Abstract
Genome-wide mutagenesis was performed in mice to identify candidate genes for male infertility, for which the predominant causes remain idiopathic. Mice were mutagenized using N-ethyl-N-nitrosourea (ENU), bred, and screened for phenotypes associated with the male urogenital system. Fifteen heritable lines were isolated and chromosomal loci were assigned using low density genome-wide SNP arrays. Ten of the fifteen lines were pursued further using higher resolution SNP analysis to narrow the candidate gene regions. Exon sequencing of candidate genes identified mutations in mice with cystic kidneys (Bicc1), cryptorchidism (Rxfp2), restricted germ cell deficiency (Plk4), and severe germ cell deficiency (Prdm9). In two other lines with severe hypogonadism candidate sequencing failed to identify mutations, suggesting defects in genes with previously undocumented roles in gonadal function. These genomic intervals were sequenced in their entirety and a candidate mutation was identified in SnrpE in one of the two lines. The line harboring the SnrpE variant retains substantial spermatogenesis despite small testis size, an unusual phenotype. In addition to the reproductive defects, heritable phenotypes were observed in mice with ataxia (Myo5a), tremors (Pmp22), growth retardation (unknown gene), and hydrocephalus (unknown gene). These results demonstrate that the ENU screen is an effective tool for identifying potential causes of male infertility.
Introduction
Male infertility affects approximately 10–15% of men (Irvine 1998). The causes are heterogeneous and include testicular dysgenesis, defects in spermatogenesis, obstructive disorders, gonadotropin deficiency, and abnormalities of testosterone synthesis or action, among other causes (Brugh and Lipshultz 2004). Although most cases of male infertility remain idiopathic, an increasing number of genetic causes are being recognized (O'Flynn O'Brien et al. 2010). Initially, chromosomal disorders such as 47/XYY, Klinefelter’s syndrome (Foresta et al. 2002) and translocations or deletions of the Y chromosome were described (Kuroda-Kawaguchi et al. 2001). More recently, specific genes involved in testis development (e.g., SRY, DAX1, SF1), descent (INSL3, RFXP2), or spermatogenesis (AZFa, AZFb, AZFc loci; CDY, TSPY) have also been identified (O'Flynn O'Brien et al. 2010; Tyler-Smith and Krausz 2009).
A large array of other genes and chromosomal loci have been associated with spermatogenesis or male fertility. There are currently over 400 mouse models with reproductive defects (Matzuk and Lamb 2008), and about 150 of these exhibit male infertility (O'Bryan and de Kretser 2006). A search of the Online Mendelian Inheritance in Man (OMIM) database yields 141 results linked to male infertility, comparable to the mice. Nonetheless, it is difficult to know with certainty how many of these genes are causative. In addition, deep sequencing of normal human tissues identified 857 genes whose expression is largely restricted to the testis (Jongeneel et al. 2005). Thus, the number of candidates to test remains very large and the number of genes identified as a cause of male infertility remains small.
In the last decade, candidate gene testing has been supplemented by genome-wide methods for identifying disease genes (Clark et al. 2004). In one approach, the mouse genome is mutagenized in an unbiased manner using N-ethyl-N-nitrosourea (ENU), a chemical that increases the natural mutation rate by a factor of 130 to 160 (Guenet 2004). The resulting animals are then bred and screened for heritable phenotypes of interest, a process referred to as forward genetics. Typically, the result of ENU mutagenesis is a nucleotide substitution (Justice et al. 1999). An advantage of this method over targeted mutagenesis is the ability of point mutations to create hypomorphic alleles, in which single amino acid substitutions result in less severe or more restricted phenotypes than gene deletion (Debruyne et al. 2006). Hypomorphs are useful for studying genes that are required for embryonic development, where a complete knockout would be lethal, or in cases where a primary phenotype masks a secondary trait of specific interest (Acevedo-Arozena et al. 2008).
Worldwide, at least three centers are performing genome-wide screens specifically for reproductive mutants (Kennedy and O'Bryan 2006). One of these, the Reproductive Genomics Group at the Jackson Laboratory, used a three generation breeding scheme to identify recessive mutations that cause infertility (Furnes and Schimenti 2007). This group screened 413 G1 males, mapping 32 lines with male infertility, 3 lines with female infertility, and 7 lines in which both sexes were infertile. This group has reported at least 6 mutant genes from this series (Bannister et al. 2007; Ching et al. 2010a; Ching et al. 2010b; Harris et al. 2007; Philipps et al. 2008; Ward et al. 2007).
In this paper, we report an ENU-based screen focused on genes that cause germ cell deficiency and hypogonadism. Using both dominant and recessive breeding schemes, we screened 1014 G1 males and mapped 15 reproductive mutants to chromosomal loci. Mutations were identified in 6 lines through candidate sequencing. In two additional cases where candidate sequencing was uninformative, genomic resequencing identified one novel gene as a candidate for hypogonadism. The remaining mutation remains unidentified.
Materials and methods
ENU Mutagenesis and Breeding
Male mice of the C57BL/6J (B6) strain were mutagenized in the Northwestern University Center for Functional Genomics as described previously (Vitaterna et al. 1994; Vitaterna et al. 2006). ENU-treated males (G0 generation) were allowed 12 weeks to recover fertility and were then crossed to untreated B6 females, yielding G1 generation males and females. For the cross designed to detect dominant mutants, G1 males and females were bred to wildtype DBA mice and the resulting F1 male progeny were screened. For the cross designed to detect recessive mutants, G1 males were bred to wildtype B6 females and the resulting G2 females were back-crossed to their G1 fathers, yielding G3 male progeny for screening. All mice to be screened were monitored for obvious defects until after puberty and sacrificed between 6 and 8 weeks of age. The kidneys, seminal vesicles, epididymides and testes were examined in situ for size and location. The testes and epididymides were then dissected and the testes were weighed. Developmental defects (e.g., cystic kidneys, cryptorchidism) and severe hypogonadism were detected at this point in the screen.
