordinate female marmoset monkeys show suppressed serum cortisol (Saltzman et al., 1994), reflecting a blunted adrenal response to hypothalamic – pituitary activation (Saltzman et al., 2006). This hyporesponsiveness, albeit different than macaque females, nonetheless reflects a dysregulation of the LHPA axis and is a common feature of humans suffering from psychopathologies such as PTSD and depression (Berga et al., 1989; Epel et al., 2001; Juster et al., 2010; McEwen, 2008). Laboratory studies that experimentally manipulate group membership and reduce opportunities for social support can effectively exacerbate the consequences of stress-induced changes in emotionality (Jarrell et al., 2008; Kaplan et al., 1991; Morgan et al., 2000; Shively et al., 1997). Thus, the extent of LHPA dysregulation and consequential stress-induced problems in macaque females are likely influenced by the amount of harassment subordinates receive and whether they have opportunities to engage in prosocial behaviors from family members or other conspecifics that may mitigate the stress (Abbott et al., 2003b). Examining females of each specific subordinate rank in future studies may help to elucidate if there is, in fact, a graded LHPA response due to specific psychosocial stress level.
Because these groups were formed by randomly taking females from the middle portion of the dominance hierarchy in their natal groups and combining them with unfamiliar females (Jarrell et al., 2008), it is unlikely that female characteristics, independent of social status, account for these differences. However, an unequivocal test of that hypothesis would require reforming groups to change the ranks of the females to ensure that, once the groups had stabilized, rank explained the distant phenotype (Shively et al., 1997). Clearly, gene polymorphisms may also increase vulnerability to the consequences of social subordination. Indeed, we have previously shown the short promoter length variant in the gene encoding the serotonin transporter exacerbates the suppression of LH secretion in subordinate females (Michopoulos et al., 2009a).
Despite the statistically distinct differences that emerged, a limitation of the present study is the rather narrow list of variables that were evaluated, as a number of systems are stress responsive. For example, immune function differs between dominant and subordinate females (Paiardini et al., 2009) and subordinate females are less behaviorally responsive to the activational effects of the ovarian hormone estradiol (Wallen, 2001). In addition, data from cynomolgus females show status differences in brain neurochemistry based on PET studies (Grand et al., 2005; Grant et al., 1998). Other neuroimaging studies could show structural and functional differences between dominant and subordinate females. Furthermore, because many of the assessments made in these ovariectomized females were done in the absence of ovarian hormone replacement, the data in some cases may be more applicable to post-menopausal women. Additional studies will need to determine how many of the phenotypes described here are modified or exacerbated by estradiol. Furthermore, we recognize that the social grouping used in our study is a “special case” with respect to normal macaque social organization. A similar analysis of adult females in large groups with multiple males and offspring may yield a somewhat different phenotypic pattern due to mitigating effects of social support from family members or other conspecifics (Abbott et al., 2003b; Ozbay et al., 2008). In addition, it is important to note that use of other nonhuman primate models may yield a different phenotypic profile between dominant and subordinate females. For example, as described above, subordinate female marmosets have a blunted adrenal cortisol response (Saltzman et al., 2006) and subordinate status is not typically associated with more harassment from more dominant females (Abbott et al., 1998). Nonetheless, the approach used in the present study shows a continual exposure to the social stress of subordination in female rhesus monkeys, an experience that has species-specific ethological validity, has lasting effects on a number of phenotypes.
In summary, the data presented here show that a number of markers in subordinate females, including alterations in LHPA axis regulation, match those observed in humans who are under chronic stress and heavy allostatic load (Berga et al., 1989; Epel et al., 2001; Juster et al., 2010; McEwen, 2008), including changes that emerge with low socioeconomic status (SES) (Marmot, 2003; Miller et al., 2009). However, studies of social status in macaques is more than a model of SES, as subordinate status reflects a condition of unresolved and unpredictable recurring stressor exposure that individuals of any SES may experience. Results assembled here suggest that social subordination in rhesus monkey females represents a unique translational model of psychosocial stress and a valuable means to investigate any number of adverse health effects experienced by women.
