Abstract
Etioplasts isolated from barley leaves and purified on a Sephadex G-50 (coarse) column were characterized by electron microscopy and nucleic acid analysis. The majority of etioplasts retained an intact outer envelope, and contamination by other fragments was extremely low. The level of gibberellin-like substances extractable from intact etioplast suspensions was enhanced within 5 min of the termination of a saturating red irradiation, and the response was far-red reversible. Ultra-sonication caused a 3-fold increase in extractable activity both in dark control suspension and suspensions treated with red light. It is concluded that phytochrome, as a function of its interconversions, probably causes the transport of gibberellin from inside the etioplast into the surrounding medium. This leads to increased production of active gibberellins, possibly by release of feedback control of late steps of the biosynthetic pathway. Dual wavelength difference spectrophotometry has demonstrated the presence of a proportion of total cellular phytochrome within the etioplast.
Keywords: photomorphogenesis, membrane permeability, hormone transport
Full text
PDF
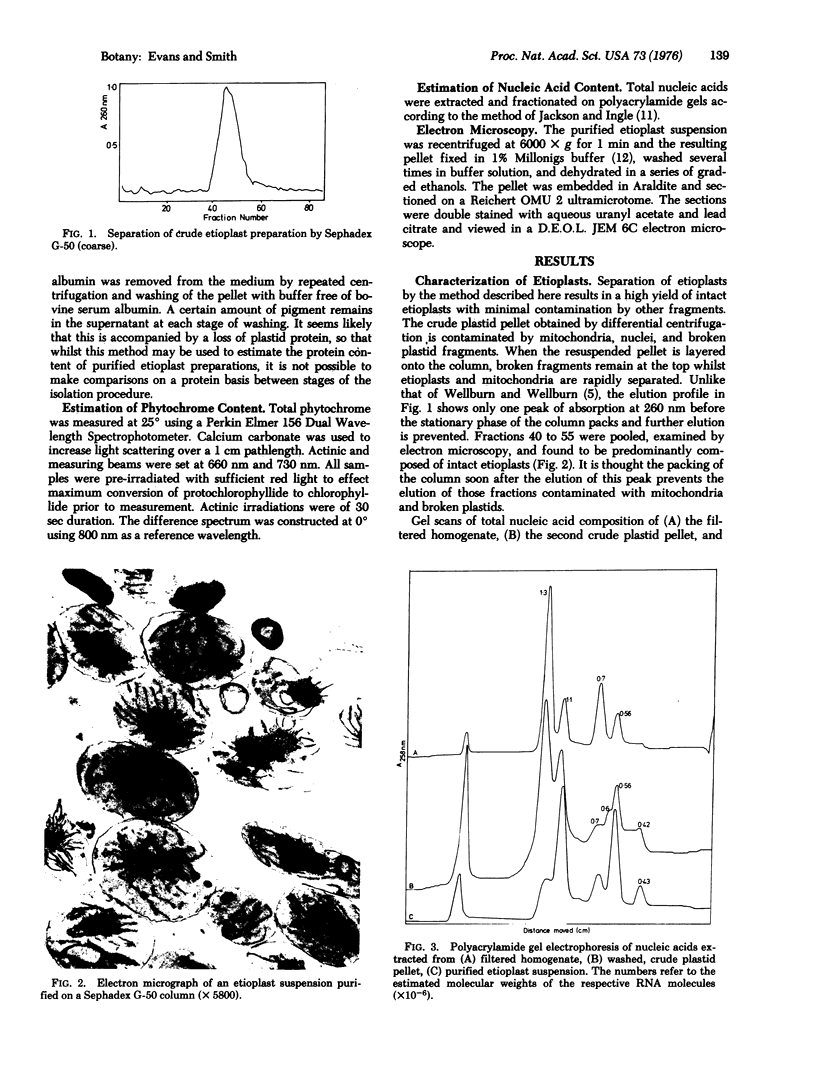
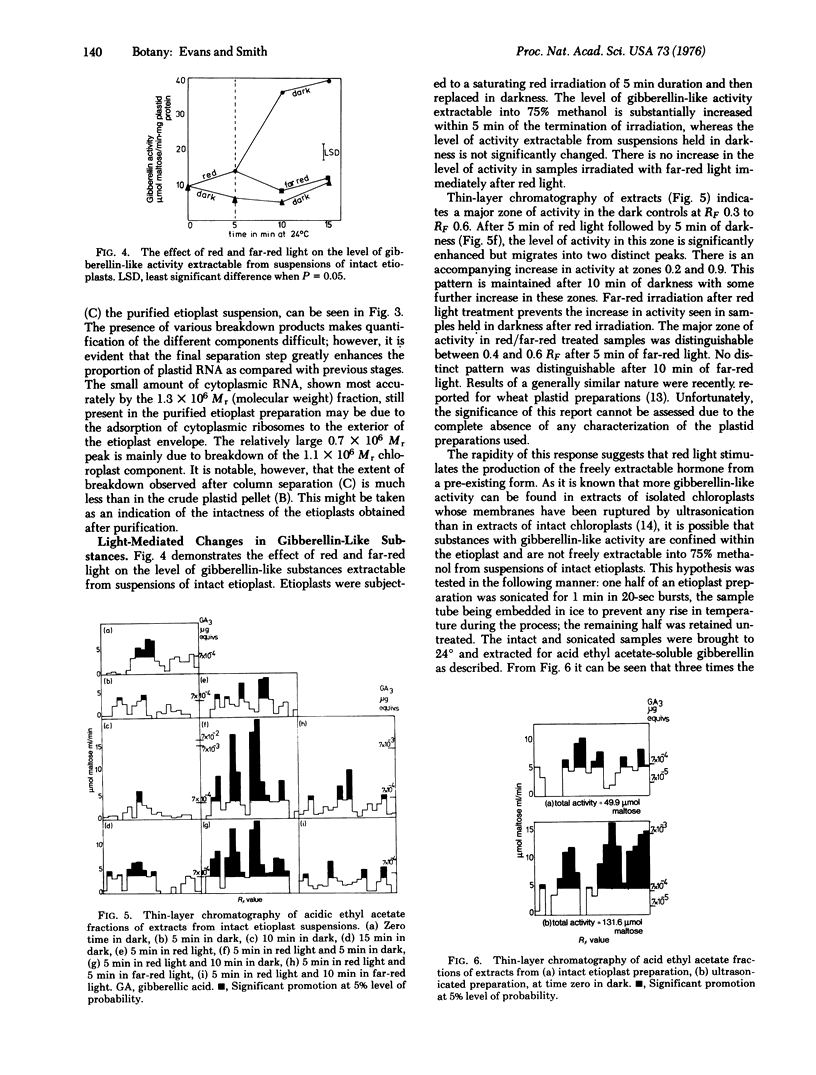
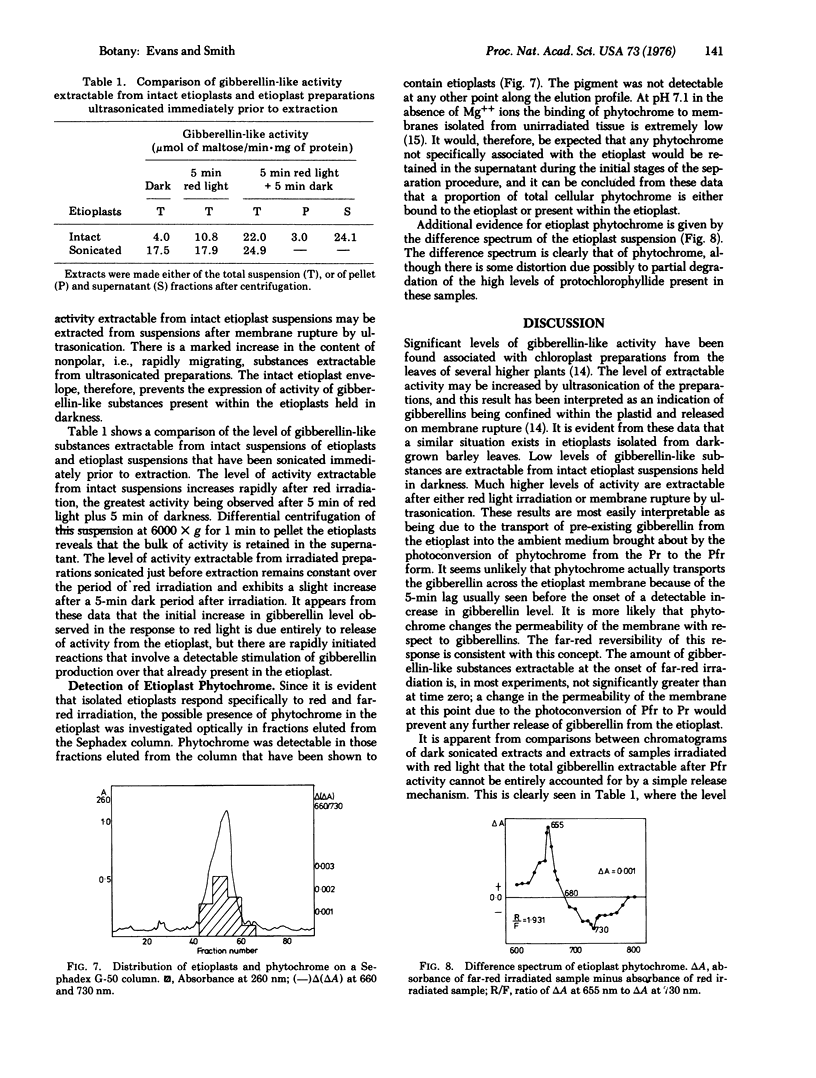

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booms in the stratosphere. Nature. 1970 Aug 15;227(5259):655–656. doi: 10.1038/227655a0. [DOI] [PubMed] [Google Scholar]
