Abstract
A growing body of evidence suggests that iron overload is associated with inferior outcomes after myeloablative allogeneic hematopoietic stem cell transplantation (HSCT). However, all of those studies used surrogate markers of iron overload, especially serum ferritin, and most had a retrospective design. We conducted a prospective observational study in patients with MDS or acute leukemia undergoing myeloablative HSCT. Forty-five patients who were followed for over 1 year, with serial measurements of serum iron parameters, as well as liver and cardiac MRI. There was no significant increase in ferritin, liver or cardiac iron content in the 12 months following HSCT. While serum ferritin still appeared to have prognostic significance, as previously reported, pre-HSCT iron overload (as reflected in liver iron content) was not associated with increased mortality, relapse, or GVHD. These results raise the possibility that the adverse prognostic impact of pre-HSCT hyperferritinemia may be related to factors independent of iron overload.
Keywords: iron overload, allogeneic stem cell transplantation, acute myeloid leukemia, myelodysplastic syndromes
INTRODUCTION
Iron overload is common in patients undergoing allogeneic stem cell transplantation (HSCT). Many studies have shown an association between elevated serum ferritin before HSCT (used as a surrogate measure of iron overload) and adverse outcomes1–14, mostly from an increase in infection risk4,6,8,9,12,14–17 and non-relapse mortality (NRM), although some of these studies also adduced an association between iron overload and acute graft-versus-host disease (GVHD)1,7,8 or hepatic veno-occlusive disease (VOD)3,14,18–20. However, these studies were retrospective and mostly based on surrogate measures of iron burden, especially serum ferritin.
We therefore conducted a prospective study in patients with acute leukemia or myelodysplastic syndrome (MDS) undergoing HSCT with myeloablative conditioning (MAC). This group of patients appeared to be at particular risk of death from iron overload in a previous retrospective study3. In this study, we followed 45 patients for 1 year following HSCT, with serial measurements of serum iron parameters, liver and cardiac MRI. We have previously reported the patients’ baseline characteristics24, and shown that liver iron overload is indeed frequent in this population (as opposed to cardiac iron overload, which was rare), with a strong correlation between liver iron content, serum ferritin, and transfusion history. Here we present the one-year follow-up of those patients.
MATERIALS AND METHODS
Patients
The details of this study have been described previously24. Briefly, we enrolled adult patients with acute myelogenous (AML) or lymphoblastic (ALL) leukemia or MDS who were scheduled to undergo allogeneic HSCT with myeloablative conditioning at the Dana-Farber/Brigham and Women’s Hospital (DF/BWH) transplant program. Measurements of serum iron parameters (including hepcidin and labile plasma iron) as well as MRI of liver and heart were obtained pre-HSCT, then at 6 and 12 months after transplantation. No chelation therapy was administered to those patients. Informed consent was obtained from all patients. IRB approval was obtained from the Office for the Protection of Research Subjects (OPRS) at Dana-Farber/Harvard Cancer Center to perform those studies. The studies were performed in accordance with the principles of the Declaration of Helsinki, and registered at ClinicalTrials.gov (NCT00954720).
