Abstract
This study investigated whether grip type and/or task goal influenced reaching and grasping performance in post-stroke hemiparesis. Sixteen adults with post-stroke hemiparesis and twelve healthy adults reached to and grasped a cylindrical object using one of two grip types (3-finger or palmar) to achieve one of two task goals (hold or lift). Performance of the stroke group was characteristic of hemiparetic limb movement during reach-to-grasp, with more curved handpaths and slower velocities compared to the control group. These effects were present regardless of grip type or task goal. Other measures of reaching (reach time and reach velocity at object contact) and grasping (peak thumb-index finger aperture during the reach and peak grip force during the grasp) were differentially affected by grip type, task goal, or both, despite the presence of hemiparesis, providing new evidence that changes in motor patterns after stroke may occur to compensate for stroke-related motor impairment.
Keywords: functional performance, motor control, rehabilitation
INTRODUCTION
Daily life requires reaching to and grasping a variety of objects that vary in size, shape, and weight, and may be used in a variety of ways. For example, reaching to grasp a coffee mug may be for lifting it to one’s mouth for drinking, or for holding it while it is being filled. Alternatively, one might reach to lift or hold a delicate glass. Both cases involve essential movement components of the upper extremity: reaching for, grasping, and moving/manipulating an object (Lang, 2011), but with different grip types and task goals. Successful task performance, therefore, relies on the ability to move in a task-specific manner. Although previous studies have quantified task-specificity of reach-to-grasp movements in healthy adults (Ansuini, Giosa, Turella, Altoe, & Castiello, 2008; Castiello, 1996; Klatzky, Fikes, & Pellegrino, 1995; Marteniuk, MacKenzie, Jeannerod, Athenes, & Dugas, 1987; Wisneski & Johnson, 2007), much less is known about task-specific motor control in the affected limb after stroke and how hemiparesis affects the ability to move in task-specific ways.
Persistent upper extremity motor impairment is common after stroke, and can significantly limit functional ability and participation in activities that involve reaching and grasping (Dromerick, et al., 2006; Lum, et al., 2009). Kinematic and kinetic analyses of reaching and grasping have been used to quantify motor performance of the affected limb across a range of time post-stroke and stroke severity (Cirstea, Mitnitski, Feldman, & Levin, 2003; Grichting, Hediger, Kaluzny, & Wiesendanger, 2000; Hermsdorfer, Hagl, Nowak, & Marquardt, 2003; Kwakkel, Kollen, van der Grond, & Prevo, 2003; Lang, DeJong, & Beebe, 2009; Lang, et al., 2005; Lang, Wagner, Edwards, Sahrmann, & Dromerick, 2006; Levin, 1996; Michaelsen, Magdalon, & Levin, 2009; Nowak, 2008; Nowak, Hermsdorfer, & Topka, 2003; Raghavan, Santello, Gordon, & Krakauer, 2010; Wenzelburger, et al., 2005) and has shown that, compared to healthy adults, upper extremity movements are slower, less coordinated, and less efficient. Thus, it is possible that stroke-related motor impairments may affect one’s ability to adjust performance given different movement contexts, potentially reducing one’s motor repertoire (Beer, Dewald, Dawson, & Rymer, 2004; Cruz, Waldinger, & Kamper, 2005; Kamper, McKenna-Cole, Kahn, & Reinkensmeyer, 2002; Lang & Schieber, 2004; Lum, Patten, Kothari, & Yap, 2004; Raghavan, Petra, Krakauer, & Gordon, 2006; Reinkensmeyer, McKenna Cole, Kahn, & Kamper, 2002). Conceptually, motor repertoires allow for diverse and adaptable motor performance because different behaviors require different control strategies (Graziano, 2006; Schieber, 1990; Schieber & Santello, 2004), yet it remains unclear how flexible movement control is after stroke across various contextual constraints.
The purpose of this study was to determine whether performance of a functional reach-to-grasp movement in people with post-stroke hemiparesis is influenced by grip type and/or task goal. Studies using single grip types or single tasks have been critical in quantifying how stroke might impair movement production, as described above, but cannot address whether stroke reduces the flexibility of movement control, because of methodological variability across studies. This study was therefore designed to directly test how stroke might alter patterns of performance when moving with multiple grip types and task goals. If movement of the affected limb depends on the grip and goal requirements of a reach-grasp task, this would indicate that the ability to modify a movement strategy in a task-specific way may be preserved in the presence of motor impairment. Alternatively, if reaching and/or grasping were invariant across these contexts, this would suggest that people with hemiparesis do not modify their movement strategy despite the different task requirements. We hypothesized that in the affected limb of stroke subjects and the limbs of healthy subjects, kinematic and kinetic measures of reaching and grasping would be different across the tested grip types and task goals, reflecting intact task-specific strategies after stroke. We therefore predicted significant effects of group, grip type, and task goal on our measures of performance. Findings from this study will further our understanding of how hemiparesis affects upper extremity motor control.
METHODS
Subjects
Sixteen adults with upper extremity hemiparesis following stroke (7 female, 9 male) participated in this study. Eleven subjects within the stroke group were right-handed, based on self-report. Stroke subjects were recruited from the Cognitive Rehabilitation Research Group and Brain Recovery Core Stroke Registries at Washington University in St. Louis based on the presence of unilateral hemiparesis. Potential stroke subjects were included if they (1) had a diagnosis of ischemic or hemorrhagic stroke by a stroke neurologist, (2) had persistent hemiparesis with a score of 1–3 on the Motor Arm item of the National Institutes of Health Stroke Scale (NIHSS), indicating mild to moderate impairment, and (3) had the ability to follow 2-step commands. Potential subjects were excluded from the study if they (1) had orthopedic or other medical conditions that limited the affected upper extremity prior to the stroke, (2) had severe hemispatial neglect as evidenced by a score of 2 on the Extinction and Inattention items of the NIHSS, (3) were unable to give informed consent, or (4) were unable to perform 3-finger and palmar grips. This study was approved by the Washington University Human Research Protection Office, and was conducted in compliance with the Helsinki Declaration. All subjects provided informed consent prior to beginning the study.
Several clinical tests were used to characterize the group of stroke subjects. Maximum grip strength (kg) of the affected and unaffected sides was measured 3 consecutive times with a dynamometer, then averaged and expressed as a percentage of the unaffected side for each stroke subject (Andrews, Thomas, & Bohannon, 1996; Schmidt & Toews, 1970). Spasticity of the elbow flexors was assessed on the affected side using the Modified Ashworth Scale (Bohannon & Smith, 1987). The Action Research Arm Test (ARAT) was used to quantify upper extremity function. This is a criterion-rated assessment of the capacity for gross movement, grasping, gripping and pinching (Hsieh, Hsueh, Chiang, & Lin, 1998; Lang, Wagner, Dromerick, & Edwards, 2006; Lyle, 1981; Van der Lee, Beckerman, Lankhorst, & Bouter, 2001; Van der Lee, De Groot, et al., 2001; Yozbatiran, Der-Yeghiaian, & Cramer, 2008). A maximum score of 57 on each side indicates normal function. Self-perceived hand function was measured using the Stroke Impact Scale (SIS) Hand Function subscale, in which subjects answered questions about their ability to use their affected hand in five daily tasks (Duncan, et al., 1999; Duncan, Wallace, Studenski, Lai, & Johnson, 2001). The score for this scale ranges from 0 to 100 (normal).
Twelve neurologically-intact adults (6 female, 6 male) also participated in this study, ten of whom were right-handed, based on self-report. These subjects served as a group of control subjects for the study, and were recruited from the Volunteer for Health Research Participant Registry at Washington University in St. Louis. Potential control subjects were included if they (1) were at least 30 years old, (2) had no known neurological disease, and (3) had no disability or injury affecting their upper extremity on either side.
