Abstract
Forkhead box P3 (FOXP3)-positive regulatory T cells (Treg) are a unique subset of T cells with immune regulatory properties. Treg cells can be induced from non-Treg CD4+ T cells (induced Treg, iTreg) by T cell receptor (TCR) triggering, IL-2 and TGF-β or retinoic acid. 1,25(OH)2 vitamin D3 (VD3) affects the functions of immune cells including T cells. 1,25(OH)2VD3 binds the nuclear vitamin D receptor (VDR) that binds target DNA sequences known as the vitamin D response element (VDRE). Although 1,25(OH)2VD3 can promote FOXP3 expression in CD4+ T cells with TCR triggering and IL-2, it is unknown whether this effect of 1,25(OH)2VD3 is mediated through direct binding of VDR to the FOXP3 gene without involving other molecules. Also, it is unclear whether FOXP3 expression in 1,25(OH)2VD3-induced Treg (VD-iTreg) cells is critical for the inhibitory function of these cells. Here we demonstrated the presence of VDREs in the intronic conserved non-coding sequence (CNS) region +1714 to +2554 of the human FOXP3 gene and the enhancement of the FOXP3 promoter activity by such VDREs in response to 1,25(OH)2VD3. In addition, VD-iTreg cells suppressed the proliferation of target CD4+ T cells and this activity was dependent on FOXP3 expression. These findings suggest that 1,25(OH)2VD3 can affect human immune responses by regulating FOXP3 expression in CD4+ T cells through direct VDR binding to the FOXP3 gene which is essential for inhibitory function of VD-iTreg cells.
Introduction
CD4+ T cells that express the transcriptional factor forkhead box P3 (FOXP3) are regulatory T (Treg) cells with the capacity to regulate immune responses (1). In addition to FOXP3, Treg cells express high levels of CD25 that serves as a cell surface marker for the identification of this cell subset (2, 3). FOXP3 is essential for the immune regulatory function of Treg cells. Transfection of FOXP3 into CD25− CD4+ T cells, which do not normally have regulatory function, conferred the immune regulatory property (4–6). Furthermore, mutations in the foxp3 gene have been found in scurfy mice with X-linked lymphoproliferative disease as well as in humans with immune dysregulation, polyendocrinopathy, enteropathy and X-linked syndrome (IPEX) (7, 8). While FOXP3+ Treg cells are normally generated in the thymus (naturally occuring Treg, nTreg), FOXP3+ Treg cells can also be induced from non-Treg CD4+ T cells (induced Treg, iTreg) in the presence of anti-CD3/CD28 Abs, IL-2 and TGF-β or retinoic acid (9–18). However, it has been controversial whether iTreg cells have inhibitory function in humans (9–14).
1,25-dihyroxyvitamin D3 (1,25(OH)2VD3), the most physiologically active VD3 metabolite, exerts an inhibitory effect on immune cells including T cells (15). Of interest, low levels of circulating 25(OH)VD3 (precursor of 1,25(OH)2VD3) are found in patients with autoimmune diseases including rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) (16–20), suggesting a potential role for this vitamin in autoimmunity. In fact, increased generation of FOXP3+CD4+ T cells was reported in mice treated with oral or topical 1,25(OH)2VD3 (21–23) although the exact mechanism(s) for this finding is yet to be determined. In humans, an increased percentage of FOXP3+CD4+ T cells was noticed in peripheral blood mononuclear cells (PBMCs) treated with 1,25(OH)2VD3 in vitro (24). Also, 1,25(OH)2VD3 enhanced FOXP3 expression in purified human CD25−CD4+ T cells in the presence of anti-CD3/CD28 antibodies (Abs) and IL-2 (25).
1,25(OH)2VD3 activates the vitamin D receptor (VDR) that heterodimerizes with retinoid X receptor (RXR) (15). The heterodimer then binds to its cognate DNA sequence known as vitamin D response elements (VDREs), leading to the regulation of gene expression. VDREs have two copies of a hexameric DNA sequence, referred to as core binding motif, with the consensus sequence RGKTSA (R = A or G, K = G or T, and S = C or G) (26). VDREs can be formed by a direct repeat (DR) of two hexameric core binding motifs with three (DR3 type, e.g. 5′ AGGTCA-NNN-AGGTCA 3′) or four (DR4) intervening nucleotides (26). Also, VDR can bind to everted repeat (ER or inverted palindrome)-type responding elements with 6, 8 or 9 spacing nucleotides (e.g. ER9 AGGTCA-(N)9-TGACCT) (26).
Despite the enhanced FOXP3 expression in CD4+ T cells by 1,25(OH)2VD3 and the capacity of this vitamin to regulate gene expression via direct binding to the genes, it is unknown whether 1,25(OH)2VD3 can directly induce FOXP3 gene expression without involving other molecules. In addition, it is unclear whether FOXP3 expressed in 1,25(OH)2VD3-treated CD25− CD4+ T cells is critical for the suppression of target T cells in that this vitamin can affect various functions of T cells such as cytokine production (15). Here we demonstrated the presence of VDREs in a conserved non-coding sequence (CNS) region that is analogous to the enhancer 1 of the mouse foxp3 gene (27). Such VDREs enhanced the FOXP3 promoter activity in the presence of 1,25(OH)2VD3. In addition, FOXP3+CD4+ T cells generated by a combination of 1,25(OH)2VD3, anti-CD3/CD28 Abs and IL-2 suppressed the proliferation of target CD4+ T cells dependent on FOXP3 and cell contact. These findings suggest that 1,25(OH)2VD3 can affect human immune responses by regulating FOXP3 expression in CD4+ T cells through direct VDR binding to the FOXP3 gene which is essential for inhibitory function of 1,25(OH)2VD3-induced FOXP3+ Treg (VD-iTreg) cells.