- Galston A. W. Microspectrophotometric evidence for phytochrome in plant nuclei. Proc Natl Acad Sci U S A. 1968 Oct;61(2):454–460. doi: 10.1073/pnas.61.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks S. B., Borthwick H. A. The function of phytochrome in regulation of plant growth. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2125–2130. doi: 10.1073/pnas.58.5.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M., Ingle J. The interpretation of studies on rapidly labeled ribonucleic Acid in higher plants. Plant Physiol. 1973 Feb;51(2):412–414. doi: 10.1104/pp.51.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Manabe K., Furuya M. Phytochrome-dependent Reduction of Nicotinamide Nucleotides in the Mitochondrial Fraction Isolated from Etiolated Pea Epicotyls. Plant Physiol. 1974 Mar;53(3):343–347. doi: 10.1104/pp.53.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmé D., Boisard J., Briggs W. R. Binding properties in vitro of phytochrome to a membrane fraction. Proc Natl Acad Sci U S A. 1973 Dec;70(12 Pt 1-2):3861–3865. doi: 10.1073/pnas.70.12.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux S. J., Yguerabide J. Photoreversible conductance changes induced by phytochrome in model lipid membranes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):762–764. doi: 10.1073/pnas.70.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanada T. A rapid photoreversible response of barley root tips in the presence of 3-indoleacetic Acid. Proc Natl Acad Sci U S A. 1968 Feb;59(2):376–380. doi: 10.1073/pnas.59.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]