Abstract
TH17 cells constitute a pro-inflammatory CD4+ T-cell subset that is important for microbial clearance, but also are implicated as propagators of various autoimmune pathologies. Evidence suggests that TH17 cells share common progenitors with immunosuppressive CD4+ inducible regulatory T-cells (iTREG), and that the developmental pathways of these two subsets are reciprocally regulated. In this study, we show evidence that the Src-family tyrosine kinase Fyn helps regulate this TH17/TREG balance. When placed under TH17-skewing conditions, CD4+ T-cells from fyn−/− mice had decreased levels of IL17, but increased expression of the TREG transcription factor Foxp3. The defect in IL17 expression occurred independently of the ectopic Foxp3 expression, and correlated with a delay in RORγt upregulation and an inability to maintain normal STAT3 activation. Fyn-deficient TH17 cells also exhibited delayed upregulation of Il23r, Il21, Rora, and Irf4, as well as aberrant expression of Socs3, suggesting that Fyn may function upstream of a variety of molecular pathways that contribute to TH17 polarization. The fyn−/− mice had fewer IL17+CD4+ T-cells in the large intestinal lamina propria compared to littermate controls. Furthermore, after transfer of either WT or fyn−/− naïve CD4+ T-cells into Rag1−/− hosts, recipients receiving fyn−/− cells had fewer IL17-producing T-cells, indicating that Fyn may also regulate TH17 differentiation in vivo. These results identify Fyn as a possible novel regulator of the developmental balance between the TH17 and TREG cell subsets.
Introduction
A major hallmark of the adaptive immune system is the ability to mount specific responses to a variety of immunological challenges. This specificity is conferred in part through the divergent differentiation of CD4+ helper T-cell subsets, the distinct functions of which allow the immune system to tailor specific responses to pathogens. For example, development of the classically described TH1 or TH2 CD4+ T-cell subsets promotes either a pro-inflammatory/cytotoxic or an antibody-mediated/humoral response, respectively (1).
TH17 cells constitute a third CD4+ T-cell subset separate from the classical TH1 and TH2 lineages; this distinction is underscored by the unique immunological functions and developmental requirements of the TH17 cell lineage (2–4). While the TH1 and TH2 subsets are regulated by the master transcription factors Tbet and Gata3, respectively (5, 6), TH17 cell differentiation depends on the transcription factor Retinoic acid-related Orphan Receptor gamma (t) (RORγt) (7). The development of TH17 cells also requires the activity of Signal Transducer and Activator of Transcription-3 (STAT3), which mediates the efficient upregulation of RORγt and other TH17-associated genes such as IL17 (8, 9). In addition to IL17, TH17 cells also produce IL21, IL22, TNF-α, and GM-CSF; these cytokines mediate the various functions of the TH17 subset, which include microbial defense, leukocyte recruitment, and autocrine positive regulation of pro-inflammatory cytokine production (10). While normal TH17-mediated inflammation is important for host defense against pathogens, it has also been implicated in a variety of autoimmune pathologies such as inflammatory bowel diseases (11), multiple sclerosis (12), and rheumatoid arthritis (13, 14). Therefore, a tight regulation of the inflammatory properties of TH17 cells is necessary in order to utilize their beneficial immune functions while curtailing their pathogenic capabilities.
One mechanism by which the immune system attenuates inflammatory mechanisms is through an additional CD4+ T-cell subset known as regulatory cells (TREG). TREG cells are regulated by the signature transcription factor Foxp3 (15, 16) and suppress the proliferation and function of effector T-cell subsets (17, 18). TREG cells are predominately divided into two subsets: the natural TREG which are derived from thymic precursors, and the inducible TREG which develop from naïve CD4+ precursors in peripheral lymphoid organs (19). Inducible TREG (henceforth referred to in this study as “TREG”) develop from the same naïve CD4+ precursors as effector T-cells, suggesting that an additional mechanism by which the adaptive immune system suppresses inflammation is by diverting the development of CD4+ precursors from an inflammatory fate to an immunosuppressive one.
Both TREG and TH17 cells are induced by the cytokine TGFβ: TGFβ alone induces Foxp3 upregulation and skewing toward a TREG phenotype (15), while the additional presence of inflammatory cytokines such as IL6 or IL21 collaborate with TGFβ to initiate the development of TH17 cells (20, 21). The reciprocal development of the TH17 and TREG lineages is also reflected at the molecular level: STAT3, a transcription factor important for the TH17 development, has been shown to inhibit the expression of Foxp3 (9, 22). Conversely, Foxp3 is capable of binding the TH17 transcription factor RORγt and inhibiting its transcriptional activity (23). These reports indicate that the development of the TH17 and TREG lineages is a dynamic process which is ultimately determined by the amalgamation of often-opposing molecular signals. Such plasticity presumably provides the immune system a mechanism by which to rapidly react to changing requirements for either a pro-inflammatory or immunosuppressive response. Many other factors have been shown to modulate TREG versus TH17 development, such as retinoic acid (24), IRF4 (25), and the Akt/phosphatidylinositol-3 kinase pathway (26, 27).
While the Src-family tyrosine kinases Fyn and Lck play a role in regulating T-cell receptor (TCR) signals (28), much less is known about their function during T-helper (TH) differentiation. Lck appears to be required for the proper TH2, but not TH1, differentiation of naïve CD4+ T-cells (29, 30). In contrast, Fyn does not play an appreciable role in promoting either TH1 or TH2 development (31). In this report, we provide evidence that the tyrosine kinase Fyn may regulate the balance between TREG and TH17 differentiation by promoting RORγt upregulation, STAT3 activation, and Foxp3 downregulation in TH17-skewed CD4+ T-cells. Our results therefore suggest a role for Fyn in modulating the homeostatic balance between the pro- and anti-inflammatory arms of the adaptive immune system.
Materials and Methods
Mice
All mice were on the C57BL/6 background, used at 6–12 weeks of age, and housed in specific pathogen-free conditions in the Center of Comparative Medicine at the Feinberg School of Medicine at Northwestern University. The fyn−/− mice (32) specifically lack the FynT isoform of Fyn, which is predominately expressed by hematopoietic cells. Animal procedures conformed to American Association for Laboratory Animal Science (AALAS) standards and were approved by Northwestern University’s Institutional Animal Care and Use Committee (IACUC).
Isolation and purification of primary CD4+ splenocytes
Spleens were homogenized in “Wash Buffer”: DMEM supplemented with 5% calf serum, 200mM L-glutamine, 50units/ml penicillin, and 50µg/ml streptomycin. Red blood cells (RBCs) were lysed using an NH4Cl solution. Bulk CD4+, CD25-depleted CD4+ cells, or naïve CD62L+ CD4+ cells were isolated using magnetic microbeads (Miltenyi Biotec). To isolate bulk CD4+ cells, RBC-lysed splenocytes were incubated with biotin-conjugated anti-mouse CD4 (eBioscience), then incubated with streptavidin-conjugated microbeads (Miltenyi Biotec); the resulting cells were routinely ≥ 95% CD4+. Alternatively, RBC-lysed splenocytes were depleted with biotin-conjugated antibodies against CD25, γδ TCR, CD8, CD11b, CD45R, and NK1.1 (all from eBioscience) with streptavidin-conjugated microbeads to enrich for CD25-depleted CD4+ cells; the resulting cells were routinely ≥ 90% CD4+CD25−. To isolate CD62L+ CD4+ cells, CD25-depleted CD4+ cells were further purified using anti-CD62L-conjugated microbeads (Miltenyi Biotec); the resulting cells were routinely ≥ 98% CD4+CD62L+.