Histology
The testes were fixed in Bouin’s fixative and the epididymides were fixed in 10% neutral buffered formalin. After fixation, one testis was cut in half longitudinally and the other testis was cut in cross-section. The four testis halves and the two epididymides were embedded in a single paraffin block such that one 5 µM section captured all six pieces of tissue. Sections were stained with hematoxylin and eosin and examined for histological defects, including spermatogenic block, loss of germ cell layers or Sertoli cell only tubules in the testes, or an absence of sperm or the presence of round germ cells in the epididymides.
Genetic mapping of putative mutants
For lines that exhibited heritable phenotypes of interest, affected animals on the B6 background were back-crossed to wildtype DBA mice to create an F1 generation. For dominant mutants, F1 mice were again crossed to wildtype DBA mice to create the N2 generation, which underwent the necessary meiotic recombination for mapping. For recessive mutants, F1 mice were inter-crossed to create the F2 generation for mapping. Mice from the F2 and N2 generations were mapped using genome-wide 384 and 768 SNP arrays (Moran et al. 2006), yielding chromosomal assignments and loci of between 9 and 77 Mb. In six lines (624, 1003, 840, 924, 1, 1105) where the phenotype had low penetrance or there was an absence of obvious candidate genes, the genes were not pursued further. Mutated genes in five other lines (1163, 1247, 1006, 1061, 708) were identified by sequencing exons of candidates in these regions (Myo5a, Pmp22, Bicc1, Rxfp2, Plk4). For the remaining four mutants (1046, 1045, 1078, 1157), high density mapping was performed using a custom 384 SNP array designed using the Illumina Golden Gate platform, including the manufacturer’s design software, protocols and reagents. This array was targeted to the four regions identified in the low density screen. SNPs were placed on average every 0.5 Mb. Between 28 and 246 samples were mapped for each of these lines, and sequencing of candidate genes in the narrowed regions identified one additional mutation (Prdm9).
Re-sequencing of genomic intervals
For lines 1078 and 1157, SNP-mapped intervals were enriched using genomic DNA from affected animals and B6 and DBA controls. Capture arrays were designed using the Agilent eArray software and libraries were prepared using Agilent SureSelect reagents. Sequencing was performed on the Applied Biosystems SOLiD platform and putative sequence variants were identified using the Applied Biosystems BioScope software. All work was performed by the Northwestern University Genomics Core Facility using the manufacturer’s protocols and reagents. Additional analysis was also undertaken in the Institute for Computational Biomedicine at Weill Cornell Medical College. The variants were confirmed using a pipeline that utilized the Burrows-Wheller Aligner (BWA) and SAMtools for variant calling.
Validation of Identified Mutations
All sequence variants were compared to public databases to identify known SNPs. For variants that were not present in available databases, background strains (B6, DBA) were sequenced to identify previously unreported SNPs. SNPs were not pursued further. The remaining variants were sequenced in complete litters from the relevant line to confirm that the variant segregated with the phenotype in affected and unaffected littermates. Variants that segregated appropriately were then sequenced in larger numbers of affected and unaffected animals from the colony to establish the frequency of transmission.
Results
Mutagenesis and genetic mapping
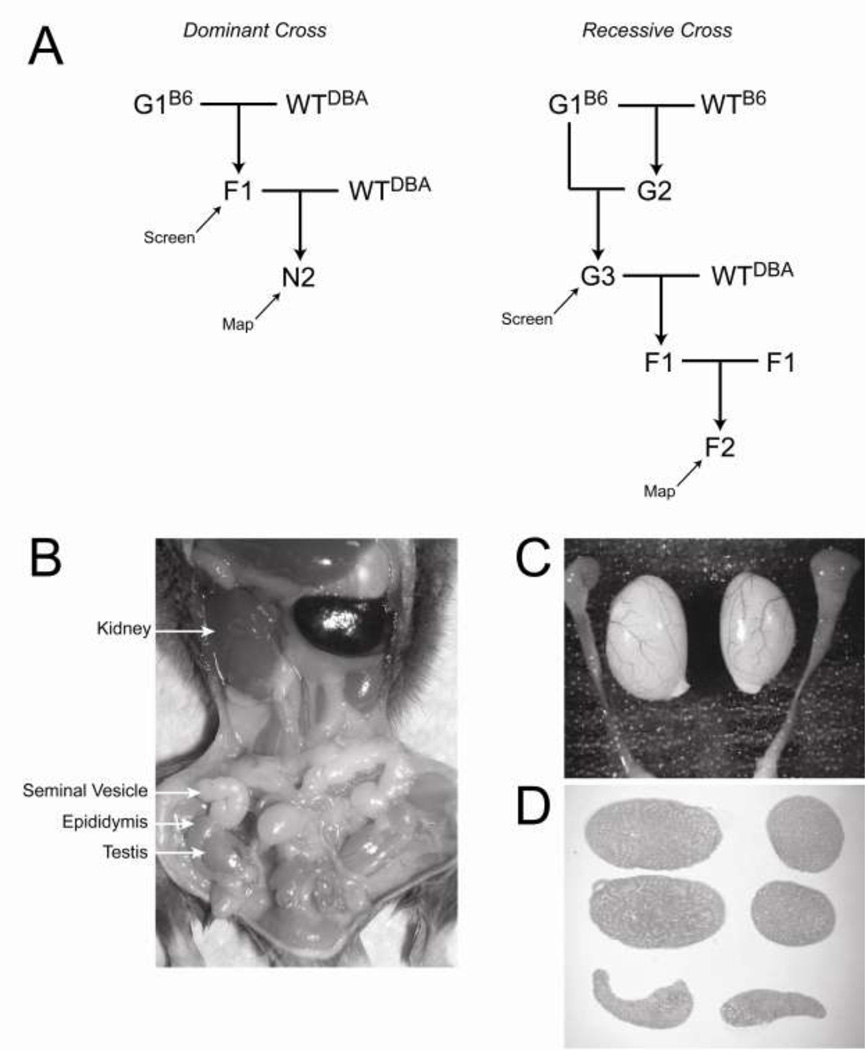
Male mice of the C57BL/6J strain (B6) were treated with ENU, allowed to recover their fertility over 12 weeks, and then mated with untreated B6 females to create the G1 generation (Vitaterna et al. 1994). The germline in ENU-treated mice is chimeric, but each G1 mouse inherits a novel set of mutations, of which no more than one generally causes a phenotype of interest. To identify dominant reproductive mutants, F1 offspring from 1014 G1 males and females mated to untreated mice were examined (Figure 1A). To identify recessive mutations, or to identify recessive traits in weakly penetrant dominant crosses, G3 offspring from 75 G1 males mated to their G2 offspring were examined. In some cases where only a small number of F1 or G3 offspring were born, mice from a subsequent generation were also screened. Males were euthanized between 6 and 8 weeks of age and the urogenital system (testes, epididymides, seminal vesicles, kidneys, bladder, ureters) was examined for morphological defects. The testes and epididymides were then dissected, weighed, fixed and embedded for histological analysis (Figure 1B–D).
