Abstract
Background
Previous studies showed that exercise in cancer patients is feasible and may reduce fatigue and improve physical fitness and quality of life. However, many previous studies had methodological weaknesses related to trial design, sample size, comparison group, outcome measures, short follow-up durations and programme content.
Purpose
This paper aims to present the rationale and design of the clinical research subprogramme of the Alpe d’HuZes Cancer Rehabilitation (A-CaRe) programme.
Method
A-CaRe Clinical Research includes four randomized controlled trials in patients: (a) after chemotherapy, (b) during chemotherapy, (c) after stem cell transplantation and (d) during childhood cancer. These trials compare high-intensity resistance and endurance exercise interventions with usual care or a waiting list control group. In two studies, a second intervention arm consisting of low-to-moderate intensity exercise is included. All four A-CaRe trials use similar methods.
Results
Outcome measures are carefully chosen based on the International Classification of Functioning Disability and Health model. Measurements will be performed prior to randomization (T0), after completion of the intervention (T1) and at follow-up (T2). The primary outcome measures are cardiorespiratory fitness, muscle strength and fatigue. Secondary outcome measures include health-related quality of life and psychosocial functioning. Furthermore, cost-effectiveness and cost-utility analyses are performed from a societal perspective.
Conclusion
We hypothesize that exercise is more effective at improving physical fitness and thereby reducing fatigue and more cost-effective compared with usual care or a waiting list control group. If so, the programmes will be implemented in the Dutch clinical practice.
Keywords: Exercise, Cancer, Rehabilitation, Physical fitness, Muscle strength, Fatigue
Introduction
Recent advances in diagnosis and treatment of cancer patients have led to improved survival rates, both for children and adults. In The Netherlands, the current 5-year survival rate across all cancers is approximately 62% for female and 56% for male patients [1]. The survival rates of childhood cancer have improved up to 75% [2, 3]. However, cancer treatment and survival are often associated with prolonged psychosocial and physical complaints [4, 5], including decreased muscle strength, reduced lean body mass, reduced cardiorespiratory fitness, bone loss and fatigue [6]. For example, approximately 70% of cancer patients report fatigue complaints during chemotherapy and/or radiotherapy [7–9], and even years after the end of therapy, fatigue is still a major problem for at least 30% of cancer survivors [9]. Feelings of fatigue may result in the avoidance of activities to reduce the discomfort. However, this may result in a self-perpetuating condition of diminished activity leading to reduced physical fitness, increased muscle wasting and consequently to easy fatigue and further physical inactivity [6, 9]. This has great impact on the patient’s quality of life [7, 9]. This is also true for survivors of childhood cancer [10]. Geenen et al. reported that 75% of childhood cancer survivors had at least one adverse health effect after a median follow-up of 17 years [11]. Late health effects include adverse general and mental health, activity limitations, functional impairments [2], reduced physical fitness, increased fatigue and reduced health-related quality of life (HRQoL) [12].
Several literature reviews summarized the available scientific literature on the effects of exercise interventions in adult cancer patients and survivors [4, 13–18]. These studies suggest that cancer patients may benefit from physical exercise both during and after treatment. The suggested beneficial effects include improved physical performance, self-reported functioning and psychological and social well-being, as well as reduced fatigue and increased quality of life [4, 13, 14, 16–18]. The majority of the studies focused on patients with breast cancer, and fewer studied colorectal, lung and prostate cancers [13, 15, 16]. A recent literature review of Liu et al. [15] reported that physical exercise interventions were also feasible to conduct in haematological cancer patients and that results on physical fitness, HRQoL and psychological well-being were encouraging [15].
However, evidence of the beneficial effects of exercise in cancer patients and survivors appeared to be limited due to poor to moderate methodological quality of the studies. Most studies were not randomized controlled trials (RCTs), did not include appropriate control groups and/or were based on small sample sizes. Furthermore, most exercise interventions were sub-optimal regarding exercise physiological aspects: Exercise programmes were relatively short in duration (less than 12 weeks) and did not systematically promote maintenance of physical activity among the patients after the programme, and most studies included only low-intensity aerobic exercise, such as walking or cycling, rather than resistance exercise and high-intensity exercise [13, 16, 19]. Since muscle atrophy is a common problem in cancer patients [20], it seems advisable to include resistance exercises as well. With appropriate training stimuli, skeletal muscles can show great adaptability even in case of severe muscle atrophy and fatigue [6].
In The Netherlands, a cancer rehabilitation programme ‘Recovery & Stability’ [21, 22] exists. This is a 12-week supervised self-management exercise programme of low-to-moderate intensity that combines endurance and resistance exercises with group sports activities. The Recovery & Stability programme showed improvements in physical fitness [21] and quality of life [23], which sustained 9 months post-intervention [23], but the study did not include a no-exercise control group. Another cancer rehabilitation programme in The Netherlands included high-intensity resistance and endurance training after chemotherapy treatment and showed improved physical performance and quality of life in cancer patients compared with a historical control group [19]. These effects persisted at 1 year follow-up [24].
In summary, previous studies showed that exercise-based rehabilitation programmes of moderate or high intensity are feasible and well tolerated by adult cancer patients and survivors. However, RCTs including adequate sample sizes, an appropriate control group and valid and reliable outcome measures are limited. Furthermore, no studies have examined the cost-effectiveness of any type of exercise programme in cancer patients. Therefore, a cancer rehabilitation research programme was proposed to and approved by the Dutch Cancer Society, financed through this society by the so-called Alpe d’HuZes foundation (www.opgevenisgeenoptie.nl), a cancer research fund. This Alpe d’HuZes Cancer Rehabilitation (A-CaRe) programme includes a clinical research subprogramme (A-CaRe Clinical Research) in addition to subprogrammes aiming at patient empowerment and public relations. The primary objectives of A-CaRe Clinical Research are to evaluate the effectiveness of state-of-the-art exercise interventions with respect to physical fitness and fatigue and secondarily HRQoL in specific cancer patient and survivor groups and to evaluate the cost-effectiveness of these interventions. The present paper describes the design of the A-CaRe Clinical Research programme.
Methods
A-CaRe Clinical Research includes four RCTs with follow-up periods up to 1 year focusing on different subgroups: (a) exercise after chemotherapy [25], (b) exercise during chemotherapy [26], (c) exercise after stem cell transplantation [27] and (d) exercise during childhood cancer [28] (Table 1).
Table 1.
Intervention and control arms of the A-CaRe trials
| 1. Exercise after chemotherapy | 2. Exercise during chemotherapy | 3. Exercise after SCT | 4. Exercise during childhood cancer | |
|---|---|---|---|---|
| Intervention duration | 12 weeks | Depending on duration of chemotherapy | 18 weeks | 12 weeks |
| Intervention arm 1 | High-intensity resistance and endurance exercise | High-intensity resistance and endurance exercise | High-intensity resistance and endurance exercise | High-intensity resistance and endurance exercise |
| Intervention arm 2 | Low-to-moderate intensity resistance and endurance exercise | Low-to-moderate intensity physical activity programme | – | – |
| Control arm | Waiting lista | Usual care | Usual careb | Usual care |
SCT stem cell transplantation
aAfter 12 weeks, patients will start with the high-intensity resistance and endurance programme or the light-to-moderate intensity exercise programme, depending on which programme they have been allocated to
bCurrently, 10–20% of patients after SCT participate in the Recovery & Stability programme, in most cases starting 6 months or longer after transplantation
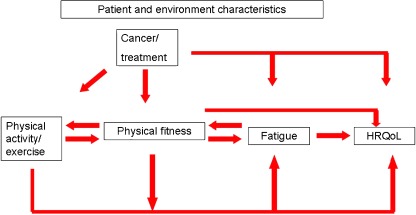
The design of the A-CaRe trials is based on a conceptual model, presented in Fig. 1. According to this model, exercise improves physical fitness (cardiorespiratory fitness and muscle strength), which improves fatigue and subsequently also physical function and HRQoL. Physical fitness may also directly influence physical function and HRQoL.