Acknowledgements
The study was conducted with the technical expertise of Jennifer Whitley, Shannon Bounar, Jodi Godfrey, Marta Checchi, Christine Marsteller, Jonathon Lowe, Desiree Sharpe, Rebecca Herman, Robert Johnston and Gregory Henry. We thank Dr. Victor May (University of Vermont) for performing the serum PACAP assays. The study was supported by NIH grants HD46501 (MW), MH081816 (DT), and RR00165, and F31MH085445 (VM). Additional support was provided by the Center for Behavioral Neuroscience through the STC Program of the National Science Foundation IBN-9876754. The YNPRC is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
Role of Funding Source Funding support for this study was provided by NIH grants HD46501 (MW), MH081816 (DT), and RR00165, and F31MH085445 (VM). The NIH had no role in the study design, the collection, analysis, and interpretation of the data, nor in writing the manuscript or the decision on where to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors Authors Michopoulos and Wilson designed and organized the studies and wrote the protocols. Authors Michopoulos, Toufexis and Wilson collected the data. Author Higgins undertook the statistical analyses. Authors Michopoulos, Toufexis and Wilson contributed to the interpretation of the data. Authors Michopoulos and Wilson wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
References
- Abbott DH, Barnett DK, Colman RJ, Yamamoto ME, Schultz-Darken NJ. Aspects of common marmoset basic biology and life history important for biomedical research. Comparative medicine. 2003a;53:339–50. [PubMed] [Google Scholar]
- Abbott DH, Hearn JP. Physical, hormonal and behavioural aspects of sexual development in the marmoset monkey, Callithrix jacchus. J Reprod Fertil. 1978;53:155–66. doi: 10.1530/jrf.0.0530155. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Sapolsky RM. Are subordinates always stressed? a comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003b;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Saltzman W, Schultz-Darken NJ, Tannenbaum PL. Adaptations to subordinate status in female marmoset monkeys. Comparative biochemistry and physiology. Part C, Pharmacology, toxicology & endocrinology. 1998;119:261–74. doi: 10.1016/s0742-8413(98)00015-2. [DOI] [PubMed] [Google Scholar]
- Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–58. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Anisman H, Matheson K. Stress, depression and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev. 2005;29:525–46. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Arce M, Michopoulos V, Shepard KN, Ha QC, Wilson ME. Diet choice, cortisol reactivity and emotional feeding in socially housed rhesus monkeys. Physiology & behavior. 2010;101:446–55. doi: 10.1016/j.physbeh.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armario A. The hypothalamic-pituitary-adrenal axis: what can it tell us about stressors? CNS Neurol Disord Drug Targets. 2006;5:485–501. doi: 10.2174/187152706778559336. [DOI] [PubMed] [Google Scholar]
- Belzung C, Anderson R. Social rank and responses to feeding competition in rhesus monkeys. Behavioral Processes. 1986;12:307–316. doi: 10.1016/0376-6357(86)90001-X. [DOI] [PubMed] [Google Scholar]
- Berga SL, Mortola JF, Girton L, Suh B, Laughlin G, Pham P, Yen SS. Neuroendocrine aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 1989;68:301–8. doi: 10.1210/jcem-68-2-301. [DOI] [PubMed] [Google Scholar]
- Bernstein I. Dominance, aggression and reproduction in primate societies. Journal of Theoretical Biology. 1976a;60:459–472. doi: 10.1016/0022-5193(76)90072-2. [DOI] [PubMed] [Google Scholar]
- Bernstein I, Gordon T. The function of aggression in primate societies. American Science. 1974a;62:304–311. [PubMed] [Google Scholar]
- Bernstein I, Gordon T, Rose R. Agression and social controls in rhesus monkeys (Macaca mulatta) groups revealed in group formation studies. Folia Primatol (Basel) 1974a;21:81–107. doi: 10.1159/000155607. [DOI] [PubMed] [Google Scholar]
- Bernstein IS. Dominance, aggression and reproduction in primate societies. J Theor Biol. 1976b;60:459–72. doi: 10.1016/0022-5193(76)90072-2. [DOI] [PubMed] [Google Scholar]
- Bernstein IS, Gordon TP. The function of aggression in primate societies. Am Sci. 1974b;62:304–11. [PubMed] [Google Scholar]
- Bernstein IS, Gordon TP. Behavioral research in breeding colonies of Old World monkeys. Lab Anim Sci. 1977;27:532–40. [PubMed] [Google Scholar]