Cell culture/TH subset skewing
Cultures were performed in 24-well plates (1×106 cells/well) with plate-bound 5µg/ml anti-mouse CD28 (hybridoma 37.51) and 0.5µg/ml anti-mouse TCRβ (eBioscience), in “T-cell media”: RPMI 1640 supplemented with 10% Fetal Bovine Serum (Foundation or Hyclone), 10mM HEPES, 1mM Sodium Pyruvate, 50µM β-mercaptoethanol, 1mM L-glutamine, and 50µg/ml gentamicin. Anti-mouse IFNγ (11B11, 5µg/ml, BioXcell), anti-mouse IL4 (XMG1.2, 5µg/ml BioXcell), anti-mouse IL12 (0.12µg/ml, eBioscience), anti-mouse IL2 (10 µg/ml, BD Pharmingen), mouse IL6 (20ng/ml unless otherwise noted, Peprotech), human TGFβ1 (1ng/ml unless otherwise noted, Peprotech), mouse IL21 (20ng/ml, Peprotech), human IL2 (20ng/ml), mouse IL12 (5ng/ml, Peprotech), mouse IL4 (10ng/ml, Peprotech), mouse IL23 (10ng/ml, R&D Systems), and SU6656 (Cayman Chemical) were added as indicated. Specific TH skewing conditions are shown in Supplementary Figure 1a.
Retrovirus production and transduction
MIG (MSCV-IRES-GFP) constructs expressing RORγt or constitutively-active STAT3 (MIG-RORγt and MIG-STAT3C, respectively) have been described previously (7, 8). MSCV-LTRmiR30-PIG (LMP) is a retroviral vector designed for the dual expression of GFP and short hairpin RNAs (shRNA) (Open Biosystems). The LMP vector expressing an shRNA targeting Foxp3 (LMP-1066) has been described previously (23). Retroviruses were packaged in Phoenix cells and virus-containing supernatant from these cultures were used for transduction of lymphocyte cultures. Briefly, cells were plated in non-skewing conditions with TCR/CD28 stimulation for 24 hours, the culture media replaced with viral supernatant containing 8µg/ml polybrene, and centrifuged at 2500 RPM for 90min at 30°C on a table-top centrifuge. Retroviral supernatant was then replaced with T-cell media containing skewing cytokines, and the cells cultured for an additional 4 to 5 days.
Cell staining and flow cytometry
For cytokine analysis, cells were stimulated for 4 hours with 500ng/ml ionomycin and 5ng/ml PMA in the presence of a protein transport inhibitor (Monensin, eBioscience or Golgistop, BD). Cells were incubated with an Fc-receptor-blocker (2.4G2 hybridoma supernatant) before staining for surface markers in Wash Buffer. Fluorochrome-conjugated AnnexinV and antibodies against CD4 and CD25 were from eBioscience. For intracellular staining, cells were treated with either eBioscience (Foxp3, RORγt) or BD (IL17A, IFNγ, IL4, IL2) fixation/permeabilization reagents and stained with the indicated fluorochrome-conjugated antibodies in Permeabilization/Wash Buffer (eBioscience): anti-IL2 (BD Pharmingen), anti-IL17 (BD Pharmingen or eBioscience), anti-Foxp3,- RORγt,- IFNγ, and -IL4 (all from eBioscience). Staining of phosphorylated STAT3 (Y705) was performed using BD Phosflow reagents, according to the manufacturer’s protocol. Samples were run on a FacsCantoII (BD) at the Northwestern University Interdepartmental ImmunoBiology Core, and data analyzed using FlowJo software (Tree Star). 7-AAD or LIVE/DEAD Fixable Dead Cell reagent (Invitrogen) was used as an indicator of cell viability. Side-scatter (SSC-W/SSC-H) and forward-scatter (FSC-W/FSC-H) plots were used to gate on singlet events prior to all subsequent analyses.
Quantitative real-time reverse transcription PCR (qRT-PCR)
RNA was isolated from 1–5 ×106 cells using Trizol reagent (Invitrogen). RNA concentration and absorbance 260/280 was determined by Nanodrop (Thermo Scientific) at the Genomics Core Facility in the Feinberg School of Medicine at Northwestern University. Complementary DNA (cDNA) was reverse-transcribed from total RNA using Superscript III (Invitrogen) and random hexamer primers. Real-time polymerase chain reaction (qRT-PCR) was performed on 15ng of cDNA in triplicate using SYBR Green Master Mix (Applied Biosystems) and an Applied Biosystems 7000 Sequence Detection System. Relative expression was determined by the ΔCt method of comparative quantification, using β-actin expression as an internal control. Primer sequences are listed in Supplementary Table I. Primers for Ror(c)γt (7) and Rora (33) were described previously.
Isolation of Lamina Proprial Lymphocytes
The cecum and colon were cleaned of adipose and mesenteric tissue, cut open lengthwise, rinsed with PBS, and cut into 2-inch segments. Epithelial cells were removed by sequential shaking in DTT- and EDTA- containing PBS solutions. The remaining tissue was digested at 37°C in T-cell media containing 200U/ml collagenase VIII (Sigma) and 150µg/ml DNase I (Sigma), and lamina proprial lymphocytes were isolated by a 40/80% Percoll gradient. Isolated cells were stimulated for 4 hours with ionomycin and PMA in the presence of a protein transport inhibitor, stained with fluorochrome-conjugated monoclonal antibodies, and analyzed by flow cytometry.
Adoptive transfer of CD45RBhigh CD4+ T-cells
CD45RBhigh CD4+ cells were isolated from the spleen of WT or fyn−/− donors, and injected through the retro-orbital route into Rag1−/− recipients. Briefly, whole spleen homogenates from donor mice were enriched for CD4+ T-cells by negative depletion using antibodies against CD8, CD11b, CD45R, and MHC Class II, followed by removal of antibody-conjugated cells using BioMag Goat Anti-Rat IgG magnetic beads (Qiagen). Viable, singlet CD45RBhighCD4+CD25− cells (about 25% of the CD4+CD25− subset with the highest CD45RB expression) were purified from this CD4+-enriched population by FACS on a MoFlo High-Speed Sorter (Beckman Coulter) at the RHLCCC Flow Cytometry Facility at Northwestern University. 0.4×106 cells in 100µl PBS were injected into age-matched Rag1−/− hosts. In order to assess cytokine expression by donor CD4+ T-cells, lymphocytes isolated from the indicated tissues were stimulated with ionomycin/PMA in the presence of a protein transport inhibitor, and IFNγ and IL17 production was assessed by intracellular staining and flow cytometry.
Data and Statistical analysis
Statistical calculations and Student’s t-tests were performed as indicated using Excel software (Microsoft).
