Abstract
Initially-neutral cues paired with rewards are thought to acquire motivational significance, as if the incentive motivational value of the reward is transferred to the cue. Such cues may serve as secondary reinforcers to establish new learning, modulate the performance of instrumental action (Pavlovian-instrumental transfer, PIT), and be the targets of approach and other cue-directed behaviors. Here we examined the effects of lesions of the ventral striatal nucleus accumbens (ACb) and the basolateral amygdala (BLA) on the acquisition of discriminative autoshaped lever-pressing in rats. Insertion of one lever into the experimental chamber was reinforced by sucrose delivery, but insertion of another lever was not reinforced. Although sucrose was delivered independently of the rats’ behavior, sham-lesioned rats rapidly came to press the reinforced but not the nonreinforced lever. Bilateral ACb lesions impaired the initial acquisition of sign-tracking but not its terminal levels. In contrast, BLA lesions produced substantial deficits in terminal levels of sign-tracking. Furthermore, whereas ACb lesions primarily affected the probability of lever press responses, BLA lesions mostly affected the rate of responding once it occurred. Finally, disconnection lesions that disrupted communication between ACb and BLA produced both sets of deficits. We suggest that ACb is important for initial acquisition of consummatory-like responses that incorporate hedonic aspects of the reward, while BLA serves to enhance such incentive salience once it is acquired.
Keywords: accumbens, amygdala, incentive salience, autoshaping, sign-tracking
1. Introduction
An important consequence of associative learning is the acquisition of emotional and motivational responses (LeDoux, 2000; Rescorla & Holland, 1982; Rescorla & Solomon, 1967). For example, many investigators have asserted that Pavlovian conditioned stimuli (CSs) that predict food unconditioned stimuli (USs) acquire “incentive salience” reflecting the transfer of incentive motivational value from the US to the CS (Berridge, 2001, 2004). Such CSs can reinforce new learning, as rats will learn to press a lever to receive CS presentations in the absence of the US (conditioned reinforcement; Mackintosh, 1974), and can modulate the performance of previously-rewarded instrumental responses (Pavlovian-instrumental transfer, PIT; Lovibond, 1983; Estes, 1948). Furthermore, certain food-paired cues can elicit approach and consummatory behaviors directed towards that CS, sometimes called “sign-tracking” (Jenkins & Moore, 1973; Brown & Jenkins, 1968; Boakes, 1977). For example, rats will approach visual cues paired with food delivery (Holland, 1977; Cardinal et al., 2002), and will approach and contact a lever whose insertion into the chamber signals food (Boakes, 1977; Flagel et al., 2008, 2009; Kearns & Weiss, 2004). In contrast, rats may also direct their behavior toward the site of US delivery upon CS presentation, otherwise known as “goal-tracking” (Boakes, 1977; Flagel et al., 2008, 2009).
Considerable attention has recently been focused on rats’ sign-tracking in an autoshaping (Brown & Jenkins, 1968) paradigm, in which the insertion of a lever into the experimental chamber is paired with the delivery of sucrose, regardless of the rats’ behavior. After repeated Pavlovian lever-sucrose pairings, rats come to press, grasp, and bite the lever as if it were sucrose itself (sign-tracking), despite the absence of any response-reward contingency (e.g., Tomie, 1996; Tomie et al., 2008). Although repeated CS-US pairings can result in both sign-tracking and goal-tracking CRs, some investigators have asserted that the sign-tracking (lever-directed) responses directly index the extent to which the lever CS becomes endowed with incentive salience (e.g., Flagel et al., 2009). For example, Robinson and Flagel (2009) reported that a lever insertion CS is more effective as a conditioned reinforcer in rats that showed high levels of sign-tracking responses during prior lever-food pairings than in rats that had primarily approached the food cup during lever insertions.
Many researchers have suggested that this paradigm may provide a valuable model for the study of incentive learning in drug addiction (e.g., Mahler & Berridge, 2009; Flagel et al., 2010; Tomie, 1996; Tomie et al., 2008). Autoshaping shares many of the behavioral characteristics associated with drug addiction such as persistence and relapse (Tomie et al., 2008), and high levels of sign-tracking responses have been associated with impulsivity, drug sensitization and other traits associated with addiction vulnerability (Flagel et al., 2010; Tomie et al., 2008). Furthermore, Flagel et al. (2011) found that sign-trackers but not goal-trackers showed increased phasic dopamine release in the nucleus accumbens (ACb) core in response to CS presentations, thus relating the sign-tracking response to brain systems frequently implicated in drug abuse and addiction (e.g., Everitt & Robbins, 2005; Robinson & Berridge, 2003).
Here we examined the roles of two brain regions known to be critical to incentive learning, ACb and the basolateral amygdala (BLA, Cador et al., 1989; Cardinal et al., 2002; Everitt et al., 2003; Parkinson et al., 2000a,b), in learning and performance of discriminative autoshaped lever pressing in rats. Because both ACb core and shell have been found to be important for various learned incentive functions (Cardinal et al., 2002; Corbit & Balleine, 2011), we elected to lesion the entire ACb as a first step in determining its role in autoshaped lever pressing. We found that ACb lesions impaired the initial acquisition of sign-tracking but not its terminal levels. In contrast, BLA lesions produced substantial deficits in terminal levels of sign-tracking. Furthermore, whereas ACb lesions primarily affected the probability of lever press responses, BLA lesions mostly affected the rate of responding once it occurred. Finally, disconnection lesions that disrupted communication between ACb and BLA produced both sets of deficits.
2. Materials and methods
2.1. Animals
The subjects were male Long-Evans rats (Charles River Laboratories, Raleigh, NC, USA), which weighed 300–325 g on arrival. Rats were individually housed in a climate controlled colony room that was illuminated from 7:00 A.M. to 7:00 P.M. Rats were given ad libitum access to food and water before and continuing two weeks after surgery. They were then placed on a food restriction schedule and maintained at 85% of their ad libitum weights throughout the autoshaping procedure.
2.2 Surgical Procedures
Surgery was performed under aseptic conditions with isoflurane anesthesia, and all infusions were made with a Hamilton 2.0-µl syringe equipped with a 26-gauge needle. ACb lesions were made with 20 mg/ml N-methyl-D-aspartate (NMDA; Sigma, St. Louis, MO, USA), 0.3 µl/site, using the coordinates 2.1mm anterior of bregma, 1.6mm from the midline, and 7.2mm ventral from the skull surface at the injection site. BLA lesions were made with NMDA (10mg/ml in Dulbecco’s PBS; Sigma) using the coordinates 2.8mm posterior of bregma, 5.1mm from the midline, and 8.7mm (0.16 µl/site) and 8.4 mm (0.08 µl/site) ventral from the skull surface at the injection site.
In Experiment 1, bilateral ACb lesion surgery was performed on 31 rats, and bilateral sham lesion surgeries on 12 rats. In Experiment 2, bilateral BLA lesion surgery was performed on 15 rats, and bilateral sham lesion surgeries on 9 rats. In each of these experiments, rats that received sham lesions underwent the same surgical procedures as their lesioned cohort, but no infusions were made once the needle was in position.
In Experiment 3, all rats received both a unilateral lesion of ACb and a unilateral lesion of BLA. Rats in the Contra condition (n = 12) received these lesions in opposite hemispheres, whereas rats in the Ipsi condition (n = 12) received both lesions in the same hemisphere. Both contra- and ipsilateral lesions were completely counterbalanced by side. Because communication between these two regions is predominantly ipsilateral, the contralateral disconnection lesion impairs functions that require communication between ACb and BLA but leaves other functions unaffected, relative to the ipsilateral control lesion, which destroys equal amounts of tissue in each structure, but leaves communication between them intact in one hemisphere.
2.3. Apparatus
The behavioral training apparatus consisted of eight individual chambers (20.5 cm × 22.0 cm × 22.5 cm) with stainless steel front and back walls, clear acrylic sides, and a floor made of 0.48-cm stainless steel rods spaced 1.9 cm apart. An illuminated clear acrylic food cup was recessed in an opening of the front wall, and photocells at the front of the food cup recorded entries and time spent in the cup. Locally-fabricated retractable levers, which were operated nearly silently by pneumatic controls, were located on either side of the food cup. Each chamber was enclosed inside a sound attenuating shell. An infrared light was located outside of each chamber, and cameras mounted within the shell allowed for television viewing.