Procedure
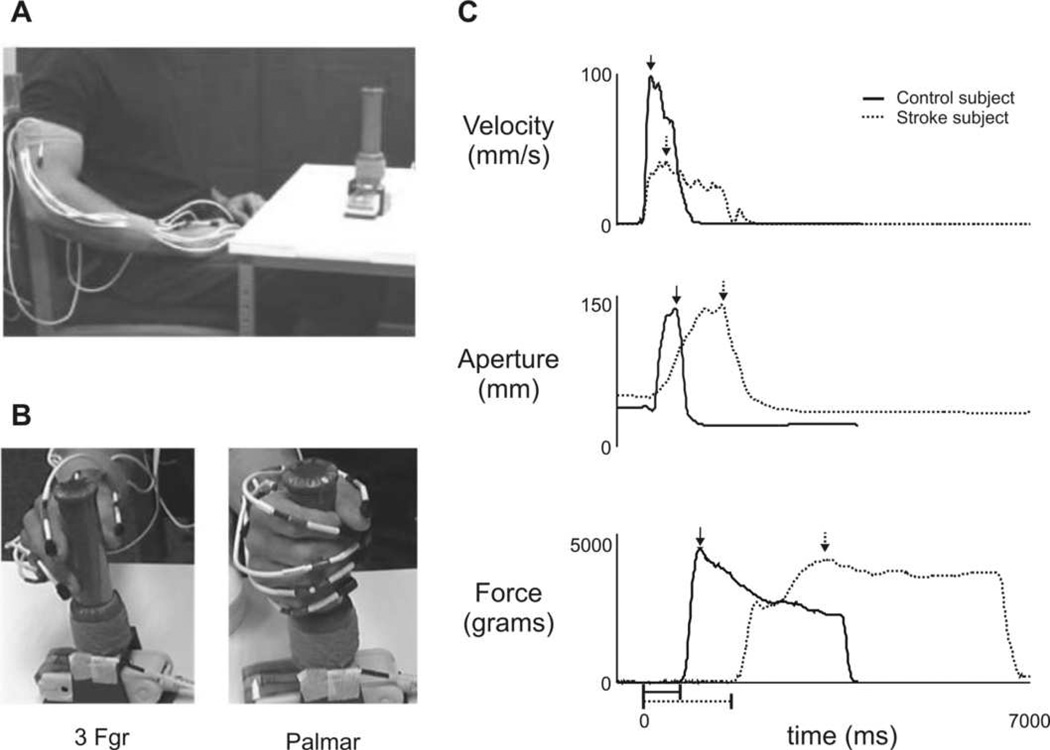
Each subject used only one upper extremity during the experiment. Stroke subjects used their affected side. Control subjects were assigned which side to use based on random selection. Seven (of 12) control subjects used their right side, and seven used their dominant side (6 right, 1 left). All subjects sat in a chair and reached for a custom-fabricated cylindrical object (10.7 cm circumference, 3.4 cm diameter, 11.3 cm height) with a rectangular base (13.4 cm by 6 cm) located on a table (Fig. 1A). The total weight of the object was 420 grams (4.12 N). The table was placed with its closest edge across the subject’s mid-thighs and the height was adjusted to be as low as possible without contacting the thighs, in order to allow clearance of the table edge while reaching. The object was placed on the table at a standardized distance from the subject (90% of the length of the arm from shoulder to wrist), aligned with the mid-clavicle in the frontal plane. At the beginning of each trial, subjects began with their hand resting on their proximal thigh, with the tips of the thumb and fingers together. Subjects were instructed to use one of 2 grip types: a 3-finger grip, which involved the thumb, index, and middle fingers, or a palmar grip (Fig. 1B). These grip types were chosen because they 1) have been well characterized as two discrete patterns of human prehension with different levels of accuracy and/or precision, and 2) represent a range of actions observed in daily life (Napier, 1956; Pouydebat, Reghem, Borel, & Gorce, 2011; Tucker & Ellis, 1998, 2004). The 3-finger grip type is typically classified as a modified pincer grip that provides additional support or strength to the grasp, when afforded by the object, and has been shown to be used more frequently in older adults compared to younger adults and children (Wong & Whishaw, 2004). Subjects were then instructed to use the specified grip type to achieve one of two task goals: To “Reach, grasp, and hold the object,” or “Reach, grasp, and lift the object.” The experimenter demonstrated each task, lifting the object approximately 15 cm above the table during the Lift task. No speed-related instructions were provided. Upon a verbal “go” signal, subjects reached at their natural, comfortable speed to grasp the object anywhere on its instrumented handle (see Fig. 1B). Four trial types (3-fgr lift, 3-fgr hold, palmar lift, palmar hold) were performed in a randomized order, and three consecutive repetitions of each trial type were collected.
Figure 1.
(A) Experimental setup. (B) Palmar and 3-finger (3Fgr) grip types are shown. (C) Sample traces of hand velocity (top), aperture (middle), and grip force (bottom) during a hold trial using a palmar grip are shown for a control subject (solid lines) and a stroke subject (dotted lines). Arrows indicate calculated peak values; horizontal lines below x-axis (time) indicate amount of elapsed time from reach start (t=0) and object contact (i.e. reach end) for both the control subject’s (solid line) and stroke subject’s (dotted line).
Data Analysis
Three-dimensional (3-D) position data of the upper extremity segments were collected with an electromagnetic tracking system with nine sensors (The Motion Monitor, Innovative Sports Training, Chicago, IL). Sensor locations were: midsternum (1 sensor); upper arm (1); forearm (1); hand (1); and fingernail of each digit (5). Kinematic data were collected at 50 Hz and low-pass filtered at 6 Hz using a second-order Butterworth filter. Motion Monitor software (Innovative Sports Training Chicago, IL) was used to calculate resultant velocity from sensor data using standard rigid body methodology (Wu et al., 2005), and custom-written software in MATLAB (The MathWorks, Natick, MA) was used for subsequent analyses.
Pressure on the object was collected with an I-Scan® pressure mapping sensor (Tekscan, Inc., Boston, MA) that was wrapped around the cylindrical surface of the object. The sensor (model 5101/3414TI/10; range 41 to 34,475 kPa) was 11.8 × 11.8 cm and comprised of two thin, flexible polyester sheets (0.1 mm total thickness) that contain electrically conductive sensing cells, or sensels™. Electrical resistance at each sensel varies inversely with applied load (normal force). The I-Scan sensor contains 1,936 sensels in a 44 × 44 array with 15.5 sensels per square cm. Sensor data were collected at 100 Hz, and were converted to units of force (grams) using calibrated Tekscan data acquisition hardware and software. Total force measured by the sensor during each trial was exported as a time-series and further analyzed using MATLAB. Pressure sensor technology is a newer method for measuring grip force (DeJong, Birkenmeier, & Lang, In Press) and was selected because it affords more natural grasping performance for subjects without requiring specific hand/finger placement on discrete sensors. A limitation of the pressure sensor system is that it measures only grip (normal) forces, not load (tangential or shear) forces. For this study, the advantage of capturing “real-life” grasping behavior during a naturalistic action outweighed the disadvantage of measuring only unidirectional load forces.
Reach start was defined as the time when the 3-D resultant velocity of the hand sensor exceeded 5 mm/s (Fig. 1C, top panel); initial contact with the object indicated reach end, and was defined as the time when the total force on the object exceeded 5 grams (0.049 N) (Fig. 1C, bottom panel). For each trial, the variables of interest for reaching performance were reach path ratio, peak reach velocity, reach time, and contact velocity. Grasping performance was further quantified using measures of peak aperture and peak grip force. Reach path ratio was calculated as total distance traveled by the wrist sensor divided by the length of a straight-line path from the reach’s starting point to ending point. A reach path ratio equal to one represents a straight reach to the object (Hollerbach & Flash, 1982; Morasso, 1981), while a ratio greater than one represents a more curved reach path. Similar indices of curvature have been shown to effectively characterize the shape of reaching trajectories (Archambault, Pigeon, Feldman, & Levin, 1999; Bastian, Martin, Keating, & Thach, 1996; Duff & Sainburg, 2007). Peak reach velocity was calculated as the maximum 3-D resultant velocity of the hand sensor during the reach (Fig. 1C, top). Reach time was the duration from reach start to reach end. Contact velocity was calculated as the 3-D resultant velocity of the hand sensor at reach end. Peak aperture was calculated as the maximum 3-D distance between the sensors on the thumbnail and the index fingernail during the reaching phase (Jeannerod, 1984) (Fig. 1C, middle); this measure reflects how wide the thumb and fingers are opened as the hand approaches an object during reach-to-grasp, and is widely viewed as a preliminary ‘sketch’ of the upcoming grasping action in healthy adults (Castiello, 2005; Smeets & Brenner, 1999) and in those with residual grasping ability following stroke (Lang, et al., 2005; Lang, Wagner, Edwards, et al., 2006; Michaelsen, Jacobs, Roby-Brami, & Levin, 2004; Michaelsen, et al., 2009), although the magnitude of peak aperture during reaching is often altered with hemiparesis. Peak grip force was the maximum grip force recorded as the object was held in place during the hold task, or lifted from the table during the lift task (Fig. 1C, bottom).
We selected these variables because they capture important and relevant characteristics of reaching and grasping behavior, and are sensitive to subtle changes in upper extremity movement both in healthy subjects and following stroke, as depicted in Figure 1C (Schaefer, Haaland, & Sainburg, 2009; Wagner, Lang, Sahrmann, Edwards, & Dromerick, 2007). Moreover, upper extremity kinematic measures have been shown to have adequate test-retest reliability in both healthy adults (Carpinella, Mazzoleni, Rabuffetti, Thorsen, & Ferrarin, 2006) and in adults with post-stroke hemiparesis (Caimmi, et al., 2008; Patterson, Bishop, McGuirk, Sethi, & Richards, 2011; Wagner, Rhodes, & Patten, 2008). Recently, Patterson et al. (2011) reported excellent reliability for handpath curvature (reach path ratio), reach time, peak velocity, and peak aperture (all Pearson r > .79; p<.05) for reaching and grasping tasks similar to those in this study, and concluded that kinematic analysis is useful and feasible for quantifying upper extremity movement after stroke.
