Abstract
Objective
Isoflurane, a volatile anesthetic agent, has been recognized for its potential neuroprotective properties and has antiapoptotic effects. We examined whether isoflurane posttreatment is protective against early brain injury (EBI) after subarachnoid hemorrhage (SAH) and determined whether this effect needs sphingosine-related pathway activation.
Design
Controlled in vivo laboratory study.
Setting
Animal research laboratory.
Subjects
179 eight-week-old male CD-1 mice weighing 30 to 38 g.
Interventions
SAH was induced in mice by endovascular perforation. Animals were randomly assigned to sham-operated, SAH-vehicle, and SAH+2% isoflurane. Neurobehavioral function and brain edema were evaluated at 24 and 72 hours. The expression of sphingosine kinase (SphK), phosphorylated Akt (p-Akt) and cleaved caspase-3 was determined by Western blotting and immunofluorescence. Neuronal cell death was examined by terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end-labeling staining. Effects of a SphK inhibitor DMS, or a sphingosine 1 phosphate receptor inhibitor VPC23019 on isoflurane’s protective action against post-SAH EBI were also examined.
Measurements and Main Results
Isoflurane significantly improved neurobehavioral function and brain edema at 24 hours but not 72 hours after SAH. At 24 hours, isoflurane attenuated neuronal cell death in the cortex, associated with an increase in SphK1 and p-Akt, and a decrease in cleaved caspase-3. The beneficial effects of isoflurane were abolished by DMS and VPC23019.
Conclusions
Isoflurane posttreatment delays the development of post-SAH EBI through antiapoptotic mechanisms including sphingosine-related pathway activation, implying its use for anesthesia during acute aneurysm surgery or intervention.
Keywords: subarachnoid hemorrhage, early brain injury, isoflurane, apoptosis, sphingosine kinase-1, sphingosine 1 phosphatereceptor-1
Introduction
Subarachnoid hemorrhage (SAH) is a life-threatening disease despite progress in diagnostics and treatment employing early neurosurgical intervention (1). Early brain injury (EBI), which occurs within 48 hours following cerebral aneurysm rupture, has been established as a factor for poor outcomes after SAH (2). Thus, identification of early neuroprotective strategies for possible clinical use is needed. Apoptosis is involved in the pathogenesis of EBI after experimental SAH (2).
Sphingosine 1 phosphate (S1P) is generated from sphingomyelin, an integral component of plasma membranes, by the sequential action of sphingomyelinase, ceramidase, and sphingosine kinase (SphK) (3). Many factors can alter SphK activity and regulate subsequent S1P levels (3). S1P regulates diverse biological processes including cell survival after binding to S1P receptor-1 to 5 (S1P1 to S1P5) (4).
Isoflurane is a volatile anesthetic and a lipophilic molecule (5). It was reported that isoflurane activated SphK in renal tubule cells and induced renal protective effects (6). We previously demonstrated that S1P1 activation was neuroprotective in cerebral ischemia via antiapoptosis (7), and that isoflurane reduced neonatal hypoxic-ischemic brain injury by a S1P1/Akt pathway (8). Therefore, activation of sphingomyelin metabolism by isoflurane can be a good candidate for neuroprotection against EBI after SAH.
In this study, we tested 2 hypotheses: 1) isoflurane posttreatment attenuates post-SAH EBI through antiapoptosis in mice, and 2) the antiapoptotic effect of isoflurane involves a sphingosine-related pathway including SphK expression and S1P receptor activation.
Materials and Methods
Experimental Design and Animal Groups
The animal and ethics review committee at Loma Linda University approved all protocols. One hundred seventy-nine 8-week-old male CD-1 mice (30–38g; Charles River, Wilmington, MA) were used for the study.
To examine whether isoflurane attenuated EBI after SAH and had an antiapoptotic effect (designated as study 1), animals were randomly divided into 3 groups, and evaluated at either 24 or 72 hours: sham-operated+30% O2+70% medical air (O2-medical air; 24-hour and 72-hour sham groups, n=16 and 4, respectively), SAH+O2-medical air (24-hour and 72-hour vehicle groups, n=22 and 7, respectively), and SAH+2% isoflurane+O2-medical air (24-hour and 72-hour treatment groups, n=21 and 10, respectively).
To determine whether this antiapoptotic effect involved SphK1 expression and S1P receptor activation (designated as study 2), we used a potent and specific SphK antagonist, N, N-dimethylsphingosine (DMS), and a S1P1- and S1P3- receptor antagonist, VPC23019. VPC23019 competitively antagonizes S1P1 receptors 10-fold more potently than S1P3 receptors on neurons (9). Animals were randomly divided into 6 groups, and evaluated at 24 hours post-SAH: dimethyl sulfoxide (DMSO; a vehicle)+sham-operated+O2-medical air (n=11), DMSO+SAH+2% isoflurane+O2-medical air (n=15), DMS in DMSO+SAH+2% isoflurane+O2-medical air (n=18), VPC23019 in DMSO+SAH+2% isoflurane+O2-medical air (n=17), DMS in DMSO+SAH+O2-medical air (n=19), and VPC23019 in DMSO+SAH+O2-medical air (n=19).
