Abstract
Tobacco smoking is a risk factor for atrial fibrillation (AF), but little is known about the impact of smoking in patients with AF. Of the 4060 patients with recurrent AF in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial, 496 (12%) reported having smoked during the past two years. Propensity scores for smoking were estimated for each of the 4060 patients using a multivariable logistic regression model and were used to assemble a matched cohort of 487 pairs of smokers and nonsmokers, who were balanced on 46 baseline characteristics. Cox and logistic regression models were used to estimate the associations of smoking with all-cause mortality and all-cause hospitalization, respectively, during over 5 years of follow-up. Matched participants had a mean age of 70 ± 9 years (± S.D.), 39% were women, and 11% were non-white. All-cause mortality occurred in 21% and 16% of matched smokers and nonsmokers, respectively (when smokers were compared with nonsmokers, hazard ratio = HR = 1.35; 95% confidence interval = 95% CI = 1.01–1.81; p = 0.046). Unadjusted, multivariable-adjusted and propensity-adjusted HR (95% CI) for all-cause mortality associated with smoking in the pre-match cohort were: 1.40 (1.13–1.72; p = 0.002), 1.45 (1.16–1.81; p = 0.001), and 1.39 (1.12–1.74; p = 0.003), respectively. Smoking had no association with all-cause hospitalization (when smokers were compared with nonsmokers, odds ratio = OR = 1.21; 95% CI = 0.94–1.57, p = 0.146). Among patients with AF, a recent history of smoking was associated with an increased risk of all-cause mortality, but had no association with all-cause hospitalization.
Keywords: Atrial fibrillation, Smoking, Mortality, Propensity score
1. Introduction
Tobacco smoking is a risk factor for cardiovascular disease (CVD) and continuing smoking is associated with poor outcomes in those with CVD (Aronow, 1971, 1978; Aronow et al., 1974; Suskin et al., 2001; Yatsuya et al., 2010). AF is also common and is associated with poor outcomes (Aronow et al., 1989). Smoking has also been described as a risk factor for AF (Goette et al., 2007; Heeringa et al., 2008). However, little is known about the impact of smoking on outcomes in AF. The objective of the current study is to evaluate the association between smoking and outcomes in the patients with AF.
2. Subjects and methods
2.1. Data source and patients
The AFFIRM was a randomized clinical trial to compare two treatment strategies in patients with AF, the details of which have been described before (The AFFIRM Investigators, 2002; Wyse et al., 2002). Briefly, 4060 patients with recurrent AF were enrolled between November 1995 and October 1999 from 213 centers and were randomized to either rate control (n = 2027) or rhythm control (n = 2033) groups. We used a public-use copy of the AFFIRM data obtained from the National Heart, Lung and Blood Institute, which also funded the study. Of the 4060 AFFIRM participants, 496 (12%) self-reported a baseline history of smoking during the past two years. Data on demographic and clinical variables including past medical history, medication use, and symptoms during AF were also collected at baseline.
2.2. Outcomes
The primary outcomes for the current analysis is all-cause mortality during about 6 years of follow-up, also the primary end point of AFFIRM. A secondary outcome was all-cause hospitalization. All events were centrally adjudicated by committees blinded to randomization.