Each variable of interest was calculated for each trial, and averaged over the three repetitions per trial type. JMP® 8.0 was used for all statistical analyses, and our criterion for statistical significance was set at p<0.05. The Shapiro-Wilk test was used to verify normal distribution of each variable. For each variable, effects were analyzed using a 2×2×2 mixed model ANOVA with grip (3-Finger versus Palmar) and goal (Hold versus Lift) as within-subject factors, and group (Control versus Stroke) as the between-subjects factor. When warranted by significant interaction effects, post hoc analyses were performed using Tukey-Kramer Honestly Significant Different (HSD) tests, which adjusted for multiple pairwise comparisons within repeated measures ANOVA (Kramer, 1956; Stoline, 1981) and is appropriate for unequal sample sizes. This method was used to detect significant differences in reaching and grasping performance between grip and task conditions and across groups. To illustrate grip-related change (Δ) on an individual basis in the stroke group, differences between palmar and 3-finger grip types (palmar minus 3-finger) were calculated for some variables. Pearson product moment correlation coefficients across subjects were calculated to determine whether there were significant relationships between Δ values and descriptive variables.
RESULTS
Group characteristics
Table 1 summarizes the characteristics of each subject group. Control and stroke groups were comparable in terms of age (t-test p=.31), gender, and hand tested. Subjects within the stroke group had a wide range of time post-stroke (14 days to 9.6 years) and mild to moderate impairment of the contralesional arm, as evidenced by reductions in grip/pinch strength and upper extremity function (ARAT and SIS scores).
Table 1.
Subject characteristics.
| Stroke group | Control group | |
|---|---|---|
| Number of subjects (n) | 16 | 12 |
| Age ( years) | 58 ± 11 | 53 ± 16 |
| Gender | 7 Female, 9 Male | 6 Female, 6 Male |
| Affected (Tested) side | 11 Dominant, 5 Nondominant (8 Right, 8 Left) |
7 Dominant, 5 Nondominant (7 Right, 5 Left) |
| Type of stroke | 14 Ischemic 2 Hemorrhagic |
|
| Days post-stroke | 657 ± 1287 | |
| Grip strengtha (affected hand) | 70.3 ± 25.1 | |
| Pinch strengtha (affected hand) | 67.7 ± 22.0 | |
| Spasticityb (affected side) | 0: n=8 (normal) | |
| 1: n=6 | ||
| 2: n=1 | ||
| 3: n=0 | ||
| 4: n=1 (rigid) | ||
| Action Research Arm Testc (affected side) |
40.2 ± 9.3 | |
| Stroke Impact Scaled – hand function subscale |
49.7 ± 21.4 | |
Unless otherwise indicated, values are mean ± SD
% of unaffected hand
Modified Ashworth scale, elbow flexors
normal (maximum) score = 57
normal (maximum) score = 100
Effect of group
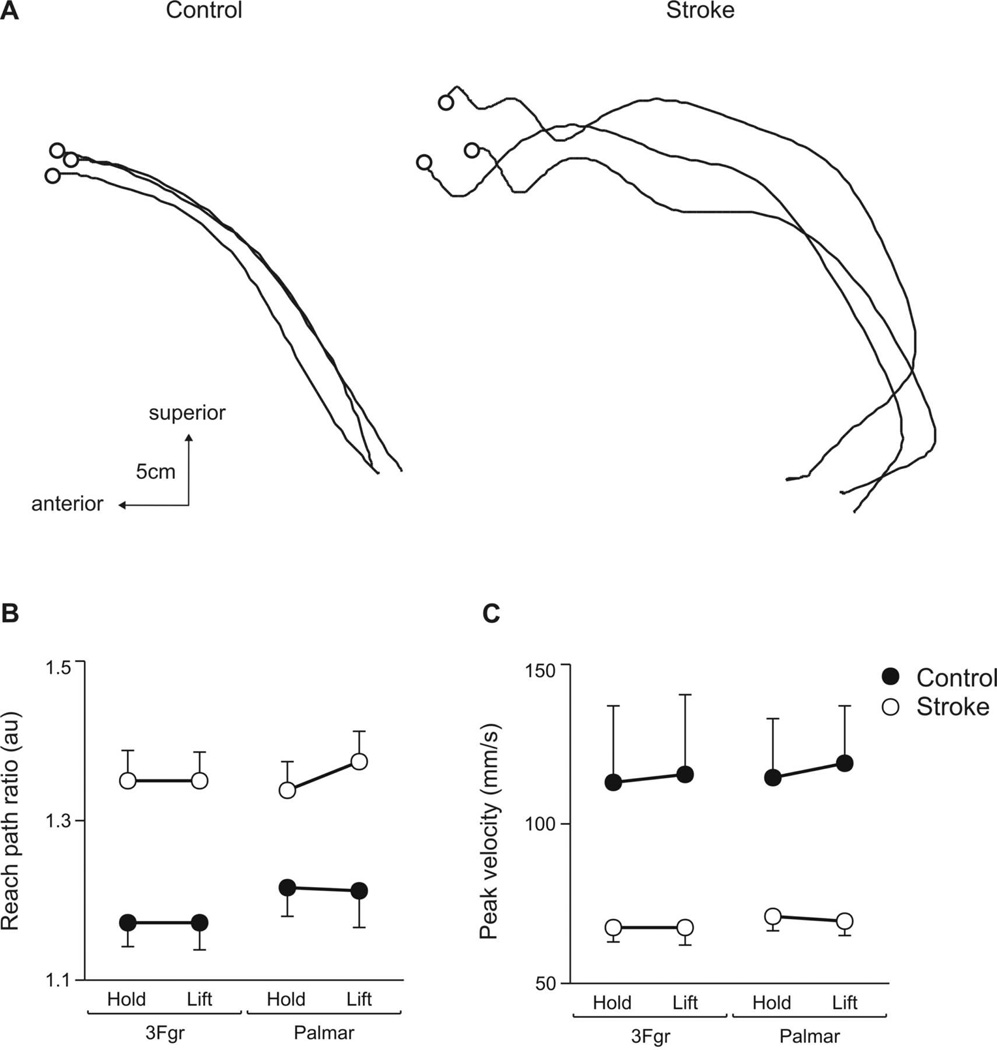
Some measures of reaching and grasping performance varied only by group (control ≠ stroke). Figure 2A shows the reach paths (medial view of wrist sensor in sagittal plane) from a control (left panel) and stroke (right panel) subject during 3 trials using a 3-finger grip in the Lift task. Reach paths are shown from movement start to object contact. Reaches by the stroke subject were more curved and had more submovements than those of the control subject. Repeated measures ANOVA revealed a main effect of group on reach path ratio (F1,26=11.1; p<.01), yet no main effect of grip (F1,26=3.06; p=.08) or goal (F1,26=0.36; p=.55). Reach path ratios were higher (i.e. reach was less straight) for the stroke group than for the control group (Fig. 2B), regardless of grip type or task goal. Likewise, peak reach velocities were lower in the stroke group (group main effect: F1,26=6.14; p<.05). As shown in Figure 2C, the magnitude of peak velocity did not vary with grip type (grip main effect: (F1,26=0.94; p=.33) or task goal (goal main effect: F1,26=0.15; p=.69).
Figure 2.
Effect of group. Right reach paths (wrist sensor, sagittal plane, medial view) from a control (left panel) and stroke (right panel) subject during 3 trials using a 3Fgr grip to lift the object. O indicates object contact. (B) Reach path ratios and (C) peak resultant hand velocities are shown for the control (filled circle) and stroke (open circle) groups when using 3Fgr and Palmar grip types during the Hold and Lift tasks. Values represent group means ± SE. Lower reach path ratios and higher peak velocities indicate more efficient, faster reach performance.
Effect of grip type
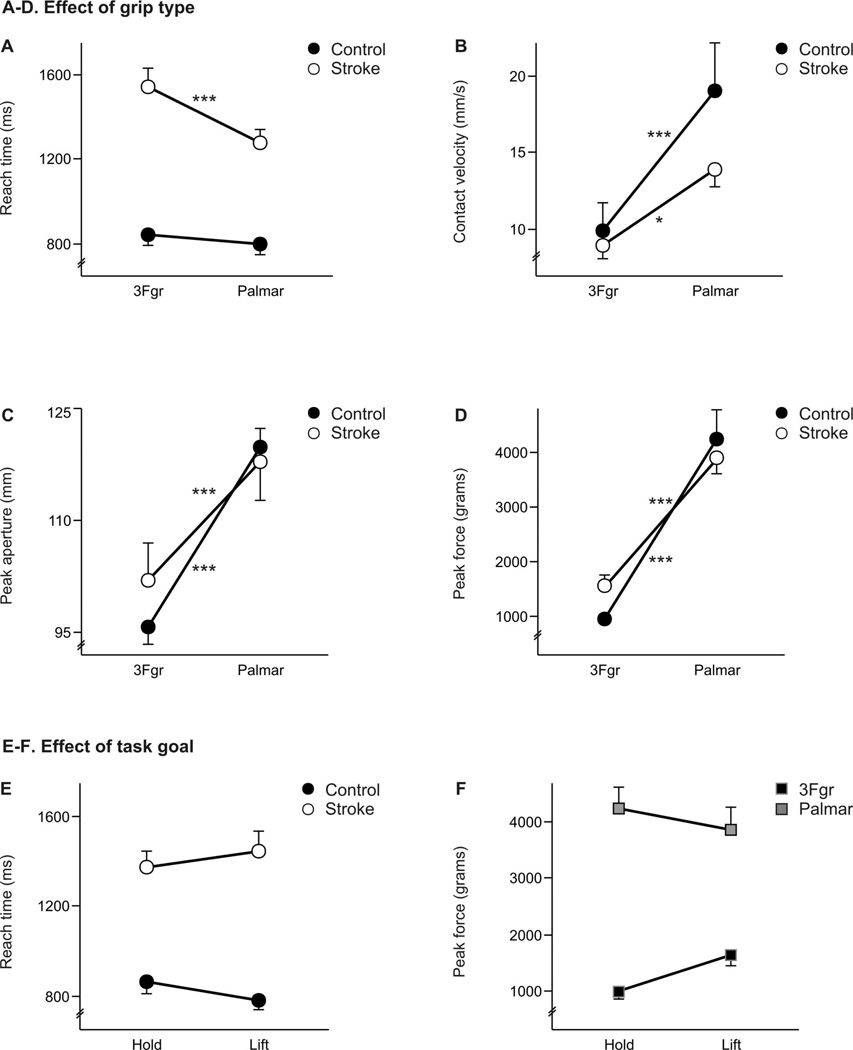
Motor performance can be significantly affected after stroke, yet an important finding of this study was that grip type also influenced some aspects of reaching and grasping performance in each group. For some variables, the effects of grip type differed between the two groups. As shown in Figure 3A, reach times were longer for the stroke group, and more so when using the 3-finger grip than the palmar grip. ANOVA results revealed a group × grip interaction effect on reach time (F1,26=8.49; p<.01). Reach time did not vary between grip types within the control group (post hoc p=.89), but were significantly longer with the 3-finger grip than with the palmar grip in the stroke group (1542 ms vs. 1280 ms, respectively) (p<.0001).