Fig. 1.
Conceptual model of the A-CaRe trials
In the A-CaRe trials, exercise interventions will be compared with either a waiting list control group or usual care. All four A-CaRe trials will use similar methods.
Study Population
Potentially eligible patients will be screened by the treating physician for the presence of comorbid conditions that would contraindicate participation in a physical exercise programme. This includes patients who are wheelchair dependent or not able to perform basic physical activities like walking or cycling, patients with contraindications for physical activity or exercise (i.e. serious orthopaedic conditions that would hamper functional recovery, serious cardiovascular or cardiopulmonary risks), patients with serious psychiatric or cognitive problems or severe emotional instability, patients suffering from malnutrition (evidenced by an unintended weight loss of more than 5% per month or more than 10% unintended weight loss during the previous 6 months), patients not being familiar with the Dutch language, patients who are unable to follow exercise instructions and patients participating in concurrent studies or rehabilitation programmes containing physical activity or exercise. Due to the focus on different patient populations, each A-CaRe study has its own inclusion criteria. Table 2 presents the inclusion criteria for each RCT, as well as additional trial-specific exclusion criteria.
Table 2.
Number of patients, participating hospitals and in- and exclusion criteria of the A-CaRe trials
| Project | # of patients | Inclusion criteria | Specific exclusion criteriaa | Participating hospitals |
|---|---|---|---|---|
| 1. Exercise after chemotherapy | 400 | Histological confirmed breast, colon, ovarian cancer or lymphomas with no indication of recurrent or progressive disease | Maxima Medical Center Veldhoven/Eindhoven, Catharina Hospital Eindhoven, Elckerlieck Hospital Helmond, Sint Anna Hospital, Geldrop, VU University Medical Center Amsterdam | |
| Aged between 18 and 70 years | ||||
| Completion of (adjuvant) chemotherapy with curative intention and completion of surgical treatment or radiotherapy | ||||
| 2. Exercise during chemotherapy | 360 | Histological confirmed primary breast of primary colon cancer who are scheduled to undergo adjuvant chemotherapy | Amstelland Hospital, Antoni van Leeuwenhoek Hospital, Bovenij Hospital, Flevohospital, Medical Center Alkmaar, Onze Lieve Vrouwe Gasthuis, Rode Kruis Hospital Beverwijk, Sint Lucas Andreas Hospital, Spaarne Hospital Hoofddorp, VU University Medical Center Amsterdam, Waterland Hospital, Zaans Medical Center | |
| 3. Exercise after SCT | 120 | Haematological malignancies undergoing high dose chemotherapy and autologous SCT | Multiple myeloma undergoing a tandem autologous–allogeneic SCT | Academic Medical Centre Amsterdam, Antoni van Leeuwenhoek Hospital Amsterdam, University Medical Center Utrecht, Antonius Hospital Nieuwegein, Haga Hospital The Hague |
| Multiple myeloma in first line; Hodgkin’s lymphoma or non-Hodgkin’s lymphoma in first relapse | Extensive osteolytic lesions with risk of fracture | |||
| Aged between 18 and 65 years | Severe infections | |||
| Sufficiently recovered from the SCT and having peripheral blood recovery | ||||
| 4. Exercise during childhood cancer | 100 | Aged 8–18 years at the time of intervention | Bone marrow transplantation | Centre for Paediatric Oncology and Haematology of VU University Medical Center, Wilhelmina Children’s Hospital University Medical Centre Utrecht, Emma Children’s Hospital Academic Medical Center Amsterdam |
| Diagnosed with any type of childhood malignancy | Growth hormone treatment | |||
| Treated with chemo- and or radiotherapy | ||||
| No longer than 12 months off treatment |
SCT Stem cell transplantation
aAll four studies exclude patients who are wheelchair dependent or not able to perform basic activities like walking or cycling, patients with contraindications for physical activity or exercise (i.e. serious orthopaedic conditions that would hamper functional recovery, serious cardiovascular or cardiopulmonary risks), patients with serious psychiatric or cognitive problems or severe emotional instability, patients suffering from malnutrition (evidenced by an unintended weight loss of more than 5% per month or more than 10% unintended weight loss during the previous 6 months), patients not being familiar with the Dutch language, patients who are unable to follow exercise instructions and patients participating in concurrent studies or rehabilitation programmes containing physical activity or exercise
Exercise Interventions
Table 1 presents the intervention and control arms of all four A-CaRe trials. In general, the exercise interventions consist of high-intensity resistance and endurance exercises under supervision of a physical therapist twice a week, with a duration of 60 min. Furthermore, all interventions include a behavioural motivation component aimed at increasing motivation and compliance to physical exercise. Patients who completed treatment trained for 12 weeks. The Recovery & Stability programme showed that an intervention duration of 12 weeks was sufficient to achieve beneficial effects on physical fitness and HRQoL [21]. Also De Backer et al. [19] showed the largest improvements in physical fitness to occur in the first 12 weeks training. However, patients after stem cell transplantation who are treated more aggressively are at increased risk for persistent complaints [29]; they are more likely to have lower levels of physical fitness and higher levels of fatigue and consequently may need more time to recover. Therefore, for these patients, the intervention duration was extended to 18 weeks.
In general, the high-intensity resistance programme will consist of exercises targeting the large muscle groups of the upper and lower extremities. Resistance exercises are performed at 65% to 80% of the one repetition maximum (1-RM), consisting of two sets of 10–15 repetitions. Every 4 weeks, the training progress is evaluated by means of an indirect 1-RM test, and the training intensity is adjusted accordingly.
The high-intensity endurance exercises are performed at an intensity of 65% of the maximal workload of the steep ramp test [30]. This corresponds to a score of 15 on the Borg scale for ratings of perceived exertion [31]. Endurance exercises are mainly performed on a cycle ergometer. Additionally, other modes of endurance, such as rowing, will be used depending on the patients’ preferences.
A behavioural motivation component is included to improve compliance and stimulate physical activity outside the exercise programme. Patients are encouraged to be moderately physically active for at least 30 min, three times per week in addition to the supervised programme. After completion of the exercise intervention, patients are encouraged to be moderately physically active for at least 30 min five times per week. Specific programme elements include the provision of general and motivational information, both verbally and via folders, about physical activity and provision of specific advice about the desired intensity of activity based on the Borg scale of rating perceived exertion.
In the studies evaluating the effectiveness of exercise interventions after and during chemotherapy, a second intervention arm is included consisting of low-to-moderate intensity exercise. Detailed descriptions of the interventions are presented elsewhere [25–28].