2.4. Behavioral Training Procedures
The behavioral training procedures were identical in all 3 experiments. Rats first received two 64-minute sessions in which they were trained to eat from the food cups. In each of these sessions, rats were given sixteen 0.1-ml deliveries of 8% (w/v) sucrose solution, with a mean intertrial interval (ITI) of 240 s. Next, rats underwent 12 sessions of the autoshaping procedure. Within each 64-min session, there were 25 CS+ and 25 CS− trials (mean ITI = 77 s), ordered so that no more than two of same trial type occurred in sequence (after Bussey et al., 1997). On CS+ trials, one lever was extended for 10 s and reinforced with 0.1 ml of 8% sucrose upon retraction and on CS− trials, the other lever was extended for 10 s, but no sucrose was delivered. For half the rats, the CS+ lever was the left lever and the CS− lever was the right lever and for the other half, the sides of the CS+ and CS− levers were reversed.
2.5. Data analysis
We analyzed two primary measures of autoshaping, the rate of lever pressing and the percentage of trials on which at least one lever press occurred, to permit distinguishing between lesion effects on the rate or persistence of responding and on the probability of an autoshaped response. We also analyzed the rate of responding on trials on which at least one response occurred, to provide additional information about response rate or persistence. Finally, we analyzed the percentage of time each rat spent with its head in the food cup during CS presentations.
To characterize the temporal distribution of responses during the CSs, we initially divided the 10-s lever presentations into two 5-s bins. Previous experiments in our laboratory (e.g., Holland, 1977) showed that when visual CSs were paired with food, cue-dependent conditioned orienting responses (ORs), such as rearing, occurred primarily in the first 5 s of a 10-s CS, whereas food cup behavior occurred more frequently during the last half of that interval. If autoshaped lever presses were an example of an OR, then rats might display peak levels of responding to the lever during the first half of CS presentations. Initially, each measure of conditioning was subjected to a 4-way mixed analysis of variance (ANOVA) with variables of lesion condition (lesion or sham), cue type (CS+ vs. CS−), interval of the CS (first vs. last 5-s interval), and session. Because these analyses revealed few interactions of interval with other variables, and higher levels of autoshaped responding during the second 5-s CS interval, we focused on this last interval in subsequent lesion × cue × sessions ANOVAs. In addition to ANOVAs including individual sessions, we also conducted analogous ANOVAs that compared performance averaged over the first vs second halves of training (sessions 1–6 and 7–12, respectively). These latter ANOVAs were especially valuable for the measure of response rate confined to trials on which at least one response occurred, because on CS− trials that measure was often undefined for individual rats in one or more sessions (because response probability was zero), making ANOVA by individual sessions inappropriate.
In all of these ANOVAs, the occurrence of discriminative autoshaping was indicated by significant effects of cue, and the effects of lesions on autoshaping was revealed in significant interactions of lesion with cue. Significant lesion × cue interactions were followed by planned individual comparisons of responding of lesioned and sham-lesioned rats during CS+ and during CS− trials.
2.6. Histological Procedures
After behavioral testing, rats were anesthetized with sodium pentobarbital (100mg/kg) and perfused intracardially with 0.9% saline, followed by 10% (v/v) Formalin in 0.1M PBS. Brains were removed and stored in 0.1M PBS and 20% (w/v) sucrose. Forty-µm slices were collected and Nissl stained to verify lesion placements.
3. Results
3.1. Experiment 1
3.1.1. Histological results
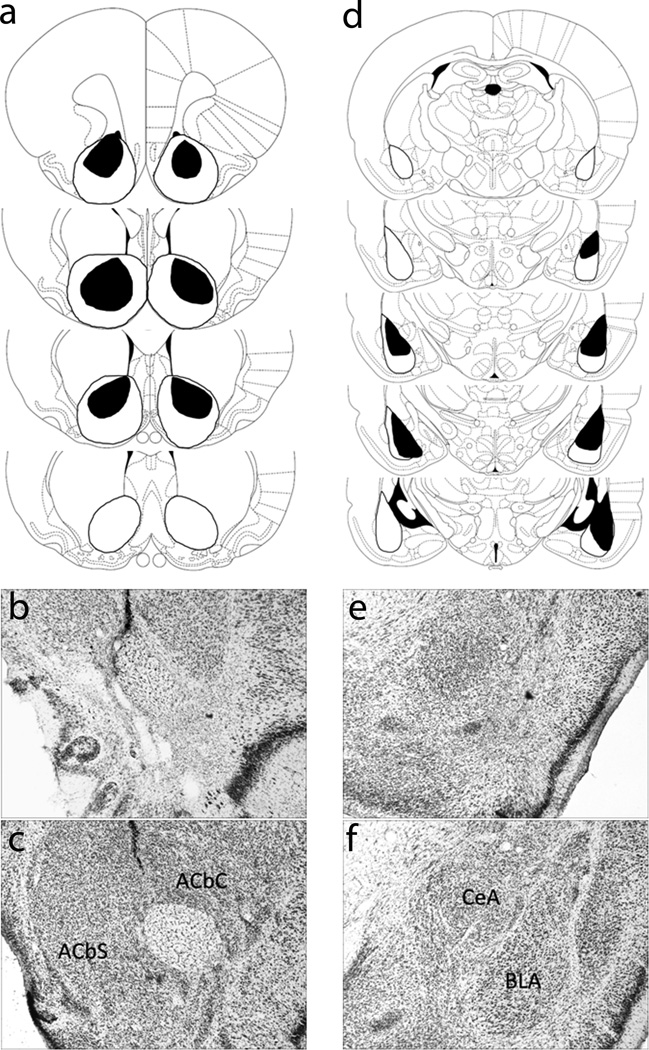
Figure 1a presents a schematic representation of neuronal damage in accepted ACb-lesioned rats (n = 11). On average, 90.0 ± 4.9% (mean ± SEM) of ACb was eliminated, consisting of 93.0 ± 2.8% damage to ACbC and 87.6 ± 6.7% damage to ACbS). Data of rats with less than 50% damage to ACb were discarded. Sham-lesioned rats (n = 12) had no observable damage other than near the needle track. Figures 1b and 1c show sample neurotoxic and sham ACb lesions, respectively.
Figure 1.
Histological results (a) Schematic representation of bilateral nucleus accumbens (ACb) lesions showing the minimum (black) and maximum (white) amount of neuronal damage. Coronal sections are 2.70 mm to 0.70 mm relative to bregma. (b) Photomicrograph of a section with a representative ACb lesion. (c) Photomicrograph of a section with a representative sham ACb lesion. (d) Schematic representation of bilateral basolateral amygdala (BLA) lesions showing the minimum (black) and maximum (white) amount of neuronal damage. Coronal sections are −1.80 mm to −3.80 mm relative to bregma. (e) Photomicrograph of a section with a representative BLA lesion. (f) Photomicrograph of a section with a representative sham BLA lesion. Diagrams from Paxinos and Watson (1998); used by permission.
3.1.2. Behavioral results
3.1.2.1. Autoshaped lever pressing
In contrast to conditioned ORs, which occur predominantly near the time of CS onset (Holland, 1977), lever press responding peaked during the last 5-s period of CS presentations, in both ACb-lesioned and sham-lesioned rats. For both lever press rate and percentage trials with a lever press, initial ANOVAs that included interval as a variable confirmed main effects of interval (Fs1,21 > 38.92, ps < .01), with no lesion × interval interactions (Fs1,21 < 2.08, ps > .10). Thus, we focused our subsequent analysis on responding during the last 5-s periods of CS presentations, when responding was at its peak. The left columns of Table 1 show the mean lever press rate and percentage of trials with a lever press of these rats during the first and second 5-s intervals of CS+ and CS− presentations, averaged over the entire training period.