Results
fyn−/− CD4+ T-cells fail to polarize normally to the TH17 lineage
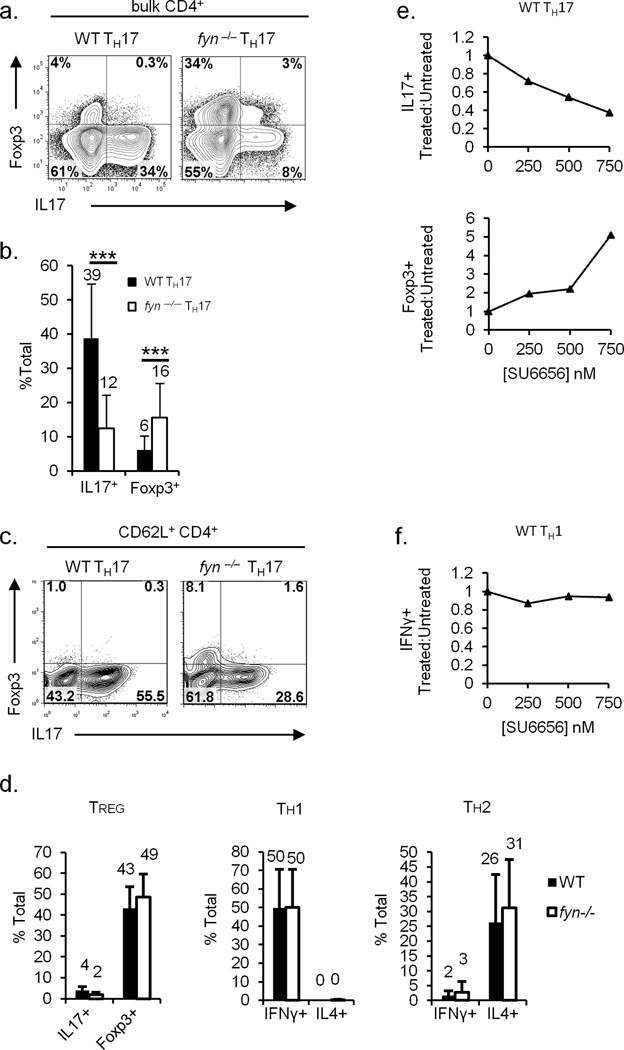
In order to assess the ability of fyn−/− CD4+ cells to polarize toward the TH17 lineage, CD4+ splenocytes were isolated from wild-type (WT) or fyn−/− mice and cultured in media containing TGFβ and IL6 (TH17 skewing conditions; Supplementary Figure 1a). WT CD4+ T-cells produced high levels of IL17 under these conditions, while fyn−/− CD4+ T-cells showed a marked reduction in IL17 expression (Figure 1a, b). Furthermore, fyn−/− CD4+ T-cells under TH17-polarizing conditions expressed high levels of Foxp3, a transcription factor associated with regulatory T-cells (TREG) (Figure 1a, b). To preclude the effect of contamination by previously activated or memory CD4+ T-cells, we performed a more stringent purification of naïve CD62L+CD4+ T-cells, and obtained similar results (Figure 1c). Like WT controls, fyn−/− TH17 cultures produced negligible levels of IFNγ and IL4 (data not shown), suggesting that the defect in TH17 polarization is not due to an aberrant presence of TH1 and TH2 cytokines that inhibit TH17 differentiation.
Figure 1. fyn−/− CD4+ T-cells fail to polarize normally to the TH17 lineage.
a, b) fyn−/− TH17 produce decreased amounts of IL17 and increased levels of Foxp3. CD4+ splenocytes were polarized in vitro under TH17-skewing conditions. IL17 and Foxp3 expression was assessed by intracellular staining and flow cytometry. Plots are gated on viable singlet CD4+ events. In (b), values above bars represent the mean value from at least 19 independent experiments. Error bars denote one standard deviation from the mean. ***: p ≤ 0.001, two-tailed unpaired Student’s t-test for equal variances.
c) Fyn promotes TH17 polarization of naïve CD4+ T-cells. Naïve CD62L+CD4+ T-cells were isolated from the spleens of WT or fyn−/− mice, and skewed under TH17-polarizing conditions. Foxp3 and IL17 expression was determined by intracellular staining and flow cytometry. Plots are gated on viable singlet CD4+ events. Results are representative of two experiments.
d) fyn−/− CD4+ T-cells polarize normally to the TREG, TH1, and TH2 lineages. WT or fyn−/− CD4+ splenocytes were skewed in vitro under TREG-, TH1-, or TH2- polarizing conditions. Foxp3 and cytokine expression in viable singlet CD4+ events was determined by intracellular staining and flow cytometry. The values above each bar indicate mean values from at least 6 experiments. Error bars denote one standard deviation from the mean.
e, f) Pharmacological inhibition of Fyn leads to a selective defect in TH17 differentiation. WT CD4+ splenocytes were skewed under TH17- (e) or TH1-polarizing (f) conditions in the presence of the indicated concentration of the Src-family kinase inhibitor SU6656. IL17, Foxp3, and IFNγ expression was assessed by intracellular staining and flow cytometry in the viable singlet CD4+ gate. The data are represented as a ratio of the percentage of cells in treated versus untreated samples that express the indicated marker. Results are representative of three (e) or two (f) experiments.
fyn−/− CD4+ T-cells polarized normally under TH1, TH2, and TREG skewing conditions, suggesting that fyn−/− CD4+ T-cells have a specific defect in polarization toward the TH17 lineage (Figure 1d). fyn−/− mice have normal percentages of CD25+Foxp3+CD4+ cells in the thymus and spleen, suggesting that natural TREG development is unaffected in the absence of Fyn (data not shown). Using Helios as a marker to distinguish natural from inducible TREGs (34), we analyzed the percentage of Foxp3+ natural (Helios+) and inducible (Helios−) TREGs in steady state fyn−/− and littermate control mice, and found comparable levels of both cell populations in the gut, spleen, and thymus (data not shown).
Lck, another Src-kinase family member with important roles in T-cell differentiation and function, plays a role in TH2 differentiation (29, 30). However, lck−/− CD4+ T-cells (35) expressed normal levels of IL17 and Foxp3 under TH17-skewing conditions (Supplementary Figure 1b).
We considered that the defect in TH17 differentiation of fyn−/− CD4+ cells could result from a non-specific alteration in T-cell development caused during the genetic deletion of fyn. Therefore, we treated WT TH17 cultures with SU6656, a Src kinase inhibitor which exhibits a 40-fold greater selectivity for Fyn than for Lck (36). SU6656 treatment of WT TH17 cultures caused a dose-dependent increase in Foxp3 and decrease in IL17 expression (Figure 1e). The inhibitor had no effect on IFNγ production by WT TH1 cultures (Figure 1f), suggesting that SU6656 does not have a general inhibitory effect on T-cells at the concentrations tested. SU6656 treatment did not affect IL17 or Foxp3 expression in fyn−/− TH17 cultures (data not shown), further suggesting that SU6656’s effect on IL17 and Foxp3 expression in WT TH17 cells is due to specific inhibition of Fyn. Therefore, both the pharmacological inhibition and the genetic deletion of Fyn support the concept that Fyn plays a specific role in TH17 differentiation.
We next considered whether differences in TGFβ or IL6 signaling might contribute to the defective TH17-polarization of fyn−/− CD4+ T-cells. To address this question, we performed a titration of IL6 and TGFβ in the TH17-skewing of WT and fyn−/− CD25-depleted CD4+ splenocytes. At every concentration of IL6 and TGFβ tested, the percentage of cells producing Foxp3 was higher in fyn−/− TH17 cells than in WT TH17 cells (Supplementary Figure 1c), suggesting that fyn−/− CD4+ T-cells have an increased propensity to express Foxp3 under TH17-polarizing conditions. Furthermore, fyn−/− TH17 cells had lower IL17 expression compared to WT cells at every concentration of TGFβ and IL6 tested (Supplementary Figure 1d). These defects in Foxp3 and IL17 expression did not appear to be mediated by changes in the expression of either the IL6 or TGFβ receptor, which was comparable between WT and fyn−/− TH17 cells at both early and late time points of in vitro skewing (Supplementary Figure 1e).