Assessments and Outcome Measures
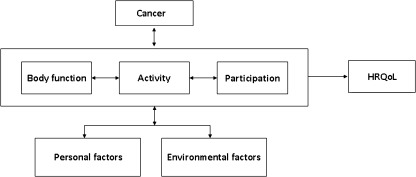
All four A-CaRe trials use similar outcome measures, which are carefully chosen based on the International Classification of Functioning, Disability and Health (ICF) of the World Health Organization [32]. The ICF provides a useful framework for classifying the components of health and consequences of a disease. According to the ICF, the consequences of a disease, in this case cancer (i.e. the type of cancer and its treatment), may concern body functions and structures, as well as the performance of activities and participation in life situations. Health states and the development of disability are modified by contextual factors including personal factors, such as sociodemographic data, and environmental factors, such as societal attitudes and social support [32]. Furthermore, cancer and its associated impairments, activity limitations and participation restrictions may have consequences for HRQoL (Fig. 2). In the ICF classification, the letters b, s, d and e refer to the following components of the classification: body functions, body structures, activities and participation and environmental factors. The hierarchical code system of the ICF consists of the abbreviation of the component and the chapter number (e.g. b4 Functions of the cardiovascular, haematological, immunological and respiratory systems), followed by the second level (e.g. b455 Exercise tolerance functions), the third level (e.g. b4551 Aerobic capacity) and possibly a fourth level. The use of a lower-level (more detailed level) category automatically implies that the higher-level category is also applicable.
Fig. 2.
The International Classification of Functioning, Disability and Health (ICF) model
In oncology rehabilitation, the ICF framework is suggested to be useful for selection of outcome measures [33]. In A-CaRe Clinical Research, the outcome measures are carefully selected to assess body functions as well as activities and participation. Table 3 presents an overview of the outcome measures and instrumentation that will be used in A-CaRe Clinical Research, classified according to the ICF. Primary outcome measures are cardiorespiratory fitness, muscle strength and fatigue. Secondary outcome measures include body composition, health-related quality of life, physical activity, mood and sleep disturbances, participation and autonomy, return to work and adverse events.
Table 3.
The outcome measures and instruments used in the A-CaRe trials, classified according to the ICF
| International Classification of Functioning, Disability and Health | |||||
|---|---|---|---|---|---|
| Second level | Third level | Adults | Children | ||
| Body functions | |||||
| Chapter 1 | Mental functions | ||||
| b122 | Global psychosocial functions | CBCL, YRS | |||
| b126 | Temperament and personality functions | CBCL, YRS, CBSA/CBSK (behavioural conduct) | |||
| b130 | Energy drive and functions | MFI, FQL | PedsQL Multidimensional Fatigue Scale Acute version | ||
| b134 | Sleep functions | PSQI | |||
| b152 | Emotional functions | HADS | CDI | ||
| b280 | Sensations of pain | EORTC QLQ-C30 symptom scale | |||
| Chapter 4 | Functions of the cardiovascular, haematological, immunological and respiratory systems | ||||
| b410–b429 | Cardiovascular system functions | Heart rate | Heart rate | ||
| b440–b449 | Respiratory system functions | Respiratory rate | Respiratory rate | ||
| b455 | Exercise tolerance functions | ||||
| b4551 | Aerobic capacity | PeakVO2 | PeakVO2 | ||
| b4552 | Fatigability | MFI | |||
| Chapter 5 | Functions related to the digestive, metabolic and respiratory systems | ||||
| b530 | Weight maintenance | BMI, skinfolds, hip and waist circumference, DEXA scan | BMI, DEXA scan | ||
| Chapter 7 | Neuromusculoskeletal and movement-related functions | ||||
| b730 | Muscle power function | ||||
| b7304 | Power of muscles of all limbs | Upper extremity: handgrip strength | Handheld dynamometer for upper and lower extremity | ||
| Lower extremity: 30 s chair stand test | |||||
| Activities and participation | |||||
| Chapter 4 | Mobility | ||||
| d410 | Changing basic body position | ||||
| d4103 | Sitting | 30-s chair stand test | |||
| d4104 | Standing | 30-s chair stand test | |||
| d450 | Walking | PASE and accelerometer | Accelerometer | ||
| d460 | Moving around in different locations | IPA (mobility and leisure) | |||
| d4600 | Moving around within the home | IPA (mobility and leisure) | |||
| d4601 | Moving around within buildings other than home | IPA (mobility and leisure) | |||
| d4602 | Moving around outside the home and other buildings | IPA (mobility and leisure) | |||
| d470 | Using transportation | d4700 | Using human-powered vehicles | PASE | |
| Chapter 5 | Self-care | ||||
| d510 | Washing oneself | IPA (autonomy in self-care) | |||
| d530 | Toileting | IPA (autonomy in self-care) | |||
| d540 | Dressing | IPA (autonomy in self-care) | |||
| d550 | Eating | IPA (autonomy in self-care) | |||
| d560 | Drinking | IPA (autonomy in self-care) | |||
| d570 | Looking after one’s health | IPA (autonomy in self-care) | |||
| Chapter 6 | Domestic life | ||||
| d620 | Acquisition of goods and services | ||||
| d6200 | Shopping | IPA (family role) | |||
| d640 | Doing housework | PASE, IPA (family role) | |||
| d6402 | Cleaning living area | IPA (family role) | |||
| d650 | Caring for household objects | IPA (family role) | |||
| Chapter 7 | Interpersonal interactions and relationships | ||||
| d750 | Informal social relationships | IPA (social relations) | CBSA/CBSK (close friendships) | ||
| d7502 | Informal relationships with co-inhabitants | IPA (social relations) | |||
| d760 | Family relationships | IPA (social relations) | |||
| d770 | |||||
| d7702 | Sexual relationships | IPA (social relations) | |||
| Chapter 8 | Major life areas | ||||
| d810–d839 | Education | Level of education | Level of education, return to school | ||
| CBSA/CBSK (scholastic competence) | |||||
| d815 | Preschool education | Level of education | Level of education, return to school | ||
| d820 | School education | Level of education | Level of education, return to school | ||
| d825 | Vocational training | Level of education | Level of education, return to school | ||
| d830 | Higher education | Level of education | Level of education, return to school | ||
| d840–d959 | Work and Employment | Type of employment, return to work | |||
| d850 | Remunerative employment | Type of employment, return to work | |||
| d850 | Self-employment | Type of employment, return to work | |||
| d8501 | Part-time employment | ||||
| d8502 | Full-time employment | Type of employment, return to work | |||
| d855 | Non-remunerative employment | Type of employment, return to work | |||
| d870 | Economic self-sufficiency | IPA (family role) | IPA (family role) | ||
| d880 | Engagement in play | Accelerometer | |||
| Chapter 9 | Community, social and civic life | ||||
| d920 | Recreation and leisure | IPA (mobility and leisure) | IPA (mobility and leisure) | ||
| d9200 | Play | Accelerometer | |||
| d9201 | Sports | PASE and accelerometer | Accelerometer | ||
BMI body mass index, CBCL Child Behaviour Checklist, CBSA self-perception profile for adolescents, CBSK self-perception profile for children, CDI Child’s Depression Inventory, DEXA dual energy X-ray, EORTC QLQ-C30 European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire C30, FQL Fatigue Quality list, HADS Hospital Anxiety and Depression Scale, IPA Impact on Participation and Autonomy, MFI Multidimensional Fatigue Inventory, PSQI Pittsburgh Sleep Quality Index, VO 2 oxygen uptake
All outcome measures are assessed at baseline, prior to randomization (T0), at completion of the intervention (T1) and at follow-up (T2). Follow-up measurements will be performed 12 months after completion of the intervention. In the RCT evaluating exercise during chemotherapy, T2 measurements take place 6 months after completion of the intervention.