- Bernstein IS, Gordon TP, Rose RM. Aggression and social controls in rhesus monkey (Macaca mulatta) groups revealed in group formation studies. Folia Primatol (Basel) 1974b;21:81–107. doi: 10.1159/000155607. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–39. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman MF, Roderick RE, Basbaum AI, Taylor BK. The effects of prior chronic stress on cardiovascular responses to acute restraint and formalin injection. Brain Res. 1998;797:313–20. doi: 10.1016/s0006-8993(98)00382-5. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C. Facilitation of hypothalamic-pituitary-adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. Horm Behav. 2003;43:158–65. doi: 10.1016/s0018-506x(02)00011-9. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J Neuroendocrinol. 2006;18:13–24. doi: 10.1111/j.1365-2826.2005.01375.x. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Sakai RR, McEwen B, Weiss SM, Blanchard RJ. Subordination stress: behavioral, brain and neuroendocrine correlates. Behav Brain Res. 1993;58:113–21. doi: 10.1016/0166-4328(93)90096-9. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20:117–34. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- Blank MS, Gordon TP, Wilson ME. Effects of capture and venipuncture on serum levels of prolactin, growth hormone and cortisol in outdoor compound-housed female rhesus monkeys (Macaca mulatta) Acta Endocrinol (Copenh) 1983;102:190–5. doi: 10.1530/acta.0.1020190. [DOI] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ulrich-Lai YM, Nguyen MM, Ostrander MM, Herman JP. The role of the posterior medial bed nucleus of the stria terminalis in modulating hypothalamic-pituitary-adrenocortical axis responsiveness to acute and chronic stress. Psychoneuroendocrinology. 2008;33:659–69. doi: 10.1016/j.psyneuen.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Nguyen MM, Tamashiro KL, Ma LY, Sakai RR, Herman JP. Chronic social stress in the visible burrow system modulates stress-related gene expression in the bed nucleus of the stria terminalis. Physiol Behav. 2006;89:301–10. doi: 10.1016/j.physbeh.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Collura LA, Hoffman JB, Wilson ME. Administration of human leptin differentially affects parameters of cortisol secretion in socially housed female rhesus monkeys. Endocrine. 2009 doi: 10.1007/s12020-009-9250-7. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Gould RW, Nader MA. Relationship between social rank and cortisol and testosterone concentrations in male cynomolgus monkeys (Macaca fascicularis) J Neuroendocrinol. 2009;21:68–76. doi: 10.1111/j.1365-2826.2008.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, Papadopoulou-Daifoti Z. Chronic mild stress impact: are females more vulnerable? Neuroscience. 2005;135:703–14. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19:275–80. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- Flak JN, Ostrander MM, Tasker JG, Herman JP. Chronic stress-induced neurotransmitter plasticity in the PVN. J Comp Neurol. 2009;517:156–65. doi: 10.1002/cne.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Molecular and cellular endocrinology. 2010;316:129–39. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Gamaro GD, Manoli LP, Torres IL, Silveira R, Dalmaz C. Effects of chronic variate stress on feeding behavior and on monoamine levels in different rat brain structures. Neurochem Int. 2003;42:107–14. doi: 10.1016/s0197-0186(02)00080-3. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Thompson RR. Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Current Opinion in Neurobiology. 2010;20:784–94. doi: 10.1016/j.conb.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Grant KA, Shively CA, Nader MA, Ehrenkaufer RL, Line SW, Morton TE, Gage HD, Mach RH. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse. 1998;29:80–3. doi: 10.1002/(SICI)1098-2396(199805)29:1<80::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Graves FC, Wallen K. Androgen-induced yawning in rhesus monkey females is reversed with a nonsteroidal anti-androgen. Horm Behav. 2006;49:233–6. doi: 10.1016/j.yhbeh.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Hambright MK, Wilson ME. Relationship between social factors and pituitary-adrenocortical activity in female rhesus monkeys (Macaca mulatta) Horm Behav. 1993;27:318–31. doi: 10.1006/hbeh.1993.1024. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Wilson ME, Ahmed-Ansari A, Brodie AR, McClure HM. Formation of a new social group of unfamiliar female rhesus monkeys affects the immune and pituitary adrenocortical systems. Brain Behav Immun. 1991;5:296–307. doi: 10.1016/0889-1591(91)90024-5. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Chandler PC, Wauford PK, Rybak RJ, Oswald KD. The role of palatable food and hunger as trigger factors in an animal model of stress induced binge eating. Int J Eat Disord. 2003;34:183–97. doi: 10.1002/eat.10168. [DOI] [PubMed] [Google Scholar]