A transient defect in STAT3 activation contributes to decreased IL17 expression by fyn−/− TH17 cells
Although the expression of the IL6 receptor was comparable between WT and fyn−/− CD4+ T-cells, it has been previously reported that Fyn and other Src family members can bind and enhance the activity of STAT3 (37, 38), a downstream mediator of the IL6 receptor and an important activator of IL17 and RORγt expression (8, 9). Therefore, we utilized a flow cytometry-based assay to quantify the level of STAT3 activation, as indicated by phosphorylation at tyrosine 705 (Y705) (39). STAT3 was rapidly activated in response to TH17-skewing cytokines, and STAT3 activation in WT and fyn−/− CD4+ T-cells was comparable during the very early stages of TH17 polarization (Figure 2a). However, fyn−/− TH17 cells exhibited a transient defect in STAT3 activation during the mid-late phase (days 1–3) of the in vitro polarization period (Figure 2b). This also correlated with increased Socs3 mRNA in the mutant, which may contribute to the attenuation of STAT3 signaling (Figure 4b). These defects were later reversed (day 4), at which point STAT3 activation in fyn−/− TH17 cells was equal to or greater than that found in WT TH17 cells. These results suggest that Fyn is transiently required to maintain STAT3 activation during the course of TH17 differentiation.
Figure 2. Transient defects in STAT3 activation contributes to decreased IL17 expression in fyn−/− TH17 cells.
a, b). fyn−/− TH17 cells have a transient defect in STAT3 activation. STAT3(phospho-Y705) was quantified by intracellular staining in WT (solid line), fyn−/− (dotted line), and STAT3-deficient (filled) CD4+ T-cells at the indicated time points after initiation of TH17 polarization. Plots are gated on viable singlet CD4+ events. Results are representative of two (a) or three (b) experiments.
c) fyn−/− CD4+ T-cells have reduced IL17 and elevated Foxp3 expression in response to IL21 and TGFβ. WT and fyn−/− CD4+ splenocytes were cultured for 5 days in the presence of TCR/CD28 stimulation and TGFβ plus IL21 or IL6. Foxp3 and IL17 expression was assessed by intracellular staining and flow cytometry. Plots are gated on viable singlet CD4+ events. Results are representative of three experiments.
d) Constitutively-active STAT3 restores IL17 expression and represses aberrant Foxp3 expression in fyn−/− TH17 cells. WT or fyn−/− CD4+ splenocytes were transduced either with an empty GFP-expressing retroviral vector (MIG; “control”), or one expressing constitutively-active STAT3 (MIG-STAT3C; “STAT3C”), then placed under TH17-polarizing conditions. Foxp3 and IL17 expression was analyzed by intracellular staining and flow cytometry. Plots are gated on viable singlet CD4+ GFP+ events. Results are representative of 3 experiments.
Figure 4. Fyn regulates the kinetics of RORγt and Foxp3 expression during TH17 differentiation.
a) RORγt upregulation and Foxp3 downregulation are delayed in fyn−/− TH17 cells. Foxp3 and RORγt expression were analyzed by flow cytometry in WT and fyn−/− CD25-depleted CD4+ T-cells at the indicated time points after the initiation of TH17 polarization. Plots are gated on viable singlet CD4+ events. Results are representative of three experiments.
b) Expression of TH17-associated genes in fyn−/− TH17 cells. Total RNA was isolated from WT and fyn−/− CD4+ cells after 48 hours (left) or 5 days (right) under TH17-polarizing conditions, and gene expression was assessed by qRT-PCR. The data for each gene represent an average of at least three independent experiments, and is depicted as a fold change over the expression of β-actin. Statistical significance between WT and fyn−/− means was determined by a two-tailed paired Student’s t-test; *: p≤0.05, **: p≤0.001. Error bars denote one standard deviation from the mean. Primer sequences are listed in Supplementary Table I.
c) Exogenous RORγt restores IL17 expression in fyn−/− TH17 cells. WT or fyn−/− CD4+ splenocytes were transduced either with an empty GFP-expressing retroviral vector (MIG; “control”), or one expressing mouse RORγt (MIG-RORγt; “RORγt”), then placed under TH17-polarizing conditions. Foxp3 and IL17 expression were analyzed by intracellular staining and flow cytometry. Plots are gated on viable singlet CD4+ GFP+ events. Results are representative of 3 experiments.
The pro-inflammatory cytokine IL21 is produced by TH17 cells and also signals through a STAT3-dependent mechanism. In combination with TGFβ, IL21 initiates an alternative pathway of TH17 differentiation in naïve CD4+ T-cells (21, 40). Defective STAT3 activation in fyn−/− CD4+ cells skewed with TGFβ and IL6 prompted us to ask whether fyn−/− CD4+ T-cells also had a defect in TH17 polarization in response to TGFβ and IL21. Indeed, under these conditions, fyn−/− CD4+ T-cells exhibited a marked reduction in IL17 production and increase in Foxp3 expression (Figure 2c, right panels). Therefore, fyn−/− CD4+ cells fail to respond normally to an alternative TH17-skewing condition which also requires STAT3 activity but is independent of IL6 receptor signaling.
STAT3 activation is necessary, though not sufficient, to drive optimal IL17 expression in naive CD4+ T-cells (8). The defective STAT3 activation observed in fyn−/− TH17 cells (Figure 2b) led us to hypothesize that Fyn is needed to maintain sufficient STAT3 activity to drive IL17 expression. We therefore transduced WT or fyn−/− CD4+ T-cells with a retrovirus encoding constitutively active STAT3 (STAT3C) prior to the initiation of TH17 skewing (Figure 2d). The introduction of exogenous STAT3 activity into fyn−/− TH17 restored IL17 production to WT levels, suggesting that the Fyn deficiency deregulates IL17 expression in TH17 cells by selectively disrupting normal STAT3 activation.
STAT3 transduction was also able to partially repress the aberrant Foxp3 expression in fyn−/− TH17 cells (Figure 2d). These results are in agreement with previous reports that STAT3 is the mediator of IL6-dependent inhibition of Foxp3 expression (9, 22), and suggest that the transient defect in STAT3 activation may contribute not only to defective IL17 expression, but also to the aberrant Foxp3 expression in fyn−/− TH17 cells.
Deficient IL17 expression in fyn−/− TH17 cells is independent of aberrant Foxp3 expression
Foxp3 can bind and inhibit RORγt, disrupting RORγt-dependent expression of TH17-associated genes (23). Intracellular staining of WT and fyn−/− TH17 cells revealed that although fyn−/− TH17 cells have only a slight reduction in the percentage of RORγt-positive cells, a greater proportion of fyn−/− TH17 cells express the TREG-associated transcription factor Foxp3 (Figure 3a, top). Because of the increased percentage of Foxp3+/RORγt+ double-positive cells present in the fyn−/− TH17 culture, we speculated that the abrogation of TH17-associated gene expression might be due to the previously demonstrated inhibitory function of Foxp3 (23). We therefore asked whether the ectopic Foxp3 expression observed in fyn−/− TH17 cells may be an additional cause of the decreased IL17 production in these cells. To address this question, we compared the IL17 production by WT and fyn−/− TH17 cells which were RORγt-single positive (RORγt SP), or RORγt/Foxp3-double positive (DP) (Figure 3a). This analysis revealed that IL17 expression by fyn−/− TH17 cells was defective in the RORγt SP subset as well as the RORγt/Foxp3 DP subset (Figure 3a, bottom). Since the RORγt SP population comprises the majority of both the WT and fyn−/− TH17 cultures, these results suggest that Foxp3-mediated inhibition of RORγt transcriptional activity is not the predominant mechanism by which IL17 expression is decreased in fyn−/− TH17 cultures.