Primary Outcome Measures
Cardiorespiratory Fitness
Cardiorespiratory fitness is measured during a maximal exercise test on an electronically braked cycle ergometer according to a ramp protocol [34], in which the resistance gradually increases every 6 s aiming to achieve the maximum within 8 to 12 min. For children and adolescents, the Godfrey protocol is used [35]. In this protocol, patients will begin pedalling at 0 W for 1 min, and the workload increases by 10, 15 or 20 W each minute depending on the patients’ height and clinical status [35]. All patients are instructed to cycle with a pedal frequency between 70 and 80 rpm and are encouraged to continue exercising until exhaustion, or inability to maintain the pedal frequency of 70 rpm. Expired gases are collected and analysed breath by breath for O2, CO2 and volume. The average values of the last 30 s of exercise are used as measures for peak oxygen uptake (peakVO2, in litres per minute), peak power output (peakW, in watt) and peak heart rate (HR). Ventilatory threshold is determined by using the oxygen equivalent method [36]. HR and respiratory exchange ratio are used as objective criteria for peak exercise.
In the RCT evaluating exercise during chemotherapy, peakVO2 cannot be determined directly, due to logistic reasons. Therefore, an estimation of peakVO2 is made based on the steep ramp test using a linear regression equation [30]. This has been shown to be a reliable (ICC = 0.996) and valid method to estimate cardiorespiratory fitness in cancer patients [30]. In the other three A-CaRe trials, the steep ramp test is performed for adjustments of training intensities.
Muscle Strength
Upper extremity muscle strength of adults is measured using a JAMAR grip strength dynamometer. Handgrip can be used to characterize general upper extremity muscle strength [37–39] and can increase after general upper extremity resistance training including exercises that did not specifically involve handgrip strength [40].
Lower extremity muscle strength of adults is tested by the functional 30-s chair stand test. This test is a valid and reliable measure of lower extremity strength in adults [41]. Patients are asked to stand upright from a chair with the arms folded across the chest, then to sit down again and repeat the action over a 30-s period. The number of times that the patient rises to a full stand from the seated position within 30 s is recorded [42–44].
For children and adolescents, upper and lower extremity muscle strength is assessed using a handheld dynamometer. Upper extremity muscles include grip, shoulder abductor and wrist extensor strength. In the lower extremity, muscle strength of the hip flexors and the knee and dorsal foot extensors is measured. Three consecutive measurements are performed using the ‘break method’, in which the examiner gradually overcomes the muscle force and stops at the moment the extremity gives way [45]. The highest value will be registered.
Fatigue
In adults, fatigue symptoms are assessed with the Multidimensional Fatigue Inventory (MFI) [46]. The MFI contains 20 items, organized into five scales: general fatigue, physical fatigue, reduced activity, reduced motivation and mental fatigue. The MFI subscales have good internal consistency (average Cronbach’s alpha = 0.84) [47].
In addition, adults’ perception and appraisal of experienced fatigue are assessed with the Fatigue Quality List (FQL) [48]. The FQL consists of 25 adjectives describing the fatigue experience, organized into four subscales: frustrating, exhausting, pleasant and frightening.
Secondary Outcome Measures
Fatigue in Children
In children, fatigue is assessed by the 18-item Paediatric Quality of Life Inventory (PedsQL) Multidimensional Fatigue Scale Acute Version, which is designed to measure both the child’s and parents’ perception of fatigue in paediatric patients [49]. It consists of three subscales: general fatigue (six items), sleep rest fatigue (six items) and cognitive fatigue. Both parent and child reports were shown to be valid and reliable in childhood cancer [49].
Mood Disturbances
In adults, mood disturbances are assessed with the 14-item Hospital Anxiety and Depression Scale (HADS) [50, 51]. It yields a total score and separate scale scores for anxiety and depression. Numerous studies have applied the HADS to assess distress among cancer patients [52–54]. Furthermore, the questionnaire has been validated for use in the Dutch population [55].
The Children’s Depression Inventory is used to assess symptoms of depression in children and adolescents with cancer. Overall, this questionnaire has good internal consistency and test–retest reliability and a positive correlation with clinicians’ independent global depression ratings [56].
Sleep Disturbances
Sleep disturbances are assessed with the Pittsburgh Sleep Quality Index (PSQI), an 18-item, self-rated questionnaire assessing the quality of sleep and sleep disturbances over a month [57]. A total score is derived as well as seven subscales that include subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication and daytime dysfunction. Scores ≥ 5 on the PSQI total scale, computed as the sum of the seven subscales are associated with clinically significant sleep disturbances [57].
Health-Related Quality of Life
In adults, HRQoL is assessed with the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30), a questionnaire specifically developed to asses HRQoL in cancer patients [58]. It consists of 30 items, organized into five functional scales (physical, role, emotional, cognitive, social), three symptom scales (pain, fatigue and emesis) and an overall quality of life scale. Additional single items address other symptoms commonly experienced by cancer patients (e.g. insomnia, diarrhoea, constipation etc.). Validity and reliability of the questionnaire have been established [58].
HRQoL in children and adolescents with cancer is assessed by child self-report and parent-proxy report using the PedsQL 4.0 Generic Core Scale and the PedsQL 3.0 Cancer module. The PedsQL 4.0 Generic Core Scale is a 23-item questionnaire encompassing physical, emotional, social and school functioning domains. The PedsQL 3.0 Cancer module is a 27-item multidimensional cancer-specific questionnaire that encompasses eight scales: pain and hurt, nausea, procedural anxiety, treatment anxiety, worry, cognitive problems, perceived physical appearance and communication. Validity and reliability of both child and parent reports of the PedsQL Generic Core Scale and the Cancer Module has been shown [49, 59].
Self-Perception and Behavioural Problems in Children and Adolescents
The Dutch version of the Self-Perception profile for children (CBSK) and adolescents (CBSA) is used to assess self-perception of scholastic competence, social acceptance, physical appearance, behavioural conduct, global self-worth and close friendships. The questionnaire has good reliability and validity when used in children 8 years and older [60, 61].
Internalizing and externalizing behavioural problems are assessed using the Dutch translated and validated Child Behaviour Checklist (CBCL) for children younger than 11 years. The CBCL is a valid and reliable instrument to assess the parents’ evaluation of internalizing and externalizing behaviour problems of children [62]. For children aged 11 to 18 years old, the Youth Self Report is used.
Functioning in Daily Life
The Impact on Participation and Autonomy (IPA) Questionnaire is used to assess functioning in daily life of adults [63]. The IPA consists of 32 items assessing perceived level of participation and autonomy, organized into five domains: autonomy in the home, family role, autonomy outside of the home, social relations and work and education. An additional nine items assess perceived problems with participation and autonomy. Internal consistency of the five domain scores range from 0.81 to 0.91.
Physical Activity
Objective levels of physical activity are assessed using an accelerometer, a small and lightweight device, which detects accelerations. Patients wear the accelerometer on the right hip attached to a belt for 4–7 days, including at least one weekend day. Although accelerometers may underestimate some activities, such as cycling and water activities, it is recognised as a reasonably valid tool to objectively assess physical activity in adults [64], as well as in children [65–67]. Accelerations are converted into activity counts per minute, indicating the level of physical activity.