2.3. Propensity score matching
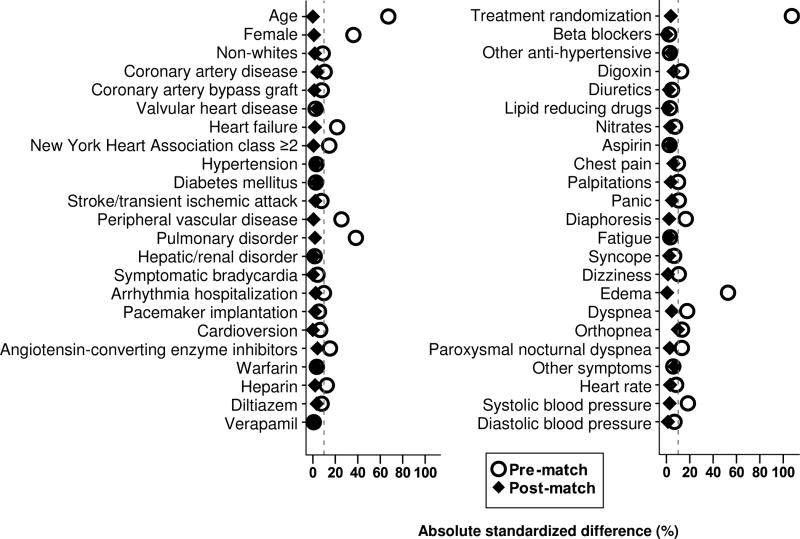
We used propensity score matching to assemble a cohort of smokers and non-smokers who would be balanced on all measured baseline characteristics (Rosenbaum and Rubin, 1983; Rubin, 2001). We estimated propensity scores for smoking for each of the 4060 patients using a non-parsimonious multivariable logistic regression model (Ahmed et al., 2006a,b, 2007, 2008; Ahmed and Aronow, 2008; Alper et al., 2009; Ekundayo et al., 2009a,b; Wahle et al., 2009, Bowling et al., 2010). In the model, we used baseline smoking as the dependent variable, and 46 other baseline characteristics displayed in Figure 1 were entered as covariates. Using a 1 to 1 greedy matching algorithm, we were able to match 487 (98% of the 496) smokers with 487 non-smokers who had similar propensity scores. Pre- and post-match absolute standardized differences are presented as Love plots (Figure 1) (Ahmed et al., 2006a,b, 2007, 2008; Ahmed and Aronow, 2008; Alper et al., 2009; Ekundayo et al., 2009; Wahle et al., 2009; Bowling et al., 2010). An absolute standardized difference of 0% on a covariate indicates no residual bias for that covariate, and values < 10% suggest inconsequential bias (Normand, 2001; Austin, 2008).
Figure 1.
Absolute standardized differences between baseline characteristics of smoker versus non-smoker older atrial fibrillation patients, before and after propensity score matching
2.4. Statistical analysis
The baseline characteristics of patients with and without a recent history of smoking were compared using Pearson’s Chi-square and Wilcoxon rank-sum tests for the pre-match cohort and McNemar’s test and paired sample t-tests for the post-match cohort as appropriate. The association of smoking with mortality was estimated using Kaplan–Meier survival analysis and Cox regression analysis. We repeated our analysis in the full pre-match cohort of 4060 participants using three different approaches: unadjusted, propensity score adjusted and multivariable adjusted. The multivariable model was adjusted for all covariates used in the propensity score model. Finally, we conducted subgroup analyses and tested interactions to determine any heterogeneity in the association between smoking and all-cause mortality. A formal sensitivity analysis was conducted to quantify the degree of a hidden bias that could invalidate our main conclusions (Rosenbaum, 2002). Due to lack of detailed time to event data for all-cause hospitalization, we used logistic regression to estimate the association of smoking with all-cause hospitalization. The mean follow-up time for smokers and nonsmokers was similar (3.4 years each). All tests were two-tailed, and a p =< 0.05 was considered to be statistically significant. SPSS for Windows (Version 18) was used for data analysis.
3. Results
3.1. Patient characteristics
Matched participants had a mean age of 70 ± 9 years, 39% were women, and 11% were non-white. Before matching, compared with nonsmokers, smokers were younger, more likely to be female, and were also more likely to have peripheral arterial disease and pulmonary disease (Table 1). These and other baseline imbalances were balanced after matching (Table 1 and Figure 1).
Table 1.