Materials and Methods
Cell preparation, culture and FOXP3 knockdown
This work was approved by the institutional review committee of Yale University. Human peripheral blood was obtained from the N.Y. Blood Center or healthy adult donors with informed consent. CD25− CD4+ T cells (>95% purity) were isolated from peripheral blood mononuclear cells using a cell purification kit (Miltenyi Biotec, Auburn, CA) or a FACSAria® (BD Bioscience, San Jose, CA). For induction of FOXP3 and T cell suppression assay, purified CD25−CD4+ T cells were suspended in RPMI 1640 medium with 10% FCS and stimulated for 5 days with 1,25(OH)2VD3 (10 nM or indicated, Sigma-Aldrich, St. Louis, MO), IL-2 (25 ng/ml, R&D Systems, Minneapolis, MN), anti-CD3 (4 μg/ml, OKT-3, National Cell Culture Center, Minneapolis, MN) and -CD28 Abs (3 μg/ml, BD Pharmingen, San Diego, CA). Cells (VD-iTreg cells) were then rested for 2 days and incubated for 5 additional days with autologous CD25−CD4+ T cells (target cells) that were stained with carboxy fluorescein diacetate succinimide ester (CFSE, Invitrogen, Carlsbad, CA) in a transwell culture system separating the two cell population or a regular culture plate in the presence of anti-CD3/CD28 Abs. To knock down FOXP3 expression, CD25−CD4+ T cells that were stimulated for 3 days with 1,25(OH)2VD3, anti-CD3/CD28 Abs and IL-2 were transfected with control or FOXP3-specific siRNA using the Amaxa® transfection system (Lonza, Walkersville, MD). Transfected cells were co-cultured for 5 days with CFSE-stained autologous CD25−CD4+ T cells in the presence of anti-CD3/CD28 Abs. Some transfected cells were stained with anti-human FOXP3 Abs (BioLegend, San Diego, CA) and analyzed on a flow cytometer. Target T cell proliferation was analyzed by flow cytometry.
Pull-down assay and immunobloting
Biotinylated DNA fragments containing putative VDRE1 or VDRE2/3 in the intronic CNS region +1714 – +2554 of the FOXP3 gene were amplified by PCR using sense (5′-biotin CTCCATATGTGGGTCCATGT-3′ for VDRE1; 5′-biotin TTTGCTGATTGTTGCTTTGC-3′ for VDRE2/3) and anti-sense primers (5′-biotin TAAAGAAGGGCAAGGTGCCA-3′; 5′-biotin CATGTGTGCGAGAGGAGG-3′ for VDRE2/3). The sequences in VDRE1, VDRE2 and VDRE3 were mutated using the GENEART Site-Directed Mutagenesis System (Invitrogen). Nuclear extracts from human CD25−CD4+ T cells that were treated for 5 days with 1,25(OH)2VD3 were obtained using a commercially available kit (Thermo Scientific, Loughborough, UK) and mixed with the biotinylated DNA fragments (27). Beads conjugated with streptoavidin were mixed with the DNA-nuclear extract reaction. VDR and RXR were detected by immunoblotting using anti-VDR and -RXR rabbit polyclonal Abs (Santa Cruz Biotechnology, Santa Cruz, CA) as previously described (27).
Chromatin Immunoprecipitation (ChIP) Assay
As previously described ChIP assay was done using a commercially available kit (Upstate Biotechnology, Inc., Lake Placid, NY). Briefly, human CD25−CD4+ T cells that were treated for 5 days with 1,25(OH)2VD3. Cells were then treated for 10 min with 1% formaldehyde at 37 °C and washed twice in ice-cold PBS containing protease inhibitors. Cell pellets were lysed in SDS lysis buffer and sonicated under conditions that reduced DNA length to between 200 and 1000 bp. The lysates were incubated overnight at 4 °C with anti-VDR rabbit polyclonal Abs and normal rabbit IgG antibody (both from Santa Cruz Biotechnology). Immune complexes were precipitated with protein G agarose (Upstate Biotechnology, Lake Placid, NY), and DNA was purified using spin columns. PCR was performed to detect VDRE1 and VDRE2/3 using the following primers: VDRE1, sense (5′-CTCCATATGTGGGTCCATGTCCAA-3′) and anti-sense (5′-TAAAGAAGGGCAAGGTGCCAGGGA-3′); and VDRE2/3, sense (5′-TTTGCTGATTGTTGCTTTGCAATA-3′) and anti-sense (5′-TGGACACATGCATGGAGAGCCAGA-3′). A 150-bp DNA fragment in the FOXP3 promoter region (−145 - +5) that did not contain a VDRE was amplified by PCR as a negative control using the following primers: sense (5′-ACCCCGTGATTATCAGCGCACACA-3′) and antisense (5′-CCTGGCTTGTGGGAAACTGTCACG-3′).
Reporter gene assay
The promoter (−511 – +176) and a highly CNS region (+1714 – +2554) of the FOXP3 gene were cloned into pGL3 basic vector (Promega, Madison, WI) followed by transfection to primary human CD25−CD4+ T cells which were stimulated for 5 days with anti-CD3/CD28 Abs and IL-2 in the presence or absence of 1,25(OH)2VD3 using the Amaxa transfection system (Lonza, Walkersville, MD) (27, 28). In some experiments, VDRE1, 2 and/or 3 sequences in the CNS region were mutated using the GENEART Site-Directed Mutagenesis System (Invitrogen) and inserted into the vector for FOXP3 gene reporter assay. Transfected cells were analyzed for luciferase activity and normalized against Renilla activity (Promega, Madison, WI).
Statistical analysis
A paired t-test was used to analyze data with a significance level of P < 0.05.