Self-reported physical activity of adults is assessed using the Physical Activity Scale for the Elderly (PASE). The PASE is a brief, self-administered 7-day recall questionnaire, which consists of questions on leisure time, household and work-related physical activities [68]. The frequency of activities is recorded as never, seldom (1 to 2 days/week), sometimes (3 to 4 days/week) or often (5–7 days/week). The duration of activities is categorized as less than 1 h, between 1 and 2 h, between 2 and 4 h or more than 4 h. Paid or unpaid work, except for work that involves mostly sitting activities such as office work, is categorized as less than 1 h, between 1 and 4 h, between 5 and 8 h or more than 8 h [69]. The total activity score is computed by multiplying the amount of time spent on each activity (in hours per week) by the empirically derived item weights and summing over all activities [68]. In healthy adults, the PASE has shown to have high test–retest reliability (r = 0.84) [68] and reasonable validity as compared with the doubly labelled water method (r = 0.58) [70]. Results from our institution showed that the PASE had good to excellent test-retest reliability in cancer patients and good content validity [71].
Body Composition
Body height and body weight are assessed in all patients, and BMI is calculated. In addition, fat mass, muscle mass and bone mineral density will be assessed by whole body dual energy X-ray scans in adults, children and adolescents, except for participants from the exercise intervention during chemotherapy, due to logistic reasons. In adults, also waist and hip circumferences and thickness of four skinfolds (biceps, triceps, suprailiacal, subscapular) are assessed.
Return to Work or School
The following indicators of return to work (RTW) or school (RTS) are measured using self-reported calendars: time to partial and to full RTW or RTS expressed in number of calendar days between the end of treatment and the first day at work, time to full RTW or RTS corrected for partial RTW or RTS and partial and full RTW or RTS rate at T0, T1 and T2.
Covariates
Sociodemographic and Clinical Data
Sociodemographic data, including age, (parental) education, marital status, living situation, work (or school) status, medication use and lifestyle variables (e.g. smoking, physical activity prior to diagnosis), are collected at baseline.
Clinical information is collected from the medical records and includes date of diagnosis, stage and subtype of disease, treatment history, type and dose of chemotherapy and/or radiotherapy and adverse events during treatment. During the follow-up period, data on disease status (response to treatment, progression or relapse) and data on any additional treatment are collected.
Moderating Variables
At baseline, a series of questions is asked to assess potential moderating variables including pre-illness lifestyle (frequency, nature and intensity of physical activity and exercise behaviour, or avoidance thereof), current attitudes towards and beliefs about exercise in general and exercise during or after treatment. Information about behavioural, normative and control beliefs about exercise, attitude towards exercise, subjective norm, perceived behavioural control and intention is collected using standardized questions as described by Courneya et al. [72, 73]. These questions have previously been used to evaluate exercise programmes in cancer patients and survivors and are based on established health behaviour theories, in particularly the Theory of Planned Behaviour [74].
Adherence
Compliance with the interventions is assessed by self-report, and attendance and exercise logs filled in by physical therapists and psychologist (e.g. observed attendance at and compliance with the exercise). Non-responders and dropouts receive a short questionnaire to assess the reason for non-participation or dropping out of the study.
Satisfaction with Intervention
After completion of the intervention programmes, patients are asked to complete a brief questionnaire addressing the perceived efficacy of and satisfaction with the programme, whether they would suggest any changes to the programme and if they would recommend it to other patients undergoing similar treatments.
Adverse Effects
Adverse effects of the rehabilitation programmes in adult patients are actively monitored during the study with a special emphasis on increased fatigue, reported by the patients, physiotherapists, sports physicians or trainers and checked in the medical records. In addition, in patients from the RCTs that evaluate training after chemotherapy and after stem cell transplantation, neurotoxicity is evaluated using the Chemotherapy-Induced Peripheral Neuropathy (CIPN20) questionnaire [75]. This is an EORTC quality of life questionnaire that is specifically developed to assess chemotherapy-induced peripheral neuropathy.
Costs from a Societal Perspective
Besides the costs of the exercise programmes, data on health care costs, patient and family costs and costs of production losses are collected using monthly cost diaries measured on a three-monthly basis during the entire follow-up period. Health care costs include the costs of oncological care, general practice care and physiotherapy, additional visits to other health care providers, prescription of medication, professional home care and hospitalization. Patient and family costs include out-of-pocket expenses such as travel expenses and costs for paid and unpaid help. Costs related to production losses include work absenteeism for patients (or parents) with paid jobs and days of inactivity for patients (or parents) without a paid job.
Utilities are measured using the EuroQol (EQ5D) [76]. The EQ5D is a health-related quality of life measure that provides a single index of an individual’s quality of life. It consists of five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety and depression. Each dimension is rated as ’no problem’, ‘some problem’ or ‘extreme problem’, resulting in 243 possible health states [76]. Dutch tariffs will be used to value these health states [77].
Statistical Analyses
Data will be analysed on an intention-to-treat basis, in which all patients will be included in the group they were allocated to by randomization. In addition, a per-protocol analysis will be performed in which we only include participants who completed the intervention they were allocated to according to the protocol. Completing the intervention is defined as having attained 75% of all training sessions.
Multi-level longitudinal regression analysis will be conducted to assess between-group differences in each outcome measure. The follow-up value will be defined as the dependent variable and the following levels will be used: (1) time of follow-up measurement (values corresponding with performance at T1 and T2), (2) training centre and (3) individual. Regression coefficients indicate differences between intervention and control groups. Regression models will be adjusted for the baseline values of the respective outcome measures. Missing values will be avoided as much as possible by asking participants to comply to the post-test and follow-up measurement even after they drop out from the exercise programme. Missing values will be accounted for in the mixed linear regression modelling.
Costs are valued using the guidelines published in the updated handbook for economic evaluation in The Netherlands, issued by the Dutch Health Care Insurance Board [78]. Both incremental cost-effectiveness and cost–utility analyses are performed. The cost-effectiveness ratio is calculated by dividing the difference between the mean total costs of the exercise and control groups by the difference in mean effects of the group(s) [79]. The primary clinical effect measures of the trials will be included in the cost-effectiveness analyses. The cost–utility ratio expresses the additional costs of the intervention compared with the control group per quality adjusted life years. Cost-effectiveness and cost–utilities ratios are estimated using bootstrapping techniques and uncertainty of these ratios graphically presented on cost-effectiveness and cost–utility planes, and acceptability curves [79, 80]. Sensitivity analyses on the most important cost drivers are performed in order to assess the robustness of results.
Discussion
This paper presents the design and methods of four randomized controlled trials on exercise-based rehabilitation programmes included in the A-CaRe Clinical Research programme. These studies are designed to evaluate the effectiveness and cost-effectiveness of exercise-based rehabilitation programmes in different cancer patient and survivor groups. The outcome measures of the studies are carefully chosen based on the ICF, and the instrumentation is standardized, valid and reliable. It is hypothesized that exercise-based rehabilitation programmes are more effective at improving cardiorespiratory fitness and muscle strength, and thereby reducing fatigue, and more cost-effective compared with usual care or a waiting list control group. In addition, we compare the results of high-intensity training to low-to-moderate intensity training.
Unlike cardiovascular diseases or other chronic conditions, exercise-based rehabilitation programmes are currently not part of standard health care for cancer patients and survivors in The Netherlands. Before being able to implement cancer rehabilitation programmes on a large scale, the efficacy of such programmes need to be established. This is the main aim of the A-CaRe programme. In addition to the evaluation of the effectiveness and cost-effectiveness of the exercise interventions, we need to gain insight in how (mediators), for whom and under what circumstances (moderators) these interventions are effective. Insight in mediators and moderators of exercise interventions is essential to be able to tailor cancer rehabilitation programmes to the needs, preferences and characteristics of individual cancer patients. With the large database resulting from the four A-CaRe trials, we will be able to explore several mediators and moderators of exercise-based cancer rehabilitation programmes.