Mouse SAH Model
SAH endovascular monofilament model was produced as described previously (10). Briefly, animals were anesthetized with an intraperitoneal injection of ketamine/xylazine (100/10 mg/kg). A sharpened 4-0 monofilament nylon suture was advanced through the internal carotid artery (ICA) to perforate the anterior cerebral artery. In the sham surgery, the filament was advanced 5mm through the ICA without perforating the artery. Body temperature was kept constant (37.5±0.5°C) during the operation.
Drug Administration
One hour after SAH induction, 2% isoflurane (Baxter, Deerfield, IL) was continuously administered for 1 hour with 30% O2 and 70% medical air.
DMS (Enzo, Plymouth Meeting, PA; final concentration, 0.17μg/0.5μL) and VPC23019 (Avanti, Alabaster, Alabama; final concentration, 0.26μg/0.5μL) were automatically infused at a rate of 0.1μL/minute intracerebroventricularly, 60 minutes before the sham surgery or SAH induction (Supplemental Digital Content 1, which shows dose determination and preparation of drugs). The vehicle groups were given the same volume (0.5μL) of DMSO (1.1g/mL/kg) diluted in phosphate-buffered saline.
Intracerebroventricular Infusion
Mice were placed in a head holder (Stoelting Stereotactic Instrument, Wood Dale, IL) and a 26s-gauge needle of a 10μL Hamilton syringe (Microliter #701; Hamilton, Reno, NV) was inserted through a burr hole perforated on the skull into the right lateral ventricle using the following coordinates relative to bregma: 0.1mm posterior; 0.9mm lateral; and 3.1mm below the horizontal plane of bregma (11). The needle was removed 10 minutes after completion of the infusion, and the burr hole was quickly plugged with bone wax.
Severity of SAH
The severity of SAH was blindly evaluated using the SAH grading scale at sacrifice (Supplemental Digital Content 1, which shows the SAH grading scale) (12). Eight mice with SAH grading scores≤7, which had no significant brain injury (10), were excluded.
Mortality and Neurological Scores
The neurological score was blindly evaluated at 24 and 72 hours after SAH, based on the scoring system of Garcia et al. (13) with modifications (Supplemental Digital Content 1, which shows neurological scoring). Mortality was calculated at 24 and 72 hours after SAH.
Brain Water Content (BWC)
Brains were quickly removed and separated into the left and right cerebral hemispheres, cerebellum, and brain stem, and weighed (wet weight) at 24 hours (n=6 per group) and at 72 hours (n=4 per group) after surgery. Next, brain specimens were dried in an oven at 105°C for 72 hours and weighed again (dry weight). The percentage of BWC was calculated as ([wet weight-dry weight]/wet weight) × 100% (10).
Western Blotting
The left cerebral hemisphere (perforation side) at 24 hours after SAH was used (n=5 per group). Western blotting was performed as previously described (7) using the following primary antibodies: anti-SphK 1 (1:250, Abgent, San Diego, CA), anti-SphK 2 (1:250, Lifespan Biosciences, Seattle, CA), anti-caspase-3 (1:200, Santa Cruz Biotechnology, Santa Cruz, CA), and anti-phospho-Akt (Ser473) (1:1000, Cell Signaling Technology, Danvers, MA) antibodies.
Immunofluorescence and Terminal Deoxynucleotidyl Transferase-mediated Uridine 5′-Triphosphate-biotin Nick End-labeling (TUNEL) Staining
Animals were euthanized 24 hours after surgery and brains were processed as previously described (10). Ten-micron-thick coronal sections at the level of bregma 1mm (caudally) were cut on a cryostat (LM3050S; Leica Microsystems, Bannockburn, Ill). Double-fluorescence labeling was performed using the following primary antibodies: anti-SphK1 (1:250, Abgent, San Diego, CA), anti-phospho-Akt (Ser473) (1:100, Cell Signaling Technology, Danvers, MA), and anti-neuronal nuclei (NeuN; 1:200, Chemicon, Temecula, CA) antibodies, and then subjected to TUNEL staining with an in situ cell death detection kit (Roche, Mannheim, Germany). TUNEL-positive cells were counted in three fields per case at ×400 magnification and expressed as the mean number of TUNEL-positive neurons/mm2 with a fluorescence microscope (10).
Statistics
Data were expressed as mean±SD. After confirming that each population being compared followed a normal distribution using Shapiro-Wilk W tests, statistical differences were analyzed using unpaired t tests or one-way analysis of variance (ANOVA) followed by the Student-Newman-Keuls method as appropriate. Differences in mortality were tested using Fisher’s exact or chi-square tests as appropriate. P<0.05 was considered statistically significant.
In the statistical analysis, we calculated the power of the tests. The number of animals per group necessary to reach the desired power of 0.800 was in the range of 4 to 6.