Baseline patient characteristics by history of smoking before and after propensity matching, mean ± S.D., n (%)
| Variable | Before propensity matching | After propensity matching | ||||
|---|---|---|---|---|---|---|
| Nonsmokers (n = 3564) | Smokers (n = 496) | p | Nonsmokers (n = 487) | Smoker (n = 487) | p | |
| Age, years | 70 ± 8 | 65 ± 8 | <0.001 | 65 ± 9 | 65 ± 8 | 1.000 |
| Female | 1472 (41) | 122 (25) | <0.001 | 122 (25) | 120 (25) | 0.935 |
| Non-whites | 392 (11) | 69 (14) | 0.055 | 69 (14) | 66 (14) | 0.852 |
| NYHAa Class II or higher | 306 (9) | 65 (13) | 0.001 | 63 (13) | 64 (13) | 1.000 |
| Randomization to rate control | 1778 (50) | 255 (51) | 0.525 | 244 (50) | 253 (52) | 0.614 |
| Past medical history | ||||||
| Hypertension | 2531 (71) | 345 (70) | 0.503 | 331 (68) | 337 (69) | 0.735 |
| Coronary artery disease | 1339 (38) | 212 (43) | 0.026 | 214 (44) | 204 (42) | 0.566 |
| Heart failure | 783 (22) | 156 (32) | <0.001 | 145 (30) | 149 (31) | 0.822 |
| Diabetes mellitus | 709 (20) | 104 (21) | 0.575 | 107 (22) | 101 (21) | 0.698 |
| Cerebrovascular events | 464 (13) | 78 (16) | 0.097 | 80 (16) | 76 (16) | 0.789 |
| Pulmonary disease | 453 (13) | 138 (28) | <0.001 | 126 (26) | 130 (27) | 0.813 |
| Valvular heart disease | 439 (12) | 65 (13) | 0.618 | 68 (14) | 62 (13) | 0.651 |
| Symptomatic bradycardia | 253 (7) | 30 (6) | 0.389 | 30 (6) | 30 (6) | 1.000 |
| Peripheral arterial disease | 215 (6) | 67 (14) | <0.001 | 63 (13) | 62 (13) | 1.000 |
| Hepatic/renal disease | 201 (6) | 30 (6) | 0.713 | 28 (6) | 29 (6) | 1.000 |
| Cardioversion | 1464 (41) | 220 (44) | 0.165 | 214 (44) | 214 (44) | 1.000 |
| Coronary bypass surgery | 457 (13) | 51 (10) | 0.109 | 53 (11) | 51 (11) | 0.914 |
| Pacemaker implantation | 225 (6) | 25 (5) | 0.269 | 23 (5) | 25 (5) | 0.885 |
| Hospitalization for arrhythmia | 1642 (46) | 253 (51) | 0.039 | 253 (52) | 247 (51) | 0.752 |
| Symptoms during atrial fibrillationb | ||||||
| Fatigue | 1982 (56) | 284 (57) | 0.489 | 274 (56) | 280 (58) | 0.745 |