Figure 1.
Overview of breeding strategy and phenotypic characterization. A, breeding schemes designed to identify dominant and recessive mutants. B, morphological examination of the urogenital system. C, dissection of the testes and epididymides. D, histological examination illustrating longitudinal and cross-sectional views of the testes and longitudinal sections of the epididymides.
Approximately ten offspring were examined for each line, and a total of 11,124 slides were scored over the course of the screen. Most animals were assessed based on a single histological section, but a subset were examined in more detail. Lines with three or more abnormal animals were assigned a “putative mutant” status and analyzed further. The dominant breeding scheme yielded 10 mutants, of which five were confirmed to transmit in a dominant manner. One additional mutant was incompletely dominant, exhibiting a milder phenotype in the heterozygous state and a more severe phenotype in the homozygous state, and one mutant was X–linked. The remaining three low penetrance mutants in the dominant breeding scheme were subsequently found to exhibit more consistent phenotypes in recessive crosses. The recessive breeding scheme yielded four additional recessive mutants and one dominant mutant.
The fifteen lines exhibiting heritable phenotypes were backcrossed to mice of the DBA strain. Using genome-wide custom SNP arrays, all lines were tentatively mapped to large chromosomal segments (Table 1). Four lines with germ cell deficiency and one line with abnormal development of the ureters exhibited decreased penetrance after crosses to other strains, precluding more detailed analyses. Four lines exhibited incidental phenotypes (growth retardation, hydrocephalus, ataxia, tremors). In two of these lines, mutations were identified by sequencing exons from candidate genes (ataxia, Myo5a; tremors, Pmp22). Mutations were identified in two lines with urogenital defects in a similar manner: a line exhibiting cystic kidneys had a mutation in Bicc1, and a line with bilateral cryptorchidism had a mutation in Rxfp2 (Harris et al. 2010).
Table 1.
Phenotypes derived from the genome-wide screen. Intervals are as initially mapped. Nucleotide references are to mouse genome build NCBI m37.
| Line | Phenotype | Inheritance | Chromosome:Interval | Gene |
|---|---|---|---|---|
| 624 | Germ Cell Deficiency | Dominant | Chr 7:60841199-71547014 | Low penetrance |
| 1003 | Germ Cell Deficiency | Dominant | Chr 7:141188625-152082670 | Low penetrance |
| 840 | Germ Cell Deficiency | Recessive | Chr 9:57100869-120927360 | Low penetrance |
| 924 | Germ Cell Deficiency | Dominant | Chr 11:70520265-99596170 | Low penetrance |
| 1 | Ureter Dysgenesis | Recessive | Chr 1:188060930-197195432 | Low penetrance |
| 1105 | Nude/Growth Retarded | X-linked | Chr X:1-33548080 | Unknown |
| 1046 | Hydrocephalus | Recessive | Chr 2:116028112-134586818 | Unknown |
| 1163 | Ataxic | Recessive | Chr 9:70880255-80855569 | Myo5a |
| 1247 | Tremors | Dominant | Chr 11:58438767-70520265 | Pmp22 |
| 1006 | Cystic Kidneys | Recessive | Chr 10:59648241-92719858 | Bicc1 |
| 1061 | Cryptorchid | Recessive | Chr 5:137393986-152537259 | Rxfp2 |
| 708 | Germ Cell Deficiency | Dominant | Chr 3:1-54853263 | Plk4 |
| 1045 | Hypogonadal | Inc. Dominant+ | Chr 17:12595938-49108450 | Prdm9 |
| 1157 | Hypogonadal | Dominant | Chr 1:74948660-151677135* | SnrpE |
| 1078 | Hypogonadal | Recessive | Chr 2:26733409-42909019* | Unknown |
Narrowed intervals from subsequent mapping are reported in the Results.
Incomplete dominant.
The remaining four lines had varying degrees of germ cell deficiency and hypogonadism. Line 708 exhibited dominant inheritance of germ cell deficiency and a mutation was found in Polo-like Kinase 4 (Plk4). The phenotype for this line is described elsewhere (Harris et al. 2011). The remaining three hypogonadal lines are described here, and illustrate the spectrum of disorders observed.