Results
Mortality and SAH Grade
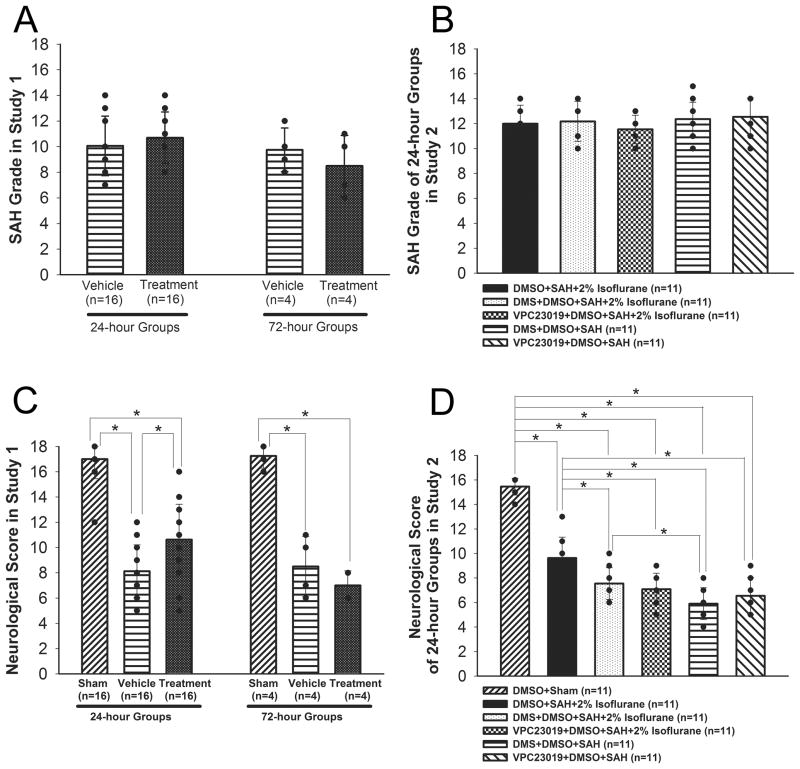
In study 1, the mortality rate was not significantly different between the vehicle and treatment groups at 24 (20%, 4 of 20 mice vs. 20%, 4 of 20 mice) and 72 hours (42.9%, 3 of 7 mice vs. 50%, 4 of 8 mice). No sham-operated mice died. There was no significant difference in SAH grade between the vehicle and treatment groups at 24 and 72 hours (Figure 1A).
Figure 1.
SAH grade (A, B) and neurological scores (C, D) in studies 1 and 2, respectively. Study 1 was evaluated at 24 and 72 hours, and study 2 was evaluated at 24 hours after SAH. Values are mean±SD; *P<0.05, ANOVA.
In study 2, no sham-operated mice died. The mortality rate was not significantly different among the DMSO+SAH+2% isoflurane (26.7%, 4 of 15 mice), DMS in DMSO+SAH+2% isoflurane (35.3%, 6 of 17 mice), VPC23019 in DMSO+SAH+2% isoflurane (35.3%, 6 of 17 mice), DMS in DMSO+SAH (38.9%, 7 of 18 mice) and VPC23019 in DMSO+SAH (38.9%, 7 of 18 mice) groups at 24 hours after SAH. SAH grade also did not show significant differences among the groups in study 2 at 24 hours (Figure 1B).
Neurological Score and BWC
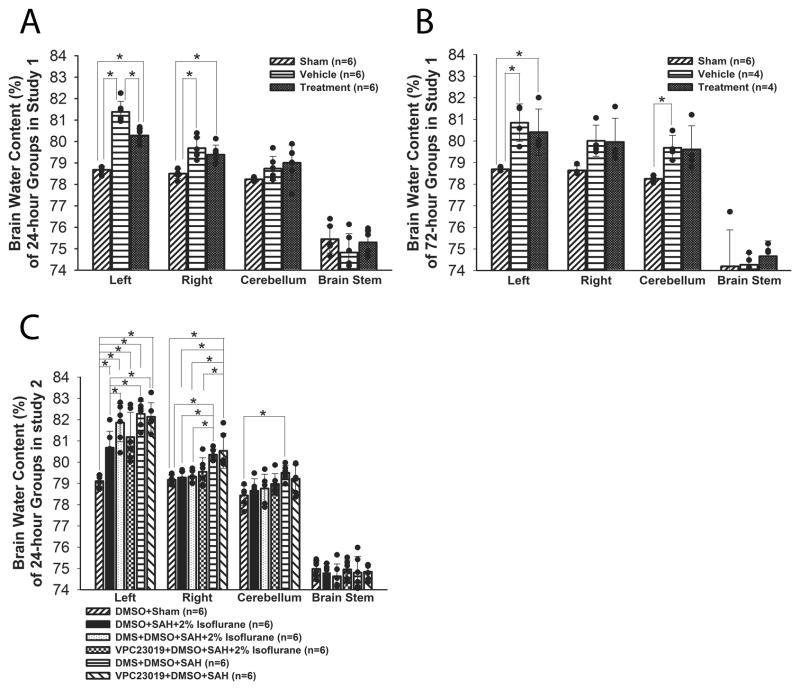
In study 1, although neurological score (Figure 1C; n=16) and BWC (ipsilateral hemisphere; Figure 2A; n=6) were significantly worse after SAH (P<0.001, P<0.001, respectively, ANOVA), a significant improvement was observed in the treatment group compared with the vehicle group at 24 hours after SAH (P=0.002, P<0.001; respectively, ANOVA). However, isoflurane did not show beneficial effects at 72 hours after SAH (P=0.456, P=0.224, respectively, ANOVA). In study 2, treatment with DMS and VPC23019 abolished isoflurane’s protective effects on neurofunction at 24 hours after SAH (P<0.001, P<0.001, respectively, ANOVA; Figure 1D). DMS but not VPC23019 also blocked isoflurane’s beneficial effects on BWC (P=0.035, P=0.274, respectively, ANOVA; Figure 2C).