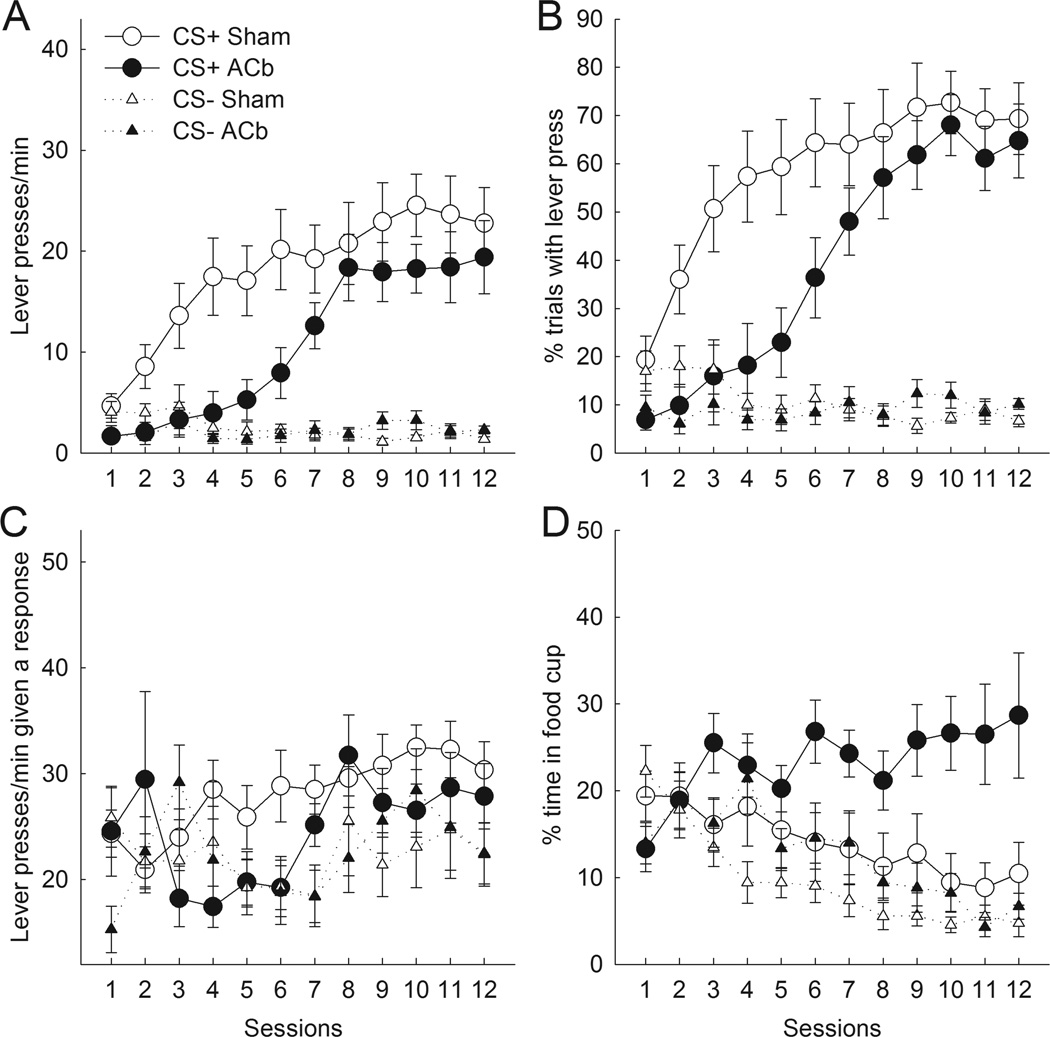
Figures 2A and 2B present the number of lever presses per minute and percentage of trials with a lever press during the last 5-s CS interval, respectively, over the course of training. Sham-lesioned rats quickly acquired sign-tracking behavior during CS+ presentations, in terms of both lever press rate and probability. In contrast, for both measures, rats with bilateral ACb lesions showed substantial deficits in lever-pressing to the CS+ during the first half (sessions 1–6) of training. However, ACb-lesioned rats overcame this initial deficit and reached levels of autoshaped responding that were comparable to sham-lesioned rats over the last half (sessions 7–12) of training. Both ACb- and sham-lesioned rats responded at minimal levels to the CS− lever.
Figure 2.
Effects of bilateral nucleus accumbens (ACb) lesions on autoshaped responding during the last 5 s of CS presentations. Compared to sham-lesioned controls, ACb-lesioned rats showed deficits in lever press response rate (A) and probability (B) across all trials during the first half but not the second half of training. (C) The rate of lever press responding on trials on which at least one response occurred did not differ as a function of lesion. The ordinate scale originates at 12 responses/min, the lowest possible value. (D) ACb-lesioned rats spent more time in the food cup during reinforced lever presentations than sham-lesioned rats. Error bars represent ±SEM.
For both measures, ANOVAs for all of training indicated main effects of cue (Fs1,21 > 58.11, ps < .01) and session (Fs11,231 > 15.96, ps < .01). Although the main effects of lesion were only marginally significant (Fs1,21 < 4.32, ps < .10), the critical lesion × cue interaction was significant for both measures (Fs1,21 > 4.77, ps < .05). The lesion × cue × session interaction was marginally significant for the rate measure (F11,231 = 1.73, p < .10) and significant for the percentage of trials measure (F11,231 = 2.72, p < .01). Separate ANOVAs of both measures of responding during the first and last six sessions of training confirmed a lesion deficit in discriminative autoshaped lever press responding during the first half of training (as evidenced by significant lesion × cue interactions, Fs1,21 > 8.13, ps < .01), but not during the second half (Fs1,21 < 1.86, ps > .10) of training sessions. An explicit contrast of the lesion × cue interaction between the first and last halves of training was significant for the probability measure (F1,21 = 5.30, p < .05), and marginally significant for the rate measure (F1,21 = 3.56, p < .10).
Analysis of response rates confined to trials on which at least one response occurred (Figure 2C) suggested that the lesion’s effect on overall response rate (Figure 2A) was driven mostly by its effect on response probability. ANOVA of lever press rates limited to trials on which rats responded, comparing the first and second halves of training, showed significant effects of cue (F1,21 = 9.05, p < .01) and training (F1,21 = 13.10, p < .01), but neither the effect of lesion nor any of its interactions was significant, ps > .20.
3.1.2.2. Food cup responding
Although bilateral ACb lesions produced substantial deficits in initial levels of autoshaped responding, they did not impair acquisition of food cup responding during CS presentations. Indeed, overall, ACb-lesioned rats showed more food cup responding to the CSs than sham-lesioned rats. Figure 2D presents the percentage of time spent in the food cup during the last 5-s of lever presentations. Whereas sham-lesioned rats decreased the amount of time spent in the food cup during the CS+ as autoshaping progressed (perhaps because of competition from autoshaped behavior), ACb rats acquired and maintained moderate levels of food cup behavior during CS+ trials. Notably, even as ACb-lesioned rats acquired normal levels of autoshaped responding to CS+ presentations during the last half of training, they continued to spend more time in the food cup than sham-lesioned rats. Both groups reduced food cup responding on CS− trials over the course of training.
ANOVA revealed main effects of lesion (F1,21 = 4.99, p < .05), cue (F1,21 = 16.55, p < .01), and session (F11,231 = 3.67, p < .01), but no lesion × cue interaction (F1,21 = 2.88, p > .10). However, the lesion × cue × session interaction was significant (F11,231 = 3.83, p < .01). ANOVAs of performance over the first vs. last 6 sessions (as above) showed that the facilitatory effect of lesion was significant in the last half of training (F1,21 = 8.66, p < .01) but not the first half (F1,21 = 1.47, p > .20).
The higher levels of food cup responding maintained in ACb-lesioned rats were not related to reduced autoshaped lever pressing in those rats (r = −.04), nor were these responses significantly correlated in sham-lesioned rats (r = −.23). It is notable that whereas some other investigators (e.g., Mahler & Berridge, 2009; Flagel et al., 2009) found only a portion of their rats to acquire sign-tracking behaviors, all of our sham-lesioned rats and 9 of our 11 ACb-lesioned rats displayed significant lever pressing. Furthermore, the 2 unsuccessful sign-trackers showed no more food cup behavior than ACb-lesioned rats that were successful sign-trackers. Thus, we did not distinguish between subpopulations of “sign-trackers” and “goal-trackers” in these experiments. Elsewhere (Chang et al., 2012a), we have discussed possible accounts for the higher incidence of sign-tracking in our studies.
3.2. Experiment 2
3.2.1. Histological results
Figure 1d presents a schematic representation of neuronal damage in BLA-lesioned rats (n = 10). On average, 77.2 ± 4.0% of BLA (range = 55.1% to 97.9%) was eliminated while leaving CeA intact. Data of rats showing less than 50% damage to BLA or substantial damage to CeA were discarded. Sham-lesioned rats (n = 9) had no observable damage other than near the needle track. Figures 1e and 1f show sample neurotoxic and sham BLA lesions, respectively.
3.2.2. Behavioral results
3.2.2.1. Autoshaped lever pressing
In contrast to rats with bilateral ACb lesions, which showed initial but not terminal deficits in autoshaped lever-pressing, rats with bilateral BLA lesions showed substantial deficits in terminal levels of autoshaped lever-pressing, but no deficit in initial acquisition. As with ACb-lesioned rats (and those rats’ sham controls), the rats in Experiment 2 showed higher levels of autoshaped responding during the last 5 s of CS presentations regardless of lesion condition. ANOVAs of both rate and probability measures confirmed main effects of interval (Fs1,17 > 25.22, ps < .01) and no lesion × interval interactions (Fs1,17 < 1.81, ps > .20). The center columns of Table 1 show the mean lever press rate and percentage of trials with a lever press of these rats during the first and second 5-s intervals of CS+ and CS− presentations, averaged over the entire training period.