Line 1078: Severe hypogonadism and germ cell loss
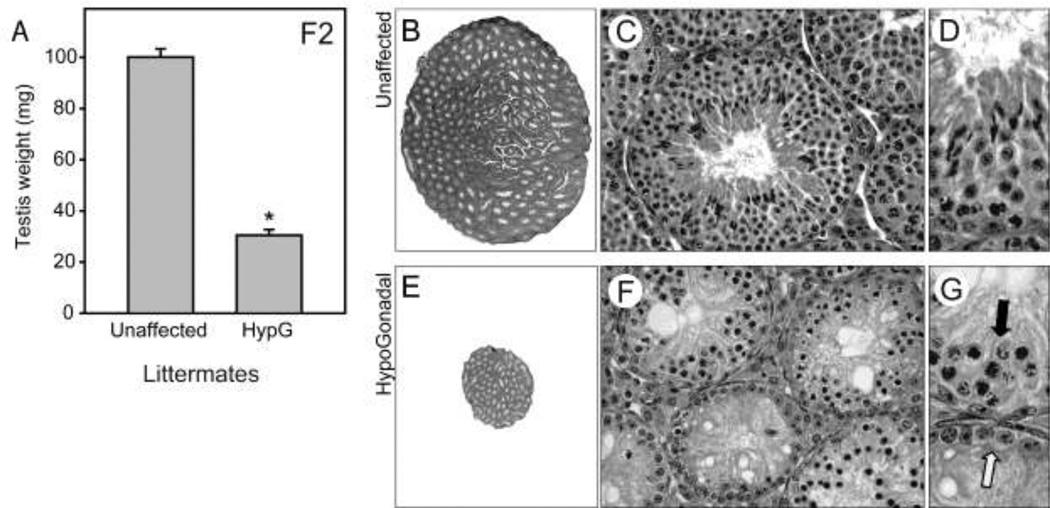
Line 1078 exhibited a mild phenotype in the dominant histological screen; scattered germ cell deficiency was observed in 4 out of 21 F1 progeny. F1 mice were then inter-crossed to identify potential recessive traits. In the F2 generation, severe hypogonadism was observed in 24% of male progeny (n=8 of 34) when considering only litters that contained at least one affected animal (i.e., both parents were obligate heterozygotes)(Figure 2A, also compare 2B & 2E), which is the expected Mendelian ratio for a recessive mutant. Histological analysis of affected mice revealed that every tubule exhibited substantial germ cell deficiency (Figure 2F). The most extensively affected tubules contain only Sertoli cells (Figure 2G, white arrow). In the other tubules, only pre-meiotic cells were observed, and many tubules contained cells with condensed, pyknotic nuclei (Figure 2G, black arrow).
Figure 2.
Recessively transmitted hypogonadism with germ cell deficiency. Line 1078; mapped to chromosome 2. A, testis weights in unaffected littermates and homozygous mutants, which exhibit hypogonadism (HypG). B, C & D, wildtype testis sections. E, F, & G, testis sections from homozygous mutants exhibit severe germ cell deficiency.
In an attempt to identify the causative gene, 63 DNA samples from affected animals were fine mapped using additional SNPs, yielding a 1.44 MB region on chromosome 2 (NCBI m37, Chr 2:27917393-29356174). The entire exome from this region was sequenced at the Broad Institute (Cambridge, MA), but no mutations were identified. The entire genomic interval was then re-sequenced at the Northwestern University Genomics Core Facility, also without identifying a mutation.
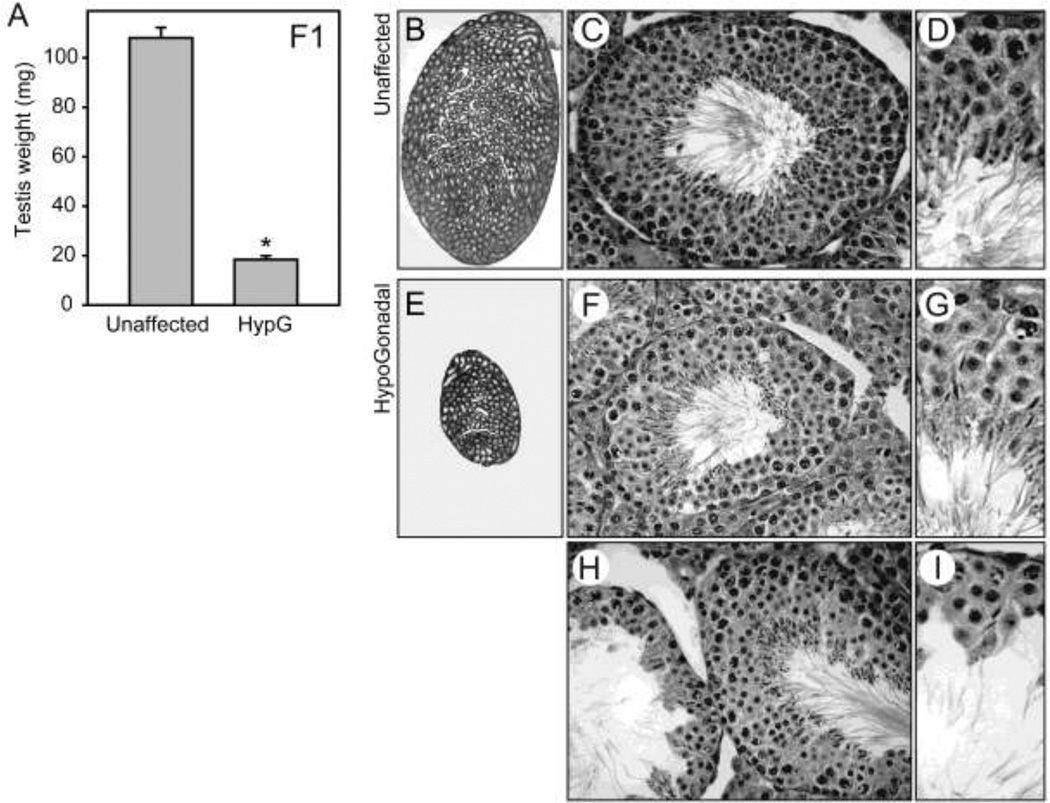
Line 1157: Severe hypogonadism with ongoing spermatogenesis
Line 1157 was identified in the dominant screen based on marked reduction in testis weight. Affected mice in the F1 generation had an average testis weight of 18 mg (n=8), compared to 108 mg for unaffected littermates (n=9)(Figure 3A, also compare 3E to 3B). Unexpectedly, histological examination of hypogonadal testes revealed ongoing spermatogenesis despite the small testis size. Compared to an unaffected littermate (Figure 3C & 3D), most tubules in the hypogonadal testes exhibited normal histology or minimal germ cell deficiency (Figure 3F & 3G), though occasional tubules had more extensive absence of round and elongating spermatids (Figure 3H). However, some elongated spermatids were present even in the most severely affected tubules (Figure 3I), and multiple stages of spermatogenesis were observed in each testis section, indicating a reduction rather than a failure of spermatogenesis in this mutant. Among the severely hypogonadal mutants observed in the screen, only line 1157 retained substantial spermatogenesis.