Results
Identification of putative VDREs in the human FOXP3 CNS region +1714 to +2554 that increase the FOXP3 promoter activity
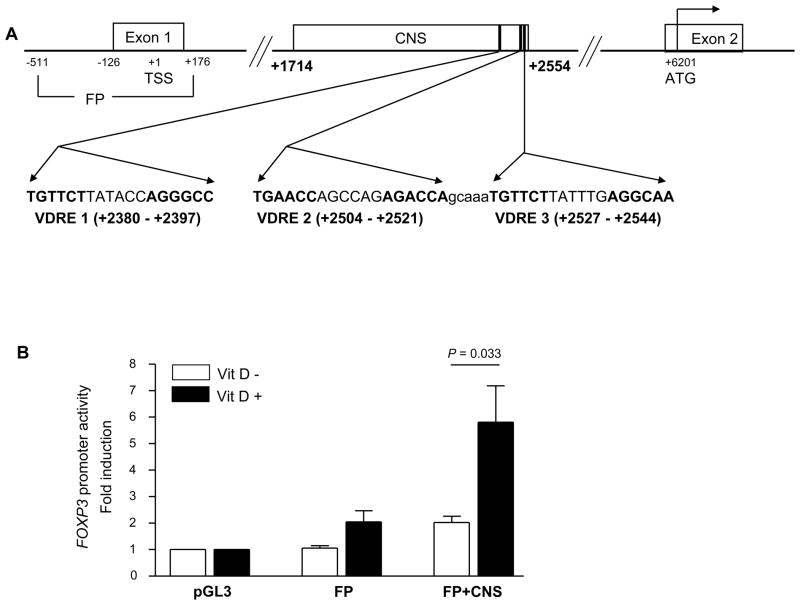
Previous studies reported enhanced induction of FOXP3 in FOXP3-negative CD25−CD4+ T cells by 1,25(OH)2VD3 in the presence of anti-CD3/CD28 Abs and IL-2 (25). However, it is unknown whether VDR binds directly to the FOXP3 gene, leading to such an up-regulation. We thus searched for potential binding sites of the VDR in the human FOXP3 gene in silico using Nubiscan (www.nubiscan.unibas.ch), an algorithm predicting nuclear receptor binding elements (29). We focused our search on the previously reported promoter (−511 ~ +176) and an intronic CNS region of the human FOXP3 gene (+1714 ~ +2554). This CNS region had been identified as an enhancer in the mouse foxp3 gene with the binding sites for Smad3 and NFAT that promoted the foxp3 gene expression (27, 28). Three potential response elements for VDR were identified in the CNS region of the human FOXP3 gene. These were ER6 types and designated as VDRE1, VDRE2 and VDRE3 (located at +2380 ~ +2397, +2504 ~ +2521 and +2527 ~ +2544 in the human FOXP3 gene, respectively) (Fig. 1A). We constructed a vector containing the FOXP3 promoter and the CNS region and then measured the promoter activity in human primary CD25−CD4+ T cells in the presence or absence of 1,25(OH)2VD3 (Fig. 1B). 1,25(OH)2VD3 increased the promoter activity in transfected CD25−CD4+ T cells in response to anti-CD3/CD28 Abs and IL-2. This suggests possible VDR binding to the putative VDREs in the CNS region.
Figure 1. Identification of putative vitamin D response elements (VDREs) in the intronic conserved non-sequencing (CNS) region +1714 to +2554 of the human FOXP3 gene that increases the FOXP3 promoter activity in response to 1,25(OH)2VD3.
(A) The locations and sequences of three putative VDREs in the intronic CNS region +1714 – +2554 of the human FOXP3 gene. The sequences of the putative VDREs are in bold. (B) The CNS region +1714 –+2554 increases the human FOXP3 promoter (FP, +151 – +176) activity in the presence of 1,25(OH)2VD3, anti-CD3/CD28 Abs and IL-2 as measured by luciferase reporter assay. Human primary CD25−CD4+ T cells were stimulated for 5 days with anti-CD3/CD28 Abs and IL-2 (25 ng/ml) in the presence or absence of 1,25(OH)2VD3 (Vit D, 10 nM) and transfected with pGL3 basic vector (PGL3), vector with an insert containing the FOXP3 promoter (FP), or vector containing the FOXP3 promoter and the CNS region +1714 – +2554 (FP+CNS). Cells were additionally incubated for 2 days in the presence of the same stimulation, with or without 1,25(OH)2VD3. Luciferase activity was measured and normalized against Renilla activity. Data represent the mean + SEM from 6 independent experiments.
VDR directly binds to VDRE1, 2, and 3 in the CNS region +1714 to +2554 of the FOXP3 gene
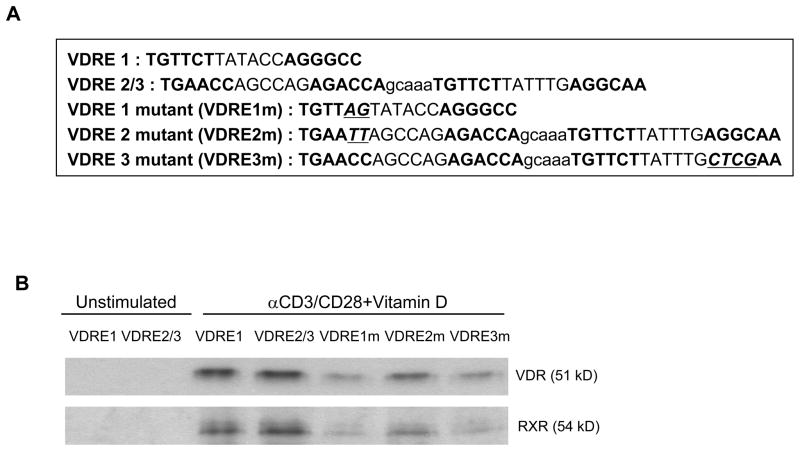
We next determined whether VDR and RXR could bind VDRE1, VDRE2 and VDRE3 using a pull-down assay. We constructed biotinylated DNA fragments that contained VDRE1, VDRE2/3, or mutated VDRE1, VDRE2 or VDRE3 (Fig. 2A). The biotinylated fragment for VDRE2/3 had both VDRE2 and 3 since these VDREs are separated by only five nucleotides. The constructed fragments were incubated with nuclear extracts of human CD25−CD4+ T cells treated with 1,25(OH)2VD3 and anti-CD3/CD28 Abs and pulled down with streptoavidin. Western blot analysis showed that the pull down with the fragment for VDRE1 or VDRE2/3 had VDR and RXR proteins while the pull down with the fragments containing mutated VDRE1, VDRE2 or VDRE3 had substantially decreased protein binding (Fig. 2B). These findings suggest VDR binding to VDRE 1 and VDRE2/3 in the CNS +1714 ~ +2554 of the human FOXP3 gene.