Study Assessments
Transfusion history was obtained from the medical records of DF/BWH blood bank and of all the institutions where the patients reported to have received transfusions. Labile plasma iron was measured by Afferix Ltd (Ashkelon, Israel). MRI studies were performed on a 1.5 Tesla magnetic resonance system (General Electric HealthCare, Excite HDx) with an 8-channel phased-array receiving surface coil. MRI included both cardiac and liver imaging with dedicated pulse sequences. Cardiac imaging included cine imaging of ventricular structure and function and imaging for quantification of myocardial iron content. Cine imaging was performed using standard cine steady-state free precession fast gradient-echo technique with the following parameters (repetition time 3 msec, echo time 1–1.3 msec, flip angle 45–55 degrees, in-plane spatial resolution 1.5×2 mm at a slice thickness of 8 mm and 0 mm interslice spacing). Quantification of myocardial iron was performed using a validated fast gradient-echo technique that acquires 8 images from a range of echo times (ranging from 2 msec to 30 msec, echo spacing 4 msec) in a mid-ventricular short-axis location19. Hepatic iron was quantified using the same pulse sequence technique but without cardiac gating and acquired in a trans-axial location of the liver. No intravenous contrast was used during MRI studies. Breath-holds were performed during cardiac imaging to minimize respiratory motions. Offline analysis was performed to obtain left ventricular volumes and ejection fraction using commercial available software (QMASS® 7.1, Medis, Leiden, The Netherlands) or iron quantification using validated research software (CineTool version 8.0, General Electric Healthcare). Liver iron content was estimated from the hepatic parenchyma T2* value using the following formula20: LIC (mg/g dry weight) = 25.4/T2* (in milliseconds) + 0.202.
Statistics
Patient characteristics were analyzed descriptively. Correlation analysis was performed using Spearman’s rank test. Changes between pre and post HSCT were tested using the Wilcoxon-signed rank test (testing a difference of greater than 1 mg/gdw for LIC and 100 ng/ml for ferritin values). Overall survival (OS) and progression-free survival (PFS) were calculated using the Kaplan-Meier method. OS was defined as the time from stem cell infusion to death from any cause. PFS was defined as the time from stem cell infusion to disease relapse or progression or death from any cause, whichever occurred first. The log-rank test was used for comparisons of Kaplan-Meier curves. Cumulative incidence curves for non-relapse death and relapse with or without death were constructed reflecting time to relapse and time to non-relapse death as competing risks. Time to relapse and time to non-relapse death were measured from the date of stem cell infusion. The difference between cumulative incidence curves in the presence of a competing risk was tested using the Gray method26. Potential prognostic factors for survival were examined in the proportional hazards model. All calculations were done using SAS 9.2 (SAS Institute Inc, Cary, NC) and R (version 2.18.1).
RESULTS
Patient characteristics
This analysis includes 45 patients who underwent myeloablative HSCT for AML, ALL or MDS. Table 1 summarizes their baseline characteristics. As previously described24, there was a strong correlation between serum ferritin and LIC prior to HSCT (Spearman r=0.71); at 6 months, the correlation coefficient between ferritin and LIC was 0.84, and at 12 months was 0.74. Both pre-HSCT ferritin and LIC were strongly associated with disease (with higher ferritin and LIC in patients with acute leukemia compared to those with MDS). However, ferritin was more strongly associated than LIC with advanced stage (p=0.011 for ferritin versus p=0.11 for LIC), and with number of prior chemotherapy regimens (Spearman correlation coefficient 0.67 versus 0.55). Neither was significantly associated with age or cytogenetics.
Table 1.