Figure 3.
A–D. Effect of grip type. (A) Reach times, (B) contact velocities, (C) peak apertures, and (D) peak forces are shown for the control (filled circle) and stroke (open circle) groups when using 3Fgr and palmar grip types. Significant post-hoc comparisons are indicated by asterisks only for differences between grip type within each group (Control and Stroke), where *p<.05 and ***p<.0001. All post-hoc results are reported in-text. E-F. Effect of task goal. (E) Reach times are shown for the control (filled circle) and stroke (open circle) groups during the Hold and Lift tasks. Within-group post-hoc: Control p=.47; Stroke p=.49. (F) Peak forces are shown for the 3Fgr (black square) and palmar (gray square) grip types during the Hold and Lift tasks. Within-grip post-hoc: 3Fgr p=.29; Palmar p=.68. Values represent group means ± SE.
Slower peak reach velocities in the stroke group during reaching for the object (see Fig. 2C) were not necessarily associated with lower velocities at object contact. Grip type also influenced the velocity at which the hand contacted the object (Fig. 3B). ANOVA results revealed a group × grip interaction effect (F1,26=3.99; p<.05), with post hoc tests indicating that contact velocities were higher for the palmar grip than for the 3-finger in both groups (control p<.0001; stroke p<.01). A group difference was evident for the palmar grip, where contact velocity was faster in the control group (p<.01), yet there was no group difference for the 3-finger grip (p=.91). This effect is illustrated in Figure 3B, such that the difference in contact velocity between the 3-finger and palmar grip types was greater in the control group than in the stroke group.
Grip type also influenced the size of peak aperture during the reach phase (Fig. 3C). Peak aperture varied by group and by grip type, indicated by a significant group × grip interaction in peak aperture (F1,26=5.57; p<.05). Peak apertures were larger for palmar grips compared to 3-finger grips in both groups (control p<.0001; stroke p<.0001). A group difference was evident for the 3-finger grip, where peak aperture was larger in the stroke group (p<.01), yet there was no group difference for the palmar grip (p=.91).
Peak grip force was higher for the palmar grip than for the 3-finger grip (Fig. 3D), with ANOVA results revealing a group × grip (F1,26=3.86; p=.05) interaction. No significant post hoc group differences in peak force were observed, however, for either the 3-finger grip (p=.29) or the palmar grip (p=.75). These results show that the stroke group scaled peak grip force to the same degree as the control group between the 3-finger and palmar grip types.
Effect of task goal
ANOVA results indicated that a few measures of performance may also vary depending on the task goal. For reach time, a significant group × goal interaction (F1,26=4.08; p<.05) showed that the control group tended to take longer in the Hold task than in the Lift task, but the stroke group tended to take longer in the Lift task as compared to the Hold task (Fig. 3E). The amount of force produced during each grip type also depended on the task goal, as indicated by a goal × grip (F1,26=4.17; p<.05) interaction in peak force. For 3-finger grips, higher peak forces were produced while lifting rather than holding the object, while for palmar grips, higher peak forces were produced while holding rather than lifting (Fig. 3F). This trend was seen in both the control and stroke groups, though it was not significant with post-hoc testing.
No relationship with time post-stroke or stroke severity
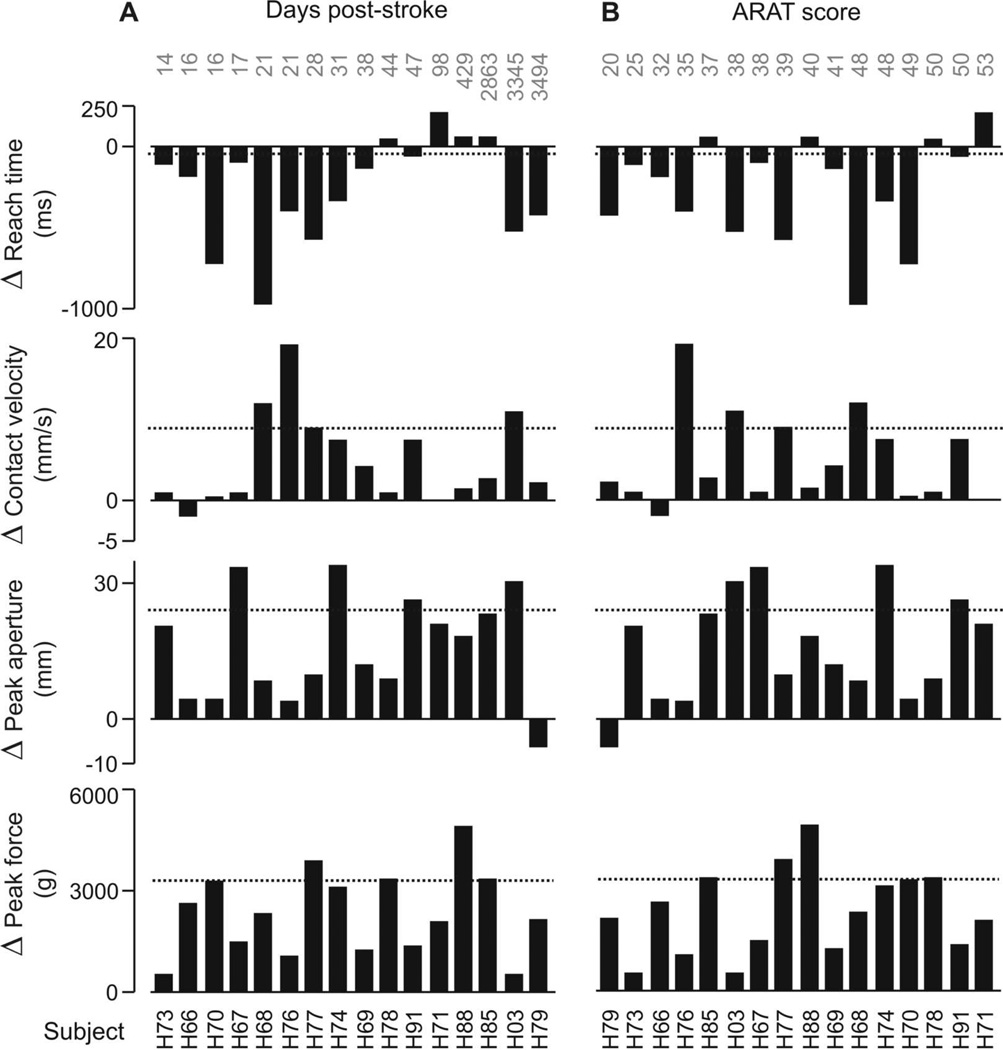
Reaching and grasping performance can vary with time post-stroke and severity of impairment; therefore, it was plausible that the group × grip interactions in reach time, contact velocity, peak aperture, and peak grip force could have depended on such factors. Figure 4 shows, however, that this was not the case within this sample of stroke subjects. Grip-related change in reach time, contact velocity, peak aperture, and peak grip force are displayed for each subject (e.g. H77, H66, etc). Change (Δ) is the difference in performance between the palmar and 3-finger grip types collapsed across task goals. Positive values indicate instances in which the values for palmar grip were greater than 3-finger; negative values indicate when palmar grip values were less than 3-finger. Subjects were sorted by the number of days post-stroke (Fig. 4A) and ARAT score (Fig. 4B), as indicated in gray text. There was no trend for the amount of change (Δ) between palmar and 3-finger grip in any given variable to increase or decrease based on an individual’s time post-stroke or stroke severity (range of Pearson r=−.026 to .31; all values p>.23). Mean change in each variable for the control group is represented by the dotted line. There was also no trend for the amount of change between task goals based on an individual’s time post-stroke or stroke severity (range of Pearson r=−.36 to .22; all values p>.18).