Figure 2. Pull-down assay shows the binding of the vitamin D receptor (VDR) to DNA fragments containing VDREs in the CNS region +1714 to +2554 of the human FOXP3 gene.
(A) Biotinylated DNA fragments that contained putative VDRE1 (+2326 – +2476) or VDRE2/3 (+2504 – +2521 and +2527 – +2544, respectively) in the intronic CNS region +1714 – +2554 of the FOXP3 gene were amplified by PCR. The sequences in VDRE1, VDRE2 and VDRE3 were mutated (VDRE1m, VDRE2m, VDRE3m) using the GENEART Site-Directed Mutagenesis System (Invitrogen). (B) Human CD25−CD4+ T cells were treated for 5 days with 1,25(OH)2VD3 (VD, 10 nM) in the presence of anti-CD3/CD28 Abs and IL-2. Nuclear extracts of the cells were mixed with biotinylated DNA fragments from (A). Beads conjugated with streptoavidin (Dynabeads M-280 Streptavidin Dynal Biotech) were mixed with the DNA-nuclear extracts. VDR and RXR were detected by immunoblotting. Representative data from 2 independent experiments.
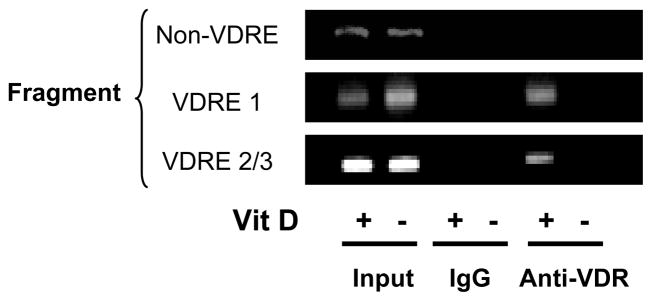
To further demonstrate VDR binding to VDRE1, 2, and 3, a chromatin immunoprecipitation (ChIP) assay was done using lysates of 1,25(OH)2VD3-treated CD25−CD4+ T cells. In the sample immunoprecipitated with anti-VDR Abs, we detected VDRE1 and VDRE2/3 by PCR (VDRE1 and VDRE2/3 fragments, respectively, Fig. 3). However, we did not detect a DNA sequence without a VDRE that exists outside the CNS (non-VDRE fragment, −145 ~ +5, negative control) in the same sample. These results of the ChIP assay further support VDR binding to VDRE1 and VDRE2/VDRE3 in the CNS +1714 to +2554 of the human FOXP3 gene.
Figure 3. Detection of VDR binding to VDRE1 and VDRE2/3 in the CNS region +1714 to +2554 of the human FOXP3 gene using chromatin immunoprecipitation (ChIP) Assay.
Human CD25−CD4+ T cells were treated for 5 days with or without 1,25(OH)2VD3 (Vit D, 10 nM) in the presence of anti-CD3/CD28 Abs and IL-2. Cell lysates were immunoprecipitated with anti-VDR Abs or control IgG. Immune complexes were collected, and PCR analysis was performed to determine the presence of VDRE1, VDRE2/3 and non-VDRE (−145 – +5, control) regions against input DNA. Representative data from 3 independent experiments.
VDRE1, 2 and 3 in the human FOXP3 CNS region +1714 to +2554 enhance the FOXP3 promoter activity in the presence of 1,25(OH)2VD3.
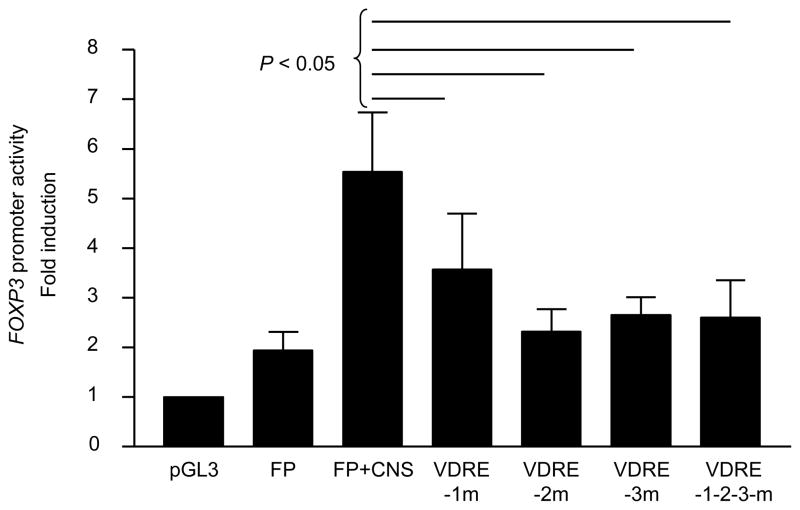
To determine the role for VDRE1, 2 and 3 in the CNS region in promoting the FOXP3 promoter activity, we constructed a vector containing the FOXP3 promoter and the CNS region with mutations in VDRE1, VDRE2 and/or VDRE3. The construct with mutated VDRE1, VDRE2 and/or VDRE3 had decreased FOXP3 promoter activity compared to the construct with the intact CNS region (Fig. 4). These findings indicate that VDRE1, VDRE2 and VDRE3 in the FOXP3 CNS region +1714 ~ + 2554 are essentially involved in promoting the FOXP3 promoter activity in the presence of 1,25(OH)2VD3.