Figure 3. The defect in IL17 expression by fyn−/− TH17 cells is independent of ectopic Foxp3 expression.
a) IL17 production is reduced in fyn−/− RORγt single-positive TH17 cells. Foxp3, RORγt, and IL17 expression was determined in WT and fyn−/− TH17 cultures by intracellular staining and flow cytometry. IL17 expression was then determined in the Foxp3 single-positive (Foxp3 SP), RORγt single-positive (RORγt SP), Foxp3/RORγt double-negative (DN) or double-positive (DP) populations. Plots are gated on viable singlet CD4+ events. Results are representative of three experiments.
b) Foxp3 knock-down does not elevate IL17 expression in fyn−/− TH17 cells. WT or fyn−/− CD4+ splenocytes were transduced either with an empty GFP-expressing retroviral vector (LMP; “control”), or one expressing a short-hairpin RNA (shRNA) targeting Foxp3 (LMP-1066; “Foxp3 KD”), then placed under TH17-polarizing conditions. Foxp3 and IL17 expression was analyzed by intracellular staining and flow cytometry. Plots are gated on viable singlet CD4+ GFP+ events. Results are representative of 3 experiments.
c) Inhibition of Foxp3 expression by IL2 neutralization does not increase IL17 expression in fyn−/− TH17 cells. WT or fyn−/− TH17 cultures were either not treated (NT), or supplemented with anti-mouse IL2 (αIL2). Foxp3 and IL17 expression was analyzed by intracellular staining and flow cytometry. Plots are gated on viable singlet CD4+ events. Results are representative of 4 experiments.
It remained possible that Foxp3-mediated inhibition of RORγt plays a role in abrogating IL17 expression in the Foxp3+/RORγt+ population of fyn−/− TH17 cells. Therefore, we inhibited Foxp3 expression in both WT and fyn−/− TH17 cells using a short-hairpin RNA (shRNA) construct targeting the mRNA transcript of Foxp3 (Figure 3b). Prior to the initiation of TH17 skewing, WT and fyn−/− CD4+ splenocytes were transduced with either an empty GFP-expressing retroviral vector (LMP; “control”), or the same vector containing an shRNA targeting Foxp3 (LMP-1066; “Foxp3-KD”). Transduction with the LMP-1066 construct (23) was able to reduce expression of Foxp3 in fyn−/− TH17 cells; however, suppression of Foxp3 had no effect on IL17 production (Figure 3b), suggesting that elevated Foxp3 expression in fyn−/− TH17 cultures is not the cause of low IL17 synthesis.
IL2 signaling contributes to Foxp3 expression in TREG cells (22, 41), and inhibits TH17 differentiation (42). fyn−/− TH17 cells had similar or lower levels of IL2 and CD25 expression, suggesting that the aberrant Foxp3 expression in fyn−/− TH17 is not caused by changes in IL2 signaling (Supplemental Figure 2a, b). However, the aberrant Foxp3 expression in fyn−/− TH17 cells was dependent on the presence of IL2, as the addition of a neutralizing antibody against IL2 (αIL2) abrogated Foxp3 expression (Figure 3c). In agreement with our observations using shRNA knockdown of Foxp3, the αIL2-mediated repression of Foxp3 expression in fyn−/− TH17 cells did not lead to an increase in IL17 expression. This further suggested that the defect in IL17 expression is not a downstream consequence of the increased Foxp3 expression (Figure 3b, c).
Delayed RORγt upregulation contributes to defective IL17 expression in fyn−/− TH17 cells
The dynamic expression of RORγt and Foxp3 during the entire course of TH17 differentiation determines the TH17/TREG fate decision (23, 43). Therefore, we next assessed RORγt and Foxp3 expression in WT and fyn−/− TH17 cells at various times after the initiation of in vitro TH17 polarization (Figure 4a). WT TH17 began to upregulate RORγt by the first day after the initiation of skewing (d1), and were nearly all RORγt-positive by the second day (d2) (Figure 4a). By comparison, fyn−/− TH17 cells exhibited a marked delay in the upregulation of RORγt, but by day 5 the percentage of RORγt-positive cells in both the WT and fyn−/− TH17 cultures were similar.
We observed a large amount of transient Foxp3 expression in both WT and fyn−/− TH17 cells during the course of the in vitro skewing process: both began to upregulate Foxp3 around day 1 after the initiation of TH17-skewing. By day 2, over 50 percent of WT and fyn−/− cells expressed Foxp3 (Figure 4a). In WT TH17, the upregulation RORγt preceded that of Foxp3 (compare d1 and d2 expression); consequently all cells transiently expressing Foxp3 also expressed RORγt, consistent with the notion that the majority of TH17 cells go through a RORγt+Foxp3+ stage (23). The transient Foxp3 expression was rapidly extinguished in WT TH17 cells; it was reduced 5-fold by day 3 and nearly undetectable by day 4. In contrast, the fyn−/− TH17 culture retained a population of Foxp3+ cells which persisted despite the presence of the pro-inflammatory cytokine IL6. While fyn−/− TH17 cells upregulated Foxp3 with kinetics similar to that of WT TH17, delayed RORγt upregulation in these cells led to an appreciable accumulation of Foxp3-single positive and Foxp3/RORγt double-negative cells, populations not observed in large numbers within WT TH17 cultures at day 2. These changes were apparent at the mRNA level as well: fyn−/− TH17 cells had a sustained elevation in aberrant Foxp3 (Foxp3) expression, while Rorc(γ)t (RORγt) expression is decreased during the early stages but is normal by day 5 of TH17 skewing (Figure 4b). Both WT and fyn−/− CD25+CD4+ splenocytes exhibited a similar lack of proliferation and high levels of apoptosis under TH17-skewing conditions, suggesting that the Foxp3+ cells in the fyn−/−TH17 culture were de novo generated and not due to abnormal outgrowth of contaminating natural TREGs (Supplemental Figure 2c).
To address whether the delayed upregulation of RORγt accounts for the defective IL17 expression in fyn−/− cells, WT and fyn−/− CD4+ T-cells were transduced with a retrovirus encoding RORγt prior to the initiation of TH17 skewing. The introduction of exogenous RORγt was able to restore IL17 to WT levels in fyn−/− TH17 cells, consistent with the notion that a defect in early RORγt expression may contribute to defective expression of IL17 in fyn−/− TH17 cells (Figure 4c). Notably, overexpression of RORγt was unable to suppress Foxp3 in the fyn−/− TH17 culture, in agreement with a previous report (23).
