Abstract
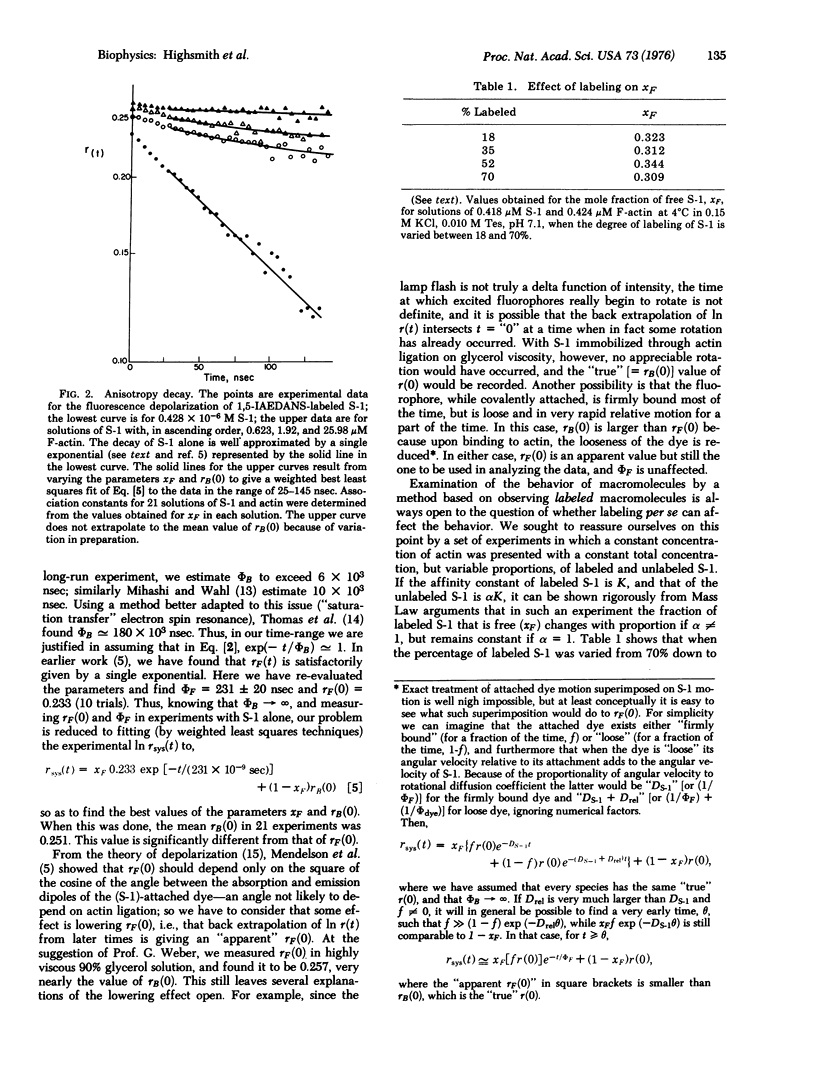
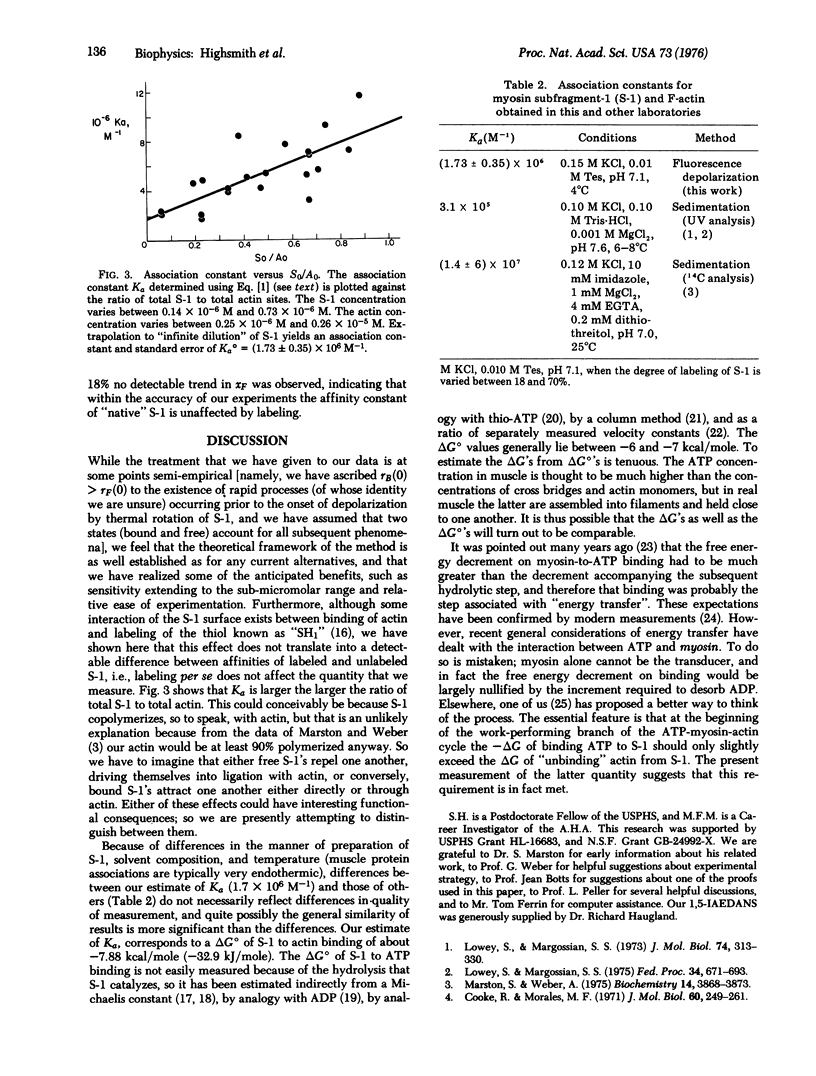
The association constant for myosin subfragment-1 (S-1) and actin was measured, using a new application of fluorescence depolarization which capitalizes on the fact that S-1 has high rotational mobility while F-actin does not. Uncoupling of the time dependences of the anisotropy decay and the association/dissociation phenomena allowed the experimentally determined anisotropy decay curve to be fitted by a sum of two terms weighted by the mole fractions of the free and bound S-1. At 4 degrees C, ionic strength 0.16 M, and pH 7.0, the association constant Ka is (1.73 +/- 0.35) X 10(6) M-1 at infinite dilution. This makes the -deltaG degrees of binding of F-actin to S-1 similar to the -deltaG degrees of binding of ATP to S-1, and the possible physiological relevance of the similarity to muscle contraction is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagshaw C. R., Trentham D. R. The characterization of myosin-product complexes and of product-release steps during the magnesium ion-dependent adenosine triphosphatase reaction. Biochem J. 1974 Aug;141(2):331–349. doi: 10.1042/bj1410331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belford G. G., Belford R. L., Weber G. Dynamics of fluorescence polarization in macromolecules. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1392–1393. doi: 10.1073/pnas.69.6.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. A new method for producing myosin subfragment-1. Biochem Biophys Res Commun. 1972 Nov 15;49(4):1021–1028. doi: 10.1016/0006-291x(72)90314-2. [DOI] [PubMed] [Google Scholar]
- Cooke R., Morales M. F. Interaction of globular actin with myosin subfragments. J Mol Biol. 1971 Sep 14;60(2):249–261. doi: 10.1016/0022-2836(71)90291-9. [DOI] [PubMed] [Google Scholar]
- Finlayson B., Lymn R. W., Taylor E. W. Studies on the kinetics of formation and dissociation of the actomyosin complex. Biochemistry. 1969 Mar;8(3):811–819. doi: 10.1021/bi00831a008. [DOI] [PubMed] [Google Scholar]
- Hudson E. N., Weber G. Synthesis and characterization of two fluorescent sulfhydryl reagents. Biochemistry. 1973 Oct 9;12(21):4154–4161. doi: 10.1021/bi00745a019. [DOI] [PubMed] [Google Scholar]