| Dyspnea | 1896 (53) | 307 (62) | <0.001 | 288 (59) | 299 (61) | 0.499 |
| Palpitation | 1741 (49) | 267 (54) | 0.038 | 250 (51) | 259 (53) | 0.615 |
| Dizziness | 1200 (34) | 192 (39) | 0.027 | 192 (39) | 189 (39) | 0.897 |
| Chest pain | 833 (23) | 137 (28) | 0.038 | 142 (29) | 129 (27) | 0.397 |
| Diaphoresis | 702 (20) | 132 (27) | <0.001 | 133 (27) | 128 (26) | 0.778 |
| Leg swelling | 671 (19) | 105 (21) | 0.214 | 104 (21) | 103 (21) | 1.000 |
| Orthopnea | 513 (14) | 96 (19) | 0.004 | 75 (15) | 93 (19) | 0.145 |
| Paroxysmal nocturnal dyspnea | 254 (7) | 54 (11) | 0.003 | 46 (9) | 50 (10) | 0.744 |
| Panic | 360 (10) | 67 (14) | 0.020 | 75 (15) | 67 (14) | 0.519 |
| Syncope | 152 (4) | 15 (3) | 0.192 | 17 (4) | 15 (3) | 0.860 |
| Other symptoms | 370 (10) | 43 (9) | 0.237 | 34 (7) | 43 (9) | 0.321 |
| Medications | ||||||
| Warfarin | 3020 (85) | 414 (84) | 0.464 | 401 (82) | 407 (84) | 0.656 |
| Digoxin | 1863 (52) | 290 (59) | 0.010 | 269 (55) | 284 (58) | 0.342 |
| Beta-blocker | 1521 (43) | 206 (42) | 0.629 | 200 (41) | 200 (41) | 1.000 |
| Diuretics | 1510 (42) | 222 (45) | 0.313 | 221 (45) | 216 (44) | 0.799 |
| ACEc inhibitors | 1355 (38) | 226 (46) | 0.001 | 209 (43) | 219 (45) | 0.565 |
| Diltiazem | 1083 (30) | 169 (34) | 0.096 | 173 (36) | 165 (34) | 0.642 |
| Verapamil | 372 (10) | 53 (11) | 0.866 | 54 (11) | 53 (11) | 1.000 |
| Aspirin | 944 (27) | 137 (28) | 0.592 | 127 (26) | 134 (28) | 0.669 |
| Lipid lowering agents | 796 (22) | 117 (24) | 0.531 | 114 (23) | 116 (24) | 0.937 |
| Nitrate | 656 (18) | 106 (21) | 0.113 | 95 (20) | 101 (21) | 0.693 |
| Heparin | 608 (17) | 109 (22) | 0.007 | 101 (21) | 105 (22) | 0.811 |
| Heart rate per minute | 73 ± 14 | 74 ± 15 | 0.081 | 74 ± 15 | 74 ± 15 | 0.619 |
| Systolic blood pressure, mm Hg | 135 ± 19 | 132 ± 19 | <0.001 | 131 ± 18 | 132 ± 19 | 0.672 |
| Diastolic blood pressure, mm Hg | 76 ± 10 | 76 ±10 | 0.148 | 76 ±10 | 76 ± 10 | 0.842 |
NYHA—New York Heart Association.