Figure 2.
Brain water content. A, study 1, 24 hours after SAH; B, study 1, 72 hours after SAH; C, study 2, 24 hours after SAH. Values are mean±SD; *P<0.05, ANOVA.
Effect of Isoflurane on Sphingosine-related Antiapoptotic Pathway
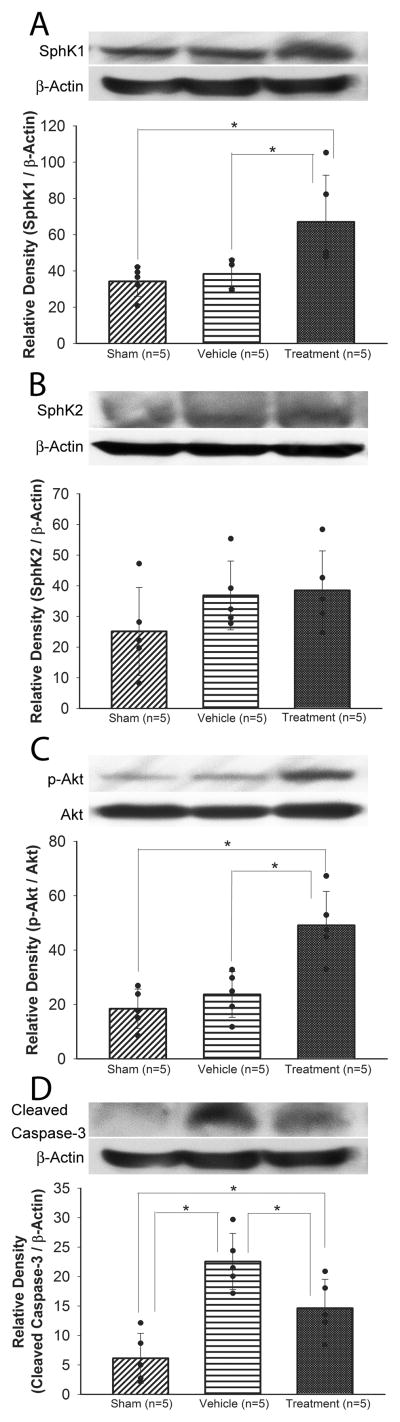
Western blot analyses showed that SphK1 and phosphorylated Akt (p-Akt) were significantly increased in the treatment group compared with the sham (P=0.020, P<0.001, respectively) and vehicle groups (P=0.017, P=0.001, respectively, ANOVA). There was no significant difference in SphK2 expression among the groups at 24 hours after SAH (Figure 3A–C). Although cleaved caspase-3 was significantly increased in the vehicle group compared with the sham group (P<0.001), isoflurane significantly reduced cleaved caspase-3 expression at 24 hours after SAH (P=0.019; Figure 3D).
Figure 3.
Representative Western blots and quantitative analysis of SphK1 (A), SphK2 (B), phosphorylated Akt (Ser473) (p-Akt; C), and cleaved caspase-3 (D) in the left cerebral hemisphere at 24 hours after SAH (study 1). The p-Akt band density values are calculated as a ratio of that of total Akt, and the other proteins band density values are calculated as a ratio of that of β-actin. Values are mean±SD; *P<0.05, ANOVA.