Table 1.
| Measure | ACb lesion | ACb sham | BLA lesion | BLA sham | Ipsi | Contra |
|---|---|---|---|---|---|---|
| Rate CS+ 1st 5s | 7.8 ± 1.9 | 13.1 ± 1.8 | 7.5 ±0.8 | 10.5 ± 1.6 | 13.7 ± 3.2 | 6.1 ± 1.4 |
| Rate CS+ 2nd 5s | 10.8 ± 2.1 | 18.0 ± 1.8 | 10.9 ± 0.9 | 16.6 ± 2.1 | 18.3 ± 3.6 | 9.1 ±1.7 |
| Rate CS− 1st 5s | 1.3 ± 0.1 | 1.2 ± 0.1 | 0.9 ± 0.2 | 1.3 ± 0.2 | 1.5 ± 0.2 | 1.0 ± 0.2 |
| Rate CS− 2nd 5s | 2.2 ± 0.2 | 2.5 ± 0.3 | 1.9 ± 0.3 | 2.4 ± 0.4 | 2.8 ± 0.5 | 1.7 ± 0.2 |
| % trials CS+ 1st 5s | 29.9 ± 5.9 | 47.9 ± 5.2 | 31.0 ± 3.9 | 40.3 ± 5.1 | 40.4 ± 7.1 | 24.2 ± 4.1 |
| % trials CS+ 2nd 5s | 39.2 ± 6.8 | 58.3 ± 4.6 | 39.9 ± 3.9 | 55.0 ± 5.6 | 51.1 ± 8.0 | 33.4 ± 4.6 |
| % trials CS− 1st 5s | 6.8 ± 0.5 | 5.9 ± 0.7 | 4.6 ± 0.8 | 5.9 ± 0.7 | 7.2 ± 1.0 | 5.0 ± 0.9 |
| % trials CS− 2nd 5s | 9.1 ± 0.6 | 10.7 ± 1.3 | 9.0 ± 1.2 | 10.3 ± 1.3 | 11.5 ± 1.6 | 7.3 ± 1.1 |
Notes. Rate = lever presses/min over all trials; % trials = percentage of trials on which at least one lever press response occurred; ACb = nucleus accumbens; BLA = basolateral amygdala; Ipsi = ipsilateral lesions of ACb and BLA; Contra = contralateral lesions of ACb and BLA. Entries are mean ± SEM, averaged over all acquisition sessions.
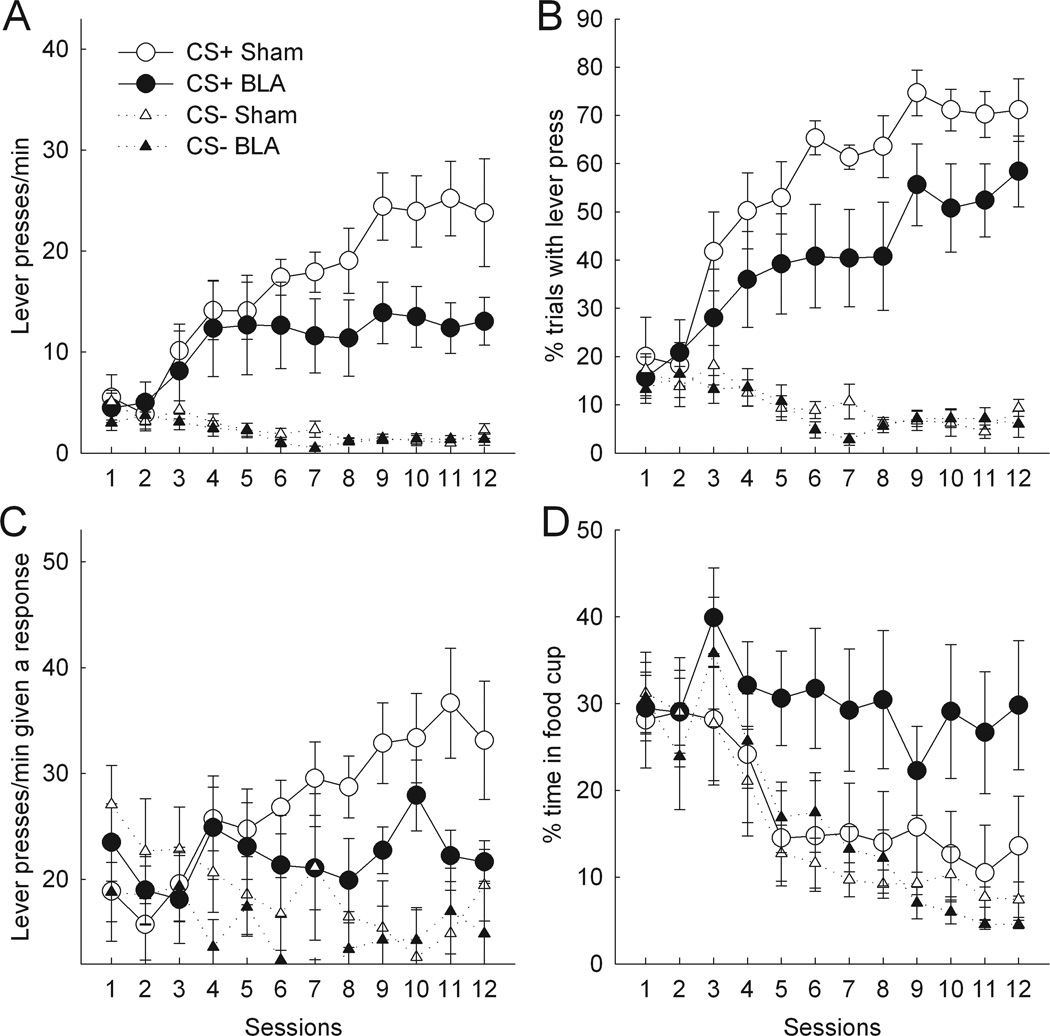
Figure 3A presents lever press rate during the last 5 s of CS presentations. BLA- and sham-lesioned rats pressed the CS+ lever at comparable rates during the first half of acquisition. However, whereas sham-lesioned rats continued to increase their level of responding over the last half of acquisition, BLA-lesioned rats maintained lower rates of lever pressing over the last half of training. Both BLA and sham rats pressed the CS− lever at comparable rates. ANOVA confirmed main effects of cue (F1,17 = 46.22, p < .01) and session (F11,187 = 6.49, p < .01). Although neither the main effect of lesion (F1,17 = 2.44, p > .10) nor the lesion × cue interaction (F1,17 = 2.27, p > .10) was significant, the lesion × cue × session interaction was significant (F11,187 = 2.83, p < .01). Separate ANOVAs of responding during the first and last six sessions revealed that although there was no lesion × cue interaction during the first six sessions (F1,17 = 0.07, p > .70), that interaction was significant during the last six sessions (F1,17 = 5.12, p < .05), indicating that the lesion effects were confined to the latter half of training. An explicit contrast of the lesion × cue interaction between the first and second halves of training was also significant (F1,17 = 6.16, p < .05).
Figure 3.
Effects of bilateral basolateral amygdala (BLA) lesions on autoshaped responding during the last 5 s of CS presentations. Compared to sham-lesioned controls, BLA-lesioned rats showed significant deficits in terminal lever press rates, both across all trials (A) and on trials on which responses occurred (C). (B) The apparent deficit in lever press probability in lesioned rats was not significant. (D) The apparent enhancements of food cup responding in lesioned rats was not significant. In (C), the ordinate scale originates at 12 responses/min, the lowest possible value. Error bars represent ±SEM.
Although these patterns of deficits were also discernible in the percentage of trials with a response measure (Figure 3B), on the whole they were not statistically significant. ANOVA showed a main effect of cue (F1,17 = 97.6, p < .01) and session (F11,187 = 8.16, p < .01), but no main effect of lesion (F1,17 = 2.74, p > .10) nor lesion × cue × session interaction (F11,187 = 1.14, p > .30). Although the lesion × cue interaction was marginally significant (F1,17 = 3.27, p < .10), an explicit contrast of the lesion × cue interaction between first and second halves of training was not significant (F1,17 = 0.84, p > .30).
The absence of a statistically significant effect of the BLA lesion on response probability despite its large effect on response rates at the end of training suggests BLA might be critical to the rate or persistence of autoshaped CRs, “amplifying” such response tendencies. Analysis of response rates confined to trials on which a response occurred supported this view. Although early in training BLA-lesioned and sham-lesioned rats did not differ in their rates of responding once they elected to respond, by the end of training BLA-lesioned rats displayed significantly lower rates of responding to the CS+ lever than shams (Figure 3C). A lesion × cue × training segment (first vs second half) ANOVA showed a significant effect of cue (F1,17 = 9.45, p < .01) and significant cue × training (F1,17 = 6.29, p < .05) and lesion × cue × training (F1,17 = 12.35, p < .01) interactions. ANOVA confined to CS+ responding showed an effect of training (F1,17 = 6.37, p < .05) and a lesion × training interaction (F1,17 = 7.08, p < .05). Individual comparisons showed the effect of lesion to be significant in the last half of training (p < .05) but not the first half (p > .90).