Baseline characteristics of the patients
| Variable | N. (%) |
|---|---|
| Number of patients | 45 |
| Age (years) Median (range) | 46 (18–63) |
| Disease | |
| MDS | 8 (18%) |
| AML | 26 (58%) |
| ALL | 11 (24%) |
| Stage at HSCT | |
| Early | 39 (87%) |
| Untreated | 8 (18%) |
| CR1 | 24 (53%) |
| CR>1 | 7 (16%) |
| Advanced | 6 (13%) |
| Induction failure/PR | 1 (2%) |
| Relapse | 5 (11%) |
| Prior treatments median (range) | 2 (0–6) |
| Cytogeneticsa | |
| Favorable | 2 (4%) |
| Intermediate | 35 (78%) |
| Adverse | 8 (18%) |
| Baseline liver iron content | |
| Median (range), mg/g dry weight | 3.0 (0.6–12.9) |
| Patients with value ≥ 5 mg/gdw | 17 (38%) |
| Patients with value ≥ 7 mg/gdw | 7 (16%) |
| Baseline serum ferritinb | |
| Median (range), ng/ml | 1432 (20–6989) |
| Baseline CRPb | |
| Median (range), mg/l | 3.3 (0.4–209.4) |
Available for 44 patients
MDS, myelodysplastic syndromes; AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; HSCT, hematopoietic stem cell transplantation; CR, complete remission; PR, partial remission; CRP, C-reactive protein (upper limit of normal 3.0 mg/l)
Change in iron burden after HSCT
The median pre-HSCT LIC was 3.0 mg/gdw (range, 0.6–12.9). Of the 32 patients alive at 6 months, 25 had a liver MRI, with median LIC of 4.5 mg/gdw (range, 1.3–12.9). Of the 28 patients alive at 12 months, 20 had a liver MRI (one patient’s MRI was obtained at 26 instead of 12 months; most of the missing data were due to patient refusal or logistical issues), with median LIC of 4.3 mg/gdw (range, 1.1–14.4). The median change in LIC between 0 and 6 months was +0.6 mg/gdw (range, −3.5 to +10.6; p=0.8), and between 0 and 12 months was +0.5 mg/gdw (range, −5.5 to +7.5; p=0.6). The median change in cardiac T2* between 0 and 6 months was −1 msec (p=0.5), and between 0 and 12 months −6 msec (p=0.6). In the 20 patients who had received fewer than 10 transfusions between 0 and 6 months after HSCT, the median change in LIC was +0.6 mg/gdw (range, −3.5 to +4.4; p=0.3), and for the 15 patients with fewer than 10 transfusions between 0 and 12 months, the median change in LIC was +0.1 mg/gdw (range, −3.3 to +5.5; p=0.7). For the 23 patients with serum ferritin values available at 12 months, the median change in ferritin between 0 and 12 months was +128 ng/ml (range, −4439 to +5539; p=0.4); for the 14 patients who had received fewer than 10 transfusions between 0 and 12 months after HSCT, the median change in serum ferritin was +121 ng/ml (range, −1941 to +881; p=1.0). There was also no apparent increased iron accumulation in the patients who developed acute GVHD. In conclusion, there was no significant change in serum ferritin or LIC in the year following HSCT, and no strong correlation between the number of transfusions between 0 and 12 months and either the change in LIC or the change in ferritin (Figure 1).
Figure 1. Post-HSCT changes in LIC and ferritin.
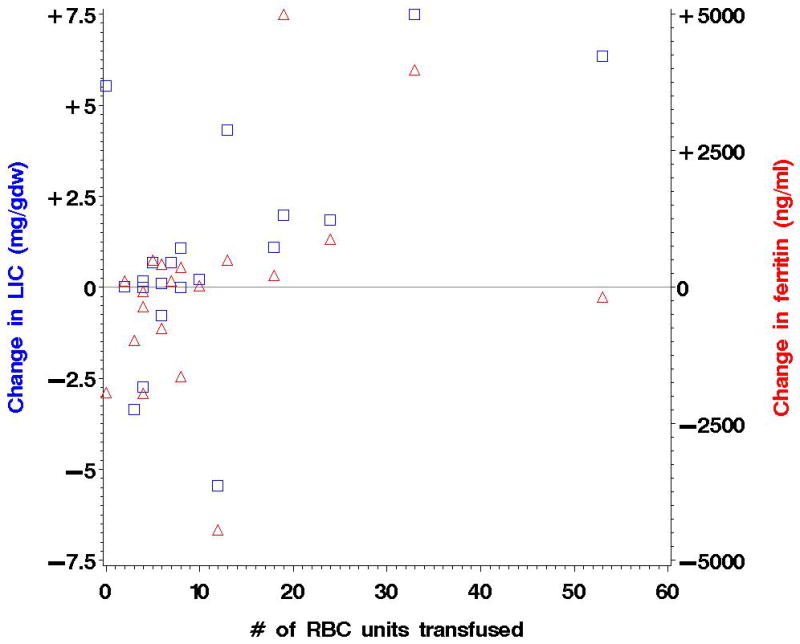
The changes in LIC (squares) and ferritin (triangles) between 0 and 12 months are plotted against the number of transfused red cell units in that period. The Spearman correlation coefficient between LIC change and red cell units was 0.5, and between ferritin change and red cell units 0.5.