In conclusion, the four A-CaRe trials evaluate the effectiveness and the cost-effectiveness of various exercise-based rehabilitation programmes in cancer patients and survivors, in comparison with usual care or no treatment.
Acknowledgments
This study is supported by the Alpe d’HuZes/KWF Fund. The research grant is provided by the Dutch Cancer Society. The contribution of L.M. Buffart was further supported by a fellowship granted by the EMGO Institute for Health and Care Research. In addition, the authors acknowledge the A-CaRe Clinical Research group (www.a-care.org): from the VU University Medical Center Amsterdam, EMGO Institute for Health and Care Research: J. Brug (Ph.D.), M.J.M. Chinapaw (Ph.D.), L.M. Buffart (Ph.D.), W. van Mechelen (MD, Ph.D.) and C.S. Kampshoff (M.Sc.); from the Department of Pediatric Oncology/Hematology: G.J.L. Kaspers (MD, Ph.D.), E. van Dulmen-den Broeder (Ph.D.), M. Veening (MD, Ph.D.) and K.I. Braam (M.Sc.); from the Department of Medical Psychology: J. Huisman (Ph.D.) and E.M. van Dijk (MA); from The Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital (NKI-AVL) Amsterdam: N.K. Aaronson (Ph.D.), W.H. van Harten (MD, Ph.D.), G. Sonke (MD, Ph.D.), M.M. Stuiver (PT, M.Sc.) and H. van Waart (M.Sc.); from the University Medical Center Utrecht, Child Development & Exercise Center: T. Takken (Ph.D.); from Máxima Medical Center Veldhoven: G. Schep (MD, Ph.D.) and S. Houterman (Ph.D.); from the Academic Medical Center, Amsterdam, Department of Rehabilitation, F. Nollet (MD, Ph.D.) and S. Persoon (M.Sc.) and from the Department of Hematology: M.J. Kersten (MD, Ph.D.).
Competing interests
The authors declare that they have no competing interests.
Disclosure of interest
All authors have no financial relationship with the organization that sponsored the research. They have full control of all primary data and that they agree to allow the journal to review their data if requested.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- A-CaRe
Alpe d’HuZes Cancer Rehabilitation
- HRQoL
Health-related quality of life
- peakVO2
Peak oxygen uptake
- RCT
Randomized controlled trial
Footnotes
Laurien Buffart and Mai Chinapaw share first authorship since they have equally contributed to this manuscript.
Trial registration
This study is registered at the Netherlands Trial Register: NTR2153 (study 1), NTR2159 (study 2), NTR2341 (study 3) and NTR1531 (study 4).
Contributor Information
Mai J. M. Chinapaw, Phone: +31-20-4448203, FAX: +31-20-4448387, Email: m.chinapaw@vumc.nl
Laurien M. Buffart, Email: l.buffart@vumc.nl
Willem van Mechelen, Email: w.vanmechelen@vumc.nl.
Goof Schep, Email: g.schep@mmc.nl.
Neil K. Aaronson, Email: n.aaronson@nki.nl
Wim H. van Harten, Email: w.v.harten@nki.nl
Martijn M. Stuiver, Email: m.stuiver@nki.nl
Marie José Kersten, Email: m.j.kersten@amc.uva.nl.
Frans Nollet, Email: f.nollet@amc.uva.nl.
Gertjan J. L. Kaspers, Email: gjl.kaspers@vumc.nl
Eline van Dulmen-den Broeder, Email: e.vanDulmen-denBroeder@vumc.nl.
Jaap Huisman, Email: drj.huisman@vumc.nl.
Tim Takken, Email: t.takken@umcutrecht.nl.
Maurits van Tulder, Email: maurits.van.tulder@falw.vu.nl.
Johannes Brug, Email: j.brug@vumc.nl.
References
- 1.IKCnet (2009) Dutch Cancer Registration. Survival. http://wwwikcnetnl
- 2.Hudson MM, Mertens AC, Yasui Y, Hobbie W, Chen H, Gurney JG, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA. 2003;290(12):1583–1592. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- 3.Reedijk AM, Janssen-Heijnen ML, Louwman MW, Snepvangers Y, Hofhuis WJ, Coebergh JW. Increasing incidence and improved survival of cancer in children and young adults in Southern Netherlands, 1973–1999. Eur J Cancer. 2005;41(5):760–769. doi: 10.1016/j.ejca.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 4.Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: a literature review. Ann Behav Med. 1999;21(2):171–179. doi: 10.1007/BF02908298. [DOI] [PubMed] [Google Scholar]
- 5.Courneya KS. Exercise in cancer survivors: an overview of research. Med Sci Sports Exerc. 2003;35(11):1846–1852. doi: 10.1249/01.MSS.0000093622.41587.B6. [DOI] [PubMed] [Google Scholar]
- 6.Lucia A, Earnest C, Perez M. Cancer-related fatigue: can exercise physiology assist oncologists? Lancet Oncol. 2003;4(10):616–625. doi: 10.1016/S1470-2045(03)01221-X. [DOI] [PubMed] [Google Scholar]
- 7.Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5(5):353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 8.de Jong N, Courtens AM, bu-Saad HH, Schouten HC. Fatigue in patients with breast cancer receiving adjuvant chemotherapy: a review of the literature. Cancer Nurs. 2002;25(4):283–297. doi: 10.1097/00002820-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Dimeo FC. Effects of exercise on cancer-related fatigue. Cancer. 2001;92(6 Suppl):1689–1693. doi: 10.1002/1097-0142(20010915)92:6+<1689::AID-CNCR1498>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 10.Smith M, Hare ML. An overview of progress in childhood cancer survival. J Pediatr Oncol Nurs. 2004;21(3):160–164. doi: 10.1177/1043454204264407. [DOI] [PubMed] [Google Scholar]
- 11.Geenen MM, Cardous-Ubbink MC, Kremer LC, van den Bos C, van der Pal HJ, Heinen RC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297(24):2705–2715. doi: 10.1001/jama.297.24.2705. [DOI] [PubMed] [Google Scholar]
- 12.Van Brussel M, Takken T, Lucia A, van der Net J, Helders PJ. Is physical fitness decreased in survivors of childhood leukemia? A systematic review. Leukemia. 2005;19(1):13–17. doi: 10.1038/sj.leu.2403547. [DOI] [PubMed] [Google Scholar]
- 13.Galvao DA, Newton RU. Review of exercise intervention studies in cancer patients. J Clin Oncol. 2005;23(4):899–909. doi: 10.1200/JCO.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 14.Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. J Clin Oncol. 2005;23(16):3830–3842. doi: 10.1200/JCO.2005.02.148. [DOI] [PubMed] [Google Scholar]
- 15.Liu RD, Chinapaw MJ, Huijgens PC, van Mechelen W. Physical exercise interventions in haematological cancer patients, feasible to conduct but effectiveness to be established: a systematic literature review. Cancer Treat Rev. 2009;35(2):185–192. doi: 10.1016/j.ctrv.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Stevinson C, Lawlor DA, Fox KR. Exercise interventions for cancer patients: systematic review of controlled trials. Cancer Causes Control. 2004;15(10):1035–1056. doi: 10.1007/s10552-004-1325-4. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomark Prev. 2005;14(7):1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 18.Watson T, Mock V. Exercise as an intervention for cancer-related fatigue. Phys Ther. 2004;84(8):736–743. [PubMed] [Google Scholar]
- 19.De Backer I, van Breda E, Vreugdenhil A, Nijziel MR, Kester AD, Schep G. High-intensity strength training improves quality of life in cancer survivors. Acta Oncol. 2007;46(8):1143–1151. doi: 10.1080/02841860701418838. [DOI] [PubMed] [Google Scholar]