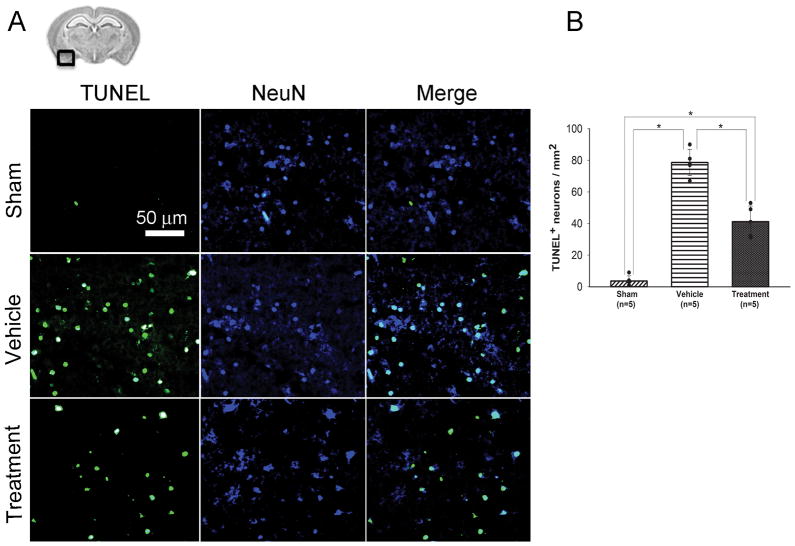
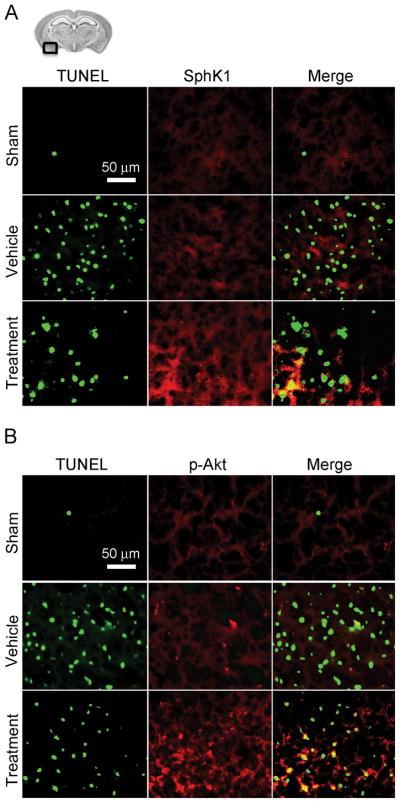
Consistent with the Western blot results, immunofluorescence analyses revealed that TUNEL-positive neurons in the left basal cortex were seen more prominent in the vehicle group compared to the sham group (Figure 4A). A significant decrease in TUNEL-positive neurons was observed in the treatment group (P<0.001, ANOVA; Figure 4B). The expression of SphK1 and p-Akt were more prominent in the treatment group than the vehicle group, and were seen even in some TUNEL-positive cells (Figure 5A and 5B).
Figure 4.
Evaluation of TUNEL-positive cells in the ipsilateral basal cortex at 24 hours after SAH (study 1). A, representative brain section and immunofluorescence images showing the colocalization of NeuN (blue) with TUNEL (green)-positive cells; B, quantitative analysis of TUNEL-positive neurons. Scale bar, 50μm; values, mean±SD; *P<0.05, ANOVA.
Figure 5.
Representative brain section and immunofluorescence images showing the relationship between TUNEL (green)-positive cells and SphK1 (red) expression (A) or phosphorylated Akt (Ser473) (p-Akt; red) expression (B) in the ipsilateral basal cortex at 24 hours after SAH (study 1). Scale bar: 50μm.
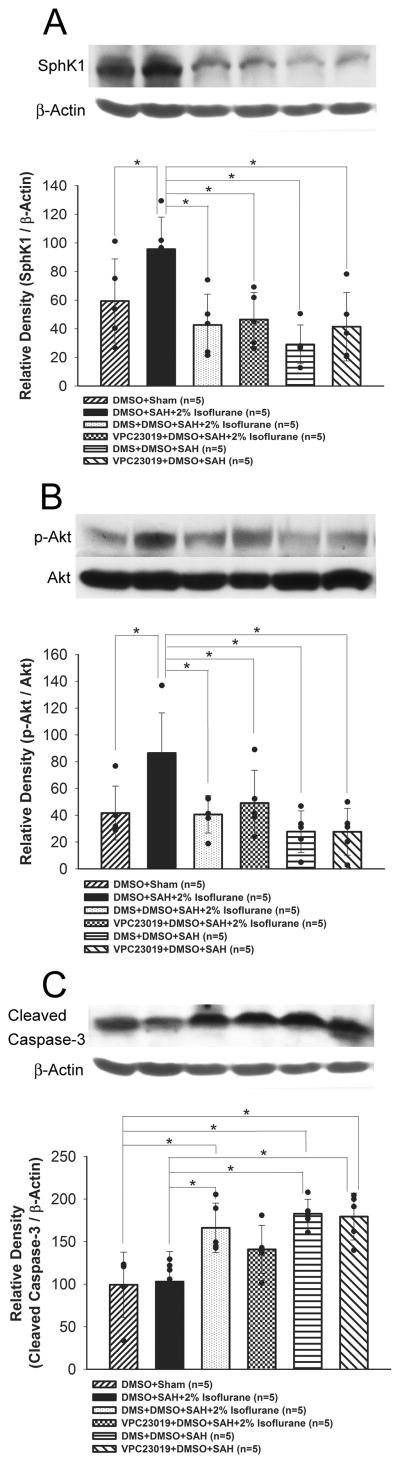
In study 2, although isoflurane treatment significantly increased SphK1 expression and Akt phosphorylation compare to the sham group (P=0.016, P=0.007, respectively), both DMS (P=0.001, P=0.002, respectively) and VPC23019 (P=0.006, P=0.002, respectively) abolished this effect (ANOVA; Figure 6A and 6B). Further, isoflurane decreased cleaved caspase-3, which was reversed by DMS (P=0.003, ANOVA; Figure 6C).
Figure 6.