- Hardy MP, Sottas CM, Ge R, McKittrick CR, Tamashiro KL, McEwen BS, Haider SG, Markham CM, Blanchard RJ, Blanchard DC, Sakai RR. Trends of reproductive hormones in male rats during psychosocial stress: role of glucocorticoid metabolism in behavioral dominance. Biol Reprod. 2002;67:1750–5. doi: 10.1095/biolreprod.102.006312. [DOI] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–90. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–80. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Homan GF, Davies M, Norman R. The impact of lifestyle factors on reproductive performance in the general population and those undergoing infertility treatment: a review. Human Reproduction Update. 2007;13:209–23. doi: 10.1093/humupd/dml056. [DOI] [PubMed] [Google Scholar]
- Huhman KL. Social conflict models: can they inform us about human psychopathology? Horm Behav. 2006;50:640–6. doi: 10.1016/j.yhbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM. Conditioned defeat in male and female Syrian hamsters. Horm Behav. 2003;44:293–9. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Jacobs A, Petit O. Social network modeling: A powerful tool for the study of group scale phenomena in primates. American Journal of Primatology. 2011;73:741–7. doi: 10.1002/ajp.20932. [DOI] [PubMed] [Google Scholar]
- Jaferi A, Bhatnagar S. Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamic-pituitary-adrenal activity in animals that habituate to repeated stress. Endocrinology. 2006;147:4917–30. doi: 10.1210/en.2005-1393. [DOI] [PubMed] [Google Scholar]
- Jankord R, Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiol Behav. 2008;93:807–19. doi: 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochman KA, Newman SM, Kalin NH, Bakshi VP. Corticotropin-releasing factor-1 receptors in the basolateral amygdala mediate stress-induced anorexia. Behav Neurosci. 2005;119:1448–58. doi: 10.1037/0735-7044.119.6.1448. [DOI] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kaplan JR. Origins and health consequences of stress-induced ovarian dysfunction. Interdiscip Top Gerontol. 2008;36:162–85. doi: 10.1159/000137709. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Clarkson TB, Manuck SB, Shively CA. Social behavior and gender in biomedical investigations using monkeys: studies in atherogenesis. Lab Anim Sci. 1991;41:334–43. [PubMed] [Google Scholar]
- Kaplan JR, Chen H, Appt SE, Lees CJ, Franke AA, Berga SL, Wilson ME, Manuck SB, Clarkson TB. Impairment of ovarian function and associated health-related abnormalities are attributable to low social status in premenopausal monkeys and not mitigated by a high-isoflavone soy diet. Hum Reprod. 2010;25:3083–94. doi: 10.1093/humrep/deq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JR, Chen H, Manuck SB. The relationship between social status and atherosclerosis in male and female monkeys as revealed by meta-analysis. Am J Primatol. 2009;71:732–41. doi: 10.1002/ajp.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB. Ovarian dysfunction, stress and disease: a primate continuum. ILAR. 2004;45:89–115. doi: 10.1093/ilar.45.2.89. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB. Ovarian dysfunction and the premenopausal origins of coronary heart disease. Menopause. 2008;15:768–76. doi: 10.1097/gme.0b013e31815eb18e. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Fontenot MB, Mann JJ. Central nervous system monoamine correlates of social dominance in cynomolgus monkeys (Macaca fascicularis) Neuropsychopharmacology. 2002;26:431–43. doi: 10.1016/S0893-133X(01)00344-X. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Ross C. Baboon (Papio anubis) social complexity-a network approach. American Journal of Primatology. 2011;73:775–89. doi: 10.1002/ajp.20967. [DOI] [PubMed] [Google Scholar]