We next assessed whether the expression of additional TH17-associated genes was altered in fyn−/− TH17 cells. Il21 (IL21) expression in TH17 cells requires STAT3 but not RORγt (8). The expression of Il21 was decreased in fyn−/− TH17 cells at 48 hours after the initiation of TH17 skewing, but was comparable to WT by day 5 (Figure 4b), suggesting that the late restoration of STAT3 activity is sufficient to restore Il21 expression in CD4+ T-cells during later stages of TH17 differentiation. The expression of Il17 (IL17) and Il23r (IL23R) require both RORγt and STAT3 (8). As with Il21, the expression of Il23r was decreased in fyn−/− TH17 cells at the early stages of TH17 differentiation, but comparable to WT levels by day 5 (Figure 4b). Therefore, fyn−/− TH17 cells are able to eventually upregulate normal levels of Il23r despite the transient defect in RORγt and STAT3 expression/activation during the early stages of TH17 differentiation. Furthermore, fyn−/− TH17 cells retained responsiveness to IL23 despite the relative decrease in Il23r expression; both WT and fyn−/− TH17 cells showed a similar fold increase in IL17 and fold decrease in Foxp3 when skewing cultures were supplemented with IL23 (Supplementary Figure 3). In contrast, the expression of Il17 in fyn−/− TH17 cells had not normalized by day 5, suggesting that the late restoration of RORγt and STAT3 expression/activity is not sufficient to drive optimal Il17 expression. The regulation of IL21 and IL17 is also dependent on the transcription factor IRF4. As was noted with IL21, Irf4 expression was reduced in fyn−/− TH17 cells at 48 hr, but normalizes by day 5 (Figure 4b). Similarly, TH17 development is partially dependent on RORα; it too is reduced at 48 hr in the fyn mutants but expression recovers by day 5 (Figure 4b). Therefore, Fyn appears to be necessary for the optimal upregulation of many intersecting molecular pathways that contribute to TH17 differentiation.
fyn−/− CD4+ T-cells have decreased TH17 differentiation in vivo
We next examined whether Fyn regulates the expression of IL17 by CD4+ T-cells in vivo. The lamina propria of intestinal tissue is a highly active lymphoid microenvironment, and is a reservoir for both TH17 (7) and TREG cells in vivo (44). We isolated lymphocytes from the large intestine lamina propria of either fyn−/− or littermate control (fyn+/− or fyn+/+) mice, and determined the Foxp3 and IL17 expression in CD4+ T-cells. WT and fyn−/− had comparable lymphocyte cell numbers in the lamina propria (data not shown). In agreement with our in vitro skewing data, CD4+ T-cells from fyn−/− mice expressed lower levels of IL17 than CD4+ T-cells from control mice (Figure 5a; b left). On the other hand, the percentage of IFNγ-producing CD4+ T-cells was comparable between fyn−/− and control mice, suggesting that the gut CD4+ T-cells from fyn−/− mice have a selective defect in IL17 expression rather than a global inhibition of inflammatory cytokine production (Figure 5b right). We did not detect any differences in the percentage of Foxp3+ cells between fyn−/− and control mice (Figure 5a). The Foxp3+ population is presumably comprised predominately of TREG cells, which develop normally in the absence of Fyn, according to our in vitro studies. No significant difference in IL17 levels was observed in the IL17-producing CD4+TCRβ− innate lymphoid tissue inducer (Lti) population (data not shown), suggesting that the Fyn selectively regulates IL17 production in the T-cell compartment.
Figure 5. fyn−/− CD4+ T-cells have decreased TH17 differentiation in vivo.
a) fyn−/− CD4+ T-cells produce decreased amounts of IL17 in the large intestinal lamina propria. Foxp3 and IL17 expression was analyzed by intracellular staining and flow cytometry in lamina proprial lymphocytes from the large intestine of fyn−/− and littermate controls (fyn+/+ or fyn+/−; CTRL). Plots are gated on viable singlet CD4+ TCRβ+ events.
b) Quantitation of the percentage of IL17+ and IFNγ+ CD4+ T-cells in the large intestine of WT and fyn−/− mice. Lamina proprial lymphocytes were isolated from the large intestine of fyn−/− mice or littermate controls, and analyzed as described in (a). Each individual experiment analyzed 6–8 week-old littermate groups; the results from separate experiments were pooled for the final analysis. Left panel: Reduced frequency of IL17+ T-cells in fyn−/− mutants. Values above bars indicate averages from 12 fyn−/− mice and 13 WT (fyn+/+ or fyn+/−) controls. Right panel: WT and fyn−/− mice have a similar frequency of IFNγ-producing T-cells. Values above bars indicate averages from 6 fyn−/− mice and 7 WT (fyn+/+ or fyn+/−) controls. Error bars indicate one standard deviation from the mean. Statistical analysis was performed using a two-tailed unpaired Student’s t-test for equal variances; **: p ≤ 0.01.
c) fyn−/− naïve CD4+ T-cells have a defect in TH17-polarization in vivo. 0.4×106 CD45RBhighCD4+ splenocytes from WT or fyn−/− donors were transferred into age-matched Rag1−/− hosts. Mice were sacrificed on day 12 after transfer, and IFNγ and IL17 production by viable CD3+CD4+ cells in various organ compartments was determined by intracellular staining and flow cytometry after ionomycin/PMA stimulation in the presence of a protein transport inhibitor. The data are representative of 2 WT and 2 fyn−/− mice. Blood: tail-vein blood; ABI LN: pooled axillary, brachial, inguinal lymph nodes; mes. LN: mesenteric lymph nodes; SI: small intestine; LI: large intestine.
In order to determine whether Fyn is necessary in vivo for the differentiation of naïve CD4+ T-cells into the TH17 subset, we adoptively transferred CD45RBhigh CD25− CD4+ splenocytes from WT or fyn−/− donors into Rag1−/− hosts. The CD45RBhighCD25− population consists of a TREG-depleted naïve subset of CD4+ T-cells; this CD4+ T-cell fraction has been shown to undergo TH1/TH17 polarization when transferred into a lymphopenic host (45–49). We assessed TH1 and TH17 effector cytokine production from lymphocytes isolated from various organ compartments of recipient Rag1−/−mice 12 days post-injection (Figure 5c). Analysis by intracellular staining and flow cytometry allowed the examination of cytokine production on a per cell basis. In all compartments, viable cells from the fyn−/− donor produced less IL17 compared to WT cells. However, fyn−/− cells produced comparable levels of IFNγ, suggesting that the diminished IL17 expression was not merely due to a general abrogation of inflammatory cytokine production. However, it should be noted that the fyn−/− CD45RBhighCD4+ T-cells appeared to have a defect in homeostatic proliferation: we consistently recovered fewer fyn−/− cells from Rag1−/− hosts compared to WT cells (data not shown). Conceivably, the mechanism of IL17 production may be tied to proliferation in a manner reminiscent of other cytokines, in which T cells undergo several rounds of division before becoming fully competent to express IL4 or IFNγ (50).
Discussion
Increasing evidence suggests that the TREG and TH17 subsets may be induced from similar precursors by divergent developmental pathways. We provide evidence that the protein tyrosine kinase Fyn may regulate the reciprocal development of the TH17 and TREG lineages by orchestrating the temporal expression or activation of STAT3, RORγt, and Foxp3. fyn−/− CD4+ splenocytes placed under TH17 polarizing conditions did not fully upregulate the TH17-associated gene Il17. Instead, fyn−/− CD4+ T-cells diverged into a TREG-like phenotype, expressing aberrant levels of Foxp3 and acquiring the ability to suppress the proliferation of naïve CD4+ T-cells in vitro (unpublished results, data not shown).