Figure 4. VDRE1, 2 and 3 in the CNS region +1714 to +2554 of the FOXP3 gene are required for enhancing the FOXP3 promoter activity in the presence of 1,25(OH)2VD3.
Human primary CD25−CD4+ T cells were incubated for 5 days with anti-CD3/CD28 Abs and IL- 2 (25 ng/ml) in the presence of 1,25(OH)2VD3 (Vit D, 10 nM) and transfected with a pGL3 basic vector (PGL3), a vector with an insert containing the FOXP3 promoter (FP), or a vector containing the FOXP3 promoter and the CNS region +1714 – +2554 (FP+CNS) with intact or mutated VDRE1, 2 and/or 3 (VDRE1m, 2m, 3m). Cells were incubated for an additional 2 days in the presence of the same stimulation. Luciferase activity was measured and normalized against Renilla activity. Data represent the mean + SEM from 7 independent experiments.
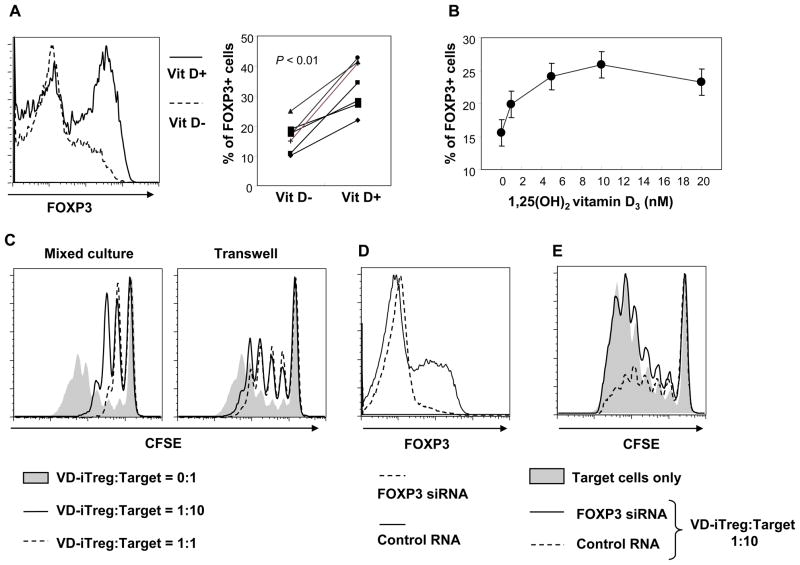
Suppression of CD4+ T cell proliferation by CD25−CD4+ T cells treated with 1,25(OH)2VD3, TCR triggering and IL-2 is dependent on FOXP3 and cell contact
Although a previous study reported the inhibitory function of FOXP3+ iTreg cells induced by the combination of 1,25(OH)2VD3, anti-CD3/CD28 Abs and IL-2 (25), it is still unknown whether FOXP3 is essential for this phenomenon. Thus, we induced FOXP3 in purified human CD25−CD4+ T cells by treating cells with anti-CD3/CD28 Abs, IL-2 and 1,25(OH)2VD3. As previously reported (25), FOXP3 expression was higher in the presence of 1,25(OH)2VD3 compared to the absence of this molecule (Fig. 5A). A dose-dependent effect of 1,25(OH)2VD3 on FOXP3 expression was observed (Fig. 5B). Also, CD25−CD4+ T cells stimulated with anti-CD3/CD28 Abs, IL-2 and 1,25(OH)2VD3 had increased expression of FOXP3 and VDR over time (Supplemental Figure 1). We then co-cultured iTreg cells induced by the combination of anti-CD3/CD28 Abs, IL-2 and 1,25(OH)2VD3 (VD-iTreg cells) with target CD25−CD4+ T cells. The former cells suppressed proliferation of the target cells (Fig. 5C, left panel). Such suppression was largely dependent on cell contact since separating the two cell populations during cell culture reduced the inhibitory effect of VD-iTreg on the target cells (Fig. 5C, right panel). To determine the direct role for FOXP3 in inhibiting the target cell proliferation, we next knocked down the FOXP3 gene in VD-iTreg cells using siRNA technique (Fig. 5D). VD-iTreg cells treated with FOXP-specific siRNA had a decreased inhibitory effect on the target cells compared to the same cells treated with control siRNA (Fig. 5E). These findings indicate that human VD-iTreg cells suppress proliferation of target CD4+ T cells and that this suppression is dependent on cell contact and FOXP3 expression.
Figure 5. 1,25(OH)2VD3 promotes FOXP3 expression in human CD25−CD4+ T cells in the presence of anti-CD3/CD28 Abs and IL-2, and these vitamin D-treated cells (VD-iTreg cells) suppress target T cell proliferation dependently of cell contact and FOXP3.
Human CD25−CD4+ T cells were purified from peripheral blood of healthy individuals. (A–B) Cells were treated for 5 days with 1,25(OH)2VD3 (10 nM or indicated), anti-CD3/CD28 Abs and IL-2 to generate 1,25(OH)2VD3-induced Treg (VD-iTreg) cells. Intracellular FOXP3 expression was determined by flow cytometry. (C) VD-iTreg cells were generated as in (A), rested for 2 days and then co-cultured for 5 days with CFSE-stained autologous CD25−CD4+ T cells (target) at different ratios in a transwell culture system separating the two cell populations or a regular culture plate in the presence of anti-CD3/CD28 Abs. (D–E) Cells were stimulated for 3 days with 1,25(OH)2VD3 (10 nM), anti-CD3/CD28 Abs and IL-2. Cells were then transfected with control or FOXP3-specific siRNA (Invitrogen, Stealth Select RN, HSS 121458) using electroporation (Amaxa Biosystems). (D) Cells were fixed, permeabilized and stained with Abs to FOXP3 or isotype controls and analyzed on a flow cytometer. (E) CD25−CD4+ T cells that were transfected with control or FOXP3-specific siRNA and treated with 1,25(OH)2VD3, anti-CD3/CD28 Abs and IL-2 were incubated for 5 days with CFSE-stained CD25−CD4+ T cells (target cells) in the presence of anti-CD3/CD28 Abs. Cells were analyzed on a flow cytometer. Representative data from 7 (A) or 3 (B–E) independent experiments (one experiment per donor).