- Lucas LR, Celen Z, Tamashiro KL, Blanchard RJ, Blanchard DC, Markham C, Sakai RR, McEwen BS. Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neuroscience. 2004;124:449–57. doi: 10.1016/j.neuroscience.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Marmot MG. Understanding social inequalities in health. Perspect Biol Med. 2003;46:S9–23. [PubMed] [Google Scholar]
- Marti O, Marti J, Armario A. Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. Physiol Behav. 1994;55:747–53. doi: 10.1016/0031-9384(94)90055-8. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–85. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. What is in a name? Integrating homeostasis, allostasis and stress. Horm Behav. 2010;57:105–11. doi: 10.1016/j.yhbeh.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire J, Herman JP, Horn PS, Sallee FR, Sah R. Enhanced fear recall and emotional arousal in rats recovering from chronic variable stress. Physiol Behav. 2010;101:474–82. doi: 10.1016/j.physbeh.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie-Quirk SD, Miczek KA. Social rank and social separation as determinants of alcohol drinking in squirrel monkeys. Psychopharmacology (Berl) 2008;201:137–45. doi: 10.1007/s00213-008-1256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. 2007;191:387–92. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Berga SL, Kaplan JR, Wilson ME. Social subordination and polymorphisms in the gene encoding the serotonin transporter enhance estradiol inhibition of luteinizing hormone secretion in female rhesus monkeys. Biol Reprod. 2009a;81:1154–63. doi: 10.1095/biolreprod.109.079038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Checchi M, Sharpe D, Wilson ME. Estradiol effects on behavior and serum oxytocin are modified by social status and polymorphisms in the serotonin transporter gene in female rhesus monkeys. Hormones and behavior. 2011a;59:528–35. doi: 10.1016/j.yhbeh.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Checchi M, Sharpe D, Wilson ME. Estradiol effects on behavior and serum oxytocin are modified by social status and polymorphisms in the serotonin transporter gene in female rhesus monkeys. Hormones and Behavior. 2011b;59:528–35. doi: 10.1016/j.yhbeh.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Checchi M, Sharpe D, Wilson ME. The prosocial effects of estradiol is modify by social subordination and polymorphisms in the gene encoding the serotonin transporter. Hormones & Behavior in review. 2011c doi: 10.1016/j.yhbeh.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Shepard KN, Arce M, Whitley J, Wilson ME. Food history and diet choice affect food intake in monkeys. Appetite. 2009b;52:848. [Google Scholar]
- Michopoulos V, Toufexis D, Wilson ME. Social stress promotes emotional feeding and interacts with diet to shape appetite in females. Psychoneuroendocrinology in review. 2011d doi: 10.1016/j.psyneuen.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Wilson ME. Body weight decreases induced by estradiol in female rhesus monkeys are dependent upon social status. Physiology & Behavior. 2011;102:382–8. doi: 10.1016/j.physbeh.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Chen E, Cole SW. Health psychology: developing biologically plausible models linking the social world and physical health. Annu Rev Psychol. 2009;60:501–24. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–74. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Prioleau OA, Nader SH, Kaplan JR, Nader MA. Predictors of social status in cynomolgus monkeys (Macaca fascicularis) after group formation. American Journal of Primatology. 2000;52:115–31. doi: 10.1002/1098-2345(200011)52:3<115::AID-AJP1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Sheps S, Arck PC. Stress and reproductive failure: past notions, present insights and future directions. Journal of assisted reproduction and genetics. 2008;25:47–62. doi: 10.1007/s10815-008-9206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbay F, Fitterling H, Charney D, Southwick S. Social support and resilience to stress across the life span: a neurobiologic framework. Curr Psychiatry Rep. 2008;10:304–10. doi: 10.1007/s11920-008-0049-7. [DOI] [PubMed] [Google Scholar]