Survival, GVHD and VOD
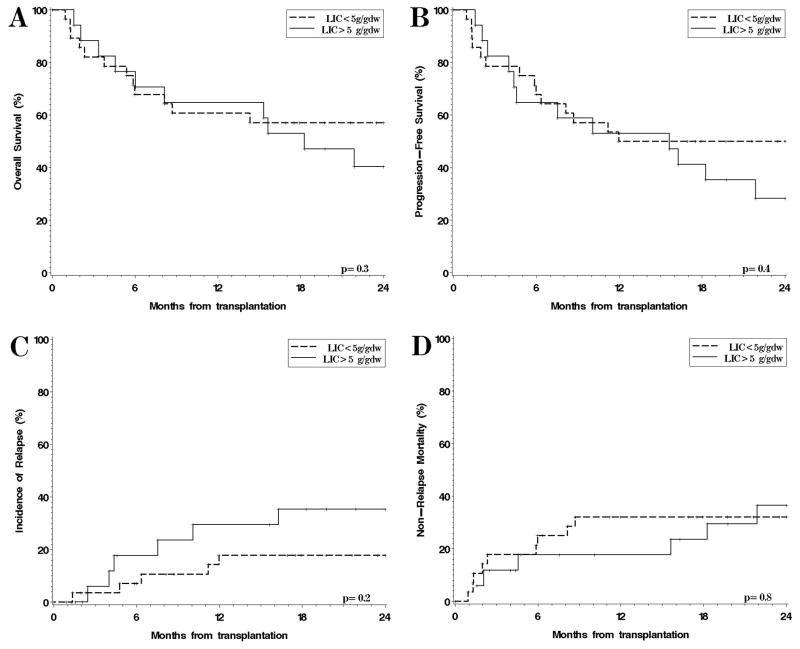
Figure 2 shows the OS and PFS, cumulative incidence of relapse (CIR) and NRM for the 45 patients on the study, stratified by pre-transplantation iron overload, using a threshold for severe iron overload of LIC ≥ 5 mg/gdw. Thirty-eight percent of the patients in the observational study had severe iron overload by this definition (Table 1). This threshold was chosen since it roughly corresponds (based on our previous work24) to a serum ferritin of 2500 ng/ml, which has been associated with worse post-HSCT survival3. Median follow-up for survivors was 27months (range, 17–36). One- and two-year OS (Figure 2A) were 61% (95% CI, 40–76) and 57% (95% CI, 37–73) respectively, in patients whose pre-HSCT LIC was <5 mg/gdw, compared to 65% (95% CI, 38–82) and 40% (95% CI, 18–62) for patients with LIC ≥ 5 g/gdw (p=0.3). At 1 year, PFS (Figure 2B) was 50% in patients with LIC < 5 mg/gdw, versus 53% in those with LIC ≥ 5 (p=0.4), CIR (Figure 2C) was 18% versus 29% (p=0.2), and NRM (Figure 2D) 32% versus 18% (p=0.8).
Figure 2. Outcomes after transplantation, stratified by severe iron overload (LIC > 5 mg/gdw) for the patients in the observational study.
(A) Overall survival; (B) Progression-free survival; (C) Cumulative incidence of relapse; (D) Cumulative incidence of non-relapse mortality.
The absence of a prognostic effect of LIC on survival was true regardless of the LIC threshold chosen. For example, there was no significant difference in outcome in patients with pre-HSCT LIC >3 mg/gdw (1y OS 58% versus 67% in patients with LIC ≤ 3 mg/gdw, p=0.16); or for patients with pre-HSCT LIC> 7 mg/gdw (1y OS 57% vs 63% in patients with LIC ≤ 7 mg/gdw, p=0.9). We did not test higher thresholds since there were too few patients in the observational study for comparison (for example, there was only 1 patient with pre-HSCT LIC above 10 mg/gdw).