Discussion
1,25(OH)2VD3 can affect the functions of immune cells including T cells (15). 1,25(OH)2VD3 binds the nuclear VDR that binds target DNA sequences known as VDRE. Although previous studies reported that 1,25(OH)2VD3 promoted FOXP3 expression in CD4+ T cells in the presence of anti-CD3/CD28 Abs and IL-2 (21–25), it is unknown whether this effect of 1,25(OH)2VD3 is mediated through direct binding of VDR to the FOXP3 gene without involving other molecules. Also, it is unclear whether FOXP3 expression in VD-iTreg cells is critical for the inhibitory function of these cells. Here we demonstrated the presence of VDREs in the intronic human FOXP3 CNS region +1714 to +2554 and the enhancement of the FOXP3 promoter activity by such VDREs in response to 1,25(OH)2VD3. Furthermore, VD-iTreg cells suppressed the proliferation of target CD4+ T cells, and this effect was dependent on FOXP3 expression and cell contact. These findings suggest that 1,25(OH)2VD3 can affect human immune responses by directly binding the FOXP3 gene and regulating its expression in CD4+ T cells and that FOXP3 is essential for the inhibitory function of VD-iTreg cells.
1,25(OH)2VD3 is a relatively small lipophilic molecule that can easily penetrate the cell membrane by simple diffusion and complex with VDR (15). The VDR then heterodimerizes with RXR and binds to VDREs in genes. In fact, 1,25(OH)2VD3 suppressed the expression of IL-2 and IFN-γ mRNA and protein in T cells by binding of VDR to the VDRE in the promoters of the IL2 and IFNG genes (30, 31). Previous studies reported increased FOXP3 expression in human and mouse T cells by treating with 1,25(OH)2VD3 (21–25). This phenomenon appeared to be secondary to the effect of this vitamin on T cells and DCs. 1,25(OH)2VD3–treated DCs induced FOXP3+ iTreg cells with suppressive activity (22) although the exact mechanism was not elucidated. In humans, increased expression of FOXP3 in CD4+ T cells was found in PBMCs treated with 1,25(OH)2VD3 (24). This effect was dependent on the production of indoleamine 2,3-dioxygenase (IDO) from DCs that could suppress immune responses and induce FOXP3+ Treg cells (32, 33). Similar to our finding, a combination of 1,25(OH)2VD3, IL-2 and anti-CD3/CD28 Abs synergistically up-regulated FOXP3 expression in human CD25−CD4+ T cells in the absence of other immune cells including DCs (25), demonstrating the direct effect of this vitamin on regulating FOXP3 expression in CD4+ T cells. Such a direct effect of 1,25(OH)2VD3 on CD4+ T cells could be secondary to altered expression of molecules that can regulate the FOXP3 promoter activity in CD4+ T cells. Alternatively, but not mutually exclusively, 1,25(OH)2VD3 could directly bind VDREs in the FOXP3 gene, leading to increased FOXP3 expression. Indeed, the results of our studies demonstrate that the intronic CNS region +1714 to +2554 of the FOXP3 gene have VDREs and that 1,25(OH)2VD3 promotes the FOXP3 gene promoter activity via directly binding to these VDREs.
In our study, we initially identified three putative VDREs in the CNS region +1714 to +2554 of the FOXP3 gene. These are VDRE1, 2 and 3 that are located at +2380 – +2397, +2504 – +2521 and +2527 – +2544, respectively. The results of our ChIP assay and pull down assay using DNA fragments containing these VDREs showed binding of VDR to VDRE1. Furthermore, mutating the sequence of VDRE1 reduced FOXP3 promoter activity in the presence of 1,25(OH)2VD3. These findings suggest the role for VDRE1 in promoting FOXP3 expression in human CD4+ T cells in response to 1,25(OH)2VD3. We also showed the binding of VDR to the region covering both VDRE2 and VDRE3. The results of the FOXP3 promoter assay indicate that both VDRE1 and VDRE2 are involved in enhancing FOXP3 promoter activity in the presence of 1,25(OH)2VD3 since mutating individual sequences reduced the promoter activity. These findings suggest that VDRE1, VDRE2 and VDRE3 are essential for the 1,25(OH)2VD3-mediated promotion of FOXP3 in human CD4+ T cells.
In our study, CD25−CD4+ T cells that were treated with 1,25(OH)2VD3 in the presence of anti-CD3/CD28 Abs and IL-2 suppressed proliferation of CD4+ T cells in a cell number dependent manner. Although a large number of these vitamin-treated CD4+ T cells expressed FOXP3, this molecule might not be necessary for the anti-proliferative effect. Of interest, cell to cell contact was required for suppressing the proliferation of target T cells by VD-iTreg cells, suggesting that soluble factor(s) are not critically involved in this phenomenon. To determine the specific role for FOXP3 in suppressing the proliferation of target T cells, we knocked down FOXP3 gene expression in CD25−CD4+ T cells treated with 1,25(OH)2VD3, anti-CD3/CD28 Abs and IL-2. Indeed, the FOXP3 knock down decreased the anti-proliferative function of such 1,25(OH)2VD3-treated cells. These findings indicate the indispensable role for FOXP3 in executing the suppressive function of VD-iTreg cells in humans.