Representative Western blots and quantitative analysis of SphK1 (A), phosphorylated Akt (Ser473) (p-Akt; B), and cleaved caspase-3 (C) in the left cerebral hemisphere at 24 hours after SAH (study 2). The p-Akt band density values are calculated as a ratio of that of total Akt, and the other proteins band density values are calculated as a ratio of that of β-actin. Values are mean±SD; *P<0.05, ANOVA.
Discussion
The present study showed that isoflurane posttreatment attenuated EBI at 24 hours after SAH, and that the neuroprotective effect was associated with decreased neuronal apoptosis. This antiapoptotic effect needed at least partly sphingosine-related pathway activation including SphK1 and S1P1/3 receptors. However, the neuroprotective effects of isoflurane were not shown at 72 hours after SAH.
Volatile anesthetics have been shown to be protective against ischemic injury in neural tissues when used as preconditioning (14, 15). Among the volatile anesthetic agents, isoflurane is the most neuroprotective. The following different mechanisms underlying the beneficial effects of isoflurane have been proposed in diverse animal models of ischemic injuries: modulation of excitotoxicity (16); induction of inducible nitric oxide synthase (15); modulation of mitochondrial KATP channels (17); and activation of S1P1 receptors and phosphatidylinositol-3 kinase pathways (6, 8, 18). It was reported that isoflurane increased SphK activity and SphK1 mRNA expression in both in vivo murine model of renal ischemia-reperfusion injury as well as an in vitro model (6). In this study, one-hour 2% isoflurane administered at 1 hour after SAH improved neurological scores, decreased BWC, and increased SphK1 expression and Akt activation, which is a well-known principal factor in antiapoptotic signaling (7, 19). Moreover, isoflurane treatment decreased cleaved caspase-3 expression and neuronal cell death at 24 hours after SAH in mice. These findings suggested that isoflurane posttreatment had a caspase-dependent antiapoptotic effect, and that this antiapoptotic effect was mediated by SphK1 and Akt activation, which were induced by S1P1 or S1P3 receptor activation. In addition, this study showed that VPC23019 suppressed SphK1 upregulation, indicating that SphK1 was downstream of S1P1/3 receptors. Because S1P is generated from sphingosine by SphK and then activates S1P receptors (3), SphK1 may work through both upstream and downstream of S1P receptors in EBI after SAH.
It was reported that isoflurane did not prevent post-ischemic neuronal apoptosis or cerebral infarction, although isoflurane reduced early neuronal death and delayed the development of cerebral infarction caused by ischemia (16, 20, 21). In this study, isoflurane also suppressed EBI at 24 hours, but failed to prevent neuronal injuries at 72 hours after SAH. Although S1P is upregulated by SphK activation, S1P is a short-lived lipid and susceptible to enzymes, such as sphingosine phosphate phosphatase-1 and S1P-lyase (22). Furthermore, in the absence of stimulus to increase SphK1 expression, the balance of sphingolipid rheostat (3) is shifted to increasing sphingosine and ceramide, so that S1P decreases and no antiapoptotic effect occurs. This might explain why one-dose or short-duration isoflurane treatment at an acute stage did not have long-term neuroprotective effects. It would be interesting to see whether short-duration isoflurane treatment over multiple days could provide prolonged protection. And also, in a clinical setting, early surgery or intervention to obliterate ruptured cerebral aneurysm is the standard therapy for aneurysmal SAH, and needs general anesthesia. Thus, this study suggests that isoflurane is useful as anesthetic agents during early aneurysm surgery or intervention to delay the development of EBI, although other strategies need to be developed to prevent neuronal injuries at later stages.
It needs to consider whether the concentration of isoflurane affects neuronal injuries after SAH. In this study, we used 2% isoflurane treatment since it is clinically relevant (8, 18). It has been reported that 1 MAC (1.4%) isoflurane is required to anesthetize 50% subjects in CD-1 mice (23). On the other hand, 1% isoflurane caused spatial learning impairment and neurodegeneration by increasing apoptosis, while 1.5 or 2% isoflurane had no adverse effects in mice (24). In cats, 3% isoflurane broke down the blood-brain barrier (25). Isoflurane’s neuroprotection may involve glutamate and/or -aminobutyric acid receptors (16), but the effects may be harmful on the developing brain (26) or by excessive exposure of isoflurane (27). Thus, further study is needed to find more effective dose and exposure time in the treatment of post-SAH brain injury. Another issue is whether isoflurane affects cerebral blood flow (CBF) after SAH. Recently, it was reported that 1.1–2.1% isoflurane dose-dependently increased CBF in rats (28). Increased CBF potentially suppresses ischemic neuronal injuries. But, we can assume that isoflurane has no or very little effect on changing the CBF given the sustained increase in intracranial pressure (29), which persists in endovascular perforation model that mimics clinical mechanism of artery rupture. However, effects of isoflurane on CBF in post-SAH brain remain undetermined.