- Paiardini M, Hoffman J, Cervasi B, Ortiz AM, Stroud F, Silvestri G, Wilson ME. T-cell phenotypic and functional changes associated with social subordination and gene polymorphisms in the serotonin reuptake transporter in female rhesus monkeys. Brain Behav Immun. 2009;23:286–93. doi: 10.1016/j.bbi.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce BN, Hemsworth PH, Rivalland ET, Wagenmaker ER, Morrissey AD, Papargiris MM, Clarke IJ, Karsch FJ, Turner AI, Tilbrook AJ. Psychosocial stress suppresses attractivity, proceptivity and pulsatile LH secretion in the ewe. Horm Behav. 2008;54:424–34. doi: 10.1016/j.yhbeh.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Pope NS, Gordon TP, Wilson ME. Age, social rank and lactational status influence ovulatory patterns in seasonally breeding rhesus monkeys. Biol Reprod. 1986;35:353–9. doi: 10.1095/biolreprod35.2.353. [DOI] [PubMed] [Google Scholar]
- Razzoli M, Carboni L, Arban R. Alterations of behavioral and endocrinological reactivity induced by 3 brief social defeats in rats: relevance to human psychopathology. Psychoneuroendocrinology. 2009;34:1405–16. doi: 10.1016/j.psyneuen.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–7. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddick NV, Czoty PW, Gage HD, Kaplan JR, Nader SH, Icenhower M, Pierre PJ, Bennett A, Garg PK, Garg S, Nader MA. Behavioral and neurobiological characteristics influencing social hierarchy formation in female cynomolgus monkeys. Neuroscience. 2009;158:1257–65. doi: 10.1016/j.neuroscience.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annual review of neuroscience. 2009;32:267–87. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosnow RL. Effect sizes for experimenting psychologists. Can J Exp Psychol. 2003;57:221–37. doi: 10.1037/h0087427. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Digby LJ, Abbott DH. Reproductive skew in female common marmosets: what can proximate mechanisms tell us about ultimate causes? Proc Biol Sci. 2008 doi: 10.1098/rspb.2008.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman W, Hogan BK, Abbott DH. Diminished cortisol levels in subordinate female marmosets are associated with altered central drive to the hypothalamic-pituitary-adrenal axis. Biol Psychiatry. 2006;60:843–9. doi: 10.1016/j.biopsych.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Schultz-Darken NJ, Scheffler G, Wegner FH, Abbott DH. Social and reproductive influences on plasma cortisol in female marmoset monkeys. Physiol Behav. 1994;56:801–10. doi: 10.1016/0031-9384(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–52. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Sassenrath EN. Increased adrenal responsiveness to social stress in rhesus monkeys. Hormones & Behavior. 1970;1:283–298. [Google Scholar]
- Schulkin J, McEwen BS, Gold PW. Allostasis, amygdala, and anticipatory angst. Neurosci Biobehav Rev. 1994;18:385–96. doi: 10.1016/0149-7634(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Shively C, Kaplan J. Effects of social factors on adrenal weight and related physiology of Macaca fascicularis. Physiol Behav. 1984;33:777–82. doi: 10.1016/0031-9384(84)90047-7. [DOI] [PubMed] [Google Scholar]
- Shively CA. Social subordination stress, behavior, and central monoaminergic function in female cynomolgus monkeys. Biological Psychiatry. 1998;44:882–91. doi: 10.1016/s0006-3223(97)00437-x. [DOI] [PubMed] [Google Scholar]
- Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biological Psychiatry. 1997;41:871–82. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- Shively CA, Mirkes SJ, Lu NZ, Henderson JA, Bethea CL. Soy and social stress affect serotonin neurotransmission in primates. Pharmacogenomics J. 2003;3:114–121. doi: 10.1038/sj.tpj.6500166. [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, Clarkson TB. Social Stress, Visceral Obesity, and Coronary Artery Atherosclerosis in Female Primates. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.74. [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, Friedman DP, Morgan TM, Thompson J, Lanier T. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis) Biol Psychol. 2005;69:67–84. doi: 10.1016/j.biopsycho.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Shively CA, Willard SL. Behavioral and neurobiological characteristics of social stress versus depression in nonhuman primates. Exp Neurol. 2011 doi: 10.1016/j.expneurol.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagin GN, Howell LA, Redmann S, Jr., Ryan DH, Harris RB. Prevention of stress-induced weight loss by third ventricle CRF receptor antagonist. Am J Physiol. 