The proportion of patients who developed grade II–IV acute GVHD was 32% among those with LIC<5 mg/gdw versus 24% among those with LIC ≥ 5 mg/gdw (p=0.7); for grade III/IV acute GVHD, the proportion was 21% versus 12% (p=0.7), for chronic GVHD 29% versus 35% (p=0.7), and for hepatic veno-occlusive disease (VOD) 18% versus 0% (p=0.14).
We also performed comparisons based on pre-HSCT ferritin instead of LIC (Figure 3). One-year OS in patients with ferritin > 2500 ng/ml was 43% versus 70% in those with ferritin ≤ 2500 ng/ml (p=0.034).d 1-year CIR was 29% in patients with ferritin >2500 ng/ml versus 20% in those with ferritin ≤ 2500 ng/ml (p=0.6), and NRM 36% versus 23% (p=0.2). A ferritin threshold of 1000 ng/ml was not prognostically useful (1y OS for patients with ferritin > 1000 ng/ml 60% versus 64% for patients below this threshold, p=0.17). The association between elevated ferritin and OS was confirmed in a multivariable model including disease stage and cytogenetics risk group, in which the hazard ratio (HR) for mortality in patients with ferritin > 2500 ng/ml was 2.4 (p=0.042). The inclusion of C-reactive protein in the model did not alter the results. In a similar model with LIC instead of ferritin, there was no association between LIC and OS (HR for mortality for LIC ≥ 5 mg/gdw was 1.4, p=0.4), which was also not influenced by the addition of CRP to the model. Moreover, the difference in OS based on serum ferritin remained apparent even among the subgroup of patients with LIC <5 mg/gdw (2y OS for patients with ferritin > 2500 ng/ml 25% versus 61%, p=0.10).
Figure 3.
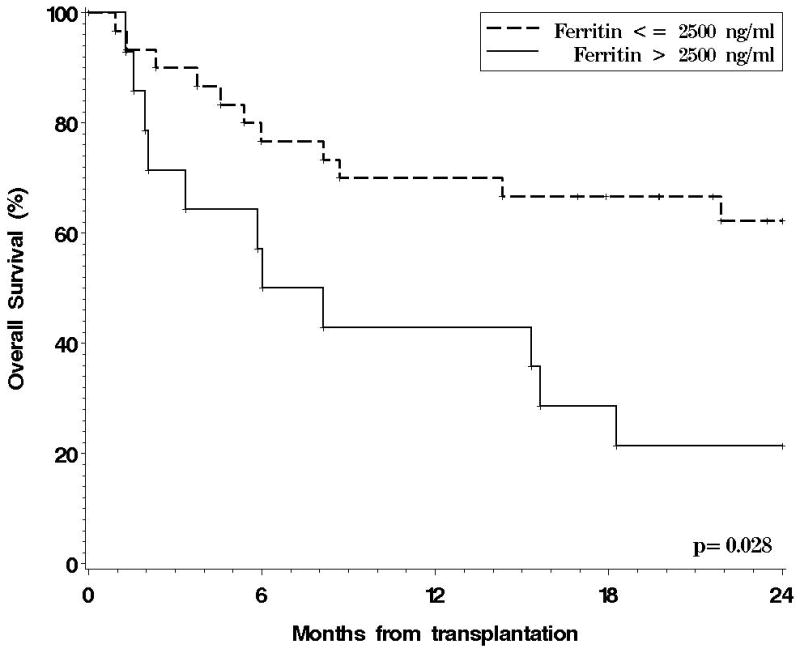
Overall survival for the 45 patients in the observational study, stratified by pre-HSCT serum ferritin.