Our results suggest that the defect in IL17 expression in fyn−/− TH17 cells occurs independently of the ectopic Foxp3 expression, and that the RORγt expressed in the later stages of fyn−/− TH17 differentiation is not sufficient to promote the normal expression of IL17. As previously reported (7, 51), WT cells rapidly upregulated RORγt when placed under TH17 skewing conditions. On the other hand, fyn−/− TH17 cells exhibited a profound delay in RORγt upregulation (Figure 4a); this early defect in RORγt expression may contribute to the later deficiency in IL17 expression. While TH17 differentiation requires RORγt expression, our results reveal that the proper timing of RORγt expression is crucial for the normal expression of TH17-associated genes. The kinetics of RORγt expression in fyn−/− TH17 cells (Figure 4a, b) suggests that RORγt is important for promoting IL17 expression during the early stages (i.e. days 1–3) of in vitro TH17 differentiation. The expression of two other transcription factors that play a role in TH17 differentiation, RORα (33)and IRF4 (25), were also reduced during early differentiation in the absence of Fyn (Figure 4b). Though it remains unclear how Fyn promotes the expression of RORα and IRF4, the global effect of Fyn deletion on these transcription factors suggests that Fyn is an upstream mediator of a variety of the molecular cascades that contribute to TH17 differentiation.
The defect in RORγt expression in fyn−/− TH17 cells was most evident between days 1–3 (Figure 4a); this corresponded to the time points when a transient defect in STAT3 activation was also observed (Figure 2a, b). We also note that SOCS3, an important negative regulator of STAT3 activity, is elevated at 48 hr (Figure 4b). This may contribute further to a reduction in STAT3 function. We therefore hypothesize that Fyn is necessary to maintain normal STAT3 activation during TH17 differentiation, and that a deregulation of STAT3 activation contributes to diminished RORγt and RORγt -dependent IL17 expression in fyn−/− TH17 cells. The role of Fyn and other Src-family kinases in STAT3 activation has been reported in cancers and cell lines (37, 38), and our current findings suggest that this pathway is also an important mediator of TH17 differentiation. During the early to middle stages (days 1–3) of the TH17 differentiation process, WT CD4+ T-cells also upregulated Foxp3, which was extinguished as the differentiation process progressed (Figure 4a). These results are in agreement with previous reports that TH17 cells transiently express Foxp3 during their development (23, 51). fyn−/− TH17, on the other hand, were unable to efficiently quench Foxp3 expression (Figure 4a). Because STAT3 mediates the IL6-dependent downregulation of Foxp3 (22, 23, 52), these results suggest that Fyn may also help orchestrate proper Foxp3 expression during TH17 differentiation by sustaining STAT3 activation.
In addition to STAT3 activation, other mechanisms downstream of Fyn may be necessary to fully extinguish the transient Foxp3 expression that occurs during TH17 differentiation. One possible mechanism is the Akt/PI3K signaling pathway, which is activated by Fyn (53), and is a negative regulator of Foxp3 expression (26, 27). Ablation of PI3K/Akt activity has been shown to promote the upregulation of Foxp3 and a TREG-like gene expression profile in newly activated naïve CD4+ T-cells (27). Similarly, the forced expression of an active Akt construct impairs the TGFβ-induced upregulation of Foxp3 in naïve CD4+ T-cells (26). Akt negatively affects Foxp3 expression by phosphorylating and blocking the nuclear localization of the Forkhead family transcription factors Foxo1 and Foxo3, positive regulators of Foxp3 gene expression (54, 55). Akt can also serve as a positive mediator of IL17 expression (56). Indeed, we have also observed that Akt activation is decreased in fyn−/− CD4+ T-cells relative to WT during the early stages of TH17 differentiation (unpublished results). Thus Fyn may be an important upstream mediator of Akt’s ability to extinguish Foxp3 and promote IL17 expression in TH17 cells. p38 MAPK, a downstream target of the PI3K/Akt pathway, has also been shown to post-transcriptionally promote IL17 production in TH17 cells (57). Therefore, it is possible that the regulation of IL17 levels by Fyn occurs at the level of protein translation as well as that of gene expression. The putative regulation of the Akt/PI3K and MAPK pathways by Fyn during TH17 differentiation requires further studies.
Our results demonstrate that a precise temporal regulation of STAT3, RORγt, and Foxp3 expression is necessary for proper TH17 differentiation. fyn−/− CD4+ T-cells had decreased IL21 and IL23R expression at 48 hours after the initiation of TH17 skewing, but the expression of these genes was comparable to WT TH17 by day 5 of differentiation (Figure 4a, b). This suggests that the recovery of STAT3 (Figure 2b) and RORγt (Figure 4a) activity/expression in fyn−/− TH17 cells during the late stages of differentiation are sufficient to drive the expression of Il21 and Il23r. On the other hand, fyn−/− TH17 cells do not express WT levels of IL17 even by day 5 (Figure 1a; Figure 4b). The precise role that early RORγt or STAT3 activity plays in promoting IL17 expression remains to be determined; the temporal requirement may indicate a role in facilitating permissive histone or chromatin modifications at the IL17 locus. IL6 and TGFβ treatment of naïve CD4+ T-cells induces permissive histone 3 hyperacetylation in the promoter and several conserved non-coding sequences (CNS) within the IL17 locus within 48 hours (58). STAT3 (59) and RORγt (33) have been shown to promote histone 3 acetylation at the promoter and CNS2, respectively, of the IL17 locus in TH17-skewed cells. It is yet unclear whether Fyn may play a role in promoting permissive chromatin restructuring of the IL17 locus during TH17 differentiation.
CD4+ T-cells isolated from the gut of fyn−/− mice also had less IL17 production than those obtained from control mice (Figure 5a–b), corroborating our in vitro data showing that Fyn supports IL17 expression in TH17 cells. Naïve CD45RBhighCD4+ T-cells adoptively transferred into Rag1−/− hosts also produced less IL17 in the absence of Fyn (Figure 5c), suggesting a T-cell-intrinsic requirement for Fyn in the promotion of IL17 expression by CD4+ T-cells in vivo. Based on these data, we hypothesize that Fyn-deficient mice may be more resistant to TH17-mediated inflammation or autoimmune disease, and that pharmacological inhibition of Fyn may be therapeutically beneficial in such disease settings.
Together, the results of this study suggest that Fyn is a mediator of TH17 differentiation, and that it modulates the temporal activation and deactivation of STAT3, RORγt, and Foxp3. We also show that the deregulation of these transcription factors has differential effects on the temporal expression of various TH17-associated genes. These findings underscore the fact that the precise regulation of myriad signaling pathways is necessary for efficient TH17 differentiation, and suggest that Fyn plays a role in orchestrating this regulation.
Supplementary Material
Acknowledgements
We thank all past and present members of the Stein and Zhou Laboratories for their helpful feedback and suggestions. Experimental support was provided by the Northwestern University Interdepartmental ImmunoBiology Flow Cytometry Core Facility, the Northwestern University Genomics Core, and the Northwestern RHLCCC Flow Cytometry Facility and Cancer Center.