Figure 4.
Grip-related change in reach time, contact velocity, peak aperture, and peak force within each subject in the stroke group, collapsed across task goals. Change (Δ) is the difference in performance between the palmar and 3-finger grip types. Positive values indicate palmar > 3-finger; negative values indicate 3-finger > palmar. Subjects (e.g. H73, H66, etc.) are sorted along x-axis by increasing (A) number of days post-stroke and (B) score on ARAT (normal = 57), shown across top of graphs. Dashed line indicates mean change in the control group.
DISCUSSION
The purpose of this study was to investigate whether grip type and task goal influenced reaching and grasping performance in people with mild to moderate hemiparesis, compared to healthy controls. We found that movement of the affected limb depended on the grip and goal requirements of a reach-grasp task, supporting the hypothesis that the ability to modify a movement strategy in a task-specific way is preserved in the presence of motor impairment. While results showed that the stroke group reached for the object with more curved handpaths and slower peak velocities compared to the control group, which is consistent with previous studies characterizing the effects of post-stroke hemiparesis on motor performance (Harvey, et al., 2001; Lang, et al., 2005; Lang, Wagner, Edwards, et al., 2006; Levin, 1996; McCrea & Eng, 2005; Schaefer, et al., 2009; Senesac, Davis, & Richards, 2010; Woodbury, et al., 2009), other measures of performance varied not only between groups but also by grip type. The stroke group’s performance when using a 3-finger grip was characterized by longer reach times, slower contact velocities, narrower peak apertures, and lower peak grip forces than when using a palmar grip. Task goal appeared to influence only reach time and peak grip force. These results supported our hypothesis that the stroke group’s performance would be influenced by grip type and task goal, and suggest a preserved ability to modify motor performance in a task-specific way.
Interaction effects observed in this study show that grip type and task goal affected movement differently in people post-stroke compared to healthy controls. These findings may be interpreted as evidence of altered movement strategies that people with stroke use to compensate, ensuring completion of the instructed task despite existing impairments. This is in contrast to the notion that changes in motor performance after stroke are only deficits in execution, which one might interpret from Figure 2. The smoothness and speed of the affected limb’s reaching performance was worse than the performance of control subjects and did not vary across grip type and task goal, which could potentially reflect a reduced motor repertoire. The significant interactions, however, revealed different task-specific performance changes across the two groups, clearly supporting the idea of compensation after stroke (Shadmehr & Krakauer, 2008). At a functional level, a “compensatory strategy” can be thought of as the appearance of alternative movement patterns during the accomplishment of a task (Levin, Kleim, & Wolf, 2009). It is likely that the changes in performance across the different grip types and task goals seen in this study reflect compensatory neural control of movement after stroke. Compensatory reaching and grasping strategies have previously been documented in people with post-stroke hemiparesis using similar kinematic analysis. During reaching, these individuals can compensate for some motor impairment with more trunk flexion (Cirstea & Levin, 2000; Levin, Michaelsen, Cirstea, & Roby-Brami, 2002; Michaelsen, et al., 2004; Murphy, Willen, & Sunnerhagen, 2011), more shoulder abduction (Malcolm, Massie, & Thaut, 2009), and wider hand apertures (Nowak, et al., 2003) than healthy adults. These individuals have also been shown to use excessive grip force when using their thumb and index finger to pick up an object (McDonnell, Hillier, Ridding, & Miles, 2006; Raghavan, Krakauer, & Gordon, 2006). More recently, Raghavan et al. (2010) compared finger kinematics of stroke subjects while grasping convex- or concave-shaped objects, and found that the stroke group used a qualitatively different strategy of finger flexion, rather than using an impaired version of the “normal” strategy seen in healthy control subjects. Our data showed similar results: If the stroke group had used the same reaching strategy as the control group, or used the same strategy regardless of which grip type was used, then one would have predicted no interaction effects, and any differences in group means would be attributed to a main effect of stroke. Instead, key reach-to-grasp variables (reach time, contact velocity, peak aperture, and peak force) were dependent on hemiparesis and grip type, as evidenced by significant group-by-grip interactions, while some variables (reach time and peak force) were dependent on hemiparesis and task goal. These results collectively suggest that in the presence of motor deficits, the stroke subjects were able to flexibly alter movement control in response to different task requirements, and that their strategies differed from those of healthy controls (Michaelsen, et al., 2004).
The contribution of this study to the understanding of compensatory motor control after stroke is its investigation of how different conditions of a functional task (grip types and goals) affect reaching and grasping variables in people with stroke compared to healthy controls. All other factors were held constant in order to effectively quantify the effects of grip type and task goal. In healthy adults, the intent to use different grip types has been shown to 1) activate different brain regions (Ehrsson, et al., 2000; Frey, Vinton, Norlund, & Grafton, 2005); 2) bias different movement strategies (Castiello, 1996; Greenwald & Knill, 2009; Ingram, Howard, Flanagan, & Wolpert, 2010; Vainio, Tucker, & Ellis, 2007); and 3) result in significantly different reaching performance (Castiello, Bennett, & Paulignan, 1992; Gentilucci, et al., 1991; Marteniuk, et al., 1987). Why, though, might some aspects of reaching and grasping performance have varied by grip or goal differently in the stroke group? The performance differences may reflect varying degrees of caution, or safety margin, encoded within the movement. For example, prolonged movement times seen in clinical populations have been interpreted as a more conservative movement strategy to compensate for underlying motor impairment (Cirstea & Levin, 2000; de los Reyes-Guzman, et al., 2010; McCrea & Eng, 2005; Smits-Engelsman, Rameckers, & Duysens, 2007; C. Y. Wu, et al., 2008). A more conservative strategy may be preferred when using a 3-finger grip than a palmar grip. Using the whole hand to grasp an object is often referred to as a ‘power’ grip, while using the tips of individual fingers is considered a more ‘precision’ grip (Jungling, Bock, & Girgenrath, 2002; Napier, 1956; Pouydebat, et al., 2011). It is plausible, therefore, that in order for the stroke group to be precise with the 3-finger grip, they used a more conservative, “safer” strategy than with the palmar grip. When using the affected limb in daily life, longer reach times, slower contact velocities, and narrower hand apertures might minimize the likelihood of knocking objects over (Rosenbaum, Meulenbroek, Vaughan, & Jansen, 1999; Saling, Alberts, Stelmach, & Bloedel, 1998; te Velde, van der Kamp, Becher, van Bennekom, & Savelsbergh, 2005; Tresilian, 1998). Higher grip force when lifting an object against gravity, especially when using a precision grip, can minimize the likelihood of dropping it (McDonnell, et al., 2006; Raghavan, et al., 2006; Visser, et al., 2003; Wenzelburger, et al., 2005; Westling & Johansson, 1984). Thus, behavioral data from this study suggest that individuals with post stroke hemiparesis may be able to select movement strategies that will optimize task completion. Future studies are needed, however, to further understand how and why specific compensatory strategies emerge, as well as their functional implications.
It is well established that reaching and grasping performance is closely related to the severity of motor impairment and dysfunction (activity limitation) after stroke (Beebe & Lang, 2008; Celik, et al., 2010; Cirstea, et al., 2003; Glymour, et al., 2007; Krebs, et al., 2008; Kwakkel, et al., 2003; Lang, et al., 2009; Leonard, Gardipee, Koontz, Anderson, & Wilkins, 2006; Levin, 1996; McDonnell, et al., 2006; Subramanian, Yamanaka, Chilingaryan, & Levin, 2010). Interestingly, modulation of task performance in this study, as indexed by changes in reach time, contact velocity, peak aperture, and peak force between grip types, did not depend on the degree of motor dysfunction nor the time post-stroke. The amount of grip-related change seen in our stroke subjects was unrelated to their scores on the Action Research Arm Test (ARAT), which has been shown to be a reliable, valid measure of upper extremity functional loss after stroke (Hsieh, et al., 1998; Lin, et al., 2009; Yozbatiran, et al., 2008). The lack of association between grip-related change (reported as Δ in Fig. 4) and dysfunction (activity limitation) is not a product of which clinical test we used to evaluate this relationship, since clinical tests of upper extremity function are known to be highly correlated (Beebe & Lang, 2009; Hsueh & Hsieh, 2002; Lin, et al., 2009; Rabadi & Rabadi, 2006). Our data instead suggest that the grip- and goal-related changes in reach time, contact velocity, peak aperture and/or peak force may be fairly constant after the first few weeks post-stroke and may be similar across the range of mild to moderate hemiparesis.
Because the task required the ability to reach and grasp an object, patients with severe hemiparesis could not participate in this study. Thus, the degree to which upper extremity motor performance varies with movement context in more severely affected individuals remains unclear. Grip type and task goal did, however, significantly affect reaching and grasping performance in those with mild-to-moderate hemiparesis, despite their varying degrees of motor capacity. Although our sample size was small, the subjects’ mild-moderate movement impairments were characteristic of those persons with stroke who have potential to recover motor function through rehabilitation (Hendricks, van Limbeek, Geurts, & Zwarts, 2002; Kwakkel, et al., 2003).