DISCUSSION
The study described here is the first to prospectively address HSCT outcomes in patients with hematologic malignancies using MRI to directly estimate iron burden. It targeted patients with acute leukemia or MDS undergoing myeloablative allogeneic HSCT, the population that appeared to be at highest risk from complications of iron overload based on prior studies. Two notable findings emerged from this work: first, while iron overload is indeed very common in this population, likely related mostly to prior transfusion burden24, there does not appear to be a significant accumulation of iron in patients after transplantation. This does not support the hypothesis that iron homeostasis is severely disturbed after transplantation.
Second, there was no suggestion in our cohort of a detrimental impact of iron overload as reflected by LIC on mortality, relapse, GVHD or VOD after HSCT. This runs deeply counter to a number of prior studies, including our own1–14. It is remarkable that there did in fact appear to be a difference in survival when pre-HSCT serum ferritin was considered (as was demonstrated in all of those prior studies), as opposed to when LIC was considered. If it is indeed the case that elevated ferritin and not elevated LIC is associated with increased mortality, it may be that ferritin is prognostic not because it reflects iron stores but because it is an acute phase reactant (reflecting inflammatory status from co-morbid conditions, advanced disease, etc). Indeed, ferritin in our cohort was more strongly associated with advanced disease stage and number of prior regimens than was LIC. It has been previously shown that other acute phase reactants (such as albumin or C-reactive protein) are indeed prognostically significant3,27,28. Naturally, it is also possible that iron overload is important in HSCT but not as parenchymal liver iron, for example as LPI or non-transferrin bound iron, or iron deposited in other tissues.
One must be cautious in interpreting these results, since the number of patients in this study was small, which may preclude the demonstration of slight survival differences. However, there was not even the suggestion of a difference in our cohort (Figure 2), whereas we found a readily apparent (and statistically significant) difference in outcome when ferritin was used instead of LIC (Figure 3). If the previously established adverse prognostic effect of hyperferritinemia reflected mostly the impact of iron overload, one might have expected to find similar results whether patients were stratified by ferritin or by LIC. The ferritin cut-off used for this analysis (2500 ng/ml) corresponds roughly to an LIC of 5 mg/gdw based on our previous correlation analysis24; since all of the previously published studies on ferritin have described an adverse effect at thresholds at or below 2500 ng/ml, we would have expected to find a difference in outcome using an LIC cutoff of 5 mg/gdw. Instead, there was no difference, and none emerged even using a higher LIC cutoff of 7 mg/gdw. Therefore, and while we cannot definitively conclude that iron overload has no impact on HSCT outcomes, our results suggest that it remains to be proven that iron overload is prognostically important in HSCT, and that such a proof will require prospective studies using direct measurements of iron overload rather than ferritin. This conclusion also has implications for the design and conduct of future chelation studies.
Acknowledgments
This work was funded in part by grants from Novartis Oncology and the National Institutes of Health, CA142106-06. PA is a recipient of a Special Fellowship in Clinical Research from the Leukemia and Lymphoma Society, a Career Development Award from the Conquer Cancer/ASCO Foundation, and a Scholar Award from the American Society of Hematology.