- 20.Argiles JM, Busquets S, Felipe A, Lopez-Soriano FJ. Molecular mechanisms involved in muscle wasting in cancer and ageing: cachexia versus sarcopenia. Int J Biochem Cell Biol. 2005;37(5):1084–1104. doi: 10.1016/j.biocel.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 21.May AM, van Weert E, Korstjens I, Hoekstra-Weebers JE, van der Schans CP, Zonderland ML, et al. Improved physical fitness of cancer survivors: a randomised controlled trial comparing physical training with physical and cognitive-behavioural training. Acta Oncol. 2008;47(5):825–834. doi: 10.1080/02841860701666063. [DOI] [PubMed] [Google Scholar]
- 22.van Weert E, Hoekstra-Weebers JE, May AM, Korstjens I, Ros WJ, van der Schans CP. The development of an evidence-based physical self-management rehabilitation programme for cancer survivors. Patient Educ Couns. 2008;71(2):169–190. doi: 10.1016/j.pec.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 23.May AM, Korstjens I, van Weert E, van den Borne B, Hoekstra-Weebers JE, van der Schans CP, et al. Long-term effects on cancer survivors’ quality of life of physical training versus physical training combined with cognitive-behavioral therapy: results from a randomized trial. Support Care Cancer. 2009;17(6):653–663. doi: 10.1007/s00520-008-0519-9. [DOI] [PubMed] [Google Scholar]
- 24.De Backer I, Vreugdenhil G, Nijziel MR, Kester AD, van Breda E, Schep G. Long-term follow-up after cancer rehabilitation using high-intensity resistance training: persistent improvement of physical performance and quality of life. Br J Cancer. 2008;99(1):30–36. doi: 10.1038/sj.bjc.6604433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kampshoff CS, Buffart LM, Schep G, van Mechelen W, Brug J, Chinapaw MJ. Design of the Resistance and Endurance exercise After ChemoTherapy (REACT) study: a randomized controlled trial to evaluate the effectiveness and cost-effectiveness of exercise interventions after chemotherapy on physical fitness and fatigue. BMC Cancer. 2010;10:658. doi: 10.1186/1471-2407-10-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Waart H, Stuiver MM, van Harten WH, Sonke GS, Aaronson NK. Design of the Physical exercise during Adjuvant Chemotherapy Effectiveness Study (PACES): a randomized controlled trial to evaluate effectiveness and cost-effectiveness of physical exercise in improving physical fitness and reducing fatigue. BMC Cancer. 2010;10:673. doi: 10.1186/1471-2407-10-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Persoon S, Kersten MJ, Chinapaw MJ, Buffart LM, Burghout H, Schep G, et al. Design of the EXercise Intervention after Stem cell Transplantation (EXIST) study: a randomized controlled trial to evaluate the effectiveness and cost-effectiveness of an individualized high intensity physical exercise program on fitness and fatigue in patients with multiple myeloma or (non-) Hodgkin’s lymphoma treated with high dose chemotherapy and autologous stem cell transplantation. BMC Cancer. 2010;10:671. doi: 10.1186/1471-2407-10-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braam KI, van Dijk EM, Veening MA, Bierings MB, Merks JH, Grootenhuis MA, et al. Design of the Quality of Life in Motion (QLIM) study: a randomized controlled trial to evaluate the effectiveness and cost-effectiveness of a combined physical exercise and psychosocial training program to improve physical fitness in children with cancer. BMC Cancer. 2010;10:624. doi: 10.1186/1471-2407-10-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gielissen MF, Schattenberg AV, Verhagen CA, Rinkes MJ, Bremmers ME, Bleijenberg G. Experience of severe fatigue in long-term survivors of stem cell transplantation. Bone Marrow Transplant. 2007;39(10):595–603. doi: 10.1038/sj.bmt.1705624. [DOI] [PubMed] [Google Scholar]
- 30.De Backer I, Schep G, Hoogeveen A, Vreugdenhil G, Kester AD, van Breda E. Exercise testing and training in a cancer rehabilitation program: the advantage of the steep ramp test. Arch Phys Med Rehabil. 2007;88(5):610–616. doi: 10.1016/j.apmr.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 32.World Health Organisation . ICF-international classification of functioning, disability and health. Geneva: WHO; 2001. [Google Scholar]
- 33.Gilchrist LS, Galantino ML, Wampler M, Marchese VG, Morris GS, Ness KK. A framework for assessment in oncology rehabilitation. Phys Ther. 2009;89(3):286–306. doi: 10.2522/ptj.20070309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anonymous. Clinical exercise testing with reference to lung diseases: indications, standardization and interpretation strategies. ERS Task Force on Standardization of Clinical Exercise Testing. European Respiratory Society. Eur Respir J. 1997;(11):2662–89. [DOI] [PubMed]
- 35.Godfrey S. Exercise testing in children. London: Saunders; 1974. [Google Scholar]
- 36.Wasserman K. Critical capillary PO2 and the role of lactate production in oxyhemoglobin dissociation during exercise. Adv Exp Med Biol. 1999;471:321–333. doi: 10.1007/978-1-4615-4717-4_39. [DOI] [PubMed] [Google Scholar]
- 37.Bohannon RW. Hand-grip dynamometry provides a valid indication of upper extremity strength impairment in home care patients. J Hand Ther. 1998;11(4):258–260. doi: 10.1016/S0894-1130(98)80021-5. [DOI] [PubMed] [Google Scholar]
- 38.Bohannon RW. Is it legitimate to characterize muscle strength using a limited number of measures? J Strength Cond Res. 2008;22(1):166–173. doi: 10.1519/JSC.0b013e31815f993d. [DOI] [PubMed] [Google Scholar]
- 39.Bohannon RW. Dynamometer measurements of grip and knee extension strength: are they indicative of overall limb and trunk muscle strength? Percept Mot Skills. 2009;108(2):339–342. doi: 10.2466/pms.108.2.339-342. [DOI] [PubMed] [Google Scholar]
- 40.Magnussen Thomas E, Sahlberg M, Svantesson U. The effect of resistance training on handgrip strength in young adults. Isokinet Exerc Sci. 2009;16:125–131. [Google Scholar]
- 41.Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7:129–161. [Google Scholar]
- 42.Csuka M, McCarty DJ. Simple method for measurement of lower extremity muscle strength. Am J Med. 1985;78(1):77–81. doi: 10.1016/0002-9343(85)90465-6. [DOI] [PubMed] [Google Scholar]
- 43.Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70(2):113–119. doi: 10.1080/02701367.1999.10608028. [DOI] [PubMed] [Google Scholar]
- 44.Rikli RE, Jones CJ. Functional fitness normative scores for community-residing older adults, ages 60–94. J Aging Phys Act. 1999;7:162–181. [Google Scholar]
- 45.Backman E. Methods for measurement of muscle function. Methodological aspects, reference values for children, and clinical applications. Scand J Rehabil Med Suppl. 1988;20:9–95. [PubMed] [Google Scholar]
- 46.Smets EM, Garssen B, Bonke B, de Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–325. doi: 10.1016/0022-3999(94)00125-O. [DOI] [PubMed] [Google Scholar]