In sum, results from this study suggest that even though the ability to move one’s arm and hand is often impaired after stroke, reaching and grasping performance can still be modified based on how and why an object will be grasped. Different movement patterns may be elicited through different grip types and task goals. Information about how different movement contexts influence performance post-stroke may assist therapists in planning how and what to practice during task-specific upper extremity training. Future training studies are needed to examine how manipulating movement contexts during therapy might impact functional recovery after stroke.
ACKNOWLEDGEMENTS
This work was supported in part by NIH R01HD055964, NIH T32HD007434 to the Program in Physical Therapy at Washington University School of Medicine, AHA 10POST4140091, and the Foundation for Physical Therapy.
REFERENCES
- Andrews AW, Thomas MW, Bohannon RW. Normative values for isometric muscle force measurements obtained with hand-held dynamometers. Phys Ther. 1996;76(3):248–259. doi: 10.1093/ptj/76.3.248. [DOI] [PubMed] [Google Scholar]
- Ansuini C, Giosa L, Turella L, Altoe G, Castiello U. An object for an action, the same object for other actions: effects on hand shaping. Exp Brain Res. 2008;185(1):111–119. doi: 10.1007/s00221-007-1136-4. [DOI] [PubMed] [Google Scholar]
- Archambault P, Pigeon P, Feldman AG, Levin MF. Recruitment and sequencing of different degrees of freedom during pointing movements involving the trunk in healthy and hemiparetic subjects. Exp Brain Res. 1999;126(1):55–67. doi: 10.1007/s002210050716. [DOI] [PubMed] [Google Scholar]
- Bastian AJ, Martin TA, Keating JG, Thach WT. Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J Neurophysiol. 1996;76(1):492–509. doi: 10.1152/jn.1996.76.1.492. [DOI] [PubMed] [Google Scholar]
- Beebe JA, Lang CE. Absence of a proximal to distal gradient of motor deficits in the upper extremity early after stroke. Clin Neurophysiol. 2008;119(9):2074–2085. doi: 10.1016/j.clinph.2008.04.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe JA, Lang CE. Relationships and responsiveness of six upper extremity function tests during the first six months of recovery after stroke. J Neurol Phys Ther. 2009;33(2):96–103. doi: 10.1097/NPT.0b013e3181a33638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer RF, Dewald JP, Dawson ML, Rymer WZ. Target-dependent differences between free and constrained arm movements in chronic hemiparesis. Exp Brain Res. 2004;156(4):458–470. doi: 10.1007/s00221-003-1807-8. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67(2):206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- Caimmi M, Carda S, Giovanzana C, Maini ES, Sabatini AM, Smania N, et al. Using kinematic analysis to evaluate constraint-induced movement therapy in chronic stroke patients. Neurorehabil Neural Repair. 2008;22(1):31–39. doi: 10.1177/1545968307302923. [DOI] [PubMed] [Google Scholar]
- Carpinella I, Mazzoleni P, Rabuffetti M, Thorsen R, Ferrarin M. Experimental protocol for the kinematic analysis of the hand: definition and repeatability. Gait Posture. 2006;23(4):445–454. doi: 10.1016/j.gaitpost.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Castiello U. Grasping a fruit: selection for action. J Exp Psychol Hum Percept Perform. 1996;22(3):582–603. doi: 10.1037//0096-1523.22.3.582. [DOI] [PubMed] [Google Scholar]
- Castiello U. The neuroscience of grasping. Nat Rev Neurosci. 2005;6(9):726–736. doi: 10.1038/nrn1744. [DOI] [PubMed] [Google Scholar]
- Castiello U, Bennett KM, Paulignan Y. Does the type of prehension influence the kinematics of reaching? Behav Brain Res. 1992;50(1–2):7–15. doi: 10.1016/s0166-4328(05)80283-9. [DOI] [PubMed] [Google Scholar]
- Celik O, O'Malley MK, Boake C, Levin HS, Yozbatiran N, Reistetter TA. Normalized movement quality measures for therapeutic robots strongly correlate with clinical motor impairment measures. IEEE Trans Neural Syst Rehabil Eng. 2010;18(4):433–444. doi: 10.1109/TNSRE.2010.2047600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000;123(Pt 5):940–953. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- Cirstea MC, Mitnitski AB, Feldman AG, Levin MF. Interjoint coordination dynamics during reaching in stroke. Exp Brain Res. 2003;151(3):289–300. doi: 10.1007/s00221-003-1438-0. [DOI] [PubMed] [Google Scholar]
- Cruz EG, Waldinger HC, Kamper DG. Kinetic and kinematic workspaces of the index finger following stroke. Brain. 2005;128(Pt 5):1112–1121. doi: 10.1093/brain/awh432. [DOI] [PubMed] [Google Scholar]
- de los Reyes-Guzman A, Gil-Agudo A, Penasco-Martin B, Solis-Mozos M, del Ama-Espinosa A, Perez-Rizo E. Kinematic analysis of the daily activity of drinking from a glass in a population with cervical spinal cord injury. J Neuroeng Rehabil. 2010;7:41. doi: 10.1186/1743-0003-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJong SL, Birkenmeier R, Lang CE. Person-specific changes in motor performance accompany upper extremity functional gains after stroke. J Appl Biomech. doi: 10.1123/jab.28.3.304. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromerick AW, Lang CE, Birkenmeier R, Hahn MG, Sahrmann SA, Edwards DF. Relationships between upper-limb functional limitation and self-reported disability 3 months after stroke. J Rehabil Res Dev. 2006;43(3):401–408. doi: 10.1682/jrrd.2005.04.0075. [DOI] [PubMed] [Google Scholar]
- Duff SV, Sainburg RL. Lateralization of motor adaptation reveals independence in control of trajectory and steady-state position. Exp Brain Res. 2007;179(4):551–561. doi: 10.1007/s00221-006-0811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30(10):2131–2140. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]
- Duncan PW, Wallace D, Studenski S, Lai SM, Johnson D. Conceptualization of a new stroke-specific outcome measure: the stroke impact scale. Top Stroke Rehabil. 2001;8(2):19–33. doi: 10.1310/BRHX-PKTA-0TUJ-UYWT. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H. Cortical activity in precision- versus power-grip tasks: an fMRI study. J Neurophysiol. 2000;83(1):528–536. doi: 10.1152/jn.2000.83.1.528. [DOI] [PubMed] [Google Scholar]
- Frey SH, Vinton D, Norlund R, Grafton ST. Cortical topography of human anterior intraparietal cortex active during visually guided grasping. Brain Res Cogn Brain Res. 2005;23(2–3):397–405. doi: 10.1016/j.cogbrainres.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Gentilucci M, Castiello U, Corradini ML, Scarpa M, Umilta C, Rizzolatti G. Influence of different types of grasping on the transport component of prehension movements. Neuropsychologia. 1991;29(5):361–378. doi: 10.1016/0028-3932(91)90025-4. [DOI] [PubMed] [Google Scholar]
- Glymour MM, Berkman LF, Ertel KA, Fay ME, Glass TA, Furie KL. Lesion characteristics, NIH stroke scale, and functional recovery after stroke. Am J Phys Med Rehabil. 2007;86(9):725–733. doi: 10.1097/PHM.0b013e31813e0a32. [DOI] [PubMed] [Google Scholar]
- Graziano M. The organization of behavioral repertoire in motor cortex. Annu Rev Neurosci. 2006;29:105–134. doi: 10.1146/annurev.neuro.29.051605.112924. [DOI] [PubMed] [Google Scholar]
- Greenwald HS, Knill DC. A comparison of visuomotor cue integration strategies for object placement and prehension. Vis Neurosci. 2009;26(1):63–72. doi: 10.1017/S0952523808080668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grichting B, Hediger V, Kaluzny P, Wiesendanger M. Impaired proactive and reactive grip force control in chronic hemiparetic patients. Clin Neurophysiol. 2000;111(9):1661–1671. doi: 10.1016/s1388-2457(00)00355-2. [DOI] [PubMed] [Google Scholar]
- Harvey M, Jackson SR, Newport R, Kramer T, Morris DL, Dow L. Is grasping impaired in hemispatial neglect? Behav Neurol. 2001;13(1–2):17–28. doi: 10.1155/2002/495854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks HT, van Limbeek J, Geurts AC, Zwarts MJ. Motor recovery after stroke: a systematic review of the literature. Arch Phys Med Rehabil. 2002;83(11):1629–1637. doi: 10.1053/apmr.2002.35473. [DOI] [PubMed] [Google Scholar]
- Hermsdorfer J, Hagl E, Nowak DA, Marquardt C. Grip force control during object manipulation in cerebral stroke. Clin Neurophysiol. 2003;114(5):915–929. doi: 10.1016/s1388-2457(03)00042-7. [DOI] [PubMed] [Google Scholar]