Figure 3.
Dominantly transmitted hypogonadism with ongoing spermatogenesis. Line 1157; mutation in the SnrpE gene. A, testis weights in unaffected littermates and heterozygous mutants, which exhibit hypogonadism (HypG). B, C & D, wildtype testis sections. E, F, & G, testis sections from unaffected/mildly affected homozygous mutants retain spermatogenesis. H & I, testis sections from moderately affected homozygous mutants exhibit variable germ cell deficiency.
In an attempt to identify the gene responsible for this novel phenotype, 253 DNA samples from affected animals were mapped, yielding a 1.34 Mb interval on chromosome 1 (NCBI m37, Chr1:134297351-135638666). Exons and intron-exon boundaries were sequenced for 13 candidate genes in this region (Cntn2, Ripk5, Etnk2, Golt1a, Kiss1, Lax1, Lemd1, Lrrn2, Ppp1r15b, Rbbp5, Ren1, Tmem81, Zc3h11a) but no mutation was identified. The entire genomic interval was then re-sequenced, which yielded a non-synonymous mutation (nucleotide T227A; amino acid E51D) in the gene for small nuclear riboprotein E (SnrpE). SnrpE is a highly conserved binding protein for the U family of small nuclear RNAs (Fautsch et al. 1992; Stanford et al. 1988).
Incomplete dominance and hypogonadism
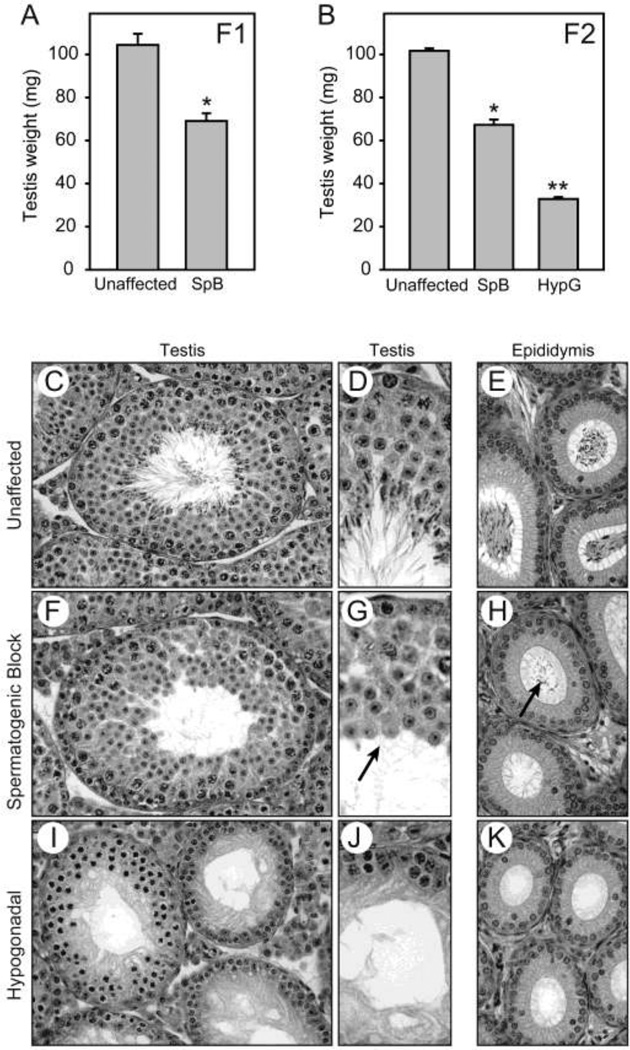
Line 1045 was identified in the dominant histological screen based on the absence of sperm in the epididymides of F1 mice, despite only mild hypogonadism in this generation (Figure 4A). For reference, figures 4C and 4D show a stage VII tubule from an unaffected littermate, with layers of differentiating germ cells culminating in elongated spermatids with their tails extending into the lumen. In the caput epididymis from the same animal (Figure 4E), columnar epithelial cells line the lumen, which is tightly packed with sperm. A testis tubule from an affected F1 littermate also shows multiple layers of round germ cells (Figure 4F), similar to the unaffected animal. However, examination at higher magnification revealed the absence of elongated spermatids (Figure 4G, arrow), suggesting that spermiogenesis failed to occur normally. The epididymis from this animal appears normal but the lumen is devoid of mature sperm, though small numbers of round germ cells are observed (Figure 4H, arrow).
Figure 4.
Incomplete dominance with spermatogenic block (heterozygous) or germ cell loss (homozygous). Line 1045; mutation in the Prdm9 gene. A, testis weights in heterozygous animals, which exhibit spermatogenic block (SpB) and mild hypogonadism in the F1 generation. B, testis weights in the F2 generation. Presumed homozygotes, identified by extensive germ cell loss, exhibit severe hypogonadism (HypG). C & D, wildtype testis sections. E, wildtype epididymis with sperm. F & G, testis sections from confirmed heterozygous mutant exhibit complete spermatogenic block, with an absence of elongated spermatids. H, epididymis from confirmed heterozygous mutant contains a small number of round germ cells and no sperm. I & J, testis section from a confirmed homozygous mutant exhibits severe germ cell loss. K, epididymis from a homozygous mutant is devoid of germ cells.
F1 mice were inter-crossed to determine if a more severe phenotype was observed in the homozygous state. Twenty-two percent of F2 progeny were severely hypogonadal (n=47 of 216), appropriate for a recessive trait, with a mean testis weight of 33 mg compared to 102 mg for unaffected littermates (Figure 4B). Germ cells in the seminiferous tubules of hypogonadal animals were all pre-meiotic (Figure 4I & J) and some nuclei were pyknotic, consistent with other severely hypogonadal ENU mutants. No germ cells were observed in the epididymis (Figure 4K).
DNA was collected from 73 animals with either spermatogenic block, predicted to be heterozygous, or severe hypogonadism, predicted to be homozygous. SNP analysis yielded a broad region on chromosome 17, and sequencing of candidate gene exons identified a non-synonymous mutation (nucleotide A739T; amino acid L247Stop) in the gene for PR domain containing 9 (Prdm9). Prdm9 was initially reported as a histone methyltransferase involved in the progression of early meiotic prophase (Hayashi et al. 2005), consistent with the histology observed here.