In addition to inducing FOXP expression in non-Treg cells, 1,25(OH)2VD3 appears to have effect on nTreg cells that express high levels of CD25 and FOXP3. In fact, 1,25(OH)2VD3 decreased the proliferation of human nTreg cells in the presence of anti-CD3/CD28 antibodies and IL-2 (34). In the same study, nTreg cells treated with this vitamin had increased production of IL-10 without any effect on their activation status and inhibitory capacity. It is disputable that 1,25(OH)2VD3 could affect a small number of non-CD25−CD4+ T cells such as nTreg cells in our study since the purity of CD25−CD4+ T cells may not be 100%. However, we believe this possibility has no significant effect on our findings in that 1,25(OH)2VD3 suppressed the expansion of nTreg cells (34). A recent mouse study that conditionally targeted VDR in T cells showed no change in the frequency of Foxp3+ CD4+ T cells although intact VDR function in hematopoietic cells was necessary for 1,25(OH)2VD3 to inhibit experimental allergic encephalitis (EAE), a mouse model of multiple sclerosis (MS) (35). These findings suggest that 1,25(OH)2VD3 may act directly on pathogenic CD4+ T cells to suppress EAE.
Decreased blood levels of vitamin D were reported in patients with autoimmune diseases including type I diabetes mellitus (DM), SLE, RA, and MS (15, 20). Given the effects of 1,25(OH)2VD3 on immune cells, it is conceivable that vitamin D deficiency may have a role in the pathogenesis of autoimmunity. In fact, 1,25(OH)2VD3 or its analogues have been tried as treatments for autoimmune diseases. For instance, in a murine model of lupus, administration of 1,25(OH)2VD3 decreased proteinuria and prolonged life span (36, 37). Also, 1,25(OH)2VD3 prevented disease or reduced disease severity in animal models of MS and type I DM (38–40). Although this therapeutic effect of 1,25(OH)2VD3 appears to be mediated by several mechanisms, it could be associated with enhanced numbers and/or functions of Treg cells in that topical 1,25(OH)2VD3 or its analogue enhanced suppressive activity of Treg cells and induced the expansion of this cell subset (21–23). Of interest, decreased numbers and/or function of FOXP3+ Treg cells were reported in patients with MS, RA and SLE (41–46), raising a possible pathogenic association of vitamin D deficiency with quantitative and qualitative defects of FOXP3+ Treg cells in these patients. However, in humans, the trial of VD3 supplement for autoimmune diseases including MS and RA showed conflicting results (20). This controversial issue on the potential therapeutic effect of vitamin D administration in autoimmunity could be clarified with additional clinical studies.
Taken together, the results of our studies demonstrate the presence of VDREs in the intronic CNS region +1714 to +2544 of the human FOXP3 gene and the enhancement of the FOXP3 promoter activity by such VDREs in response to 1,25(OH)2VD3. In addition, VD-iTreg cells suppressed the proliferation of target CD4+ T cells, and this effect was dependent on FOXP3 expression and cell contact. These findings suggest that 1,25(OH)2VD3 can affect human immune responses by regulating FOXP3 expression in CD4+ T cells through direct VDR binding to the FOXP3 gene which is essential for the inhibitory function of VD-iTreg cells.
Supplementary Material
Acknowledgments
The source of support
This work was supported in part by grants from the National Institute of Health (AT005241, AG028069, AI075157, U19AI082713 all to IK).
We thank Dr. Alexia Belperron for critical review of this manuscript. Insoo Kang and Wan-Uk Kim are participants of the World Class University Program of Republic of Korea.
Abbreviations used in this article
- CNS
conserved non-coding sequence
- FOXP3
Forkhead box P3
- Treg
regulatory T cells
- iTreg
induced Treg
- VD
vitamin D
- VDR
vitamin D receptor
- VDRE
vitamin D response element
- nTreg
naturally occurring Treg
- SLE
systemic lupus erythematosus
- RA
rheumatoid arthritis
- RXR
retinoid X receptor
- DR
direct repeat
- ER
everted repeat
- ChIP
chromatin immunoprecipitation
- EAE
experimental allergic encephalitis
- MS
multiple sclerosis
- DM
diabetes mellitus
Footnotes
Disclosure. The authors have no competing financial interests in this work.
References
- 1.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 2.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Valencia X, Lipsky PE. CD4+CD25+FoxP3+ regulatory T cells in autoimmune diseases. Nat Clin Pract Rheumatol. 2007;3:619–626. doi: 10.1038/ncprheum0624. [DOI] [PubMed] [Google Scholar]
- 4.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 5.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 6.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 7.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 8.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 9.Pillai V, Ortega SB, Wang CK, Karandikar NJ. Transient regulatory T-cells: a state attained by all activated human T-cells. Clin Immunol. 2007;123:18–29. doi: 10.1016/j.clim.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 12.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol. 2007;179:3724–3733. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 13.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 14.Schambach F, Schupp M, Lazar MA, Reiner SL. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur J Immunol. 2007;37:2396–2399. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- 15.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008 doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huisman AM, White KP, Algra A, Harth M, Vieth R, Jacobs JW, Bijlsma JW, Bell DA. Vitamin D levels in women with systemic lupus erythematosus and fibromyalgia. J Rheumatol. 2001;28:2535–2539. [PubMed] [Google Scholar]
- 17.Muller K, Kriegbaum NJ, Baslund B, Sorensen OH, Thymann M, Bentzen K. Vitamin D3 metabolism in patients with rheumatic diseases: low serum levels of 25-hydroxyvitamin D3 in patients with systemic lupus erythematosus. Clin Rheumatol. 1995;14:397–400. doi: 10.1007/BF02207671. [DOI] [PubMed] [Google Scholar]