- Hollerbach MJ, Flash T. Dynamic interactions between limb segments during planar arm movement. Biol Cybern. 1982;44(1):67–77. doi: 10.1007/BF00353957. [DOI] [PubMed] [Google Scholar]
- Hsieh CL, Hsueh IP, Chiang FM, Lin PH. Inter-rater reliability and validity of the action research arm test in stroke patients. Age Ageing. 1998;27(2):107–113. doi: 10.1093/ageing/27.2.107. [DOI] [PubMed] [Google Scholar]
- Hsueh IP, Hsieh CL. Responsiveness of two upper extremity function instruments for stroke inpatients receiving rehabilitation. Clin Rehabil. 2002;16(6):617–624. doi: 10.1191/0269215502cr530oa. [DOI] [PubMed] [Google Scholar]
- Ingram JN, Howard IS, Flanagan JR, Wolpert DM. Multiple grasp-specific representations of tool dynamics mediate skillful manipulation. Curr Biol. 2010;20(7):618–623. doi: 10.1016/j.cub.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannerod M. The timing of natural prehension movements. J Mot Behav. 1984;16(3):235–254. doi: 10.1080/00222895.1984.10735319. [DOI] [PubMed] [Google Scholar]
- Jungling S, Bock O, Girgenrath M. Speed-accuracy trade-off of grasping movements during microgravity. Aviat Space Environ Med. 2002;73(5):430–435. [PubMed] [Google Scholar]
- Kamper DG, McKenna-Cole AN, Kahn LE, Reinkensmeyer DJ. Alterations in reaching after stroke and their relation to movement direction and impairment severity. Arch Phys Med Rehabil. 2002;83(5):702–707. doi: 10.1053/apmr.2002.32446. [DOI] [PubMed] [Google Scholar]
- Klatzky RL, Fikes TG, Pellegrino JW. Planning for hand shape and arm transport when reaching for objects. Acta Psychol (Amst) 1995;88(3):209–232. doi: 10.1016/0001-6918(93)e0068-d. [DOI] [PubMed] [Google Scholar]
- Kramer CY. Extension of Multiple Range Tests to Group Means with Unequal Numbers of Replications. Biometrics. 1956;12(3):307–310. [Google Scholar]
- Krebs HI, Mernoff S, Fasoli SE, Hughes R, Stein J, Hogan N. A comparison of functional and impairment-based robotic training in severe to moderate chronic stroke: a pilot study. NeuroRehabilitation. 2008;23(1):81–87. [PMC free article] [PubMed] [Google Scholar]
- Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34(9):2181–2186. doi: 10.1161/01.STR.0000087172.16305.CD. [DOI] [PubMed] [Google Scholar]
- Lang CE. Impaired motor control. In: Guccione AA, Wong RA, Avers D, editors. Geriatric Physical Therapy. 3rd ed. St. Louis, MO: Elsevier; 2011. [Google Scholar]
- Lang CE, DeJong SL, Beebe JA. Recovery of thumb and finger extension and its relation to grasp performance after stroke. J Neurophysiol. 2009;102(1):451–459. doi: 10.1152/jn.91310.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CE, Schieber MH. Reduced muscle selectivity during individuated finger movements in humans after damage to the motor cortex or corticospinal tract. J Neurophysiol. 2004;91(4):1722–1733. doi: 10.1152/jn.00805.2003. [DOI] [PubMed] [Google Scholar]
- Lang CE, Wagner JM, Bastian AJ, Hu Q, Edwards DF, Sahrmann SA, et al. Deficits in grasp versus reach during acute hemiparesis. Exp Brain Res. 2005;166(1):126–136. doi: 10.1007/s00221-005-2350-6. [DOI] [PubMed] [Google Scholar]
- Lang CE, Wagner JM, Dromerick AW, Edwards DF. Measurement of upper-extremity function early after stroke: properties of the action research arm test. Arch Phys Med Rehabil. 2006;87(12):1605–1610. doi: 10.1016/j.apmr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Lang CE, Wagner JM, Edwards DF, Sahrmann SA, Dromerick AW. Recovery of grasp versus reach in people with hemiparesis poststroke. Neurorehabil Neural Repair. 2006;20(4):444–454. doi: 10.1177/1545968306289299. [DOI] [PubMed] [Google Scholar]
- Leonard CT, Gardipee KA, Koontz JR, Anderson JH, Wilkins SA. Correlation between impairment and motor performance during reaching tasks in subjects with spastic hemiparesis. J Rehabil Med. 2006;38(4):243–249. doi: 10.1080/16501970600609808. [DOI] [PubMed] [Google Scholar]
- Levin MF. Interjoint coordination during pointing movements is disrupted in spastic hemiparesis. Brain. 1996;119(Pt 1):281–293. doi: 10.1093/brain/119.1.281. [DOI] [PubMed] [Google Scholar]
- Levin MF, Kleim JA, Wolf SL. What do motor "recovery" and "compensation" mean in patients following stroke? Neurorehabil Neural Repair. 2009;23(4):313–319. doi: 10.1177/1545968308328727. [DOI] [PubMed] [Google Scholar]
- Levin MF, Michaelsen SM, Cirstea CM, Roby-Brami A. Use of the trunk for reaching targets placed within and beyond the reach in adult hemiparesis. Exp Brain Res. 2002;143(2):171–180. doi: 10.1007/s00221-001-0976-6. [DOI] [PubMed] [Google Scholar]
- Lin JH, Hsu MJ, Sheu CF, Wu TS, Lin RT, Chen CH, et al. Psychometric comparisons of 4 measures for assessing upper-extremity function in people with stroke. Phys Ther. 2009;89(8):840–850. doi: 10.2522/ptj.20080285. [DOI] [PubMed] [Google Scholar]
- Lum PS, Mulroy S, Amdur RL, Requejo P, Prilutsky BI, Dromerick AW. Gains in upper extremity function after stroke via recovery or compensation: Potential differential effects on amount of real-world limb use. Top Stroke Rehabil. 2009;16(4):237–253. doi: 10.1310/tsr1604-237. [DOI] [PubMed] [Google Scholar]
- Lum PS, Patten C, Kothari D, Yap R. Effects of velocity on maximal torque production in poststroke hemiparesis. Muscle Nerve. 2004;30(6):732–742. doi: 10.1002/mus.20157. [DOI] [PubMed] [Google Scholar]
- Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. 1981;4(4):483–492. doi: 10.1097/00004356-198112000-00001. [DOI] [PubMed] [Google Scholar]
- Malcolm MP, Massie C, Thaut M. Rhythmic auditory-motor entrainment improves hemiparetic arm kinematics during reaching movements: a pilot study. Top Stroke Rehabil. 2009;16(1):69–79. doi: 10.1310/tsr1601-69. [DOI] [PubMed] [Google Scholar]
- Marteniuk RG, MacKenzie CL, Jeannerod M, Athenes S, Dugas C. Constraints on human arm movement trajectories. Can J Psychol. 1987;41(3):365–378. doi: 10.1037/h0084157. [DOI] [PubMed] [Google Scholar]
- McCrea PH, Eng JJ. Consequences of increased neuromotor noise for reaching movements in persons with stroke. Exp Brain Res. 2005;162(1):70–77. doi: 10.1007/s00221-004-2106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell MN, Hillier SL, Ridding MC, Miles TS. Impairments in precision grip correlate with functional measures in adult hemiplegia. Clin Neurophysiol. 2006;117(7):1474–1480. doi: 10.1016/j.clinph.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Michaelsen SM, Jacobs S, Roby-Brami A, Levin MF. Compensation for distal impairments of grasping in adults with hemiparesis. Exp Brain Res. 2004;157(2):162–173. doi: 10.1007/s00221-004-1829-x. [DOI] [PubMed] [Google Scholar]
- Michaelsen SM, Magdalon EC, Levin MF. Grip aperture scaling to object size in chronic stroke. Motor Control. 2009;13(2):197–217. doi: 10.1123/mcj.13.2.197. [DOI] [PubMed] [Google Scholar]
- Morasso P. Spatial control of arm movements. Exp Brain Res. 1981;42(2):223–227. doi: 10.1007/BF00236911. [DOI] [PubMed] [Google Scholar]
- Murphy MA, Willen C, Sunnerhagen KS. Kinematic variables quantifying upper-extremity performance after stroke during reaching and drinking from a glass. Neurorehabil Neural Repair. 2011;25(1):71–80. doi: 10.1177/1545968310370748. [DOI] [PubMed] [Google Scholar]
- Napier JR. The prehensile movements of the human hand. J Bone Joint Surg Br. 1956;38-B(4):902–913. doi: 10.1302/0301-620X.38B4.902. [DOI] [PubMed] [Google Scholar]
- Nowak DA. The impact of stroke on the performance of grasping: usefulness of kinetic and kinematic motion analysis. Neurosci Biobehav Rev. 2008;32(8):1439–1450. doi: 10.1016/j.neubiorev.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Hermsdorfer J, Topka H. Deficits of predictive grip force control during object manipulation in acute stroke. J Neurol. 2003;250(7):850–860. doi: 10.1007/s00415-003-1095-z. [DOI] [PubMed] [Google Scholar]
- Patterson TS, Bishop MD, McGuirk TE, Sethi A, Richards LG. Reliability of upper extremity kinematics while performing different tasks in individuals post stroke. J Mot Behav. 2011;43:121–130. doi: 10.1080/00222895.2010.548422. [DOI] [PubMed] [Google Scholar]