Symptoms associated with clinical diagnosis of atrial fibrillation.
ACE—angiotensin-converting enzyme.
3.2. Association of age and mortality
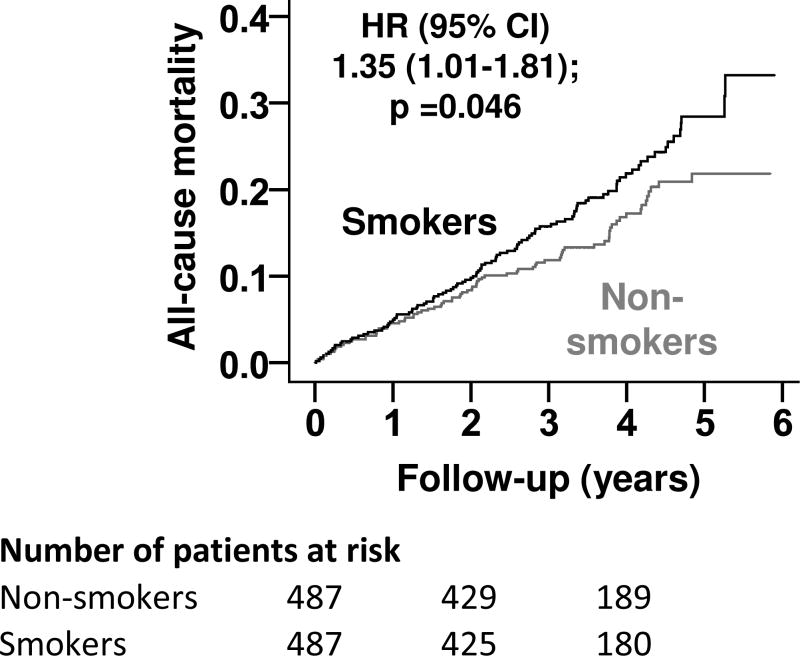
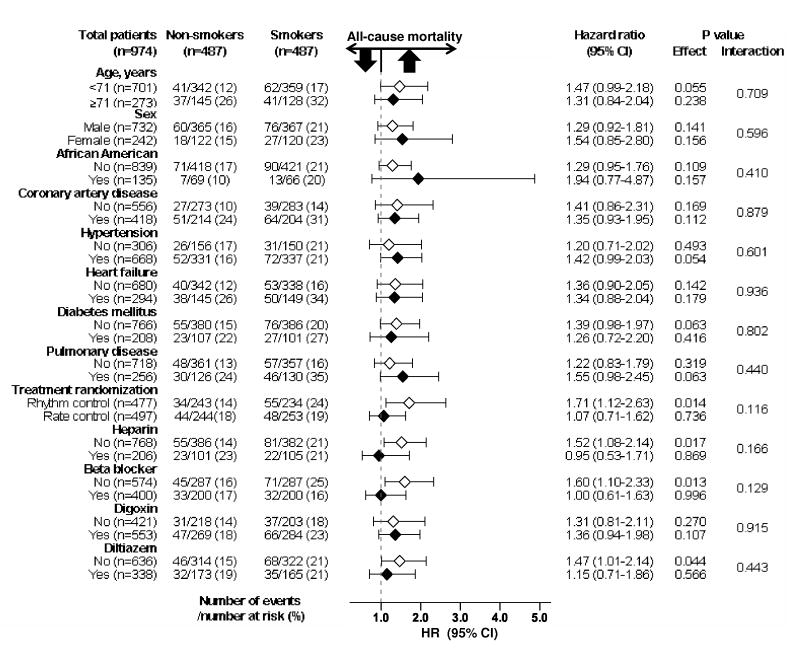
All-cause mortality occurred in 21% and 16% of matched smokers and nonsmokers, respectively (when smokers were compared with nonsmokers, hazard ratio = HR = 1.35; 95% confidence interval (CI) = 1.01–1.81; p = 0.046; Table 2 and Figure 2). In the absence of a hidden confounder, a sign-score test for matched data with censoring provides strong evidence (p < 0.0001) that non-smokers clearly outlived smokers. A hidden covariate that would be a near- perfect predictor of mortality could potentially explain away this association if it also increases the odds ratio = (OR) of smoking by 28.5%. The association between smoking and mortality was homogeneous across various subgroups of patients (Figure 3). Unadjusted, multivariable-adjusted and propensity-adjusted HR (95% CI) for all-cause mortality associated with smoking in the pre-match cohort were: 1.40 (1.13–1.72; p = 0.002), 1.45 (1.16–1.81; p = 0.001), and 1.39 (1.12–1.74; p = 0.003), respectively (Table 2). Smoking had no association with all-cause hospitalization (when smokers were compared with nonsmokers, OR = 1.21; 95% CI = 0.94– 1.57; p = 0.146).
Table 2.
Association of history of smoking with all cause mortality
| All-cause mortality | Events (%)
|
Absolute risk difference (%)a | Hazard ratio (95%CI) | p | |
|---|---|---|---|---|---|
| Nonsmokers | Smokers | ||||
| Pre-match | n = 3564 | n = 496 | |||
| Unadjusted | 560 (16%) | 106 (21%) | + 5 | 1.40 (1.13–1.72) | 0.002 |
| Multivariable-adjusted | --- | --- | -- | 1.45 (1.16–1.81) | 0.001 |
| Propensity-adjusted | --- | --- | --- | 1.39 (1.12–1.74) | 0.003 |
| Post-match | n = 487 | n = 487 | |||
| Propensity-matched | 78 (16%) | 103 (21%) | + 5 | 1.35 (1.01–1.81) | 0.046 |
Absolute risk differences were calculated by subtracting the events in non-smokers from those in smokers.