This study failed to show isoflurane’s neuroprotective effects in terms of neurological scores and BWC at 72 hours post-SAH. However, we cannot exclude the possibility that these 2 tests could not detect isoflurane’s neuroprotective effects at 72 hours. More importantly, isoflurane treatment was conducted only once and we do not know if multiple treatments at different time courses will be effective. To address this issue, it will be worthwhile to perform other more detailed neurobehavioral tests at later stages with more than one treatment. In addition, isoflurane may have other protective effects that were not evaluated in this study. For example, immunofluorescence used in this study prevented us from assessing morphologically intact neurons and necrotic cell loss, which are potentially affected by isoflurane (30, 31). Also, isoflurane may suppress activation of microglia or astroglia, leading to less gliosis. These studies are beyond the score of this project and therefore warrant further investigation.
In conclusion, this study demonstrated that isoflurane posttreatment has an antiapoptotic effect on neurons at least for 24 hours post-SAH, and that this effect involves the sphingosine-related pathway. The identification of anesthetics with properties that help to delay or attenuate neuronal damage as a consequence of aneurysmal rupture is clinically highly relevant, and this study suggested that isoflurane is a good candidate for it. This is because the first step for intensive care of aneurysmal SAH patients is aneurysmal obliteration under general anesthesia. Further studies are warranted to elaborate the dose and duration of isoflurane treatment and to test the combination with other neuroprotectants for EBI after SAH.
Supplementary Material
Acknowledgments
Funding: This study is partially supported by NIH NS060936 to JT and NS053407 to JHZ.
Footnotes
The authors have not disclosed any potential conflict of interest.
Supplemental Digital Content 1. Supplemental Materials and Methods.docx.
1. Dose Determination and Preparation of Drugs, which shows dose determination and preparation of DMS and VPC23019.
2. Severity of SAH, which shows the SAH grading scale.
3. Neurological Scores, which shows modified Garcia’s score.
Contributor Information
Orhan Altay, Department of Physiology, Loma Linda University School of Medicine, Loma Linda, CA, 92354, USA.
Yu Hasegawa, Department of Physiology, Loma Linda University School of Medicine, Loma Linda, CA, 92354, USA.
Prativa Sherchan, Department of Physiology, Loma Linda University School of Medicine, Loma Linda, CA, 92354, USA.
Hidenori Suzuki, Department of Physiology, Loma Linda University School of Medicine, Loma Linda, CA, 92354, USA.
Nikan H. Khatibi, Department of Physiology, Loma Linda University School of Medicine, Loma Linda, CA, 92354, USA.
Jiping Tang, Department of Physiology, Loma Linda University School of Medicine, Loma Linda, CA, 92354, USA.
John H. Zhang, Department of Physiology and Neurosurgery, Loma Linda University School of Medicine, Loma Linda, CA, 92354, USA.
References
- 1.Van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain. 2001;124:249–278. doi: 10.1093/brain/124.2.249. [DOI] [PubMed] [Google Scholar]
- 2.Cahill J, Zhang JH. Subarachnoid hemorrhage: is it time for a new direction? Stroke. 2009;40:S86–S87. doi: 10.1161/STROKEAHA.108.533315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maceyka M, Payne SG, Milstein S, et al. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta. 2002;1585:193–201. doi: 10.1016/s1388-1981(02)00341-4. [DOI] [PubMed] [Google Scholar]
- 4.Dev KK, Mullerhausen F, Mattes H, et al. Brain sphingosine-1-phosphate receptors: implication for FTY720 in the treatment of multiple sclerosis. Pharmacol Ther. 2008;117:77–93. doi: 10.1016/j.pharmthera.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Antkowiak B. How do general anaesthetics work? Naturwissenschaften. 2001;88:201–213. doi: 10.1007/s001140100230. [DOI] [PubMed] [Google Scholar]
- 6.Kim M, Kim M, Kim N, et al. Isoflurane mediates protection from renal ischemia-reperfusion injury via sphingosine kinase and sphingosine-1-phosphate-dependent pathways. Am J Physiol Renal Physiol. 2007;293:F1827–1835. doi: 10.1152/ajprenal.00290.2007. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa Y, Suzuki H, Sozen T, et al. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke. 2010;41:368–374. doi: 10.1161/STROKEAHA.109.568899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Y, Lekic T, Fathali N, et al. Isoflurane posttreatment reduces neonatal hypoxic-ischemic brain injury in rats by the sphingosine-1-phosphate/phosphatidylinositol-3-kinase/Akt pathway. Stroke. 2010;41:1521–1527. doi: 10.1161/STROKEAHA.110.583757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis MD, Clemens JJ, Macdonald TL, et al. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem. 2005;280:9833–9841. doi: 10.1074/jbc.M412356200. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa Y, Suzuki H, Altay O, et al. Preservation of tropomyosin-related kinase B (TrkB) signaling by sodium orthovanadate attenuates early brain injury after subarachnoid hemorrhage in rats. Stroke. 2011;42:477–483. doi: 10.1161/STROKEAHA.110.597344. [DOI] [PubMed] [Google Scholar]