Discussion
ENU mutagenesis has evolved rapidly in the last 15 years. This acceleration reflects several parallel events: 1) investment by funding agencies in mutagenesis centers (Boles et al. 2009; Justice et al. 1999; Moldin et al. 2001), building on early successes in the identification of disease-specific genes; 2) development of genome-wide and tailored SNP arrays, which greatly simplify the mapping of loci in mutant mouse lines; and 3) advances in DNA sequencing, which provided the human and mouse reference genomes (Durbin et al. 2010; Lander et al. 2001; Venter et al. 2001; Waterston et al. 2002), as well as the ability to quickly identify base changes responsible for observed phenotypes (Boles et al. 2009).
The current screen was designed to identify genes required for spermatogenesis and related urogenital functions. Mutations in these genes are potential causes of male infertility. One concern when planning this screen was whether mutations in genes that impair male germ cell development would have similar effects in the female germline, thus precluding propagation of the mutants through either sex. In this respect the ReproGenomics screen at the Jackson Laboratory was informative. When searching for genes required for fertility, this group found a preponderance of mutants that caused male-only infertility (Handel et al. 2006), with far fewer genes causing infertility in both sexes. Our own experience is consistent with these results. Three dominant lines with germ cell loss (624, 1003, 924), all derived from male G1 founders, could not be pursued due to poor breeding and transmission. Of the two dominant lines in which mutations were successfully identified, one (line 708; Plk4) had a mild phenotype (Harris et al. 2011) and the other (line 1157; SnrpE) was derived from a female G1. We included female G1 founders in the screen specifically to address this concern about fertility, based in part on the results from the previous screen. G1 males are more commonly used in ENU screens to allow trio or harem breeding, which expedites the generation of progeny for screening.
Several other adjustments were also made to enhance efficiency. The current screen was primarily designed to detect mutations with dominant inheritance, owing to the simplified breeding compared to a recessive screen. In an attempt to capture a subset of the recessive alleles, dominant crosses that yielded a mild phenotype or which exhibited low penetrance were selectively inbred to identify potential recessive traits. The incomplete dominance in line 1045 (Prdm9) was identified in this manner, as was the severe hypogonadism in line 1078. As noted, however, this strategy was not universally successful. Overall we encountered 5 lines for which the transmission rate was too low in either cross to allow the lines to be studied beyond low resolution mapping (Table 1). Generating enough affected animals would have required very large colonies. In some of these lines transmission was low in the initial B6 crosses, possibly reflecting a multi-genic etiology. In others we observed a loss of penetrance specifically when back-crossing to the DBA strain for mapping. In the latter case affected mice were crossed to several additional strains without a rescue of the phenotype. These data are consistent with the known sensitivity of the B6 strain to disruption of the reproductive axis, an effect we have also observed with targeted knockouts (Raverot et al. 2005).
Identified mutations were confirmed in additional progeny from the same line. Initially, sequencing was performed in related animals to allow direct comparison of littermates. Subsequently, larger groups of affected and unaffected mice were sequenced to confirm the correlation of genotype to phenotype. We identified a number of SNPs, either synonymous or in non-coding regions, that did not track with the phenotype. However, all of the mutations reported here were transmitted in tight association with the phenotype. Two of the mutations (Plk4, Rxfp2) were further demonstrated to be causative using genetic complementation assays (Harris et al. 2010; Harris et al. 2011). The remaining genetic variants remain candidates until they are confirmed with additional complementation assays or by replication of the phenotype after introducing the mutation into wildtype mice using standard gene targeting techniques.
To estimate the completeness of the screen we used several known metrics and calculated the genomic coverage using a computational simulation. The mice used in this study were produced as part of an established mutagenesis program at Northwestern University (Vitaterna et al. 1994), in which the estimated forward mutation rate was 0.0015 per locus per gamete, or about 1 in 700. Using a figure of 20,210 protein coding genes in the mouse genome (Church et al. 2009), it was estimated that each G1 mouse harbored 29 independent mutations, on average. Beginning with the 1014 gametes (G1 mice) screened and simulating the mutagenesis process over 100 trials to account for random variability yielded an estimated coverage of 15,262 genes, or 77% of the mouse genome. By extension, to mutate each gene at least once with 95% confidence would have required screening of 6,537 gametes. This simple approximation does not account for fold coverage, variability of the forward mutation rate across the genome, or several other factors. However, based on this estimate we would have expected a diminished screening efficiency beyond the initial one thousand G1 mice examined under the specific conditions used in this study.
The genes ultimately identified in this screen were informative. Although several were previously known to be involved in spermatogenesis, notable insights were still achieved. The Prdm9 gene is a good example in this regard. Initially known as Meisetz, Prdm9 was shown to be a histone H3 methyltransferase necessary for meiotic prophase. Mice with targeted deletion of this gene were sterile in both sexes due to severe impairment of the double-stranded DNA break repair pathway, deficient pairing of homologous chromosomes, and impaired sex body formation (Hayashi et al. 2005). However, heterozygous null animals were fertile. Prdm9 was subsequently shown to be a hybrid sterility gene that plays a role in mammalian speciation (Mihola et al. 2009) by controlling meiotic recombination hotspots (Baudat et al. 2010; Parvanov et al. 2010), the first such gene identified in a vertebrate species. In the present study, it was observed that haploinsufficiency of Prdm9 results in a late spermatogenic block, precluding spermiogenesis. Notably, this defect was manifest subsequent to meiosis and was therefore not observed in the previous reports. Thus, the ENU allele unmasked a second requirement for Prdm9 in spermatogenesis and extends the known roles of this gene.