3.2.2.2. Food cup responding
Although rats with BLA lesions showed substantial deficits in autoshaped lever-pressing compared to rats with sham lesions during the last six sessions of autoshaping, BLA lesions did not reduce food cup behavior during the CSs. Both BLA- and sham-lesioned rats spent comparable amounts of time in the food cup during the first and last 5 s of CS presentations; although the main effect of interval was marginally significant (F1,17 = 3.80, p < .10), the lesion × interval interaction (F1,17 = 1.12, p >.30) was not significant. Figure 3D presents the percentage of time spent in the food cup during the last 5 s of CS lever presentations. The pattern of data observed resembled that seen in Experiment 1: Although sham-lesioned rats showed a decrease in the amount of time spent in the food cup on CS+ trials as they increased their rates of lever pressings, BLA-lesioned rats maintained their level of food cup behavior to CS+ over the course of autoshaping. However, due to substantial variation in food cup responding among individual BLA-lesioned rats, that trend was not significant. ANOVA revealed a main effect of cue (F1,17 = 6.62, p < .05) and session (F11,187 = 10.1, p < .01), but no effect of lesion (F1,17 = 2.12, p >.10), lesion × cue interaction (F1,17 = 2.88, p > .10), or lesion × cue × session interaction (F11,187 = 1.32, p > .20).
3.3. Experiment 3
3.3.1 Histological results
Eight rats in the Contra condition had acceptable lesions of both ACb and BLA, averaging 91.7 ± 4.8% damage to ACb (93.2 ± 3.6% damage to ACbC and 90.4 ± 6.2% damage to ACbS) and 83.7 ± 1.7% damage to BLA. Nine rats in the Ipsi condition had acceptable lesions of both structures, averaging 84.6 ± 3.5% damage to Acb (82.9 ± 4.4% damage to ACbC and 85.9 ± 3.0% damage to ACbS), and 90.4 ± 3.1% damage to BLA. ANOVAs confirmed that there were no significant differences between Contra and Ipsi rats in the amount of damage to ACb, ACbC, ACbS, or BLA (Fs1,15 < 3.27, ps > .05). Although the size of ACb lesions (ACb, ACbC, and ACbS) in these rats did not differ significantly from that observed in Experiment 1 (averaged across hemispheres, Fs1,26 < 1.38, ps > .20), the BLA lesions in the present experiment were significantly larger than those of Experiment 2 (averaged across hemispheres, F1,25 = 6.49, p < .05). In addition, in the present experiment the damage extended beyond BLA in several rats in both lesion conditions, including damage to the posterior amygdala central nucleus (CeA) and piriform cortex. However, it is notable that we have shown that bilateral CeA lesions have no effect on autoshaped responding using these same procedures (Chang, Wheeler, & Holland, 2011).
3.3.2. Behavioral results
3.3.2.1. Autoshaped lever pressing
Compared to rats with ipsilateral BLA-ACb control lesions, rats with contralateral BLA-ACb disconnection lesions showed impaired autoshaped responding throughout training. Thus, these rats displayed both the early-stage deficit shown by ACb-lesioned rats in Experiment 1 and the late-stage deficit shown by the BLA-lesioned rats in Experiment 2. As with bilateral ACb- and BLA-lesioned rats, both Contra and Ipsi rats showed peak levels of autoshaped responding during the last 5 s of CS presentations. Preliminary ANOVAs of both lever press rate and probability measures confirmed main effects of interval (Fs1,15 > 18.00, ps < .01) and no lesion × interval interaction (Fs1,15 < 2.62, ps > .10). The right columns of Table 1 show the mean lever press rate and percentage of trials with a lever press of these rats during the first and second 5-s intervals of CS+ and CS− presentations, averaged over the entire training period.
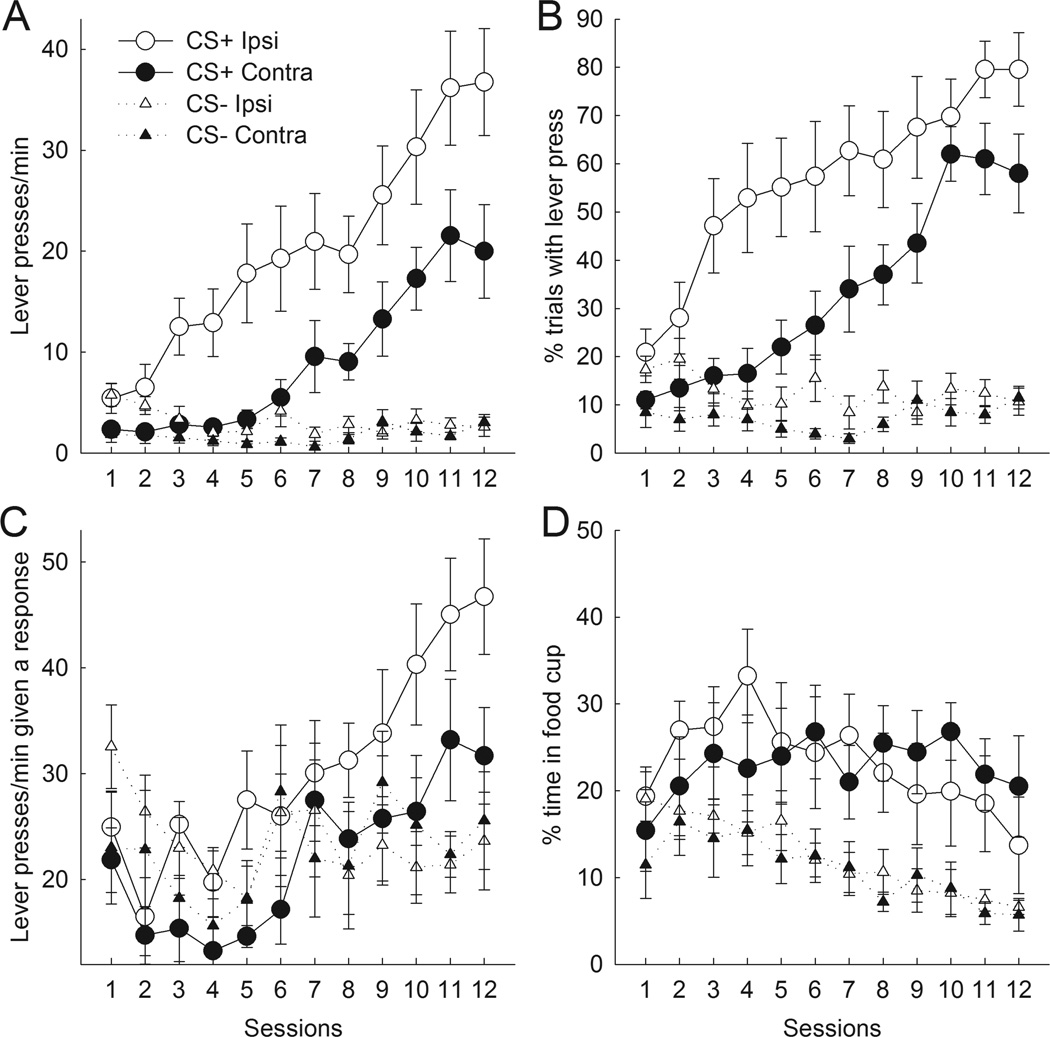
Figure 4A presents lever press rates during the last 5 s of CS presentations. Ipsi control rats rapidly acquired and maintained autoshaped responding to the CS+ lever. By contrast, although rats in the Contra condition did acquire such responding over the course of training, their performance remained lower than that of the Ipsi control rats throughout. Both Contra and Ipsi rats showed comparable, minimal rates of responding to the CS− lever. ANOVA confirmed main effects of lesion (F1,15 = 8.50, p = .01), cue (F1,15 = 35.91, p < .01), and session (F11,165 = 22.48, p < .01). Most importantly, there was a significant lesion × cue interaction (F1,15 = 5.65, p < .05), but no lesion × session interaction (F11,165 = 0.80, p > .60). Unlike in Experiments 1 and 2, in which the lesion × cue interaction was confined to either the first or second halves of training, that interaction was significant in both halves of training, Fs(1,15) > 4.67, ps < .05, and an explicit contrast of the lesion × cue interaction between first and second halves was not significant, F(1, 15) = 1.91, p > .10.
Figure 4.