- 18.Kerr GS, Sabahi I, Richards JS, Caplan L, Cannon GW, Reimold A, Thiele GM, Johnson D, Mikuls TR. Prevalence of vitamin d insufficiency/deficiency in rheumatoid arthritis and associations with disease severity and activity. J Rheumatol. 2011;38:53–59. doi: 10.3899/jrheum.100516. [DOI] [PubMed] [Google Scholar]
- 19.Haque UJ, Bartlett SJ. Relationships among vitamin D, disease activity, pain and disability in rheumatoid arthritis. Clin Exp Rheumatol. 2010;28:745–747. [PubMed] [Google Scholar]
- 20.Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis. 2007;66:1137–1142. doi: 10.1136/ard.2007.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324:23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 22.Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, Adorini L. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106:3490–3497. doi: 10.1182/blood-2005-05-2044. [DOI] [PubMed] [Google Scholar]
- 23.Gorman S, Kuritzky LA, Judge MA, Dixon KM, McGlade JP, Mason RS, Finlay-Jones JJ, Hart PH. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+CD25+ cells in the draining lymph nodes. J Immunol. 2007;179:6273–6283. doi: 10.4049/jimmunol.179.9.6273. [DOI] [PubMed] [Google Scholar]
- 24.Correale J, Ysrraelit MC, Gaitan MI. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain. 2009;132:1146–1160. doi: 10.1093/brain/awp033. [DOI] [PubMed] [Google Scholar]
- 25.Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, Walker LS, Lammas DA, Raza K, Sansom DM. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlberg C, Seuter S. The vitamin D receptor. Dermatol Clin. 2007;25:515–523. viii. doi: 10.1016/j.det.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 28.Mantel PY, Ouaked N, Ruckert B, Karagiannidis C, Welz R, Blaser K, Schmidt-Weber CB. Molecular mechanisms underlying FOXP3 induction in human T cells. J Immunol. 2006;176:3593–3602. doi: 10.4049/jimmunol.176.6.3593. [DOI] [PubMed] [Google Scholar]
- 29.Turunen MM, Dunlop TW, Carlberg C, Vaisanen S. Selective use of multiple vitamin D response elements underlies the 1 alpha,25-dihydroxyvitamin D3-mediated negative regulation of the human CYP27B1 gene. Nucleic Acids Res. 2007;35:2734–2747. doi: 10.1093/nar/gkm179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alroy I, Towers TL, Freedman LP. Transcriptional repression of the interleukin-2 gene by vitamin D3: direct inhibition of NFATp/AP-1 complex formation by a nuclear hormone receptor. Mol Cell Biol. 1995;15:5789–5799. doi: 10.1128/mcb.15.10.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cippitelli M, Santoni A. Vitamin D3: a transcriptional modulator of the interferon-gamma gene. Eur J Immunol. 1998;28:3017–3030. doi: 10.1002/(SICI)1521-4141(199810)28:10<3017::AID-IMMU3017>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 32.Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, Mellor AL. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475–2483. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung DJ, Rossi M, Romano E, Ghith J, Yuan J, Munn DH, Young JW. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood. 2009;114:555–563. doi: 10.1182/blood-2008-11-191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khoo AL, Joosten I, Michels M, Woestenenk R, Preijers F, He XH, Netea MG, van der Ven AJ, Koenen HJ. 1,25-Dihydroxyvitamin D3 inhibits proliferation but not the suppressive function of regulatory T cells in the absence of antigen-presenting cells. Immunology. 2011;134:459–468. doi: 10.1111/j.1365-2567.2011.03507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayne CG, Spanier JA, Relland LM, Williams CB, Hayes CE. 1,25-Dihydroxyvitamin D3 acts directly on the T lymphocyte vitamin D receptor to inhibit experimental autoimmune encephalomyelitis. Eur J Immunol. 2011;41:822–832. doi: 10.1002/eji.201040632. [DOI] [PubMed] [Google Scholar]
- 36.Abe J, Nakamura K, Takita Y, Nakano T, Irie H, Nishii Y. Prevention of immunological disorders in MRL/l mice by a new synthetic analogue of vitamin D3: 22-oxa-1 alpha,25-dihydroxyvitamin D3. J Nutr Sci Vitaminol (Tokyo) 1990;36:21–31. doi: 10.3177/jnsv.36.21. [DOI] [PubMed] [Google Scholar]
- 37.Lemire JM, Ince A, Takashima M. 1,25-Dihydroxyvitamin D3 attenuates the expression of experimental murine lupus of MRL/l mice. Autoimmunity. 1992;12:143–148. doi: 10.3109/08916939209150321. [DOI] [PubMed] [Google Scholar]
- 38.Lemire JM, Archer DC. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest. 1991;87:1103–1107. doi: 10.1172/JCI115072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spach KM, Nashold FE, Dittel BN, Hayes CE. IL-10 signaling is essential for 1,25-dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:6030–6037. doi: 10.4049/jimmunol.177.9.6030. [DOI] [PubMed] [Google Scholar]
- 40.Mathieu C, Gysemans C, Giulietti A, Bouillon R. Vitamin D and diabetes. Diabetologia. 2005;48:1247–1257. doi: 10.1007/s00125-005-1802-7. [DOI] [PubMed] [Google Scholar]
- 41.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venken K, Hellings N, Thewissen M, Somers V, Hensen K, Rummens JL, Medaer R, Hupperts R, Stinissen P. Compromised CD4+ CD25(high) regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology. 2008;123:79–89. doi: 10.1111/j.1365-2567.2007.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huan J, Culbertson N, Spencer L, Bartholomew R, Burrows GG, Chou YK, Bourdette D, Ziegler SF, Offner H, Vandenbark AA. Decreased FOXP3 levels in multiple sclerosis patients. J Neurosci Res. 2005;81:45–52. doi: 10.1002/jnr.20522. [DOI] [PubMed] [Google Scholar]
- 44.Lyssuk EY, Torgashina AV, Soloviev SK, Nassonov EL, Bykovskaia SN. Reduced number and function of CD4+CD25highFoxP3+ regulatory T cells in patients with systemic lupus erythematosus. Adv Exp Med Biol. 2007;601:113–119. [PubMed] [Google Scholar]
- 45.Zhang B, Zhang X, Tang F, Zhu L, Liu Y. Reduction of forkhead box P3 levels in CD4(+)CD25(high) T cells in patients with new-onset systemic lupus erythematosus. Clin Exp Immunol. 2008 doi: 10.1111/j.1365-2249.2008.03686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.