- Lowey S., Luck S. M. Equilibrium binding of adenosine diphosphate to myosin. Biochemistry. 1969 Aug;8(8):3195–3199. doi: 10.1021/bi00836a010. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Substructure of the myosin molecule. IV. Interactions of myosin and its subfragments with adenosine triphosphate and F-actin. J Mol Biol. 1973 Mar 5;74(3):313–330. doi: 10.1016/0022-2836(73)90376-8. [DOI] [PubMed] [Google Scholar]
- Marston S., Weber A. The dissociation constant of the actin-heavy meromyosin subfragment-1 complex. Biochemistry. 1975 Aug 26;14(17):3868–3873. doi: 10.1021/bi00688a021. [DOI] [PubMed] [Google Scholar]
- Mendelson R. A., Morales M. F., Botts J. Segmental flexibility of the S-1 moiety of myosin. Biochemistry. 1973 Jun 5;12(12):2250–2255. doi: 10.1021/bi00736a011. [DOI] [PubMed] [Google Scholar]
- Mihashi K., Wahl P. Nanosecond pulsefluorometry in polarized light of G-actin-epsilon-ATP and F-actin-epsilon-ADP. FEBS Lett. 1975 Mar 15;52(1):8–12. doi: 10.1016/0014-5793(75)80625-9. [DOI] [PubMed] [Google Scholar]
- Murphy A. J., Morales M. F. Number and location of adenosine triphosphatase sites of myosin. Biochemistry. 1970 Mar 31;9(7):1528–1532. doi: 10.1021/bi00809a008. [DOI] [PubMed] [Google Scholar]
- Nihel T., Mendelson R. A., Botts J. The site of force generation in muscle contraction as deduced from fluorescence polarization studies. Proc Natl Acad Sci U S A. 1974 Feb;71(2):274–277. doi: 10.1073/pnas.71.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliselfeld L. H., Bárány M. The binding of adenosine triphosphate to myosin. Biochemistry. 1968 Sep;7(9):3206–3213. doi: 10.1021/bi00849a024. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Stryer L. Fluorescence spectroscopy of proteins. Science. 1968 Nov 1;162(3853):526–533. doi: 10.1126/science.162.3853.526. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Seidel J. C., Hyde J. S., Gergely J. Motion of subfragment-1 in myosin and its supramolecular complexes: saturation transfer electron paramagnetic resonance. Proc Natl Acad Sci U S A. 1975 May;72(5):1729–1733. doi: 10.1073/pnas.72.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl P. Measure de la décroissange de la fluorescence polarisée de la gamma-globuline-1-sulfonyl-5-diméthylaminonaphthalène. Biochim Biophys Acta. 1969 Feb 4;175(1):55–64. [PubMed] [Google Scholar]