Effects of unilateral lesions of both nucleus accumbens (ACb) and basolateral amygdale (BLA) on autoshaped responding during the last 5 s of CS presentations. Rats received these lesions in either the same (Ipsi) or contralateral (Contra) hemispheres. Contralateral lesions disrupted communication between the two regions whereas ipsilateral lesions left it intact in the unlesioned hemisphere. Compared to Ipsi controls, Contra rats showed deficits in both lever press response rate (A, C) and probability (B) throughout training. (D) Ipsi and Contra rats did not differ in their performance of food cup repsonses. In (C), the ordinate scale originates at 12 responses/min, the lowest possible value. Error bars represent ±SEM.
Although Contra rats displayed lower probabilities of responding than Ipsi rats throughout training (Figure 4B), as with BLA-lesioned rats in Experiment 2, the deficit observed was less pronounced than with the rate measure. ANOVA confirmed main effects of lesion (F1,15 = 7.97, p < .01), cue (F1,15 = 57.24, p < .01), and session (F11,165 = 20.17, p < .01), but the lesion × cue interaction was only of marginal significance (F1,15 = 3.73, p < .10.). However, it is notable that the magnitude of that marginally significant interaction was comparable in the first and last halves of training (F1,15 = .01, p > .90). Furthermore, ANOVA confined to CS+ responding alone showed significant effects of lesion (F1,15 = 5.99, p < .05) and sessions (F11,165 = 31.14, p < .01), but no difference in the size of the lesion effect in the first vs second halves of training (F1,15 = 0.59, p > .40).
ANOVA of response rates confined to trials on which at least one response occurred (Figure 4C) showed significant effects of lesion (F1,15 = 4.93 p < .05) and training segment (F1,15 = 26.79, p < .01). However, unlike in Experiment 2, the lesion effect was found throughout training; the lesion × training interaction was not significant (F1,15 = .26, p > .60).
3.3.2.2. Food cup responding
Although compared to Ipsi rats, Contra rats showed substantial deficits in autoshaped responding to CS+ presentations throughout training, both Contra and Ipsi rats showed comparable levels of food cup responding during CS presentations. In contrast to lever presses/min and percentage of trials with a lever press, both Contra and Ipsi rats spent comparable amounts of time in the food cup during the first and last 5 s of CS presentations. Preliminary ANOVA showed only a marginal effect of interval (F1,15 = 3.58, p < .10) and no lesion × interval interaction (F1,15 = 0.08, p > .70). Figure 4D shows the percentage of time spent in the food cup during the last 5 s of lever presentations. As training progressed, Contra and Ipsi rats maintained higher levels of food cup behavior during CS+ presentations and decreased their levels of food cup behavior to CS− presentations. Thus, Contra rats acquired the CS-US association despite showing substantially lower levels of autoshaped responding compared to Ipsi rats. ANOVA confirmed main effects of cue (F1,15 = 24.56, p < .01) and session (F11,165 = 3.88, p < 0.01), but no effect of lesion (F1,15 = 0.05, p > .80). Additionally, there was no lesion × cue (F1,15 = 0.07, p > .80) or lesion × cue × session (F11,165 = 1.24, p > .20) interaction.
4.0 Discussion
Our results show that ACb and BLA made distinct contributions to autoshaped lever pressing. Although rats with bilateral lesions of either ACb or BLA showed impairments in their acquisition or expression of autoshaping, they differed in both the timing and nature of those impairments (Experiments 1 and 2). Lesions of ACb reduced the probability of responding to the reinforced lever, but only early in training; by the end of training there was no significant difference in the probability of lever pressing of ACb-lesioned and sham-lesioned rats. Furthermore, despite the ACb-lesioned rats’ deficit in response probability, they did not show significantly lower rates of responding than sham-lesioned rats on trials on which they did respond. By contrast, lesions of BLA primarily reduced the rate of responding once the lever was contacted, but only at later stages of training.
Although the contributions of ACb and BLA were distinct, they were not independent. In Experiment 3, rats with contralateral disconnection lesions of ACb and BLA showed deficits in both the probability and rate of responding, both early and late in training. Thus, rats in which each structure was intact in one hemisphere but normal communication between them was compromised showed deficits characteristic of both ACb and BLA lesions. Notably, these performance impairments were relative to rats that had received equivalent unilateral damage to ACb and BLA ipsilaterally, which preserved communication between these regions in one hemisphere. Thus, functional connection of ACb and BLA was critical to normal acquisition of autoshaping throughout training, in terms of both the probability and rate of lever pressing. Furthermore, although in Experiment 3 we did not compare the performance of ipsilaterally lesioned control rats to sham-lesioned controls, casual comparison of the performances of those rats with that of sham-lesioned rats in Experiments 1 and 2 suggest that unilateral function and communication between ACb and BLA was sufficient for normal autoshaping.
These data are generally consistent with prior assertions that ACb is important for the acquisition or expression of incentive salience to cues paired with food (e.g., Kelley et al., 2005; Parkinson et al., 2000b). By contrast, a primary role for BLA appeared to be the enhancement or magnification of responding to lever cues that had already acquired incentive salience. Lesions of BLA alone produced response rate deficits late in training, and throughout training when combined with contralateral ACb lesions. Notably, using a discrete-trial instrumental task, Ambroggi et al. (2008) found that functional disconnection of BLA and ACb interfered with both the acquisition and asymptotic levels of discriminative lever pressing. Furthermore, using a similar discrete-trial instrumental task, Jones et al. (2010) found that inactivation of BLA reduced both conditioned approach to the reward-predictive cue and the amount of dopamine release in ACb core to in response to that cue. Our BLA-ACb disconnection results are consistent with these findings, suggesting that modulation of ACb dopamine by the dense glutamatergic projection from BLA to ACb (Kelley et al., 1982), which overlaps substantially with dopamine projections originating from the midbrain ventral tegmental area (VTA), is an important mediator of the assignment of incentive salience to reward-predictive cues. In particular, it may be a route by which incentive salience is enhanced.
The eventual acquisition of autoshaped responding to normal levels in ACb-lesioned rats in Experiment 1 deserves further comment. What might be the nature of compensation for ACb damage? It seems unlikely that surviving portions of ACb were responsible; the lesions were very large, averaging over 90% damage to the extent of both core and shell regions. One possibility is that BLA subsumed both acquisition and amplification roles in the absence of normal ACb activity. Although BLA lesions had no effect on initial acquisition in Experiment 2, those rats had intact ACb function and hence no need for ACb compensation. In this case, rats with bilateral lesions of both ACb and BLA might show profound deficits in autoshaping. However, if BLA function could compensate for losses in ACb function, one might expect that rats with contralateral ACb and BLA lesions in Experiment 3 would show at least as much compensation (if not more) than rats with ipsilateral lesions. Another candidate region that may compensate for the lack of ACb function is the orbitofrontal cortex (OFC), an area that is important for encoding and updating cue-outcome associations (Gallagher et al., 1999; Pickens et al., 2003, Saddoris et al., 2005). As with ACb, OFC receives projections from BLA (Kita & Kitai, 1990). Thus, OFC might mediate acquisition of the autoshaping response over the second half of training, and BLA could enhance the rate of responding to sham control levels.
The effectiveness of BLA lesions in attenuating asymptotic responding is novel and contrasts with Blundell et al.’s (2003) observation that BLA lesions had no effect on autoshaped lever pressing. However, this discrepancy is unsurprising if the BLA functions as a maximum response enhancer, as appears to be the case in our Experiment 2. Blundell et al.(2003) found that sucrose-reinforced autoshaped lever pressing was acquired slowly and occurred only at low rates; despite double the number of training sessions as used here, their sham-lesioned rats attained response rates only 60% of those found here. Given the relatively weak autoshaping in their study, it is likely that the role of the BLA was minimal in comparison to our study. Alternatively, it is also possible that lesion size is critical, as our lesions in Experiment 2 were considerably larger than those used by Blundell et al. (2003).
The effects we observed with autoshaped lever pressing differ considerably from those observed with other examples of cue-directed responding in appetitive conditioning. First, responding in the present experiments did not show much in common with conditioned ORs reported previously from this laboratory. After cue-food pairings, conditioned ORs occurred almost exclusively near cue onset (Holland, 1977), whereas in the present studies autoshaped lever pressing occurred throughout the CS-US interval but especially near the end of that interval. Furthermore, the pattern of lesion effects on autoshaped lever pressing and conditioned ORs differ considerably. Here, aspects of autoshaping were impaired by both BLA and ACb lesions, but conditioned ORs are not specifically affected by either BLA (e.g., Hatfield et al., 1996; Setlow et al., 2002) or ACb lesions (Chang et al., 2012b). Conversely, although rats with lesions of CeA fail to acquire conditioned ORs (e.g., Gallagher et al., 1990; Groshek et al., 2005; McDannald et al., 2004), CeA lesions have no effect on the acquisition or expression of autoshaping in procedures identical to those used here (Chang et al., 2012a).