Figure 2.
Kaplan-Meier plot for all-cause mortality (HR=hazard ratio; CI=confidence interval)
Figure 3.
Association of recent smoking history with all-cause mortality in subgroups of propensity score-matched participants in the AFFIRM (HR = hazard ratio; CI = confidence interval)
4. Discussion
4.1. Key findings
Findings from the current study demonstrate that among patients with recurrent AF, a history of recent smoking was associated with increased all-cause mortality but had no association with all-cause hospitalization. The increased risk of death without an associated increased risk of hospitalization suggests that smoking-associated mortality may have been sudden in nature. Findings from our study suggest that in patients with AF, smoking continues to have an adverse effect on mortality. These findings are important as AF is common among older adults and is one of the few CVD with increasing prevalence.
4.2. Explanation of study findings
The observed association between smoking and mortality may be explained by residual or unmeasured confounding or it may imply a true association. The magnitude of the unadjusted association between smoking and mortality was rather similar to that observed after multivariable risk adjustment and propensity matching suggesting that despite many imbalances in measured baseline covariates between smokers and nonsmokers, they played minimal confounding roles. This is probably due to fact that the confounders were equally shared by smokers and non-smokers. For example, compared to smokers, nonsmokers were older and compared to nonsmokers, smokers were more likely to be male and have heart failure. It is also possible that the observed association between smoking and mortality may be due to an unmeasured confounder. However, findings from our sensitivity analysis suggest that this association was rather insensitive to an unmeasured confounder. The consistent finding of a strong, significant and rather insensitive association suggests that smoking may have an intrinsic association with mortality in patients with AF.
4.3. Literature comparison
Smoking is known to be associated with poor prognosis in patients with established CVD (Aronow, 1971; Aronow et al., 1998; Suskin et al., 2001). Smoking has been shown to inflict damage on the cardiovascular system through diverse mechanisms that include platelet dysfunction, hypercoagulative effect, lipid peroxidation, increased myocardial oxygen demand and vasomotor dysfunction (Ambrose and Barua, 2004). Smoking has also been shown to be associated with myocardial ischemia, ventricular arrhythmias, and sudden cardiac death (Davis et al., 1984; Hayano et al., 1990; Kinoshita et al., 2009).
4.4. Clinical and public health implications
Although we had no data on cause-specific mortality or hospitalization, the lack of an association between smoking and hospitalization despite a strong association with all-cause mortality suggests that smoking-related deaths may have been sudden in nature thus precluding hospitalization. Although the prevalence of coronary artery disease (CAD) was balanced in the post-match cohort, it is possible that smokers had more severe CAD or for longer duration. Interestingly, findings from our subgroup analysis suggest that the effect of smoking was rather homogeneous regardless of a history of CAD. Findings from our subgroup analyses also suggest that smoking had no association with mortality in the patients with AF who were receiving beta-blockers or were randomized to rate control strategy. Nearly 70% of the patients in the rate control group received beta-blockers at any time (vs. 50% for those in the rhythm control group) (Wyse et al., 2002). Although there was no significant interaction, these findings may have clinical implications as smoking-associated hemodynamic changes have been shown to be mediated through adrenergic mechanisms, which has been shown to be prevented by adrenergic blockade (Cryer et al., 1976; Hung et al., 1995).
4.5. Limitations
There are a few limitations to our study, which should be acknowledged. We had no data on the number of pack-years of smoking or passive smoking exposure. It is also possible that some of smokers may have quit during follow-up while some non-smokers became smokers. However, this regression dilution may have underestimated the true associations observed in our study (Clarke et al., 1999).