1999;276:R1461–8. doi: 10.1152/ajpregu.1999.276.5.R1461. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Jankord R, Flak JN, Herman JP. Chronic stress, energy balance and adiposity in female rats. Physiol Behav. 2011;102:84–90. doi: 10.1016/j.physbeh.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon MB, Karom MC, Huhman KL. Sex and estrous cycle differences in the display of conditioned defeat in Syrian hamsters. Horm Behav. 2007;52:211–9. doi: 10.1016/j.yhbeh.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Spranger J, Kroke A, Mohlig M, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–8. doi: 10.1016/S0140-6736(03)12255-6. [DOI] [PubMed] [Google Scholar]
- Stavisky RC, Adams MR, Watson SL, Kaplan JR. Dominance, cortisol, and behavior in small groups of female cynomolgus monkeys (Macaca fascicularis) Hormones & Behavior. 2001;39:232–8. doi: 10.1006/hbeh.2001.1650. [DOI] [PubMed] [Google Scholar]
- Steckler T. Developing small molecule nonpeptidergic drugs for the treatment of anxiety disorders: is the challenge still ahead? Curr Top Behav Neurosci. 2010;2:415–28. doi: 10.1007/7854_2009_14. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Hegeman MA, Sakai RR. Chronic social stress in a changing dietary environment. Physiol Behav. 2006;89:536–42. doi: 10.1016/j.physbeh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Nguyen MM, Fujikawa T, Xu T, Yun Ma L, Woods SC, Sakai RR. Metabolic and endocrine consequences of social stress in a visible burrow system. Physiol Behav. 2004;80:683–93. doi: 10.1016/j.physbeh.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Nguyen MM, Ostrander MM, Gardner SR, Ma LY, Woods SC, Sakai RR. Social stress and recovery: implications for body weight and body composition. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1864–74. doi: 10.1152/ajpregu.00371.2007. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Nguyen MM, Sakai RR. Social stress: from rodents to primates. Front Neuroendocrinol. 2005;26:27–40. doi: 10.1016/j.yfrne.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Tannenbaum BM, Brindley DN, Tannenbaum GS, Dallman MF, McArthur MD, Meaney MJ. High-fat feeding alters both basal and stress-induced hypothalamic-pituitary-adrenal activity in the rat. The American journal of physiology. 1997;273:E1168–77. doi: 10.1152/ajpendo.1997.273.6.E1168. [DOI] [PubMed] [Google Scholar]
- Troisi A. Displacement activities as a behavioral measure of stress in nonhuman primates and human subjects. Stress. 2002;5:47–54. doi: 10.1080/102538902900012378. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009 doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ML, Gordon TP, Wilson ME. Reproductive performance in capture-acclimated female rhesus monkeys (Macaca mulatta) J Med Primatol. 1982;11:291–302. [PubMed] [Google Scholar]
- Wallen K. Sex and context: hormones and primate sexual motivation. Horm Behav. 2001;40:339–57. doi: 10.1006/hbeh.2001.1696. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, McKittrick CR, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Effects of chronic social stress on tyrosine hydroxylase mRNA and protein levels. Brain Res Mol Brain Res. 1995;32:176–80. doi: 10.1016/0169-328x(95)00081-3. [DOI] [PubMed] [Google Scholar]
- Wilson JF, Kopitzke EJ. Stress and infertility. Curr Womens Health Rep. 2002;2:194–9. [PubMed] [Google Scholar]
- Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, Bartness TJ. Quantifying food intake in socially housed monkeys: social status effects on caloric consumption. Physiology & Behavior. 2008;94:586–594. doi: 10.1016/j.physbeh.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Kinkead B. Gene-environment interactions, not neonatal growth hormone deficiency, time puberty in female rhesus monkeys. Biol Reprod. 2008;78:736–43. doi: 10.1095/biolreprod.107.065953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Current status of cortisol findings in post-traumatic stress disorder. Psychiatr Clin North Am. 2002;25:341–68. vii. doi: 10.1016/s0193-953x(02)00002-3. [DOI] [PubMed] [Google Scholar]
- Zehr JL, Van Meter PE, Wallen K. Factors regulating the timing of puberty onset in female rhesus monkeys (Macaca mulatta): role of prenatal androgens, social rank, and adolescent body weight. Biol Reprod. 2005;72:1087–94. doi: 10.1095/biolreprod.104.027755. [DOI] [PubMed] [Google Scholar]