The cryptorchid mutant (line 1061), which has a mutation in Rfxp2, also extends our understanding of the function of this gene. The human homolog, RXFP2, is a cell surface receptor that is well known to cause cryptorchidism when mutated in patients (Ferlin et al. 2003). For this reason, its biochemical mechanism has been studied in detail. Fortuitously, aspartic acid 275 in human RXFP2, which is structurally equivalent to the D294 residue mutated in the current ENU mouse mutant, has been examined in vitro. Mutation of human D275 did not affect cell surface expression but precluded binding of its ligand, INSL3. In contrast, mutation of mouse D294 decreased trafficking of the receptor to the cell surface but there was normal signaling by the relatively small amounts of receptor that did localize to the membrane (Harris et al. 2010). The presence or absence of the receptor on the cell surface is important relative to any attempt to manipulate RXFP2 using agonists or antagonists in cryptorchid patients. Thus, ENU mutants can be informative even when the relevant gene has been well-studied, and in this case highlights an evolutionary divergence.
The final two lines identified in this study highlight the promise (line 1157) and also the remaining challenges (line 1078) in mutagenesis screening. In both cases, the SNP arrays were followed by manual mapping with additional SNPs, thus narrowing the regions carrying the mutation to 1.34 Mb and 1.44 Mb, respectively. In line 1157, the exons and intron-exon boundaries were sequenced for 13 genes considered candidates based on known physiological or biochemical activity. In line 1078, all of the exons and intron-exon boundaries in the region were sequenced as part of a pilot program at the Broad Institute. In both cases the background strains (B6 and DBA) were also sequenced using DNA from the same colonies as the affected animals. This method allowed direct comparisons to identify sequence differences, rather than relying on genomic databases. In neither case did exon sequencing reveal the causative mutation, indicating that the mutations were either in a regulatory sequence, an intron, or in a gene lacking any known activity relevant to germ cells or the testis.
Using recently available methods, both intervals were re-sequenced after genomic enrichment and an aspartic acid to glutamic acid substitution was identified in the SnrpE gene in line 1157. Although the E51D substitution is biochemically conservative, we note that the SnrpE amino acid sequence is 100% conserved between humans, mice and chickens (Fautsch et al. 1992). These data suggest that amino acid substitutions, however conservative, are strongly selected against. SnrpE belongs to a group of proteins that associate with the U family of small nuclear RNAs, which are involved in RNA processing (Stanford et al. 1988). Despite its participation in this fundamental cellular activity, there is no reported example of a specific physiological function that is dependent on SnrpE. Thus, in this case mutagenesis screening has likely identified an entirely novel cause of male infertility.
In line 1078 we were unable to identify a candidate for the causative mutation. It is unlikely that this mutation resides in a coding sequence as the relevant exome was sequenced twice independently, once at the Broad Institute and once during genomic re-sequencing. A more plausible explanation is that the mutation resides in an intervening sequence that was not well-covered in our re-sequencing. Approximately 91.5% of the interval containing the mutation in line 1078 was sequenced at a coverage of 10-fold or higher, a useful target for separation of valid polymorphisms from technical artifacts in the sequencing reads. Approximately 3.9% of the interval failed to sequence at all. Notably, in an exhaustive re-sequencing study involving 41 ENU mutants, Boles et. al. found that 61% of their identified mutations were located outside of the coding regions (Boles et al. 2009). While only a fraction of those sequence changes may ultimately be proven causative, this study further highlights the importance of mutations in structural and regulatory regions. It is also possible that this line harbors a natural mutation of a different class (e.g., a mobile element insertion), and care must be taken in the analysis not to overlook mutations that are different from the expected single base changes.
In summary, we find that ENU mutagenesis is an effective tool for identifying candidate genes for male infertility and milder reproductive phenotypes. While some of these genes are novel, this pool will likely diminish as the human and mouse genomes become more completely annotated. However, insights may still be gained from assigning new roles to genes previously associated with non-reproductive functions, as described above for Prdm9 and Rxfp2.
Acknowledgements
This work was supported by NIH grant U01 HD043425 (JLJ), NIH grant UO1 HD43430 (DRB), by the Northwestern University Genomics Core which is supported by a Cancer Center Support Grant (NCI CA060553), and by the Northwestern University Biostatistics Collaboration Center which is supported by a grant from the National Center for Research Resources (UL1 RR025741). The authors thank Timothy Barrett for exceptional technical assistance.
References
- Acevedo-Arozena A, et al. ENU mutagenesis, a way forward to understand gene function. Annu Rev Genomics Hum Genet. 2008;9:49–69. doi: 10.1146/annurev.genom.9.081307.164224. [DOI] [PubMed] [Google Scholar]
- Bannister LA, et al. A dominant, recombination-defective allele of Dmc1 causing male-specific sterility. PLoS biology. 2007;5:e105. doi: 10.1371/journal.pbio.0050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudat F, et al. PRDM9 is a major determinant of meiotic recombination hotspots in humans and mice. Science. 2010;327:836–840. doi: 10.1126/science.1183439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles MK, et al. Discovery of candidate disease genes in ENU-induced mouse mutants by large-scale sequencing, including a splice-site mutation in nucleoredoxin. PLoS Genet. 2009;5:e1000759. doi: 10.1371/journal.pgen.1000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugh VM, 3rd, et al. Male factor infertility: evaluation and management. Med Clin North Am. 2004;88:367–385. doi: 10.1016/S0025-7125(03)00150-0. [DOI] [PubMed] [Google Scholar]