Second, lesions of ACb, CeA and BLA had very different effects on autoshaped lever pressing in our studies than have been observed in conditioned approach procedures (e.g., Parkinson et al., 2000a,b). Whereas those authors found that conditioned approach to visual CSs (illuminated rectangles presented on video monitors) paired with food was disrupted by lesions of CeA but not of BLA, we found that asymptotic levels of autoshaped lever pressing were affected by lesions of BLA (Experiment 2) but not by lesions of CeA (Chang et al., 2012a). Furthermore, although both conditioned approach (Parkinson et al., 2000b) and autoshaped lever pressing were disrupted by lesions of ACb, the pattern of deficits differed. Over extended training, our ACb-lesioned rats recovered from large initial deficits to match the asymptotic performance levels of sham-lesioned controls, whereas Parkinson et al.’s (2000b) rats did not display such a trend. However, we provided three times as many training trials as those investigators; their rats might have displayed this same pattern with additional training.
Third, the pattern of lesion effects on autoshaped responding observed here and by Chang et al. (2012a) also differed from lesion effects on PIT, another phenomenon often attributed to learned incentive processes. Although here we found large asymptotic effects of BLA lesions on the rate of autoshaped lever pressing, such lesions have no effect on PIT when training is conducted with a single reinforcer (Corbit & Balleine, 2005; Gallagher & Holland, 2003). By contrast, although rats with CeA lesions typically fail to display PIT when trained with a single reinforcer (e.g., Corbit & Balleine, 2005; Hall et al., 2001; Holland & Gallagher, 2003), Chang et al. (2012a) found no significant deficit in autoshaped lever pressing in such rats. Finally, whereas here we found that ACb lesions produced substantial initial deficits in autoshaped lever pressing, the effects of ACb lesions on PIT are less certain. Although lesions of ACb core have been found to affect single-reinforcer PIT (Corbit & Balleine, 2011; de Borchgrave et al., 2002; Hall et al., 2001), no such effect has been reported with lesions of ACb shell (Corbit & Balleine, 2011; Hall et al., 2001), and we (Chang et al., 2012b) have found no disruption of PIT after lesions that encompassed both shell and core regions of ACb, like those in the current study.
Given these different patterns of lesion effects on various putative sequellae of incentive learning, and even on different examples of cue-directed responding, it is important to consider how ACb and BLA contribute to autoshaped lever pressing in particular. Autoshaped lever pressing differs from other examples of learned incentive because lever-insertion CSs, unlike typical auditory or visual CSs, support acquisition of consummatory-like behaviors, such as biting, grasping, and handling, which may reflect transfer of hedonic properties of the US to the CS. Our data show that ACb is critical for the initial acquisition of autoshaped responding. Notably, previous studies have shown that ACbS is particularly important in processing the hedonic component of reward. Using a taste reactivity procedure, Berridge and colleagues showed that microinjections of the opiate agonist DAMGO (Pecina & Berridge, 2005) or the endogenous cannabinoid anandamide (Mahler et al., 2007) into particular sites within the anterior medial ACbS can enhance the hedonic impact (“liking”) of sucrose. If autoshaped lever pressing reflects the transfer of hedonic properties from the food reinforcer to the lever CS+ (unlike in other cases of cue-directed responding), then lesions and other manipulations of ACb shell would be expected to influence autoshaped lever pressing (as we found with large ACb lesions), but not simple approach to a non-manipulable visual CS. Indeed, Parkinson et al. (1999) found that the acquisition of visual approach CRs was impaired by ACb core but not ACb shell lesions.
In this regard it is interesting to note that the pattern of lesion effects we have observed with autoshaped lever pressing most resembles that found with conditioned reinforcement, the ability of a food-paired cue to serve as a reinforcer in the acquisition of new learning. As with our autoshaped lever pressing, BLA but not CeA lesions disrupt conditioned reinforcement in both Pavlovian (Hatfield el al., 1996; Setlow et al., 2002) and instrumental (Burns et al., 1993; Robledo et al., 1996) training procedures. Similarly, elsewhere we (Chang et al., 2012b) found that large ACb lesions disrupted Pavlovian conditioned reinforcement (but see de Borchgrave et al., 2002, and Parkinson et al., 1999, who found no effect of lesions limited to ACb core on instrumental conditioned reinforcement). In this context, it is of special interest that Robinson and Flagel (2009) found that lever presentation was a more powerful conditioned reinforcer of nose-poking in rats that had previously responded to lever presentation by lever contact and pressing responses (that is, which were sign-trackers) than in rats that had shown goal-tracking responses. Moreover, Johnson and Gallagher (2011) found that effort-induced variations in reinforcer palatability (as measured by patterns of lick microstructure) were correlated with variations in conditioned reinforcement power of cues previously paired with those reinforcers, again supporting a relation between conditioned reinforcement and transfer of hedonic properties from US to CS.
Autoshaping shares many of the behavioral characteristics associated with drug addiction (Tomie et al, 2008; Tomie, 1996). In particular, autoshaping has been used as a model for drug relapse, as rats rapidly engage in sign-tracking behavior after long retention intervals, and they show spontaneous recovery and rapid reacquisition after responding is extinguished from presentations of the lever by itself once CS-US pairings are re-established (Tomie et al., 2008). In addition, sign-trackers have also been shown to be more impulsive than goal-trackers, a trait that can enhance addiction vulnerability (Flagel et al., 2010; Tomie et al., 2008). For example, Tomie et al. (1998) found that rats that previously showed enhanced sign-tracking behavior were more likely to choose small immediate rewards over large delayed rewards in a two-choice lever-press operant delay-of-reward procedure than rats that showed less sign-tracking behavior. Similarly, Flagel et al. (2010) found that rats selectively bred for greater locomotor activity in response to a novel environment were more likely to become sign-trackers in an autoshaping procedure and were less able to withhold responding in a differential reinforcement of low rates of responding (DRL) task that cancelled delivery of a reinforcer if rats failed to withhold lever pressing for a certain period of time. In contrast, selectively bred rats that showed less locomotor activity in response to a novel environment were more likely to became goal-trackers and lever-pressed more efficiently than sign-trackers in the DRL task.
Sign-tracking also shares several neurobiological characteristics associated with drug addiction (Tomie et al., 2008). Here, we found that lesions of ACb, a brain region frequently implicated in drug addiction as well as the attribution of incentive salience to reward-paired cues (Robinson & Berridge, 2003) interfered with the acquisition of autoshaped lever presses to lever presentation (sign-tracking), but not food source approach (goal-tracking). Using both outbred rats and the same selectively bred lines of rats as Flagel et al. (2010), Flagel et al. (2011) demonstrated that sign-trackers but not goal-trackers showed increased phasic dopamine release in ACb core in response to CS presentations. Similarly, Flagel et al. (2011) showed that dopamine transmission is important for the acquisition of sign-tracking but not goal-tracking, as injections of dopamine antagonist flupenthixol impaired the development of sign-tracking but not goal-tracking in selectively bred rat lines. Finally, sign-tracking has been shown to have stress-related effects that also occur with drug abuse, such as the increase in corticosterone levels associated with increased alcohol intake and self-administration of drugs of abuse (Tomie et al., 2008). Rats that show greater levels of sign-tracking have higher corticosterone levels than rats that show lower levels of sign-tracking (Tomie et al., 2000). Moreover, rats that receive lever-food pairings also show higher corticosterone levels than rats that receive random presentations of those events (Tomie et al., 2002, 2004), analogous to the increases in corticosterone levels observed with administration of drugs of abuse (Tomie et al., 2008).
The autoshaping preparation provides a valuable window on incentive learning processes that may involve transfer of hedonic properties to cues through associative learning. The extent to which subjects show genuine consummatory behavior toward a cue that is paired with a gustatory outcome might indicate a somewhat different form of incentive transfer (or engage different brain structures) than mere approach. This sort of mimetic transfer appears to involve similar neural circuitry as that involved in conditioned reinforcement, in which a cue takes on some of the reinforcing properties of the original reinforcer. Future research will be directed toward teasing apart these potentially different forms of incentive learning, and determining the sort of incentive learning that can best advance the understanding of risk factors associated with drug abuse and relapse.
Highlights.