- 47.Smith KB, Pukall CF. An evidence-based review of yoga as a complementary intervention for patients with cancer. Psychooncology. 2009;18(5):465–475. doi: 10.1002/pon.1411. [DOI] [PubMed] [Google Scholar]
- 48.Gielissen MF, Knoop H, Servaes P, Kalkman JS, Huibers MJ, Verhagen S, et al. Differences in the experience of fatigue in patients and healthy controls: patients’ descriptions. Health Qual Life Outcomes. 2007;5:36. doi: 10.1186/1477-7525-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer. 2002;94(7):2090–2106. doi: 10.1002/cncr.10428. [DOI] [PubMed] [Google Scholar]
- 50.Snaith P, Zigmond AS. Anxiety and depression in general medical settings. BMJ. 1988;297(6662):1544. doi: 10.1136/bmj.297.6662.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 52.Adamsen L, Quist M, Midtgaard J, Andersen C, Moller T, Knutsen L, et al. The effect of a multidimensional exercise intervention on physical capacity, well-being and quality of life in cancer patients undergoing chemotherapy. Support Care Cancer. 2006;14(2):116–127. doi: 10.1007/s00520-005-0864-x. [DOI] [PubMed] [Google Scholar]
- 53.Browall M, Ahlberg K, Karlsson P, Danielson E, Persson LO, Gaston-Johansson F. Health-related quality of life during adjuvant treatment for breast cancer among postmenopausal women. Eur J Oncol Nurs. 2008;12(3):180–189. doi: 10.1016/j.ejon.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Thorsen L, Skovlund E, Stromme SB, Hornslien K, Dahl AA, Fossa SD. Effectiveness of physical activity on cardiorespiratory fitness and health-related quality of life in young and middle-aged cancer patients shortly after chemotherapy. J Clin Oncol. 2005;23(10):2378–2388. doi: 10.1200/JCO.2005.04.106. [DOI] [PubMed] [Google Scholar]
- 55.Spinhoven P, Ormel J, Sloekers PP, Kempen GI, Speckens AE, van Hemert AM. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27(2):363–370. doi: 10.1017/S0033291796004382. [DOI] [PubMed] [Google Scholar]
- 56.Kovacs M. Rating scales to assess depression in school-aged children. Acta Paedopsychiatr. 1981;46(5–6):305–315. [PubMed] [Google Scholar]
- 57.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 58.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 59.Varni JW, Limbers CA, Burwinkle TM. Impaired health-related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5:43. doi: 10.1186/1477-7525-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Treffers PDA, Goedhart AW, Veerman JW, van den Berg BRH, Ackaert L, De Rycke L. De competentiebelevingsschaal voor adolescenten CBSA. Handleiding. Lisse: Swets Test; 2003. [Google Scholar]
- 61.Veerman JW, Straathof MAE, Treffers PDA, van den Berg BRH, Ten Brink LT. De competentiebelevingsschaal voor kinderen CBSK. Handleiding. Lisse: Swets & Zeitlinger; 1997. [Google Scholar]
- 62.Verhulst FC, Koot JM, Akkerhuis GW, Veerman JW. Praktische handleiding voor de CBCL (Child Behavior Checklist) Assen/Maastricht: van Gorcum; 1990. [Google Scholar]
- 63.Cardol M, Beelen A, van den Bos GA, De Jong BA, de Groot IJ, de Haan RJ. Responsiveness of the Impact on Participation and Autonomy questionnaire. Arch Phys Med Rehabil. 2002;83(11):1524–1529. doi: 10.1053/apmr.2002.35099. [DOI] [PubMed] [Google Scholar]
- 64.Bassett DR, Jr, Ainsworth BE, Swartz AM, Strath SJ, O’Brien WL, King GA. Validity of four motion sensors in measuring moderate intensity physical activity. Med Sci Sports Exerc. 2000;32(9 Suppl):S471–S480. doi: 10.1097/00005768-200009001-00006. [DOI] [PubMed] [Google Scholar]
- 65.Esliger DW, Tremblay MS. Technical reliability assessment of three accelerometer models in a mechanical setup. Med Sci Sports Exerc. 2006;38(12):2173–2181. doi: 10.1249/01.mss.0000239394.55461.08. [DOI] [PubMed] [Google Scholar]
- 66.Pfeiffer KA, McIver KL, Dowda M, Almeida MJ, Pate RR. Validation and calibration of the Actical accelerometer in preschool children. Med Sci Sports Exerc. 2006;38(1):152–157. doi: 10.1249/01.mss.0000183219.44127.e7. [DOI] [PubMed] [Google Scholar]
- 67.Puyau MR, Adolph AL, Vohra FA, Butte NF. Validation and calibration of physical activity monitors in children. Obes Res. 2002;10(3):150–157. doi: 10.1038/oby.2002.24. [DOI] [PubMed] [Google Scholar]
- 68.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 69.Washburn RA, Zhu W, McAuley E, Frogley M, Figoni SF. The physical activity scale for individuals with physical disabilities: development and evaluation. Arch Phys Med Rehabil. 2002;83(2):193–200. doi: 10.1053/apmr.2002.27467. [DOI] [PubMed] [Google Scholar]
- 70.Schuit AJ, Schouten EG, Westerterp KR, Saris WH. Validity of the Physical Activity Scale for the Elderly (PASE): according to energy expenditure assessed by the doubly labeled water method. J Clin Epidemiol. 1997;50(5):541–546. doi: 10.1016/S0895-4356(97)00010-3. [DOI] [PubMed] [Google Scholar]
- 71.Liu RD, Buffart LM, Kersten MJ, Spiering M, Brug J, van Mechelen W, Chinapaw MJ. Psychometric properties of two physical activity questionnaires, the AQuAA and the PASE, in cancer patients. BMC Med Res Methodol 2011;11:30. [DOI] [PMC free article] [PubMed]
- 72.Courneya KS. Understanding readiness for regular physical activity in older individuals: an application of the theory of planned behavior. Health Psychol. 1995;14(1):80–87. doi: 10.1037/0278-6133.14.1.80. [DOI] [PubMed] [Google Scholar]
- 73.Courneya KS, Friedenreich CM. Utility of the theory of planned behavior for understanding exercise during breast cancer treatment. Psychooncology. 1999;8(2):112–122. doi: 10.1002/(SICI)1099-1611(199903/04)8:2<112::AID-PON341>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 74.Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process. 1999;50:179–211. doi: 10.1016/0749-5978(91)90020-T. [DOI] [Google Scholar]
- 75.Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer. 2005;41(8):1135–1139. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 76.Uyl-de Groot CA, Rutten FF, Bonsel GJ. Measurement and valuation of quality of life in economic appraisal of cancer treatment. Eur J Cancer. 1994;30A(1):111–117. doi: 10.1016/S0959-8049(05)80030-9. [DOI] [PubMed] [Google Scholar]
- 77.Lamers LM, Stalmeier PF, McDonnell J, Krabbe PF, van Busschbach JJ. Measuring the quality of life in economic evaluations: the Dutch EQ-5D tariff. Ned Tijdschr Geneeskd. 2005;149(28):1574–1578. [PubMed] [Google Scholar]
- 78.Oostenbrink JB. Handbook for cost studies: methods and standard costs for economic evaluation in health care. Updated version. The Hague: Dutch Health Insurance Counsel; 2004. [Google Scholar]
- 79.Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. New York: Oxford University Press; 2005. [Google Scholar]
- 80.Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Mak. 1998;18(2 Suppl):S68–S80. doi: 10.1177/0272989X9801800209. [DOI] [PubMed] [Google Scholar]