- Ching YH, et al. High resolution mapping and positional cloning of ENU-induced mutations in the Rw region of mouse chromosome 5. BMC Genet. 2010a;11:106. doi: 10.1186/1471-2156-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching YH, et al. An allele separating skeletal patterning and spermatogonial renewal functions of PLZF. BMC Dev Biol. 2010b;10:33. doi: 10.1186/1471-213X-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church DM, et al. Lineage-specific biology revealed by a finished genome assembly of the mouse. PLoS Biol. 2009;7:e1000112. doi: 10.1371/journal.pbio.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AT, et al. Implementing large-scale ENU mutagenesis screens in North America. Genetica. 2004;122:51–64. doi: 10.1007/s10709-004-1436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruyne JP, et al. A clock shock: mouse CLOCK is not required for circadian oscillator function. Neuron. 2006;50:465–477. doi: 10.1016/j.neuron.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Durbin RM, et al. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fautsch MP, et al. Conservation of coding and transcriptional control sequences within the snRNP E protein gene. Genomics. 1992;14:883–890. doi: 10.1016/s0888-7543(05)80109-0. [DOI] [PubMed] [Google Scholar]
- Ferlin A, et al. The INSL3-LGR8/GREAT ligand-receptor pair in human cryptorchidism. J Clin Endocrinol Metab. 2003;88:4273–4279. doi: 10.1210/jc.2003-030359. [DOI] [PubMed] [Google Scholar]
- Foresta C, et al. Guidelines for the appropriate use of genetic tests in infertile couples. Eur J Hum Genet. 2002;10:303–312. doi: 10.1038/sj.ejhg.5200805. [DOI] [PubMed] [Google Scholar]
- Furnes B, et al. Fast forward to new genes in mammalian reproduction. J Physiol. 2007;578:25–32. doi: 10.1113/jphysiol.2006.119164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenet JL. Chemical mutagenesis of the mouse genome: an overview. Genetica. 2004;122:9–24. [PubMed] [Google Scholar]
- Handel MA, et al. Mutagenesis as an unbiased approach to identify novel contraceptive targets. Mol Cell Endocrinol. 2006;250:201–205. doi: 10.1016/j.mce.2005.12.046. [DOI] [PubMed] [Google Scholar]
- Harris RM, et al. A missense mutation in LRR8 of RXFP2 is associated with cryptorchidism. Mamm Genome. 2010;21:442–449. doi: 10.1007/s00335-010-9291-5. [DOI] [PubMed] [Google Scholar]
- Harris RM, et al. Male Hypogonadism and Germ Cell Loss Caused by a Mutation in Polo-Like Kinase 4. Endocrinology. 2011 doi: 10.1210/en.2011-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T, et al. Sperm motility defects and infertility in male mice with a mutation in Nsun7, a member of the Sun domain-containing family of putative RNA methyltransferases. Biol Reprod. 2007;77:376–382. doi: 10.1095/biolreprod.106.058669. [DOI] [PubMed] [Google Scholar]
- Hayashi K, et al. A histone H3 methyltransferase controls epigenetic events required for meiotic prophase. Nature. 2005;438:374–378. doi: 10.1038/nature04112. [DOI] [PubMed] [Google Scholar]
- Irvine DS. Epidemiology and aetiology of male infertility. Hum Reprod. 1998;13(Suppl 1):33–44. doi: 10.1093/humrep/13.suppl_1.33. [DOI] [PubMed] [Google Scholar]
- Jongeneel CV, et al. An atlas of human gene expression from massively parallel signature sequencing (MPSS) Genome Res. 2005;15:1007–1014. doi: 10.1101/gr.4041005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice MJ, et al. Mouse ENU mutagenesis. Hum Mol Genet. 1999;8:1955–1963. doi: 10.1093/hmg/8.10.1955. [DOI] [PubMed] [Google Scholar]
- Kennedy CL, et al. N-ethyl-N-nitrosourea (ENU) mutagenesis and male fertility research. Hum Reprod Update. 2006;12:293–301. doi: 10.1093/humupd/dmk004. [DOI] [PubMed] [Google Scholar]
- Kuroda-Kawaguchi T, et al. The AZFc region of the Y chromosome features massive palindromes and uniform recurrent deletions in infertile men. Nat Genet. 2001;29:279–286. doi: 10.1038/ng757. [DOI] [PubMed] [Google Scholar]
- Lander ES, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, et al. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14:1197–1213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihola O, et al. A mouse speciation gene encodes a meiotic histone H3. methyltransferase. Science. 2009;323:373–375. doi: 10.1126/science.1163601. [DOI] [PubMed] [Google Scholar]
- Moldin SO, et al. Trans-NIH neuroscience initiatives on mouse phenotyping and mutagenesis. Mamm Genome. 2001;12:575–581. doi: 10.1007/s00335-001-4005-7. [DOI] [PubMed] [Google Scholar]
- Moran JL, et al. Utilization of a whole genome SNP panel for efficient genetic mapping in the mouse. Genome Res. 2006;16:436–440. doi: 10.1101/gr.4563306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Bryan MK, et al. Mouse models for genes involved in impaired spermatogenesis. Int J Androl. 2006;29:76–89. doi: 10.1111/j.1365-2605.2005.00614.x. discussion 105-108. [DOI] [PubMed] [Google Scholar]
- O'Flynn O'Brien KL, et al. The genetic causes of male factor infertility: a review. Fertil Steril. 2010;93:1–12. doi: 10.1016/j.fertnstert.2009.10.045. [DOI] [PubMed] [Google Scholar]
- Parvanov ED, et al. Prdm9 controls activation of mammalian recombination hotspots. Science. 2010;327:835. doi: 10.1126/science.1181495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipps DL, et al. The dual bromodomain and WD repeat-containing mouse protein BRWD1 is required for normal spermiogenesis and the oocyte-embryo transition. Developmental biology. 2008;317:72–82. doi: 10.1016/j.ydbio.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raverot G, et al. Sox3 expression in undifferentiated spermatogonia is required for the progression of spermatogenesis. Developmental biology. 2005;283:215–225. doi: 10.1016/j.ydbio.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Stanford DR, et al. The complete primary structure of the human snRNP E protein. Nucleic Acids Res. 1988;16:10593–10605. doi: 10.1093/nar/16.22.10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler-Smith C, et al. The will-o'-the-wisp of genetics--hunting for the azoospermia factor gene. N Engl J Med. 2009;360:925–927. doi: 10.1056/NEJMe0900301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter JC, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Vitaterna MH, et al. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaterna MH, et al. Large-scale mutagenesis and phenotypic screens for the nervous system and behavior in mice. Trends Neurosci. 2006;29:233–240. doi: 10.1016/j.tins.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JO, et al. Mutation in mouse hei10, an e3 ubiquitin ligase, disrupts meiotic crossing over. PLoS genetics. 2007;3:e139. doi: 10.1371/journal.pgen.0030139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterston RH, et al. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]