Rats with lesions of nucleus accumbens showed reduced probability of autoshaped lever pressing but only early in training
Rats with lesions of basolateral amygdala showed normal acquisition but reduced terminal rates of autoshaped lever pressing
Rats with asymmetrical disconnection lesions of both structures showed both deficits
Acknowledgment
This work was supported by NIH grant MH53667.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Reward learning: Reinforcement, incentives, and expectations. Psychology of Learning and Motivation: Advances in Research and Theory. 2001;40:223–278. [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiology & Behavior. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Blundell P, Hall G, Killcross S. Preserved sensitivity to outcome value after lesions of the basolateral amygdala. Journal of Neuroscience. 2003;23:7702–7709. doi: 10.1523/JNEUROSCI.23-20-07702.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boakes R. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz H, editors. Operant-Pavlovian interactions. Hillsdale, NJ: Lawrence Erlbaum Associates; 1977. pp. 67–97. [Google Scholar]
- Brown PL, Jenkins HM. Auto-shaping of the pigeon’s key-peck. Journal of the Experimental Analysis of Behavior. 1968;11:1–8. doi: 10.1901/jeab.1968.11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns LH, Robbins TW, Everitt BJ. Differential effects of excitotoxic lesions of the basolateral amygdala, ventral subiculum and medial prefrontal cortex on responding with conditioned reinforcement and locomotor-activity potentiated by intraaccumbens infusions of D-amphetamine. Behavioural Brain Research. 1993;55:167–183. doi: 10.1016/0166-4328(93)90113-5. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Everitt BJ, Robbins TW. Dissociable effects of cingulated and medial frontal cortex lesions on stimulus-reward learning using a novel Pavlovian autoshaping procedure for the rat: Implications for the neurobiology of emotion. Behavioral Neuroscience. 1997;111:908–919. doi: 10.1037//0735-7044.111.5.908. [DOI] [PubMed] [Google Scholar]
- Cador M, Robbins TW, Everitt BJ. Involvement of the amygdala in stimulus-reward associations: Interaction with the ventral striatum. Neuroscience. 1989;30:77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience and Biobehavioral Reviews. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Chang SE, Wheeler DS, Holland PC. Effects of lesions of the amygdala central nucleus on autoshaped lever pressing. Brain Research. 2012a doi: 10.1016/j.brainres.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Wheeler DS, McDannald MA, Setlow B, Holland PC. Effects of lesions of ventral striatum on several measures of incentive learning. 2012 (Unpublished observations, in preparation for publication). [Google Scholar]
- Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer. Journal of Neuroscience. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The general and outcome-specific forms of Pavlovian-instrumental transfer are differentially mediated by the nucleus accumbens core and shell. Journal of Neuroscience. 2011;31:11786–11794. doi: 10.1523/JNEUROSCI.2711-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Borchgrave R, Rawlins JNP, Dickinson A, Balleine BW. Effects of cytotoxic nucleus accumbens lesions on instrumental conditioning in rats. Experimental Brain Research. 2002;144:50–68. doi: 10.1007/s00221-002-1031-y. [DOI] [PubMed] [Google Scholar]
- Estes WK. Discriminative conditioning: effects of a Pavlovian conditioned stimulus upon a subsequently established operant response. Journal of Experimental Psychology. 1948;38:173–177. doi: 10.1037/h0057525. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: Impact of amygdala-dependent mechanisms of emotional learning. Annals of the New York Academy of Sciences. 2003;985:233–250. [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to compulsion. Nature Neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56:139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PEM, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PEM, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: Implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to a reward-related cue: Influence on cocaine sensitization. Behavioural Brain Research. 2008;186:48–56. doi: 10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. Journal of Neuroscience. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groshek F, Kerfoot E, McKenna V, Polackwich AS, Gallagher M, Holland PC. Amygdala central nucleus function is necessary for learning, but not expression, of conditioned auditory orienting. Behavioral Neuroscience. 2005;119:202–212. doi: 10.1037/0735-7044.119.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: Lesions impair one class of conditioned behavior. Journal of Neuroscience. 1990;10:1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. European Journal of Neuroscience. 2001;13:1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. Journal of Neuroscience. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3:77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. European Journal of Neuroscience. 2003;17:1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- Jenkins HM, Moore BR. Form of auto-shaped response with food or water reinforcers. Journal of the Experimental Analysis of Behavior. 1973;20:163–181. doi: 10.1901/jeab.1973.20-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW, Gallagher M. Greater effort boosts the affective taaste properties of food. Proceedings of the Royal Society (B) 2011;278:1450–1456. doi: 10.1098/rspb.2010.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Day JJ, Aragona BJ, Wheeler RA, Wightman RM, Carelli RM. Basolateral amygdala modulates terminal dopamine release in the nucleus accumbens and conditioned responding. Biological Psychiatry. 2010;67:737–744. doi: 10.1016/j.biopsych.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns DN, Weiss SJ. Sign-tracking (autoshaping) in rats: A comparison of cocaine and food as unconditioned stimuli. Learning & Behavior. 2004;32:463–476. doi: 10.3758/bf03196042. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: Integration of energy, action, and reward. Physiology and Behavior. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, Nauta WJH. The amydalostriatal projection in the rat – An anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7:615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- Kita H, Kita ST. Amygdaloid projections to the frontal cortex and the striatum in the rat. Journal of Comparative Neurology. 1990;298:40–49. doi: 10.1002/cne.902980104. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lovibond PF. Facilitation of instrumental behavior by a Pavlovian appetitive conditioned-stimulus. Journal of Experimental Psychology: Animal Behavior Processes. 1983;9:225–247. [PubMed] [Google Scholar]
- Mackintosh NM. The psychology of animal learning. New York: Academic Press; 1974. [Google Scholar]
- Mahler SV, Berridge KC. Which cue to “want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. Journal of Neuroscience. 2009;29:6500–6513. doi: 10.1523/JNEUROSCI.3875-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: Anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology. 2007;32:2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- McDannald M, Kerfoot EC, Gallagher M, Holland PC. Amygdala central nucleus function is necessary for learning but not expression of conditioned visual orienting. European Journal of Neuroscience. 2004;20:240–248. doi: 10.1111/j.0953-816X.2004.03458.x. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive Pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine. Journal of Neuroscience. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Robbins TW, Everitt BJ. Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. European Journal of Neuroscience. 2000a;12:405–413. doi: 10.1046/j.1460-9568.2000.00960.x. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Willoughby PJ, Robbins TW, Everitt BJ. Disconnection of the anterior cingulated cortex and nucleus accumbens core impairs Pavlovian approach behavior: Further evidence for limbic cortical-ventral striatopallidal systems. Behavioral Neuroscience. 2000b;114:42–63. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Edition 4. San Diego CA USA: Academic Press; 1998. [Google Scholar]
- Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: Where do muopioids cause increased hedonic impact of sweetness? Journal of Neuroscience. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. Journal of Neuroscience. 2003;23:11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Holland PC. Behavioral studies of associative learning in animals. Annual Review of Psychology. 1982;33:265–308. [Google Scholar]
- Rescorla RA, Solomon RL. Two process learning theory: Relationships between classical conditioning and instrumental learning. Psychological Review. 1967;74:151–182. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annual Review of Psychology. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biological Psychiatry. 2009;65:869–873. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo P, Robbins TW, Everitt BJ. Effects of excitotoxic lesions of the central amygdaloid nucleus on the potentiation of reward-related stimuli by intra-accumbens amphetamine. Behavioral Neuroscience. 1996;110:981–990. doi: 10.1037//0735-7044.110.5.981. [DOI] [PubMed] [Google Scholar]
- Saddoris MP, Gallagher M, Schoenbaum G. Rapid associative encoding in basolateral amygdala depends on connections with orbitofrontal cortex. Neuron. 2005;46:321–331. doi: 10.1016/j.neuron.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Setlow B, Gallagher M, Holland PC. The basolateral complex of the amygdala is necessary for acquisition but not expression of CS motivational value in appetitive Pavlovian second-order conditioning. European Journal of Neuroscience. 2002;15:1841–1853. doi: 10.1046/j.1460-9568.2002.02010.x. [DOI] [PubMed] [Google Scholar]
- Tomie A. Locating reward cue at response manipulandum (CAM) induces symptoms of drug abuse. Neuroscience and Biobehavioral Reviews. 1996;20:505–535. doi: 10.1016/0149-7634(95)00023-2. [DOI] [PubMed] [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Research Reviews. 2008;58:121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A, Silberman Y, Williams K, Pohorecky LA. Pavlovian autoshaping procedures increase plasma corticosterone levels in rats. Pharmacology, Biochemistry, and Behavior. 2002;72:507–513. doi: 10.1016/s0091-3057(01)00781-x. [DOI] [PubMed] [Google Scholar]
- Tomie A, Tirado AD, Yu L, Pohorecky LA. Pavlovian autoshaping procedures increase plasma corticosterone and levels of norepinephrine and serotonin in prefrontal cortex in rats. Behavioural Brain Research. 2004;153:97–105. doi: 10.1016/j.bbr.2003.11.006. [DOI] [PubMed] [Google Scholar]