References
- 1.Alessandrino EP, Della Porta MG, Bacigalupo A, et al. Prognostic impact of pre-transplantation transfusion history and secondary iron overload in patients with myelodysplastic syndrome undergoing allogeneic stem cell transplantation: a GITMO study. Haematologica. 2010;95:476–484. doi: 10.3324/haematol.2009.011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altes A, Remacha AF, Sureda A, et al. Iron overload might increase transplant-related mortality in haematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29:987–989. doi: 10.1038/sj.bmt.1703570. [DOI] [PubMed] [Google Scholar]
- 3.Armand P, Kim HT, Cutler CS, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007;109:4586–4588. doi: 10.1182/blood-2006-10-054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kataoka K, Nannya Y, Hangaishi A, et al. Influence of pretransplantation serum ferritin on nonrelapse mortality after myeloablative and nonmyeloablative allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:195–204. doi: 10.1016/j.bbmt.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Lee JW, Kang HJ, Kim EK, Kim H, Shin HY, Ahn HS. Effect of iron overload and iron-chelating therapy on allogeneic hematopoietic SCT in children. Bone Marrow Transplant. 2009;44:793–797. doi: 10.1038/bmt.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahindra A, Bolwell B, Sobecks R, et al. Elevated pretransplant ferritin is associated with a lower incidence of chronic graft-versus-host disease and inferior survival after myeloablative allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2009;146:310–316. doi: 10.1111/j.1365-2141.2009.07774.x. [DOI] [PubMed] [Google Scholar]
- 7.Platzbecker U, Bornhauser M, Germing U, et al. Red blood cell transfusion dependence and outcome after allogeneic peripheral blood stem cell transplantation in patients with de novo myelodysplastic syndrome (MDS) Biol Blood Marrow Transplant. 2008;14:1217–1225. doi: 10.1016/j.bbmt.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pullarkat V, Blanchard S, Tegtmeier B, et al. Iron overload adversely affects outcome of allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2008;42:799–805. doi: 10.1038/bmt.2008.262. [DOI] [PubMed] [Google Scholar]
- 9.Storey JA, Connor RF, Lewis ZT, et al. The transplant iron score as a predictor of stem cell transplant survival. J Hematol Oncol. 2009;2:44. doi: 10.1186/1756-8722-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YR, Kim JS, Cheong JW, Song JW, Min YH. Transfusion-associated iron overload as an adverse risk factor for transplantation outcome in patients undergoing reduced-intensity stem cell transplantation for myeloid malignancies. Acta Haematol. 2008;120:182–189. doi: 10.1159/000187646. [DOI] [PubMed] [Google Scholar]
- 11.Lim ZY, Fiaccadori V, Gandhi S, et al. Impact of pre-transplant serum ferritin on outcomes of patients with myelodysplastic syndromes or secondary acute myeloid leukaemia receiving reduced intensity conditioning allogeneic haematopoietic stem cell transplantation. Leuk Res. 2009;34:723–727. doi: 10.1016/j.leukres.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 12.Chow JK, Werner BG, Ruthazer R, Snydman DR. Increased serum iron levels and infectious complications after liver transplantation. Clin Infect Dis. 2010;51:e16–23. doi: 10.1086/654802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bazuave GN, Buser A, Gerull S, Tichelli A, Stern M. Prognostic impact of iron parameters in patients undergoing allo-SCT. Bone Marrow Transplant. 2011 doi: 10.1038/bmt.2011.13. [DOI] [PubMed] [Google Scholar]
- 14.Sucak GT, Yegin ZA, Ozkurt ZN, Aki SZ, Yagci M. Iron overload: predictor of adverse outcome in hematopoietic stem cell transplantation. Transplant Proc. 2010;42:1841–1848. doi: 10.1016/j.transproceed.2009.11.049. [DOI] [PubMed] [Google Scholar]
- 15.Kontoyiannis DP, Chamilos G, Lewis RE, et al. Increased bone marrow iron stores is an independent risk factor for invasive aspergillosis in patients with high-risk hematologic malignancies and recipients of allogeneic hematopoietic stem cell transplantation. Cancer. 2007;110:1303–1306. doi: 10.1002/cncr.22909. [DOI] [PubMed] [Google Scholar]