Footnotes
This work was supported by a Cancer Research Institute Investigator Award (LZ) and awards from the American Heart Association (AU), Chicago Baseball Cancer Charities (AU) and the National Institutes of Health (CA868687 to PLS; AI089954 and AI091962 to LZ). Liang Zhou, M.D., Ph.D., is a Pew Scholar in Biomedical Sciences, supported by the Pew Charitable Trusts.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 2.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 3.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 5.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 6.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 7.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 8.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 9.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 10.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 11.Abraham C, Cho J. Interleukin-23/Th17 pathways and inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1090–1100. doi: 10.1002/ibd.20894. [DOI] [PubMed] [Google Scholar]
- 12.Zepp J, Wu L, Li X. IL-17 receptor signaling and T helper 17-mediated autoimmune demyelinating disease. Trends Immunol. 2011;32:232–239. doi: 10.1016/j.it.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, Miossec P. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42:963–970. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 14.Lubberts E. Th17 cytokines and arthritis. Semin Immunopathol. 2010;32:43–53. doi: 10.1007/s00281-009-0189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 17.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 18.Rudensky AY, Campbell DJ. In vivo sites and cellular mechanisms of T reg cell-mediated suppression. J Exp Med. 2006;203:489–492. doi: 10.1084/jem.20060214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horwitz DA, Zheng SG, Gray JD. Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other. Trends Immunol. 2008;29:429–435. doi: 10.1016/j.it.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, Hennighausen L, Wu C, O'Shea JJ. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-β-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORγt function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 26.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O'Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salmond RJ, Filby A, Qureshi I, Caserta S, Zamoyska R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol Rev. 2009;228:9–22. doi: 10.1111/j.1600-065X.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- 29.Kemp KL, Levin SD, Bryce PJ, Stein PL. Lck mediates Th2 differentiation through effects on T-bet and GATA-3. J Immunol. 2010;184:4178–4184. doi: 10.4049/jimmunol.0901282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamashita M, Hashimoto K, Kimura M, Kubo M, Tada T, Nakayama T. Requirement for p56(lck) tyrosine kinase activation in Th subset differentiation. Int Immunol. 1998;10:577–591. doi: 10.1093/intimm/10.5.577. [DOI] [PubMed] [Google Scholar]
- 31.Tamura T, Igarashi O, Hino A, Yamane H, Aizawa S, Kato T, Nariuchi H. Impairment in the expression and activity of Fyn during differentiation of naive CD4+ T cells into the Th2 subset. J Immunol. 2001;167:1962–1969. doi: 10.4049/jimmunol.167.4.1962. [DOI] [PubMed] [Google Scholar]
- 32.Appleby MW, Gross JA, Cooke MP, Levin SD, Qian X, Perlmutter RM. Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell. 1992;70:751–763. doi: 10.1016/0092-8674(92)90309-z. [DOI] [PubMed] [Google Scholar]
- 33.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trobridge PA, Levin SD. Lck plays a critical role in Ca(2+) mobilization and CD28 costimulation in mature primary T cells. Eur J Immunol. 2001;31:3567–3579. doi: 10.1002/1521-4141(200112)31:12<3567::aid-immu3567>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 36.Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L, Courtneidge SA. SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol. 2000;20:9018–9027. doi: 10.1128/mcb.20.23.9018-9027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schreiner SJ, Schiavone AP, Smithgall TE. Activation of STAT3 by the Src family kinase Hck requires a functional SH3 domain. J Biol Chem. 2002;277:45680–45687. doi: 10.1074/jbc.M204255200. [DOI] [PubMed] [Google Scholar]
- 38.Yu CL, Meyer DJ, Campbell GS, Larner AC, Carter-Su C, Schwartz J, Jove R. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science. 1995;269:81–83. doi: 10.1126/science.7541555. [DOI] [PubMed] [Google Scholar]
- 39.Kaptein A, Paillard V, Saunders M. Dominant negative stat3 mutant inhibits interleukin-6-induced Jak-STAT signal transduction. J Biol Chem. 1996;271:5961–5964. doi: 10.1074/jbc.271.11.5961. [DOI] [PubMed] [Google Scholar]
- 40.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 41.Murawski MR, Litherland SA, Clare-Salzler MJ, Davoodi-Semiromi A. Upregulation of Foxp3 expression in mouse and human Treg is IL-2/STAT5 dependent: implications for the NOD STAT5B mutation in diabetes pathogenesis. Ann N Y Acad Sci. 2006;1079:198–204. doi: 10.1196/annals.1375.031. [DOI] [PubMed] [Google Scholar]
- 42.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O'Shea J J. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 44.Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, Grigg ME, Berzofsky JA, Belkaid Y. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–649. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 46.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 47.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morrissey PJ, Charrier K. Induction of wasting disease in SCID mice by the transfer of normal CD4+/CD45RBhi T cells and the regulation of this autoreactivity by CD4+/CD45RBlo T cells. Res Immunol. 1994;145:357–362. doi: 10.1016/s0923-2494(94)80200-9. [DOI] [PubMed] [Google Scholar]
- 50.Bird JJ, Brown DR, Mullen AC, Moskowitz NH, Mahowald MA, Sider JR, Gajewski TF, Wang CR, Reiner SL. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–237. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 51.Ichiyama K, Yoshida H, Wakabayashi Y, Chinen T, Saeki K, Nakaya M, Takaesu G, Hori S, Yoshimura A, Kobayashi T. Foxp3 inhibits RORgammat-mediated IL-17A mRNA transcription through direct interaction with RORgammat. J Biol Chem. 2008;283:17003–17008. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- 52.Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, Vollmar P, Stritesky GL, Kaplan MH, Waisman A, Kuchroo VK, Oukka M. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105:18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang X, Feng Y, Ye K. Src-family tyrosine kinase fyn phosphorylates phosphatidylinositol 3-kinase enhancer-activating Akt, preventing its apoptotic cleavage and promoting cell survival. Cell Death Differ. 2007;14:368–377. doi: 10.1038/sj.cdd.4402011. [DOI] [PubMed] [Google Scholar]
- 54.Ouyang W, Beckett O, Ma Q, Paik JH, DePinho RA, Li MO. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol. 2010;11:618–627. doi: 10.1038/ni.1884. [DOI] [PubMed] [Google Scholar]
- 55.Harada Y, Elly C, Ying G, Paik JH, DePinho RA, Liu YC. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. J Exp Med. 2010;207:1381–1391. doi: 10.1084/jem.20100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim KW, Cho ML, Park MK, Yoon CH, Park SH, Lee SH, Kim HY. Increased interleukin-17 production via a phosphoinositide 3-kinase/Akt and nuclear factor kappaB-dependent pathway in patients with rheumatoid arthritis. Arthritis Res Ther. 2005;7:R139–R148. doi: 10.1186/ar1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Noubade R, Krementsov DN, Del Rio R, Thornton T, Nagaleekar V, Saligrama N, Spitzack A, Spach K, Sabio G, Davis RJ, Rincon M, Teuscher C. Activation of p38 MAPK in CD4 T cells controls IL-17 production and autoimmune encephalomyelitis. Blood. 2011;118:3290–3300. doi: 10.1182/blood-2011-02-336552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akimzhanov AM, Yang XO, Dong C. Chromatin remodeling of interleukin-17 (IL-17)-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J Biol Chem. 2007;282:5969–5972. doi: 10.1074/jbc.C600322200. [DOI] [PubMed] [Google Scholar]
- 59.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, Sun HW, Wei L, Vahedi G, Kanno Y, O'Shea JJ, Laurence A. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.