5. Conclusion
In the patients with AF, a recent history of smoking was associated with increased mortality but had no association with hospitalization. Patients with AF should be informed of these risks, encouraging smokers to quit and nonsmokers to maintain abstinence.
Acknowledgments
The AFFIRM is conducted and supported by the NHLBI in collaboration with the AFFIRM Investigators. This manuscript was prepared using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the AFFIRM or the NHLBI.
Dr. A. Ahmed is supported by the National Institutes of Health through grants (R01-HL085561 and R01-HL097047) from the National Heart, Lung, and Blood Institute and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama.
Footnotes
Conflict of interest statement: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed A, Aronow WS. A propensity-matched study of the association of physical function and outcomes in geriatric heart failure. Arch Gerontol Geriatr. 2008;46:161–172. doi: 10.1016/j.archger.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Husain A, Love TE, Gambassi G, Dell’Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006a;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Perry GJ, Fleg JL, Love TE, Goff DC, Jr, Kitzman DW. Outcomes in ambulatory chronic systolic and diastolic heart failure: a propensity score analysis. Am Heart J. 2006b;152:956–966. doi: 10.1016/j.ahj.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, Love TE, Aban IB, Shlipak MG. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–398. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Allman RM, Fonarow GC, Love TE, Zannad F, Dell’italia LJ, White M, Gheorghiade M. Incident heart failure hospitalization and subsequent mortality in chronic heart failure: a propensity-matched study. J Card Fail. 2008;14:211–218. doi: 10.1016/j.cardfail.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper AB, Campbell RC, Anker SD, Bakris G, Wahle C, Love TE, Hamm LL, Mujib M, Ahmed A. A propensity-matched study of low serum potassium and mortality in older adults with chronic heart failure. Int J Cardiol. 2009;137:1–8. doi: 10.1016/j.ijcard.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- Aronow WS. The effect of smoking cigarettes on the apexcardiogram in coronary heart disease. Chest. 1971;59:365–368. doi: 10.1378/chest.59.4.365. [DOI] [PubMed] [Google Scholar]
- Aronow WS. Effect of passive smoking on angina pectoris. N Engl J Med. 1978;299:21–24. doi: 10.1056/NEJM197807062990105. [DOI] [PubMed] [Google Scholar]
- Aronow WS, Cassidy J, Vangrow JS, March H, Kern JC, Goldsmith JR, Khemka M, Pagano J, Vawter M. Effect of cigarette smoking and breathing carbon monoxide on cardiovascular hemodynamics in anginal patients. Circulation. 1974;50:340–347. doi: 10.1161/01.cir.50.2.340. [DOI] [PubMed] [Google Scholar]
- Aronow WS, Gutstein H, Hsieh FY. Risk factors for thromboembolic stroke in elderly patients with chronic atrial fibrillation. Am J Cardiol. 1989;63:366–367. doi: 10.1016/0002-9149(89)90349-4. [DOI] [PubMed] [Google Scholar]
- Austin PC. Primer on statistical interpretation or methods report card on propensity-score matching in the cardiology literature from 2004 to 2006: a systematic review. Circ Cardiovasc Qual Outcomes. 2008;1:62–67. doi: 10.1161/CIRCOUTCOMES.108.790634. [DOI] [PubMed] [Google Scholar]
- Bowling CB, Pitt B, Ahmed MI, Aban IB, Sanders PW, Mujib M, Campbell RC, Love TE, Aronow WS, Allman RM, Bakris GL, Ahmed A. Hypokalemia and outcomes in patients with chronic heart failure and chronic kidney disease: findings from propensity-matched studies. Circ Heart Fail. 2010;3:253–260. doi: 10.1161/CIRCHEARTFAILURE.109.899526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]