- 16.Ozyilmaz E, Aydogdu M, Sucak G, et al. Risk factors for fungal pulmonary infections in hematopoietic stem cell transplantation recipients: the role of iron overload. Bone Marrow Transplant. 2010 doi: 10.1038/bmt.2009.383. Epub. [DOI] [PubMed] [Google Scholar]
- 17.Tachibana T, Tanaka M, Takasaki H, et al. Pretransplant serum ferritin is associated with bloodstream infections within 100 days of allogeneic stem cell transplantation for myeloid malignancies. Int J Hematol. 2011;93:368–374. doi: 10.1007/s12185-011-0784-0. [DOI] [PubMed] [Google Scholar]
- 18.Morado M, Ojeda E, Garcia-Bustos J, et al. BMT: Serum Ferritin as Risk Factor for Veno-occlusive Disease of the Liver. Prospective Cohort Study. Hematol. 2000;4:505–512. [PubMed] [Google Scholar]
- 19.Maradei SC, Maiolino A, de Azevedo AM, Colares M, Bouzas LF, Nucci M. Serum ferritin as risk factor for sinusoidal obstruction syndrome of the liver in patients undergoing hematopoietic stem cell transplantation. Blood. 2009;114:1270–1275. doi: 10.1182/blood-2009-03-212282. [DOI] [PubMed] [Google Scholar]
- 20.Lee SH, Yoo KH, Sung KW, et al. Hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation: incidence, risk factors, and outcome. Bone Marrow Transplant. 2009 doi: 10.1038/bmt.2009.349. EPub. [DOI] [PubMed] [Google Scholar]
- 21.Sahlstedt L, Ebeling F, von Bonsdorff L, Parkkinen J, Ruutu T. Non-transferrin-bound iron during allogeneic stem cell transplantation. Br J Haematol. 2001;113:836–838. doi: 10.1046/j.1365-2141.2001.02820.x. [DOI] [PubMed] [Google Scholar]
- 22.Sahlstedt L, von Bonsdorff L, Ebeling F, Parkkinen J, Juvonen E, Ruutu T. Non-transferrin-bound iron in haematological patients during chemotherapy and conditioning for autologous stem cell transplantation. Eur J Haematol. 2009;83:455–459. doi: 10.1111/j.1600-0609.2009.01310.x. [DOI] [PubMed] [Google Scholar]
- 23.Porter JB, Abeysinghe RD, Marshall L, Hider RC, Singh S. Kinetics of removal and reappearance of non-transferrin-bound plasma iron with deferoxamine therapy. Blood. 1996;88:705–713. [PubMed] [Google Scholar]
- 24.Armand P, Kim HT, Rhodes J, et al. Iron overload in patients with acute leukemia or MDS undergoing myeloablative stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:852–860. doi: 10.1016/j.bbmt.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter JB, Jaswon MS, Huehns ER, East CA, Hazell JW. Desferrioxamine ototoxicity: evaluation of risk factors in thalassaemic patients and guidelines for safe dosage. Br J Haematol. 1989;73:403–409. doi: 10.1111/j.1365-2141.1989.tb07761.x. [DOI] [PubMed] [Google Scholar]
- 26.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. The Annals of Statistics. 1988;16:1140–1154. [Google Scholar]
- 27.Artz AS, Wickrema A, Dinner S, et al. Pretreatment C-reactive protein is a predictor for outcomes after reduced-intensity allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:1209–1216. doi: 10.1016/j.bbmt.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanda J, Mizumoto C, Ichinohe T, et al. Pretransplant serum ferritin and C-reactive protein as predictive factors for early bacterial infection after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2011;46:208–216. doi: 10.1038/bmt.2010.108. [DOI] [PubMed] [Google Scholar]
- 29.Armand P, Kim HT, Zhang MJ, et al. Classifying Cytogenetics in Patients with Acute Myelogenous Leukemia in Complete Remission Undergoing Allogeneic Transplantation: A Center for International Blood and Marrow Transplant Research Study. Biol Blood Marrow Transplant. 2011 doi: 10.1016/j.bbmt.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armand P, Deeg HJ, Kim HT, et al. Multicenter validation study of a transplantation-specific cytogenetics grouping scheme for patients with myelodysplastic syndromes. Bone Marrow Transplant. 2010;45:877–885. doi: 10.1038/bmt.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]