- Pouydebat E, Reghem E, Borel A, Gorce P. Diversity of grip in adults and young humans and chimpanzees (Pan troglodytes) Behav Brain Res. 2011;218(1):21–28. doi: 10.1016/j.bbr.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Rabadi MH, Rabadi FM. Comparison of the action research arm test and the Fugl-Meyer assessment as measures of upper-extremity motor weakness after stroke. Arch Phys Med Rehabil. 2006;87(7):962–966. doi: 10.1016/j.apmr.2006.02.036. [DOI] [PubMed] [Google Scholar]
- Raghavan P, Krakauer JW, Gordon AM. Impaired anticipatory control of fingertip forces in patients with a pure motor or sensorimotor lacunar syndrome. Brain. 2006;129(Pt 6):1415–1425. doi: 10.1093/brain/awl070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan P, Petra E, Krakauer JW, Gordon AM. Patterns of impairment in digit independence after subcortical stroke. J Neurophysiol. 2006;95(1):369–378. doi: 10.1152/jn.00873.2005. [DOI] [PubMed] [Google Scholar]
- Raghavan P, Santello M, Gordon AM, Krakauer JW. Compensatory motor control after stroke: an alternative joint strategy for object-dependent shaping of hand posture. J Neurophysiol. 2010;103(6):3034–3043. doi: 10.1152/jn.00936.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinkensmeyer DJ, McKenna Cole A, Kahn LE, Kamper DG. Directional control of reaching is preserved following mild/moderate stroke and stochastically constrained following severe stroke. Exp Brain Res. 2002;143(4):525–530. doi: 10.1007/s00221-002-1055-3. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DA, Meulenbroek RG, Vaughan J, Jansen C. Coordination of reaching and grasping by capitalizing on obstacle avoidance and other constraints. Exp Brain Res. 1999;128(1–2):92–100. doi: 10.1007/s002210050823. [DOI] [PubMed] [Google Scholar]
- Saling M, Alberts J, Stelmach GE, Bloedel JR. Reach-to-grasp movements during obstacle avoidance. Exp Brain Res. 1998;118(2):251–258. doi: 10.1007/s002210050279. [DOI] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy. Neuropsychologia. 2009;47(13):2953–2966. doi: 10.1016/j.neuropsychologia.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH. How might the motor cortex individuate movements? Trends Neurosci. 1990;13(11):440–445. doi: 10.1016/0166-2236(90)90093-p. [DOI] [PubMed] [Google Scholar]
- Schieber MH, Santello M. Hand function: peripheral and central constraints on performance. J Appl Physiol. 2004;96(6):2293–2300. doi: 10.1152/japplphysiol.01063.2003. [DOI] [PubMed] [Google Scholar]
- Schmidt RT, Toews JV. Grip strength as measured by the Jamar dynamometer. Arch Phys Med Rehabil. 1970;51(6):321–327. [PubMed] [Google Scholar]
- Senesac CR, Davis S, Richards L. Generalization of a modified form of repetitive rhythmic bilateral training in stroke. Hum Mov Sci. 2010;29(1):137–148. doi: 10.1016/j.humov.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185(3):359–381. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets JB, Brenner E. A new view on grasping. Motor Control. 1999;3(3):237–271. doi: 10.1123/mcj.3.3.237. [DOI] [PubMed] [Google Scholar]
- Smits-Engelsman BC, Rameckers EA, Duysens J. Children with congenital spastic hemiplegia obey Fitts' Law in a visually guided tapping task. Exp Brain Res. 2007;177(4):431–439. doi: 10.1007/s00221-006-0698-x. [DOI] [PubMed] [Google Scholar]
- Stoline MR. The Status of Multiple Comparisons: Simultaneous Estimation of All Pairwise Comparisons in One-Way ANOVA Designs. Am Stat. 1981;35(3):134–141. [Google Scholar]
- Subramanian SK, Yamanaka J, Chilingaryan G, Levin MF. Validity of movement pattern kinematics as measures of arm motor impairment poststroke. Stroke. 2010;41(10):2303–2308. doi: 10.1161/STROKEAHA.110.593368. [DOI] [PubMed] [Google Scholar]
- te Velde AF, van der Kamp J, Becher JG, van Bennekom C, Savelsbergh GJ. Planning and control in a manual collision avoidance task by children with hemiparesis. Motor Control. 2005;9(4):417–438. doi: 10.1123/mcj.9.4.417. [DOI] [PubMed] [Google Scholar]
- Tresilian JR. Attention in action or obstruction of movement? A kinematic analysis of avoidance behavior in prehension. Exp Brain Res. 1998;120(3):352–368. doi: 10.1007/s002210050409. [DOI] [PubMed] [Google Scholar]
- Tucker M, Ellis R. On the relations between seen objects and components of potential actions. J Exp Psychol Hum Percept Perform. 1998;24(3):830–846. doi: 10.1037//0096-1523.24.3.830. [DOI] [PubMed] [Google Scholar]
- Tucker M, Ellis R. Action priming by briefly presented objects. Acta Psychol (Amst) 2004;116(2):185–203. doi: 10.1016/j.actpsy.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Vainio L, Tucker M, Ellis R. Precision and power grip priming by observed grasping. Brain Cogn. 2007;65(2):195–207. doi: 10.1016/j.bandc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Van der Lee JH, Beckerman H, Lankhorst GJ, Bouter LM. The responsiveness of the Action Research Arm test and the Fugl-Meyer Assessment scale in chronic stroke patients. J Rehabil Med. 2001;33(3):110–113. doi: 10.1080/165019701750165916. [DOI] [PubMed] [Google Scholar]
- Van der Lee JH, De Groot V, Beckerman H, Wagenaar RC, Lankhorst GJ, Bouter LM. The intra- and interrater reliability of the action research arm test: a practical test of upper extremity function in patients with stroke. Arch Phys Med Rehabil. 2001;82(1):14–19. doi: 10.1053/apmr.2001.18668. [DOI] [PubMed] [Google Scholar]
- Visser B, de Looze MP, Veeger DH, Douwes M, Groenesteijn L, de Korte E, et al. The effects of precision demands during a low intensity pinching task on muscle activation and load sharing of the fingers. J Electromyogr Kinesiol. 2003;13(2):149–157. doi: 10.1016/s1050-6411(02)00108-6. [DOI] [PubMed] [Google Scholar]
- Wagner JM, Lang CE, Sahrmann SA, Edwards DF, Dromerick AW. Sensorimotor impairments and reaching performance in subjects with poststroke hemiparesis during the first few months of recovery. Phys Ther. 2007;87(6):751–765. doi: 10.2522/ptj.20060135. [DOI] [PubMed] [Google Scholar]
- Wagner JM, Rhodes JA, Patten C. Reproducibility and minimal detectable change of three-dimensional kinematic analysis of reaching tasks in people with hemiparesis after stroke. Phys Ther. 2008;88(5):652–663. doi: 10.2522/ptj.20070255. [DOI] [PubMed] [Google Scholar]
- Wenzelburger R, Kopper F, Frenzel A, Stolze H, Klebe S, Brossmann A, et al. Hand coordination following capsular stroke. Brain. 2005;128(Pt 1):64–74. doi: 10.1093/brain/awh317. [DOI] [PubMed] [Google Scholar]
- Westling G, Johansson RS. Factors influencing the force control during precision grip. Exp Brain Res. 1984;53(2):277–284. doi: 10.1007/BF00238156. [DOI] [PubMed] [Google Scholar]
- Wisneski KJ, Johnson MJ. Quantifying kinematics of purposeful movements to real, imagined, or absent functional objects: implications for modelling trajectories for robot-assisted ADL tasks. J Neuroeng Rehabil. 2007;4:7. doi: 10.1186/1743-0003-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury ML, Howland DR, McGuirk TE, Davis SB, Senesac CR, Kautz S, et al. Effects of trunk restraint combined with intensive task practice on poststroke upper extremity reach and function: a pilot study. Neurorehabil Neural Repair. 2009;23(1):78–91. doi: 10.1177/1545968308318836. [DOI] [PubMed] [Google Scholar]
- Wong YJ, Whishaw IQ. Precision grasps of children and young and old adults: individual differences in digit contact strategy, purchase pattern, and digit posture. Behav Brain Res. 2004;154:113–123. doi: 10.1016/j.bbr.2004.01.028. [DOI] [PubMed] [Google Scholar]
- Wu CY, Chou SH, Kuo MY, Chen CL, Lu TW, Fu YC. Effects of object size on intralimb and interlimb coordination during a bimanual prehension task in patients with left cerebral vascular accidents. Motor Control. 2008;12(4):296–310. doi: 10.1123/mcj.12.4.296. [DOI] [PubMed] [Google Scholar]
- Wu G, van der Helm FC, Veeger HE, Makhsous M, Van Roy P, Anglin C, et al. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion--Part II: shoulder, elbow, wrist and hand. J Biomech. 2005;38(5):981–992. doi: 10.1016/j.jbiomech.2004.05.042. [DOI] [PubMed] [Google Scholar]
- Yozbatiran N, Der-Yeghiaian L, Cramer SC. A standardized approach to performing the action research arm test. Neurorehabil Neural Repair. 2008;22(1):78–90. doi: 10.1177/1545968307305353. [DOI] [PubMed] [Google Scholar]