- Cryer PE, Haymond MW, Santiago JV, Shah SD. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemo-dynamic and metabolic events. N Engl J Med. 1976;295:573–577. doi: 10.1056/NEJM197609092951101. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Hockings BE, El Dessouky MA, Hajar HA, Taylor RR. Cigarette smoking and ventricular arrhythmia in coronary heart disease. Am J Cardiol. 1984;54:282–285. doi: 10.1016/0002-9149(84)90183-8. [DOI] [PubMed] [Google Scholar]
- Ekundayo OJ, Allman RM, Sanders PW, Aban I, Love TE, Arnett D, Ahmed A. Isolated systolic hypertension and incident heart failure in older adults: a propensity-matched study. Hypertension. 2009a;53:458–465. doi: 10.1161/HYPERTENSIONAHA.108.119792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekundayo OJ, Markland A, Lefante C, Sui X, Goode PS, Allman RM, Ali M, Wahle C, Thornton PL, Ahmed A. Association of diuretic use and overactive bladder syndrome in older adults: a propensity score analysis. Arch Gerontol Geriatr. 2009b;49:64–68. doi: 10.1016/j.archger.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goette A, Lendeckel U, Kuchenbecker A, Bukowska A, Peters B, Klein HU, Huth C, Rocken C. Cigarette smoking induces atrial fibrosis in humans via nicotine. Heart. 2007;93:1056–1063. doi: 10.1136/hrt.2005.087171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano J, Yamada M, Sakakibara Y, Fujinami T, Yokoyama K, Watanabe Y, Takata K. Short- and long-term effects of cigarette smoking on heart rate variability. Am J Cardiol. 1990;65:84–88. doi: 10.1016/0002-9149(90)90030-5. [DOI] [PubMed] [Google Scholar]
- Heeringa J, Kors JA, Hofman A, Van Rooij FJ, Witteman JC. Cigarette smoking and risk of atrial fibrillation: the Rotterdam Study. Am Heart J. 2008;156:1163–1169. doi: 10.1016/j.ahj.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Hung J, Lam JY, Lacoste L, Letchacovski G. Cigarette smoking acutely increases platelet thrombus formation in patients with coronary artery disease taking aspirin. Circulation. 1995;92:2432–2436. doi: 10.1161/01.cir.92.9.2432. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Herges RM, Hodge DO, Friedman L, Ammash NM, Bruce CJ, Somers V, Malouf JF, Askelin J, Gilles JA, Gersh BJ, Friedman PA. Role of smoking in the recurrence of atrial arrhythmias after cardioversion. Am J Cardiol. 2009;104:678–682. doi: 10.1016/j.amjcard.2009.04.041. [DOI] [PubMed] [Google Scholar]
- Normand S, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- Rosenbaum PR. Sensitivity to hidden bias. In: Rosenbaum PR, editor. Observational Studies. Vol. 1. Springer-Verlag; New York: 2002. pp. 105–170. [Google Scholar]
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2:169–188. [Google Scholar]
- Suskin N, Sheth T, Negassa A, Yusuf S. Relationship of current and past smoking to mortality and morbidity in patients with left ventricular dysfunction. J Am Coll Cardiol. 2001;37:1677–1682. doi: 10.1016/s0735-1097(01)01195-0. [DOI] [PubMed] [Google Scholar]
- The AFFIRM Investigators. Baseline characteristics of patients with atrial fibrillation: the AFFIRM Study. Am Heart J. 2002;143:991–1001. doi: 10.1067/mhj.2002.122875. [DOI] [PubMed] [Google Scholar]
- Wahle C, Adamopoulos C, Ekundayo OJ, Mujib M, Aronow WS, Ahmed A. A propensity-matched study of outcomes of chronic heart failure (HF) in younger and older adults. Arch Gerontol Geriatr. 2009;49:165–171. doi: 10.1016/j.archger.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- Yatsuya H, Folsom AR ARIC Investigators. Risk of incident cardiovascular disease among users of smokeless tobacco in the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2010;172:600–605. doi: 10.1093/aje/kwq191. [DOI] [PMC free article] [PubMed] [Google Scholar]