- 11.Hirt L, Badaut J, Thevenet J, et al. D-JNKI1, a cell-penetrating c-Jun-N-terminal kinase inhibitor, protects against cell death in severe cerebral ischemia. Stroke. 2004;35:1738–1743. doi: 10.1161/01.STR.0000131480.03994.b1. [DOI] [PubMed] [Google Scholar]
- 12.Sugawara T, Ayer R, Jadhav V, et al. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167:327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia JH, Liu KF, Ho KL. Neuronal necrosis after middle cerebral artery occlusion in Wistar rats progresses at different time intervals in the caudoputamen and the cortex. Stroke. 1995;26:636–642. doi: 10.1161/01.str.26.4.636. [DOI] [PubMed] [Google Scholar]
- 14.Zhang HP, Yuan LB, Zhao RN, et al. Isoflurane preconditioning induces neuroprotection by attenuating ubiquitin-conjugated protein aggregation in a mouse model of transient global cerebral ischemia. Anesth Analg. 2010;111:506–514. doi: 10.1213/ANE.0b013e3181e45519. [DOI] [PubMed] [Google Scholar]
- 15.Zhao P, Zuo Z. Isoflurane preconditioning induces neuroprotection that is inducible nitric oxide synthase-dependent in neonatal rats. Anesthesiology. 2004;101:695–702. doi: 10.1097/00000542-200409000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Kawaguchi M, Furuya H, Patel PM. Neuroprotective effects of anesthetic agents. J Anesth. 2005;19:150–156. doi: 10.1007/s00540-005-0305-5. [DOI] [PubMed] [Google Scholar]
- 17.Lee JJ, Li L, Jung HH, et al. Postconditioning with isoflurane reduced ischemia-induced brain injury in rats. Anesthesiology. 2008;108:1055–1062. doi: 10.1097/ALN.0b013e3181730257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiari PC, Bienengraeber MW, Pagel PS, et al. Isoflurane protects against myocardial infarction during early reperfusion by activation of phosphatidylinositol-3-kinase signal transduction: evidence for anesthetic induced postconditioning in rabbits. Anesthesiology. 2005;102:102–109. doi: 10.1097/00000542-200501000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Gao X, Zhang H, Steinberg G, Zhao H. The Akt pathway is involved in rapid ischemic tolerance in focal ischemia in rats. Translational Stroke Research. 2010;1:202–209. doi: 10.1007/s12975-010-0017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawaguchi M, Drummond JC, Cole DJ, et al. Effect of isoflurane on neuronal apoptosis in rats subjected to focal cerebral ischemia. Anesth Analg. 2004;98:798–805. doi: 10.1213/01.ane.0000105872.76747.f6. [DOI] [PubMed] [Google Scholar]
- 21.Inoue S, Drummond JC, Davis DP, Cole DJ, Patel PM. Combination of isoflurane and caspase inhibition reduces cerebral injury in rats subjected to focal cerebral ischemia. Anesthesiology. 2004;101:75–81. doi: 10.1097/00000542-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Mandala SM, Thornton R, Galve-Roperh I, et al. Molecular cloning and characterization of a lipid phosphohydrolase that degrades sphingosine-1- phosphate and induces cell death. Proc Natl Acad Sci. 2000;97:7859–7864. doi: 10.1073/pnas.120146897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lochhead KM, Zager RA. Fluorinated anesthetic exposure “activates” the renal cortical sphingomyelinase cascade. Kidney Int. 1998;54:373–381. doi: 10.1046/j.1523-1755.1998.00022.x. [DOI] [PubMed] [Google Scholar]
- 24.Valentim AM, Di Giminiani P, Ribeiro PO, et al. Lower isoflurane concentration affects spatial learning and neurodegeneration in adult mice compared with higher concentrations. Anesthesiology. 2010;113:1099–1108. doi: 10.1097/ALN.0b013e3181f79c7c. [DOI] [PubMed] [Google Scholar]
- 25.Tétrault S, Chever O, Sik A, et al. Opening of the blood-brain barrier during isoflurane anaesthesia. Eur J Neurosci. 2008;28:1330–1341. doi: 10.1111/j.1460-9568.2008.06443.x. [DOI] [PubMed] [Google Scholar]
- 26.Stratmann G, Sall JW, May LD, et al. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–848. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 27.Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masamoto K, Fukuda M, Vazquez A, et al. Dose-dependent effect of isoflurane on neurovascular coupling in rat cerebral cortex. Eur J Neurosci. 2009;30:242–250. doi: 10.1111/j.1460-9568.2009.06812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JY, Sagher O, Keep R, et al. Comparison of experimental rat models of early brain injury after subarachnoid hemorrhage. Neurosurgery. 2009;65:331–343. doi: 10.1227/01.NEU.0000345649.78556.26. [DOI] [PubMed] [Google Scholar]
- 30.Kim M, Kim M, Park SW, et al. Isoflurane protects human kidney proximal tubule cells against necrosis via sphingosine kinase and sphingosine-1-phosphate generation. Am J Nephrol. 2010;31:353–362. doi: 10.1159/000298339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bekker A, Shah R, Quartermain D, et al. Isoflurane preserves spatial working memory in adult mice after moderate hypoxia. Anesth Analg. 2006;102:1134–1138. doi: 10.1213/01.ane.0000198637.36539.c1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.